In Silico and In Vitro Analyses of Strawberry-Derived Extracts in Relation to Key Compounds’ Metabolic and Anti-Tumor Effects
Abstract
1. Introduction
2. Results
2.1. Analytical Results
2.2. In Silico SwissADME Computational Results
2.3. In Silico Molecular CLC Docking Results
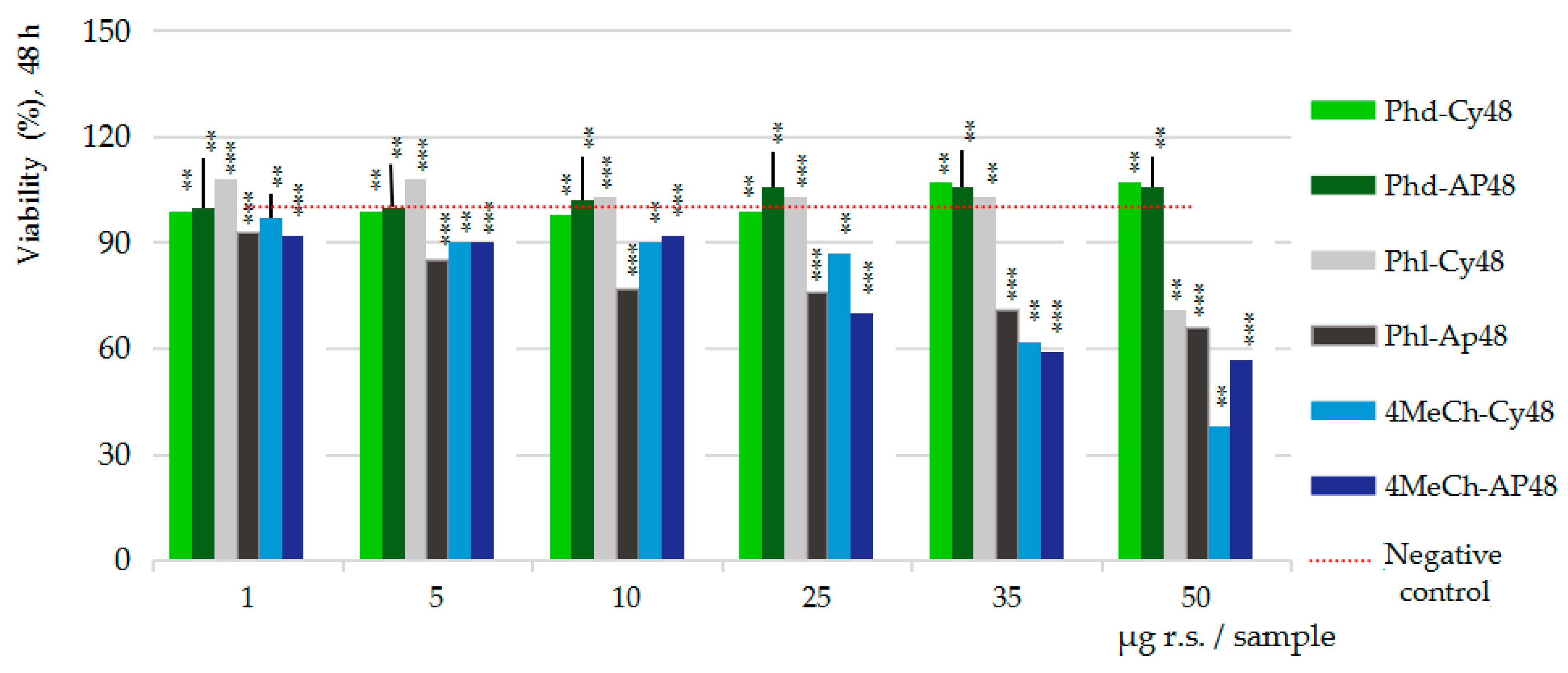
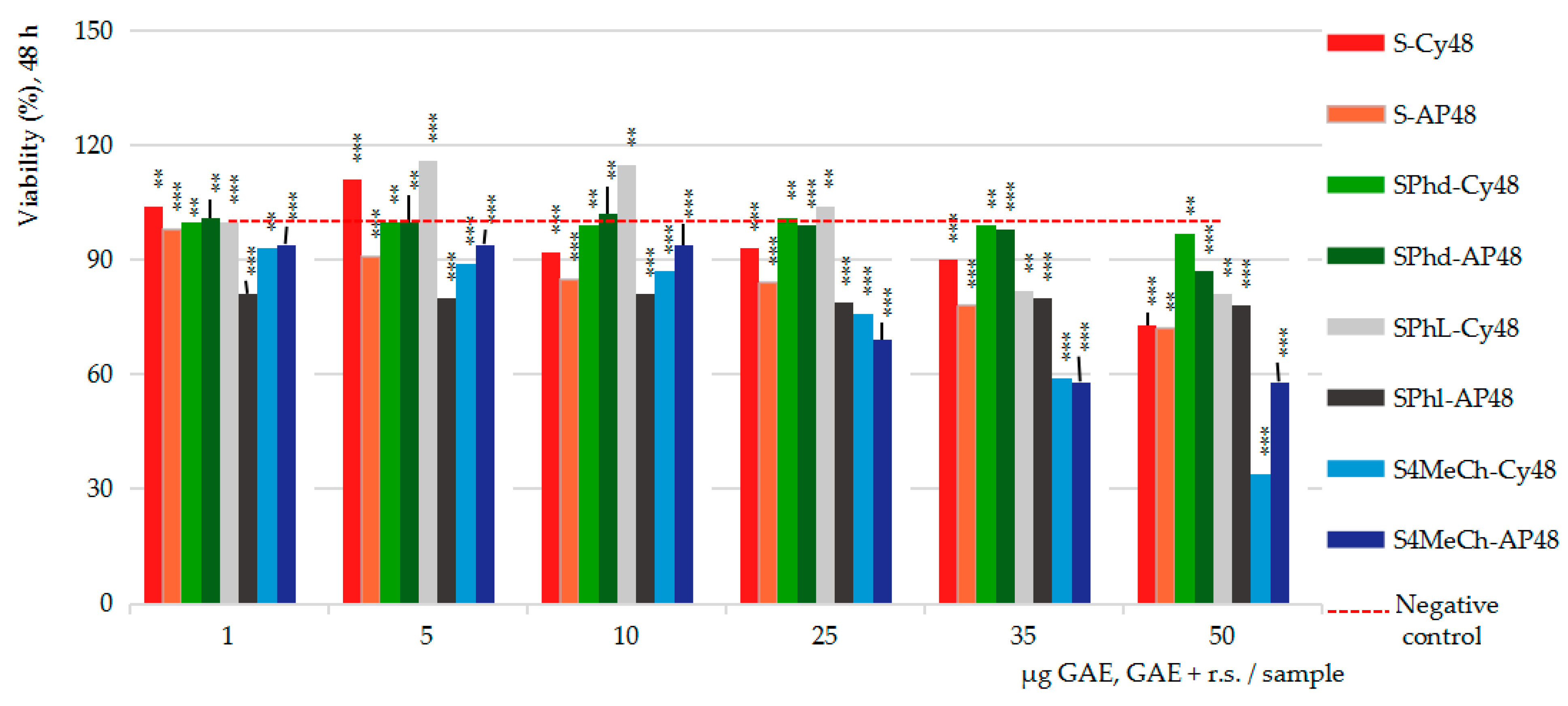
2.4. Pharmacological Results
In Vitro MTS Cytotoxicity and Anti-Proliferative Assessments
3. Discussion
4. Materials and Methods
4.1. Preparation of the Strawberry Extract
4.2. Preparation of the Reference Substances
4.3. Analytical Studies on the Strawberry Extracts
4.4. In Silico Studies
4.5. In Vitro Pharmacological Studies
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhang, Y.L.; Sun, S.J.; Zeng, L. Biological effects and mechanisms of dietary chalcones: Latest research progress, future research strategies, and challenges. Food Funct. 2024, 21, 10582–10599. [Google Scholar] [CrossRef]
- Kretzer, C.; Jordan, P.M.; Meyer, K.P.L.; Hoff, D.; Werner, M.; Hofstetter, R.K.; Koeberle, A.; Cala Peralta, A.; Viault, G.; Seraphin, D.; et al. Natural chalcones elicit formation of specialized pro-resolving mediators and related 15-lipoxygenase products in human macrophages. Biochem. Pharmacol. 2022, 195, 114825. [Google Scholar] [CrossRef]
- Carrillo, M.F.; Ortiz-Rojas, L.Y.; Chaves-Bedoya, G. Antibacterial properties of ethanolic extracts from Melochia pyramidata L. Rev. Colomb. Cienc. Hortícolas 2024, 18, e16252. [Google Scholar] [CrossRef]
- Rodríguez-Cepeda, R.; Alvarez-Suarez, N.Y. Atividade antimicrobiano do extrato hidroalcohólico de Calendula officinalis L. Rev. ION 2021, 34, 97–110. [Google Scholar] [CrossRef]
- Beppu, Y.; Komura, H.; Izumo, T.; Horii, Y.; Shen, J.; Tanida, M.; Nakashima, T.; Tsuruoka, N.; Nagai, K. Identificaton of 2,3-dihydro-3,5-dihydroxy-6-methyl-4H-pyran-4-one isolated from Lactobacillus pentosus strain S-PT84 culture supernatants as a compound that stimulates autonomic nerve activities in rats. J. Agric. Food Chem. 2012, 60, 11044–11049. [Google Scholar] [CrossRef] [PubMed]
- UKCS. UK Chemical Suppliers. Available online: https://www.ukchemicalsuppliers.co.uk (accessed on 7 February 2025).
- Hallur, R.L.S.; Motamarri, C.V.N.L.; Ramamoorthy, P.K.T.; Murthy, C.D.; Siddappa, R.P.H.; Bramhanakonda, V.N. Gas chromatography-mass spectrometry fingerprint and in vitro cytotoxic studies of Rubus steudneri leaf fractions against Michigan cancer foundation-7 breast cancer cell line. Pharmacogn. Mag. 2021, s54–s62. [Google Scholar] [CrossRef]
- Ravi, L.; Krishan, K. Cytotoxic Potential of N-hexadecanoic Acid Extracted from Kigelia pinnata Leaves. Asian J. Biol. 2017, 12, 20–27. [Google Scholar] [CrossRef]
- Hernández-Martínez, N.R.; Blanchard, C.; Wells, D.; Salazar-Gutiérrez, M.R. Current state and future perspectives of commercial strawberry production: A review. Sci. Hortic. 2023, 312, 111893. [Google Scholar] [CrossRef]
- FoodData Central. Available online: https://fdc.nal.usda.gov/food-details/2346409/nutrients (accessed on 29 February 2025).
- The European Market Potential for Strawberries. Available online: https://www.cbi.eu/market-information/fresh-fruit-vegetables/strawberries/market-potential (accessed on 28 February 2025).
- Aaby, K.; Mazur, S.; Nes, A.; Skrede, G. Phenolic compounds in strawberry (Fragaria x ananassa Duch.) fruits: Composition in 27 cultivars and changes during ripening. Food Chem. 2012, 132, 86–97. [Google Scholar] [CrossRef]
- Amatori, S.; Mazzon, L.; Alvarez-Suarez, J.M.; Giampieri, F.; Gasparrini, M.; Forbes-Hernandez, T.Y.; Afrin, S.; Provenzano, A.E.; Persico, G.; Mezzetti, B.; et al. Polyphenol-rich strawberry extract (PRSE) shows in vitro and in vivo biological activity against invasive breast cancer cells. Sci. Rep. 2016, 6, 30917. [Google Scholar] [CrossRef]
- Gampieri, F.; Tulipan, S.; Alvarez-Suarez, J.M.; Quiles, J.L.; Mezzetti, B.; Battino, M. The strawberry: Composition, nutritional quality, and impact on human health. Nutrition 2012, 28, 9–19. [Google Scholar] [CrossRef]
- Fierascu, R.C.; Temocico, G.; Fierascu, I.; Ortan, A.; Babeanu, N.E. Fragaria Genus: Chemical Composition and Biological Activities. Molecules 2020, 25, 498. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Feucht, W.; Schmid, M. Bioactive Compounds of Strawberry and Blueberry and Their Potential Health Effects Based on Human Intervention Studies: A Brief Overview. Nutrients 2019, 11, 1510. [Google Scholar] [CrossRef]
- Hilt, P.; Schieber, A.; Yildirim, C.; Arnold, G.; Klaiber, I.; Conrad, J.; Beifuss, U.; Carle, R. Detection of Phloridzin in Strawberries (Fragaria × ananassa Duch.) by HPLC−PDA−MS/MS and NMR Spectroscopy. J. Agric. Food Chem. 2003, 10, 2896–2899. [Google Scholar] [CrossRef]
- Rabab, W.M.; Khaled, M.E. Chemical quality and nutrient composition of strawberry fruits treated by γ-irradiation. J. Radiat. Res. Appl. Sci. 2017, 10, 80–87. [Google Scholar] [CrossRef]
- Remsberg, C.M.; Yáñez, J.A.; Vega-Villa, K.; Miranda, N.D.; Andrews, P.K. HPLC-UV Analysis of Phloretin in Biological Fluids and Application to Pre-Clinical Pharmacokinetic Studies. J. Chromatogr. Sep. Technol. 2010, 1, 101. [Google Scholar] [CrossRef]
- Zhao, Y.Y.; Fan, Y.; Wang, M.; Wang, J.; Cheng, J.X.; Zou, J.B.; Zhang, X.F.; Shi, Y.J.; Guo, D.Y. Studies on pharmacokinetic properties and absorption mechanism of phloretin: In vivo and in vitro. Biomed. Pharmacother. 2020, 132, 110809. [Google Scholar] [CrossRef]
- Behzad, S.; Sureda, A.; Barreca, D.; Nabavi, S.F.; Rastrelli, L.; Nabavi, S.M. Health effects of phloretin: From chemistry to medicine. Phytochem. Rev. 2017, 16, 527–533. [Google Scholar] [CrossRef]
- Salehi, B.; Quispe, C.; Chamkhi, I.; El Omari, N.; Balahbib, A.; Sharifi-Rad, J.; Bouyahva, A.; Akram, M.; Iqbal, M.; Docea, A.O.; et al. Pharmacological Properties of Chalcones: A Review of Preclinical Including Molecular Mechanisms and Clinical Evidence. Front. Pharmacol. 2021, 11, 592654. [Google Scholar] [CrossRef]
- Tuli, H.S.; Rath, P.; Chauhan, A.; Ramniwas, S.; Vashishth, K.; Varol, M.; Jaswal, V.S.; Haque, S.; Sak, K. Phloretin, as a Potent Anticancer Compound: From Chemistry to Cellular Interactions. Molecules 2022, 27, 8819. [Google Scholar] [CrossRef]
- Kartik, T.N.; Hemant, B.; Rajesh, C.; Kalyani, S.; Yogeeta, O.A.; Charu, S.; Shreesh, O.; Sameer, N.G. Therapeutic Potential and Pharmaceutical Development of a Multitargeted Flavonoid Phloretin. Nutrients 2022, 14, 3638. [Google Scholar] [CrossRef] [PubMed]
- Moreira, J.; Almeida, J.; Saraiva, L.; Cidade, H.; Pinto, M. Chalcones as Promising Antitumor Agents by Targeting the p53 Pathway: An Overview and New Insights in Drug-Likeness. Molecules 2021, 26, 3737. [Google Scholar] [CrossRef] [PubMed]
- Syed, M.D.R.; Manjunatha, P.M.; Sateesha, S.B.; Talib, H.; Turki, H.; Marwa, H.A.; El-Sayed, K.; Arshad, H.; Fahad, A.Y.A.; Amr, S.A.L. The flavonoid hesperidin methyl chalcone as a potential therapeutic agent for cancer therapy: Molecular docking, in vitro cytotoxicity, and in vivo antitumor activity. Arab. J. Chem. 2023, 16, 104769. [Google Scholar] [CrossRef]
- Yang, Z.; Liu, Z.; Ablise, M.; Maimaiti, A.; Aihaiti, A.; Alimujiang, Y. Design and Synthesis of Novel α-Methylchalcone Derivatives, Anti-Cervical Cancer Activity, and Reversal of Drug Resistance in HeLa/DDP Cells. Molecules 2023, 28, 7697. [Google Scholar] [CrossRef]
- Tang, J.; Gong, Y. Synergistic Effect of Phloretin Combined with Radiotherapy on Lung Cancer. Int. J. Radiat. Oncol.*Biol.*Phys. 2021, 111, e238. [Google Scholar] [CrossRef]
- Zhou, K.; Luo, X.; Liu, F.; Li, B.; Tian, J. Synergistic Inhibitory Effects of Myricetin and Phloretin on Microorganism Survival and Their Application in the Fresh Noodles. SSRN. Tomorrows’ Research Today. Preprint, 40 Pages, Posted: 4 Feb 2025. Available online: https://ssrn.com/abstract=5124686 (accessed on 22 March 2025). [CrossRef]
- Wang, S.Y.; Jiao, H. Scavenging Capacity of Berry Crops on Superoxide Radicals, Hydrogen Peroxide, Hydroxyl Radicals, and Singlet Oxygen. J. Agric. Food Chem. 2000, 48, 5677–5684. [Google Scholar] [CrossRef]
- Wang, S.Y.; Lin, H.S. Antioxidant activity in fruits and leaves of blackberry, raspberry, and strawberry varies with cultivar and developmental stage. J. Agric. Food Chem. 2000, 48, 140–146. [Google Scholar] [CrossRef]
- Wang, S.Y.; Zheng, W.; Galletta, G.J. Cultural System Affects Fruit Quality and Antioxidant Capacity in Strawberries. J. Agric. Food Chem. 2002, 50, 6534–6542. [Google Scholar] [CrossRef]
- Heo, H.J.; Lee, C.Y. Strawberry and its anthocyanins reduce oxidative stress-induced apoptosis s in PC12 cells. J. Agric. Food Chem. 2005, 53, 1984–1989. [Google Scholar] [CrossRef]
- Cvijetic, I.N.; Karaconji, I.B.; Juric, A.; Jurica, K.; Milojkovic-Opsenica, D. In silico study of the tyrosinase inhibitory potential of arbutin and other bioactive compounds from strawberry tree (Arbutus unedo L.). In Proceedings of the 1st European Symposium on Phytochemicals in Medicine and Food (1-EuSPMF), Belgrade, Serbia, 7–9 September 2022; University of Belgrade: Belgrade, Serbia, 2022. [Google Scholar]
- Ramlal, A.; Bhat, I.; Subramanian, S.; Saini, M.; Fawzy, I.M.; Raju, D.; Rajendran, A. In silico analyses of strawberry and soybean bioactive compounds’ inhibitory effects against angiotensin-converting enzyme (ACE). In Proceedings of the 3rd International Electronic Conference on Biomolecules Session Bioinformatics and Computational Biology, Online, 23–25 April 2024. [Google Scholar]
- Rocha, S.; Ribeiro, D.; Fernandes, E.; Freitas, M. A systematic review on anti-diabetic properties of chalcones. Curr. Med. Chem. 2020, 27, 2257–2321. [Google Scholar]
- Croom, E. Chapter Three—Metabolism of Xenobiotics of Human Environments. In Progress in Molecular Biology and Translational Science; Hodgson, E., Ed.; Academic Press: Cambridge, MA, USA, 2012; Volume 112, pp. 31–38. [Google Scholar] [CrossRef]
- Sayyed, Y.; Rehman, K.; Akash, M.S.H.; Kamran, S.H.; Badran, M. Chapter 13—Xenobiotics and drug-metabolizing enzymes: Challenges and strategies. In Biochemistry of Drug Metabolizing Enzymes; Academic Press: Cambridge, MA, USA, 2022; pp. 305–321. [Google Scholar] [CrossRef]
- SwissADME. SwissDrugDesign. Available online: http://www.swissadme.ch (accessed on 18 December 2024).
- Daina, A.; Michielin, O.; Zoete, V. iLOGP: A simple, robust, and efficient description of n-octanol/water partition coefficient for drug design using the GB/SA approach. J. Chem. Inf. Model. 2014, 54, 3284–3301. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Zoete, V. A BOILED-Egg to predict gastrointestinal absorption and brain penetration of small molecules. Chem. Med. Chem. 2016, 11, 1117–1121. [Google Scholar] [CrossRef] [PubMed]
- Daina, A.; Michielin, O.; Zoete, V. SwissADME: A free web tool to evaluate pharmacokinetics, drug-likeness and medicinal chemistry friendliness of small molecules. Sci. Rep. 2017, 7, 42717. [Google Scholar] [CrossRef]
- Bugnon, M.; Goullieux, M.; Röhrig, U.F.; Perez, M.A.S.; Daina, A.; Michielin, O.; Zoete, V. SwissParam 2023: A modern web-based tool for efficient small molecule parameterization. J. Chem. Inf. Model. 2023, 63, 6469–6475. [Google Scholar] [CrossRef]
- Zoete, V.; Cuendet, M.A.; Grosdidier, A.; Michielin, O. SwissParam: A fast force field generation tool for small organic molecules. J. Comput. Chem. 2011, 32, 2359–2368. [Google Scholar] [CrossRef] [PubMed]
- Ritchie, T.J.; Macdonald, S.J.F.; Peace, S.; Pickett, S.D.; Luscombe, C.N. Increasing small molecule drug developability in sub-optimal chemical space. Med. Chem. Commun. 2013, 4, 673. [Google Scholar] [CrossRef]
- Martin, Y.C. A Bioavailability Score. J. Med. Chem. 2005, 48, 3164–3170. [Google Scholar] [CrossRef]
- Molinspiration Cheminformatics Free Web Services. Slovensky Grob, Slovakia. Available online: https://www.molinspiration.com (accessed on 16 July 2024).
- Ghose, K.; Pritchett, A.; Crippen, G.M. Atomic physicochemical parameters for three dimensional structure directed quantitative structure-activity relationships III: Modeling hydrophobic interactions. J. Comput. Chem. 1988, 9, 80–90. [Google Scholar] [CrossRef]
- Leo, A.; Hansch, C.; Elkins, D. Partition Coefficients and their uses. Chem. Rev. 1971, 6, 525–616. [Google Scholar] [CrossRef]
- Hansch, C.; Björkrot, J.B.; Leo, A. Hydrophobicity and Central Nervous System Agents: On the Principle of Minimal Hydrophobicity in Drug Design. J. Pharm. Sci. 1987, 76, 663–687. [Google Scholar] [CrossRef]
- Bhal, S.K. LogP—MakingSenseoftheValue; Advanced Chemistry Development, Inc.: Toronto, ON, Canada, 2007; Available online: https://www.acdlabs.com (accessed on 14 August 2024).
- Stefaniu, A.; Neagu, G.; Albulescu, A.; Radu, N.; Pirvu, L. In silico comparison of quantum and bioactivity parameters of a series of natural diphenyl acetone analogues; in vitro Caco-2 studies on three main chalcone derivatives. Symmetry 2024, 16, 1383. [Google Scholar] [CrossRef]
- Feyereisen René. Insect CYP Genes and P450 Enzymes. In Reference Module in Life Sciences; Elsevier: Amsterdam, The Netherlands, 2019. [Google Scholar] [CrossRef]
- Ortiz de Montellano, P.R. (Ed.) Cytochrome P450: Structure, Mechanism, and Biochemistry, 4th ed.; Springer: New York, NY, USA, 2015. [Google Scholar]
- Thorn, C.F.; Aklillu, E.; Klein, T.; Altman, R.B. PharmGKB summary: Very important pharmacogene information for CYP1A2. Pharmacogenet Genom. 2012, 22, 73–77. [Google Scholar] [CrossRef]
- Fontana, R.J.; Lown, K.S.; Paine, M.F.; Fortlage, L.; Santella, R.M.; Felton, J.S.; Knize, M.G.; Greenberg, A.; Watkins, P.B. Effects of a chargrilled meat diet on expression of CYP3A, CYP1A, and P-glycoprotein levels in healthy volunteers. Gastroenterology 1999, 117, 89–98. [Google Scholar] [CrossRef]
- Cornelis, M.C.; El-Sohemy, A.; Kabagambe, E.K.; Campos, H. Coffee, CYP1A2 genotype, and risk of myocardial infarction. JAMA 2006, 295, 1135–1141. [Google Scholar] [CrossRef]
- Fleming, I. The pharmacology of the cytochrome P450 epoxygenase/soluble epoxide hydrolase axis in the vasculature and cardiovascular disease. Pharmacol. Rev. 2014, 66, 1106–1140. [Google Scholar] [CrossRef]
- Boobis, A.R.; Lynch, A.M.; Murray, S.; de la Torre, R.; Solans, A.; Farré, M.; Segura, J.; Gooderham, N.J.; Davies, D.S. CYP1A2-catalyzed conversion of dietary heterocyclic amines to their proximate carcinogens is their major route of metabolism in humans. Cancer Res. 1994, 54, 89–94. [Google Scholar]
- Rettie, A.E.; Jones, J.P. Clinical and toxicological relevance of CYP2C9: Drug-drug interactions and pharmacogenetics. Annu. Rev. Pharmacol. Toxicol. 2005, 45, 477–494. [Google Scholar] [CrossRef]
- Spector, A.A.; Kim, H.Y. Cytochrome P450 epoxygenase pathway of polyunsaturated fatty acid metabolism. Biochim. Biophys. Acta 2015, 1851, 356–365. [Google Scholar] [CrossRef]
- Colorado Center for Personalized Medicine (CCPM) Biobank Research Study. Available online: https://medschool.cuanschutz.edu/cobiobank/return-of-results/pgx/cyp2c9 (accessed on 9 January 2025).
- Miners, J.O.; Birkett, D.J. Cytochrome P4502C9: An enzyme of major importance in human drug metabolism. Br. J. Clin. Pharmacol. 1998, 45, 525–538. [Google Scholar] [CrossRef] [PubMed]
- CYP3A4 Cytochrome P450 Family 3 Subfamily A Member 4 [Homo sapiens (Human)], Gene ID: 1576, Updated on 27-Dec-2024. Available online: https://www.ncbi.nlm.nih.gov/gene/1576 (accessed on 4 January 2025).
- Schmiedlin-Ren, P.; Edwards, D.J.; Fitzsimmons, M.E.; He, K.; Lown, K.S.; Woster, P.M.; Rahman, A.; Thummel, K.E.; Fisher, J.M.; Hollenberg, P.F.; et al. Mechanisms of enhanced oral availability of CYP3A4 substrates by grapefruit constituents. Decreased enterocyte CYP3A4 concentration and mechanism-based inactivation by furanocoumarins. Drug Metab. Dispos. 1997, 25, 1228–1333. [Google Scholar] [PubMed]
- Stresser, D.M.; Kupfer, D. Prosubstrates of CYP3A4, the Major Human Hepatic Cytochrome P450: Transformation into Substrates by Other p450 Isoforms. Biochem. Pharmacol. 1998, 55, 1861–1871. [Google Scholar] [CrossRef]
- Zanger, U.M.; Schwab, M. Cytochrome P450 enzymes in drug metabolism: Regulation of gene expression, enzyme activities, and impact of genetic variation. Pharmacol. Ther. 2013, 138, 103–142. [Google Scholar] [CrossRef]
- Fujino, C.; Sanoh, S.; Katsura, T. Variation in Expression of Cytochrome P450 3A Isoforms and Toxicological Effects: Endo- and Exogenous Substances as Regulatory Factors and Substrates. Biol. Pharm. Bull. 2021, 44, 1617–1634. [Google Scholar] [CrossRef]
- Klyushova, L.S.; Perepechaeva, M.L.; Grishanova, A.Y. The Role of CYP3A in Health and Disease. Biomedicines 2022, 10, 2686. [Google Scholar] [CrossRef]
- MedlinePlus [Internet]. Bethesda (MD): National Library of Medicine (US). Available online: https://medlineplus.gov/genetics/gene/cyp2c19/ (accessed on 7 January 2025).
- CYP2C19 Cytochrome P450 Family 2 Subfamily C Member 19 [Homo sapiens (Human)], Gene ID: 1557. Available online: https://www.ncbi.nlm.nih.gov/gene/1557 (accessed on 7 January 2025).
- Yao, L.; Wang, H.C.; Liu, J.Z.; Xiong, Z.M. Quantitative assessment of the influence of cytochrome P450 2C19 gene polymorphisms and digestive tract cancer risk. Tumour Biol. 2013, 34, 3083–3091. [Google Scholar] [CrossRef]
- Wang, B.; Yang, L.P.; Zhang, X.Z.; Huang, S.Q.; Bartlam, M.; Zhou, S.F. New insights into the structural characteristics and functional relevance of the human cytochrome P450 2D6 enzyme. Drug Metab. Rev. 2009, 41, 573–643. [Google Scholar] [CrossRef]
- The, L.K.; Bertilsson, L. Pharmacogenomics of CYP2D6: Molecular genetics, interethnic differences and clinical importanc. Drug Metab. Pharmacokinet. 2012, 27, 55–67. [Google Scholar] [CrossRef]
- Walko, C.M.; McLeod, H. Use of CYP2D6 genotyping in practice: Tamoxifen dose adjustmen. Pharmacogenomics 2012, 13, 691–697. [Google Scholar] [CrossRef]
- Wang, X.; Li, J.; Dong, G.; Yue, J. The endogenous substrates of brain CYP2D. Eur. J. Pharmacol. 2014, 724, 211–218. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.H.; Yamazaki, M. Role of P-glycoprotein in pharmacokinetics: Clinical implications. Clin. Pharmacokinet. 2003, 42, 59–98. [Google Scholar] [CrossRef] [PubMed]
- Baell, J.B.; Holloway, G.A. New substructure filters for removal of pan assay interference compounds (PAINS) from screening libraries and for their exclusion in bioassays. J. Med. Chem. 2010, 53, 2719–2740. [Google Scholar] [CrossRef]
- Baell, J.; Walters, M.A. Chemistry: Chemical con artists foil drug discovery. Nature 2014, 513, 481–483. [Google Scholar] [CrossRef]
- Brenk, R.; Schipani, A.; James, D.; Krasowski, A.; Gilbert, I.H.; Frearson, J.; Wyatt, P.G. Lessons learnt from assembling screening libraries for drug discovery for neglected diseases. ChemMedChem 2008, 3, 435–444. [Google Scholar] [CrossRef]
- Andrés, C.M.C.; Pérez de la Lastra, J.M.; Bustamante Munguira, E.; Andrés Juan, C.; Pérez-Lebeña, E. Michael Acceptors as Anti-Cancer Compounds: Coincidence or Causality? Int. J. Mol. Sci. 2024, 25, 6099. [Google Scholar] [CrossRef]
- Polinsky, A. Lead-Likeness and Drug-Likeness. In The Practice of Medicinal Chemistry, 3rd ed.; Wermuth, C.G., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 244–254. [Google Scholar] [CrossRef]
- Ursu, O.; Rayan, A.; Goldblum, A.; Oprea, T.I. Understanding drug-likeness. WIREs Comput. Mol. Sci. 2011, 1, 760–781. [Google Scholar] [CrossRef]
- Jia, C.-Y.; Li, J.-Y.; Hao, G.-F.; Yang, G.-F. A drug-likeness toolbox facilitates ADMET study in drug discovery. Drug Discov. Today 2020, 25, 248–258. [Google Scholar] [CrossRef] [PubMed]
- Oprea, T.I.; Hasselgren, C. Predicting Target and Chemical Druggability. In Comprehensive Medicinal Chemistry III; Chackalamannil, S., Rotella, D., Ward, S.E., Eds.; Elsevier: Amsterdam, The Netherlands, 2017; pp. 429–439. [Google Scholar] [CrossRef]
- Lipinski, C.A.; Lombardo, F.; Dominy, B.W.; Feeney, P.J. Experimental and computational approaches to estimate solubility and permeability in drug discovery and development settings. Adv. Drug Deliv. Rev. 1997, 23, 3–25. [Google Scholar] [CrossRef]
- Lipinski, C.A. Drug-like properties, and the causes of poor solubility and poor permeability. J. Pharmacol. Toxicol. Methods 2000, 44, 235–249.50. [Google Scholar] [CrossRef]
- Ghose, A.K.; Viswanathan, V.N.; Wendoloski, J.J. Aknowledge-based approach in designing combinatorial or medicinal chemistry libraries for drug discovery. 1. Qualitative and quantitative characterization of known drug databases. J. Comb. Chem. 1999, 1, 55–68.51. [Google Scholar] [CrossRef]
- Veber, D.F.; Johnson, S.R.; Cheng, H.Y.; Smith, B.R.; Ward, K.W.; Kopple, K.D. Molecular properties that influence the oral bioavailability of drug candidates. J. Med. Chem. 2002, 4, 2615–2623. [Google Scholar] [CrossRef]
- Egan, W.J.; Merz, K.M.; Baldwin, J.J. Prediction of drug absorption using multivariate statistics. J. Med. Chem. 2000, 43, 3867–3877.52. [Google Scholar]
- Muegge, I.; Heald, S.L.; Brittelli, D. Simple selection criteria for the drug-like chemical matter. J. Med. Chem. 2001, 44, 1841–1846. [Google Scholar]
- Muegge, I. Selection criteria for drug-like compounds. Med. Res. Rev. 2003, 23, 302–321. [Google Scholar]
- Pirvu, L.; Stefaniu, A.; Neagu, G.; Albu, B.; Pintilie, L. In Vitro Cytotoxic and Antiproliferative Activity of Cydonia oblonga flower petals, leaf and fruit pellet ethanolic extracts. Docking simulation of the active flavonoids on anti-apoptotic protein Bcl-2. Open Chem. 2018, 16, 591–604. [Google Scholar] [CrossRef]
- Pirvu, L.; Stefaniu, A.; Neagu, G.; Pintilie, L. Studies on Anemone nemorosa L. extracts; polyphenols profile, antioxidant activity, and effects on Caco-2 cells by in vitro and in silico studies. Open Chem. 2022, 20, 299–312. [Google Scholar]
- PDB. Protein Data Bank. Available online: https://www.rcsb.org (accessed on 20 February 2025).
- Bruncko, M.; Oost, T.K.; Belli, B.A.; Ding, H.; Joseph, M.K.; Kunzer, A.; Martineau, D.; McClellan, W.J.; Mitten, M.; Ng, S.C.; et al. Studies leading to potent, dual inhibitors of Bcl-2 and Bcl-xL. J. Med. Chem. 2007, 50, 641–662. [Google Scholar] [CrossRef] [PubMed]
- Johannes, J.W.; Almeida, L.L.; Barlaam, B.; Boriack-Sjodin, P.A.; Casella, R.; Croft, R.A.; Dishington, A.P.; Gingipalli, L.; Gu, C.; Hawkins, J.L.; et al. Pyrimidinone Nicotinamide Mimetics as Selective Tankyrase and Wnt Pathway Inhibitors Suitable for in Vivo Pharmacology. ACS Med. Chem. Lett. 2015, 6, 254–259. [Google Scholar] [CrossRef]
- Rowlinson, S.W.; Kiefer, J.R.; Prusakiewicz, J.J.; Pawlitz, J.L.; Kozak, K.R.; Kalgutkar, A.S.; Stallings, W.C.; Kurumbail, R.G.; Marnett, L.J. A Novel Mechanism of Cyclooxygenase-2 Inhibition Involving Interactions with Ser-530 and Tyr-385. J. Biol. Chem. 2003, 278, 45763–45769. [Google Scholar]
- Shao, Y.; Molnar, L.F.; Jung, Y.; Kussmann, J.; Ochsenfeld, C.; Brown, S.T.; Gilbert, A.T.B.; Slipchenko, L.V.; Levchenko, S.V.; O’Neill, D.P.; et al. Advances in methods and algorithms in a modern quantum chemistry program package. Phys. Chem. Chem. Phys. 2006, 8, 3172–3191. [Google Scholar]
- Protocols & Applications Guide. Available online: https://www.promega.com (accessed on 20 September 2023).
- Forbes-Hernandez, T.Y.; Gasparrini, M.; Afrin, S.; Mazzoni, L.; Reboredo, P.; Giampieri, F. A comparative study on cytotoxic effects of strawberry extract on different cellular models. J. Berry Res. 2016, 6, 263–275. [Google Scholar] [CrossRef]
- Pirvu, L.; Rusu, N.; Bazdoaca, C.; Androne, E.; Neag, G.; Albulescu, A. A View on the Chemical and Biological Attributes of Five Edible Fruits after Finishing Their Shelf Life: Studies on Caco-2 Cells. Int. J. Mol. Sci. 2024, 25, 4848. [Google Scholar] [CrossRef] [PubMed]
- Hong, J.Y.; Song, S.H.; Park, H.J.; Cho, Y.J.; Pyee, J.H.; Lee, S.K. Antioxidant, Antiinflamatory, and Antiproliferative Activities of Strawberry Extracts. Biomol. Ther. 2008, 16, 286–292. [Google Scholar] [CrossRef]
- Meyers, K.J.; Watkins, C.B.; Pritts, M.P.; Liu, R.H. Antioxidant and antiproliferative activities of strawberries. J. Agric. Food Chem. 2003, 51, 6887–6892. [Google Scholar] [CrossRef] [PubMed]
- Olsson, M.E.; Andersson, C.S.; Oredsson, S.; Berglund, R.H.; Gustavsson, K.E. Antioxidant level sand inhibition of cancer cell proliferation in vitro by extracts from organically and conventionally cultivated strawberries. J. Agric. Food Chem. 2006, 54, 1248–1255. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.; Taskar, K.S.; Zamek-Gliszczynski, M.J. Importance of Hepatic Transporters in Clinical Disposition of Drugs and Their Metabolites. J. Clin. Pharmacol. 2016, 56 (Suppl. S7), S23–S39. [Google Scholar] [CrossRef]
- Iacopetta, D.; Ceramella, J.; Catalano, A.; Scali, E.; Scumaci, D.; Pellegrino, M.; Aquaro, S.; Saturnino, C.; Sinicropi, M.S. Impact of Cytochrome P450 Enzymes on the Phase I Metabolism of Drugs. Appl. Sci. 2023, 13, 6045. [Google Scholar] [CrossRef]
- Rowland, A.; Miners, J.O.; Mackenzie, P.I. The UDP-glucuronosyltransferases: Their role in drug metabolism and detoxification. Int. J. Biochem. Cell Biol. 2013, 45, 1121–1132. [Google Scholar] [CrossRef]
- Glaeser, H. Importance of P-glycoprotein for drug-drug interactions. Handb. Exp. Pharmacol. 2011, 201, 285–297. [Google Scholar] [CrossRef]
- P-Glycoprotein Substrate ADMET Feature Prediction. Available online: https://dev.drugbank.com/guides/terms/p-glycoprotein-substrate (accessed on 9 January 2025).
- Horn, J.R.; Hansten, P.D. Drug interactions with digoxin: The role of p-glycoprotein. Pharm. Times 2004, 70, 45–46. [Google Scholar]
- Fenner, K.S.; Troutman, M.D.; Kempshall, S.; Cook, J.A.; Ware, J.A.; Smith, D.A.; Lee, C.A. Drug-drug Interactions mediated through p-glycoprotein: Clinical relevance and in vitro-in vivo correlation using digoxin as a probe drug’. Clin. Pharmacol. Therapeut. 2008, 85, 173–181. [Google Scholar]
- Lee, C.A.; Cook, J.A.; Reyner, E.L.; Smith, D.A. P-glycoprotein related drug interactions: Clinical importance and a consideration of disease states. Expert Opin. Drug Metab. Toxicol. 2010, 6, 603–619. [Google Scholar] [CrossRef] [PubMed]
- Sawyer, J.; Sharp, J.; Baxter, K. p-glycoprotein: Why is it significant? Pharm. J. 2011, 286, 595–596. [Google Scholar]
- Richard, J.Y.; Andreas, S. The BCL-2 protein family: Opposing activities that mediate cell death. Nat. Rev. Mol. Cell Biol. 2008, 9, 47–59. [Google Scholar] [CrossRef]
- Bock, F.J.; Tait, S.W.G. Mitochondria as multifaceted regulators of cell death. Nat. Rev. Mol. Cell Biol. 2020, 21, 85–100. [Google Scholar] [CrossRef] [PubMed]
- Lishner, M.; Lalkin, A.; Klein, A.; Yarkoni, S.; Manor, Y.; Fejgin, M.; Leytin, V.; Ravid, M.; Amiel, A. The BCL-1, BCL-2, and BCL-3 oncogenes are involved in chronic lymphocytic leukemia. Detection by fluorescence in situ hybridization. Cancer Genet. Cytogenet. 1995, 85, 118–123. [Google Scholar] [CrossRef]
- Barker, N.; Van Es, J.H.; Kuipers, J.; Kujala, P.; Van den Born, M.; Cozijnsen, M.; Haegebarth, A.; Korving, J.; Begthel, H.; Peters, P.J.; et al. Identification of stem cells in small intestine and colon by marker gene Lgr5. Nature 2007, 449, 1003–1007. [Google Scholar] [CrossRef]
- Ramesh, P.; Medema, J.P. BCL-2 family deregulation in colorectal cancer: Potential for BH3 mimetics in therapy. Apoptosis 2020, 25, 305–320. [Google Scholar] [CrossRef]
- Sinicrope, F.A.; Ruan, S.B.; Cleary, K.R.; Stephens, L.C.; Lee, J.J.; Levin, B. bcl-2 and p53 oncoprotein expression during colorectal tumorigenesis. Cancer Res. 1995, 15, 237–241. [Google Scholar]
- Sorenson, C.M. Bcl-2 family members and disease. Biochim. Biophys. Acta 2004, 1644, 169–177. [Google Scholar] [CrossRef]
- MedChemExpress. Master of Bioactive Molecules. Available online: https://www.medchemexpress.com (accessed on 22 January 2025).
- Korinek, V.; Barker, N.; Morin, P.J.; Van Wichen, D.; De Weger, R.; Kinzler, K.W.; Vogelstein, B.; Clevers, H. Constitutive transcriptional activation by a β-catenin-Tcf complex in APC−/− colon carcinoma. Science 1997, 275, 1784–1787. [Google Scholar] [CrossRef]
- Smith, S.; Giriat, I.; Schmitt, A.; De Lange, T. Tankyrase, a poly(ADP-ribose) polymerase at human telomeres. Science 1998, 282, 1484–1487. [Google Scholar] [CrossRef] [PubMed]
- Salahshor, S.; Woodgett, J.R. The links between axin and carcinogenesis. J. Clin. Pathol. 2005, 58, 225–236. [Google Scholar] [CrossRef]
- Hans, C. Wnt/β-Catenin Signaling in Develepment and Disease. Cell 2006, 127, 469–480. [Google Scholar]
- Huang, S.M.; Mishina, Y.; Liu, S.; Cheung, A.; Stegmeier, F.; Michaud, G.A.; Charlat, O.; Wiellette, E.; Zhang, Y.; Wiessner, S.; et al. Tankyrase inhibition stabilizes axin and antagonizes Wnt signalling. Nature 2009, 461, 614–620. [Google Scholar] [CrossRef] [PubMed]
- Valenta, T.; Hausmann, G.; Basler, K. The many faces and functions of β-catenin. EMBO J. 2012, 31, 2714–2736. [Google Scholar] [CrossRef] [PubMed]
- Croy, H.E.; Fuller, C.N.; Giannotti, J.; Robinson, P.; Foley, A.V.A.; Yamulla, R.J.; Cosgriff, S.; Greaves, B.D.; von Kleeck, R.A.; An, H.H.; et al. The Poly(ADP-ribose) Polymerase Enzyme Tankyrase Antagonizes Activity of the β-Catenin Destruction Complex through ADP-ribosylation of Axin and APC2. J. Biol. Chem. 2016, 291, 12747–12760. [Google Scholar] [CrossRef]
- Zamudio-Martinez, E.; Herrera-Campos, A.B.; Muñoz, A.; Rodríguez-Vargas, J.M.; Oliver, F.J. Tankyrases as modulators of pro-tumoral functions: Molecular insights and therapeutic opportunities. J. Exp. Clin. Cancer Res. 2021, 40, 144. [Google Scholar] [CrossRef]
- Slade, D. PARP and PARG inhibitors in cancer treatment. Genes Dev. 2020, 34, 360–394. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular characterization of human colon and rectal cancer. Nature 2012, 487, 330–337. [CrossRef]
- Zhan, T.; Rindtorff, N.; Boutros, M. Wnt Signaling in Cancer. Oncogene 2017, 36, 1461–1473. [Google Scholar] [CrossRef]
- Waaler, J.; Machon, O.; Tumova, L.; Dinh, H.; Korinek, V.; Wilson, S.R.; Paulsen, J.E.; Pedersen, N.M.; Eide, T.J.; Machonova, O.; et al. A novel tankyrase inhibitor decreases canonical Wnt signaling in colon carcinoma cells and reduces tumor growth in conditional APC mutant mice. Cancer Res. 2012, 72, 2822–2832. [Google Scholar] [CrossRef] [PubMed]
- Zhu, L.; Li, X.; Yuan, Y.; Dong, C.; Yang, M. APC Promoter Methylation in Gastrointestinal REVIEW article. Front. Oncol. 2021, 11, 2021. [Google Scholar] [CrossRef]
- Wu, X.; Luo, F.; Li, J.; Zhong, X.; Liu, K. Tankyrase 1 inhibitior XAV939 increases chemosensitivity in colon cancer cell lines via inhibition of the Wnt signaling pathway. Int. J. Oncol. 2016, 48, 1333–1340. [Google Scholar] [CrossRef]
- Lau, T.; Chan, E.; Callow, M.; Waaler, J.; Boggs, J.; Blake, R.A.; Magnuson, S.; Sambrone, A.; Schutten, M.; Firestein, R.; et al. A novel tankyrase small-molecule inhibitor suppresses APC mutation-driven colorectal tumor growth. Cancer Res. 2013, 73, 3132–3144. [Google Scholar] [CrossRef]
- Zhu, H.; Gao, Y.; Liu, L.; Tao, M.; Lin, X.; Cheng, Y.; Shen, Y.; Xue, H.; Guan, L.; Zhao, H.; et al. A novel TNKS/USP25 inhibitor blocks the Wnt pathway to overcome multi-drug resistance in TNKS-overexpressing colorectal cancer. Acta Pharm. Sin. B 2024, 14, 207–222. [Google Scholar] [CrossRef]
- Haikarainen, T.; Krauss, S.; Lehtio, L. Tankyrases: Structure, function and therapeutic implications in cancer. Curr. Pharm. Des. 2014, 20, 6472–6488. [Google Scholar] [CrossRef]
- Kim, M.K. Novel insight into the function of tankyrase. Oncol. Lett. 2018, 16, 6895–6902. [Google Scholar] [CrossRef]
- Bakhle, Y.S.; Botting, R.M. Cyclooxygenase-2 and its regulation in inflammation. Mediat. Inflamm. 1996, 5, 305–323. [Google Scholar] [CrossRef] [PubMed]
- Bakhle, Y.S. COX-2 and cancer: A new approach to an old problem. Br. J. Pharmacol. 2001, 134, 1137–1150. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Menter, D.G.; Schilsky, R.L.; DuBois, R.N. Cyclooxygenase-2 and cancer treatment: Understanding the risk should be worth the reward. Clin. Cancer Res. 2010, 16, 1384–1390. [Google Scholar] [CrossRef]
- Lord, R.V.; Danenberg, K.D.; Danenberg, P.V. Cyclo-oxygenase-2 in Barrett’s esophagus, Barrett’s adenocarcinomas, and esophageal SCC: Ready for clinical trials. Am. J. Gastroenterol. 1999, 94, 2313–2315. [Google Scholar] [PubMed]
- Dubois, R.N.; Giardiello, F.M.; Smalley, W.E. Nonsteroidal anti-inflammatory drugs, eicosanoids, and colorectal cancer prevention. Gastroenterol. Clin. N. Am. 1996, 25, 773–791. [Google Scholar]
- Steinbach, G.; Lynch, P.M.; Phillips, R.K.; Walace, M.H.; Hawk, E.; Gordon, G.B.; Wakayashi, N.; Saunders, B.; Shen, Y.; Fujimura, T.; et al. The effect of Celecoxib, a cyclo-oxygenase inhibitor, in Familial Adenomatous Polyposis. N. Engl. J. Med. 2000, 342, 1946–1952. [Google Scholar]
- Cuendet, M.; Pezzuto, J.M. The role of cyclooxygenase and lipoxygenase in cancer chemoprevention. Drug Metabol. Drug Interact. 2000, 17, 109–157. [Google Scholar] [PubMed]
- Gately, S. The contributions of cyclooxygenase-2 to tumor angiogenesis. Cancer Metastasis Rev. 2000, 19, 19–27. [Google Scholar] [PubMed]
- Tari, A.M.; Simeone, A.M.; Li, Y.J.; Gutierrez-Puente, Y.; Lai, S.; Symmans, W.F. Cyclooxygenase-2 protein reduces tamoxifen and N-(4-hydroxyphenyl)retinamide inhibitory effects in breast cancer cells. Lab. Investig. 2005, 85, 1357–1367. [Google Scholar]
- Saito, S.; Ozawa, H.; Imanishi, Y.; Sekimizu, M.; Watanabe, Y.; Ito, F.; Ikari, Y.; Nakahara, N.; Kameyama, K.; Ogawa, K. Cyclooxygenase-2 expression is associated with chemoresistance through cancer stemness property in hypopharyngeal carcinoma. Oncol. Lett. 2021, 22, 33. [Google Scholar] [CrossRef]
- Kurumbail, R.G.; Stevens, A.M.; Gierse, J.K.; McDonald, J.J.; Stegeman, R.A.; Pak, J.Y.; Gildehaus, D.; Miyashiro, J.M.; Penning, T.D.; Seibert, K.; et al. Structural basis for selective inhibition of cyclo-oxygenase-2 by anti-inflammatory agents. Nature 1996, 384, 644–648. [Google Scholar]
- Luong, C.; Miller, A.; Barnett, J.; Chow, J.; Ramesha, C.; Browner, F. Flexibility of the NSAID binding site in the structure of human cyclooxygenase-2. Nat. Struct. Biol. 1996, 3, 927–933. [Google Scholar]
- Chan, C.C.; Boyce, S.; Brideau, C. Rofecoxib (Vioxx, MK-0966; 4-(4′-methylsulfonylphenyl)-3-phenyl-2(5H)-furanone: A potent and orally active cyclooxygenase inhibitor. Pharmacological and biochemical profiles. J. Pharm. Exp. Ther. 1999, 290, 551–560. [Google Scholar]
- Talley, J.J.; Brown, D.L.; Carter, J.S.; Graneto, M.J.; Koboldt, C.M.; Masferrer, J.; Perkins, W.E.; Rogers, R.S.; Shaffer, A.F.; Zhang, Y.Y.; et al. 4-[5-Methyl-3-phenylisoxazol-4-yl]-benzenesulfonamide, Valdecoxib: A potent and selective inhibitor of COX-2. J. Med. Chem. 2000, 43, 775–777. [Google Scholar] [CrossRef]
- Riendeau, D.; Percival, M.D.; Brideau, C.; Charleson, S.; Dube, D.; Ethier, D.; Falgueyret, J.P.; Friesen, R.W.; Gordon, R.; Greig, G.; et al. Etoricoxib (MK-0663): Preclinical profile and comparison with other agents that selectively inhibit cyclooxygenase-2. J. Pharm. Exp. Ther. 2001, 296, 558–566. [Google Scholar] [CrossRef]
- Hawkey, C.J. COX-1 and COX-2 inhibitors. Best Pract. Res. Clin. Gastroenterol. 2001, 15, 801–820. [Google Scholar] [CrossRef] [PubMed]
- Li, S.; Jiang, M.; Wang, L.; Yu, S. Combined chemotherapy with cyclooxygenase-2 (COX-2) inhibitors in treating human cancers: Recent advancement. Biomed. Pharmacother. 2020, 129, 110389. [Google Scholar] [CrossRef] [PubMed]
- Roelofs, H.M.; te Morsche, R.H.; van Heumen, B.W.; Nagengast, F.M.; Peters, W.H.M. Over-expression of COX-2 mRNA in colorectal cancer. BMC Gastroenterol. 2014, 14, 9005. [Google Scholar] [CrossRef]
- Venkatachala, S.; Rajendran, M. Correlation of COX-2 Expression in Colorectal Carcinoma with Clinicopathological Features. Turk Patoloji Derg 2017, 33, 228–234. [Google Scholar] [CrossRef] [PubMed]
- Sheng, J.; Sun, H.; Yu, F.B.; Li, B.; Zhang, Y.; Zhu, Y.T. The Role of Cyclooxygenase-2 in Colorectal Cancer. Int. J. Med. Sci. 2020, 17, 1095–1101. [Google Scholar] [CrossRef]
- Purnama, A.; Lukman, K.; Rudiman, R.; Prasetyo, D.; Fuadah, Y.; Nugraha, P.; Candrawinata, V.S. The prognostic value of COX-2 in predicting metastasis of patients with colorectal cancer: A systematic review and meta analysis. Heliyon 2023, 9, e21051. [Google Scholar] [CrossRef]
- Hu, T.; Liu, C.J.; Yin, X.; Tang, W.; Yin, L.; Bai, H.; Liu, F.; Wang, D.; Li, Y. Selective COX-2 inhibitors do not increase gastrointestinal reactions after colorectal cancer surgery: A systematic review and meta-analysis. BMC Gastroenterol. 2023, 23, 281. [Google Scholar] [CrossRef]
- Berbecka, M.; Forma, A.; Baj, J.; Furtak-Niczyporuk, M.; Maciejewski, R.; Sitarz, R. A Systematic Review of the Cyclooxygenase-2 (COX-2) Expression in Rectal Cancer Patients Treated with Preoperative Radiotherapy or Radiochemotherapy. J. Clin. Med. 2021, 10, 4443. [Google Scholar] [CrossRef]
- Villa, S.M.; Heckman, J.; Bandyopadhyay, D. Medicinally Privileged Natural Chalcones: Abundance, Mechanisms of Action, and Clinical Trials. Int. J. Mol. Sci. 2024, 5, 9623. [Google Scholar] [CrossRef]
- Mariadoss, A.V.A.; Vinayagam, R.; Senthilkumar, V.; Paulpandi, M.; Murugan, K.; Xu, B.; Gothandam, K.M.; Kotakadi, V.S.; David, E. Phloretin loaded chitosan nanoparticles augments the pH-dependent mitochondrial-mediated intrinsic apoptosis in human oral cancer cells. Int. J. Biol. Macromol. 2019, 130, 997–1008. [Google Scholar] [CrossRef] [PubMed]
- Lu, M.; Kong, Q.; Xu, X.; Lu, H.; Lu, Z.; Yu, W.; Zuo, B.; Su, J.; Guo, R. Evaluation of apoptotic and growth inhibitory activity of phloretin in BGC823 gastric cancer cell. Trop. J. Pharm. Res. 2015, 14, 27–31. [Google Scholar] [CrossRef]
- Roy, S.; Mondru, A.K.; Chakraborty, T.; Das, A.; Dasgupta, S. Apple polyphenol phloretin complexed with ruthenium is capable of reprogramming the breast cancer microenvironment through modulation of PI3K/Akt/mTOR/VEGF pathways. Toxicol. Appl. Pharmacol. 2022, 434, 115822. [Google Scholar] [CrossRef]
- Wu, C.H.; Ho, Y.S.; Tsai, C.Y.; Wang, Y.J.; Tseng, H.; Wei, P.L.; Lee, C.H.; Liu, R.S.; Lin, S.Y. In vitro and in vivo study of phloretin-induced apoptosis in human liver cancer cells involving inhibition of type II glucose transporter. Int. J. Cancer 2009, 124, 2210–2219. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.; Wang, D.; Pu, Y.; Pan, D.; Guan, W.; Ma, Y. Phloretin induced apoptosis of human hepatoma cells SMMC-7721 and its correlative biological mechanisms. Afr. J. Pharm. Pharmacol. 2012, 6, 648–659. [Google Scholar]
- Kang, Y.; Park, M.-A.; Heo, S.-W.; Park, S.-Y.; Kang, K.W.; Park, P.-H.; Kim, J.-A. The radio-sensitizing effect of xanthohumol is mediated by STAT3 and EGFR suppression in doxorubicin-resistant MCF-7 human breast cancer cells. Biochim. Biophys. Acta (BBA)-Gen. Subj. 2013, 1830, 2638–2648. [Google Scholar] [CrossRef]
- Michalkova, R.; Mirossay, L.; Kello, M.; Mojzisova, G.; Baloghova, J.; Podracka, A.; Mojzis, J. Anticancer Potential of Natural Chalcones: In Vitro and In Vivo Evidence. Int. J. Mol. Sci. 2023, 24, 10354. [Google Scholar] [CrossRef]
- Zhou, X.; Zhou, R.; Li, Q.; Jie, X.; Hong, J.; Zong, Y.; Dong, X.; Zhang, S.; Li, Z.; Wu, G. Cardamonin Inhibits the Proliferation and Metastasis of Non-Small-Cell Lung Cancer Cells by Suppressing the PI3K/Akt/mTOR Pathway. Anti-Cancer Drugs 2019, 30, 241–250. [Google Scholar] [CrossRef]
- Wang, P.; Jin, J.M.; Liang, X.H.; Yu, M.Z.; Yang, C.; Huang, F.; Wu, H.; Zhang, B.B.; Fei, X.Y.; Wang, Z.T.; et al. Helichrysetin Inhibits Gastric Cancer Growth by Targeting c-Myc/PDHK1 Axis-Mediated Energy Metabolism Reprogramming. Acta Pharmacol. Sin. 2022, 43, 1581–1593. [Google Scholar] [CrossRef]
- ClinicalTrials.gov. Available online: https://clinicaltrials.gov/study/NCT02907320 (accessed on 2 April 2025).
- Collective Work. Romanian Pharmacopoeia, Xth ed.; (FRX); Medicala: Bucharest, Romania, 1993. [Google Scholar]
- Kapoor, S.; Padwad, Y.S. Phloretin suppresses intestinal inflammation and maintained epithelial tight junction integrity by modulating cytokines secretion in in vitro model of gut inflammation. Cell. Immunol. 2023, 391–392, 104754. [Google Scholar] [CrossRef]
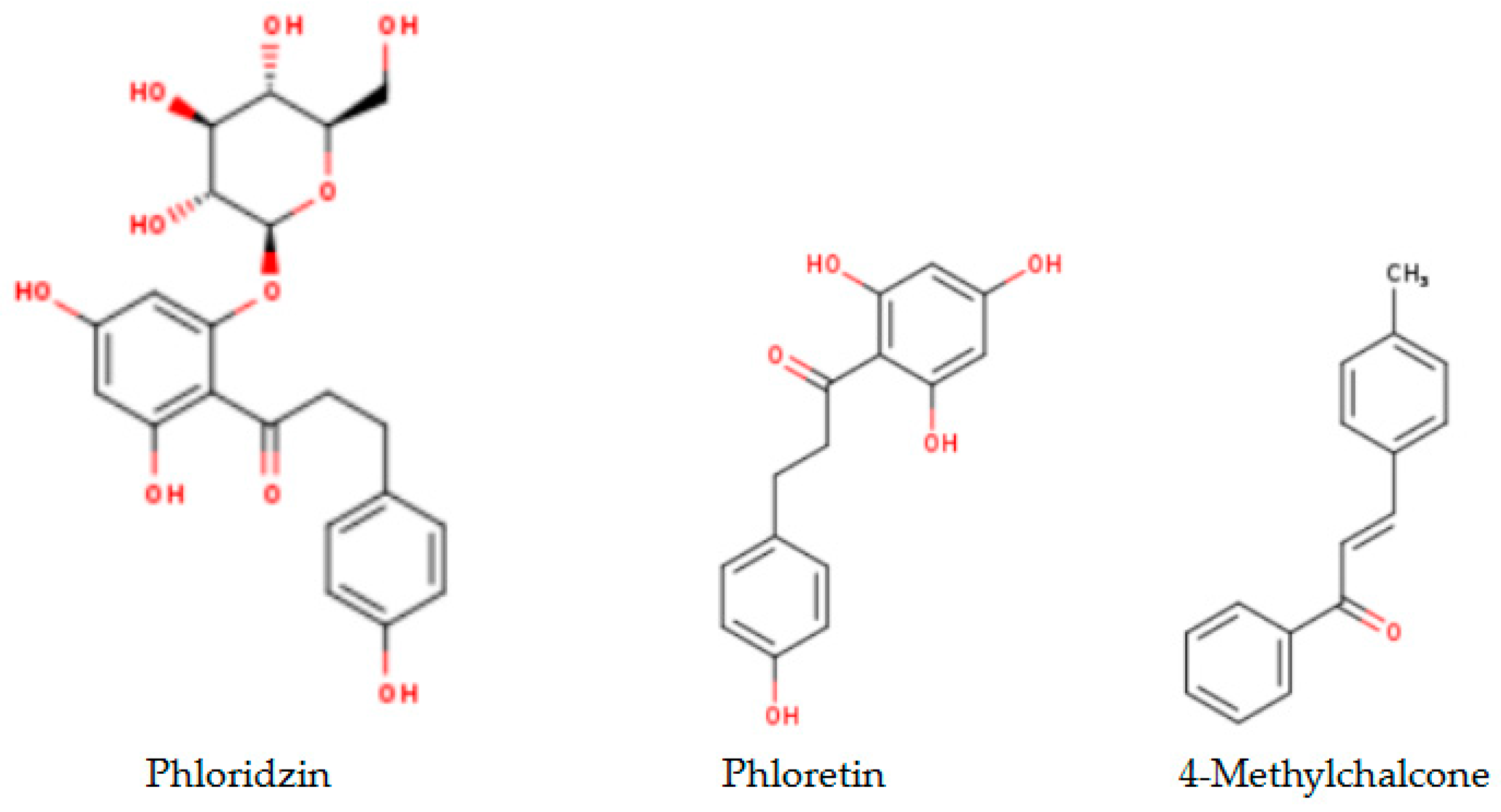
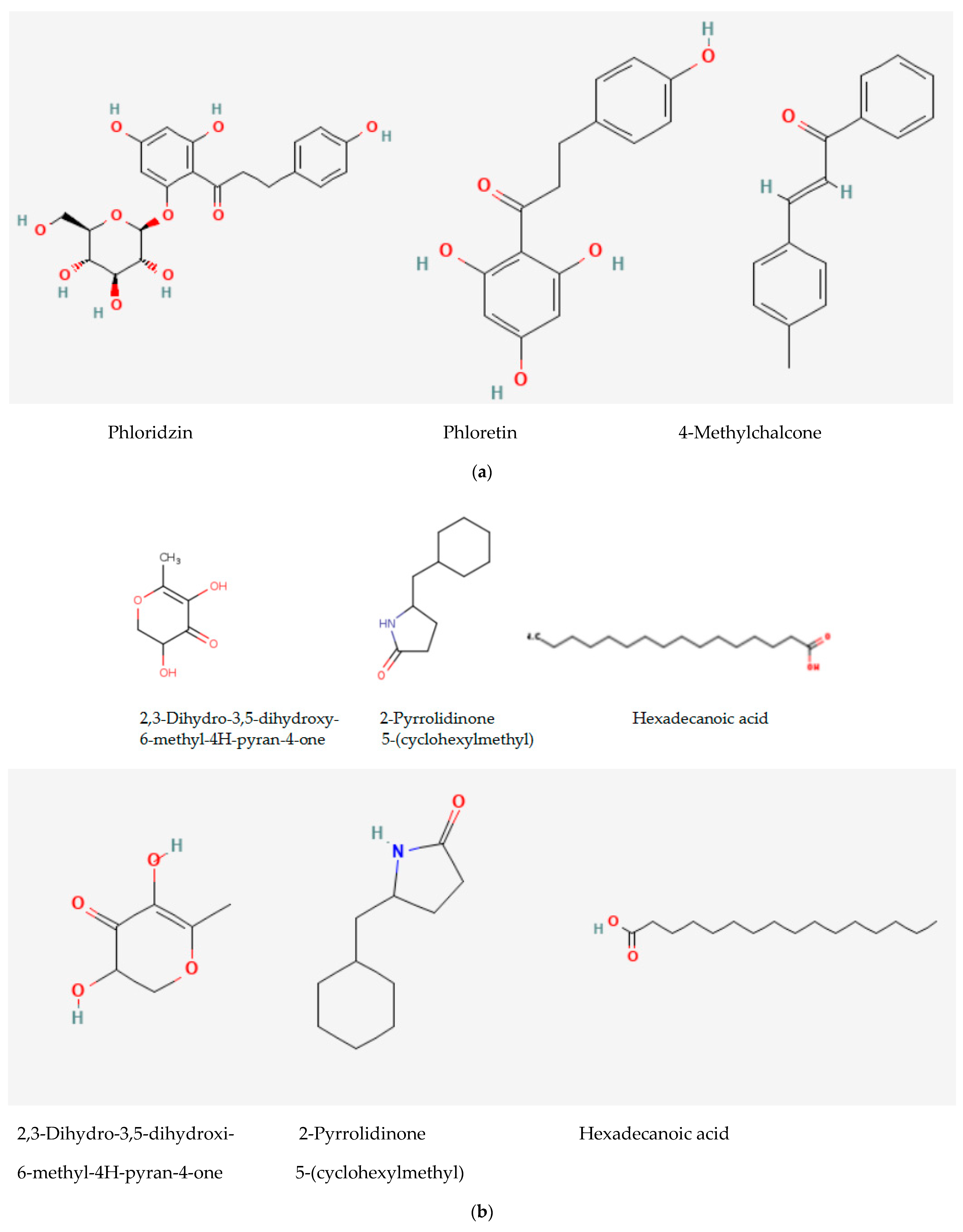
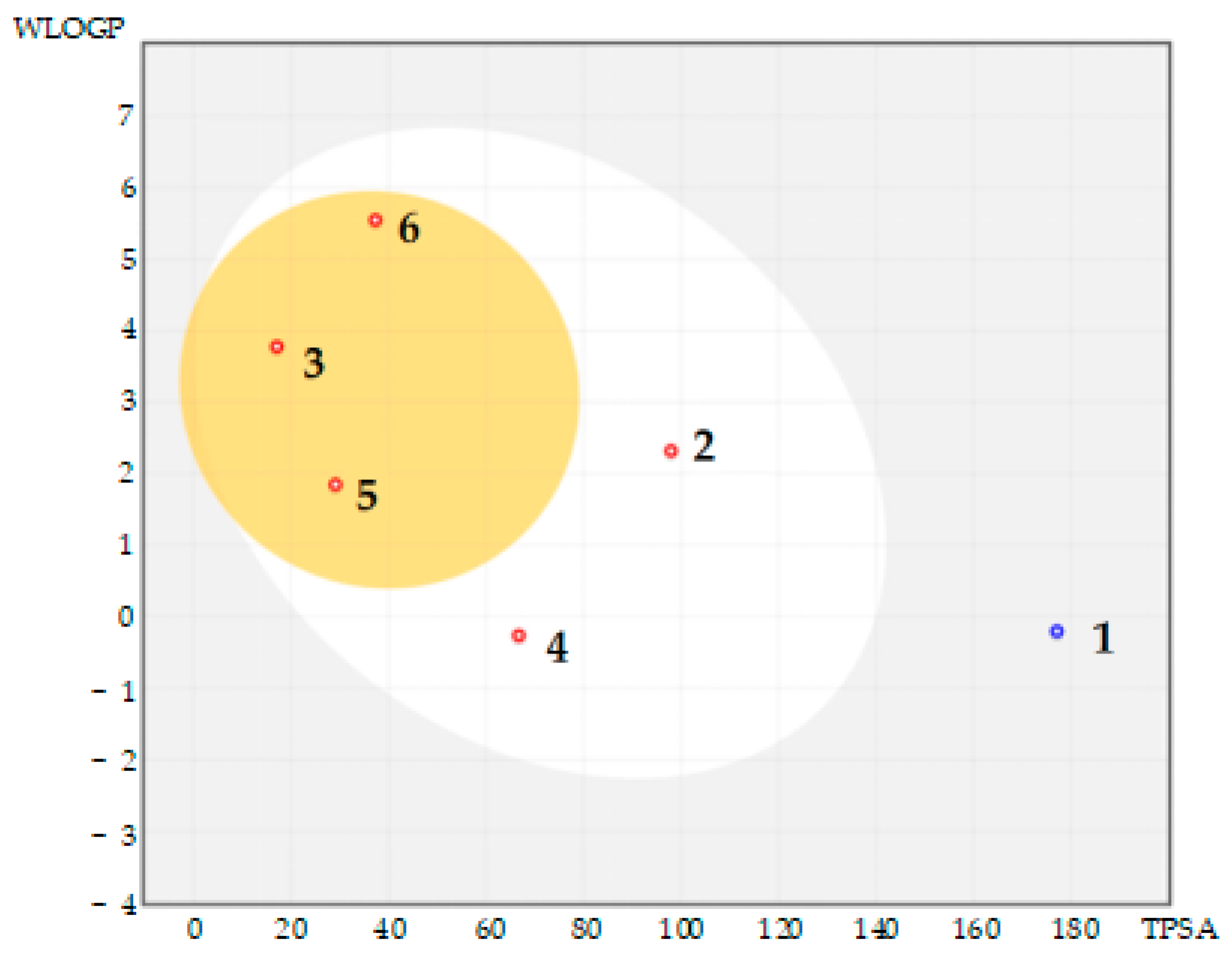
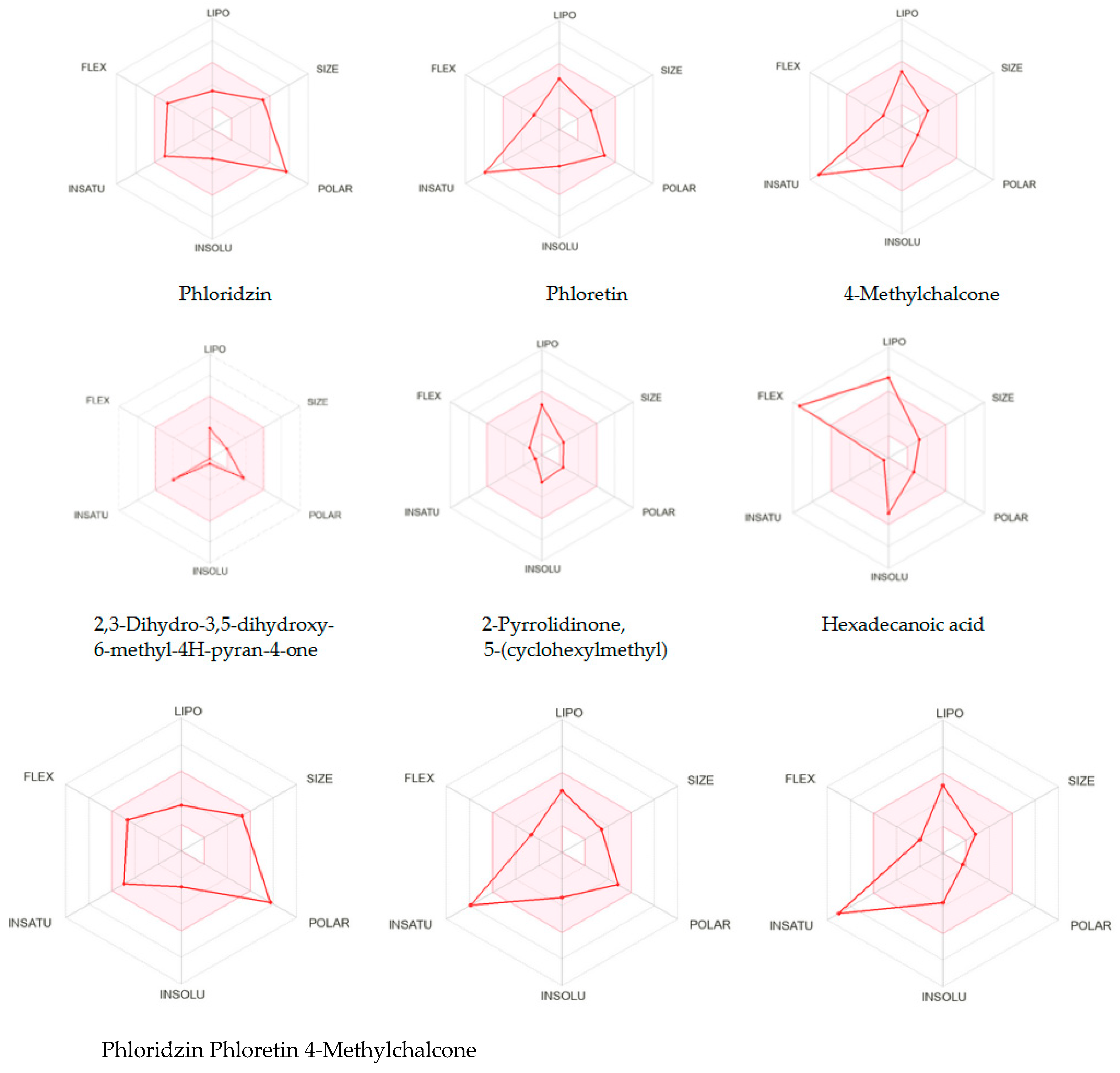
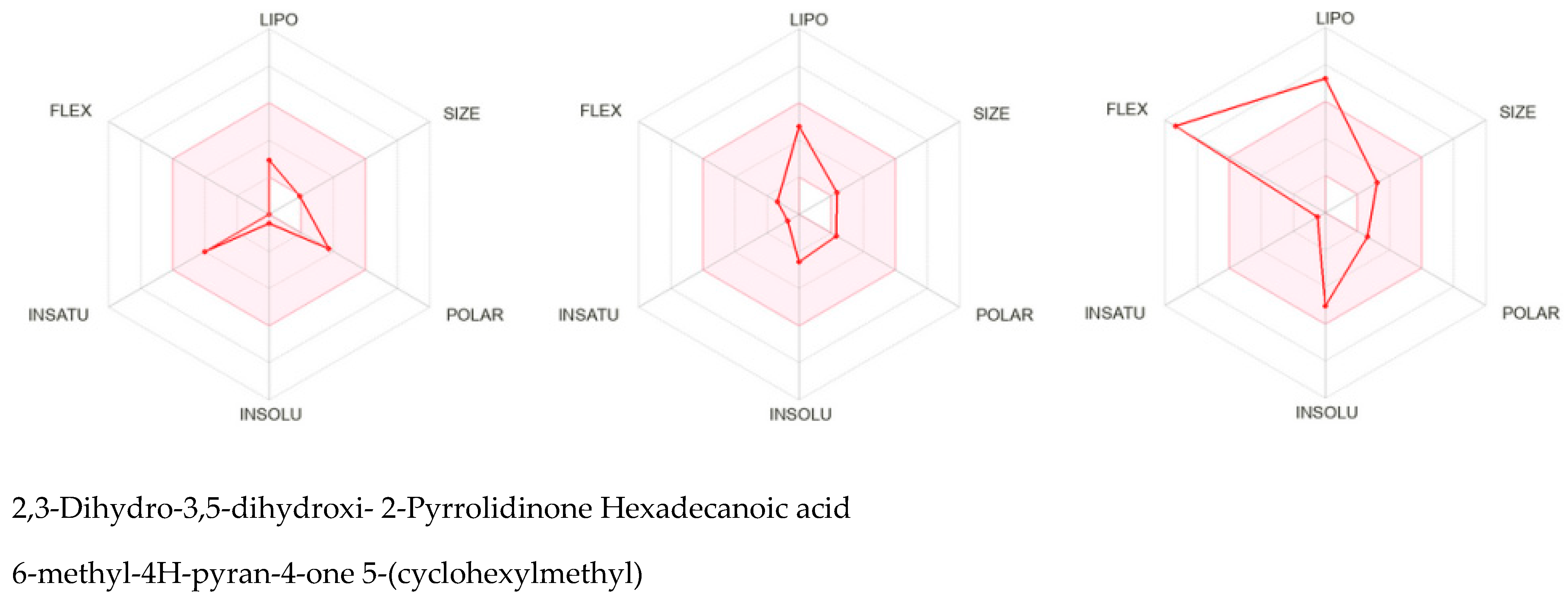

| Test Compound | MW | Formula | Peak Area | Retention Time (min) | Probability (%) | Concentration in Sample (%) |
|---|---|---|---|---|---|---|
| 2,3-dihidro-3,5-dihidroxi- 6-metil-4H-piran-4-ona | 144 | C6H8O4 | 782,240,832 | 8.50 | 93.4 | 11.03 |
| 2-pyrrolidinone 5-(cyclohexylmethyl) | 181 | C11H19ON | 138,499,424 | 12.54 | 95.5 | 1.95 |
| Acid n-hexadecanoic | 256 | C16H32O2 | 44,022,120 | 19.87 | 93.7 | 0.62 |
| (a) | |||
| Test Compound | Phloridzin | Phloretin | 4-Methylchalcone |
| Physical-chemical properties | |||
| Formula | C21H24O10 | C15H14O5 | C16H14O |
| Molecular weight | 436.41 g/mol | 274.27 g/mol | 222.28 g/mol |
| Num. heavy atoms | 31 | 20 | 17 |
| Num. arom. heavy atoms | 12 | 12 | 12 |
| Fraction Csp3 | 0.38 | 0.13 | 0.06 |
| Num. rotatable bonds | 7 | 4 | 3 |
| Num. H-bond acceptors | 10 | 5 | 1 |
| Num. H-bond donors | 7 | 4 | 0 |
| Molar refractivity | 106.14 | 74.02 | 71.21 |
| TPSA | 177.14 Å2 | 97.99 Å2 | 17.07 Å2 |
| Lipophilicity | |||
| Log Po/w (iLOGP) | 1.25 | 1.41 | 2.78 |
| Log Po/w (XLOGP3) | 0.54 | 2.63 | 3.44 |
| Log Po/w (WLOGP) | −0.20 | 2.32 | 3.78 |
| Log Po/w (MLOGP) | −1.42 | 1.10 | 3.69 |
| Log Po/w (SILICOS-IT) | 0.13 | 2.17 | 4.45 |
| Consensus Log Po/w | 0.06 | 1.93 | 3.63 |
| Water solubility | |||
| Log S (ESOL) | −2.71 | −3.38 | −3.71 |
| Log S (Ali) | −3.83 | −4.34 | −3.48 |
| Log S (SILICOS-IT) | −1.66 | −3.37 | −5.35 |
| Pharmacokinetic | |||
| GI absorption | Low | High | High |
| BBB permeant | No | No | Yes |
| P-gp substrate | Yes | No | No |
| CYP1A2 inhibitor | No | Yes | No |
| CYP2C19 inhibitor | No | No | Yes |
| CYP2C9 inhibitor | No | Yes | No |
| CYP2D6 inhibitor | No | No | Yes |
| CYP3A4 inhibitor | No | Yes | No |
| Medicinal Chemistry | |||
| PAINS | 0 alert | 0 alert | 0 alert |
| Brenk | 0 alert | 0 alert | 1 alert: Michael_acceptor_1 |
| Synthetic accessibility | 4.93 | 1.88 | 2.53 |
| Lead-likeness | No, 1 violation: MW > 350 | Yes | No, 1 violation: MW < 250 |
| Drug-likeness | |||
| Lipinski | Yes, 1 violation: NH or OH > 5 | Yes, 0 violation | Yes, 0 violation |
| Ghose | Yes | Yes | Yes |
| Veber | No, 1 violation: TPSA > 140 | Yes | Yes |
| Egan | No, 1 violation: TPSA > 131. | Yes | Yes |
| Muegge | No, 2 violations: | Yes | No, 1 violation: |
| TPSA > 150, H-don > 5 | Heteroatoms < 2 | ||
| Bioavailability score | 0.55 | 0.55 | 0.55 |
| (b) | |||
| Test Compound | 2,3-Dihydro-3,5-dihydroxy- 6-methyl-4H-pyran-4-one | 2-Pyrrolidinone 5-(cyclohexylmethyl) | Hexadecanoic Acid |
| Physical-chemical properties | |||
| Formula | C6H8O4 | C11H19NO | C16H32O2 |
| Molecular weight | 144.13 g/mol | 181.27 g/mol | 256.42 g/mol |
| Num. heavy atoms | 10 | 13 | 18 |
| Num. arom. heavy atoms | 0 | 0 | 0 |
| Fraction Csp3 | 0.50 | 0.91 | 0.94 |
| Num. rotatable bonds | 0 | 2 | 14 |
| Num. H-bond acceptors | 4 | 1 | 2 |
| Num. H-bond donors | 2 | 1 | 1 |
| Molar refractivity | 32.39 | 57.68 | 80.80 |
| TPSA | 66.76 Å2 | 29.10 Å2 | 37.30 Å2 |
| Lipophilicity | |||
| Log Po/w (iLOGP) | 1.19 | 2.39 | 3.85 |
| Log Po/w (XLOGP3) | −0.37 | 2.77 | 7.17 |
| Log Po/w (WLOGP) | −0.26 | 1.85 | 5.55 |
| Log Po/w (MLOGP) | −1.77 | 2.07 | 4.19 |
| Log Po/w (SILICOS-IT) | 0.13 | 2.61 | 5.25 |
| Consensus log Po/w | −0.22 | 2.34 | 5.20 |
| Water solubility | |||
| Log S (ESOL) | −0.50 | −2.58 | −5.02 |
| Log S (Ali) | −0.57 | −3.04 | −7.77 |
| Log S (SILICOS-IT) | 0.15 | −2.54 | −5.31 |
| Pharmacokinetic | |||
| GI absorption | High | High | High |
| BBB permeant | No | Yes | Yes |
| P-gp substrate | No | No | No |
| CYP1A2 inhibitor | No | No | Yes |
| CYP2C19 inhibitor | No | No | No |
| CYP2C9 inhibitor | No | No | Yes |
| CYP2D6 inhibitor | No | No | No |
| CYP3A4 inhibitor | No | No | No |
| Medicinal chemistry | |||
| PAINS | 0 alert | 0 alert | 0 alert |
| Brenk | 0 alert | 0 alert | 0 alert |
| Synthetic accessibility | 3.60 | 1.99 | 2.31 |
| Lead-likeness | No, 1 violation: | No, 1 violation: | No, 2 violations: |
| MW < 250 | MW < 250 | Rotors > 7, XLOGP3 > 3.5 | |
| Drug-likeness | |||
| Lipinski | Yes, 0 violation | Yes, 0 violation | Yes, 1 violation: MLOGP > 4.15 |
| Ghose | No, 3 violations: MW < 160, | Yes | Yes |
| MR < 40, no. atoms < 20 | |||
| Veber | Yes | Yes | No, 1 violation: Rotors > 10 |
| Egan | Yes | Yes | Yes |
| Muegge | No, 1 violation: | No, 1 violation: | No, 1 violation: |
| MW < 200 | MW < 200 | XLOGP3 > 5 | |
| Bioavailability score | 0.85 | 0.55 | 0.85 |
| Test Pairs | The Docking Image of the Test Ligand Interacting with the Amino Acid Residues in the Binding Site, Chain A | The Punctual Hydrogen Bonds: Å | Score/RMSD |
|---|---|---|---|
| 2O2F in complex with co-crystallized LI0 A 1000 | 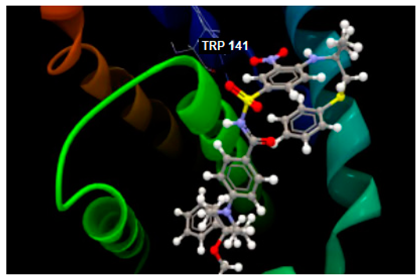 | O sp2–N sp2 TRP141: 3.281 | −82.97/2.02 [93] |
| 2O2F in complex with phloridzin | 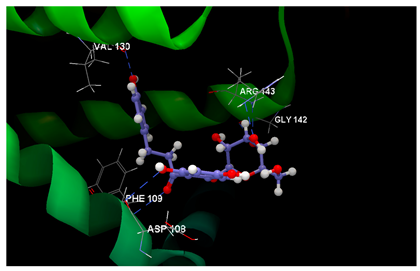 | O7 sp2–N sp2 PHE109: 3.066 O6 sp3–O sp2 ASP108: 3.330 O2 sp3–N sp2 ARG143: 2.619 O3 sp3–O sp2 GLY142: 3.176 O3 sp3–N sp2 ARG143: 3.351 O9 sp3–O sp2 VAL130: 2.874 | −62.85/0.09 |
| 2O2F in complex with phloretin | 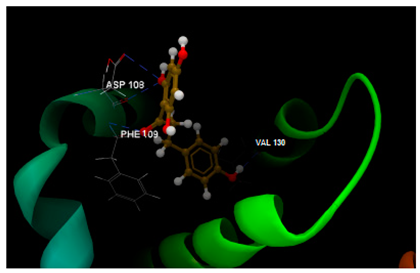 | O0 sp2–N sp2 PHE109: 3.238 O4 sp3–O sp2 VAL130: 2.062 O1 sp3–O sp2 ASP108: 3.093 O1 sp3–O sp2 ASP108: 3.235 | −51.97/0.15 |
| 2O2F in complex with 4-methylchalcone | no bonds | −52.09/0.10 |
| Test Pairs | The Docking Image of the Test Ligand Interacting with the Amino Acid Residues in the Binding Site | The Punctual Hydrogen Bonds: Å | Score/RMSD |
|---|---|---|---|
| 4W6E in complex with co-crystallized 3J5A | 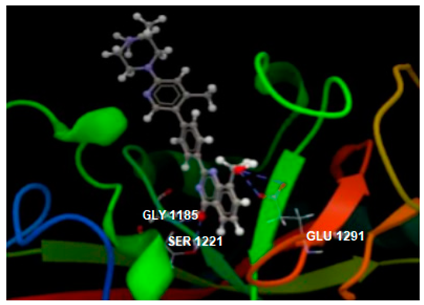 | O1 sp3–O sp2 GLU1291: 3.393 O1 sp3–O sp2 GLU1291: 3.250 O sp2–O sp3 SER1221: 2.778 O sp2–N sp2 GLY1185: 2.895 | −104.15/0.16 [52] |
| 4W6E in complex with phloridzin | 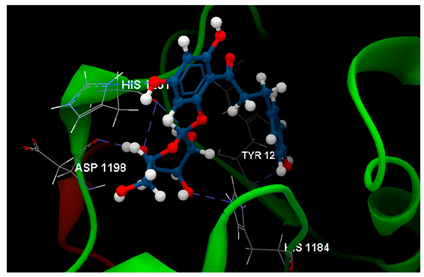 | O9 sp3–Osp2 TYR1213: 2.977 O8 sp3–Nsp2 HIS1201: 2.688 O3 sp3–Nsp2 HIS1184: 2.752 O4 sp3–Osp2 HIS1201: 3.156 O2 sp3–Osp2 HIS1201: 3.002 O2 sp3–Osp2 ASP1198: 2.678 | −74.58/0.82 |
| 4W6E in complex with phloretin | 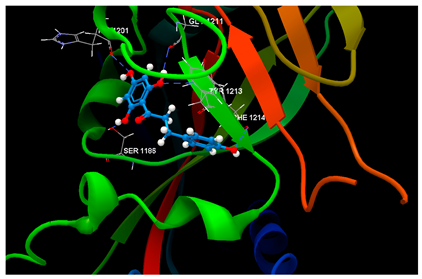 | O1 sp3–Osp3 SER1186: 3.261 O3 sp3–Osp2 HIS1201: 3.119 O2 sp3–Osp2 GLY1211: 3.167 O2 sp3–Nsp2 TYR1213: 3.049 O4 sp3–Osp2 PHE1214: 3.269 | −67.28/0.52 |
| 4W6E in complex with 4-methylchalcone | no bonds | −67.80/0.12 |
| Test Pairs | The Docking Image of the Test Ligand Interacting with the Amino Acid Residues in the Binding Site | The Punctual Hydrogen Bonds: Å | Score/RMSD |
|---|---|---|---|
| 1PXX in complex with diclofenac | 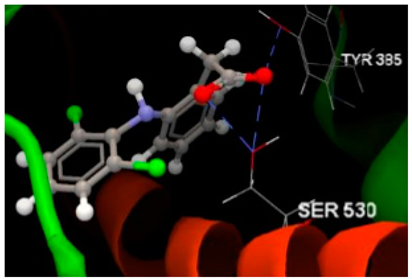 | O sp2–O sp3 SER530 (2.653) O sp2–O sp3 SER530 (2.905) O sp2–O sp3 TYR385 (2.729) | −68.76/0.15 [94] |
| 1PXX in complex with phloridzin | 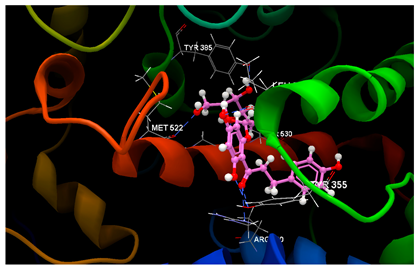 | O7 sp2–O sp3 TYR355 (3.175) O7 sp2–N sp2 ARG120 (3.155) O9 sp3–O sp3 TYR355 (2.577) O8 sp3–O sp2 LEU352 (2.715) O4 sp3–O sp3 SER530 (3.124) O2 sp3–O sp2 GLY526 (2.460) O5 sp3–O sp2 MET522 (2.984) | −52.56/0.34 |
| 1PXX in complex with phloretin | 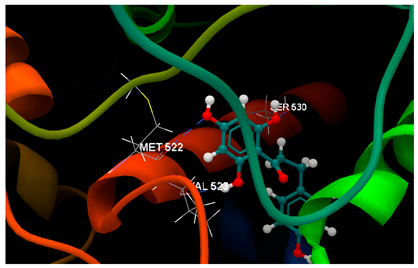 | O1 sp3–O sp2 VAL523 (3.126) O2 sp3–O sp3 SER530 (2.934) O3 sp3–O sp2 MET522 (3.244) | −57.24/0.07 |
| 1PXX in complex with 4-methylchalcone | no bonds | −58.38/0.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pirvu, L.C.; Stefaniu, A.; Nita, S.; Radu, N.; Neagu, G. In Silico and In Vitro Analyses of Strawberry-Derived Extracts in Relation to Key Compounds’ Metabolic and Anti-Tumor Effects. Int. J. Mol. Sci. 2025, 26, 3492. https://doi.org/10.3390/ijms26083492
Pirvu LC, Stefaniu A, Nita S, Radu N, Neagu G. In Silico and In Vitro Analyses of Strawberry-Derived Extracts in Relation to Key Compounds’ Metabolic and Anti-Tumor Effects. International Journal of Molecular Sciences. 2025; 26(8):3492. https://doi.org/10.3390/ijms26083492
Chicago/Turabian StylePirvu, Lucia Camelia, Amalia Stefaniu, Sultana Nita, Nicoleta Radu, and Georgeta Neagu. 2025. "In Silico and In Vitro Analyses of Strawberry-Derived Extracts in Relation to Key Compounds’ Metabolic and Anti-Tumor Effects" International Journal of Molecular Sciences 26, no. 8: 3492. https://doi.org/10.3390/ijms26083492
APA StylePirvu, L. C., Stefaniu, A., Nita, S., Radu, N., & Neagu, G. (2025). In Silico and In Vitro Analyses of Strawberry-Derived Extracts in Relation to Key Compounds’ Metabolic and Anti-Tumor Effects. International Journal of Molecular Sciences, 26(8), 3492. https://doi.org/10.3390/ijms26083492








