3D-Bioprinted Oil-Based Hydrogels: A Sustainable Approach for Bone and Dental Regeneration
Abstract
1. Introduction
1.1. Pathophysiology of Dental Tissue and Periodontitis
1.2. Pathophysiology of Bone Tissue
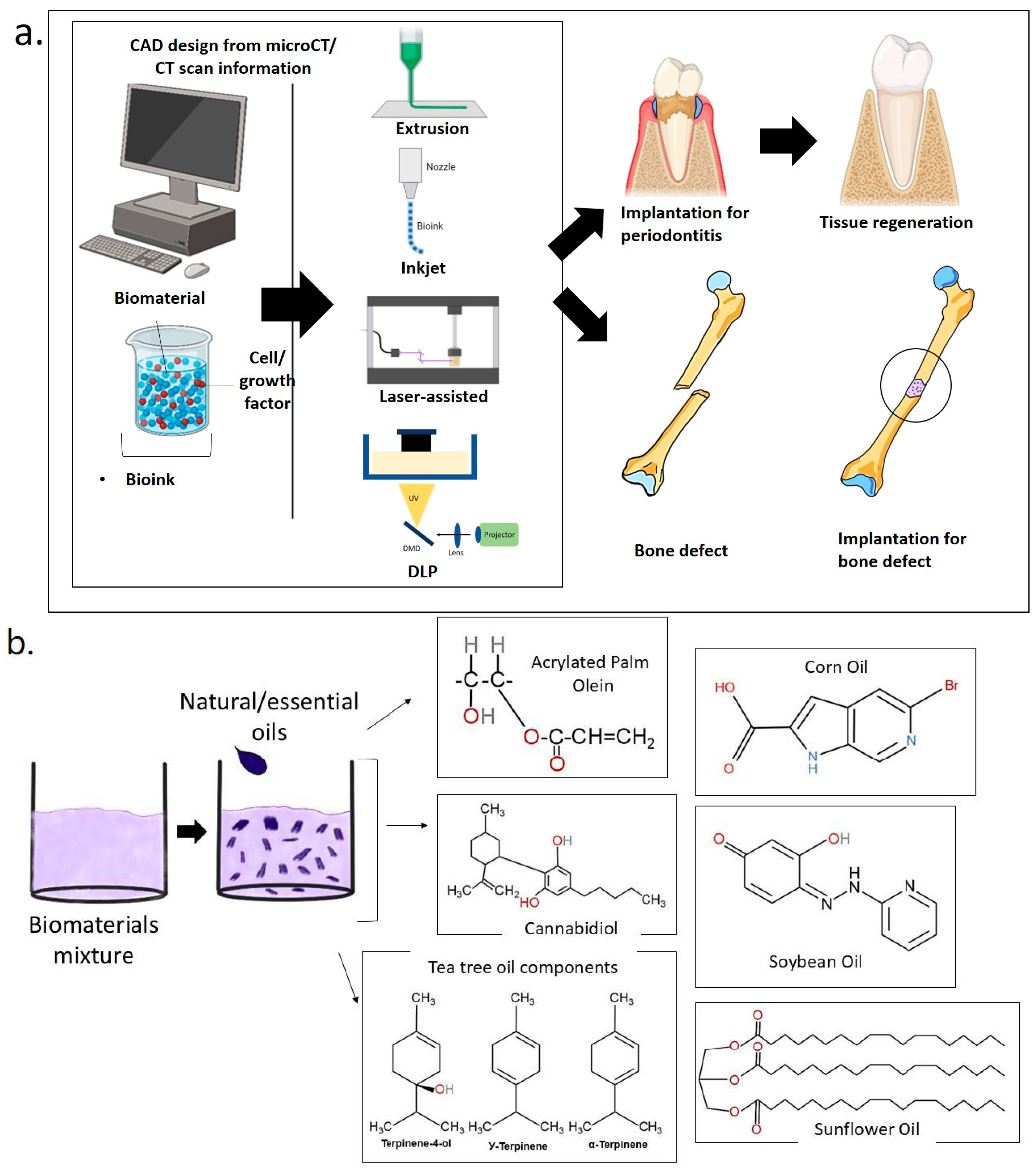
1.3. D Bioprinting for Medical and Dental Tissue Engineering
1.3.1. Laser-Assisted Bioprinting (LAB)
1.3.2. Extrusion-Based 3D Bioprinting
1.3.3. Inkjet-Based 3D Bioprinting
2. A Sustainable Approach for Bone and Dental Regeneration
2.1. Soybean Oil
2.2. Corn Oil
2.3. Sunflower Oil
2.4. Tea Tree Oil
2.5. Cannabis Sativa Oil
2.6. Acrylated Palm Olein
3. Hydrogels Enriched with Natural/Essential Oil for Bone and Dental Regeneration
3.1. Rheological Properties of 3D-Bioprinted Oil-Based Hydrogels
3.2. Physical Properties of 3D-Bioprinted Oil-Based Hydrogels
3.3. Chemical Characterization of 3D-Bioprinted Oil-Based Hydrogels
3.4. Thermal Properties of 3D-Bioprinted Oil-Based Hydrogels
3.5. Mechanical Properties of 3D-Bioprinted Oil-Based Hydrogels
4. Overview of 3D Bioprinting in Bone and Dental Applications
4.1. Application of Oil-Based Hydrogels for Bone and Dental
4.2. Characterization of Oil-Based Hydrogels
5. Strength and Limitations
6. Future Directions
7. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Matichescu, A.; Ardelean, L.C.; Rusu, L.C.; Craciun, D.; Bratu, E.A.; Babucea, M.; Leretter, M. Advanced biomaterials and techniques for oral tissue engineering and regeneration—A review. Materials 2020, 13, 5303. [Google Scholar] [CrossRef]
- Xu, H.; Zhang, Y.; Zhang, Y.; Zhao, Z.; Xue, T.; Wang, J.; Li, M.; Zhao, S.; Zhang, H.; Ding, Y. 3D bioprinting advanced biomaterials for craniofacial and dental tissue engineering—A review. Mater. Des. 2024, 241, 112886. [Google Scholar] [CrossRef]
- Jazayeri, H.E.; Lee, S.M.; Kuhn, L.; Fahimipour, F.; Tahriri, M.; Tayebi, L. Polymeric scaffolds for dental pulp tissue engineering: A review. Dent. Mater. 2020, 36, e47–e58. [Google Scholar] [CrossRef]
- Tang, G.; Tan, Z.; Zeng, W.; Wang, X.; Shi, C.; Liu, Y.; He, H.; Chen, R.; Ye, X. Recent Advances of Chitosan-Based Injectable Hydrogels for Bone and Dental Tissue Regeneration. Front. Bioeng. Biotechnol. 2020, 8, 587658. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Fauzi, M.B.; Abu Kasim, N.H. In Vitro and In Vivo Biological Assessments of 3D-Bioprinted Scaffolds for Dental Applications. Int. J. Mol. Sci. 2023, 24, 12881. [Google Scholar] [CrossRef]
- Haugen, H.J.; Basu, P.; Sukul, M.; Mano, J.F.; Reseland, J.E. Injectable biomaterials for dental tissue regeneration. Int. J. Mol. Sci. 2020, 21, 3442. [Google Scholar] [CrossRef]
- Cheah, C.W.; Al-Namnam, N.M.; Lau, M.N.; Lim, G.S.; Raman, R.; Fairbairn, P.; Ngeow, W.C. Synthetic material for bone, periodontal, and dental tissue regeneration: Where are we now, and where are we heading next? Materials 2021, 14, 6123. [Google Scholar] [CrossRef]
- Salgado, C.L.; Barrias, C.C.; Monteiro, F.J.M. Clarifying the Tooth-Derived Stem Cells Behavior in a 3D Biomimetic Scaffold for Bone Tissue Engineering Applications. Front. Bioeng. Biotechnol. 2020, 8, 724. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Abu Kasim, N.H. Current Advances of Three-Dimensional Bioprinting Application in Dentistry: A Scoping Review. Materials 2022, 15, 6398. [Google Scholar] [CrossRef]
- Kim, S.; Hwangbo, H.; Chae, S.J.; Lee, H. Biopolymers and Their Application in Bioprinting Processes for Dental Tissue Engineering. Pharmaceutics 2023, 15, 2118. [Google Scholar] [CrossRef]
- Sarna-Boś, K.; Skic, K.; Boguta, P.; Adamczuk, A.; Vodanovic, M.; Chałas, R. Elemental mapping of human teeth enamel, dentine and cementum in view of their microstructure. Micron 2023, 172, 103485. [Google Scholar] [CrossRef]
- Williams, C.; Wu, Y.; Bowers, D.F. ImageJ analysis of dentin tubule distribution in human teeth. Tissue Cell 2015, 47, 343–348. [Google Scholar] [CrossRef]
- Cho, Y.S.; Ryu, C.H.; Won, J.H.; Vang, H.; Oh, S.B.; Ro, J.Y.; Bae, Y.C. Rat odontoblasts may use glutamate to signal dentin injury. Neuroscience 2016, 335, 54–63. [Google Scholar] [CrossRef]
- Won, J.; Vang, H.; Kim, J.H.; Lee, P.R.; Kang, Y.; Oh, S.B. TRPM7 Mediates Mechanosensitivity in Adult Rat Odontoblasts. J. Dent. Res. 2018, 97, 1039–1046. [Google Scholar] [CrossRef]
- Slots, J. Periodontitis: Facts, fallacies and the future. Periodontol. 2000 2017, 75, 7–23. [Google Scholar] [CrossRef]
- Papapanou, P.N.; Sanz, M.; Buduneli, N.; Dietrich, T.; Feres, M.; Fine, D.H.; Flemmig, T.F.; Garcia, R.; Giannobile, W.V.; Graziani, F.; et al. Periodontitis: Consensus report of workgroup 2 of the 2017 World Workshop on the Classification of Periodontal and Peri-Implant Diseases and Conditions. J. Periodontol. 2018, 89, S173–S182. [Google Scholar] [CrossRef]
- Kwon, T.H.; Lamster, I.B.; Levin, L. Current Concepts in the Management of Periodontitis. Int. Dent. J. 2021, 71, 462–476. [Google Scholar] [CrossRef]
- Kornman, K.S. Mapping the Pathogenesis of Periodontitis: A New Look. J. Periodontol. 2008, 79, 1560–1568. [Google Scholar] [CrossRef]
- Bernabe, E.; Marcenes, W.; Hernandez, C.R.; Bailey, J.; Abreu, L.G.; Alipour, V.; Amini, S.; Arabloo, J.; Arefi, Z.; Arora, A.; et al. Global, Regional, and National Levels and Trends in Burden of Oral Conditions from 1990 to 2017: A Systematic Analysis for the Global Burden of Disease 2017 Study. J. Dent. Res. 2020, 99, 362–373. [Google Scholar] [CrossRef]
- Lobprise, H.B.; Johnson, J. Periodontal Disease. Pet-Specif. Care Vet. Team 2021, 6, 271–275. [Google Scholar] [CrossRef]
- White, D.A.; Tsakos, G.; Pitts, N.B.; Fuller, E.; Douglas, G.V.A.; Murray, J.J.; Steele, J.G. Adult Dental Health Survey 2009: Common oral health conditions and their impact on the population. Br. Dent. J. 2012, 213, 567–572. [Google Scholar] [CrossRef]
- Yucel-Lindberg, T.; Båge, T. Inflammatory mediators in the pathogenesis of periodontitis. Expert Rev. Mol. Med. 2013, 15, e7. [Google Scholar] [CrossRef] [PubMed]
- Fine, D.H.; Patil, A.G.; Loos, B.G. Classification and diagnosis of aggressive periodontitis. J. Periodontol. 2018, 89, S103–S119. [Google Scholar] [CrossRef] [PubMed]
- Dietrich, T.; Ower, P.; Tank, M.; West, N.X.; Walter, C.; Needleman, I.; Hughes, F.J.; Wadia, R.; Milward, M.R.; Hodge, P.J.; et al. Periodontal diagnosis in the context of the 2017 classification system of periodontal diseases and conditions—Implementation in clinical practice. Br. Dent. J. 2019, 226, 16–22. [Google Scholar] [CrossRef]
- Graetz, C.; Mann, L.; Krois, J.; Sälzer, S.; Kahl, M.; Springer, C.; Schwendicke, F. Comparison of periodontitis patients’ classification in the 2018 versus 1999 classification. J. Clin. Periodontol. 2019, 46, 908–917. [Google Scholar] [CrossRef]
- Pihlstrom, B.L.; Michalowicz, B.S.; Johnson, N.W. Periodontal diseases. Lancet 2005, 366, 1809–1820. [Google Scholar] [CrossRef] [PubMed]
- Ivanovski, S.; Vaquette, C.; Gronthos, S.; Hutmacher, D.W.; Bartold, P.M. Multiphasic scaffolds for periodontal tissue engineering. J. Dent. Res. 2014, 93, 1212–1221. [Google Scholar] [CrossRef]
- Thattaruparambil Raveendran, N.; Vaquette, C.; Meinert, C.; Samuel Ipe, D.; Ivanovski, S. Optimization of 3D bioprinting of periodontal ligament cells. Dent. Mater. 2019, 35, 1683–1694. [Google Scholar] [CrossRef]
- Traill, Z.; Richards, M.A.; Moore, N.R. Magnetic resonance imaging of metastatic bone disease. Clin. Orthop. Relat. Res. 1995, 312, 76–88. [Google Scholar] [CrossRef]
- Clarke, B. Normal bone anatomy and physiology. Clin. J. Am. Soc. Nephrol. 2008, 3 (Suppl. S3), 131–139. [Google Scholar] [CrossRef]
- Feng, X.; McDonald, J.M. Disorders of bone remodeling. Annu. Rev. Pathol. Mech. Dis. 2011, 6, 121–145. [Google Scholar] [CrossRef] [PubMed]
- Järvinen, T.L.N.; Sievänen, H.; Jokihaara, J.; Einhorn, T.A. Revival of bone strength: The bottom line. J. Bone Miner. Res. 2005, 20, 717–720. [Google Scholar] [CrossRef] [PubMed]
- Fogelman, I.; Van Der Wall, H.; Gnanasegaran, G. (Eds.) Physiology of Bone Formation Remodeling, and Metabolism. In Radionuclide and Hybrid Bone Imaging; Springer: Berlin/Heidelberg, Germany, 2012; pp. 29–57. [Google Scholar] [CrossRef]
- Black, J.; Mattson, R.; Korostoff, E. Haversian osteons: Size, distribution, internal structure, and orientation. J. Biomed. Mater. Res. 1974, 8, 299–319. [Google Scholar] [CrossRef]
- Kobayashi, S.; Takahashi, H.E.; Ito, A.; Saito, N.; Nawata, M.; Horiuchi, H.; Ohta, H.; Iorio, R.; Yamamoto, N.; Takaoka, K. Trabecular minimodeling in human iliac bone. Bone 2003, 32, 163–169. [Google Scholar] [CrossRef] [PubMed]
- Mori, S.; Burr, D. Increased Intracortical Remodeling Following Fatigue Damage. Bone 2017, 14, 103–109. [Google Scholar] [CrossRef]
- Kenkre, J.S.; Bassett, J.H.D. The bone remodelling cycle. Ann. Clin. Biochem. 2018, 55, 308–327. [Google Scholar] [CrossRef]
- Willie, B.M.; Petersen, A.; Schmidt-Bleek, K.; Cipitria, A.; Mehta, M.; Strube, P.; Lienau, J.; Wildemann, B.; Fratzl, P.; Duda, G. Designing biomimetic scaffolds for bone regeneration: Why aim for a copy of mature tissue properties if nature uses a different approach? Soft Matter 2010, 6, 4976–4987. [Google Scholar] [CrossRef]
- Manzini, B.M.; Machado, L.M.R.; Noritomi, P.Y.; da Silva, J.V.L. Advances in Bone tissue engineering: A fundamental review. J. Biosci. 2021, 46, 17. [Google Scholar] [CrossRef]
- Grayson, W.L.; Bunnell, B.A.; Martin, E.; Frazier, T.; Hung, B.P.; Gimble, J.M. Stromal cells and stem cells in clinical bone regeneration. Nat. Rev. Endocrinol. 2015, 11, 140–150. [Google Scholar] [CrossRef]
- Wang, C.; Huang, W.; Zhou, Y.; He, L.; He, Z.; Chen, Z.; He, X.; Tian, S.; Liao, J.; Lu, B.; et al. 3D printing of bone tissue engineering scaffolds. Bioact. Mater. 2020, 5, 82–91. [Google Scholar] [CrossRef]
- Mohd, N.; Razali, M.; Ghazali, M.J.; Kasim, N.H.A. 3D-Printed Hydroxyapatite and Tricalcium Phosphates-Based Scaffolds for Alveolar Bone Regeneration in Animal Models: A Scoping Review. Materials 2022, 15, 2621. [Google Scholar] [CrossRef] [PubMed]
- Chen, Y.; Jia, Z.; Shafiq, M.; Xie, X.; Xiao, X.; Castro, R.; Rodrigues, J.; Wu, J.; Zhou, G.; Mo, X. Gas foaming of electrospun poly(L-lactide-co-caprolactone)/silk fibroin nanofiber scaffolds to promote cellular infiltration and tissue regeneration. Colloids Surf. B Biointerfaces 2021, 201, 111637. [Google Scholar] [CrossRef]
- Sola, A.; Bertacchini, J.; D’Avella, D.; Anselmi, L.; Maraldi, T.; Marmiroli, S.; Messori, M. Development of solvent-casting particulate leaching (SCPL) polymer scaffolds as improved three-dimensional supports to mimic the bone marrow niche. Mater. Sci. Eng. C 2019, 96, 153–165. [Google Scholar] [CrossRef] [PubMed]
- Dey, M.; Ozbolat, I.T. 3D bioprinting of cells, tissues and organs. Sci. Rep. 2020, 10, 10–12. [Google Scholar] [CrossRef]
- Amoli, M.S.; Ezeldeen, M.; Jacobs, R.; Bloemen, V. Materials for Dentoalveolar Bioprinting: Current State of the Art. Biomedicines 2022, 10, 71. [Google Scholar] [CrossRef]
- Guzzi, E.A.; Tibbitt, M.W. Additive Manufacturing of Precision Biomaterials. Adv. Mater. 2020, 32, e1901994. [Google Scholar] [CrossRef] [PubMed]
- Gruene, M.; Unger, C.; Koch, L.; Deiwick, A.; Chichkov, B. Dispensing pico to nanolitre of a natural hydrogel by laser-assisted bioprinting. Biomed. Eng. Online 2011, 10, 9–12. [Google Scholar] [CrossRef]
- Zhang, Z.; Xiong, R.; Mei, R.; Huang, Y.; Chrisey, D.B. Time-Resolved Imaging Study of Jetting Dynamics during Laser Printing of Viscoelastic Alginate Solutions. Langmuir 2015, 31, 6447–6456. [Google Scholar] [CrossRef]
- Dou, C.; Perez, V.; Qu, J.; Tsin, A.; Xu, B.; Li, J. A State-of-the-Art Review of Laser-Assisted Bioprinting and its Future Research Trends. ChemBioEng Rev. 2021, 8, 517–534. [Google Scholar] [CrossRef]
- Suamte, L.; Tirkey, A.; Barman, J.; Jayasekhar Babu, P. Various manufacturing methods and ideal properties of scaffolds for tissue engineering applications. Smart Mater. Manuf. 2023, 1, 100011. [Google Scholar] [CrossRef]
- Kérourédan, O.; Bourget, J.M.; Rémy, M.; Crauste-Manciet, S.; Kalisky, J.; Catros, S.; Thébaud, N.B.; Devillard, R. Micropatterning of endothelial cells to create a capillary-like network with defined architecture by laser-assisted bioprinting. J. Mater. Sci. Mater. Med. 2019, 30, 28. [Google Scholar] [CrossRef] [PubMed]
- Kérourédan, O.; Hakobyan, D.; Rémy, M.; Ziane, S.; Dusserre, N.; Fricain, J.-C.; Delmond, S.; Thébaud, N.B.; Devillard, R. In situ prevascularization designed by laser-assisted bioprinting: Effect on bone regeneration. Int. Soc. Biofabrication 2019, 11, 45002. [Google Scholar] [CrossRef] [PubMed]
- Masri, S.; Fauzi, M.B. Current insight of printability quality improvement strategies in natural-based bioinks for skin regeneration and wound healing. Polymers 2021, 13, 1011. [Google Scholar] [CrossRef] [PubMed]
- Unagolla, J.M.; Jayasuriya, A.C. Hydrogel-based 3D bioprinting: A comprehensive review on cell-laden hydrogels, bioink formulations, and future perspectives. Appl. Mater. Today 2020, 18, 100479. [Google Scholar] [CrossRef]
- Chen, Y.-S.; Chang, S.-S.; Ng, H.Y.; Huang, Y.-X.; Chen, C.-C.; Shie, M.-Y. Additive manufacturing of astragaloside-containing polyurethane nerve conduits influenced Schwann cell inflammation and regeneration. Processes 2021, 9, 353. [Google Scholar] [CrossRef]
- Mandrycky, C.; Wang, Z.; Kim, K.; Kim, D.H. 3D bioprinting for engineering complex tissues. Biotechnol. Adv. 2016, 34, 422–434. [Google Scholar] [CrossRef]
- Nakamura, M.; Kobayashi, A.; Takagi, F.; Watanabe, A.; Hiruma, Y.; Ohuchi, K.; Iwasaki, Y.; Horie, M.; Morita, I.; Takatani, S. Biocompatible inkjet printing technique for designed seeding of individual living cells. Tissue Eng. 2004, 11, 1658–1666. [Google Scholar] [CrossRef] [PubMed]
- Kačarević, Ž.P.; Rider, P.M.; Alkildani, S.; Retnasingh, S.; Smeets, R.; Jung, O.; Ivanišević, Z.; Barbeck, M. An introduction to 3D bioprinting: Possibilities, challenges and future aspects. Materials 2018, 11, 2199. [Google Scholar] [CrossRef]
- Li, J.; Chen, M.; Fan, X.; Zhou, H. Recent advances in bioprinting techniques: Approaches, applications and future prospects. J. Transl. Med. 2016, 14, 271. [Google Scholar] [CrossRef]
- Cui, X.; Boland, T.; D’Lima, D.D.; Lotz, M.K. Thermal Inkjet Printing in Tissue Engineering and Regenerative Medicine. Recent Pat. Drug Deliv. Formul. 2012, 6, 149–155. [Google Scholar] [CrossRef]
- Li, X.; Liu, B.; Pei, B.; Chen, J.; Zhou, D.; Peng, J.; Zhang, X.; Jia, W.; Xu, T. Inkjet Bioprinting of Biomaterials. Chem. Rev. 2020, 120, 10793–10833. [Google Scholar] [CrossRef]
- Wijshoff, H. The dynamics of the piezo inkjet printhead operation. Phys. Rep. 2010, 491, 77–177. [Google Scholar] [CrossRef]
- Xu, T.; Gregory, C.A.; Molnar, P.; Cui, X.; Jalota, S.; Bhaduri, S.B.; Boland, T. Viability and electrophysiology of neural cell structures generated by the inkjet printing method. Biomaterials 2006, 27, 3580–3588. [Google Scholar] [CrossRef] [PubMed]
- Axpe, E.; Oyen, M.L. Applications of alginate-based bioinks in 3D bioprinting. Int. J. Mol. Sci. 2016, 17, 1976. [Google Scholar] [CrossRef] [PubMed]
- Chircov, C.; Miclea, I.I.; Grumezescu, V.; Grumezescu, A.M. Essential oils for bone repair and regeneration—Mechanisms and applications. Materials 2021, 14, 1867. [Google Scholar] [CrossRef] [PubMed]
- Muresan, S.M.C.; Dreanca, A.; Repciuc, C.; Dejescu, C.; Rotar, O.; Pop, R.A.; Pantea, S.; Pall, E.; Ciotlaus, I.; Sarosi, C.; et al. Dental Hydrogels with Essential Oils with Potential Activity in Periodontitis. Appl. Sci. 2023, 13, 1787. [Google Scholar] [CrossRef]
- Correa, G.T.B.; Veranio, G.A.C.; Silva, L.E.; Hirata Junior, R.; Coil, J.M.; Scelza, M.F.Z. Cytotoxicity evaluation of two root canal sealers and a commercial calcium hydroxide paste on thp1 cell line by trypan blue assay. J. Appl. Oral Sci. 2009, 17, 457–461. [Google Scholar] [CrossRef]
- United States Department of Agriculture Foreign Agricultural Service. Oilseeds: World Markets and Trade; USDA: Washington, DC, USA, 2024.
- Ribeiro, A.R.; Silva, S.S.; Reis, R.L. Challenges and opportunities on vegetable oils derived systems for biomedical applications. Biomater. Adv. 2022, 134, 112720. [Google Scholar] [CrossRef]
- Farooq, M.; Azadfar, E.; Trif, M.; Jabaleh, R.A.; Rusu, A.; Bahrami, Z.; Sharifi, M.; Bangar, S.P.; Ilyas, N.; Ștefănescu, B.E.; et al. Soybean oil enriched with antioxidants extracted from watermelon (Citrullus colocynthis) skin sap and coated in hydrogel beads via ionotropic gelation. Coatings 2021, 11, 1370. [Google Scholar] [CrossRef]
- Miao, S.; Zhu, W.; Castro, N.J.; Nowicki, M.; Zhou, X.; Cui, H.; Fisher, J.P.; Zhang, L.G. 4D printing smart biomedical scaffolds with novel soybean oil epoxidized acrylate. Sci. Rep. 2016, 6, 27226. [Google Scholar] [CrossRef]
- Gupta, S.; Kumar, S. Experimental Investigation of Bio-Diesel With Corn. Int. J. Mech. Product. Eng. 2015, 3, 73–78. [Google Scholar]
- Barrera-Arellano, D.; Badan-Ribeiro, A.P.; Serna-Saldivar, S.O. Corn Oil: Composition, Processing, and Utilization, 3rd ed.; Elsevier Inc.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Alameldin, H.; Izadi-Darbandi, A.; Smith, S.A.; Balan, V.; Jones, A.D.; Ebru Orhun, G.; Sticklen, M. Metabolic engineering to increase the corn seed storage lipid quantity and change its compositional quality. Crop Sci. 2017, 57, 1854–1864. [Google Scholar] [CrossRef]
- Chen, F.; Zeng, Y.; Cheng, Q.; Xiao, L.; Ji, J.; Hou, X.; Huang, Q.; Lei, Z. Tissue culture and Agrobacterium-mediated genetic transformation of the oil crop sunflower. PLoS ONE 2024, 19, e0298299. [Google Scholar] [CrossRef]
- Temme, A.A.; Kerr, K.L.; Masalia, R.R.; Burke, J.M.; Donovan, L.A. Key traits and genes associate with salinity tolerance independent from vigor in cultivated sunflower. Plant Physiol. 2020, 184, 865–880. [Google Scholar] [CrossRef] [PubMed]
- Jaganathan, S.K.; Mani, M.P.; Nageswaran, G.; Krishnasamy, N.P.; Ayyar, M. Single stage electrospun multicomponent scaffold for bone tissue engineering application. Polym. Test. 2018, 70, 244–254. [Google Scholar] [CrossRef]
- Pal, D. Sunflower (Helianthus annuus L.) Seeds in Health and Nutrition; Elsevier Inc.: Amsterdam, The Netherlands, 2011. [Google Scholar] [CrossRef]
- Monja-Mio, K.M.; Herrera-Alamillo, M.A.; Sánchez-Teyer, L.F.; Robert, M.L. Breeding strategies to improve production of agave (Agave spp.). In Advances in Plant Breeding Strategies: Industrial and Food Crops; Springer: Cham, Switzerland, 2019; Volume 6. [Google Scholar] [CrossRef]
- Cooper, D.; Doucet, L.; Pratt, M. Understanding in multinational organizations. J. Organ. Behav. 2017, 28, 303–325. [Google Scholar] [CrossRef]
- Moreno, M.; Armentano, I.; Fortunati, E.; Mattioli, S.; Torre, L.; Lligadas, G.; Ronda, J.C.; Galià, M.; Cádiz, V. Cellulose nano-biocomposites from high oleic sunflower oil-derived thermosets. Eur. Polym. J. 2016, 79, 109–120. [Google Scholar] [CrossRef]
- Carson, C.F.; Hammer, K.A.; Riley, T.V.; Carson, C.F.; Hammer, K.A.; Riley, T.V. Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties Melaleuca alternifolia (Tea Tree) Oil: A Review of Antimicrobial and Other Medicinal Properties. Clin. Microbiol. Rev. 2006, 19, 50–62. [Google Scholar] [CrossRef]
- Pazyar, N.; Yaghoobi, R.; Bagherani, N.; Kazerouni, A. A review of applications of tea tree oil in dermatology. Int. J. Dermatol. 2013, 52, 784–790. [Google Scholar] [CrossRef]
- Edwards-Jones, V.; Buck, R.; Shawcross, S.G.; Dawson, M.M.; Dunn, K. The effect of essential oils on methicillin-resistant Staphylococcus aureus using a dressing model. Burns 2004, 30, 772–777. [Google Scholar] [CrossRef]
- Graziano, T.S.; Calil, C.M.; Sartoratto, A.; Franco, G.C.N.; Groppo, F.C.; Cogo-Müller, K. In vitro effects of Melaleuca alternifolia essential oil on growth and production of volatile sulphur compounds by oral bacteria. J. Appl. Oral Sci. 2016, 24, 582–589. [Google Scholar] [CrossRef]
- Yadav, E.; Kumar, S.; Mahant, S.; Khatkar, S.; Rao, R. Tea tree oil: A promising essential oil. J. Essent. Oil Res. 2017, 29, 201–213. [Google Scholar] [CrossRef]
- Castro, J.I.; Valencia-Llano, C.H.; Zapata, M.E.V.; Restrepo, Y.J.; Hernandez, J.H.M.; Navia-Porras, D.P.; Valencia, Y.; Valencia, C.; Grande-Tovar, C.D. Chitosan/polyvinyl alcohol/tea tree essential oil composite films for biomedical applications. Polymers 2021, 13, 3753. [Google Scholar] [CrossRef]
- Grande-Tovar, C.D.; Castro, J.I.; Valencia Llano, C.H.; Tenorio, D.L.; Saavedra, M.; Zapata, P.A.; Chaur, M.N. Polycaprolactone (PCL)-Polylactic Acid (PLA)-Glycerol (Gly) Composites Incorporated with Zinc Oxide Nanoparticles (ZnO-NPs) and Tea Tree Essential Oil (TTEO) for Tissue Engineering Applications. Pharmaceutics 2023, 15, 43. [Google Scholar] [CrossRef] [PubMed]
- Chandra, S.; Lata, H.; ElSohly, M.A. Propagation of Cannabis for Clinical Research: An Approach Towards a Modern Herbal Medicinal Products Development. Front. Plant Sci. 2020, 11, 958. [Google Scholar] [CrossRef] [PubMed]
- Gunasekera, B.; Davies, C.; Martin-Santos, R.; Bhattacharyya, S. The Yin and Yang of Cannabis: A Systematic Review of Human Neuroimaging Evidence of the Differential Effects of Δ9-Tetrahydrocannabinol and Cannabidiol. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2021, 6, 636–645. [Google Scholar] [CrossRef]
- Weigelt, M.A.; Sivamani, R.; Lev-Tov, H. The therapeutic potential of cannabinoids for integumentary wound management. Exp. Dermatol. 2021, 30, 201–211. [Google Scholar] [CrossRef]
- Blaskovich, M.A.T.; Kavanagh, A.M.; Elliott, A.G.; Zhang, B.; Ramu, S.; Amado, M.; Lowe, G.J.; Hinton, A.O.; Pham, D.M.T.; Zuegg, J.; et al. The antimicrobial potential of cannabidiol. Commun. Biol. 2021, 4, 7. [Google Scholar] [CrossRef]
- Antezana, P.E.; Municoy, S.; Orive, G.; Desimone, M.F. Design of a New 3D Gelatin—Alginate Scaffold Loaded with Cannabis sativa Oil. Polymers 2022, 14, 4506. [Google Scholar] [CrossRef]
- Aung, M.M.; Yaakob, Z.; Abdullah, L.C.; Rayung, M.; Li, W.J. A comparative study of acrylate oligomer on Jatropha and Palm oil-based UV-curable surface coating. Ind. Crops Prod. 2015, 77, 1047–1052. [Google Scholar] [CrossRef]
- Tan, C.P.; Nehdi, I.A. The Physicochemical Properties of Palm Oil and Its Components; AOCS Press: Champaign, IL, USA, 2012. [Google Scholar] [CrossRef]
- Tajau, R.; Rohani, R.; Alias, M.S.; Mudri, N.H.; Abdul Halim, K.A.; Harun, M.H.; Isa, N.M.; Ismail, R.C.; Faisal, S.M.; Talib, M.; et al. Emergence of polymeric material utilising sustainable radiation curable palm oil-based products for advanced technology applications. Polymers 2021, 13, 1865. [Google Scholar] [CrossRef]
- Serbinova, E.; Kagan, V.; Han, D.; Packer, L. Free radical recycling and intramembrane mobility in the antioxidant properties of alpha-tocopherol and alpha-tocotrienol. Free Radic. Biol. Med. 1991, 10, 263–275. [Google Scholar] [CrossRef] [PubMed]
- Tajau, R.; Rohani, R.; Salleh, M.Z. Physicochemical and Thermal Properties of Acrylated Palm Olein as a Promising Biopolymer. J. Polym. Environ. 2020, 28, 2734–2748. [Google Scholar] [CrossRef]
- Tajau, R.; Rohani, R.; Abdul Hamid, S.S.; Adam, Z.; Mohd Janib, S.N.; Salleh, M.Z. Surface functionalisation of poly-APO-b-polyol ester cross-linked copolymers as core–shell nanoparticles for targeted breast cancer therapy. Sci. Rep. 2020, 10, 21704. [Google Scholar] [CrossRef] [PubMed]
- Stavila, E.; Yuliati, F.; Adharis, A.; Laksmono, J.A.; Iqbal, M. Recent advances in synthesis of polymers based on palm oil and its fatty acids. RSC Adv. 2023, 13, 14747–14775. [Google Scholar] [CrossRef]
- Rao, U.; Sridhar, R.; Sehgal, P.K. Biosynthesis and biocompatibility of poly(3-hydroxybutyrate-co-4-hydroxybutyrate) produced by Cupriavidus necator from spent palm oil. Biochem. Eng. J. 2010, 49, 13–20. [Google Scholar] [CrossRef]
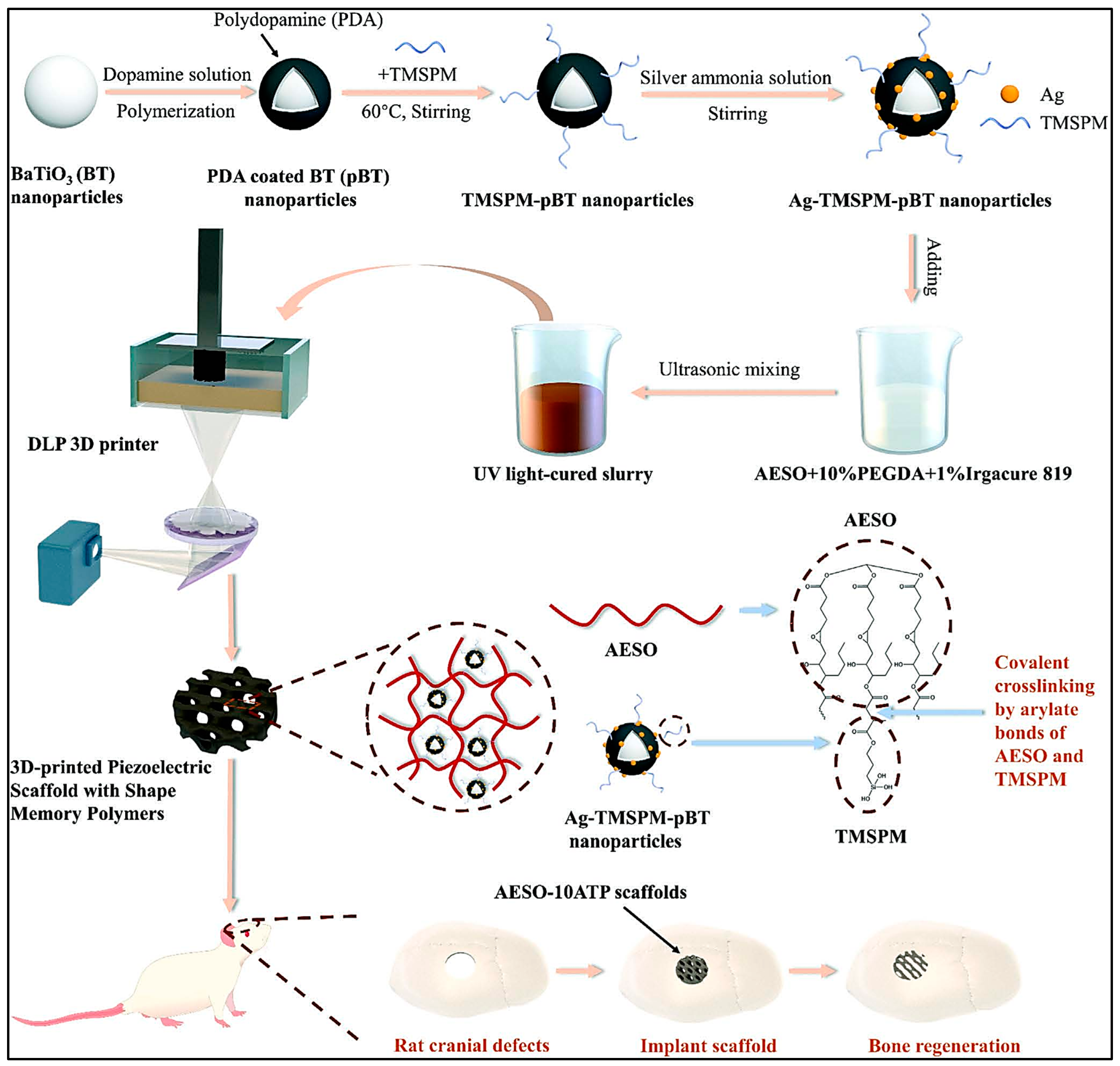
- Li, G.; Li, Z.; Min, Y.; Chen, S.; Han, R.; Zhao, Z. 3D-Printed Piezoelectric Scaffolds with Shape Memory Polymer for Bone Regeneration. Small 2023, 19, e2302927. [Google Scholar] [CrossRef]
- Liu, Q.; Li, T.; Gan, S.W.; Chang, S.Y.; Yen, C.C.; Zhai, W. Controlling the hierarchical microstructure of bioceramic scaffolds by 3D printing of emulsion inks. Addit. Manuf. 2023, 61, 103332. [Google Scholar] [CrossRef]
- Ahmed, J.; Mulla, M.; Joseph, A.; Ejaz, M.; Maniruzzaman, M. Zinc oxide/clove essential oil incorporated type B gelatin nanocomposite formulations: A proof-of-concept study for 3D printing applications. Food Hydrocoll. 2020, 98, 105256. [Google Scholar] [CrossRef]
- Mondal, D.; Srinivasan, A.; Comeau, P.; Toh, Y.C.; Willett, T.L. Acrylated epoxidized soybean oil/hydroxyapatite-based nanocomposite scaffolds prepared by additive manufacturing for bone tissue engineering. Mater. Sci. Eng. C 2021, 118, 111400. [Google Scholar] [CrossRef]
- Kim, W.J.; Jang, C.H.; Kim, G.H. Bone tissue engineering supported by bioprinted cell constructs with endothelial cell spheroids. Theranostics 2022, 12, 5404–5417. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.J.; Kim, G.H. Highly bioactive cell-laden hydrogel constructs bioprinted using an emulsion bioink for tissue engineering applications. Biofabrication 2022, 14, 45018. [Google Scholar] [CrossRef] [PubMed]
- Obregon, F.; Vaquette, C.; Ivanovski, S.; Hutmacher, D.W.; Bertassoni, L.E. Three-Dimensional Bioprinting for Regenerative Dentistry and Craniofacial Tissue Engineering. J. Dent. Res. 2015, 94, 143S–152S. [Google Scholar] [CrossRef] [PubMed]
- Naghieh, S.; Chen, X. Printability–A key issue in extrusion-based bioprinting. J. Pharm. Anal. 2021, 11, 564–579. [Google Scholar] [CrossRef]
- Gao, G.; Cui, X. Three-dimensional bioprinting in tissue engineering and regenerative medicine. Biotechnol. Lett. 2016, 38, 203–211. [Google Scholar] [CrossRef]
- Melchels, F.P.W.; Domingos, M.A.N.; Klein, T.J.; Malda, J.; Bartolo, P.J.; Hutmacher, D.W. Additive manufacturing of tissues and organs. Prog. Polym. Sci. 2012, 37, 1079–1104. [Google Scholar] [CrossRef]
- Rodriguez-Salvador, M.; Ruiz-Cantu, L. Revealing emerging science and technology research for dentistry applications of 3D bioprinting. Int. J. Bioprinting 2019, 5, 170. [Google Scholar] [CrossRef]
- Heo, D.N.; Castro, N.J.; Lee, S.-J.; Noh, H.; Zhu, W.; Zhang, L.G. Enhanced bone tissue regeneration using 3D printed microstructure incorporated with hybrid nano hydrogel Dong. Physiol. Behav. 2018, 176, 139–148. [Google Scholar]
- Hernandez, I.; Kumar, A.; Joddar, B. A bioactive hydrogel and 3d printed polycaprolactone system for bone tissue engineering. Gels 2017, 3, 26. [Google Scholar] [CrossRef]
- Adepu, S.; Dhiman, N.; Laha, A.; Sharma, C.S.; Ramakrishna, S.; Khandelwal, M. Three-dimensional bioprinting for bone tissue regeneration. Curr. Opin. Biomed. Eng. 2017, 2, 22–28. [Google Scholar] [CrossRef]
- Kyburz, K.A.; Anseth, K.S. Synthetic Mimics of the Extracellular Matrix: How Simple is Complex Enough? Ann. Biomed. Eng. 2015, 43, 489–500. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Yu, F.; Zheng, L.; Wang, R.; Yan, W.; Wang, Z.; Xu, J.; Wu, J.; Shi, D.; Zhu, L.; et al. Natural hydrogels for cartilage regeneration: Modification, preparation and application. J. Orthop. Transl. 2019, 17, 26–41. [Google Scholar] [CrossRef] [PubMed]
- Syed, I.; Garg, S.; Sarkar, P. Entrapment of Essential Oils in Hydrogels for Biomedical Applications; Elsevier Ltd.: Amsterdam, The Netherlands, 2018. [Google Scholar] [CrossRef]
- Kuo, P.J.; Lin, Y.H.; Huang, Y.X.; Lee, S.Y.; Huang, H.M. Effects of Sapindus mukorossi Seed Oil on Bone Healing Efficiency: An Animal Study. Int. J. Mol. Sci. 2024, 25, 6749. [Google Scholar] [CrossRef] [PubMed]

| No | Reference | Study Design | Type of 3D Bioprinting | Scaffold | Applications | Outcomes |
|---|---|---|---|---|---|---|
| 1. | Ahmed et al. (2020) [105] |
| Extrusion-based 3D bioprinting | Bovine skin gelatin (BSG)/zinc oxide (ZnO)/clove essential oil (CEO) with alginate. | Tissue engineering | A gelatin-based antimicrobial film was adapted into 3D-printable ink for applications like food printing. This approach combines semi-solid extrusion with 3D printing, reducing development time and steps. The platform can be used with other drugs and biomaterials for personalized products. |
| 2. | Mondal et al. (2021) [106] |
| Extrusion-based 3D bioprinting | Acrylated epoxidized soybean oil (AESO), Poly(ethylene glycol) diacrylate (PEGDA), and nano-hydroxyapatite (nHA) | Bone | The study developed 3D-printed nanocomposite scaffolds using AESO, nHA rods, and either HEA or PEGDA. The scaffolds showed good viscosity, particle dispersion, and mechanical strength. HEA improved shear yield strength, printability, cell adhesion, proliferation, and osteogenic differentiation, supporting bone tissue growth after 14 and 21 days. |
| 3. | Antezana et al. (2022) [94] |
| Extrusion-based 3D bioprinting | Gelatin–Alginate Bioink with Cannabis sativa oil | NA | The study developed a gelatin–alginate bioink optimized for 3D bioprinting. The scaffolds can be lyophilized for storage without losing structure and have high absorption capacity for loading therapeutic molecules. Adding Cannabis sativa oil enhanced antioxidant and antimicrobial activity, making it a promising alternative to conventional treatments. |
| 4. | Kim et al. (2022) [107] |
| Extrusion-based 3D bioprinting (pneumatic pressure) | Porcine bone-derived dECM (BdECM) hydrgel-Ecs steroid in the mineral oi | Bone | Hybrid cell constructs with EC spheroids and hASC-laden dECM/β-TCP struts enhance bone formation and angiogenic activities, potentially serving as a therapeutic biomaterial for bone tissue induction. |
| 5. | Kim and Kim (2022) [108] |
| Extrusion-based 3D bioprinting (pneumatic pressure) | Methacrylated collagen (CMA) bioink–mineral oil | Bone | The study developed a CMA-MO emulsion bioink for 3D cell constructs with human adenocarcinoma stem cells (hASCs). The bioink offers a stable structure with hierarchical pores, enhancing cell growth and cytoskeleton reorganization. It also delivers KGN and BMP-2, promoting chondrogenic and osteogenic differentiation, making it promising for improving cellular activities. |
| 6. | Liu et al. (2023) [104] |
| Extrusion-based 3D bioprinting | (1) Hydroxyappatite (HA)-sunflower oil. (2) Hydroxyappatite (HA)-Pluronic® F-127. | Bone | This study developed biomimetic hpHA scaffolds for bone tissue engineering using DIW. The scaffolds have interconnected macropores and micropores, with strength similar to cancellous bone. They enhance stem cell attachment, spreading, and growth, making DIW a promising method for BTE scaffold optimization. |
| No | Reference | Type of Crosslinker | Rheological Properties | Physical Properties | Chemical Characterization |
|---|---|---|---|---|---|
| 1. | Ahmed et al. (2020) [105] | Cross-linked with 100 mM calcium chloride (CaCl2) solution | The print resolution of the printing materials was achieved to have layer height ~100 μm and the construct directly printed in a Petri dish containing 100 mM CaCl2 solution exhibited a good crosslinking (curing) behavior of the alginate at room temperature. | SEM: The addition of clove essential oil (CEO) into the bovine skin gelatin (BSG) matrix generated porosity, which could be related with the evaporation of the CEO during drying. | XRD: The neat gelatin film showed no distinct peaks, but zinc oxide (ZnO) and CEO composite films had characteristic peaks at 2θ of 10.4, 12.7, 22.8, 32.2, 34.8, 36.8, and 56.5. FTIR: All samples showed similar amide-band peaks (A, B, I, II, and III), with differences in wavenumber and peak intensity. The band at 1035 cm−1 indicated interactions between the film structure and glycerol’s O–H group vibrations. |
| 2. | Mondal et al. (2021) [106] | Photocrosslinking | Adding 2-Hydroxyethyl Acrylate (HEA) and Polyethylene Glycol Diacrylate (PEGDA) reduced the viscosity of nanocomposite inks compared to pure Acrylated Epoxidized Soybean Oil (AESO)-based ones. HEA significantly lowered viscosity from 40.4 ± 0.88 Pa·s to 0.83 ± 0.24 Pa·s and increased shear yield stress from 11.33 ± 0.48 Pa to 68.33 ± 17.15 Pa. | SEM: The incorporation of HEA and PEGDA resulted in open-faced, well-defined, and interconnected porous networks. | FTIR: The spectra of the nanocomposites demonstrated successful curing as there were no peaks associated with vinyl groups remaining after curing. |
| 3. | Antezana et al. (2022) [94] | Calcium chloride (CaCl2) | NA | SEM: The material had a compact, smooth surface with an average pore size of 15.0 ± 2.3 μm. Biodegradation: The gelatin– alginate (GEL–ALG) scaffold fully degraded in 5 h, while the gelatin–alginate–Cannabis sativa (GEL–ALG–CS) scaffold lasted up to 20 h, retaining 20% of its weight. | FTIR: Presence of characteristic peaks of CS at 2924 and 2850 cm−1, which is not observed in GEL-ALG. |
| 4. | Kim et al. (2022) [107] | NA | NA | NA | NA |
| 5. | Kim and Kim (2022) [108] | NA | The rheological properties abruptly decreased when the oil volume fraction in the emulsion bioink was above 30 v/v%. | SEM: Methacrylated collagen/mineral oil (CMA/MO) had a higher porosity (97.9 ± 0.3%) than CMA (96.4 ± 0.3%). Wettability: Dextran diffused faster in CMA/MO than in CMA, indicating better wettability. CMA/MO also showed higher protein absorption. | NA |
| 6. | Liu et al. (2023) [104] | NA | All inks were shear-thinning. Hydroxyapatite (HA)-stabilized emulsions without Pluronic® F-127 had higher viscosity, storage modulus, and yield stress after emulsification. More oil improved these properties but reduced HA content, limiting the viscosity increase. | SEM: 3D-printed HA scaffolds had pores of 300–400 μm horizontally and ~100 μm vertically. hpHA-P100 had more and larger micropores than F100, with higher oil content increasing micropores in the struts. | NA |
| No | Reference | Thermal Stability | Mechanical Characterization |
|---|---|---|---|
| 1. | Ahmed et al. (2020) [105] | Bovine skin gelatin/zinc oxide/clove essential oil (BSG/ZnO/CEO) films showed three weight loss stages: 37.35% (197–256 °C), 31.51% (267–367 °C), and 18.41% (400–460 °C). ZnO reinforcement improved the thermal stability of the film. | Adding ZnO and CEO reduced the tensile strength of BSG films from 36.9 ± 2.8 to 32.7 ± 2.3 MPa (p < 0.05) but increased elongation at break from 15.05 ± 1.3% to 19.1 ± 1.8%. |
| 2. | Mondal et al. (2021) [106] | NA | The representative stress-strain curves of S20 and SP20 nanocomposites demonstrate their higher tensile strengths compared to the SH20 nanocomposites. The tensile elastic moduli for S20, SH20, and SP20 were 689.95 ± 189.45 MPa, 198.72 ± 36.65 MPa, and 645.34 ± 149.78 MPa, respectively. |
| 3. | Antezana et al. (2022) [94] | NA | NA |
| 4. | Kim et al. (2022) [107] | NA | NA |
| 5. | Kim and Kim (2022) [108] | NA | NA |
| 6. | Liu et al. (2023) [104] | NA | dHA scaffolds had the highest compressive strength and Young’s modulus due to their dense struts and low porosity, exceeding those of cancellous bone (yield strength: 2–12 MPa, Young’s modulus: 50–500 MPa). |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Masri, S.; Mohd, N.; Abu Kasim, N.H.; Razali, M. 3D-Bioprinted Oil-Based Hydrogels: A Sustainable Approach for Bone and Dental Regeneration. Int. J. Mol. Sci. 2025, 26, 3510. https://doi.org/10.3390/ijms26083510
Masri S, Mohd N, Abu Kasim NH, Razali M. 3D-Bioprinted Oil-Based Hydrogels: A Sustainable Approach for Bone and Dental Regeneration. International Journal of Molecular Sciences. 2025; 26(8):3510. https://doi.org/10.3390/ijms26083510
Chicago/Turabian StyleMasri, Syafira, Nurulhuda Mohd, Noor Hayaty Abu Kasim, and Masfueh Razali. 2025. "3D-Bioprinted Oil-Based Hydrogels: A Sustainable Approach for Bone and Dental Regeneration" International Journal of Molecular Sciences 26, no. 8: 3510. https://doi.org/10.3390/ijms26083510
APA StyleMasri, S., Mohd, N., Abu Kasim, N. H., & Razali, M. (2025). 3D-Bioprinted Oil-Based Hydrogels: A Sustainable Approach for Bone and Dental Regeneration. International Journal of Molecular Sciences, 26(8), 3510. https://doi.org/10.3390/ijms26083510







