A Biomimetic Human Multi-Cellular In Vitro Model of the Blood–Brain Barrier
Abstract
1. Introduction
2. Results
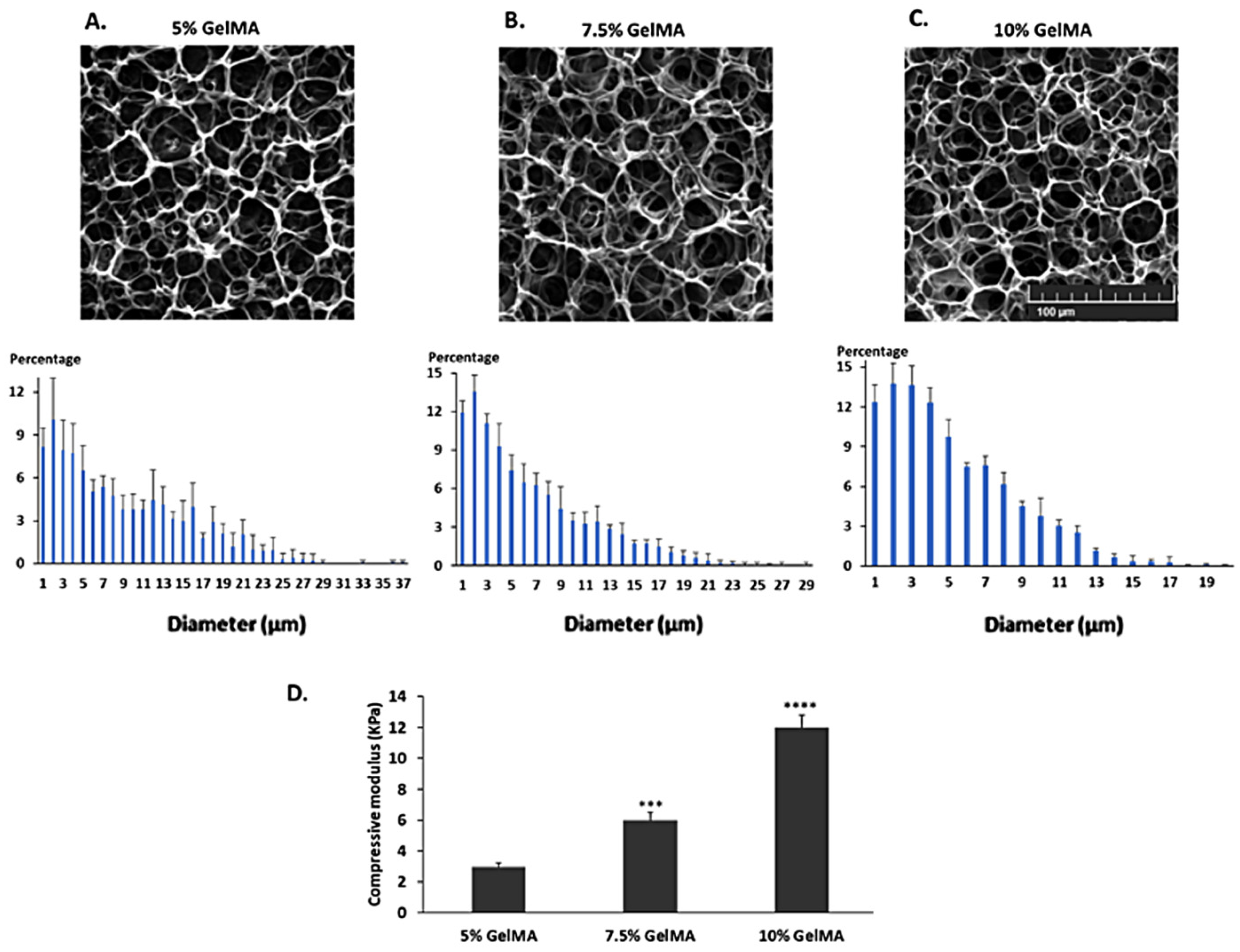
2.1. Characterization of the GelMA Hydrogels
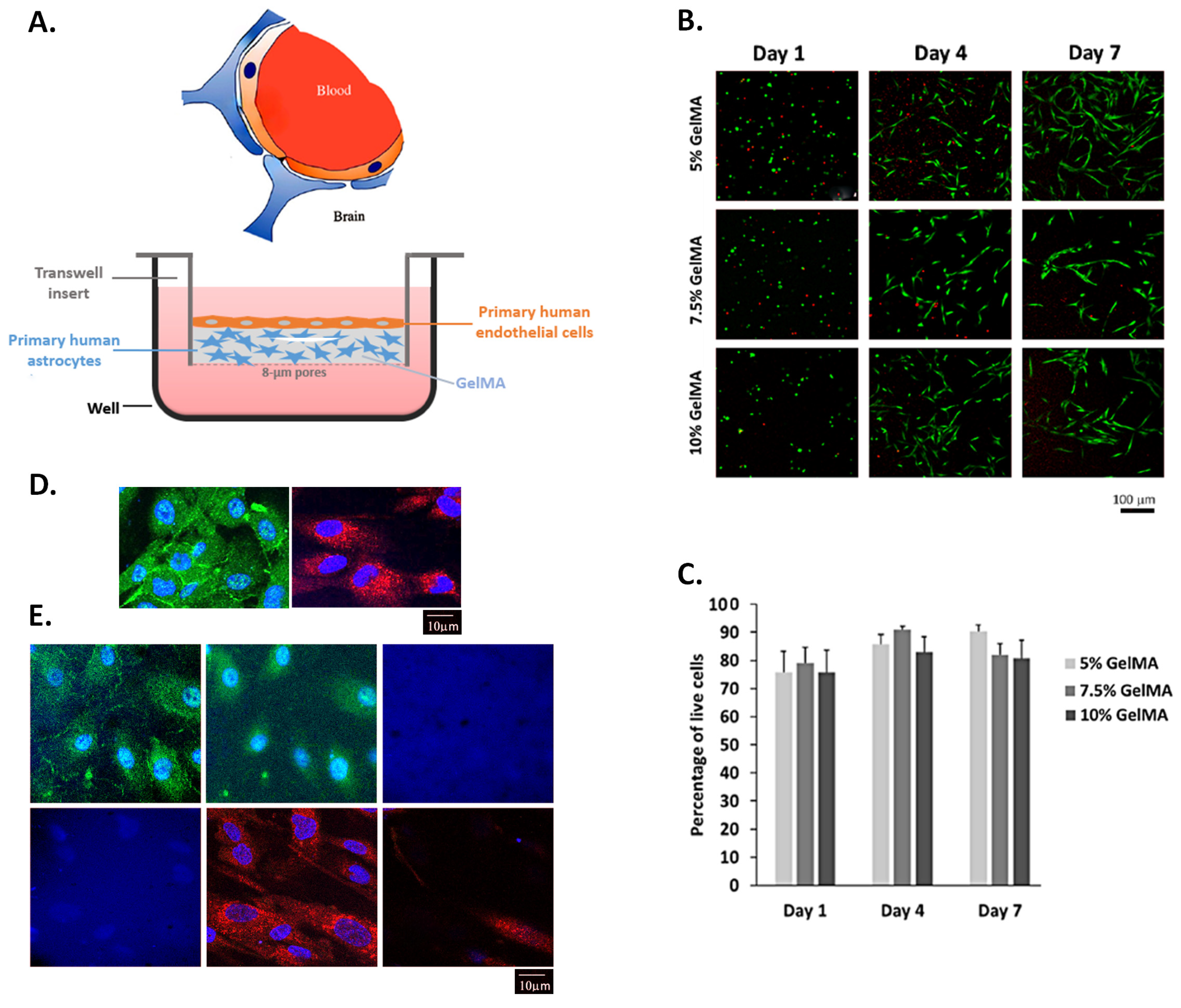
2.2. Human Astrocytes Cultured in GelMA Hydrogels Are Viable
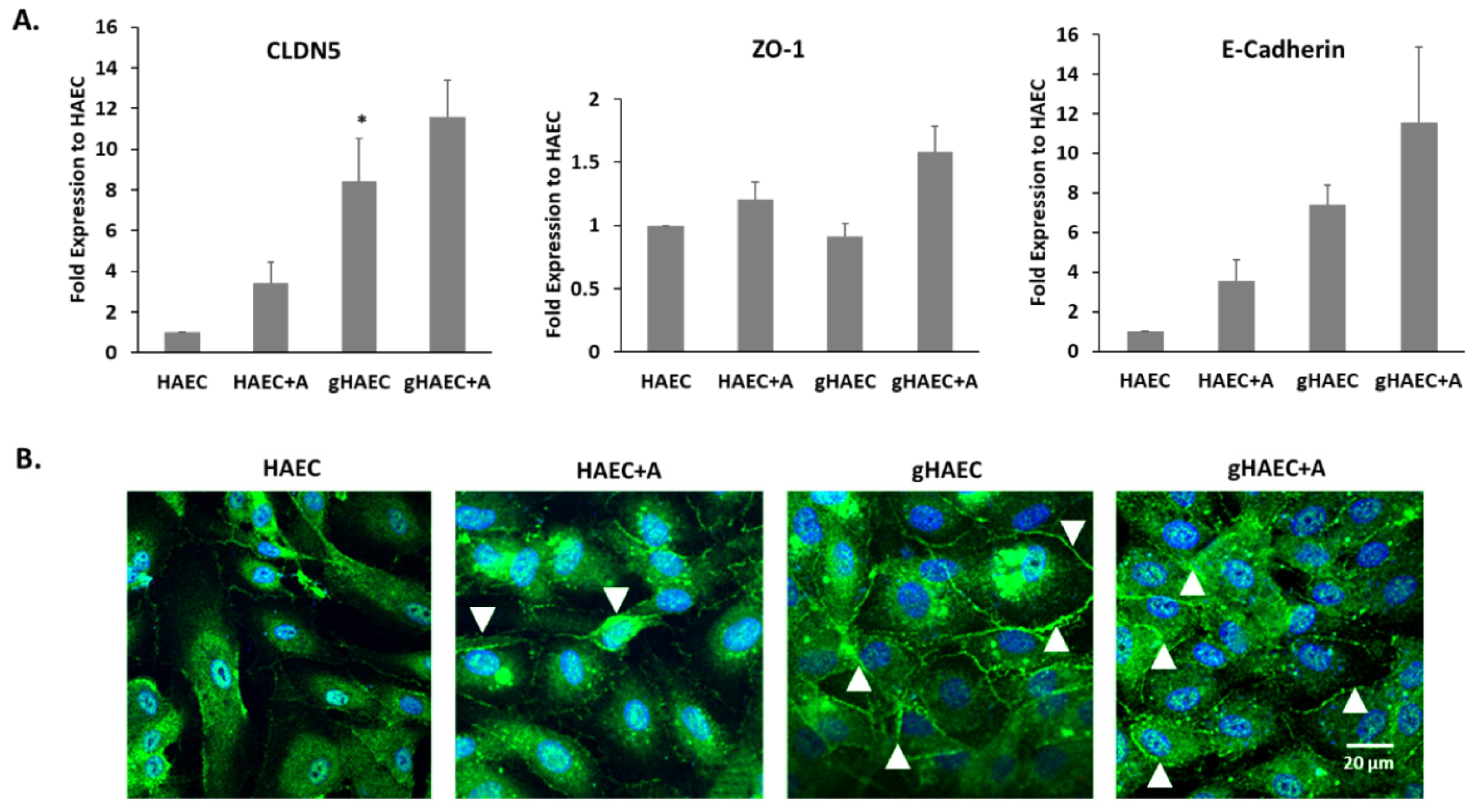
2.3. Tight and Adherens Junction Proteins Are Expressed in This Model of the BBB
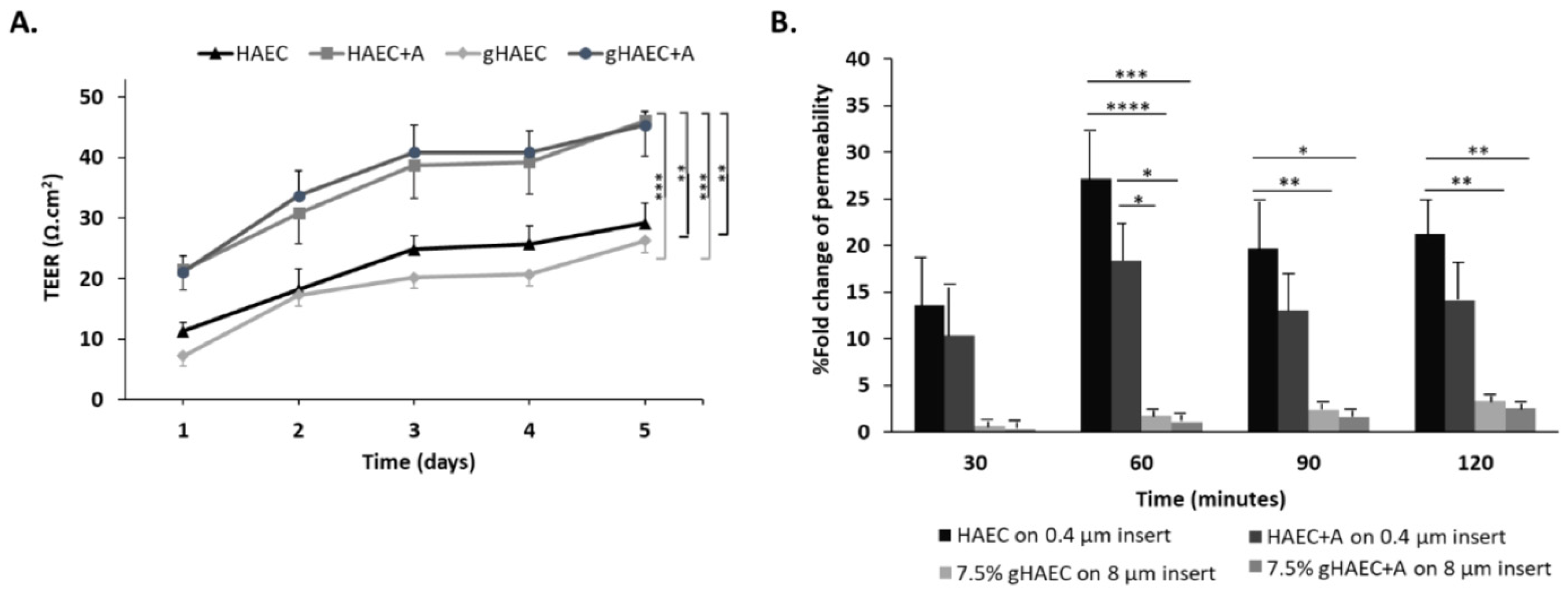
2.4. Efficiency of the Barrier Function
3. Discussion
4. Materials and Methods
4.1. GelMA Synthesis
4.2. Scanning Electron Microscopy
4.3. Cell Culture
4.4. Assessment of Cell Viability
4.5. Assessment of Gene Expression
4.6. Expression and Localization of ZO-1 and GFAP by Immunofluorescence
4.7. Measurement of TEER
4.8. Measurement of Permeability
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Zlokovic, B.V. The Blood-Brain Barrier in Health and Chronic Neurodegenerative Disorders. Neuron 2008, 57, 178–201. [Google Scholar] [CrossRef] [PubMed]
- Gabathuler, R. Approaches to transport therapeutic drugs across the blood-brain barrier to treat brain diseases. Neurobiol. Dis. 2010, 37, 48–57. [Google Scholar] [CrossRef] [PubMed]
- Obermeier, B.; Daneman, R.; Ransohoff, R.M. Development, maintenance and disruption of the blood-brain barrier. Nat. Med. 2013, 19, 1584. [Google Scholar] [CrossRef] [PubMed]
- Pardridge, W.M. Blood–brain barrier delivery. Drug Discov. Today 2007, 12, 54–61. [Google Scholar] [CrossRef]
- Lin, X.; DeAngelis, L.M. Treatment of Brain Metastases. J. Clin. Oncol. 2015, 33, 3475–3484. [Google Scholar] [CrossRef]
- Kersten, K.; de Visser, K.E.; van Miltenburg, M.H.; Jonkers, J. Genetically engineered mouse models in oncology research and cancer medicine. EMBO Mol. Med. 2017, 9, 137–153. [Google Scholar] [CrossRef]
- Hiratsuka, K.; Miyoshi, T.; Kroll, K.T.; Gupta, N.R.; Valerius, M.T.; Ferrante, T.; Yamashita, M.; Lewis, J.A.; Morizane, R. Organoid-on-a-chip model of human ARPKD reveals mechanosensing pathomechanisms for drug discovery. Sci. Adv. 2022, 8, eabq0866. [Google Scholar] [CrossRef]
- Phan, D.T.T.; Wang, X.; Craver, B.M.; Sobrino, A.; Zhao, D.; Chen, J.C.; Lee, L.Y.N.; George, S.C.; Lee, A.P.; Hughes, C.C.W. A vascularized and perfused organ-on-a-chip platform for large-scale drug screening applications. Lab Chip 2017, 17, 511–520. [Google Scholar] [CrossRef]
- Skardal, A.; Aleman, J.; Forsythe, S.; Rajan, S.; Murphy, S.; Devarasetty, M.; Pourhabibi Zarandi, N.; Nzou, G.; Wicks, R.; Sadri-Ardekani, H.; et al. Drug compound screening in single and integrated multi-organoid body-on-a-chip systems. Biofabrication 2020, 12, 025017. [Google Scholar] [CrossRef]
- Weng, K.C.; Kurokawa, Y.K.; Hajek, B.S.; Paladin, J.A.; Shirure, V.S.; George, S.C. Human Induced Pluripotent Stem-Cardiac-Endothelial-Tumor-on-a-Chip to Assess Anticancer Efficacy and Cardiotoxicity. Tissue Eng. Part C Methods 2020, 26, 44–55. [Google Scholar] [CrossRef]
- Hachey, S.J.; Hughes, C.C.W. Applications of tumor chip technology. Lab Chip 2018, 18, 2893–2912. [Google Scholar] [CrossRef] [PubMed]
- Drolez, A.; Vandenhaute, E.; Julien, S.; Gosselet, F.; Burchell, J.; Cecchelli, R.; Delannoy, P.; Dehouck, M.-P.; Mysiorek, C. Selection of a relevant In vitro blood-brain barrier model to investigate pro-metastatic features of human breast cancer cell lines. PLoS ONE 2016, 11, e0151155. [Google Scholar] [CrossRef] [PubMed]
- Kaisar, M.A.; Sajja, R.K.; Prasad, S.; Abhyankar, V.V.; Liles, T.; Cucullo, L. New experimental models of the blood-brain barrier for CNS drug discovery. Expert Opin. Drug Discov. 2017, 12, 89–103. [Google Scholar] [CrossRef] [PubMed]
- Vandenhaute, E.; Drolez, A.; Sevin, E.; Gosselet, F.; Mysiorek, C.; Dehouck, M.-P. Adapting coculture in vitro models of the blood–brain barrier for use in cancer research: Maintaining an appropriate endothelial monolayer for the assessment of transendothelial migration. Lab. Investig. 2016, 96, 588. [Google Scholar] [CrossRef]
- Singh, N.R.; Gromnicova, R.; Brachner, A.; Kraev, I.; Romero, I.A.; Neuhaus, W.; Male, D. A hydrogel model of the human blood-brain barrier using differentiated stem cells. PLoS ONE 2023, 18, e0283954. [Google Scholar] [CrossRef]
- Gopinadhan, A.; Hughes, J.M.; Conroy, A.L.; John, C.C.; Canfield, S.G.; Datta, D. A human pluripotent stem cell-derived in vitro model of the blood–brain barrier in cerebral malaria. Fluids Barriers CNS 2024, 21, 38. [Google Scholar] [CrossRef]
- Augustine, R.; Zahid, A.A.; Mraiche, F.; Alam, K.; Al Moustafa, A.E.; Hasan, A. Gelatin-methacryloyl hydrogel based in vitro blood-brain barrier model for studying breast cancer-associated brain metastasis. Pharm. Dev. Technol. 2021, 26, 490–500. [Google Scholar] [CrossRef]
- Sreekanthreddy, P.; Gromnicova, R.; Davies, H.; Phillips, J.; Romero, I.A.; Male, D. A three-dimensional model of the human blood-brain barrier to analyse the transport of nanoparticles and astrocyte/endothelial interactions. F1000Research 2015, 4, 1279. [Google Scholar] [CrossRef]
- Brandl, S.; Reindl, M. Blood-Brain Barrier Breakdown in Neuroinflammation: Current In Vitro Models. Int. J. Mol. Sci. 2023, 24, 12699. [Google Scholar] [CrossRef]
- Eigenmann, D.E.; Xue, G.; Kim, K.S.; Moses, A.V.; Hamburger, M.; Oufir, M. Comparative study of four immortalized human brain capillary endothelial cell lines, hCMEC/D3, hBMEC, TY10, and BB19, and optimization of culture conditions, for an in vitro blood–brain barrier model for drug permeability studies. Fluids Barriers CNS 2013, 10, 33. [Google Scholar] [CrossRef]
- Hatherell, K.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Pilkington, G.J. Development of a three-dimensional, all-human in vitro model of the blood–brain barrier using mono-, co-, and tri-cultivation Transwell models. J. Neurosci. Methods 2011, 199, 223–229. [Google Scholar] [CrossRef] [PubMed]
- National Heart, Lung and Blood InstituteNational Heart, Lung and Blood Institute. Future of Medicine: Lab-on-a-Chip Devices Starting to Make an Impact; National Heart, Lung and Blood Institute, Ed.; 2021. Available online: https://www.nhlbi.nih.gov/news/2021/future-medicine-lab-chip-devices-starting-make-impact (accessed on 7 April 2025).
- Agafonova, A.; Cosentino, A.; Musso, N.; Prinzi, C.; Russo, C.; Pellitteri, R.; Anfuso, C.D.; Lupo, G. Hypoxia-Induced Inflammation in In Vitro Model of Human Blood-Brain Barrier: Modulatory Effects of the Olfactory Ensheathing Cell-Conditioned Medium. Mol. Neurobiol. 2025, 62, 4008–4022. [Google Scholar] [CrossRef] [PubMed]
- Ling, L.; Zhou, G.; Zhang, X.; Mao, B.; Wan, S.; Bao, Y. A novel histone deacetylase inhibitor protects the blood-brain barrier by regulating NF-κB and Nrf2 signaling pathways in OGD/R injury. Arch. Gerontol. Geriatr. 2025, 131, 105739. [Google Scholar] [CrossRef] [PubMed]
- Tega, Y.; Kawauchi, Y.; Akanuma, S.I.; Inagaki, M.; Tachikawa, M.; Hosoya, K.I. In vitro characterization of taurine transport using the human brain microvascular endothelial cell line as a human blood-brain barrier model. Drug Metab. Pharmacokinet. 2025, 61, 101040. [Google Scholar] [CrossRef]
- Ma, S.H.; Lepak, L.A.; Hussain, R.J.; Shain, W.; Shuler, M.L. An endothelial and astrocyte co-culture model of the blood–brain barrier utilizing an ultra-thin, nanofabricated silicon nitride membrane. Lab Chip 2005, 5, 74–85. [Google Scholar] [CrossRef]
- Fallenstein, G.; Hulce, V.D.; Melvin, J.W. Dynamic mechanical properties of human brain tissue. J. Biomech. 1969, 2, 217–226. [Google Scholar] [CrossRef]
- Cader, Z. Human Blood-Brain-Barrier In Vitro Models: Overview and Applications. Handb. Exp. Pharmacol. 2022, 273, 205–222. [Google Scholar] [CrossRef]
- Jagtiani, E.; Yeolekar, M.; Naik, S.; Patravale, V. In vitro blood brain barrier models: An overview. J. Control Release 2022, 343, 13–30. [Google Scholar] [CrossRef]
- Saliba, J.; Daou, A.; Damiati, S.; Saliba, J.; El-Sabban, M.; Mhanna, R. Development of Microplatforms to Mimic the In Vivo Architecture of CNS and PNS Physiology and Their Diseases. Genes 2018, 9, 285. [Google Scholar] [CrossRef]
- Abbott, N.J. Physiology of the blood–brain barrier and its consequences for drug transport to the brain. Int. Congr. Ser. 2005, 1277, 3–18. [Google Scholar] [CrossRef]
- Cardoso, F.L.; Brites, D.; Brito, M.A. Looking at the blood–brain barrier: Molecular anatomy and possible investigation approaches. Brain Res. Rev. 2010, 64, 328–363. [Google Scholar] [CrossRef] [PubMed]
- Osório, L.A.; Silva, E.; Mackay, R.E. A Review of Biomaterials and Scaffold Fabrication for Organ-on-a-Chip (OOAC) Systems. Bioengineering 2021, 8, 113. [Google Scholar] [CrossRef] [PubMed]
- Aralekallu, S.; Boddula, R.; Singh, V. Development of glass-based microfluidic devices: A review on its fabrication and biologic applications. Mater. Des. 2023, 225, 111517. [Google Scholar] [CrossRef]
- Aura, S.; Sikanen, T.; Kotiaho, T.; Franssila, S. Novel hybrid material for microfluidic devices. Sens. Actuators B Chem. 2008, 132, 397–403. [Google Scholar] [CrossRef]
- Haase, K.; Offeddu, G.S.; Gillrie, M.R.; Kamm, R.D. Endothelial Regulation of Drug Transport in a 3D Vascularized Tumor Model. Adv. Funct. Mater. 2020, 30, 2002444. [Google Scholar] [CrossRef]
- Offeddu, G.S.; Haase, K.; Gillrie, M.R.; Li, R.; Morozova, O.; Hickman, D.; Knutson, C.G.; Kamm, R.D. An on-chip model of protein paracellular and transcellular permeability in the microcirculation. Biomaterials 2019, 212, 115–125. [Google Scholar] [CrossRef]
- Liu, T.; Weng, W.; Zhang, Y.; Sun, X.; Yang, H. Applications of Gelatin Methacryloyl (GelMA) Hydrogels in Microfluidic Technique-Assisted Tissue Engineering. Molecules 2020, 25, 5305. [Google Scholar] [CrossRef]
- Xiao, S.; Zhao, T.; Wang, J.; Wang, C.; Du, J.; Ying, L.; Lin, J.; Zhang, C.; Hu, W.; Wang, L.; et al. Gelatin Methacrylate (GelMA)-Based Hydrogels for Cell Transplantation: An Effective Strategy for Tissue Engineering. Stem Cell Rev. Rep. 2019, 15, 664–679. [Google Scholar] [CrossRef]
- Delsing, L.; Herland, A.; Falk, A.; Hicks, R.; Synnergren, J.; Zetterberg, H. Models of the blood-brain barrier using iPSC-derived cells. Mol. Cell Neurosci. 2020, 107, 103533. [Google Scholar] [CrossRef]
- Vatine, G.D.; Barrile, R.; Workman, M.J.; Sances, S.; Barriga, B.K.; Rahnama, M.; Barthakur, S.; Kasendra, M.; Lucchesi, C.; Kerns, J.; et al. Human iPSC-Derived Blood-Brain Barrier Chips Enable Disease Modeling and Personalized Medicine Applications. Cell Stem Cell 2019, 24, 995–1005.e1006. [Google Scholar] [CrossRef]
- Appelt-Menzel, A.; Oerter, S.; Mathew, S.; Haferkamp, U.; Hartmann, C.; Jung, M.; Neuhaus, W.; Pless, O. Human iPSC-Derived Blood-Brain Barrier Models: Valuable Tools for Preclinical Drug Discovery and Development? Curr. Protoc. Stem Cell Biol. 2020, 55, e122. [Google Scholar] [CrossRef] [PubMed]
- Campisi, M.; Shin, Y.; Osaki, T.; Hajal, C.; Chiono, V.; Kamm, R.D. 3D self-organized microvascular model of the human blood-brain barrier with endothelial cells, pericytes and astrocytes. Biomaterials 2018, 180, 117–129. [Google Scholar] [CrossRef] [PubMed]
- Hajal, C.; Offeddu, G.S.; Shin, Y.; Zhang, S.; Morozova, O.; Hickman, D.; Knutson, C.G.; Kamm, R.D. Engineered human blood-brain barrier microfluidic model for vascular permeability analyses. Nat. Protoc. 2022, 17, 95–128. [Google Scholar] [CrossRef] [PubMed]
- Petrovskaya, A.V.; Barykin, E.P.; Tverskoi, A.M.; Varshavskaya, K.B.; Mitkevich, V.A.; Petrushanko, I.Y.; Makarov, A.A. Blood-Brain Barrier Transwell Modeling. Mol. Biol. 2022, 56, 1086–1094. [Google Scholar] [CrossRef]
- Cameron, T.; Bennet, T.; Rowe, E.M.; Anwer, M.; Wellington, C.L.; Cheung, K.C. Review of design considerations for brain-on-a-chip models. Micromachines 2021, 12, 441. [Google Scholar] [CrossRef]
- Herland, A.; van der Meer, A.D.; FitzGerald, E.A.; Park, T.-E.; Sleeboom, J.J.; Ingber, D.E. Distinct contributions of astrocytes and pericytes to neuroinflammation identified in a 3D human blood-brain barrier on a chip. PLoS ONE 2016, 11, e0150360. [Google Scholar] [CrossRef]
- Mármol, I.; Abizanda-Campo, S.; Ayuso, J.M.; Ochoa, I.; Oliván, S. Towards Novel Biomimetic In Vitro Models of the Blood-Brain Barrier for Drug Permeability Evaluation. Bioengineering 2023, 10, 572. [Google Scholar] [CrossRef]
- Takeuchi, L.E.; Kalia, L.V.; Simmons, C.A. Vascular models of Alzheimer’s disease: An overview of recent in vitro models of the blood-brain barrier. Neurobiol. Dis. 2025, 208, 106864. [Google Scholar] [CrossRef]
- Félétou, M. The Endothelium: Part 1: Multiple Functions of the Endothelial Cells—Focus on Endothelium-Derived Vasoactive Mediators. Chapter 1, Introduction; Morgan & Claypool Life Sciences: San Rafael, CA, USA, 2011. [Google Scholar]
- Oberheim, N.A.; Takano, T.; Han, X.; He, W.; Lin, J.H.; Wang, F.; Xu, Q.; Wyatt, J.D.; Pilcher, W.; Ojemann, J.G.; et al. Uniquely hominid features of adult human astrocytes. J. Neurosci. 2009, 29, 3276–3287. [Google Scholar] [CrossRef]
- Stone, N.L.; England, T.J.; O’Sullivan, S.E. A novel transwell blood brain barrier model using primary human cells. Front. Cell. Neurosci. 2019, 13, 230. [Google Scholar] [CrossRef]
- Brown, A.; He, H.; Trumper, E.; Valdez, J.; Hammond, P.; Griffith, L.G. Engineering PEG-based hydrogels to foster efficient endothelial network formation in free-swelling and confined microenvironments. Biomaterials 2020, 243, 119921. [Google Scholar] [CrossRef]
- Moon, J.J.; Saik, J.E.; Poché, R.A.; Leslie-Barbick, J.E.; Lee, S.-H.; Smith, A.A.; Dickinson, M.E.; West, J.L. Biomimetic hydrogels with pro-angiogenic properties. Biomaterials 2010, 31, 3840–3847. [Google Scholar] [CrossRef] [PubMed]
- Vigen, M.; Ceccarelli, J.; Putnam, A.J. Protease-sensitive PEG hydrogels regulate vascularization in vitro and in vivo. Macromol. Biosci. 2014, 14, 1368–1379. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Hajal, C.; Benjamin, D.C.; Yu, C.; Azizgolshani, H.; Hynes, R.O.; Kamm, R.D. Inflamed neutrophils sequestered at entrapped tumor cells via chemotactic confinement promote tumor cell extravasation. Proc. Natl. Acad. Sci. USA 2018, 115, 7022–7027. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.B.; Whisler, J.A.; Fröse, J.; Yu, C.; Shin, Y.; Kamm, R.D. On-chip human microvasculature assay for visualization and quantification of tumor cell extravasation dynamics. Nat. Protoc. 2017, 12, 865–880. [Google Scholar] [CrossRef]
- Shamul, J.G.; Wang, Z.; Gong, H.; Ou, W.; White, A.M.; Moniz-Garcia, D.P.; Gu, S.; Clyne, A.M.; Quiñones-Hinojosa, A.; He, X. Meta-analysis of the make-up and properties of in vitro models of the healthy and diseased blood–brain barrier. Nat. Biomed. Eng. 2024. [Google Scholar] [CrossRef]
- Nakagawa, S.; Deli, M.A.; Kawaguchi, H.; Shimizudani, T.; Shimono, T.; Kittel, A.; Tanaka, K.; Niwa, M. A new blood-brain barrier model using primary rat brain endothelial cells, pericytes and astrocytes. Neurochem. Int. 2009, 54, 253–263. [Google Scholar] [CrossRef]
- Rizzi, E.; Deligne, C.; Dehouck, L.; Bilardo, R.; Sano, Y.; Shimizu, F.; Kanda, T.; Resmini, M.; Gosselet, F.; Dehouck, M.P.; et al. A Triple Culture Cell System Modeling the Human Blood-Brain Barrier. J. Vis. Exp. 2021. [Google Scholar] [CrossRef]
- Ahmad, N.; Kiriako, G.; Saliba, J.; Abla, K.; El-Sabban, M.; Mhanna, R. Engineering a 3D Biomimetic Peptides Functionalized-Polyethylene Glycol Hydrogel Model Cocultured with Endothelial Cells and Astrocytes: Enhancing In Vitro Blood-Brain Barrier Biomimicry. Mol. Pharm. 2024, 21, 4664–4672. [Google Scholar] [CrossRef]
- Hajal, C.; Le Roi, B.; Kamm, R.D.; Maoz, B.M. Biology and models of the blood–brain barrier. Annu. Rev. Biomed. Eng. 2021, 23, 359–384. [Google Scholar] [CrossRef]
- Goldim, M.P.S.; Della Giustina, A.; Petronilho, F. Using Evans Blue Dye to Determine Blood-Brain Barrier Integrity in Rodents. Curr. Protoc. Immunol. 2019, 126, e83. [Google Scholar] [CrossRef]
- Leung, C.M.; De Haan, P.; Ronaldson-Bouchard, K.; Kim, G.-A.; Ko, J.; Rho, H.S.; Chen, Z.; Habibovic, P.; Jeon, N.L.; Takayama, S. A guide to the organ-on-a-chip. Nat. Rev. Methods Primers 2022, 2, 33. [Google Scholar] [CrossRef]
- Lee, B.H.; Shirahama, H.; Cho, N.-J.; Tan, L.P. Efficient and controllable synthesis of highly substituted gelatin methacrylamide for mechanically stiff hydrogels. RSC Adv. 2015, 5, 106094–106097. [Google Scholar] [CrossRef]
- Wang, Z.; Huang, C.; Shi, Z.; Liu, H.; Han, X.; Chen, Z.; Li, S.; Wang, Z.; Huang, J. Coaxial bioprinting of a three-layer vascular structure exhibiting blood-brain barrier function for neuroprotective drug screening. Colloids Surf. B Biointerfaces 2025, 249, 114494. [Google Scholar] [CrossRef] [PubMed]
- El-Harakeh, M.; Saliba, J.; Sharaf Aldeen, K.; Haidar, M.; El Hajjar, L.; Awad, M.K.; Hashash, J.G.; Shirinian, M.; El-Sabban, M. Expression of the methylcytosine dioxygenase ten-eleven translocation-2 and connexin 43 in inflammatory bowel disease and colorectal cancer. World J. Gastroenterol. 2022, 28, 5845–5864. [Google Scholar] [CrossRef]
- Radu, M.; Chernoff, J. An in vivo assay to test blood vessel permeability. J. Vis. Exp. 2013, e50062. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saliba, J.; Saliba, J.; El-Sabban, M.; Mhanna, R. A Biomimetic Human Multi-Cellular In Vitro Model of the Blood–Brain Barrier. Int. J. Mol. Sci. 2025, 26, 3592. https://doi.org/10.3390/ijms26083592
Saliba J, Saliba J, El-Sabban M, Mhanna R. A Biomimetic Human Multi-Cellular In Vitro Model of the Blood–Brain Barrier. International Journal of Molecular Sciences. 2025; 26(8):3592. https://doi.org/10.3390/ijms26083592
Chicago/Turabian StyleSaliba, John, Jessica Saliba, Marwan El-Sabban, and Rami Mhanna. 2025. "A Biomimetic Human Multi-Cellular In Vitro Model of the Blood–Brain Barrier" International Journal of Molecular Sciences 26, no. 8: 3592. https://doi.org/10.3390/ijms26083592
APA StyleSaliba, J., Saliba, J., El-Sabban, M., & Mhanna, R. (2025). A Biomimetic Human Multi-Cellular In Vitro Model of the Blood–Brain Barrier. International Journal of Molecular Sciences, 26(8), 3592. https://doi.org/10.3390/ijms26083592





