A Cold-Induced LEA3 Protein, DohD, Confers Cryoprotective Protection Against Low-Temperature Stress in Deinococcus radiodurans
Abstract
1. Introduction
2. Results
2.1. DohD Is a Hydrophilic LEA3 Protein Containing a Disordered Domain
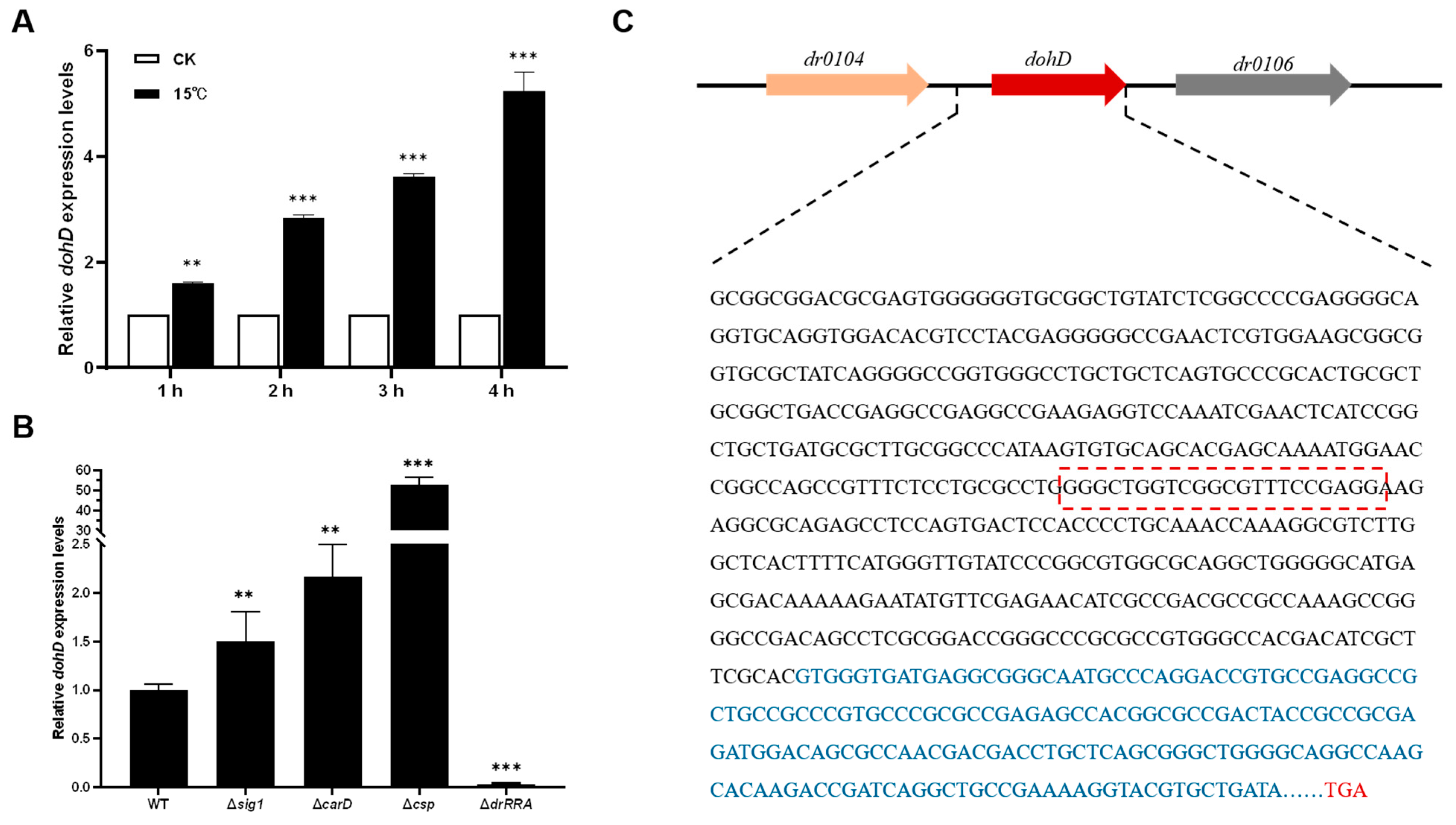
2.2. Low-Temperature Stress Induces DohD Expression
2.3. The Role of Secondary Structural Changes in the DohD Protein in Low-Temperature Stress Resistance
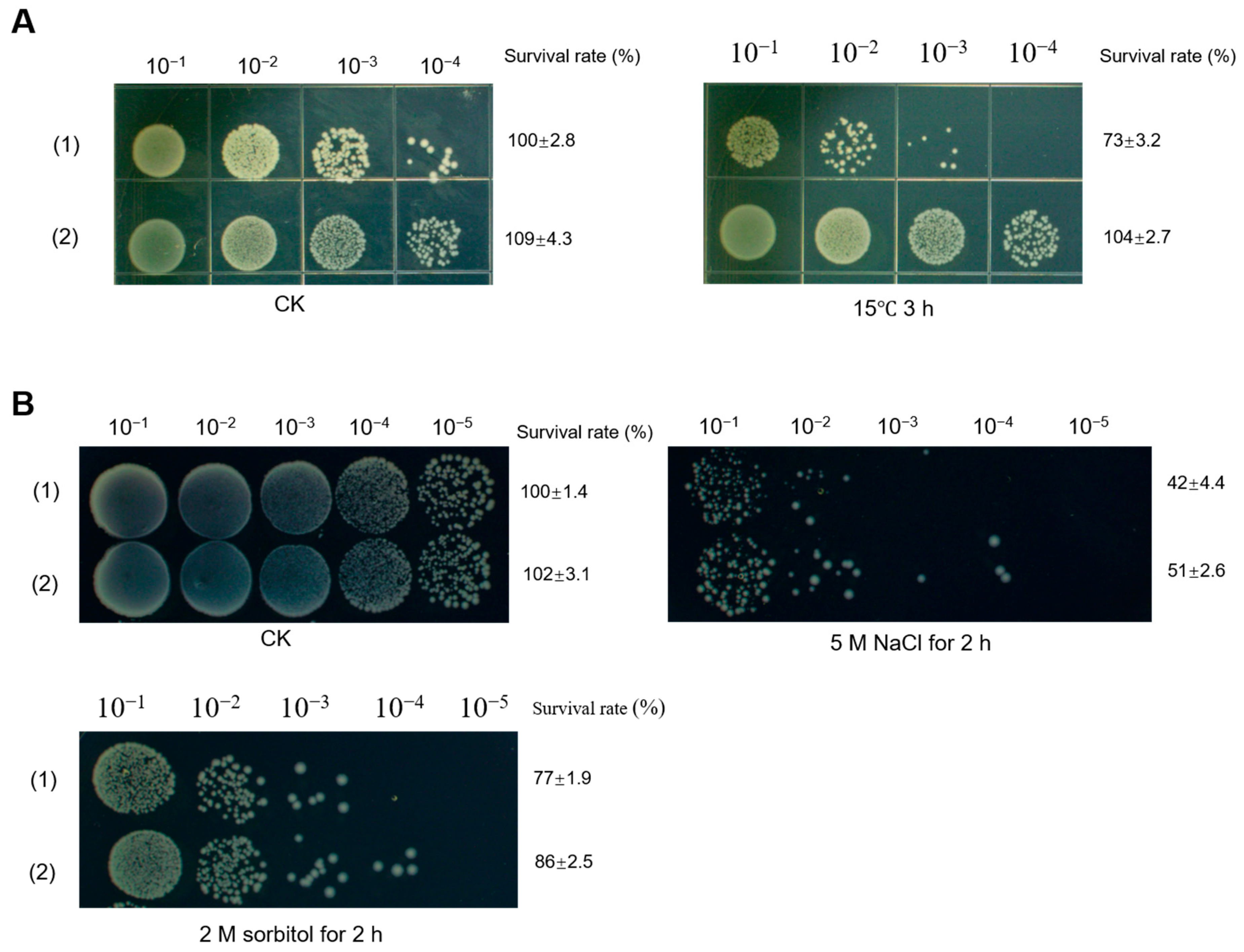
2.4. Deletion of dohD Decreases Abiotic Stress Tolerances of D. radiodurans
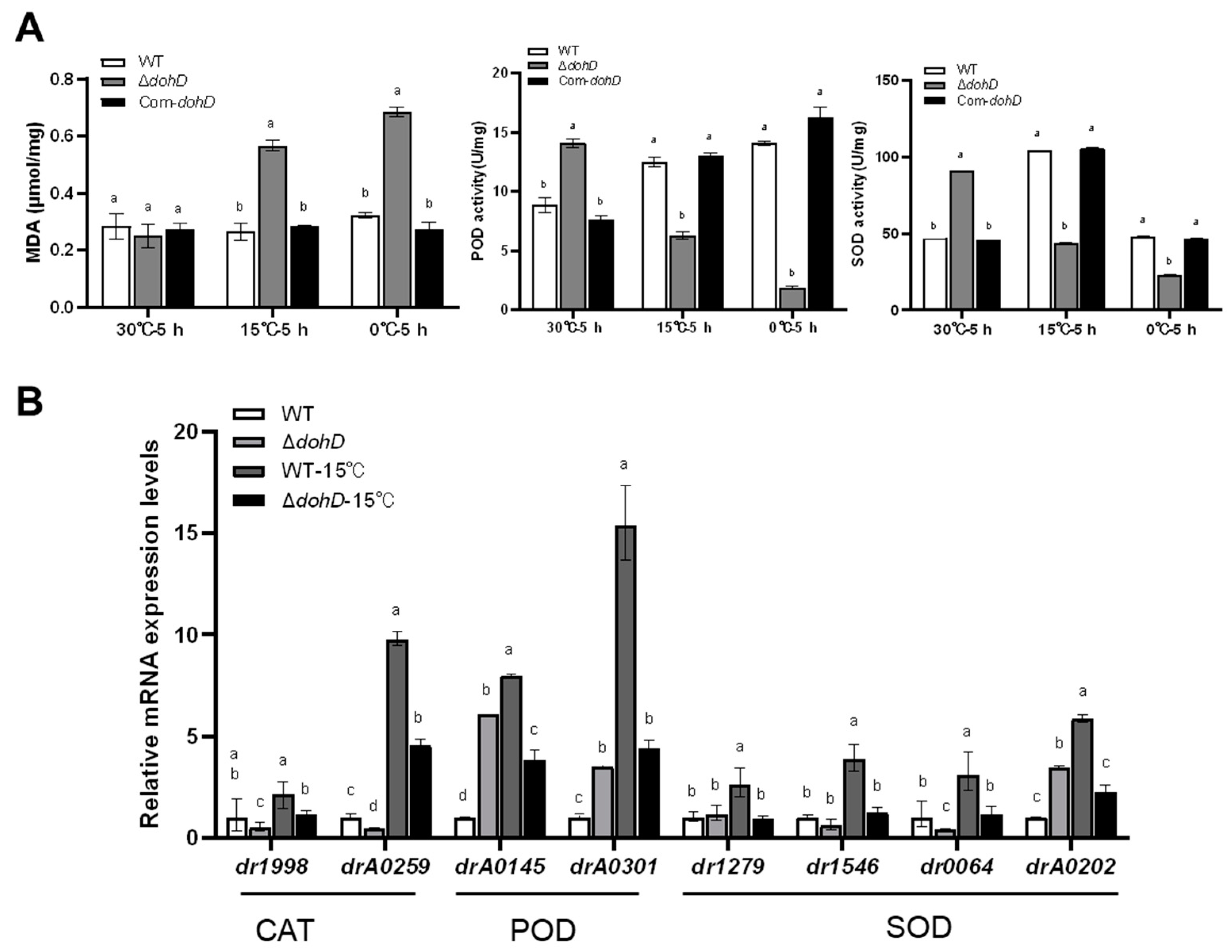
2.5. DohD Alleviates Cold-Induced Oxidative Damage by Maintaining Antioxidant Enzyme Activity
2.6. DohD Enhances the Low-Temperature Stress Tolerance of E. coli
3. Discussion
3.1. Structural Plasticity of the Cold-Inducible Protein DohD Drives Its Cold Adaptation Mechanism
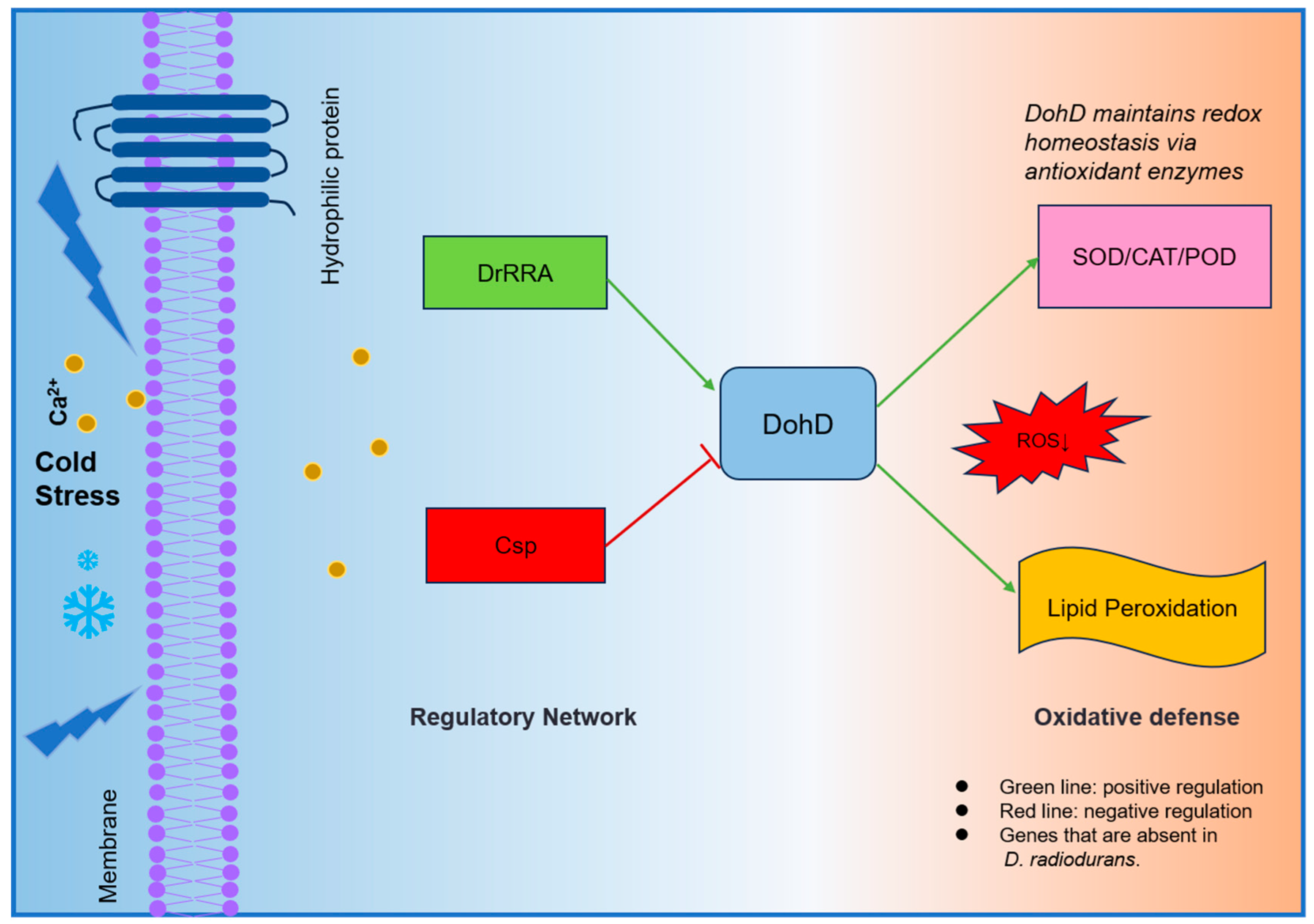
3.2. Regulatory Circuitry and Oxidative Defense
3.3. DohD Integrates Antioxidant Defense with Membrane Stabilization
3.4. Cross-Species Functionality Highlights Evolutionary Conservation
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Media
4.2. Construction of DohD Deletion Mutants and Complementary Strains
4.3. Bioinformatics Analysis
4.4. Real-Time Fluorescence Quantitative PCR for Gene Expression
4.5. Activity Measurement of Major Antioxidant Enzymes
4.6. Abiotic Stress-Resistance Assays
4.7. DohD Expression and Purification
4.8. Microscopy
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Guo, P.; Liu, A.; Qi, Y.; Wang, X.; Fan, X.; Guo, X.; Yu, C.; Tian, C. Genome-wide identification of cold shock proteins (CSPs) in sweet cherry (Prunus avium L.) and exploring the differential responses of PavCSP1 and PavCSP3 to low temperature and salt stress. Genes Genom. 2024, 46, 1023–1036. [Google Scholar] [CrossRef]
- Derzelle, S.; Hallet, B.; Ferain, T.; Delcour, J.; Hols, P. Improved adaptation to cold-shock, stationary-phase, and freezing stresses in Lactobacillus plantarum overproducing cold-shock proteins. Appl. Environ. Microb. 2003, 69, 4285–4290. [Google Scholar] [CrossRef]
- Lindquist, J.A.; Mertens, P.R. Cold shock proteins: From cellular mechanisms to pathophysiology and disease. Cell Commun. Signal. 2018, 16, 63. [Google Scholar] [CrossRef]
- Lee, H.J.; Alirzayeva, H.; Koyuncu, S.; Rueber, A.; Noormohammadi, A.; Vilchez, D. Cold temperature extends longevity and prevents disease-related protein aggregation through PA28gamma-induced proteasomes. Nat. Aging 2023, 3, 546–566. [Google Scholar] [CrossRef]
- Liu, M.; Bai, S.; Jiang, Z.; Li, H.; Tu, Z.; Liao, T.; Yu, W.; Qiu, L. Identification and isolation of a novel antifreeze peptide from crayfish shells. LWT 2024, 198, 116030. [Google Scholar] [CrossRef]
- Yu, M.; Luobu, Z.; Zhuoga, D.; Wei, X.; Tang, Y. Advances in plant response to low-temperature stress. Plant Growth Regul. 2024, 105, 167–185. [Google Scholar] [CrossRef]
- Liu, X.; Li, L.; Zhao, G.; Xiong, P. Optimization strategies for CO2 biological fixation. Biotechnol. Adv. 2024, 73, 108364. [Google Scholar] [CrossRef]
- Xiong, R.; Wang, H.; Mao, F.; Tao, L.; Tan, X.; Pan, X.; Zeng, Y.; Zeng, Y. The mechanism of carbon and nitrogen metabolism under low temperature and low light stress after heading in late indica rice. Plant Physiol. Biochem. 2025, 218, 109316. [Google Scholar] [CrossRef]
- Bo, Y.; Zhang, H.; Tong, Y.; Jia, Y.; Liu, X.; Yang, L.; Zuo, Z.; Wang, Y. Growth, antioxidant enzyme activity and transcriptome response to low-temperature induction of flowering in cultivated strawberry. Plant Stress 2024, 12, 100453. [Google Scholar] [CrossRef]
- Rekadwad, B.N.; Shouche, Y.S.; Jangid, K. Exploring glaciers and glacier environments as potential habitats for cold-loving bacterial taxa with diverse industrial and environmental implications. Environ. Sustain. 2024, 7, 279–286. [Google Scholar] [CrossRef]
- Shen, L.; Liu, Y.; Chen, L.; Lei, T.; Ren, P.; Ji, M.; Song, W.; Lin, H.; Su, W.; Wang, S.; et al. Genomic basis of environmental adaptation in the widespread poly-extremophilic Exiguobacterium group. ISME J. 2024, 18, wrad020. [Google Scholar] [CrossRef]
- Margesin, R.; Collins, T. Microbial ecology of the cryosphere (glacial and permafrost habitats): Current knowledge. Appl. Microbiol. Biot. 2019, 103, 2537–2549. [Google Scholar] [CrossRef]
- Shen, L.; Hu, J.; Zhang, L.; Wu, Z.; Chen, L.; Adhikari, N.P.; Ji, M.; Chen, S.; Peng, F.; Liu, Y. Genomics-based identification of a cold adapted clade in Deinococcus. BMC Biol. 2024, 22, 145. [Google Scholar] [CrossRef]
- Slade, D.; Radman, M. Oxidative stress resistance in Deinococcus radiodurans. Microbiol. Mol. Biol. Rev. 2011, 75, 133–191. [Google Scholar] [CrossRef]
- Wang, L.; Xu, G.; Chen, H.; Zhao, Y.; Xu, N.; Tian, B.; Hua, Y. DrRRA: A novel response regulator essential for the extreme radioresistance of Deinococcus radiodurans. Mol. Microbiol. 2008, 67, 1211–1222. [Google Scholar] [CrossRef]
- Battista, J.R. Against all odds: The survival strategies of Deinococcus radiodurans. Annu. Rev. Microbiol. 1997, 51, 203–224. [Google Scholar] [CrossRef]
- Aziz, M.A.; Sabeem, M.; Kutty, M.S.; Rahman, S.; Alneyadi, M.K.; Alkaabi, A.B.; Almeqbali, E.S.; Brini, F.; Vijayan, R.; Masmoudi, K. Enzyme stabilization and thermotolerance function of the intrinsically disordered LEA2 proteins from date palm. Sci. Rep. 2023, 13, 11878. [Google Scholar] [CrossRef]
- Liu, Y.; Liang, J.; Sun, L.; Yang, X.; Li, D. Group 3 LEA Protein, ZmLEA3, Is Involved in Protection from Low Temperature Stress. Front. Plant Sci. 2016, 7, 1011. [Google Scholar] [CrossRef][Green Version]
- Li, L.; Bi, X.; Wu, X.; Chen, Z.; Cao, Y.; Zhao, G. Improving vitrification efficiency of human in vitro matured oocytes by the addition of LEA proteins. Hum. Reprod. 2024, 39, 1275–1290. [Google Scholar] [CrossRef]
- Makarova, K.S.; Aravind, L.; Wolf, Y.I.; Tatusov, R.L.; Minton, K.W.; Koonin, E.V.; Daly, M.J. Genome of the extremely radiation-resistant bacterium Deinococcus radiodurans viewed from the perspective of comparative genomics. Microbiol. Mol. Biol. Rev. 2001, 65, 44–79. [Google Scholar] [CrossRef]
- Omelchenko, M.V.; Wolf, Y.I.; Gaidamakova, E.K.; Matrosova, V.Y.; Vasilenko, A.; Zhai, M.; Daly, M.J.; Koonin, E.V.; Makarova, K.S. Comparative genomics of Thermus thermophilus and Deinococcus radiodurans: Divergent routes of adaptation to thermophily and radiation resistance. BMC Evol. Biol. 2005, 5, 57. [Google Scholar] [CrossRef] [PubMed]
- Im, S.; Joe, M.; Kim, D.; Park, D.; Lim, S. Transcriptome analysis of salt-stressed Deinococcus radiodurans and characterization of salt-sensitive mutants. Res. Microbiol. 2013, 164, 923–932. [Google Scholar] [CrossRef] [PubMed]
- Jones, D.T. Protein secondary structure prediction based on position-specific scoring matrices. J. Mol. Biol. 1999, 292, 195–202. [Google Scholar] [CrossRef]
- Oldfield, C.J.; Dunker, A.K. Intrinsically disordered proteins and intrinsically disordered protein regions. Annu. Rev. Biochem. 2014, 83, 553–584. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Zhou, X.; Chen, Z.; Cao, Y.; Zhao, G. The Group 3 LEA proteins of Artemia franciscana for cryopreservation. Cryobiology 2022, 106, 1–12. [Google Scholar] [CrossRef]
- Ujjainkar, N.R.; Panpatil, A.U.; Chimote, V.P.; Pawar, B.D.; Kale, A.A. Molecular characterization of late embryogenesis abundant proteins encoding genes from wild sorghum genotype IS-18,909 expressed under drought stress. Vegetos 2024. [Google Scholar] [CrossRef]
- Koubaa, S.; Brini, F. Functional analysis of a wheat group 3 late embryogenesis abundant protein (TdLEA3) in Arabidopsis thaliana under abiotic and biotic stresses. Plant Physiol. Biochem. 2020, 156, 396–406. [Google Scholar] [CrossRef]
- Huang, P.S.; Oberdorfer, G.; Xu, C.; Pei, X.Y.; Nannenga, B.L.; Rogers, J.M.; DiMaio, F.; Gonen, T.; Luisi, B.; Baker, D. High thermodynamic stability of parametrically designed helical bundles. Science 2014, 346, 481–485. [Google Scholar] [CrossRef]
- Cardoza, E.; Singh, H. Involvement of CspC in response to diverse environmental stressors in Escherichia coli. J. Appl. Microbiol. 2022, 132, 785–801. [Google Scholar] [CrossRef]
- von Konig, K.; Kachel, N.; Kalbitzer, H.R.; Kremer, W. RNA and DNA Binding Epitopes of the Cold Shock Protein TmCsp from the Hyperthermophile Thermotoga maritima. Protein J. 2020, 39, 487–500. [Google Scholar] [CrossRef]
- Li, S.; Meng, H.; Yang, Y.; Zhao, J.; Xia, Y.; Wang, S.; Wang, F.; Zheng, G.; Li, J. Overexpression of AtruLEA1 from Acer truncatum Bunge Enhanced Arabidopsis Drought and Salt Tolerance by Improving ROS-Scavenging Capability. Plants 2025, 14, 117. [Google Scholar] [CrossRef]
- Jia, H.; Wang, X.; Shi, Y.; Wu, X.; Wang, Y.; Liu, J.; Fang, Z.; Li, C.; Dong, K. Overexpression of Medicago sativa LEA4-4 can improve the salt, drought, and oxidation resistance of transgenic Arabidopsis. PLoS ONE 2020, 15, e234085. [Google Scholar] [CrossRef]
- Jiang, S.; Wang, J.; Liu, X.; Liu, Y.; Guo, C.; Zhang, L.; Han, J.; Wu, X.; Xue, D.; Gomaa, A.E.; et al. DrwH, a novel WHy domain-containing hydrophobic LEA5C protein from Deinococcus radiodurans, protects enzymatic activity under oxidative stress. Sci. Rep. 2017, 7, 9281. [Google Scholar] [CrossRef]
- Turell, L.; Zeida, A.; Trujillo, M. Mechanisms and consequences of protein cysteine oxidation: The role of the initial short-lived intermediates. Essays Biochem. 2020, 64, 55–66. [Google Scholar] [CrossRef]
- Huang, Y.; Xiong, K.; Wang, A.; Wang, Z.; Cui, Q.; Xie, H.; Yang, T.; Fan, X.; Jiang, W.; Tan, X.; et al. Cold stress causes liver damage by inducing ferroptosis through the p38 MAPK/Drp1 pathway. Cryobiology 2023, 113, 104563. [Google Scholar] [CrossRef]
- Ali, S.; Tyagi, A.; Bae, H. ROS interplay between plant growth and stress biology: Challenges and future perspectives. Plant Physiol. Biochem. 2023, 203, 108032. [Google Scholar] [CrossRef]
- Xiang, Z.; Zhang, L.; Zhang, M.; Yao, Y.; Qian, Q.; Wei, Z.; Cui, B.; Wang, D.; Quan, C.; Lu, M.; et al. OsNCED5 confers cold stress tolerance through regulating ROS homeostasis in rice. Plant Physiol. Biochem. 2025, 220, 109455. [Google Scholar] [CrossRef]
- Zhao, Q.; Li, S.; Wang, F.; Lu, J.; Tan, G.; Wang, N.; Qi, F.; Zhang, C.; Deyholos, M.K.; Zang, Z.; et al. Transcriptome analysis and physiological response to heat and cold stress in flax (Linum usitatissimum L.) at the seedling stage. Environ. Exp. Bot. 2025, 229, 106076. [Google Scholar] [CrossRef]
- Cordiano, R.; Di Gioacchino, M.; Mangifesta, R.; Panzera, C.; Gangemi, S.; Minciullo, P.L. Malondialdehyde as a Potential Oxidative Stress Marker for Allergy-Oriented Diseases: An Update. Molecules 2023, 28, 5979. [Google Scholar] [CrossRef] [PubMed]
- Nabih, G.A.; Sheshtawy, N.E.; Mikkawy, D.M.E.E.; Kamel, M.A. Serum malondialdehyde as a marker of oxidative stress in rheumatoid arthritis. Egypt. Rheumatol. Rehabil. 2024, 51, 43. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, F.; Ma, M.; Gong, J.; Wang, Q.; Jia, P.; Zheng, G.; Liu, H. Overexpression of AtLEA3-3 confers resistance to cold stress in Escherichia coli and provides enhanced osmotic stress tolerance and ABA sensitivity in Arabidopsis thaliana. Mol. Biol. 2011, 45, 851–862. [Google Scholar] [CrossRef]
- Xu, G.; Wang, L.; Chen, H.; Lu, H.; Ying, N.; Tian, B.; Hua, Y. RecO Is Essential for DNA Damage Repair in Deinococcus radiodurans. J. Bacteriol. 2008, 190, 2624–2628. [Google Scholar] [CrossRef] [PubMed]
- Bradshaw, W.J.; Kirby, J.M.; Roberts, A.K.; Shone, C.C.; Acharya, K.R. The molecular structure of the glycoside hydrolase domain of Cwp19 from Clostridium difficile. FEBS J. 2017, 284, 4343–4357. [Google Scholar] [CrossRef]
- Fredriksen, L.; Stokke, R.; Jensen, M.S.; Westereng, B.; Jameson, J.K.; Steen, I.H.; Eijsink, V. Discovery of a Thermostable GH10 Xylanase with Broad Substrate Specificity from the Arctic Mid-Ocean Ridge Vent System. Appl. Environ. Microbiol. 2019, 85, e02970-18. [Google Scholar] [CrossRef] [PubMed]
 : The target protein of this study.
: The target protein of this study.
 : The target protein of this study.
: The target protein of this study.




| Localization | Sequence |
|---|---|
| 59–74 | ADTQEAAQN⋯⋯AREKAQD |
| 77–92 | ANVHESAQD⋯⋯FRAGAQE |
| 99–114 | ADARDAAAQ⋯⋯ARDHAQN |
| 117–136 | TDVHEKAQD⋯⋯AHETAQN |
| 150–163 | ADVREI⋯⋯D⋯⋯RRSKNQD |
| Treatment | Helix % | Antiparallel % | Parallel % | β-Turn % | Random Coil % |
|---|---|---|---|---|---|
| 0 °C | 23.5 | 31.3 | 9.1 | 21.1 | 25.7 |
| 15 °C | 20.4 | 41.1 | 10.1 | 22.3 | 27.2 |
| 25 °C | 20.1 | 42.1 | 10.3 | 22.4 | 27.9 |
| 35 °C | 19.6 | 45.1 | 10.3 | 22.8 | 27.2 |
| 50 °C | 18.7 | 50.8 | 10.3 | 23.6 | 26.3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, W.; Qi, Z.; Yan, C.; Zhou, Z.; Wang, J. A Cold-Induced LEA3 Protein, DohD, Confers Cryoprotective Protection Against Low-Temperature Stress in Deinococcus radiodurans. Int. J. Mol. Sci. 2025, 26, 3511. https://doi.org/10.3390/ijms26083511
Wang W, Qi Z, Yan C, Zhou Z, Wang J. A Cold-Induced LEA3 Protein, DohD, Confers Cryoprotective Protection Against Low-Temperature Stress in Deinococcus radiodurans. International Journal of Molecular Sciences. 2025; 26(8):3511. https://doi.org/10.3390/ijms26083511
Chicago/Turabian StyleWang, Wenxiu, Zhi Qi, Chunxia Yan, Zhengfu Zhou, and Jin Wang. 2025. "A Cold-Induced LEA3 Protein, DohD, Confers Cryoprotective Protection Against Low-Temperature Stress in Deinococcus radiodurans" International Journal of Molecular Sciences 26, no. 8: 3511. https://doi.org/10.3390/ijms26083511
APA StyleWang, W., Qi, Z., Yan, C., Zhou, Z., & Wang, J. (2025). A Cold-Induced LEA3 Protein, DohD, Confers Cryoprotective Protection Against Low-Temperature Stress in Deinococcus radiodurans. International Journal of Molecular Sciences, 26(8), 3511. https://doi.org/10.3390/ijms26083511






