Metabolomics and Antioxidant Activity of Valonea from Quercus variabilis Produced in Different Geographical Regions in China
Abstract
1. Introduction
2. Results and Discussion
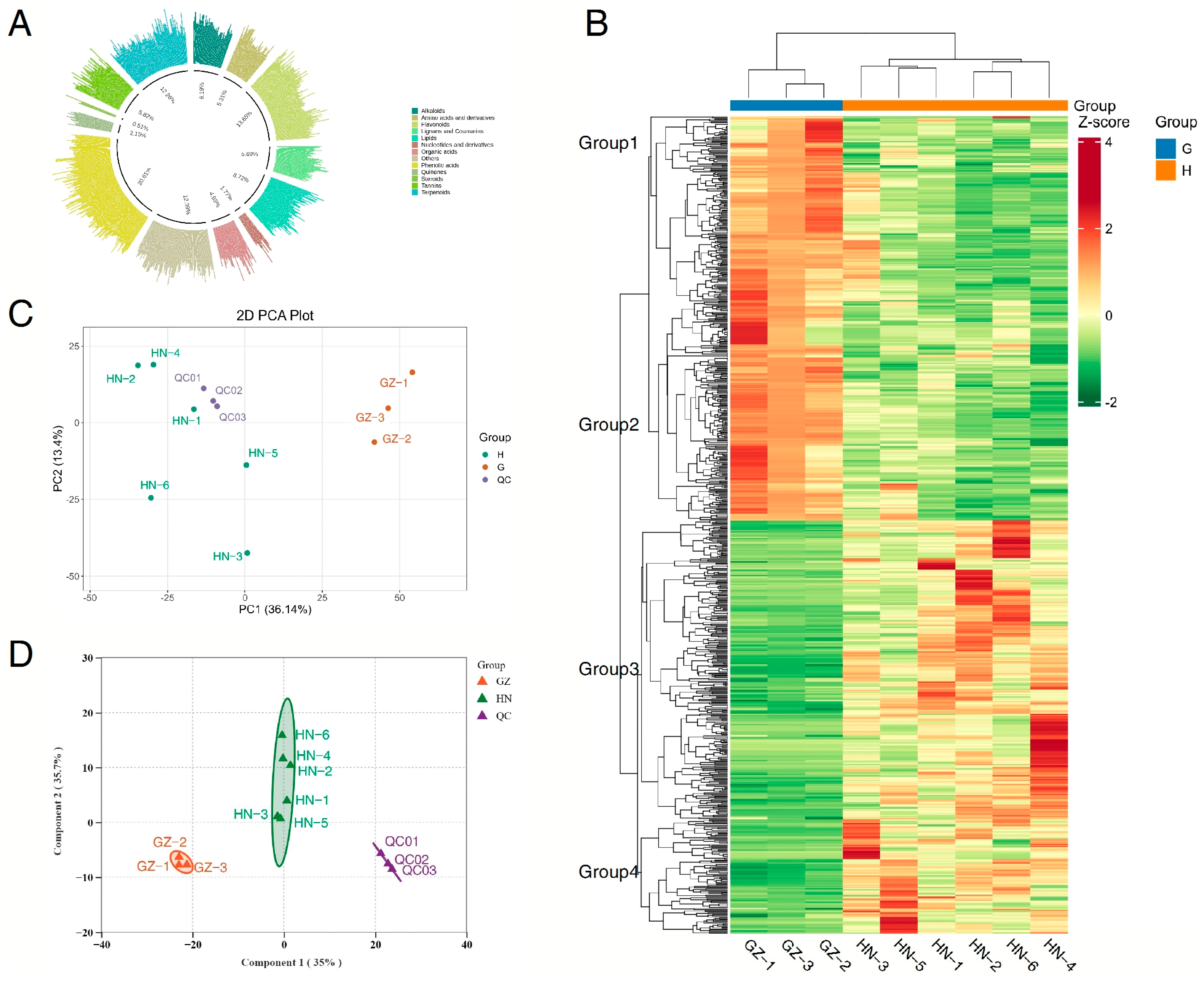
2.1. An Overview of the Metabolites
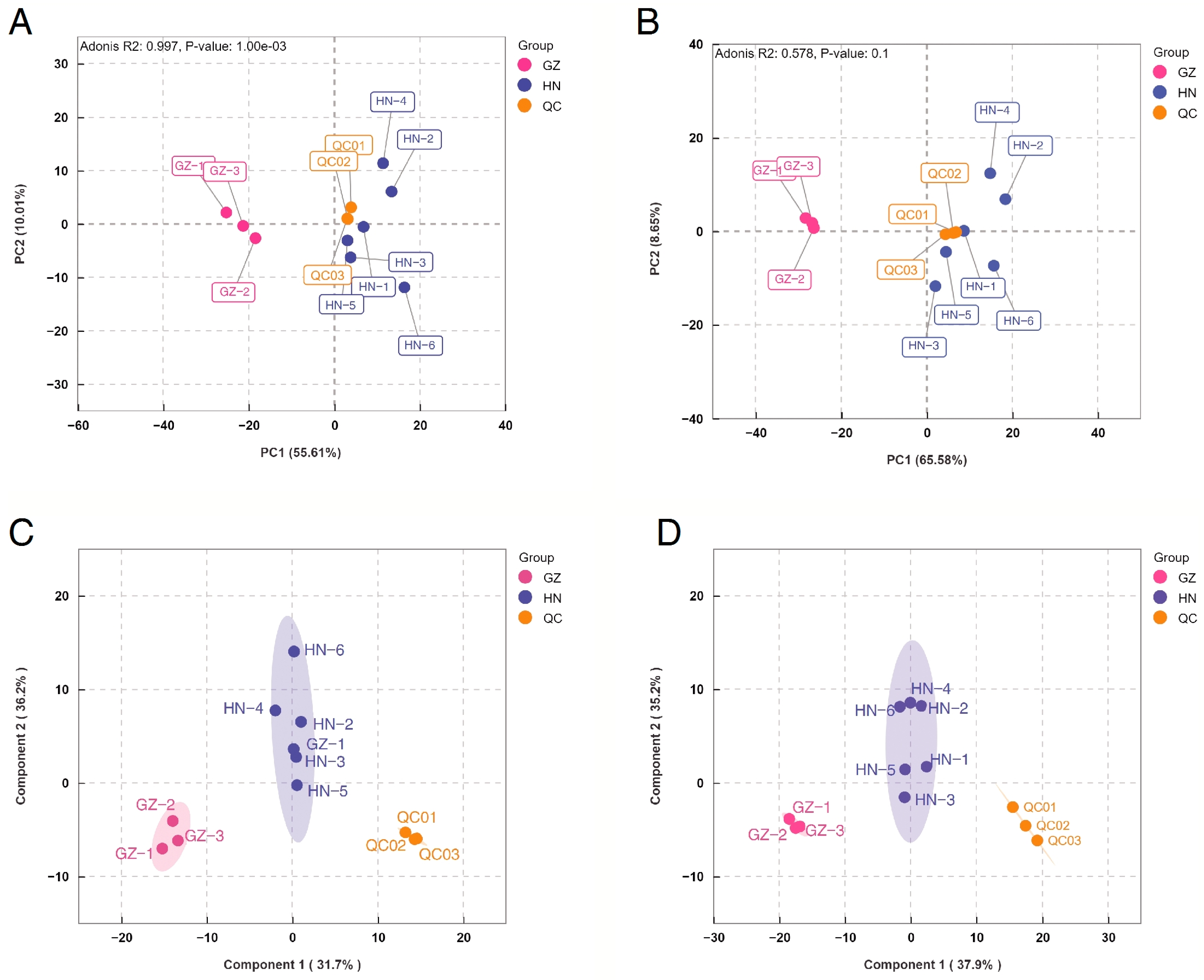
2.2. PCA and OPLS-DA of Samples from the Two Regions
2.3. HCA of Valonea Samples from the Three Regions
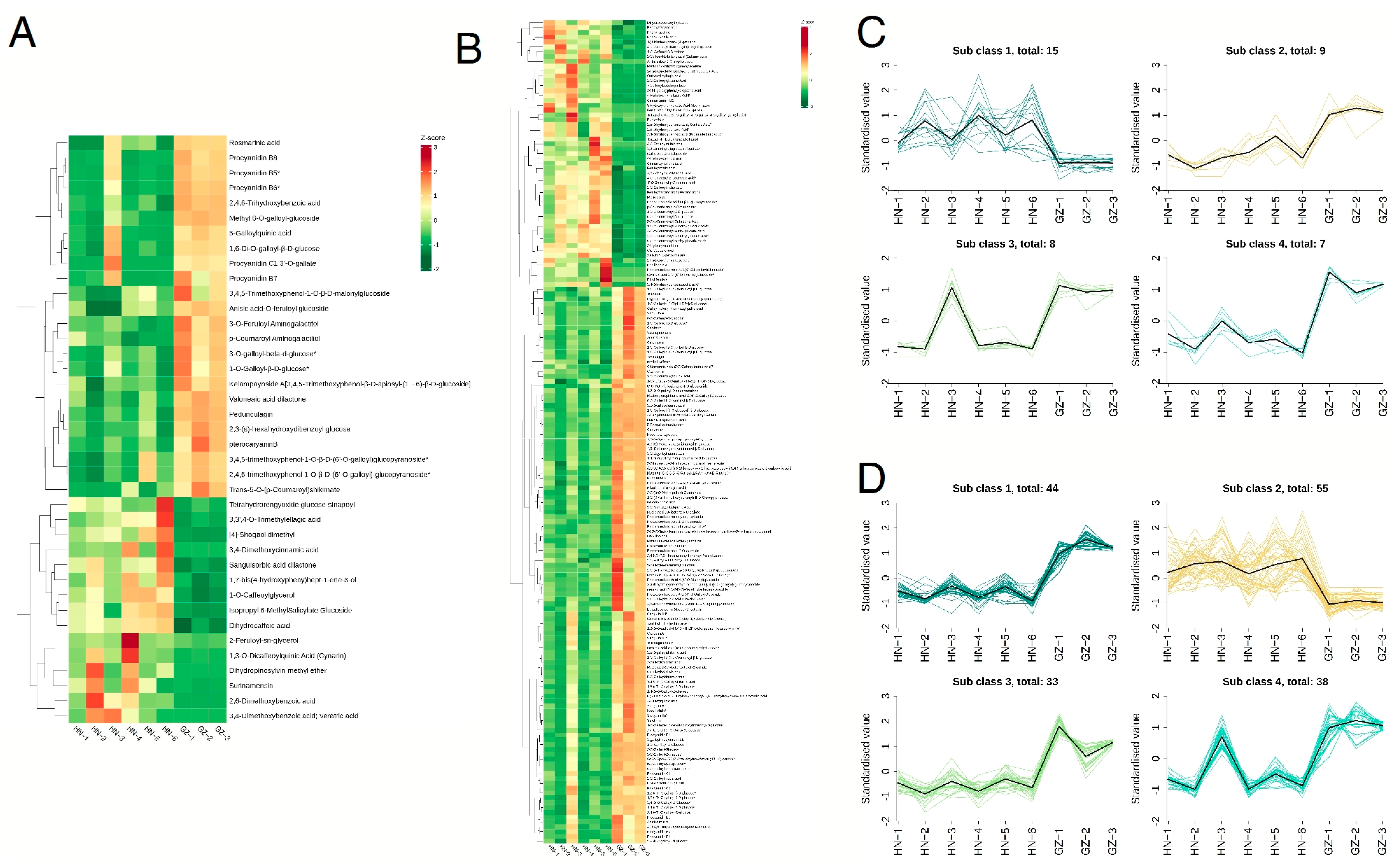
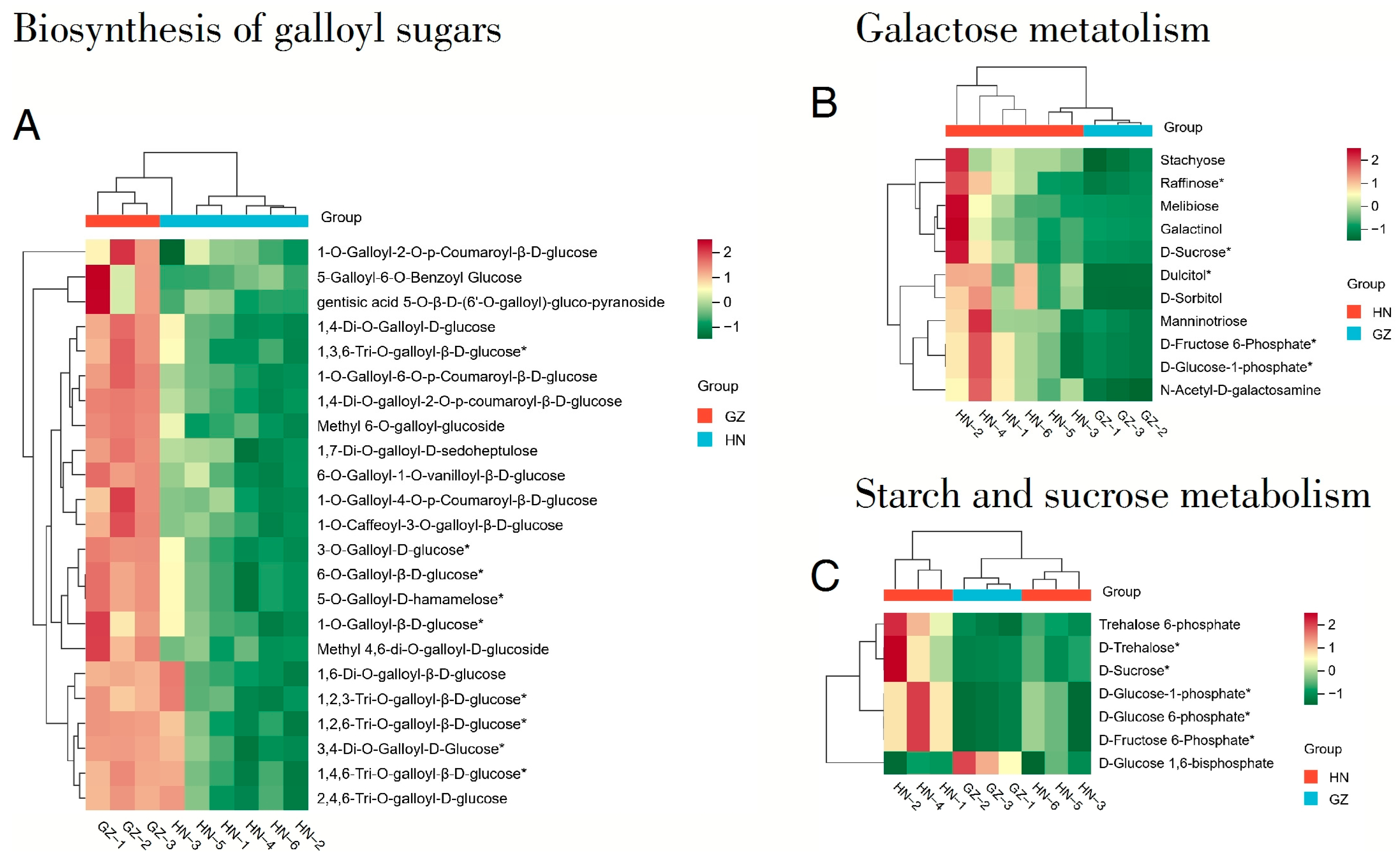
2.4. Identification and Analysis of Candidate Differential Metabolites
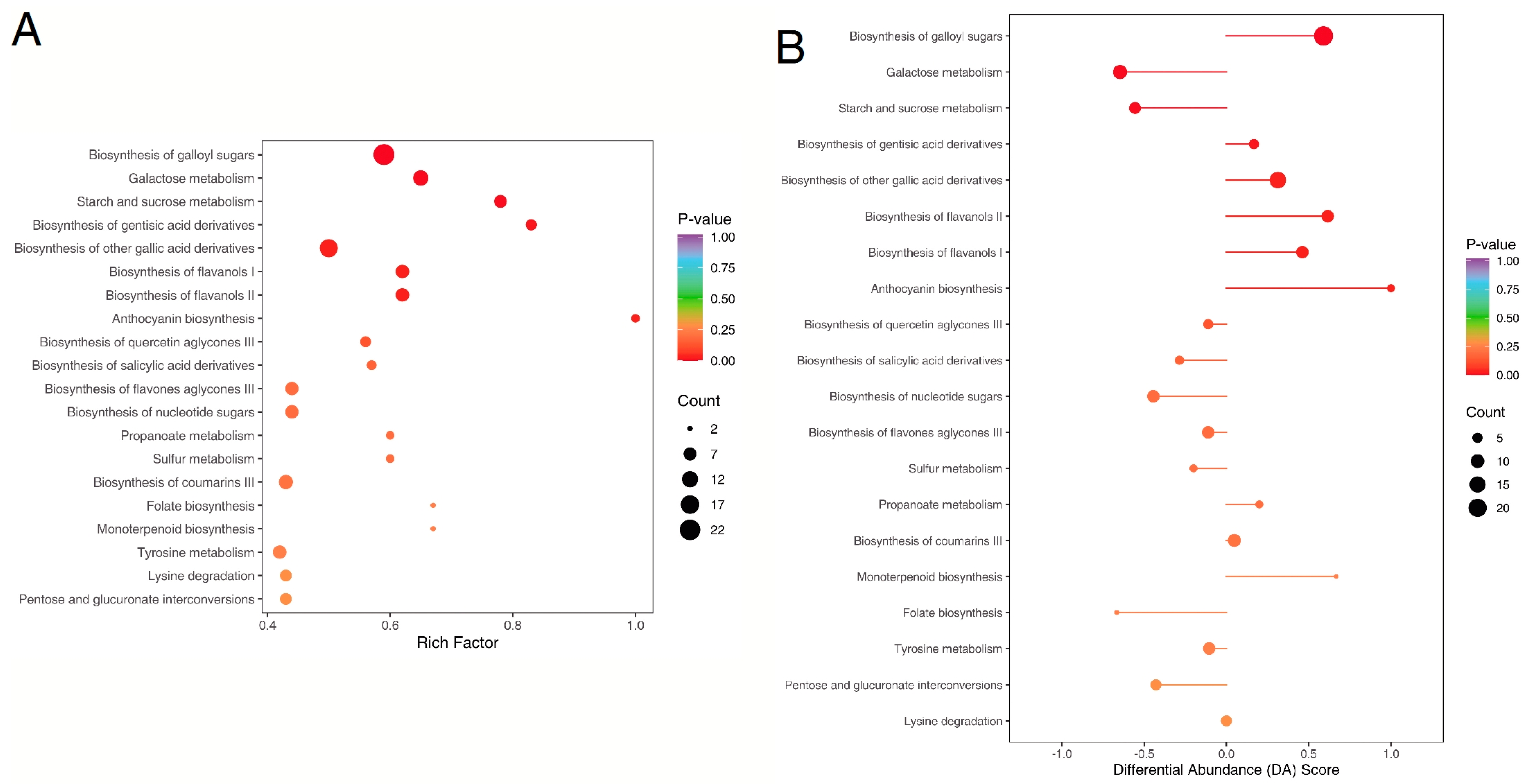
2.5. KEGG Annotation
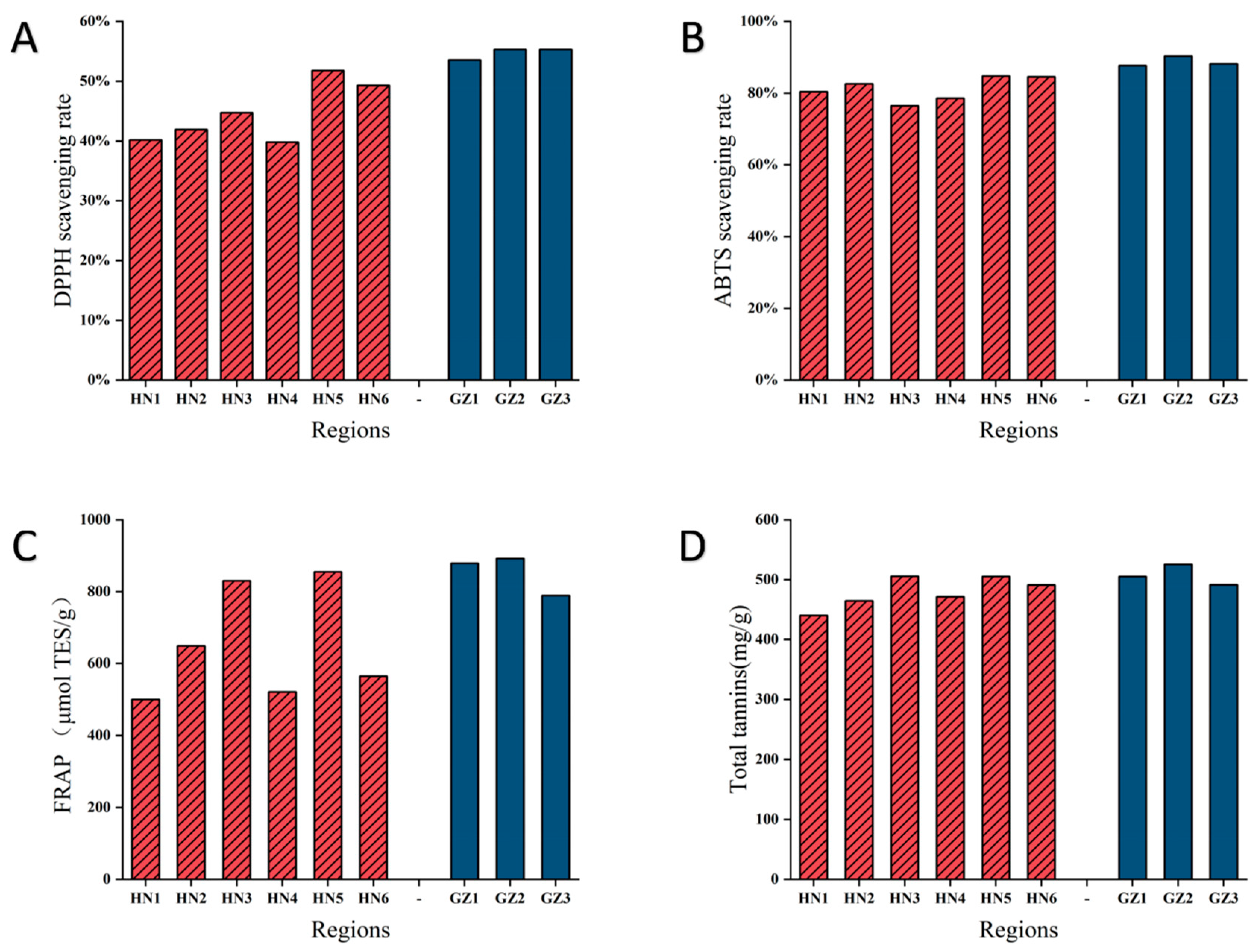
2.6. In Vitro Antioxidant Activities and Total Tannins Analysis
3. Materials and Methods
3.1. Plant Materials, Chemicals, and Reagents
3.2. Sample Processing and Quality Control
3.3. UPLC-ESI-MS/MS Analysis
3.4. Metabolite Profiling Analysis
3.5. In Vitro Antioxidant Activity Assay
3.6. Determination of Total Tannins Content
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hipp, A.L.; Manos, P.S.; Hahn, M.; Avishai, M.; Bodenes, C.; Cavender-Bares, J.; Crowl, A.A.; Deng, M.; Denk, T.; Fitz-Gibbon, S.; et al. Genomic landscape of the global oak phylogeny. New Phytol. 2020, 226, 1198–1212. [Google Scholar] [CrossRef] [PubMed]
- Cavender-Bares, J. Diversification, adaptation, and community assembly of the American oaks (Quercus), a model clade for integrating ecology and evolution. New Phytol. 2019, 221, 669–692. [Google Scholar] [CrossRef]
- Liang, Y.Y.; Shi, Y.; Yuan, S.; Zhou, B.F.; Chen, X.Y.; An, Q.Q.; Ingvarsson, P.K.; Plomion, C.; Wang, B. Linked selection shapes the landscape of genomic variation in three oak species. New Phytol. 2022, 233, 555–568. [Google Scholar] [CrossRef] [PubMed]
- Crowl, A.A.; Manos, P.S.; McVay, J.D.; Lemmon, A.R.; Lemmon, E.M.; Hipp, A.L. Uncovering the genomic signature of ancient introgression between white oak lineages (Quercus). New Phytol. 2020, 226, 1158–1170. [Google Scholar] [CrossRef]
- Yang, X.; Zhang, Q.; Yang, N.; Chang, M.; Ge, Y.; Zhou, H.; Li, G. Traits variation of acorns and cupules during maturation process in Quercus variabilis and Quercus aliena. Plant Physiol. Biochem. 2023, 196, 531–541. [Google Scholar] [CrossRef] [PubMed]
- Smeriglio, A.; Barreca, D.; Bellocco, E.; Trombetta, D. Proanthocyanidins and hydrolysable tannins: Occurrence, dietary intake and pharmacological effects. Br. J. Pharmacol. 2017, 174, 1244–1262. [Google Scholar] [CrossRef]
- Dufourc, E.J. Wine tannins, saliva proteins and membrane lipids. Biochim. Biophys. Acta-Biomembr. 2021, 1863, 183670. [Google Scholar] [CrossRef]
- Melo, L.; Aquino-Martins, V.; Silva, A.; Oliveira, R.H.; Scortecci, K.C. Biological and pharmacological aspects of tannins and potential biotechnological applications. Food Chem. 2023, 414, 135645. [Google Scholar] [CrossRef]
- China, C.R.; Maguta, M.M.; Nyandoro, S.S.; Hilonga, A.; Kanth, S.V.; Njau, K.N. Alternative tanning technologies and their suitability in curbing environmental pollution from the leather industry: A comprehensive review. Chemosphere 2020, 254, 126804. [Google Scholar] [CrossRef]
- Das, N.; Shende, A.P.; Mandal, S.K.; Ojha, N. Biologia Futura: Treatment of wastewater and water using tannin-based coagulants. Biol. Futura 2022, 73, 279–289. [Google Scholar] [CrossRef]
- Gao, S.; Liu, X.; Liu, Y.; Cao, B.; Chen, Z.; Xu, K. The Spectral Irradiance, Growth, Photosynthetic Characteristics, Antioxidant System, and Nutritional Status of Green Onion (Allium fistulosum L.) Grown Under Different Photo-Selective Nets. Front. Plant Sci. 2021, 12, 650471. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.F.; Liu, J.F.; Gao, W.Q.; Deng, Y.P.; Ni, Y.Y.; Xiao, Y.H.; Kang, F.F.; Wang, Q.; Lei, J.P.; Jiang, Z.P. Defense pattern of Chinese cork oak across latitudinal gradients: Influences of ontogeny, herbivory, climate and soil nutrients. Sci. Rep. 2016, 6, 27269. [Google Scholar] [CrossRef]
- Li, Y.; Wang, X.; Sa, Y.; Li, L.; Wang, W.; Yang, L.; Ding, S.; Wilson, G.; Yang, Y.; Zhang, Y.; et al. A comparative UHPLC-QTOF-MS/MS-based metabolomics approach reveals the metabolite profiling of wolfberry sourced from different geographical origins. Food Chem. X 2024, 21, 101221. [Google Scholar] [CrossRef] [PubMed]
- Jing, Y.; Chen, W.; Qiu, X.; Qin, S.; Gao, W.; Li, C.; Quan, W.; Cai, K. Exploring Metabolic Characteristics in Different Geographical Locations and Yields of Nicotiana tabacum L. Using Gas Chromatography-Mass Spectrometry Pseudotargeted Metabolomics Combined with Chemometrics. Metabolites 2024, 14, 176. [Google Scholar] [CrossRef]
- He, L.; Hu, Q.; Zhang, J.; Xing, R.; Zhao, Y.; Yu, N.; Chen, Y. An integrated untargeted metabolomic approach reveals the quality characteristics of black soybeans from different geographical origins in China. Food Res. Int. 2023, 169, 112908. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Li, J.; Li, J.; Yang, L.; Wu, G.; Liu, S. Comparative Metabolomics Study of Chaenomeles speciosa (Sweet) Nakai from Different Geographical Regions. Foods 2022, 11, 1019. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.W.; Liu, Z.W.; Sun, Y.Z.; Liu, L.F. Comparison of Semen Aesculi species from different geographical origins and horse chestnut based on metabolomics and chemometrics analysis. Phytochem. Anal. 2023, 34, 830–841. [Google Scholar] [CrossRef]
- Singh, S.; Sharma, P.C. Gas chromatography-mass spectrometry (GC-MS) profiling reveals substantial metabolome diversity in seabuckthorn (Hippophae rhamnoides L.) berries originating from different geographical regions in the Indian Himalayas. Phytochem. Anal. 2022, 33, 214–225. [Google Scholar] [CrossRef]
- Liu, X.; Zhong, C.; Xie, J.; Liu, H.; Xie, Z.; Zhang, S.; Jin, J. Geographical region traceability of Poria cocos and correlation between environmental factors and biomarkers based on a metabolomic approach. Food Chem. 2023, 417, 135817. [Google Scholar] [CrossRef]
- Zhang, S.; Li, C.; Gu, W.; Qiu, R.; Chao, J.; Pei, L.; Ma, L.; Guo, Y.; Tian, R. Metabolomics analysis of dandelions from different geographical regions in China. Phytochem. Anal. 2021, 32, 899–906. [Google Scholar] [CrossRef]
- Zhao, G.; Zhao, W.; Han, L.; Ding, J.; Chang, Y. Metabolomics analysis of sea cucumber (Apostichopus japonicus) in different geographical origins using UPLC-Q-TOF/MS. Food Chem. 2020, 333, 127453. [Google Scholar] [CrossRef] [PubMed]
- Long, W.; Bai, X.; Wang, S.; Chen, H.; Yin, X.L.; Gu, H.W.; Yang, J.; Fu, H. UHPLC-QTOF-MS-based untargeted metabolomics and mineral element analysis insight into the geographical differences of Chrysanthemum morifolium Ramat cv. “Hangbaiju” from different origins. Food Res. Int. 2023, 163, 112186. [Google Scholar] [CrossRef]
- Yang, Q.; Li, J.; Wang, Y.; Wang, Z.; Pei, Z.; Street, N.R.; Bhalerao, R.P.; Yu, Z.; Gao, Y.; Ni, J.; et al. Genomic basis of the distinct biosynthesis of beta-glucogallin, a biochemical marker for hydrolyzable tannin production, in three oak species. New Phytol. 2024, 242, 2702–2718. [Google Scholar] [CrossRef]
- Buyse, K.; Delezie, E.; Goethals, L.; Van Noten, N.; Van Poucke, C.; Devreese, M.; Antonissen, G.; Janssens, G.; Lourenco, M. Chestnut Wood Tannins in Broiler Diets: Pharmacokinetics, Serum Levels during Rearing, and Intestinal Absorption Pattern of Gallic Acid. J. Agric. Food. Chem. 2024, 72, 2648–2656. [Google Scholar] [CrossRef] [PubMed]
- Wang, F.; Cheng, Y.; Yin, L.; Liu, S.; Li, X.; Xie, M.; Li, J.; Chen, J.; Fu, C. Dietary supplementation with ellagic acid improves the growth performance, meat quality, and metabolomics profile of yellow-feathered broiler chickens. Poult. Sci. 2024, 103, 103700. [Google Scholar] [CrossRef] [PubMed]
- Yu, J.; Song, Y.; Yu, B.; He, J.; Zheng, P.; Mao, X.; Huang, Z.; Luo, Y.; Luo, J.; Yan, H.; et al. Tannic acid prevents post-weaning diarrhea by improving intestinal barrier integrity and function in weaned piglets. J. Anim. Sci. Biotechnol. 2020, 11, 87. [Google Scholar] [CrossRef]
- Aguerre, M.J.; Duval, B.; Powell, J.M.; Vadas, P.A.; Wattiaux, M.A. Effects of feeding a quebracho-chestnut tannin extract on lactating cow performance and nitrogen utilization efficiency. J. Dairy Sci. 2020, 103, 2264–2271. [Google Scholar] [CrossRef] [PubMed]
- Garcia-Conesa, M.T.; Chambers, K.; Combet, E.; Pinto, P.; Garcia-Aloy, M.; Andres-Lacueva, C.; de Pascual-Teresa, S.; Mena, P.; Konic, R.A.; Hollands, W.J.; et al. Meta-Analysis of the Effects of Foods and Derived Products Containing Ellagitannins and Anthocyanins on Cardiometabolic Biomarkers: Analysis of Factors Influencing Variability of the Individual Responses. Int. J. Mol. Sci. 2018, 19, 694. [Google Scholar] [CrossRef]
- Thompson, K.; Hosking, H.; Pederick, W.; Singh, I.; Santhakumar, A.B. The effect of anthocyanin supplementation in modulating platelet function in sedentary population: A randomised, double-blind, placebo-controlled, cross-over trial. Br. J. Nutr. 2017, 118, 368–374. [Google Scholar] [CrossRef]
- Cechinel-Zanchett, C.C.; Bolda, M.L.; Schlickmann, F.; Cechinel-Filho, V.; de Souza, P. In vitro effects of two bioactive compounds, gallic acid and methyl gallate, on urolithiasis. Actas Urol. Esp. (Engl. Ed.) 2021, 45, 604–608. [Google Scholar] [CrossRef]
- Mittasch, J.; Bottcher, C.; Frolova, N.; Bonn, M.; Milkowski, C. Identification of UGT84A13 as a candidate enzyme for the first committed step of gallotannin biosynthesis in pedunculate oak (Quercus robur). Phytochemistry 2014, 99, 44–51. [Google Scholar] [CrossRef] [PubMed]
- Majeed, M.; Mundkur, L.; Paulose, S.; Nagabhushanam, K. Novel Emblica officinalis extract containing beta-glucogallin vs. metformin: A randomized, open-label, comparative efficacy study in newly diagnosed type 2 diabetes mellitus patients with dyslipidemia. Food Funct. 2022, 13, 9523–9531. [Google Scholar] [CrossRef]
- Puppala, M.; Ponder, J.; Suryanarayana, P.; Reddy, G.B.; Petrash, J.M.; LaBarbera, D.V. The isolation and characterization of beta-glucogallin as a novel aldose reductase inhibitor from Emblica officinalis. PLoS ONE 2012, 7, e31399. [Google Scholar] [CrossRef] [PubMed]
- Cao, T.; Wang, J.; Wu, Y.; Wang, L.; Zhang, H. Antiglaucoma Potential of beta-Glucogallin Is Mediated by Modulating Mitochondrial Responses in Experimentally Induced Glaucoma. Neuroimmunomodulation 2020, 27, 142–151. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.N.; Singh, R.; Bhattacharya, A.; Chakravarti, R.; Roy, S.; Ravichandiran, V.; Ghosh, D. A Short Review on Glucogallin and its Pharmacological Activities. Mini-Rev. Med. Chem. 2022, 22, 2820–2830. [Google Scholar] [CrossRef]
- Vesa, C.M.; Bungau, S.G. Novel Molecules in Diabetes Mellitus, Dyslipidemia and Cardiovascular Disease. Int. J. Mol. Sci. 2023, 24, 4029. [Google Scholar] [CrossRef]
- Jan, R.; Asaf, S.; Numan, M.; Lubna; Kim, K. Plant Secondary Metabolite Biosynthesis and Transcriptional Regulation in Response to Biotic and Abiotic Stress Conditions. Agronomy 2021, 11, 968. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Zakhary, N.I.; Aleya, L.; Bungau, S.G.; Bohara, R.A.; Siddiqi, N.J. Aging, Metabolic, and Degenerative Disorders: Biomedical Value of Antioxidants. Oxidative Med. Cell. Longev. 2018, 2018, 2098123. [Google Scholar] [CrossRef]
- Abdel-Daim, M.M.; Abo-El-Sooud, K.; Aleya, L.; Bungau, S.G.; Najda, A.; Saluja, R. Alleviation of Drugs and Chemicals Toxicity: Biomedical Value of Antioxidants. Oxidative Med. Cell. Longev. 2018, 2018, 6276438. [Google Scholar] [CrossRef]
- Valero-Galvan, J.; Gonzalez-Fernandez, R.; Jorrin-Novo, J.V. Interspecific Variation between the American Quercus virginiana and Mediterranean Quercus Species in Terms of Seed Nutritional Composition, Phytochemical Content, and Antioxidant Activity. Molecules 2021, 26, 2351. [Google Scholar] [CrossRef]
- Cantos, E.; Espin, J.C.; Lopez-Bote, C.; de la Hoz, L.; Ordonez, J.A.; Tomas-Barberan, F.A. Phenolic compounds and fatty acids from acorns (Quercus spp.), the main dietary constituent of free-ranged Iberian pigs. J. Agric. Food. Chem. 2003, 51, 6248–6255. [Google Scholar] [CrossRef] [PubMed]
- Alvarez, S.A.; Rocha-Guzman, N.E.; Sanchez-Burgos, J.A.; Gallegos-Infante, J.A.; Moreno-Jimenez, M.R.; Gonzalez-Laredo, R.F.; Solis-Gonzalez, S. Analysis of Antioxidant Constituents of Filtering Infusions from Oak (Quercus sideroxyla Bonpl. and Quercus eduardii Trel.) and Yerbaniz (Tagetes lucida (Sweet) Voss) as Monoamine Oxidase Inhibitors. Molecules 2023, 28, 5167. [Google Scholar] [CrossRef] [PubMed]
- Du, J.; Han, Z.; Ran, L.; Zhang, T.; Li, J.; Li, H. Correction: Du et al. Extraction Condition Optimization, Quantitative Analysis, and Anti-AD Bioactivity Evaluation of Acorn Polyphenols from Quercus variabilis, Quercus aliena, and Quercus dentata. Int. J. Mol. Sci. 2024, 25, 10536. Int. J. Mol. Sci. 2025, 26, 2015. [Google Scholar] [CrossRef]
- Schrimpe-Rutledge, A.C.; Codreanu, S.G.; Sherrod, S.D.; McLean, J.A. Untargeted Metabolomics Strategies-Challenges and Emerging Directions. J. Am. Soc. Mass Spectrom. 2016, 27, 1897–1905. [Google Scholar] [CrossRef] [PubMed]
- Sumner, L.W.; Amberg, A.; Barrett, D.; Beale, M.H.; Beger, R.; Daykin, C.A.; Fan, T.W.; Fiehn, O.; Goodacre, R.; Griffin, J.L.; et al. Proposed minimum reporting standards for chemical analysis Chemical Analysis Working Group (CAWG) Metabolomics Standards Initiative (MSI). Metabolomics 2007, 3, 211–221. [Google Scholar] [CrossRef]
- Kubica, P.; Kokotkiewicz, A.; Malinowska, M.A.; Synowiec, A.; Gniewosz, M.; Hussain, S.; Yaqoob, M.; Bonn, G.K.; Jakschitz, T.; Mahmoud, E.A.; et al. Phenylpropanoid Glycoside and Phenolic Acid Profiles and Biological Activities of Biomass Extracts from Different Types of Verbena officinalis Microshoot Cultures and Soil-Grown Plant. Antioxidants 2022, 11, 409. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, X.; Li, J.; Pei, Z.; Chen, Y.; Liu, P.; Yu, Z.; Liu, Y.; Li, G. HB26, a member of HD-Zip I subfamily, is involved in the regulation of hydrolysable tannin biosynthesis in the cupules of Quercus variabilis by transactivation of UGT84A13. Ind. Crop. Prod. 2023, 200, 116866. [Google Scholar] [CrossRef]

| Province | Area | Longitude (°E) | Latitude (°N) | Size (cm) | Degree of Ripeness | Climatic Conditions | The Color of the Acorn | Range of the Valonea Package |
|---|---|---|---|---|---|---|---|---|
| Henan | HN-1 | 111.48 | 33.28 | 3–4 | fully mature | Warm Temperate Continental Monsoon Climate | dark red | 2/3 |
| HN-2 | 112.49 | 33.29 | 3–4 | fully mature | Warm Temperate Continental Monsoon Climate | dark brown | 2/3 | |
| HN-3 | 111.49 | 33.14 | 3–4 | fully mature | Warm Temperate Continental Monsoon Climate | dark brown | 2/3 | |
| HN-4 | 111.62 | 33.78 | 2–3 | fully mature | Warm Temperate Continental Monsoon Climate | dark red | 2/3 | |
| HN-5 | 112.47 | 34.15 | 2–3 | fully mature | Warm Temperate Continental Monsoon Climate | dark brown | 2/3 | |
| HN-6 | 112.14 | 33.02 | 3–4 | fully mature | Warm Temperate Continental Monsoon Climate | dark brown | 2/3 | |
| Guizhou | GZ-1 | 106.09 | 25.17 | 2–3 | fully mature | Subtropical Humid Monsoon Climate | dark red | 3/4 |
| GZ-2 | 106.75 | 25.43 | 2–3 | fully mature | Subtropical Humid Monsoon Climate | dark brown | 3/4 | |
| GZ-3 | 106.45 | 26.03 | 2–3 | fully mature | Subtropical Humid Monsoon Climate | dark red | 3/4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tong, Z.; Zhou, H.; Qi, Z.; Jiang, J.; Li, W.; Wang, C. Metabolomics and Antioxidant Activity of Valonea from Quercus variabilis Produced in Different Geographical Regions in China. Int. J. Mol. Sci. 2025, 26, 3599. https://doi.org/10.3390/ijms26083599
Tong Z, Zhou H, Qi Z, Jiang J, Li W, Wang C. Metabolomics and Antioxidant Activity of Valonea from Quercus variabilis Produced in Different Geographical Regions in China. International Journal of Molecular Sciences. 2025; 26(8):3599. https://doi.org/10.3390/ijms26083599
Chicago/Turabian StyleTong, Zhenkai, Hao Zhou, Zhiwen Qi, Jianxin Jiang, Wenjun Li, and Chengzhang Wang. 2025. "Metabolomics and Antioxidant Activity of Valonea from Quercus variabilis Produced in Different Geographical Regions in China" International Journal of Molecular Sciences 26, no. 8: 3599. https://doi.org/10.3390/ijms26083599
APA StyleTong, Z., Zhou, H., Qi, Z., Jiang, J., Li, W., & Wang, C. (2025). Metabolomics and Antioxidant Activity of Valonea from Quercus variabilis Produced in Different Geographical Regions in China. International Journal of Molecular Sciences, 26(8), 3599. https://doi.org/10.3390/ijms26083599






