CD4-Positive T-Cell Responses to MOG Peptides in MOG Antibody-Associated Disease
Abstract
1. Introduction
2. Results
2.1. Clinical Demographics of Study Participants
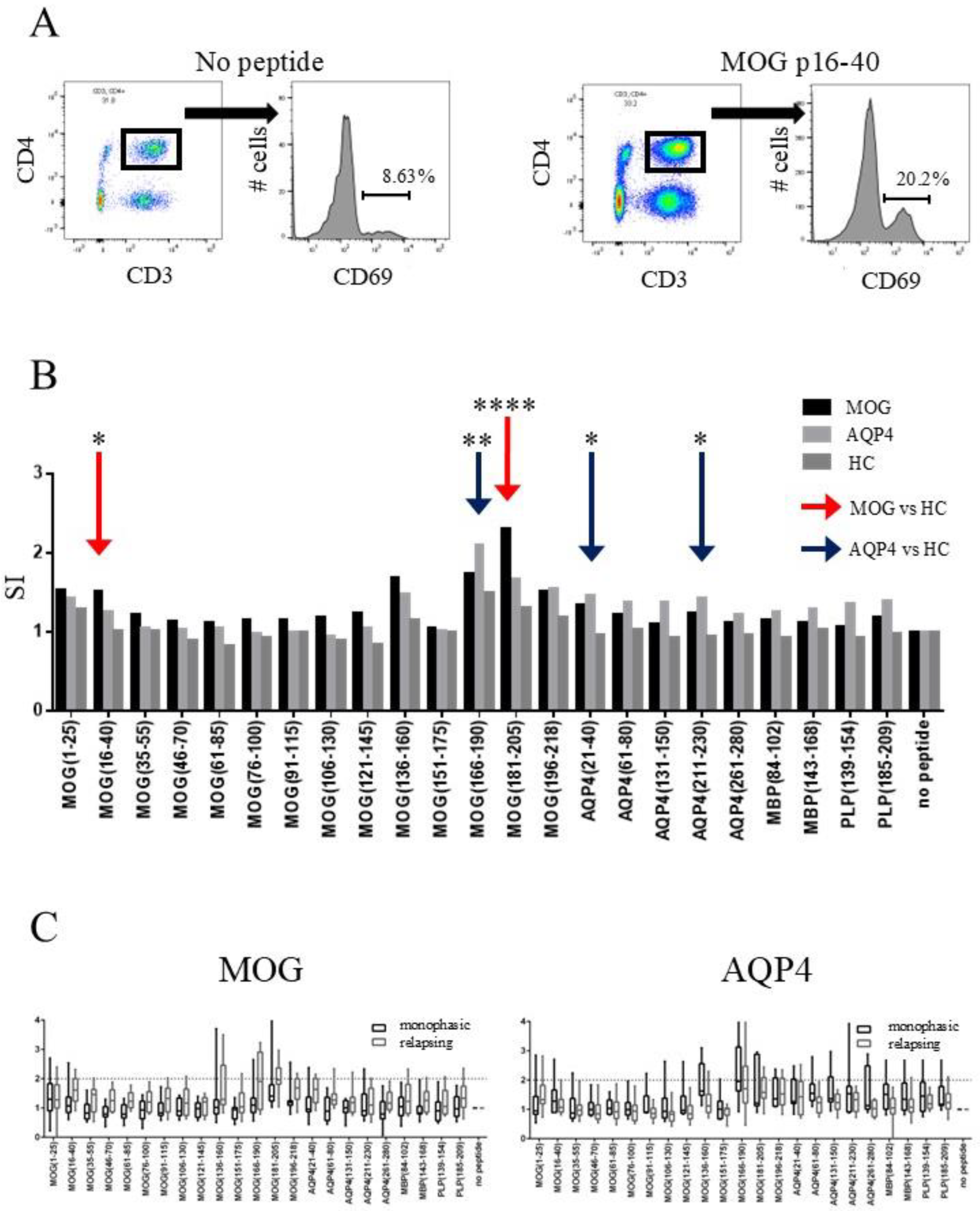
2.2. CD4-Positive T-Cells of MOGAD Patients Respond to MOG p16–40 and MOG p181–205
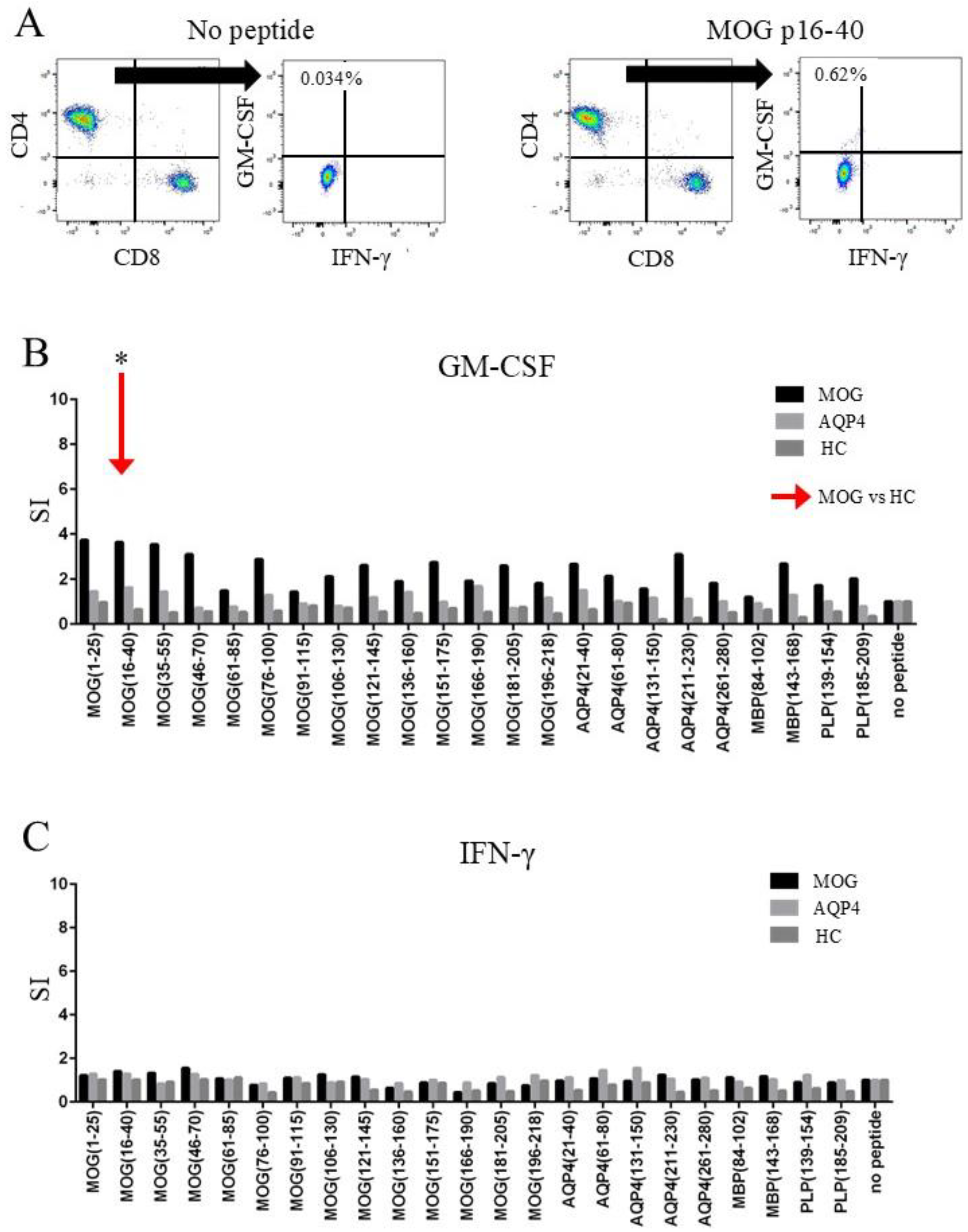
2.3. CD4+ T-Cells of MOGAD Patients Produced GM-CSF in Response to MOG p16–40
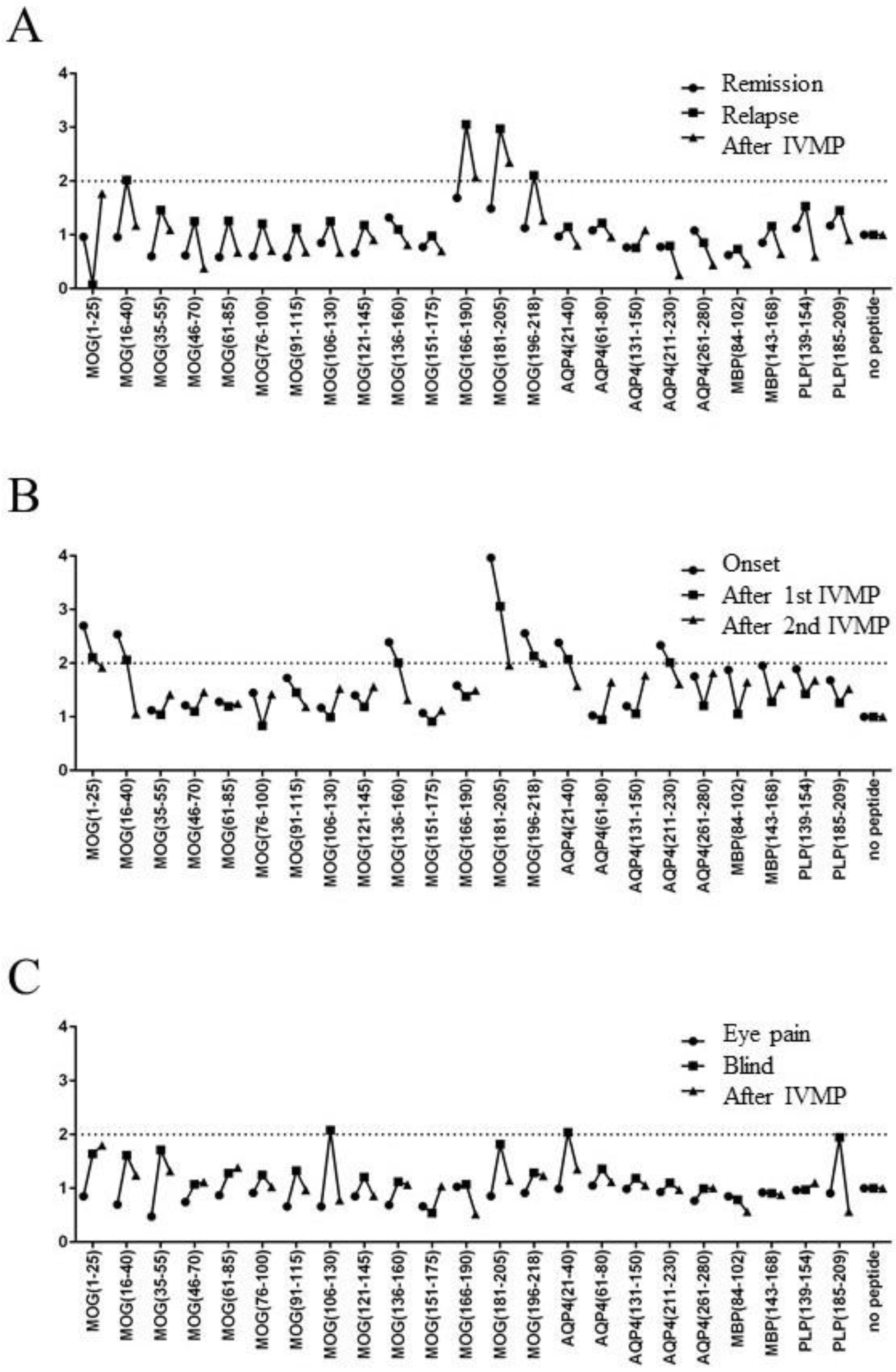
2.4. Relationship Between Disease Activity and T-Cell Response to MOG Peptides in Three Patients with MOGAD
3. Discussion
4. Methods
4.1. Selection of Patients and Controls
4.2. Standard Protocol Approval, Registration, and Patient Consent
4.3. Peptides
4.4. Cell Preparation and Culture
4.5. Flow Cytometry Analysis
4.6. Statistical Analysis of Flow Cytometry Data
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Johns, T.G.; Bernard, C.C.A. The Structure and Function of Myelin Oligodendrocyte Glycoprotein. J. Neurochem. 1999, 72, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Mayer, M.C.; Meinl, E. Glycoproteins as targets of autoantibodies in CNS inflammation: MOG and more. Ther. Adv. Neurol. Disord. 2012, 5, 147–159. [Google Scholar] [CrossRef] [PubMed]
- Waters, P.; Woodhall, M.; O’Connor, K.C.; Reindl, M.; Lang, B.; Sato, D.K.; Juryńczyk, M.; Tackley, G.; Rocha, J.; Takahashi, T.; et al. MOG cell-based assay detects non-MS patients with inflammatory neurologic disease. Neurol. Neuroimmunol. Neuroinflamm. 2015, 2, e89. [Google Scholar] [CrossRef]
- Reindl, M.; Waters, P. Myelin oligodendrocyte glycoprotein antibodies in neurological disease. Nat. Rev. Neurol. 2018, 15, 89–102. [Google Scholar] [CrossRef]
- Yandamuri, S.S.; Filipek, B.; Obaid, A.H.; Lele, N.; Thurman, J.M.; Makhani, N.; Nowak, R.J.; Guo, Y.; Lucchinetti, C.F.; Flanagan, E.P.; et al. MOGAD patient autoantibodies induce complement, phagocytosis, and cellular cytotoxicity. J. Clin. Investig. 2023, 8, e165373. [Google Scholar] [CrossRef]
- Huda, S.; Whittam, D.; Jackson, R.; Karthikeayan, V.; Kelly, P.; Linaker, S.; Mutch, K.; Kneen, R.; Woodhall, M.; Murray, K.; et al. Predictors of relapse in MOG antibody associated disease: A cohort study. BMJ Open 2021, 11, e055392. [Google Scholar] [CrossRef]
- Takai, Y.; Misu, T.; Kaneko, K.; Chihara, N.; Narikawa, K.; Tsuchida, S.; Nishida, H.; Komori, T.; Seki, M.; Komatsu, T.; et al. Myelin oligodendrocyte glycoprotein antibody-associated disease: An immunopathological study. Brain 2020, 143, 1431–1446. [Google Scholar] [CrossRef]
- Moseley, C.E.; Virupakshaiah, A.; Forsthuber, T.G.; Steinman, L.; Waubant, E.; Zamvil, S.S. MOG CNS Autoimmunity and MOGAD. Neurol. Neuroimmunol. Neuroinflamm. 2024, 11, e200275. [Google Scholar]
- Matsuya, N.; Komori, M.; Nomura, K.; Nakane, S.; Fukudome, T.; Goto, H.; Shiraishi, H.; Wandinger, K.-P.; Matsuo, H.; Kondo, T. Increased T-cell immunity against aquaporin-4 and proteolipid protein in neuromyelitis optica. Int. Immunol. 2011, 23, 565–573. [Google Scholar] [CrossRef]
- Varrin-Doyer, M.; Spencer, C.M.; Schulze-Topphoff, U.; Nelson, P.A.; Stroud, R.M.; Cree, B.A.C.; Zamvil, S.S. Aquaporin 4-specific T cells in neuromyelitis optica exhibit a Th17 bias and recognize Clostridium ABC transporter. Ann. Neurol. 2012, 72, 53–64. [Google Scholar] [CrossRef]
- Sospedra, M.; Martin, R. Immunology of Multiple Sclerosis. Annu. Rev. Immunol. 2005, 23, 683–747. [Google Scholar] [CrossRef] [PubMed]
- Varrin-Doyer, M.; Shetty, A.; Spencer, C.M.; Schulze-Topphoff, U.; Weber, M.S.; Bernard, C.C.; Forsthuber, T.; Cree, B.A.; Slavin, A.J.; Zamvil, S.S. MOG transmembrane and cytoplasmic domains contain highly stimulatory T-cell epitopes in MS. Neurol. Neuroimmunol. Neuroinflamm. 2014, 1, e20. [Google Scholar] [CrossRef]
- Kalman, B.; Lublin, F.D. The genetics of multiple sclerosis. A review. Biomed. Pharmacother. 1999, 53, 358–370. [Google Scholar] [CrossRef] [PubMed]
- Weissert, R.; Kuhle, J.; de Graaf, K.L.; Wienhold, W.; Herrmann, M.M.; Müller, C.; Forsthuber, T.G.; Wiesmüller, K.-H.; Melms, A. High Immunogenicity of Intracellular Myelin Oligodendrocyte Glycoprotein Epitopes. J. Immunol. 2002, 169, 548–556. [Google Scholar] [CrossRef] [PubMed]
- Koehler, N.K.U.; Genain, C.P.; Giesser, B.; Hauser, S.L. The Human T Cell Response to Myelin Oligodendrocyte Glycoprotein: A Multiple Sclerosis Family-Based Study. J. Immunol. 2002, 168, 5920–5927. [Google Scholar] [CrossRef]
- Raddassi, K.; Kent, S.C.; Yang, J.; Bourcier, K.; Bradshaw, E.M.; Seyfert-Margolis, V.; Nepom, G.T.; Kwok, W.W.; Hafler, D.A. Increased Frequencies of Myelin Oligodendrocyte Glycoprotein/MHC Class II-Binding CD4 Cells in Patients with Multiple Sclerosis. J. Immunol. 2011, 187, 1039–1046. [Google Scholar] [CrossRef]
- Lampasona, V.; Franciotta, D.; Furlan, R.; Zanaboni, S.; Fazio, R.; Bonifacio, E.; Comi, G.; Martino, G. Similar low frequency of anti-MOG IgG and IgM in MS patients and healthy subjects. Neurology 2004, 62, 2092–2094. [Google Scholar] [CrossRef]
- Sun, X.; Qiu, W.; Wang, J.; Wang, S.; Wang, Y.; Zhong, X.; Liu, C.; Cui, C.; Hong, H.; Yang, H.; et al. Myelin oligodendrocyte glycoprotein-associated disorders are associated with HLA subtypes in a Chinese paediatric-onset cohort. J. Neurol. Neurosurg. Psychiatry 2020, 91, 733–739. [Google Scholar] [CrossRef]
- Hofer, L.S.; Ramberger, M.; Gredler, V.; Pescoller, A.S.; Rostásy, K.; Sospedra, M.; Hegen, H.; Berger, T.; Lutterotti, A.; Reindl, M. Comparative Analysis of T-Cell Responses to Aquaporin-4 and Myelin Oligodendrocyte Glycoprotein in Inflammatory Demyelinating Central Nervous System Diseases. Front. Immunol. 2020, 11, 1188. [Google Scholar] [CrossRef]
- Lotfi, N.; Thome, R.; Rezaei, N.; Zhang, G.-X.; Rezaei, A.; Rostami, A.; Esmaeil, N. Roles of GM-CSF in the Pathogenesis of Autoimmune Diseases: An Update. Front. Immunol. 2019, 10, 1265. [Google Scholar] [CrossRef]
- Mcqualter, J.L.; Darwiche, R.; Ewing, C.; Onuki, M.; Kay, T.W.; Hamilton, J.A.; Reid, H.H.; Bernard, C.C. Granulocyte Macrophage Colony-stimulating Factor: A New Putative Therapeutic Target in Multiple Sclerosis. J. Exp. Med. 2001, 194, 873–881. [Google Scholar]
- Ponomarev, E.D.; Shriver, L.P.; Maresz, K.; Pedras-Vasconcelos, J.; Verthelyi, D.; Dittel, B.N. GM-CSF Production by Autoreactive T Cells Is Required for the Activation of Microglial Cells and the Onset of Experimental Autoimmune Encephalomyelitis. J. Immunol. 2007, 178, 39–48. [Google Scholar] [CrossRef] [PubMed]
- Kaneko, K.; Sato, D.K.; Nakashima, I.; Ogawa, R.; Akaishi, T.; Takai, Y.; Nishiyama, S.; Takahashi, T.; Misu, T.; Kuroda, H.; et al. CSF cytokine profile in MOG-IgG+ neurological disease is similar to AQP4-IgG+ NMOSD but distinct from MS: A cross-sectional study and potential therapeutic implications. J. Neurol. Neurosurg. Psychiatry 2018, 89, 927–936. [Google Scholar] [CrossRef]
- Lassmann, H.; Brunner, C.; Bradl, M.; Linington, C. Experimental allergic encephalomyelitis: The balance between encephalitogenic T lymphocytes and demyelinating antibodies determines size and structure of demyelinated lesions. Acta Neuropathol. 1988, 75, 566–576. [Google Scholar] [CrossRef]
- Bettelli, E.; Baeten, D.; Jäger, A.; Sobel, R.A.; Kuchroo, V.K. Myelin oligodendrocyte glycoprotein–specific T and B cells cooperate to induce a Devic-like disease in mice. J. Clin. Investig. 2006, 116, 2393–2402. [Google Scholar] [CrossRef] [PubMed]
- Krishnamoorthy, G.; Lassmann, H.; Wekerle, H.; Holz, A. Spontaneous opticospinal encephalomyelitis in a double-transgenic mouse model of autoimmune T cell/B cell cooperation. J. Clin. Investig. 2006, 116, 2385–2392. [Google Scholar] [CrossRef]
- Winklmeier, S.; Schlüter, M.; Spadaro, M.; Thaler, F.S.; Vural, A.; Gerhards, R.; Macrini, C.; Mader, S.; Kurne, A.; Inan, B.; et al. Identification of circulating MOG-specific B cells in patients with MOG antibodies. Neurol. Neuroimmunol. NeuroInflamm. 2019, 6, e625. [Google Scholar]
- The Australasian and New Zealand MOG Study Group; Tea, F.; Lopez, J.A.; Ramanathan, S.; Merheb, V.; Lee, F.X.Z.; Zou, A.; Pilli, D.; Patrick, E.; van der Walt, A.; et al. Characterization of the human myelin oligodendrocyte glycoprotein antibody response in demyelination. Acta Neuropathol. Commun. 2019, 7, 145. [Google Scholar] [CrossRef]
- Ota, K.; Matsui, M.; Edgar, L.M.; Mackin, G.A.; Weiner, H.L.; Hafler, D.A. T-cell recognition of an immunodominant myelin basic protein epitope in multiple sclerosis. Nature 1990, 346, 183–187. [Google Scholar] [CrossRef]
- Jiang, W.; Birtley, J.R.; Hung, S.-C.; Wang, W.; Chiou, S.-H.; Macaubas, C.; Kornum, B.; Tian, L.; Huang, H.; Adler, L.; et al. In vivo clonal expansion and phenotypes of hypocretin-specific CD4+ T cells in narcolepsy patients and controls. Nat. Commun. 2019, 10, 5247. [Google Scholar] [CrossRef]
- Banwell, B.; Bennett, J.L.; Marignier, R.; Kim, H.J.; Brilot, F.; Flanagan, E.P.; Ramanathan, S.; Waters, P.; Tenembaum, S.; Graves, J.S.; et al. Diagnosis of myelin oligodendrocyte glycoprotein antibody-associated disease: International MOGAD Panel proposed criteria. Lancet Neurol. 2023, 22, 268–282. [Google Scholar] [CrossRef] [PubMed]
- Wingerchuk, D.M.; Banwell, B.; Bennett, J.L.; Cabre, P.; Carroll, W.; Chitnis, T.; De Seze, J.; Fujihara, K.; Greenberg, B.; Jacob, A.; et al. International consensus diagnostic criteria for neuromyelitis optica spectrum disorders. Neurology 2015, 85, 177–189. [Google Scholar] [CrossRef] [PubMed]
- Sato, D.K.; Callegaro, D.; Lana-Peixoto, M.A.; Waters, P.J.; de Haidar Jorge, F.M.; Takahashi, T.; Nakashima, I.; Apostolos-Pereira, S.L.; Talim, N.; Simm, R.F.; et al. Distinction between MOG antibody-positive and AQP4 antibody-positive NMO spectrum disorders. Neurology 2014, 82, 474–481. [Google Scholar] [CrossRef] [PubMed]
- Zeka, B.; Hastermann, M.; Hochmeister, S.; Kögl, N.; Kaufmann, N.; Schanda, K.; Mader, S.; Misu, T.; Rommer, P.; Fujihara, K.; et al. Highly encephalitogenic aquaporin 4-specific T cells and NMO-IgG jointly orchestrate lesion location and tissue damage in the CNS. Acta Neuropathol. 2015, 130, 783–798. [Google Scholar] [CrossRef]
| MOGAD (n = 24) | NMOSD (n = 20) | HC (n = 17) | |
|---|---|---|---|
| sex ratio (male/female) | 12:12 | 1:19 | 3:14 |
| age, years (mean ± SD) | 42.1 ± 13.2 | 57.0 ± 12.1 | 33.8 ± 4.4 |
| disease duration, year, median (range) | 5.1 (0.1–27) | 12.2 (0.1–25) | - |
| number of attacks, median (range) | 2.1 (1–5) | 2.7 (1–13) | - |
| time interval between blood sampling and last attack, year, median (range) | 1.0 (0–14) | 6.5 (1–14) | - |
| number of patients under oral steroid treatment | 11 | 19 | - |
| dose of oral steroid, mg/day, median | 0 | 5.0 | - |
| immunosuppressive treatment | AZA (n = 3), MTX (n = 1), mesalazine (n = 1) | AZA (n = 1) | - |
| Peptides | Sequence | AA Length | |
|---|---|---|---|
| MOG | MOG (1–25) | GQFRVIGPRHPIRALVGDEVELPCR | 25 |
| MOG (16–40) | VGDEVELPCRISPGKNATGMEVGWY | 25 | |
| MOG (35–55) | MEVGWYRPPFSRVVHLYRNGK | 21 | |
| MOG (46–70) | RVVHLYRNGKDQDGDQAPEYRGRTE | 25 | |
| MOG (61–85) | QAPEYRGRTELLKDAIGEGKVTLRI | 25 | |
| MOG (76–100) | IGEGKVTLRIRNVRFSDEGGFTCFF | 25 | |
| MOG (91–115) | SDEGGFTCFFRDHSYQEEAAMELKV | 25 | |
| MOG (106–130) | QEEAAMELKVEDPFYWVSPGVLVLL | 25 | |
| MOG (121–145) | WVSPGVLVLLAVLPVLLLQITVGLV | 25 | |
| MOG (136–160) | LLLQITVGLVFLCLQYRLRGKLRAE | 25 | |
| MOG (151–175) | YRLRGKLRAEIENLHRTFDPHFLRV | 25 | |
| MOG (166–190) | RTFDPHFLRVPCWKITLFVIVPVLG | 25 | |
| MOG (181–205) | TLFVIVPVLGPLVALIICYNWLHRR | 25 | |
| MOG (196–218) | IICYNWLHRRLAGQFLEELRNPF | 23 | |
| AQP4 | AQP4 (21–40) | NIMVAFKGVWTQAFWKAVTA | 20 |
| AQP4 (61–80) | GTEKPLPVDMVLISLCFGLS | 20 | |
| AQP4 (131–150) | GILYLVTPPSVVGGLGVTMV | 20 | |
| AQP4 (211–230) | SMNPARSFGPAVIMGNWENH | 20 | |
| AQP4 (261–280) | RFKEAFSKAAQQTKGSYMEV | 20 | |
| PLP | PLP (139–154) | HCLGKWLGHPDKFVGI | 16 |
| PLP (185–209) | SIAFPSKTSASIGSLCADARMYGVL | 25 | |
| MBP | MBP (84–102) | DENPVVHFFKNIVTPRTPP | 19 |
| MBP (143–168) | FKGVDAQGTLSKIFKLGGRD | 20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ono, H.; Misu, T.; Namatame, C.; Matsumoto, Y.; Takai, Y.; Nishiyama, S.; Kuroda, H.; Takahashi, T.; Nakashima, I.; Fujihara, K.; et al. CD4-Positive T-Cell Responses to MOG Peptides in MOG Antibody-Associated Disease. Int. J. Mol. Sci. 2025, 26, 3606. https://doi.org/10.3390/ijms26083606
Ono H, Misu T, Namatame C, Matsumoto Y, Takai Y, Nishiyama S, Kuroda H, Takahashi T, Nakashima I, Fujihara K, et al. CD4-Positive T-Cell Responses to MOG Peptides in MOG Antibody-Associated Disease. International Journal of Molecular Sciences. 2025; 26(8):3606. https://doi.org/10.3390/ijms26083606
Chicago/Turabian StyleOno, Hirohiko, Tatsuro Misu, Chihiro Namatame, Yuki Matsumoto, Yoshiki Takai, Shuhei Nishiyama, Hiroshi Kuroda, Toshiyuki Takahashi, Ichiro Nakashima, Kazuo Fujihara, and et al. 2025. "CD4-Positive T-Cell Responses to MOG Peptides in MOG Antibody-Associated Disease" International Journal of Molecular Sciences 26, no. 8: 3606. https://doi.org/10.3390/ijms26083606
APA StyleOno, H., Misu, T., Namatame, C., Matsumoto, Y., Takai, Y., Nishiyama, S., Kuroda, H., Takahashi, T., Nakashima, I., Fujihara, K., & Aoki, M. (2025). CD4-Positive T-Cell Responses to MOG Peptides in MOG Antibody-Associated Disease. International Journal of Molecular Sciences, 26(8), 3606. https://doi.org/10.3390/ijms26083606





