1. Introduction
Introduced by Nan and colleagues in 2013 [
1],
Prunus xueluoensis represents a novel species within the Prunus genus, distinguished by its unique characteristics. It is distributed in China [
2] and is considered a significant genetic resource within cherry plants of the Rosaceae family. Like other ornamental species, such as the Chinese jujube (
Ziziphus jujuba) [
3] and sweet cherry (
Prunus avium) [
4], which are known for their short juvenile phase and early flowering,
Prunus xueluoensis is postulated to exhibit similar characteristics, including a short juvenile phase, early flowering, abundant flowers, elegant coloration, and a low-growing habit. These traits make it valuable for genetic research due to its unique characteristics and rapid flowering and fruiting. These traits would make it an excellent material for bonsai cultivation and an attractive option for genetic research and breeding programs aimed at creating novel ornamental varieties. The genus
Prunus encompasses approximately 150 species with over 500 varieties, exhibiting considerable genetic diversity [
5]. Among them,
P. xueluoensis stands out due to its extensive natural distribution and the availability of numerous cultivated varieties. Despite its ornamental potential, the natural distribution of
P. xueluoensis is limited to specific mountainous regions at altitudes of 1100 to 1500 m. Additionally, its seed germination rate under natural conditions is low, which is further complicated by the inefficiency of its regeneration system and the lack of an established genetic transformation protocol. These factors have hindered the advancement of molecular studies and restricted the application and popularization of this species. Therefore, there is an urgent need to establish efficient tissue culture and genetic transformation systems for
P. xueluoensis. Such advancements would greatly support efforts in resource conservation, germplasm innovation, and molecular breeding programs. Previous studies have already made significant strides in understanding the tissue culture and genetic transformation of various
Prunus species, including
Prunus cerasifera [
6],
Prunus avium [
7],
P. persica [
8], and
P. mume [
9]. These investigations have provided invaluable insights into the factors that influence regeneration and transformation efficiency within the
Prunus genus.
Tissue culture techniques offer a promising avenue for the rapid propagation and conservation of rare and endangered plant species [
10]. By employing plant growth regulators and optimized media compositions, researchers can induce callus formation, adventitious [
11] shoot regeneration, and rooting, thereby establishing in vitro regeneration systems [
12]. Previous studies have successfully established tissue culture protocols for various
Prunus species, demonstrating the feasibility of this approach for
P. xueluoensis as well [
13,
14,
15]. Genetic transformation, particularly through
Agrobacterium-mediated methods, has emerged as a powerful tool for introducing desirable traits into plant genomes [
16,
17]. This technique leverages the natural ability of
Agrobacterium tumefaciens to transfer DNA into plant cells, offering advantages such as high efficiency, low cost, and clear transfer fragments. By integrating foreign genes into the plant genome, researchers can confer resistance to biotic and abiotic stresses, enhance growth characteristics, and improve the overall value of the crop [
18]. Despite the potential benefits, the genetic transformation of woody plants, including
P. xueluoensis, remains challenging due to factors such as genotype sensitivity, explant type, and the complex interaction between
Agrobacterium and plant cells. Previous studies on genetic transformation in
Prunus species have primarily focused on herbaceous model plants or economically important fruit crops, with limited research on ornamental tree species like
P. xueluoensis. Therefore, there is a need to systematically investigate the key factors affecting the efficiency of
Agrobacterium-mediated genetic transformation in
P. xueluoensis and to establish an optimized protocol suitable for this species.
To the best of our knowledge, the establishment of an efficient genetic transformation system for
P. xueluoensis remains understudied, with most research focusing on its living environment, seed characteristics, phenotypic and genetic variation, gene cloning, and leaf regeneration systems [
1,
19]. Currently, challenges such as the selection of suitable explants, low regeneration rates, and difficulties in disinfecting explant surfaces hinder methodological advancements. Notably, there have been no prior reports on the successful development of a genetic transformation system for
P. xueluoensis using stem segments as explants [
20]. In the present study, we utilized stem segments from aseptic seedlings of
P. xueluoensis as explants and established a highly efficient and stable regeneration system by optimizing the types and concentrations of plant growth regulators, as well as culture conditions. We also explored the feasibility of
Agrobacterium-mediated genetic transformation using the green fluorescent protein (
GFP) gene as a reporter. By systematically investigating various factors influencing transformation efficiency, including the optical density of
Agrobacterium [
21,
22] tumefaciens (OD
600), infection time [
23], the duration of pre-cultivation and co-cultivation [
24], and the concentration of the selective agent [
25], this study provides a reliable regeneration and transient genetic transformation system for
P. xueluoensis, thereby facilitating genetic improvement for breeding and laying the groundwork for the creation of varieties with superior market potential for this valuable ornamental species.
3. Discussion
In this study, the efficient in vitro regeneration and Agrobacterium-mediated transient genetic transformation systems for P. xueluoensis were systematically investigated. The results obtained provide valuable insights into the genetic engineering of this endangered species and offer a solid foundation for future functional genomics studies.
3.1. Effect of Plant Growth Regulators on Tissue Culture
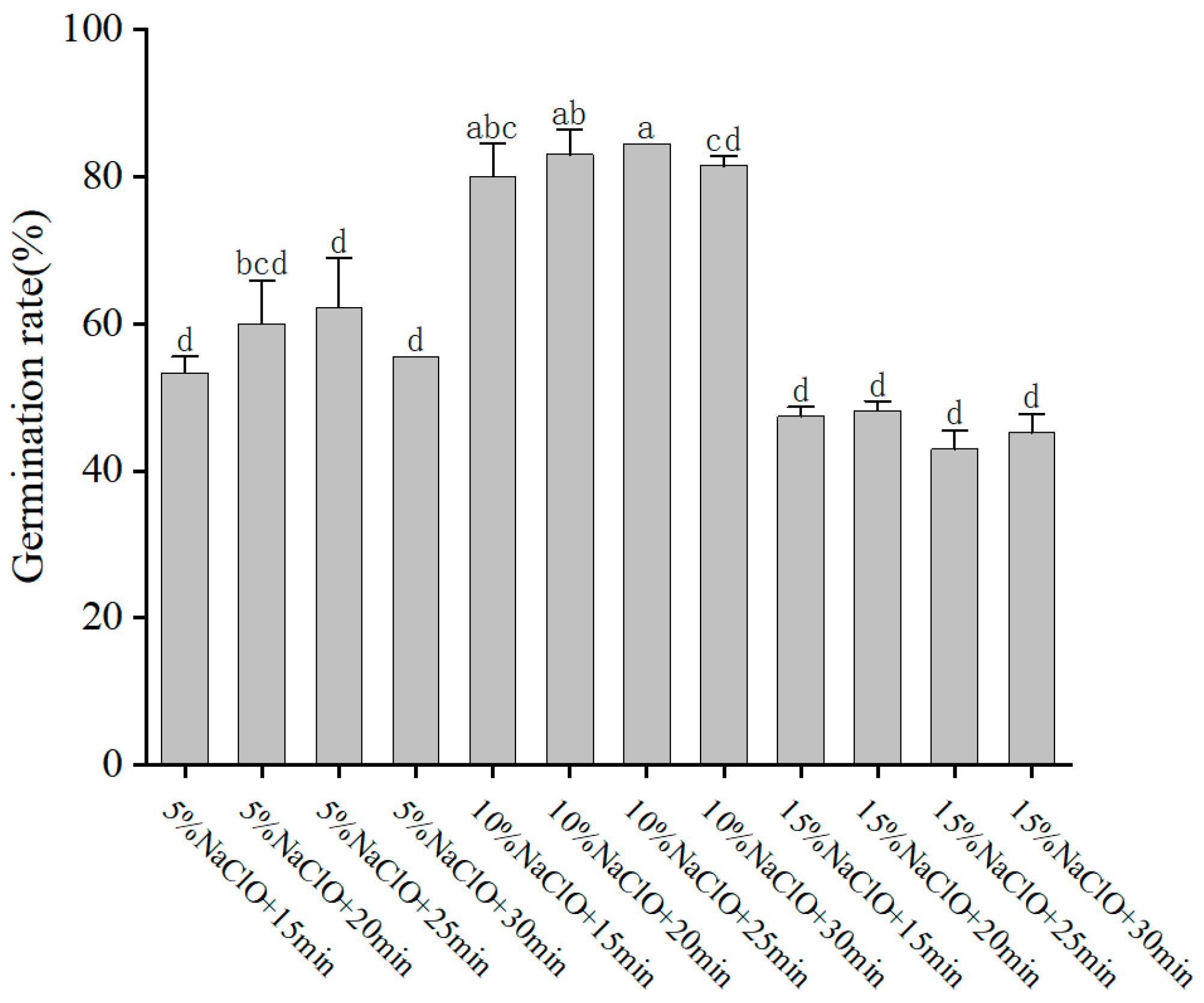
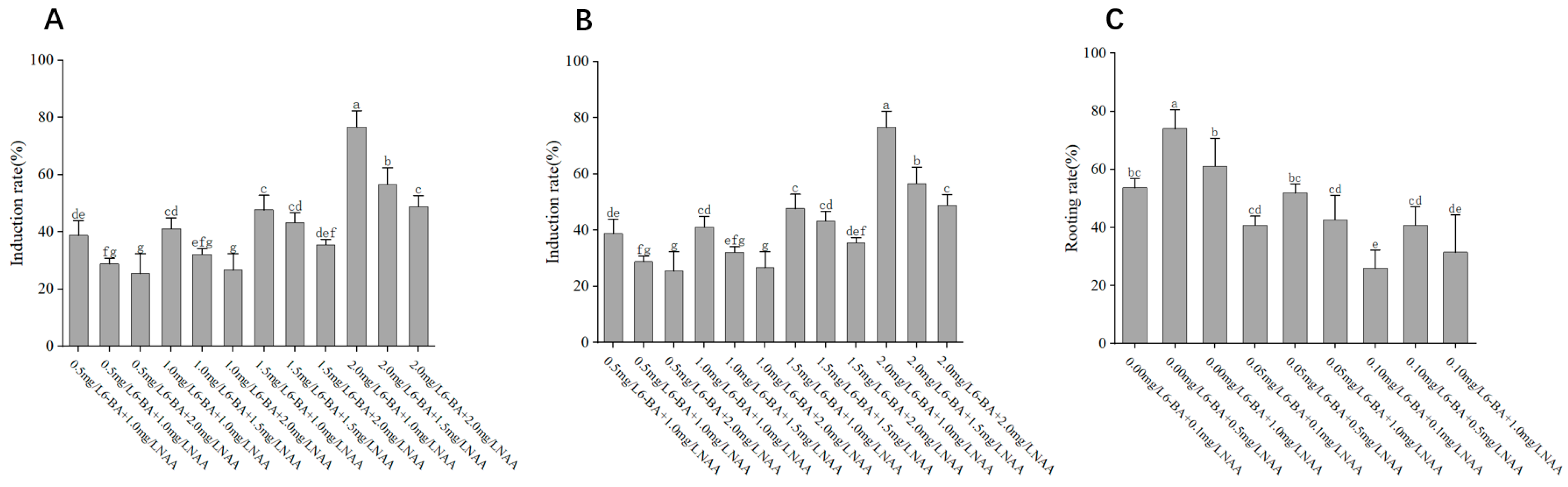
The selection and concentration of plant growth regulators (PGRs) are crucial for successful tissue culture and plant regeneration. In our study, the combination of MS medium supplemented with 200 mg/L GA3 and 4 mg/L 6-BA was found to be optimal for seedling germination, achieving a high germination rate of 94.44%. During initial experiments on disinfection methods, we found that the germination rate of embryos reached up to 84.44% when grown on medium without auxins. Upon introducing NAA at specific concentrations, we noted variations in the germination patterns. Notably, the inclusion of 1.00 mg/L NAA suggested a possible enhancement in germination, although this improvement did not reach statistical significance. These preliminary findings underscore the need for further investigation to conclusively determine the role of auxin in promoting embryo germination, as the current data suggest a trend rather than a definitive conclusion. These results are consistent with previous studies indicating that Nan Chenghui [
1] found that adding GA3 and 6-BA to the germination culture of
P. discoidea could better break dormancy and promote sprouting. Similarly, the medium with MS and a combination of 2.00 mg/L 6-BA, 1.00 mg/L NAA, and 200 mg/L VC was most effective for callus induction, yielding a callus induction rate of 76.66%. This indicates that cytokinin is a key factor in callus induction, while auxin plays a secondary role. These results are consistent with previous studies indicating that an appropriate balance between cytokinins and auxins is essential for callus formation and shoot regeneration in woody plants [
26,
27].
For adventitious shoot differentiation, the medium containing MS with 1.00 mg/L 6-BA, 0.10 mg/L NAA, and 200 mg/L VC promoted the highest differentiation rate of 75.92%. In contrast, the rooting medium composed of 3/4 MS and 0.50 mg/L NAA was optimal for rooting, achieving a rooting rate of 74.08%. These findings suggest that lower concentrations of auxins favor root development, while higher concentrations promote shoot formation, which aligns with previous reports on cherry tissue culture [
28].
3.2. Antibiotic Concentration Screening and Bacteriostatic Concentration Screening
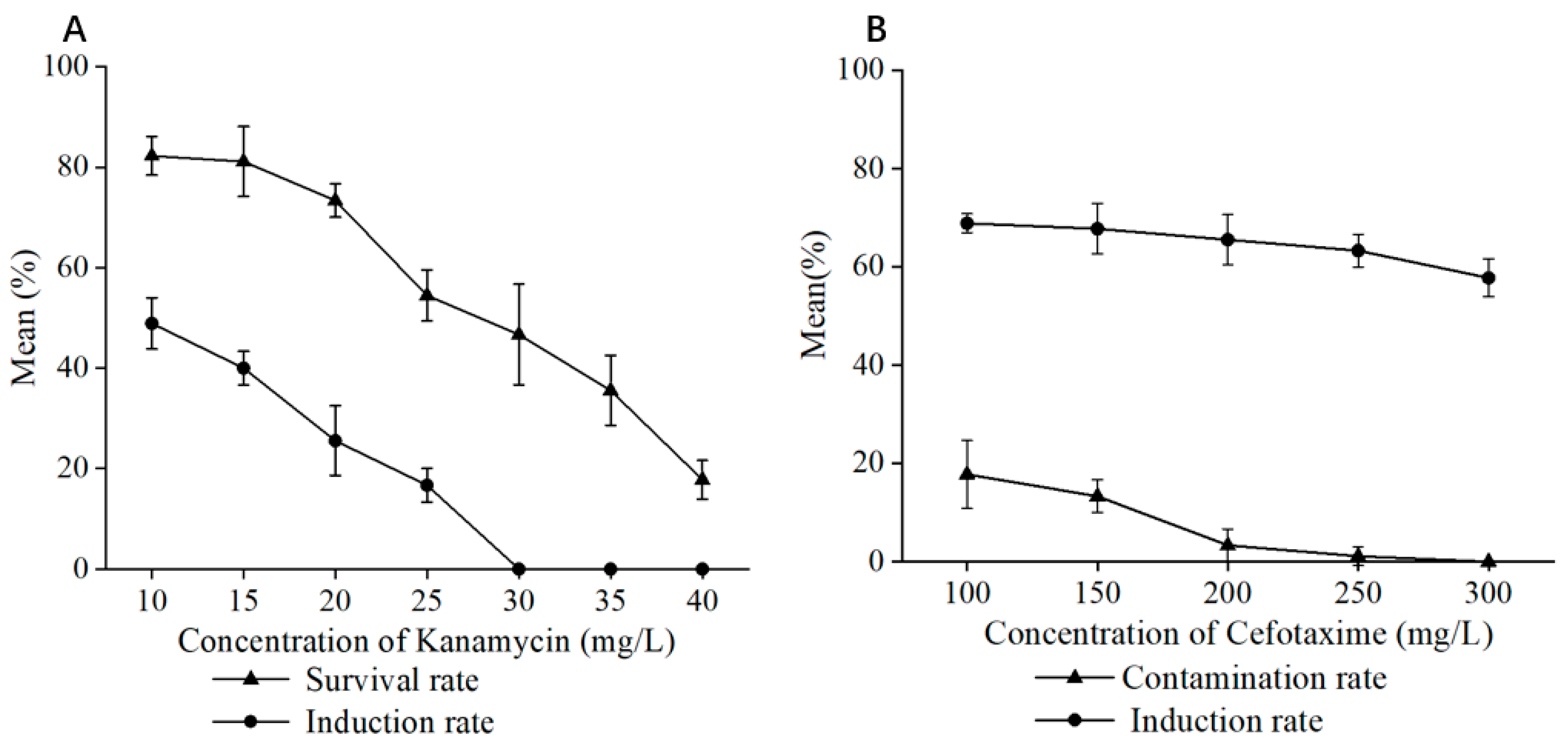
In the process of establishing the genetic transformation system for
P. xueluoensis, screening suitable concentrations of antibiotics and bacteriostatic agents is crucial for the successful selection of transgenic plants and the prevention of contamination caused by
Agrobacterium. The present study systematically investigated the effects of different concentrations of kanamycin (Kan) and cefotaxime (Cef) on the growth and transformation efficiency of
P. xueluoensis explants. Kanamycin, commonly used as a selective agent in plant genetic transformation, effectively inhibits the growth of non-transgenic tissues while allowing transgenic plants to thrive. Our results revealed that a Kan concentration of 30 mg/L was optimal for screening transformed
P. xueluoensis stem segments, as it effectively suppressed the growth of non-transformed tissues without adversely affecting the transformed explants. This concentration is comparable to those reported for other woody plants, although it may vary depending on the species and explant type [
29]. For instance, studies on the genetic transformation of lilies have demonstrated the utility of antibiotics like hygromycin, kanamycin, and glyphosate for resistance screening [
25]. It is important to note that the selection of the appropriate Kan concentration must balance effectively eliminating non-transformed tissues and minimizing the negative impact on transformed explants [
30].
In addition to selective antibiotics, bacteriostatic antibiotics play a crucial role in genetic transformation by preventing post-infection contamination of the receptor material by Agrobacterium and mitigating the inhibitory effects of Agrobacterium growth on the transformation process, thereby reducing the likelihood of recipient cell death and regeneration difficulties. Cephalosporins, known for their broad-spectrum antibacterial activity and relatively low toxicity to plant cells, are commonly employed as bacteriostatic agents in plant genetic transformation. Furthermore, this study revealed that the concentration of the bacteriostatic agent is intertwined with the concentration of Agrobacterium used for infection. Specifically, at lower Agrobacterium concentrations, minimal or no bacterial colonies were observed, suggesting an interplay between the two that warrants careful consideration during experimental design.
3.3. Factors Influencing Genetic Transformation Efficiency
The efficiency of
Agrobacterium-mediated genetic transformation is influenced by various factors, including pre-culture time,
Agrobacterium concentration, infection time, and co-culture time. In our study, the optimal conditions for transforming
P. xueluoensis were determined to be a pre-culture time of 2 days, an
Agrobacterium concentration of OD
600 = 0.6, an infection time of 30 min, and a co-culture time of 3 days. Under these conditions, the genetic transformation efficiency reached a maximum of 10.42%, as determined by PCR and
GFP fluorescence detection. This efficiency is comparable to that of
Prunus cerasifera [
6] but lower than that of herbaceous models [
17], reflecting inherent challenges in woody plant transformation.
Pre-culture has been shown to enhance the susceptibility of explants to
Agrobacterium infection by priming the cells for transformation [
25]. Our study determined that a 2-day pre-culture period is optimal, as it enables cells to enter a phase of active division, thereby increasing their receptivity to T-DNA transfer. This duration likely primes explant cells for T-DNA uptake by enhancing mitotic activity, as evidenced in
Prunus avium [
4].
The concentration of
Agrobacterium plays a critical role in transformation efficiency. An OD
600 value of 0.6 was found to be optimal, as higher concentrations could cause excessive damage to the explants, leading to reduced transformation efficiency. This finding is consistent with previous studies that report that moderate
Agrobacterium concentrations are essential for successful transformation [
31].
Infection time is another important parameter affecting transformation efficiency. An infection period of 30 min was determined to be optimal in our study [
32]. Shorter infection times may not allow sufficient time for T-DNA transfer, while longer times can cause excessive damage to the explants [
33].
Co-culture time also significantly impacts transformation success. A co-culture period of 3 days was found to be ideal, as it allowed sufficient time for T-DNA integration into the plant genome without causing excessive
Agrobacterium growth and subsequent explant damage. These findings are in agreement with previous reports emphasizing the balance between T-DNA transfer and explant health during co-culture [
34]. It is important to note that the genetic transformation achieved in this study is transient, meaning that the introduced T-DNA is not stably integrated into the plant genome. The 3 days of co-cultivation were found to be sufficient for T-DNA delivery and initial expression of the
GFP gene, as evidenced by
GFP fluorescence detection. However, further studies are needed to achieve stable genetic transformation in
Prunus xueluoensis.
3.4. Implications and Future Directions
To contextualize the efficiency of our regeneration and transient transformation system, we compared our results with previously reported studies on other
Prunus species (
Table 6). For instance, regeneration efficiencies in
Prunus cerasifera [
6] (76.66% callus induction) and
P. avium [
7] (65–70% shoot regeneration) align closely with our findings for
P. xueluoensis (76.66% callus induction, 75.92% adventitious bud differentiation). However, the transient transformation efficiency in our study (10.42%) exceeds the values reported for
P. mume [
9] (6.5%) and
P. persica [
8] (3–5%), likely due to optimized
Agrobacterium infection and co-cultivation conditions. Notably, the transformation of
P. xueluoensis using stem segments as explants represents a methodological advancement, as earlier studies predominantly used cotyledons or leaves. These comparisons underscore the robustness of our protocol and its applicability to understudied ornamental species within the
Prunus genus.
The establishment of an efficient regeneration and transient genetic transformation system for
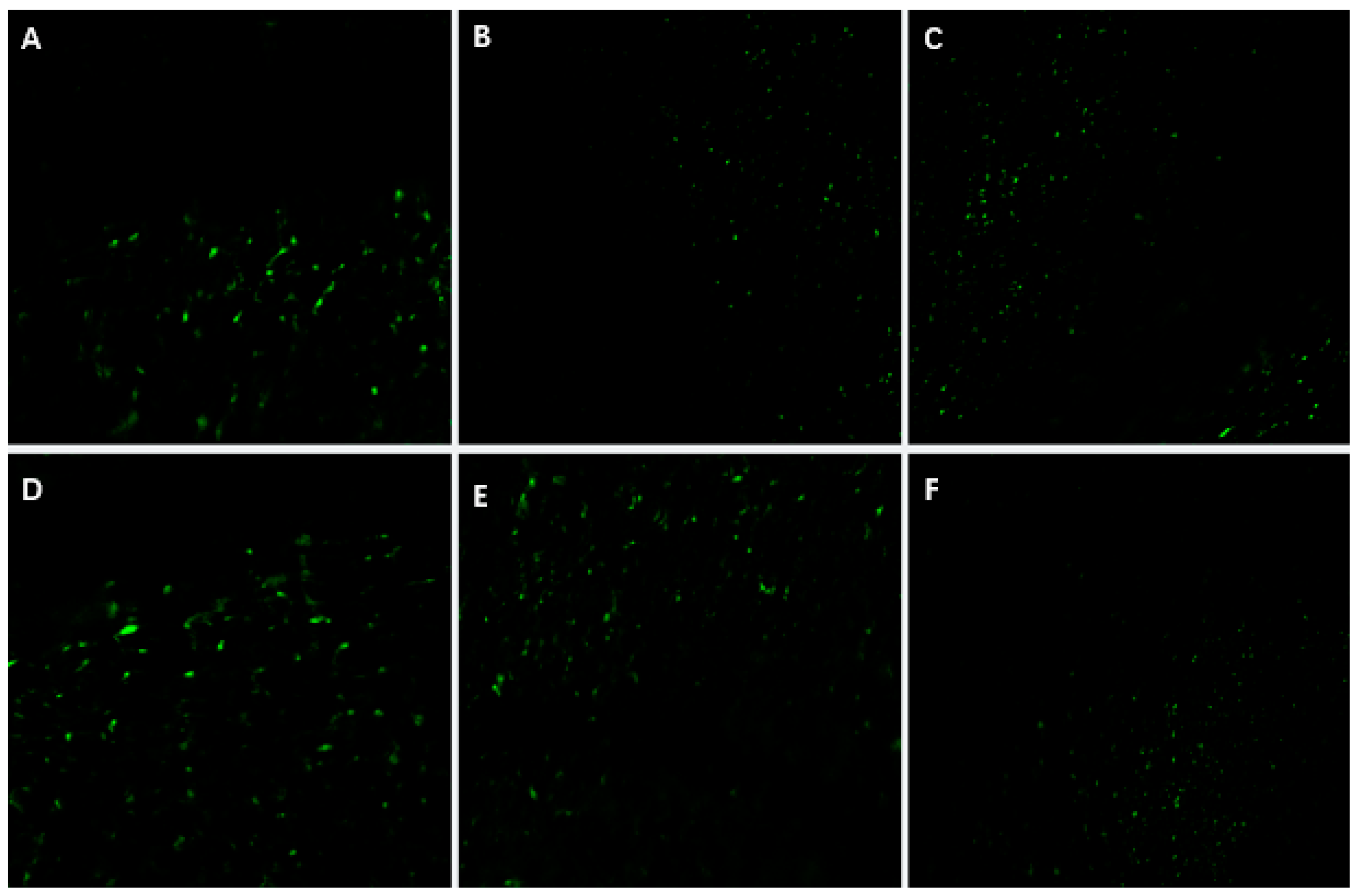
P. xueluoensis has significant implications. First, it enables the rapid propagation of this rare species through tissue culture, aiding conservation efforts. Second, it provides a foundation for genetic improvement, such as introducing stress resistance or enhancing ornamental traits. The punctate
GFP fluorescence observed in leaves (
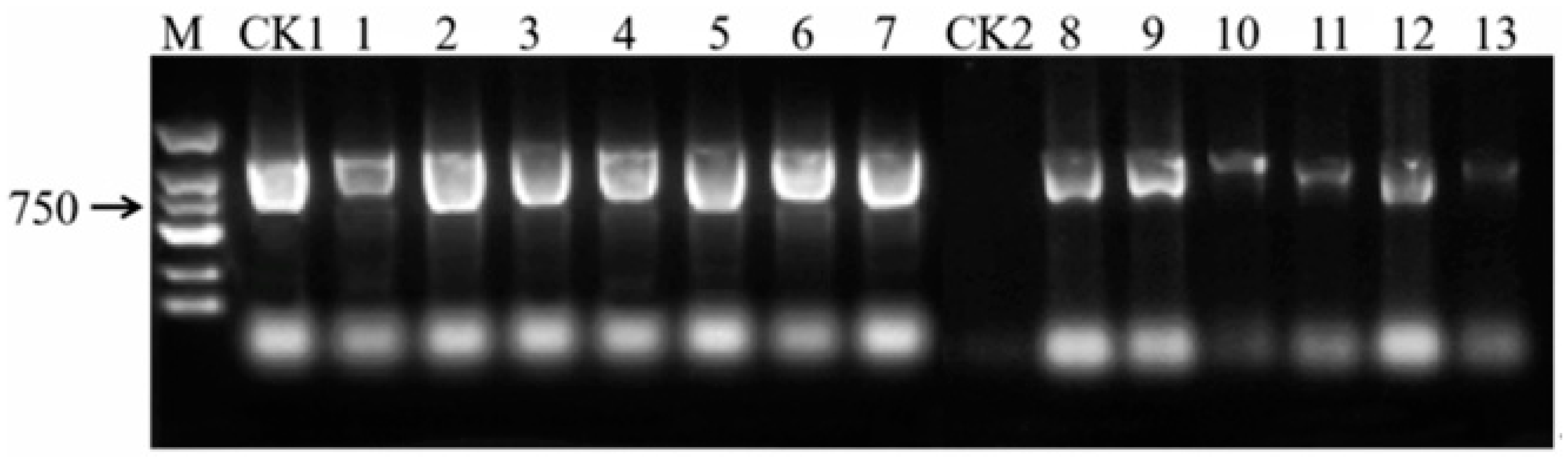
Figure 6), validated by PCR amplification of the
GFP gene (
Figure 7), confirms transient expression, which is consistent with prior studies in
Prunus species [
6,
9]. While southern blot analysis remains essential for verifying stable integration, our focus here is on transient transformation, which does not require genomic integration for validation.
The transient nature of this system is evident in the localized GFP signals, which reflect T-DNA expression in individual cells rather than stable genome integration. Future studies will prioritize stable transformation by optimizing selection markers, regeneration media, and alternative methods (e.g., protoplast transformation). Additionally, advanced imaging techniques (e.g., confocal microscopy with cellular markers) will resolve the subcellular localization of GFP signals, addressing current limitations in spatial resolution.
5. Conclusions
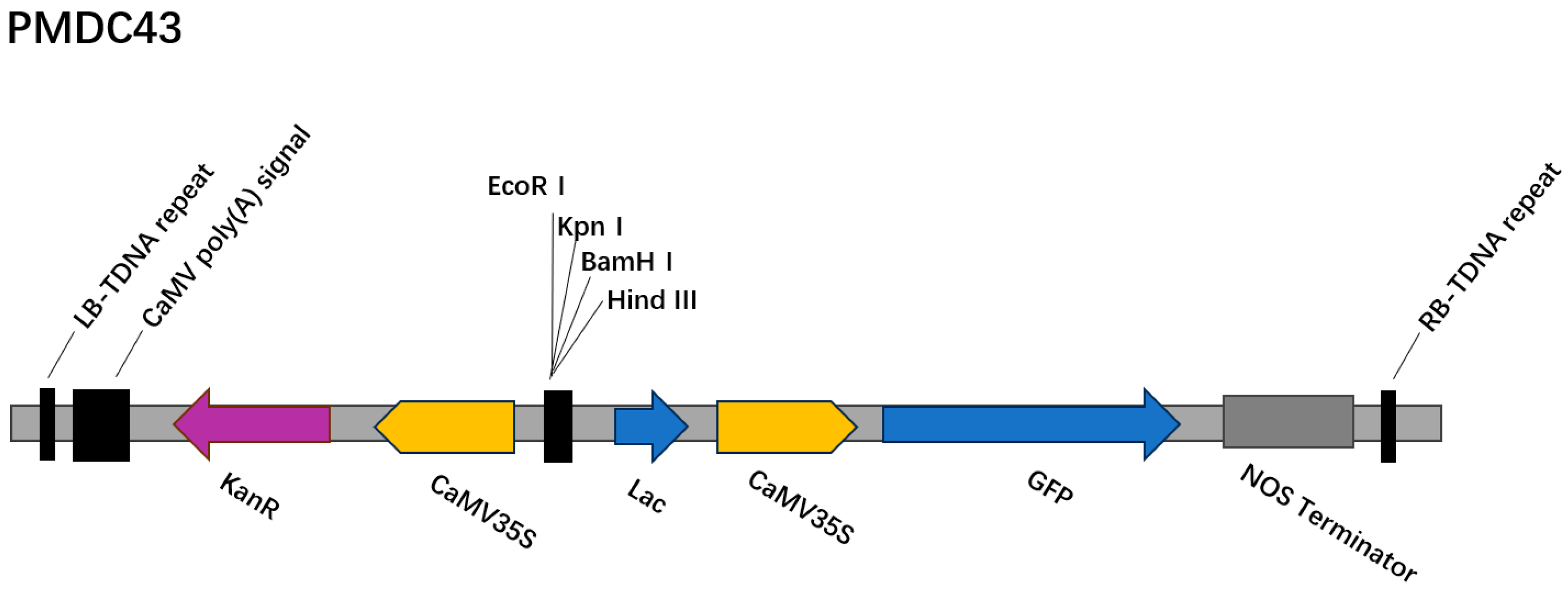
In this study, an efficient regeneration and Agrobacterium-mediated transient genetic transformation system was successfully established for P. xueluoensis, a rare and valuable ornamental cherry species endemic to China. By optimizing the combination of PGRs, the most effective medium for callus induction and adventitious bud regeneration from seed embryos was identified as MS supplemented with 200 mg/L GA3 and 4 mg/L 6-BA. Furthermore, the regeneration system was employed to develop a genetic transformation protocol using the PMDC43 vector harboring the GFP reporter gene. Key factors influencing transformation efficiency, including pre-cultivation time, Agrobacterium concentration, infection duration, and co-cultivation period, were systematically investigated. The optimal transformation conditions were determined to be a pre-cultivation time of 2 days, an Agrobacterium concentration of OD600 = 0.6, an infection duration of 30 min, and a co-cultivation period of 3 days. Under these conditions, a transformation efficiency of 10.42% was achieved, as confirmed by both GFP fluorescence detection and PCR analysis. This study not only provides a reliable protocol for the regeneration and genetic manipulation of P. xueluoensis but also lays a solid foundation for future genetic improvement and molecular breeding of this unique cherry species. While the current study focused on establishing a transient genetic transformation system, future research will explore methods for achieving stable integration of T-DNA, and we will focus on optimizing conditions for stable transformation, including southern blot analysis to confirm genomic integration, to advance the genetic improvement of P. xueluoensis, which is essential for creating varieties with superior market potential.