The Small RNA Landscape in Azoospermia: Implications for Male Infertility and Sperm Retrieval—A Preliminary Study
Abstract
1. Introduction
2. Results
2.1. Patients’ Characteristics
2.2. Small RNA Sequencing and Data Quality
2.3. Identification of Known and Novel miRNAs
2.4. Differential Expression Profiles of miRNAs
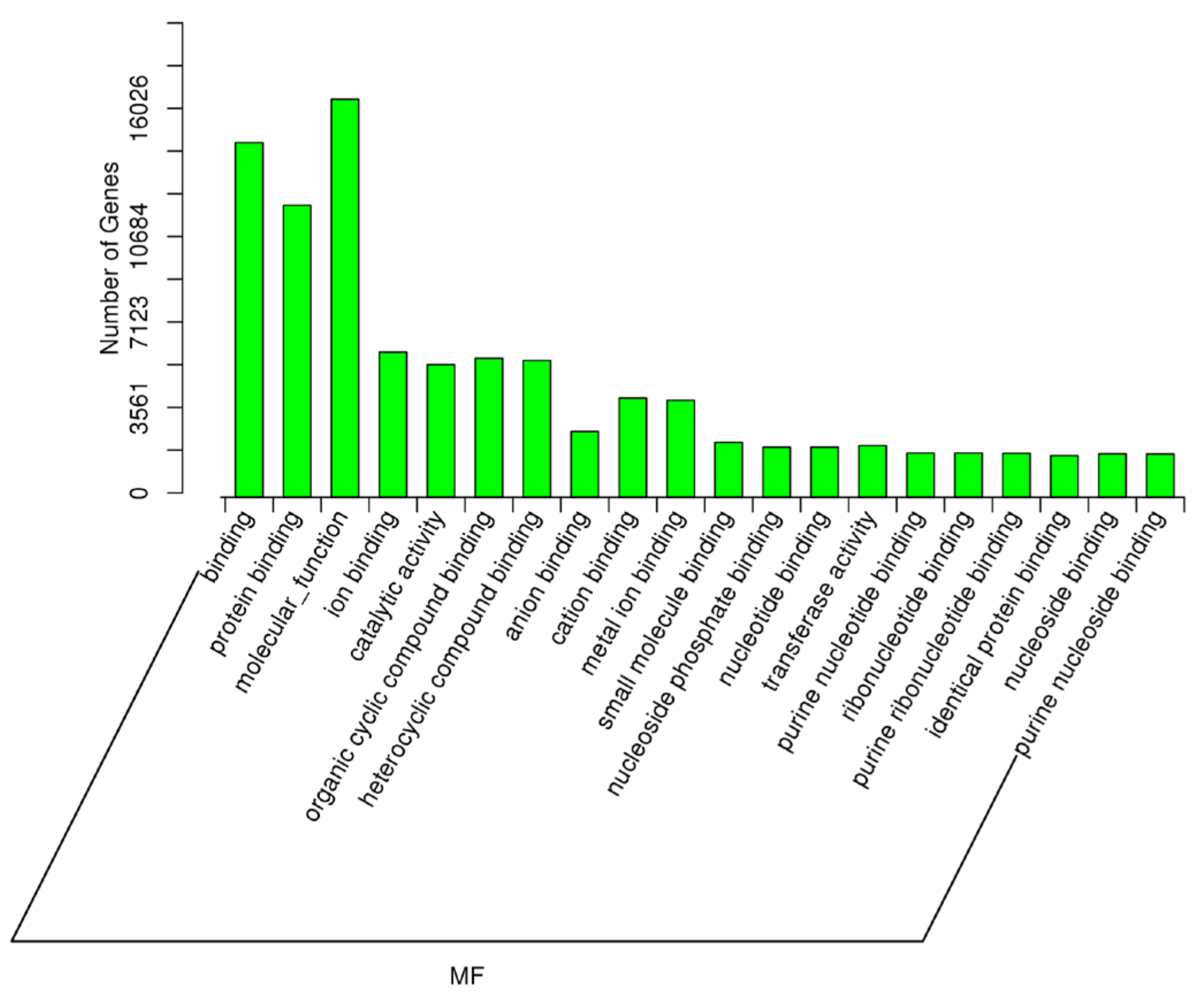
2.5. Target Prediction of Differentially Expressed miRNAs—GO and KEGG Analyses
3. Discussion
3.1. miRNA-Mediated Dysregulation in NOSPZ vs. HIGHSPZ
3.2. miRNA-Mediated Dysregulation in RSPZNP vs. RSPZP
3.3. miRNAs as Male Infertility Biomarkers and the Role of miR-34c-5p
3.4. Potential Functional Roles of Novel miRNAs Identified
3.5. Strengths and Limitations—Future Directions
4. Materials and Methods
4.1. Patient Recruitment
4.2. RNA Extraction and Sample Preparation
4.3. Small RNA Library Construction and Sequencing
4.4. Analysis of Differentially Expressed miRNAs and Functional Enrichment of Their Target Genes
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AR | Androgen Receptor |
| ART | Assisted Reproductive Technologies |
| BMI | Body Mass Index |
| CRYPTO | Cryptozoospermia |
| DAG | Diacylglycerol |
| DE | Differentially Expressed |
| ESR | Estrogen Receptor |
| GO | Gene Ontology |
| HIGHSPZ | High presence of Spermatozoa |
| ICSI | Intra-Cytoplasmic Sperm Injection |
| IL-4 | Interleukin-4 |
| IL-13 | Interleukin-13 |
| IVF | In Vitro Fertilization |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
| miRNA | MicroRNA |
| NOA | Non-obstructive Azoospermia |
| NOSPZ | No Presence of Spermatozoa |
| OA | Obstructive Azoospermia |
| PI3K | Phosphoinositide-3-Kinase |
| RAR | Retinoic Acid Receptor |
| RSPZNP | Rare Presence of Spermatozoa—No Pregnancy |
| RSPZP | Rare Presence of Spermatozoa—Pregnancy |
| RTK | Receptor Tyrosine Kinases |
| SSC | Spermatogonial Stem Cell |
| sRNA | Small RNA |
| TESA | Testicular Sperm Aspiration |
| TESE | Testicular Sperm Extraction |
| TGFB | Transforming Growth Factor-Beta |
| WHO | World Health Organization |
References
- Hull, M.G.R.; Glazener, C.M.A.; Kelly, N.J.; Conway, D.I.; Foster, P.A.; Hinton, R.A.; Coulson, C.; Lambert, P.A.; Watt, E.M.; Desai, K.M. Population Study of Causes, Treatment, and Outcome of Infertility. Br. Med. J. (Clin. Res. Ed.) 1985, 291, 1693. [Google Scholar] [CrossRef] [PubMed]
- Boitrelle, F.; Shah, R.; Saleh, R.; Henkel, R.; Kandil, H.; Chung, E.; Vogiatzi, P.; Zini, A.; Arafa, M.; Agarwal, A. The Sixth Edition of the WHO Manual for Human Semen Analysis: A Critical Review and SWOT Analysis. Life 2021, 11, 1368. [Google Scholar] [CrossRef] [PubMed]
- Cerván-Martín, M.; Castilla, J.A.; Palomino-Morales, R.J.; Carmona, F.D. Genetic Landscape of Nonobstructive Azoospermia and New Perspectives for the Clinic. J. Clin. Med. 2020, 9, 300. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, S.; Wang, M.; Yang, J. Analysis of Genes Implicated in Non-Obstructive Azoospermia. Steroids 2025, 216, 109583. [Google Scholar] [CrossRef]
- Palermo, G.; Joris, H.; Devroey, P.; Van Steirteghem, A.C. Pregnancies after Intracytoplasmic Injection of Single Spermatozoon into an Oocyte. Lancet 1992, 340, 17–18. [Google Scholar] [CrossRef]
- Devroey, P.; Liu, J.; Nagy, Z.; Goossens, A.; Tournaye, H.; Camus, M.; Van Steirteghem, A.; Silber, S. Pregnancies after Testicular Sperm Extraction and Intracytoplasmic Sperm Injection in Non-Obstructive Azoospermia. Hum. Reprod. 1995, 10, 1457–1460. [Google Scholar] [CrossRef]
- Practice Committee of the American Society for Reproductive Medicine. Management of Nonobstructive Azoospermia: A Committee Opinion. Fertil. Steril. 2018, 110, 1239–1245. [Google Scholar] [CrossRef]
- Esteves, S.C.; Miyaoka, R.; Orosz, J.E.; Agarwal, A. An Update on Sperm Retrieval Techniques for Azoospermic Males. Clinics 2013, 68, 99. [Google Scholar] [CrossRef]
- Schlegel, P.N.; Li, P.S. Microdissection TESE: Sperm Retrieval in Non-Obstructive Azoospermia. Hum. Reprod. Update 1998, 4, 439. [Google Scholar] [CrossRef]
- Khurana, K.K.; Sabanegh, E.S. Office-Based Sperm Retrieval for Treatment of Infertility. Urol. Clin. N. Am. 2013, 40, 569–579. [Google Scholar] [CrossRef]
- Vloeberghs, V.; Verheyen, G.; Haentjens, P.; Goossens, A.; Polyzos, N.P.; Tournaye, H. How Successful Is TESE-ICSI in Couples with Non-Obstructive Azoospermia? Hum. Reprod. 2015, 30, 1790–1796. [Google Scholar] [CrossRef] [PubMed]
- Marinaro, J.; Goldstein, M. Microsurgical Management of Male Infertility: Compelling Evidence That Collaboration with Qualified Male Reproductive Urologists Enhances Assisted Reproductive Technology (ART) Outcomes. J. Clin. Med. 2022, 11, 4593. [Google Scholar] [CrossRef] [PubMed]
- Deruyver, Y.; Vanderschueren, D.; Van der Aa, F. Outcome of Microdissection TESE Compared with Conventional TESE in Non-obstructive Azoospermia: A Systematic Review. Andrology 2014, 2, 20–24. [Google Scholar] [CrossRef] [PubMed]
- Tsujimura, A. Microdissection Testicular Sperm Extraction: Prediction, Outcome, and Complications. Int. J. Urol. 2007, 14, 883–889. [Google Scholar] [CrossRef]
- Krausz, C.; Riera-Escamilla, A.; Moreno-Mendoza, D.; Holleman, K.; Cioppi, F.; Algaba, F.; Pybus, M.; Friedrich, C.; Wyrwoll, M.J.; Casamonti, E.; et al. Genetic Dissection of Spermatogenic Arrest through Exome Analysis: Clinical Implications for the Management of Azoospermic Men. Genet. Med. 2020, 22, 1956–1966. [Google Scholar] [CrossRef]
- Eliveld, J.; van Wely, M.; Meicßner, A.; Repping, S.; van der Veen, F.; van Pelt, A.M.M. The risk of TESE-Induced Hypogonadism: A Systematic Review and Meta-Analysis. Hum. Reprod. Update 2018, 24, 442–454. [Google Scholar] [CrossRef]
- Eken, A.; Gulec, F. Microdissection Testicular Sperm Extraction (Micro-TESE): Predictive Value of Preoperative Hormonal Levels and Pathology in Non-Obstructive Azoospermia. Kaohsiung J. Med. Sci. 2018, 34, 103–108. [Google Scholar] [CrossRef]
- Kaltsas, A.; Stavros, S.; Kratiras, Z.; Zikopoulos, A.; Machairiotis, N.; Potiris, A.; Dimitriadis, F.; Sofikitis, N.; Chrisofos, M.; Zachariou, A. Predictors of Successful Testicular Sperm Extraction: A New Era for Men with Non-Obstructive Azoospermia. Biomedicines 2024, 12, 2679. [Google Scholar] [CrossRef]
- Klami, R.; Tomás, C.; Mankonen, H.; Perheentupa, A. ICSI Outcome after Microdissection Testicular Sperm Extraction, Testicular Sperm Aspiration and Ejaculated Sperm. Reprod. Biol. 2024, 24, 100825. [Google Scholar] [CrossRef]
- Lacey, L.; Henderson, I.; Hassan, S.; Hunter, H.; Sajjad, Y.; Akhtar, M.A. Can Preoperative Parameters Predict Successful Sperm Retrieval and Live Birth in Couples Undergoing Testicular Sperm Extraction and Intracytoplasmic Sperm Injection for Azoospermia? Middle East Fertil. Soc. J. 2021, 26, 6. [Google Scholar] [CrossRef]
- Gao, S.; Yang, X.; Xiao, X.; Yin, S.; Guan, Y.; Chen, J.; Chen, Y. Outcomes and Affecting Factors for ICSI and MicroTESE Treatments in Nonobstructive Azoospermia Patients with Different Etiologies: A Retrospective Analysis. Front. Endocrinol. 2022, 13, 1006208. [Google Scholar] [CrossRef] [PubMed]
- Kim, V.N. Small RNAs: Classification, Biogenesis, and Function. Mol. Cells 2005, 19, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Shi, J.; Zhou, T.; Chen, Q. Exploring the Expanding Universe of Small RNAs. Nat. Cell Biol. 2022, 24, 415–423. [Google Scholar] [CrossRef] [PubMed]
- Yadav, R.P.; Kotaja, N. Small RNAs in Spermatogenesis. Mol. Cell. Endocrinol. 2014, 382, 498–508. [Google Scholar] [CrossRef]
- Joshi, M.; Sethi, S.; Mehta, P.; Kumari, A.; Rajender, S. Small RNAs, Spermatogenesis, and Male Infertility: A Decade of Retrospect. Reprod. Biol. Endocrinol. 2023, 21, 106. [Google Scholar] [CrossRef]
- Chan, S.Y.; Wan, C.W.T.; Law, T.Y.S.; Chan, D.Y.L.; Fok, E.K.L. The Sperm Small RNA Transcriptome: Implications beyond Reproductive Disorder. Int. J. Mol. Sci. 2022, 23, 15716. [Google Scholar] [CrossRef]
- Mukherjee, A.; Koli, S.; Reddy, K.V.R. Regulatory Non-Coding Transcripts in Spermatogenesis: Shedding Light on “Dark Matter”. Andrology 2014, 2, 360–369. [Google Scholar] [CrossRef]
- Zhou, G.; Zhang, M.; Zhang, J.; Feng, Y.; Xie, Z.; Liu, S.; Zhu, D.; Luo, Y. The Gene Regulatory Role of Non-Coding RNAs in Non-Obstructive Azoospermia. Front. Endocrinol. 2022, 13, 959487. [Google Scholar] [CrossRef]
- Shi, Z.; Yu, M.; Guo, T.; Sui, Y.; Tian, Z.; Ni, X.; Chen, X.; Jiang, M.; Jiang, J.; Lu, Y.; et al. MicroRNAs in Spermatogenesis Dysfunction and Male Infertility: Clinical Phenotypes, Mechanisms and Potential Diagnostic Biomarkers. Front. Endocrinol. 2024, 15, 1293368. [Google Scholar] [CrossRef]
- Sinaei, R.; Jamebozorgi, K.; Mirshekarpour, H.; Poormasoumi, H.; Mahdizadeh, A.; Akbari, Z.; Taghizadeh, E. The Role of MiRNAs in the Diagnosis and Treatment of Male Infertility: A Review Study. Egypt. J. Med. Hum. Genet. 2023, 24, 40. [Google Scholar] [CrossRef]
- Salas-Huetos, A.; James, E.R.; Aston, K.I.; Carrell, D.T.; Jenkins, T.G.; Yeste, M. The Role of MiRNAs in Male Human Reproduction: A Systematic Review. Andrology 2020, 8, 7–26. [Google Scholar] [CrossRef] [PubMed]
- Du, L.; Chen, W.; Zhang, D.; Cui, Y.; He, Z. The Functions and Mechanisms of PiRNAs in Mediating Mammalian Spermatogenesis and Their Applications in Reproductive Medicine. Cell. Mol. Life Sci. 2024, 81, 379. [Google Scholar] [CrossRef] [PubMed]
- Masone, M.C. PiRNA Pathway Disruption in Human Infertility. Nat. Rev. Urol. 2024, 21, 577. [Google Scholar] [CrossRef] [PubMed]
- Condrat, C.E.; Thompson, D.C.; Barbu, M.G.; Bugnar, O.L.; Boboc, A.; Cretoiu, D.; Suciu, N.; Cretoiu, S.M.; Voinea, S.C. MiRNAs as Biomarkers in Disease: Latest Findings Regarding Their Role in Diagnosis and Prognosis. Cells 2020, 9, 276. [Google Scholar] [CrossRef]
- Chen, J.; Han, C. In Vivo Functions of MiRNAs in Mammalian Spermatogenesis. Front. Cell Dev. Biol. 2023, 11, 1154938. [Google Scholar] [CrossRef]
- Lian, J.; Zhang, X.; Tian, H.; Liang, N.; Wang, Y.; Liang, C.; Li, X.; Sun, F. Altered MicroRNA Expression in Patients with Non-Obstructive Azoospermia. Reprod. Biol. Endocrinol. 2009, 7, 13. [Google Scholar] [CrossRef]
- Piryaei, F.; Mozdarani, H.; Sadighi Gilani, M.A.; Rajender, S.; Finelli, R.; Darestanifarahani, M.; Sarli, A.; Mehta, P.; Agarwal, A. Global Analysis in Nonobstructive Azoospermic Testis Identifies MiRNAs Critical to Spermatogenesis. Andrologia 2023, 2023, 2074931. [Google Scholar] [CrossRef]
- Chen, X.; Che, D.; Zhang, P.; Li, X.; Yuan, Q.; Liu, T.; Guo, J.; Feng, T.; Wu, L.; Liao, M.; et al. Profiling of MiRNAs in Porcine Germ Cells during Spermatogenesis. Reproduction 2017, 154, 789–798. [Google Scholar] [CrossRef]
- Sree, S.; Radhakrishnan, K.; Indu, S.; Kumar, P.G. Dramatic Changes in 67 MiRNAs during Initiation of First Wave of Spermatogenesis in Mus Musculus Testis: Global Regulatory Insights Generated by MiRNA-MRNA Network Analysis. Biol. Reprod. 2014, 91, 1–11. [Google Scholar] [CrossRef]
- Abu-Halima, M.; Hammadeh, M.; Backes, C.; Fischer, U.; Leidinger, P.; Lubbad, A.M.; Keller, A.; Meese, E. Panel of Five MicroRNAs as Potential Biomarkers for the Diagnosis and Assessment of Male Infertility. Fertil. Steril. 2014, 102, 989–997. [Google Scholar] [CrossRef]
- Fontana, L.; Sirchia, S.M.; Pesenti, C.; Colpi, G.M.; Miozzo, M.R. Non-Invasive Biomarkers for Sperm Retrieval in Non-Obstructive Patients: A Comprehensive Review. Front. Endocrinol. 2024, 15, 1349000. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Yang, F.; Dong, L.; Chang, D.; Yu, X. Seminal Plasma Biomarkers for Predicting Successful Sperm Retrieval in Patients with Nonobstructive Azoospermia: A Narrative Review of Human Studies. Basic Clin. Androl. 2023, 33, 9. [Google Scholar] [CrossRef] [PubMed]
- Willems, M.; Devriendt, C.; Olsen, C.; Caljon, B.; Janssen, T.; Gies, I.; Vloeberghs, V.; Tournaye, H.; Van Saen, D.; Goossens, E. Micro RNA in Semen/Urine from Non-Obstructive Azoospermia Patients as Biomarkers to Predict the Presence of Testicular Spermatozoa and Spermatogonia. Life 2023, 13, 616. [Google Scholar] [CrossRef] [PubMed]
- Chen, K.Q.; Wei, B.H.; Hao, S.L.; Yang, W.X. The PI3K/AKT Signaling Pathway: How Does It Regulate Development of Sertoli Cells and Spermatogenic Cells? Histol. Histopathol. 2022, 37, 621–636. [Google Scholar] [CrossRef]
- Deng, C.-Y.; Lv, M.; Luo, B.-H.; Zhao, S.-Z.; Mo, Z.-C.; Xie, Y.-J. The Role of the PI3K/AKT/MTOR Signalling Pathway in Male Reproduction. Curr. Mol. Med. 2021, 21, 539–548. [Google Scholar] [CrossRef]
- Zhao, Y.; Yang, W. The Effects of Hormone-Mediated PI3K/AKT Signaling on Spermatogenesis in Sertoli Cells. Biocell 2023, 47, 1709–1725. [Google Scholar] [CrossRef]
- Martello, A.; Mellis, D.; Meloni, M.; Howarth, A.; Ebner, D.; Caporali, A.; Al Haj Zen, A. Phenotypic MiRNA Screen Identifies MiR-26b to Promote the Growth and Survival of Endothelial Cells. Mol. Ther. Nucleic Acids 2018, 13, 29. [Google Scholar] [CrossRef]
- Xing, X.; Guo, S.; Zhang, G.; Liu, Y.; Bi, S.; Wang, X.; Lu, Q. MiR-26a-5p Protects against Myocardial Ischemia/Reperfusion Injury by Regulating the PTEN/PI3K/AKT Signaling Pathway. Braz. J. Med. Biol. Res. 2020, 53, e9106. [Google Scholar] [CrossRef]
- Ran, M.; Weng, B.; Cao, R.; Li, Z.; Peng, F.; Luo, H.; Gao, H.; Chen, B. MiR-26a Inhibits Proliferation and Promotes Apoptosis in Porcine Immature Sertoli Cells by Targeting the PAK2 Gene. Reprod. Domest. Anim. 2018, 53, 1375–1385. [Google Scholar] [CrossRef]
- Teng, F.; Hu, F.; Zhang, M. MicroRNA-125a-5p Modulates the Proliferation and Apoptosis of TM4 Sertoli Cells by Targeting RAB3D and Regulating the PI3K/AKT Signaling Pathway. Mol. Hum. Reprod. 2021, 27, gaab049. [Google Scholar] [CrossRef]
- Fu, K.; Zhang, L.; Liu, R.; Shi, Q.; Li, X.; Wang, M. MiR-125 Inhibited Cervical Cancer Progression by Regulating VEGF and PI3K/AKT Signaling Pathway. World J. Surg. Oncol. 2020, 18, 115. [Google Scholar] [CrossRef] [PubMed]
- Cooke, P.S.; Nanjappa, M.K.; Ko, C.; Prins, G.S.; Hess, R.A. Estrogens in Male Physiology. Physiol. Rev. 2017, 97, 995. [Google Scholar] [CrossRef] [PubMed]
- Carreau, S.; de Vienne, C.; Galeraud-Denis, I. Aromatase and Estrogens in Man Reproduction: A Review and Latest Advances. Adv. Med. Sci. 2008, 53, 139–144. [Google Scholar] [CrossRef]
- Dostalova, P.; Zatecka, E.; Dvorakova-Hortova, K. Of Oestrogens and Sperm: A Review of the Roles of Oestrogens and Oestrogen Receptors in Male Reproduction. Int. J. Mol. Sci. 2017, 18, 904. [Google Scholar] [CrossRef]
- Hess, R.A. Estrogen in the Adult Male Reproductive Tract: A Review. Reprod. Biol. Endocrinol. 2003, 1, 52. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, C.; Wang, J.; Xiao, J.; Gatalica, Z.; Recker, R.R.; Xiao, G.G. Let-7 Family MiRNAs Regulate Estrogen Receptor Alpha Signaling in Estrogen Receptor Positive Breast Cancer. Breast Cancer Res. Treat. 2011, 127, 69–80. [Google Scholar] [CrossRef]
- Abhari, A.; Zarghami, N.; Shahnazi, V.; Barzegar, A.; Farzadi, L.; Karami, H.; Zununi Vahed, S.; Nouri, M.; Vahed, Z.S.; Significance, N.M. Significance of MicroRNA Targeted Estrogen Receptor in Male Fertility. Iran. J. Basic Med. Sci. 2014, 17, 81. [Google Scholar]
- Guarducci, E.; Nuti, F.; Becherini, L.; Rotondi, M.; Balercia, G.; Forti, G.; Krausz, C. Estrogen Receptor Alpha Promoter Polymorphism: Stronger Estrogen Action Is Coupled with Lower Sperm Count. Hum. Reprod. 2006, 21, 994–1001. [Google Scholar] [CrossRef]
- Christian, M.; W-F Lam, E.; SC Wilson, M.; J Brosens, J. FOXO Transcription Factors and Their Role in Disorders of the Female Reproductive Tract. Curr. Drug Targets 2011, 12, 1291–1302. [Google Scholar] [CrossRef]
- Goertz, M.J.; Wu, Z.; Gallardo, T.D.; Hamra, F.K.; Castrillon, D.H. Foxo1 Is Required in Mouse Spermatogonial Stem Cells for Their Maintenance and the Initiation of Spermatogenesis. J. Clin. Investig. 2011, 121, 3456–3466. [Google Scholar] [CrossRef]
- Duwe, L.; Munoz-Garrido, P.; Lewinska, M.; Lafuente-Barquero, J.; Satriano, L.; Høgdall, D.; Taranta, A.; Nielsen, B.S.; Ghazal, A.; Matter, M.S.; et al. MicroRNA-27a-3p Targets FoxO Signalling to Induce Tumour-like Phenotypes in Bile Duct Cells. J. Hepatol. 2023, 78, 364–375. [Google Scholar] [CrossRef] [PubMed]
- Young, J.C.; Wakitani, S.; Loveland, K.L. TGF-β Superfamily Signaling in Testis Formation and Early Male Germline Development. Semin. Cell Dev. Biol. 2015, 45, 94–103. [Google Scholar] [CrossRef] [PubMed]
- Moreno, S.G.; Attali, M.; Allemand, I.; Messiaen, S.; Fouchet, P.; Coffigny, H.; Romeo, P.H.; Habert, R. TGFβ Signaling in Male Germ Cells Regulates Gonocyte Quiescence and Fertility in Mice. Dev. Biol. 2010, 342, 74–84. [Google Scholar] [CrossRef]
- Loveland, K.L.; Hogarth, C.; Mendis, S.; Efthymiadis, A.; Ly, J.; Itman, C.; Meachem, S.; Brown, C.W.; Jans, D.A. Drivers of Germ Cell Maturation. Ann. N. Y. Acad. Sci. 2005, 1061, 173–182. [Google Scholar] [CrossRef]
- Liu, W.; Du, L.; Li, J.; He, Y.; Tang, M. Microenvironment of Spermatogonial Stem Cells: A Key Factor in the Regulation of Spermatogenesis. Stem Cell Res. Ther. 2024, 15, 294. [Google Scholar] [CrossRef]
- Abé, K.; Eto, K.; Abé, S.I. Epidermal Growth Factor Mediates Spermatogonial Proliferation in Newt Testis. Reprod. Biol. Endocrinol. 2008, 6, 7. [Google Scholar] [CrossRef]
- Aminmalek, M.; Mashayekhi, F.; Salehi, Z. Epidermal Growth Factor +61A/G (RS4444903) Promoter Polymorphism and Serum Levels Are Linked to Idiopathic Male Infertility. Br. J. Biomed. Sci. 2021, 78, 92–94. [Google Scholar] [CrossRef]
- Saucedo, L.; Buffa, G.N.; Rosso, M.; Guillardoy, T.; Góngora, A.; Munuce, M.J.; Vazquez-Levin, M.H.; Marín-Briggiler, C. Fibroblast Growth Factor Receptors (FGFRs) in Human Sperm: Expression, Functionality and Involvement in Motility Regulation. PLoS ONE 2015, 10, e0127297. [Google Scholar] [CrossRef]
- Cotton, L.M.; O’Bryan, M.K.; Hinton, B.T. Cellular Signaling by Fibroblast Growth Factors (FGFs) and Their Receptors (FGFRs) in Male Reproduction. Endocr. Rev. 2008, 29, 193–216. [Google Scholar] [CrossRef]
- Metallinou, C.; Staneloudi, C.; Nikolettos, K.; Asimakopoulos, B. NGF, EPO, and IGF-1 in the Male Reproductive System. J. Clin. Med. 2024, 13, 2918. [Google Scholar] [CrossRef]
- Kierszenbaum, A.L. Tyrosine Protein Kinases and Spermatogenesis: Truncation Matters. Mol. Reprod. Dev. 2006, 73, 399–403. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wang, H.; Qi, N.; Wu, H.; Xiong, W.; Ma, J.; Lu, Q.; Han, D. Functions of TAM RTKs in Regulating Spermatogenesis and Male Fertility in Mice. Reproduction 2009, 138, 655–666. [Google Scholar] [CrossRef] [PubMed]
- Xiong, W.; Chen, Y.; Wang, H.; Wang, H.; Wu, H.; Lu, Q.; Han, D. Gas6 and the Tyro 3 Receptor Tyrosine Kinase Subfamily Regulate the Phagocytic Function of Sertoli Cells. Reproduction 2008, 135, 77–87. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Wei, H.-F.; Song, C.-W.; Zhu, C.-C. Discoidin Domain Receptor 2 Expression Increases Phagocytotic Capacity in Sertoli Cells of Sertoli Cell-Only Syndrome Testes. Am. J. Transl. Res. 2022, 14, 6067. [Google Scholar]
- Kuang, H.; Zhang, C.; Zhang, W.; Cai, H.; Yang, L.; Yuan, N.; Yuan, Y.; Yang, Y.; Zuo, C.; Zhong, F. Electroacupuncture Improves Intestinal Motility through Exosomal MiR-34c-5p Targeting SCF/c-Kit Signaling Pathway in Slow Transit Constipation Model Rats. Evid.-Based Complement. Alternat. Med. 2022, 2022, 8043841. [Google Scholar] [CrossRef]
- Feng, H.L.; Sandlow, J.I.; Sparks, A.E.T.; Sandra, A.; Zheng, L.J. Decreased Expression of the C-Kit Receptor is Associated with Increased Apoptosis in Subfertile Human Testes. Fertil. Steril. 1999, 71, 85–89. [Google Scholar] [CrossRef]
- Qin, W.Y.; Feng, S.C.; Sun, Y.Q.; Jiang, G.Q. MiR-96-5p Promotes Breast Cancer Migration by Activating MEK/ERK Signaling. J. Gene Med. 2020, 22, e3188. [Google Scholar] [CrossRef]
- Zolfaghari, N.; Soheili, Z.S.; Samiei, S.; Latifi-Navid, H.; Hafezi-Moghadam, A.; Ahmadieh, H.; Rezaei-Kanavi, M. MicroRNA-96 Targets the INS/AKT/GLUT4 Signaling Axis: Association with and Effect on Diabetic Retinopathy. Heliyon 2023, 9, e15539. [Google Scholar] [CrossRef]
- Lackey, B.R.; Gray, S.L. Second Messengers, Steroids and Signaling Cascades: Crosstalk in Sperm Development and Function. Gen. Comp. Endocrinol. 2015, 224, 294–302. [Google Scholar] [CrossRef]
- Loveland, K.L.; Klein, B.; Pueschl, D.; Indumathy, S.; Bergmann, M.; Loveland, B.E.; Hedger, M.P.; Schuppe, H.C. Cytokines in Male Fertility and Reproductive Pathologies: Immunoregulation and Beyond. Front. Endocrinol. 2017, 8, 307. [Google Scholar] [CrossRef]
- Syriou, V.; Papanikolaou, D.; Kozyraki, A.; Goulis, D.G. Cytokines and Male Infertility. Eur. Cytokine Netw. 2018, 29, 73–82. [Google Scholar] [CrossRef] [PubMed]
- Dutta, S.; Sengupta, P.; Slama, P.; Roychoudhury, S. Oxidative Stress, Testicular Inflammatory Pathways, and Male Reproduction. Int. J. Mol. Sci. 2021, 22, 10043. [Google Scholar] [CrossRef]
- Hu, J.; Huang, S.; Liu, X.; Zhang, Y.; Wei, S.; Hu, X. MiR-155: An Important Role in Inflammation Response. J. Immunol. Res. 2022, 2022, 7437281. [Google Scholar] [CrossRef]
- Xu, J.; He, C.; Fang, Y.W.; Hu, Z.Y.; Peng, M.L.; Chen, Y.Y.; Su, Y.F.; Liu, C.Y.; Zhang, H.P.; Zhao, K. Testicular Exosomes Disturb the Immunosuppressive Phenotype of Testicular Macrophages Mediated by MiR-155-5p in Uropathogenic Escherichia Coli-Induced Orchitis. Asian J. Androl. 2022, 25, 389. [Google Scholar] [CrossRef]
- Doherty, C.M.; Tarchala, S.M.; Radwanska, E.; de Jonge, C.J. Characterization of Two Second Messenger Pathways and Their Interactions in Eliciting the Human Sperm Acrosome Reaction. J. Androl. 1995, 16, 36–46. [Google Scholar] [CrossRef]
- Xu, K.; Chen, C.; Wu, Y.; Wu, M.; Lin, L. Advances in MiR-132-Based Biomarker and Therapeutic Potential in the Cardiovascular System. Front. Pharmacol. 2021, 12, 751487. [Google Scholar] [CrossRef]
- Stewart, T.A.; Davis, F.M. An Element for Development: Calcium Signaling in Mammalian Reproduction and Development. Biochim. Biophys. Acta (BBA)-Mol. Cell Res. 2019, 1866, 1230–1238. [Google Scholar] [CrossRef]
- Finkelstein, M.; Etkovitz, N.; Breitbart, H. Ca2+ Signaling in Mammalian Spermatozoa. Mol. Cell. Endocrinol. 2020, 516, 110953. [Google Scholar] [CrossRef]
- Guido, C.; Perrotta, I.; Panza, S.; Middea, E.; Avena, P.; Santoro, M.; Marsico, S.; Imbrogno, P.; Andò, S.; Aquila, S. Human Sperm Physiology: Estrogen Receptor Alpha (ERα) and Estrogen Receptor Beta (ERβ) Influence Sperm Metabolism and May Be Involved in the Pathophysiology of Varicocele-Associated Male Infertility. J. Cell. Physiol. 2011, 226, 3403–3412. [Google Scholar] [CrossRef]
- Hess, R.A.; Cooke, P.S. Estrogen in the Male: A Historical Perspective. Biol. Reprod. 2018, 99, 27–44. [Google Scholar] [CrossRef]
- Ded, L.; Dostalova, P.; Dorosh, A.; Dvorakova-Hortova, K.; Peknicova, J. Effect of Estrogens on Boar Sperm Capacitation in Vitro. Reprod. Biol. Endocrinol. 2010, 8, 87. [Google Scholar] [CrossRef] [PubMed]
- Martinot, E.; Sedes, L.; Baptissart, M.; Holota, H.; Angelique, D.H.; Beaudoin, C.; Volle, D.H. Nuclear Receptor Networks in Male Fertility. Endocr. Abstr. 2018, 54, IS12. [Google Scholar] [CrossRef]
- Monrose, M.; Thirouard, L.; Garcia, M.; Holota, H.; De Haze, A.; Caira, F.; Beaudoin, C.; Volle, D.H. New Perspectives on PPAR, VDR and FXRα as New Actors in Testicular Pathophysiology. Mol. Aspects Med. 2021, 78, 100886. [Google Scholar] [CrossRef] [PubMed]
- O’Hara, L.; Smith, L.B. Androgen Receptor Roles in Spermatogenesis and Infertility. Best Pract. Res. Clin. Endocrinol. Metab. 2015, 29, 595–605. [Google Scholar] [CrossRef]
- Dohle, G.R.; Smit, M.; Weber, R.F.A. Androgens and Male Fertility. World J. Urol. 2003, 21, 341–345. [Google Scholar] [CrossRef]
- Zhao, Y.; Deng, S.; Li, C.; Cao, J.; Wu, A.; Chen, M.; Ma, X.; Wu, S.; Lian, Z. The Role of Retinoic Acid in Spermatogenesis and Its Application in Male Reproduction. Cells 2024, 13, 1092. [Google Scholar] [CrossRef]
- Pakpahan, C.; Margiana, R.; Pangestu, M. A Systematic Review of Retinoic Acid in the Journey of Spermatogonium to Spermatozoa: From Basic to Clinical Application. F1000Research 2022, 11, 552. [Google Scholar] [CrossRef]
- Villani, M.T.; Morini, D.; Spaggiari, G.; Falbo, A.I.; Melli, B.; La Sala, G.B.; Romeo, M.; Simoni, M.; Aguzzoli, L.; Santi, D. Are Sperm Parameters Able to Predict the Success of Assisted Reproductive Technology? A Retrospective Analysis of over 22,000 Assisted Reproductive Technology Cycles. Andrology 2021, 10, 310. [Google Scholar] [CrossRef]
- Conflitti, A.C.; Cicolani, G.; Buonacquisto, A.; Pallotti, F.; Faja, F.; Bianchini, S.; Blaconà, G.; Bruno, S.M.; Linari, A.; Lucarelli, M.; et al. Sperm DNA Fragmentation and Sperm-Borne MiRNAs: Molecular Biomarkers of Embryo Development? Int. J. Mol. Sci. 2023, 24, 1007. [Google Scholar] [CrossRef]
- Abu-Halima, M.; Backes, C.; Leidinger, P.; Keller, A.; Lubbad, A.M.; Hammadeh, M.; Meese, E. MicroRNA Expression Profiles in Human Testicular Tissues of Infertile Men with Different Histopathologic Patterns. Fertil. Steril. 2014, 101, 78–86. [Google Scholar] [CrossRef]
- Dorostghoal, M.; Galehdari, H.; Hemadi, M.; Davoodi, E. Sperm MiR-34c-5p Transcript Content and Its Association with Sperm Parameters in Unexplained Infertile Men. Reprod. Sci. 2022, 29, 84–90. [Google Scholar] [CrossRef] [PubMed]
- Finocchi, F.; Pelloni, M.; Balercia, G.; Pallotti, F.; Radicioni, A.F.; Lenzi, A.; Lombardo, F.; Paoli, D. Seminal Plasma MiRNAs in Klinefelter Syndrome and in Obstructive and Non-Obstructive Azoospermia. Mol. Biol. Rep. 2020, 47, 4373–4382. [Google Scholar] [CrossRef] [PubMed]
- Momeni, A.; Najafipour, R.; Hamta, A.; Jahani, S.; Moghbelinejad, S. Expression and Methylation Pattern of Hsa-MiR-34 Family in Sperm Samples of Infertile Men. Reprod. Sci. 2020, 27, 301–308. [Google Scholar] [CrossRef] [PubMed]
- Fang, N.; Cao, C.; Wen, Y.; Wang, X.; Yuan, S.; Huang, X. MicroRNA Profile Comparison of Testicular Tissues Derived from Successful and Unsuccessful Microdissection Testicular Sperm Extraction Retrieval in Non-Obstructive Azoospermia Patients. Reprod. Fertil. Dev. 2019, 31, 671–682. [Google Scholar] [CrossRef]
- Cui, L.; Fang, L.; Shi, B.; Qiu, S.; Ye, Y. Spermatozoa Micro Ribonucleic Acid–34c Level Is Correlated with Intracytoplasmic Sperm Injection Outcomes. Fertil. Steril. 2015, 104, 312–317. [Google Scholar] [CrossRef]
- Pantos, K.; Grigoriadis, S.; Tomara, P.; Louka, I.; Maziotis, E.; Pantou, A.; Nitsos, N.; Vaxevanoglou, T.; Kokkali, G.; Agarwal, A.; et al. Investigating the Role of the MicroRNA-34/449 Family in Male Infertility: A Critical Analysis and Review of the Literature. Front. Endocrinol. 2021, 12, 709943. [Google Scholar] [CrossRef]
- Coultas, L.; Bouillet, P.; Loveland, K.L.; Meachem, S.; Perlman, H.; Adams, J.M.; Strasser, A. Concomitant Loss of Proapoptotic BH3-Only Bcl-2 Antagonists Bik and Bim Arrests Spermatogenesis. EMBO J. 2005, 24, 3963–3973. [Google Scholar] [CrossRef]
- Zhang, L.; Tang, J.; Haines, C.J.; Feng, H.L.; Lai, L.; Teng, X.; Han, Y. C-Kit and Its Related Genes in Spermatogonial Differentiation. Spermatogenesis 2011, 1, 186. [Google Scholar] [CrossRef]
- Hirsch, M.N.; Pallotti, F.; Faja, F.; Buonacquisto, A.; Cicolani, G.; Conflitti, A.C.; Di Chiano, S.; Lenzi, A.; Lombardo, F.; Paoli, D. Bladder Neck Obstruction: Experience and Management in a Sperm Bank. Life 2023, 13, 842. [Google Scholar] [CrossRef]
- Mekhaimar, A.; Goble, M.; Brunckhorst, O.; Alnajjar, H.M.; Ralph, D.; Muneer, A.; Ahmed, K. A Systematic Review of Transurethral Resection of Ejaculatory Ducts for the Management of Ejaculatory Duct Obstruction. Turk. J. Urol. 2020, 46, 335–347. [Google Scholar] [CrossRef]
- Achermann, A.P.P.; Esteves, S.C. Diagnosis and Management of Infertility Due to Ejaculatory Duct Obstruction: Summary Evidence. Int. Braz. J. Urol. Off. J. Braz. Soc. Urol. 2020, 47, 868. [Google Scholar] [CrossRef] [PubMed]
- Jarvi, K.; Lo, K.; Grober, E.; Mak, V.; Fischer, A.; Grantmyre, J.; Zini, A.; Chan, P.; Patry, G.; Chow, V.; et al. The Workup and Management of Azoospermic Males. Can. Urol. Assoc. J. 2015, 9, 229–235. [Google Scholar] [CrossRef] [PubMed]
- Du Plessis, S.S.; Agarwal, A.; Sabanegh, E.S. Male Infertility: A Complete Guide to Lifestyle and Environmental Factors; Springer: Berlin, Germany, 2014; p. 268. [Google Scholar]
- Chen, S.; An, G.; Wang, H.; Wu, X.; Ping, P.; Hu, L.; Chen, Y.; Fan, J.; Cheng, C.Y.; Sun, F. Human Obstructive (Postvasectomy) and Nonobstructive Azoospermia–Insights from ScRNA-Seq and Transcriptome Analysis. Genes Dis. 2022, 9, 766–776. [Google Scholar] [CrossRef] [PubMed]
- Oraiopoulou, C.; Vorniotaki, A.; Taki, E.; Papatheodorou, A.; Christoforidis, N.; Chatziparasidou, A. The Impact of Fresh and Frozen Testicular Tissue Quality on Embryological and Clinical Outcomes. Andrologia 2021, 53, e14040. [Google Scholar] [CrossRef]
- Bolger, A.M.; Lohse, M.; Usadel, B. Trimmomatic: A Flexible Trimmer for Illumina Sequence Data. Bioinformatics 2014, 30, 2114. [Google Scholar] [CrossRef]
- Langmead, B.; Salzberg, S.L. Fast Gapped-Read Alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef]
- Langmead, B.; Wilks, C.; Antonescu, V.; Charles, R. Scaling Read Aligners to Hundreds of Threads on General-Purpose Processors. Bioinformatics 2019, 35, 421–432. [Google Scholar] [CrossRef]
- Kozomara, A.; Birgaoanu, M.; Griffiths-Jones, S. MiRBase: From MicroRNA Sequences to Function. Nucleic Acids Res. 2019, 47, D155–D162. [Google Scholar] [CrossRef]
- Friedländer, M.R.; MacKowiak, S.D.; Li, N.; Chen, W.; Rajewsky, N. MiRDeep2 Accurately Identifies Known and Hundreds of Novel MicroRNA Genes in Seven Animal Clades. Nucleic Acids Res. 2012, 40, 37–52. [Google Scholar] [CrossRef]
- Aparicio-Puerta, E.; Lebrón, R.; Rueda, A.; Gómez-Martín, C.; Giannoukakos, S.; Jaspez, D.; Medina, J.M.; Zubkovic, A.; Jurak, I.; Fromm, B.; et al. SRNAbench and SRNAtoolbox 2019: Intuitive Fast Small RNA Profiling and Differential Expression. Nucleic Acids Res. 2019, 47, W530–W535. [Google Scholar] [CrossRef]
- Ontiveros-Palacios, N.; Cooke, E.; Nawrocki, E.P.; Triebel, S.; Marz, M.; Rivas, E.; Griffiths-Jones, S.; Petrov, A.I.; Bateman, A.; Sweeney, B. Rfam 15: RNA Families Database in 2025. Nucleic Acids Res. 2013, 1, 13–14. [Google Scholar] [CrossRef] [PubMed]
- Wen, M.; Shen, Y.; Shi, S.; Tang, T. MiREvo: An Integrative MicroRNA Evolutionary Analysis Platform for Next-Generation Sequencing Experiments. BMC Bioinform. 2012, 13, 140. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated Estimation of Fold Change and Dispersion for RNA-Seq Data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Storey, J.D. The Positive False Discovery Rate: A Bayesian Interpretation and the q-Value. Ann. Stat. 2003, 31, 2013–2035. [Google Scholar] [CrossRef]
- Betel, D.; Wilson, M.; Gabow, A.; Marks, D.S.; Sander, C. The MicroRNA.Org Resource: Targets and Expression. Nucleic Acids Res. 2008, 36, D149–D153. [Google Scholar] [CrossRef]
- Ashburner, M.; Ball, C.A.; Blake, J.A.; Botstein, D.; Butler, H.; Cherry, J.M.; Davis, A.P.; Dolinski, K.; Dwight, S.S.; Eppig, J.T.; et al. Gene Ontology: Tool for the Unification of Biology. Nat. Genet. 2000, 25, 25–29. [Google Scholar] [CrossRef]
- Consortium, T.G.O.; Aleksander, S.A.; Balhoff, J.; Carbon, S.; Cherry, J.M.; Drabkin, H.J.; Ebert, D.; Feuermann, M.; Gaudet, P.; Harris, N.L.; et al. The Gene Ontology Knowledgebase in 2023. Genetics 2023, 224, iyad031. [Google Scholar] [CrossRef]
- Kanehisa, M.; Sato, Y.; Kawashima, M.; Furumichi, M.; Tanabe, M. KEGG as a Reference Resource for Gene and Protein Annotation. Nucleic Acids Res. 2016, 44, D457–D462. [Google Scholar] [CrossRef]
- Kanehisa, M.; Furumichi, M.; Sato, Y.; Kawashima, M.; Ishiguro-Watanabe, M. KEGG for Taxonomy-Based Analysis of Pathways and Genomes. Nucleic Acids Res. 2023, 51, D587–D592. [Google Scholar] [CrossRef]
- Wu, J.; Mao, X.; Cai, T.; Luo, J.; Wei, L. KOBAS Server: A Web-Based Platform for Automated Annotation and Pathway Identification. Nucleic Acids Res. 2006, 34, W720. [Google Scholar] [CrossRef]
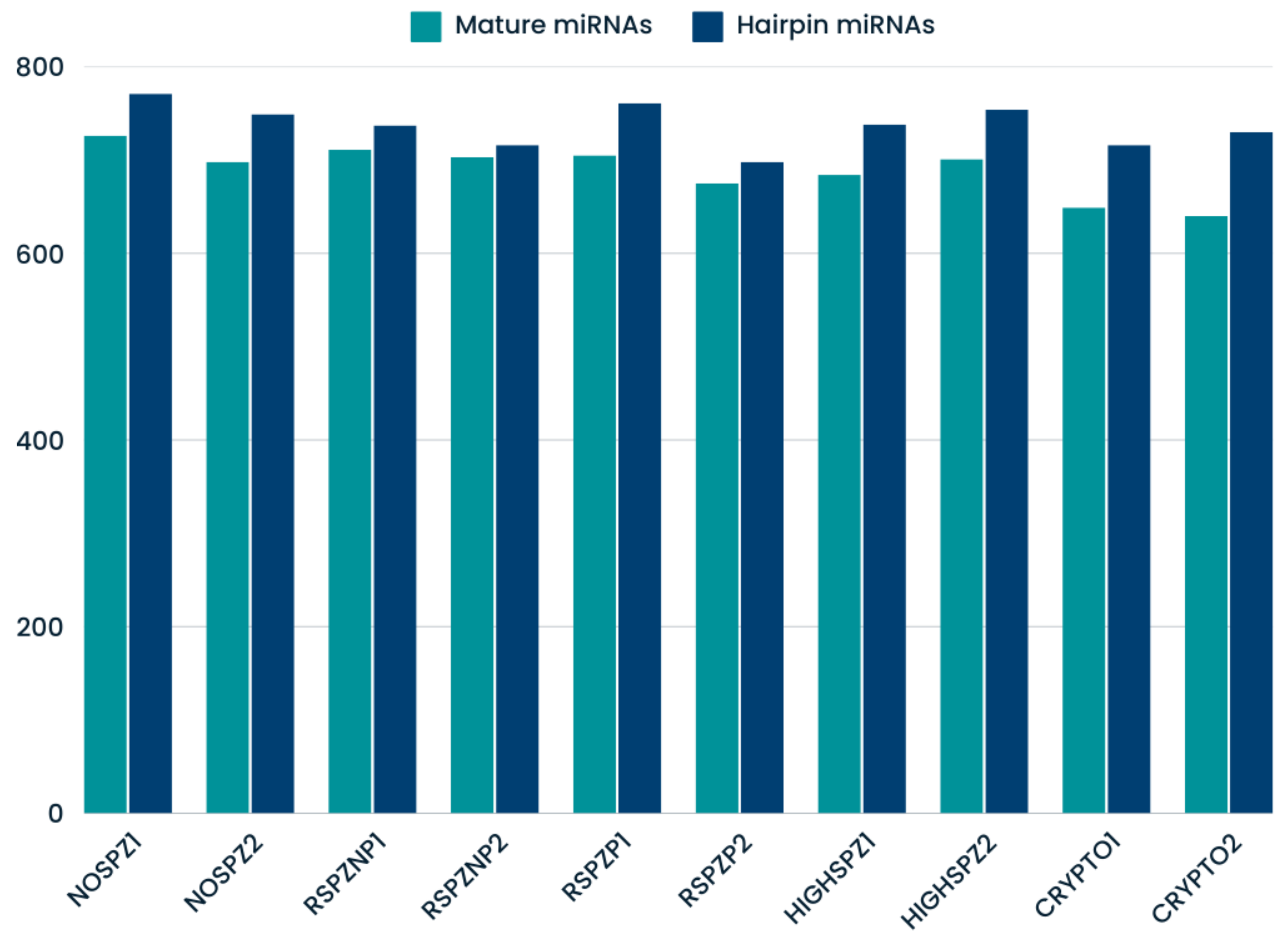

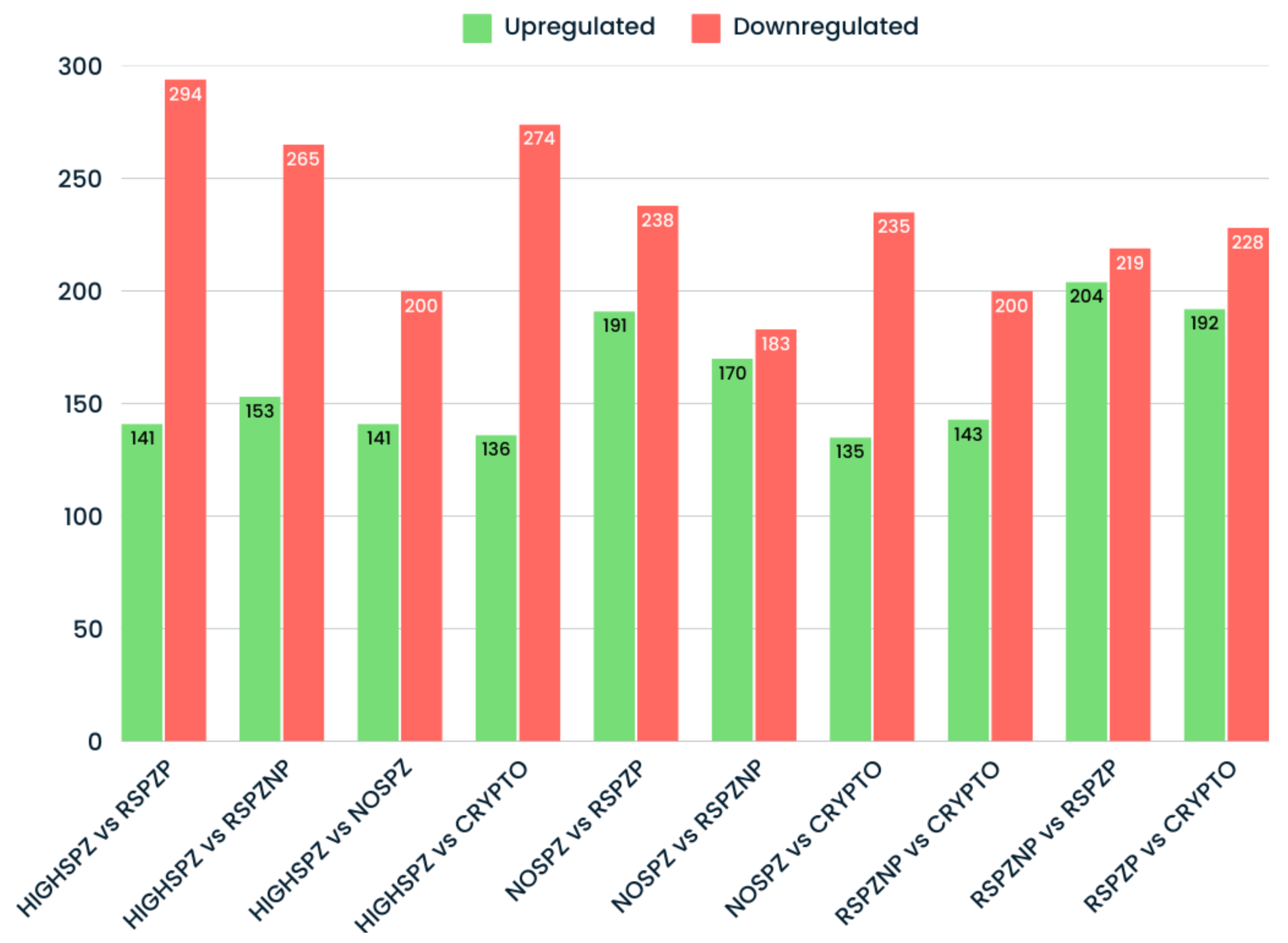
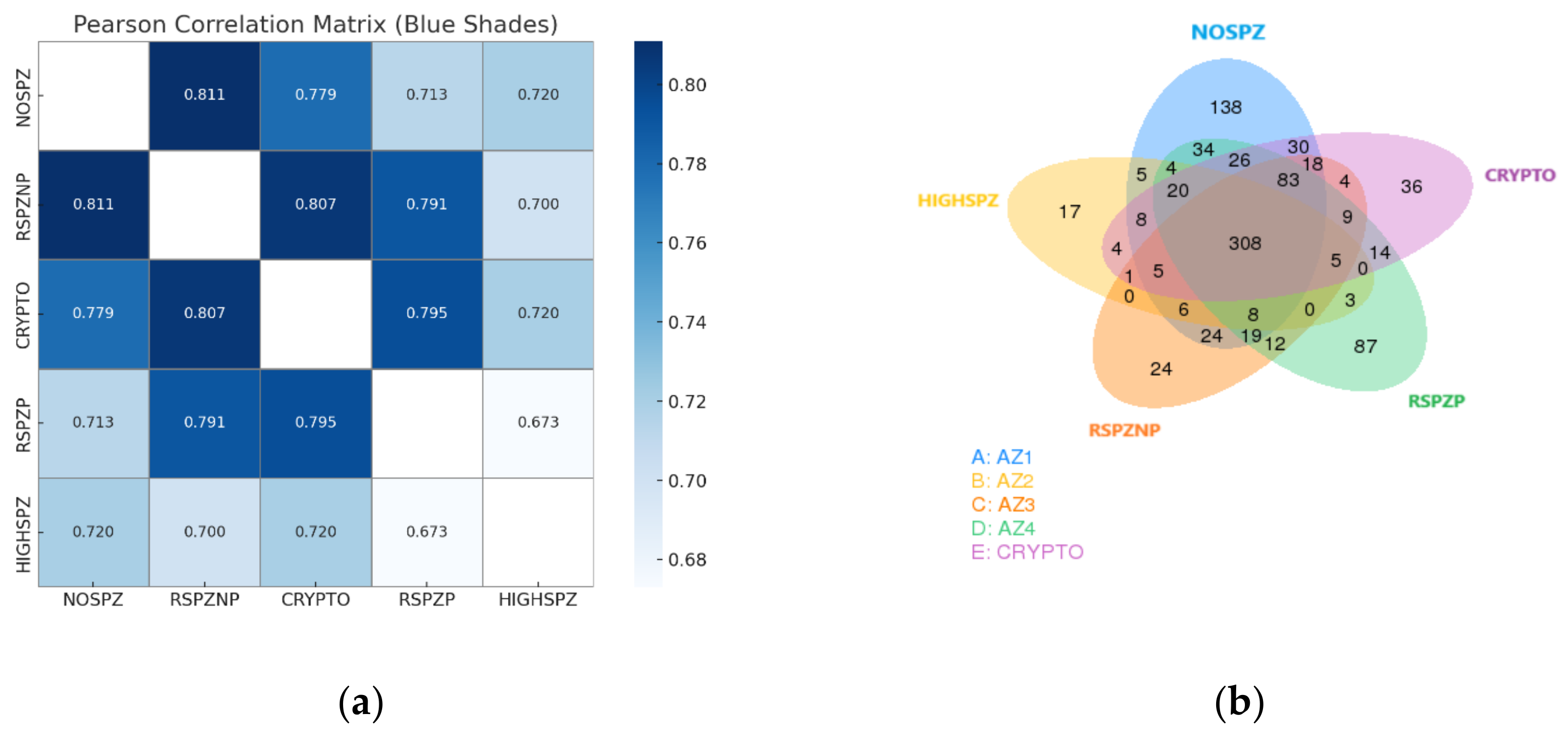
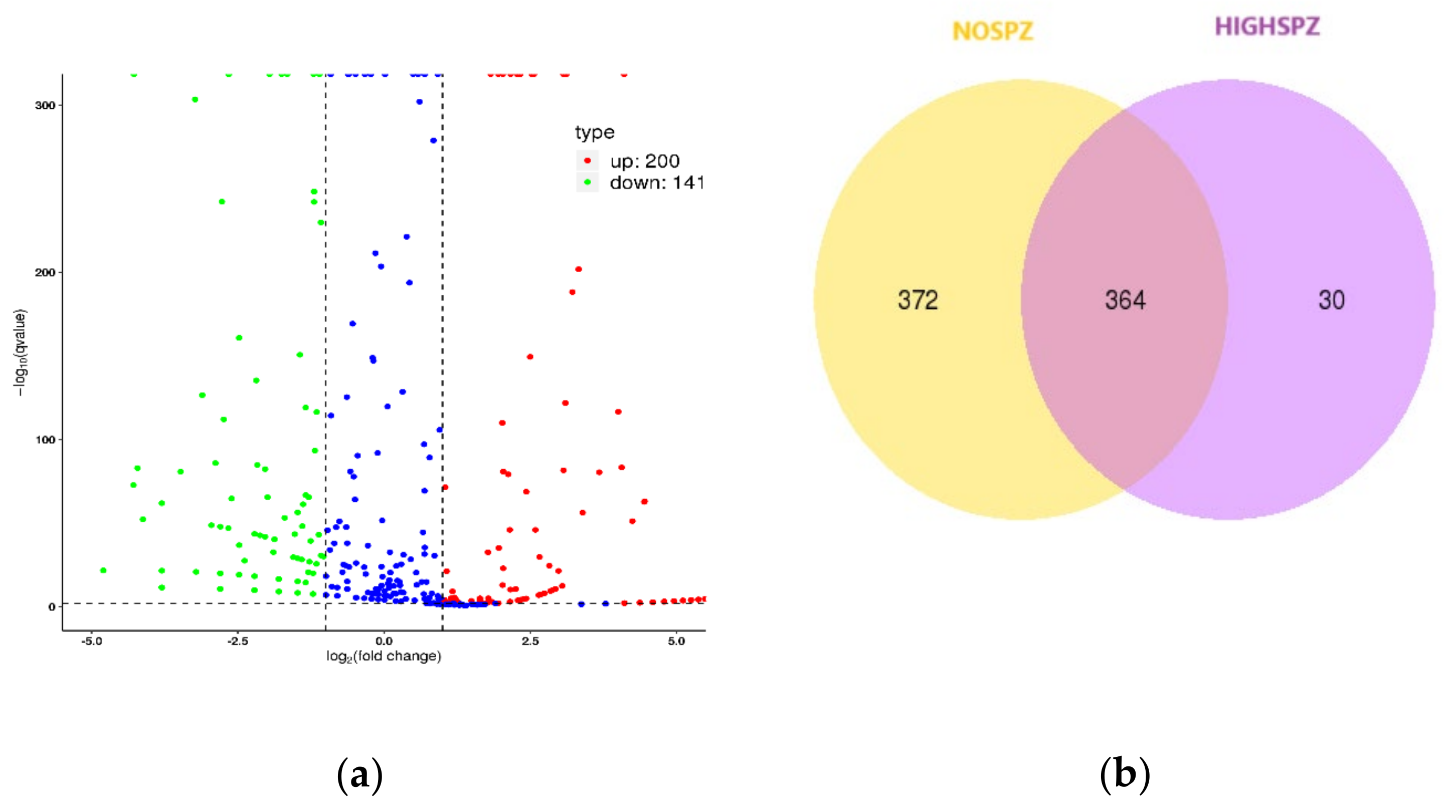

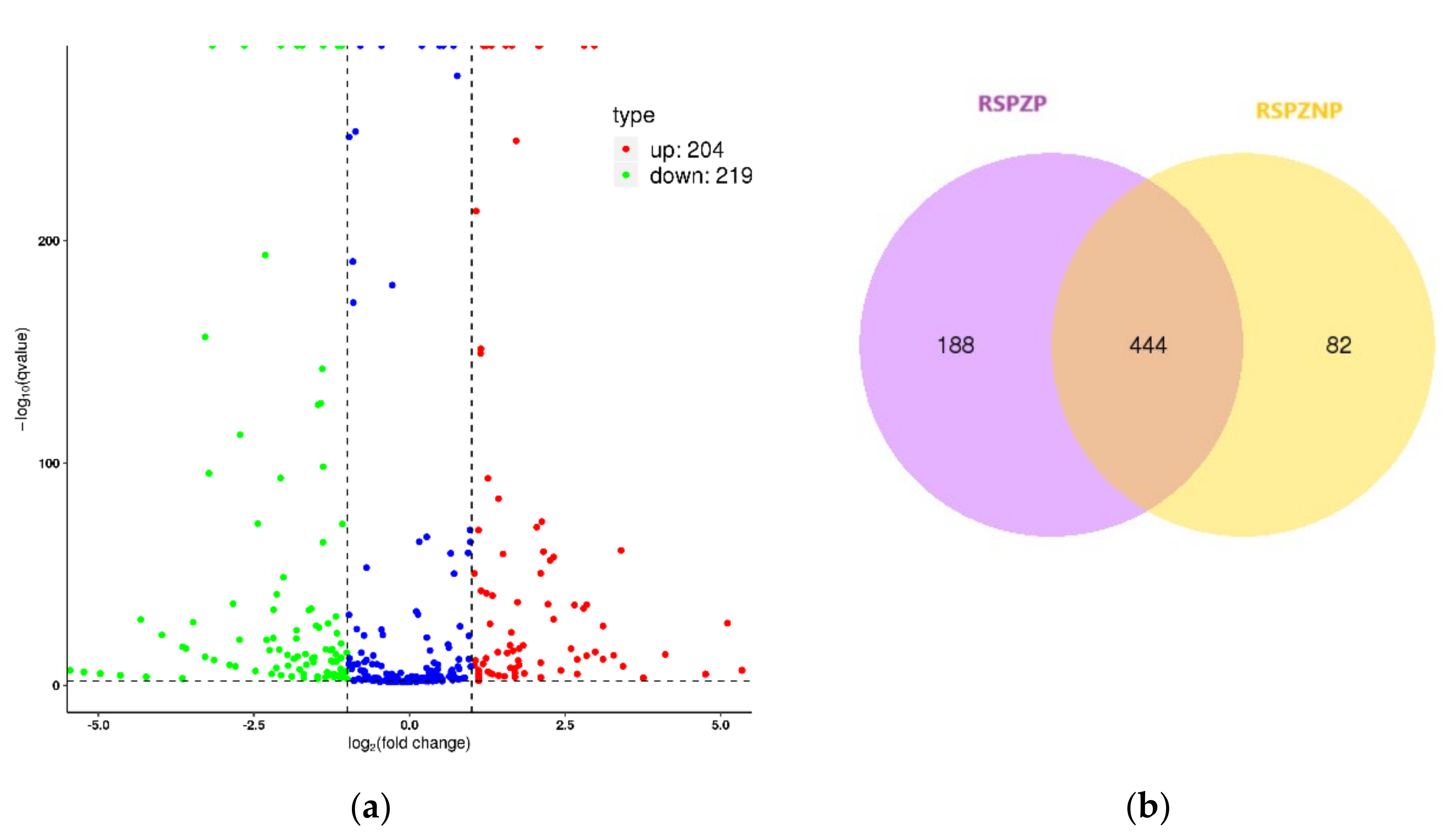
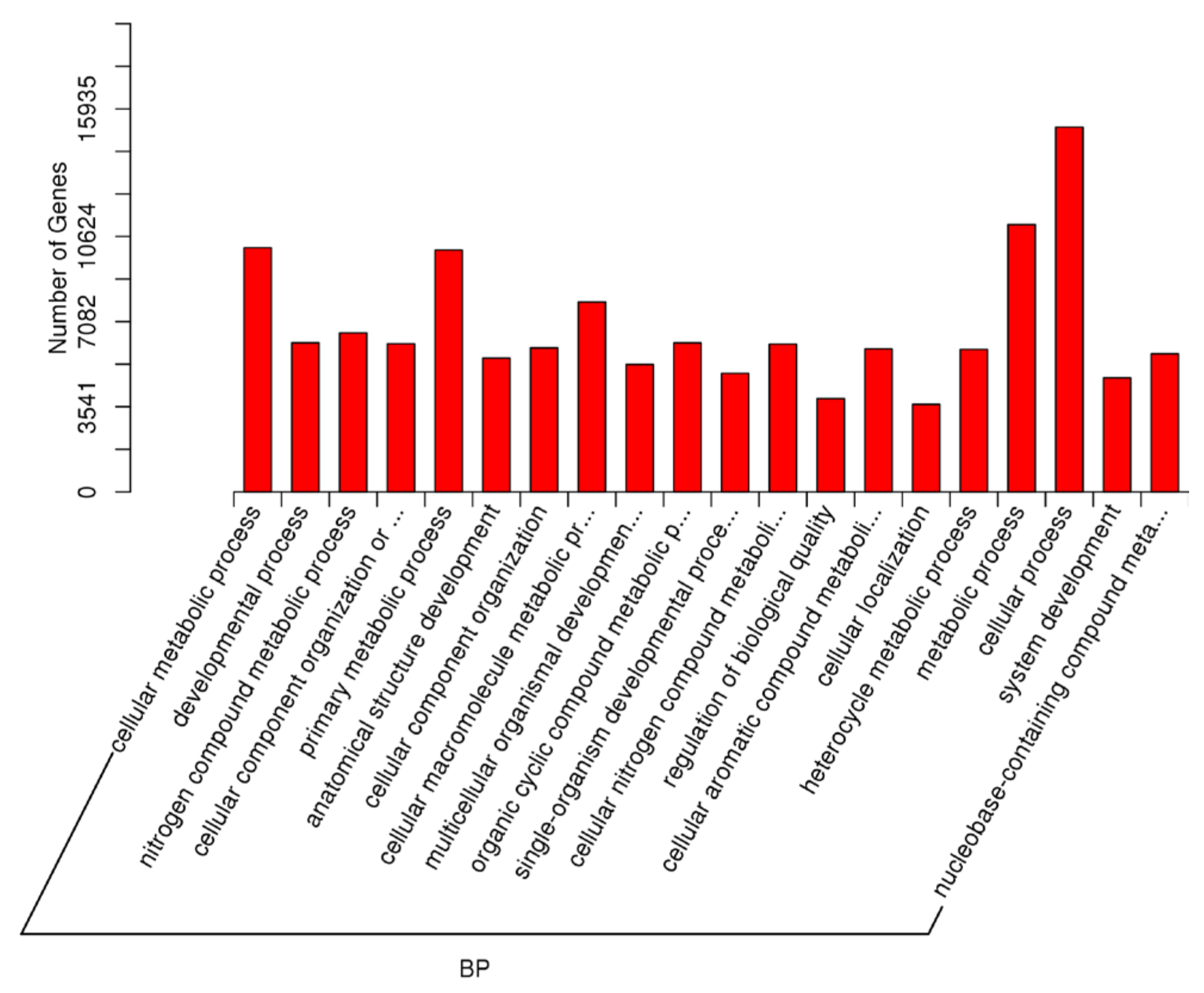

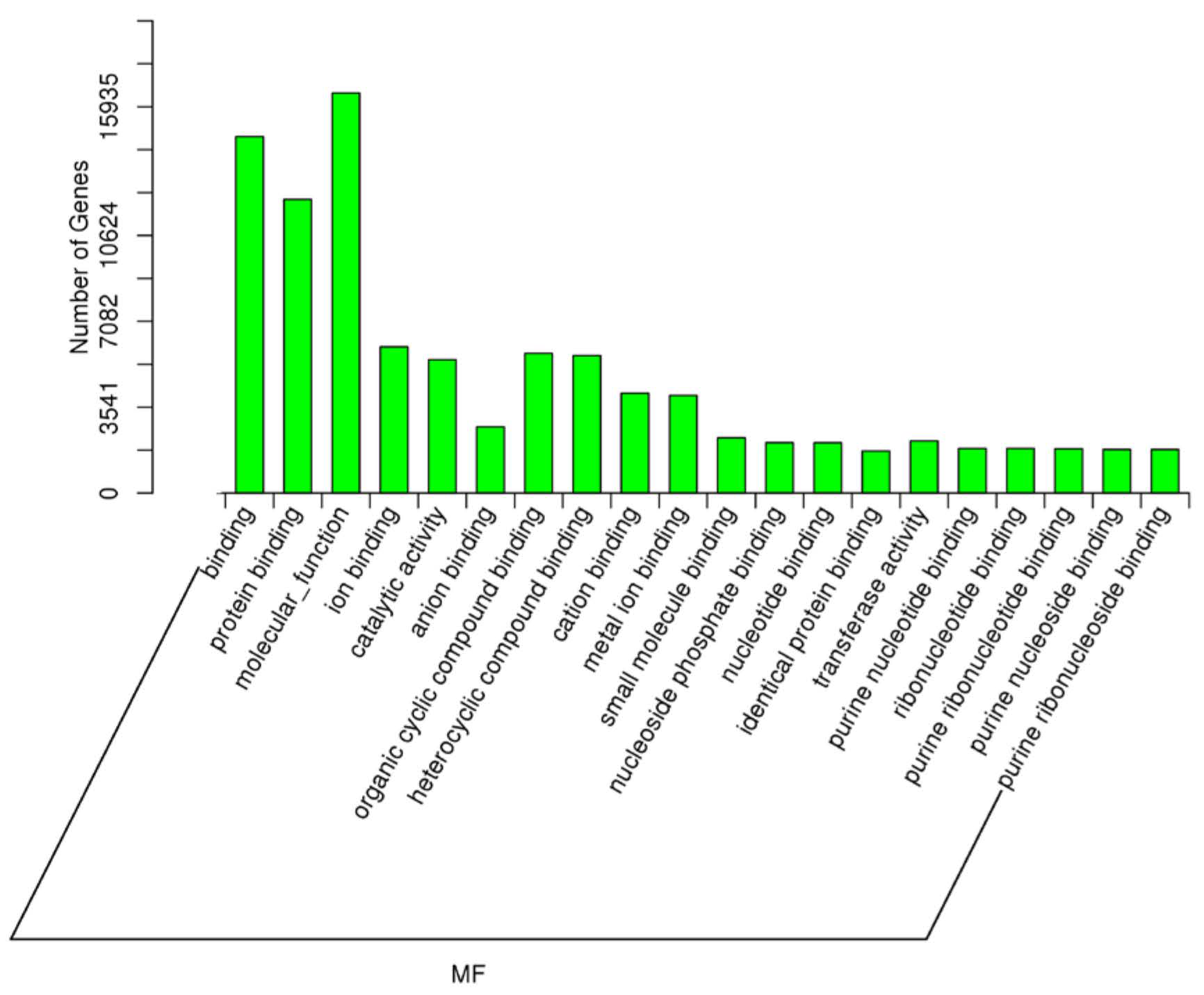

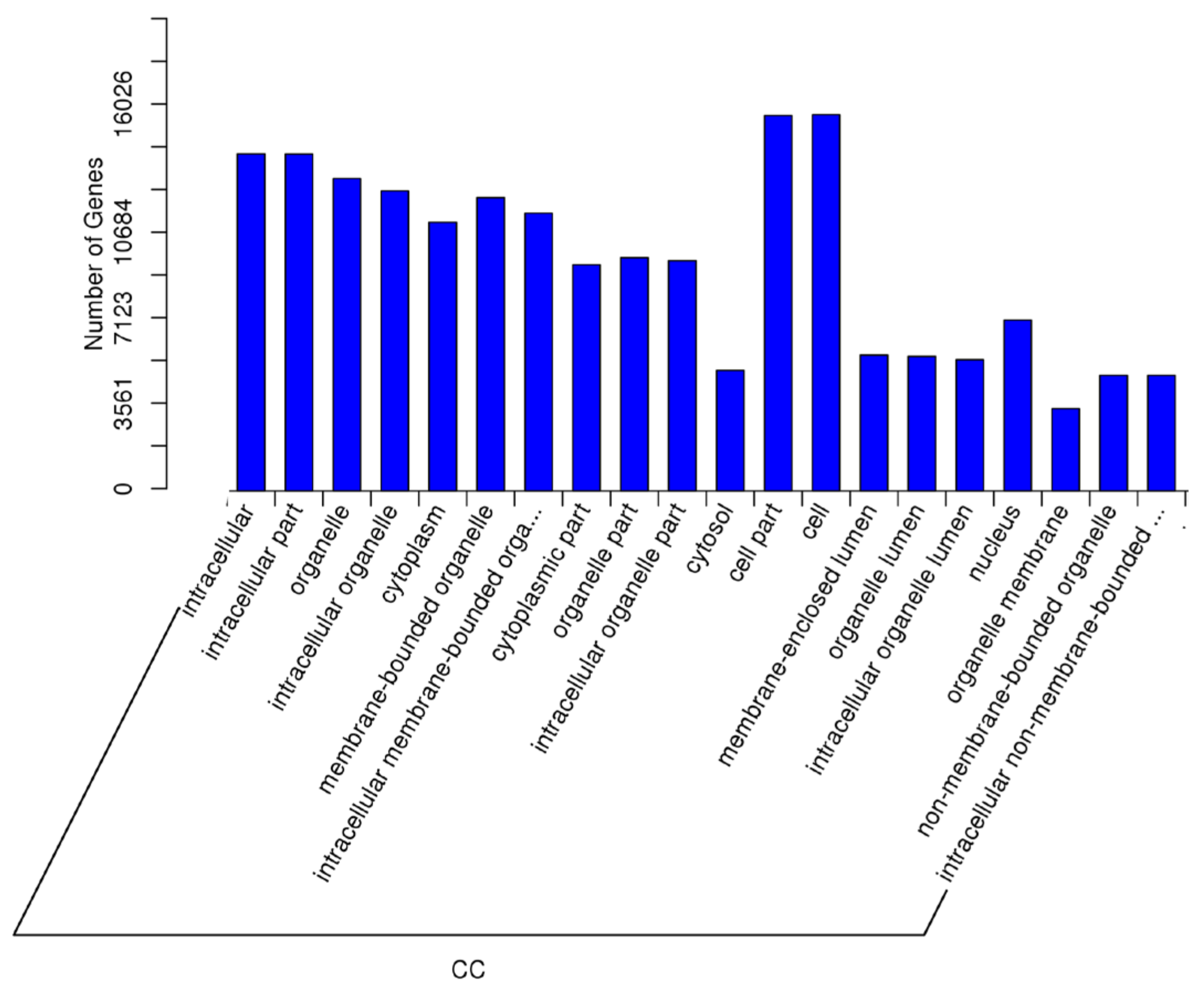

| Cryptozoospermia —CRYPTO (n = 8) | No Presence of Spermatozoa—NOSPZ (n = 8) | Rare Presence of Spermatozoa—No Pregnancy—RSPZNP (n = 8) | Rare Presence of Spermatozoa—Pregnancy—RSPZP (n = 8) | High Presence of Spermatozoa—HIGHSPZ (n = 8) | p-Value | |
|---|---|---|---|---|---|---|
| Age | 31–44 Mean = 37.5 (SD = 6.45) | 29–39 Mean = 33.75 (SD = 4.57) | 24–45 Mean = 34.5 (SD = 9.04) | 31–41 Mean = 36.25 (SD = 4.27) | n28–41 Mean = 34.5 (SD = 6.45) | 0.917 (ANOVA) |
| Body Mass Index (BMI) | 22.8–28.1 Mean = 25.9 (SD = 2.64) | 22.9–33.2 Mean = 29.38 (SD =4.56) | 20.6–34 Mean = 27.22 (SD = 5.48) | 18.5–30.1 Mean = 25.55 (SD = 5.27) | 22.5–30.8 Mean = 27.88 (SD = 4.51) | 0.769 (ANOVA) |
| Smoking | 50% No, 50% Yes | 50% No, 50% Yes | 60% No, 40% Yes | 50% No, 50% Yes | 60% No,40% Yes | 0.870 (chi-square test) |
| Alcohol Consumption | 100% ≤ 2 drinks/week | 80% ≤ 2 drinks/week | 80% ≤ 2 drinks/week | 100% ≤ 2 drinks/week | 80% ≤ 2 drinks/week | 0.671 (chi-square test) |
| Sample Name | Total Reads | Clean Reads | Percentage | Mapping Rate |
|---|---|---|---|---|
| NOSPZ1 | 22,823,882 | 21,824,598 | 95.6% | 69.6% |
| NOSPZ2 | 23,245,844 | 21,639,163 | 93.1% | 71.9% |
| RSPZNP1 | 23,522,135 | 22,201,524 | 94.4% | 73.43% |
| RSPZNP2 | 23,968,227 | 21,964,941 | 91.6% | 74.1% |
| RSPZP1 | 23,808,843 | 22,429,360 | 94.2% | 79.34 |
| RSPZP2 | 23,072,834 | 21,966,578 | 95.2% | 77.8% |
| HIGHSPZ1 | 21,980,184 | 20,449,851 | 93% | 78.00% |
| HIGHSPZ2 | 21,604,874 | 20,557,389 | 95.2% | 76.5% |
| CRYPTO1 | 22,930,151 | 21,852,645 | 95.3% | 75.3% |
| CRYPTO2 | 21,886,205 | 20,179,995 | 92.2% | 72.8% |
| miRNA | Dysregulation | Log2 Fold Change | q-Value |
|---|---|---|---|
| hsa-miR-450b-5p | Up | 95.876 | 1.50 × 10−52 |
| hsa-miR-615-3p | Up | 91.265 | 1.78 × 10−39 |
| hsa-miR-129-1-3p | Up | 84.732 | 8.96 × 10−26 |
| hsa-miR-135a-5p | Up | 82.785 | 1.41 × 10−22 |
| hsa-miR-506-5p | Up | 82.785 | 1.41 × 10−22 |
| hsa-miR-29b-3p | Up | 80.721 | 1.49 × 10−19 |
| hsa-miR-433-3p | Up | 76.936 | 7.11 × 10−15 |
| hsa-miR-708-3p | Up | 76.936 | 7.11 × 10−15 |
| hsa-miR-891a-5p | Up | 76.693 | 1.31 × 10−14 |
| hsa-miR-494-3p | Up | 76.196 | 4.54 × 10−14 |
| miRNA | Dysregulation | Log2 Fold Change | q-Value |
|---|---|---|---|
| novel_30 | Down | −94.856 | 1.05 × 10−57 |
| hsa-miR-1-3p | Down | −92.529 | 2.15 × 10−50 |
| hsa-miR-193b-5p | Down | −86.315 | 9.65 × 10−35 |
| hsa-miR-125b-2-3p | Down | −82.529 | 1.76 × 10−27 |
| hsa-miR-891a-5p | Down | −80.185 | 1.14 × 10−23 |
| hsa-miR-3614-5p | Down | −78.379 | 4.17 × 10−21 |
| hsa-miR-378c | Down | −78.379 | 4.17 × 10−21 |
| hsa-miR-708-3p | Down | −78.379 | 4.17 × 10−21 |
| hsa-miR-7706 | Down | −78.379 | 4.17 × 10−21 |
| hsa-miR-129-1-3p | Down | −77.384 | 8.79 × 10−20 |
| miRNA | Dysregulation | Log2 Fold Change | q-Value |
|---|---|---|---|
| hsa-miR-146b-3p | Down | −76.007 | 6.32 × 10−19 |
| hsa-miR-1247-5p | Down | −75.045 | 9.33 × 10−18 |
| hsa-miR-659-5p | Down | −71.059 | 1.68 × 10−13 |
| hsa-miR-206 | Down | −69.684 | 2.88 × 10−12 |
| hsa-miR-450b-5p | Up | 69.255 | 8.73 × 10−13 |
| hsa-miR-3148 | Down | −65.534 | 4.93 × 10−9 |
| hsa-miR-6511b-5p | Down | −64.539 | 2.23 × 10−8 |
| novel_41 | Down | −62.315 | 5.00 × 10−7 |
| novel_45 | Down | −62.315 | 5.00 × 10−7 |
| hsa-miR-369-3p | Up | 60.775 | 8.89 × 10−7 |
| Upregulated | Downregulated |
|---|---|
| PI3K/AKT Signaling | Signaling by receptor tyrosine kinases |
| ESR-mediated signaling | Generic transcription pathway |
| PIP3-activated AKT signaling | RNA polymerase II transcription |
| FOXO-mediated transcription | Diseases of signal transduction by growth factor receptors and second messengers |
| Signaling by TGFB family members | Gene expression (transcription) |
| Upregulated | Downregulated |
|---|---|
| Interleukin-4 and Interleukin-13 signaling | ESR-mediated signaling |
| PIP3 activates AKT signaling | Signaling by nuclear receptors |
| Signaling by TGFB family members | Generic transcription pathway |
| Intracellular signaling by second messengers | RNA polymerase II transcription |
| Signaling by receptor tyrosine kinases | Gene expression (transcription) |
| KEGG Term | Corrected p-Value |
|---|---|
| Metabolic pathways | 1.20602675899 × 10−72 |
| PI3K-Akt signaling pathway | 1.87222793907 × 10−20 |
| Cytokine–cytokine receptor interaction | 2.21189356028 × 10−15 |
| MAPK signaling pathway | 8.85018316676 × 10−15 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kyrgiafini, M.-A.; Kaltsas, A.; Chatziparasidou, A.; Mamuris, Z. The Small RNA Landscape in Azoospermia: Implications for Male Infertility and Sperm Retrieval—A Preliminary Study. Int. J. Mol. Sci. 2025, 26, 3537. https://doi.org/10.3390/ijms26083537
Kyrgiafini M-A, Kaltsas A, Chatziparasidou A, Mamuris Z. The Small RNA Landscape in Azoospermia: Implications for Male Infertility and Sperm Retrieval—A Preliminary Study. International Journal of Molecular Sciences. 2025; 26(8):3537. https://doi.org/10.3390/ijms26083537
Chicago/Turabian StyleKyrgiafini, Maria-Anna, Aris Kaltsas, Alexia Chatziparasidou, and Zissis Mamuris. 2025. "The Small RNA Landscape in Azoospermia: Implications for Male Infertility and Sperm Retrieval—A Preliminary Study" International Journal of Molecular Sciences 26, no. 8: 3537. https://doi.org/10.3390/ijms26083537
APA StyleKyrgiafini, M.-A., Kaltsas, A., Chatziparasidou, A., & Mamuris, Z. (2025). The Small RNA Landscape in Azoospermia: Implications for Male Infertility and Sperm Retrieval—A Preliminary Study. International Journal of Molecular Sciences, 26(8), 3537. https://doi.org/10.3390/ijms26083537







