Genetic and Genomic Analysis Identifies bcltf1 as the Transcription Factor Coding Gene Mutated in Field Isolate Bc116, Deficient in Light Responses, Differentiation and Pathogenicity in Botrytis cinerea
Abstract
1. Introduction
2. Results
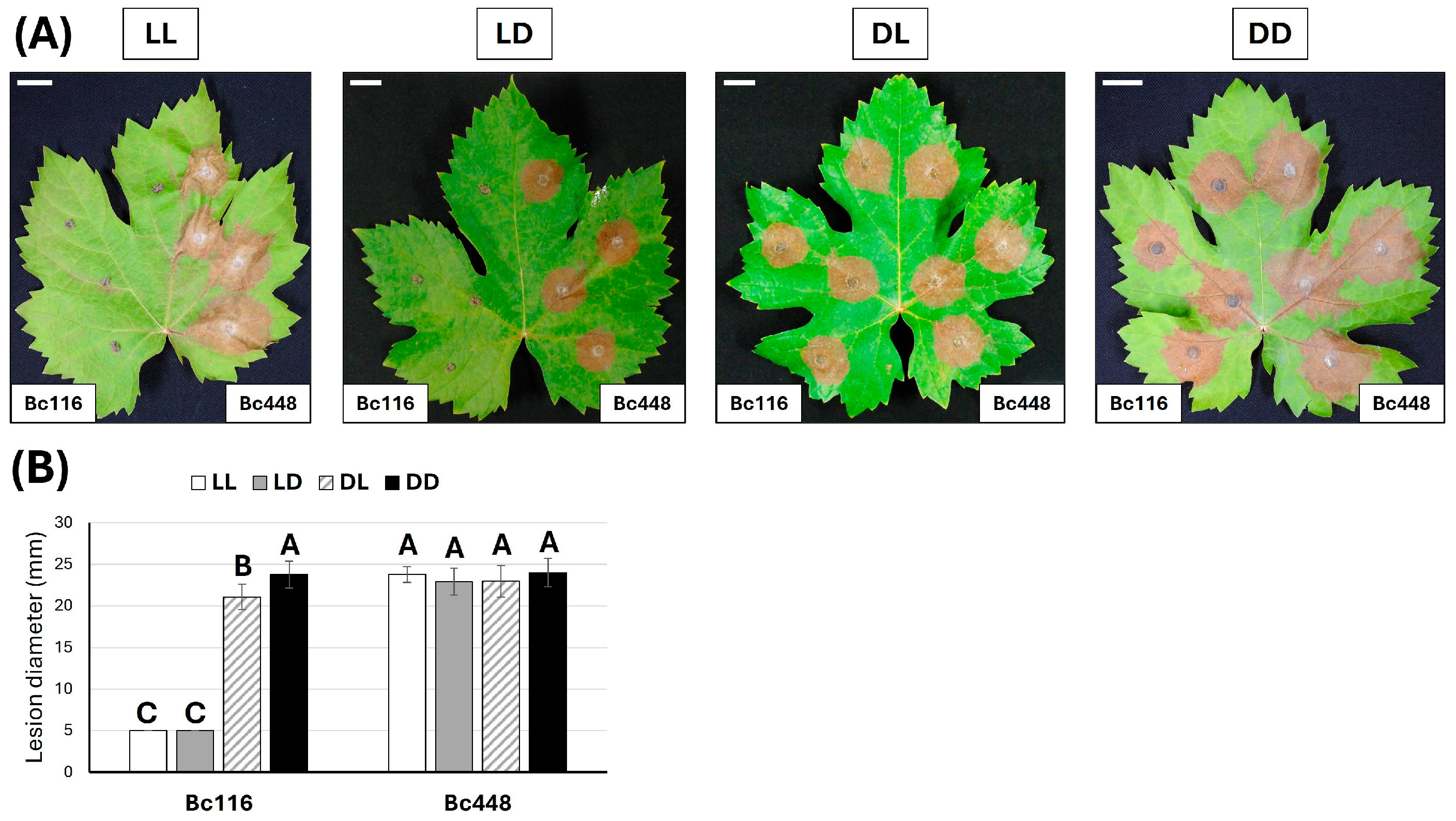
2.1. The Pathogenicity of Bc116 Is Light Dependent
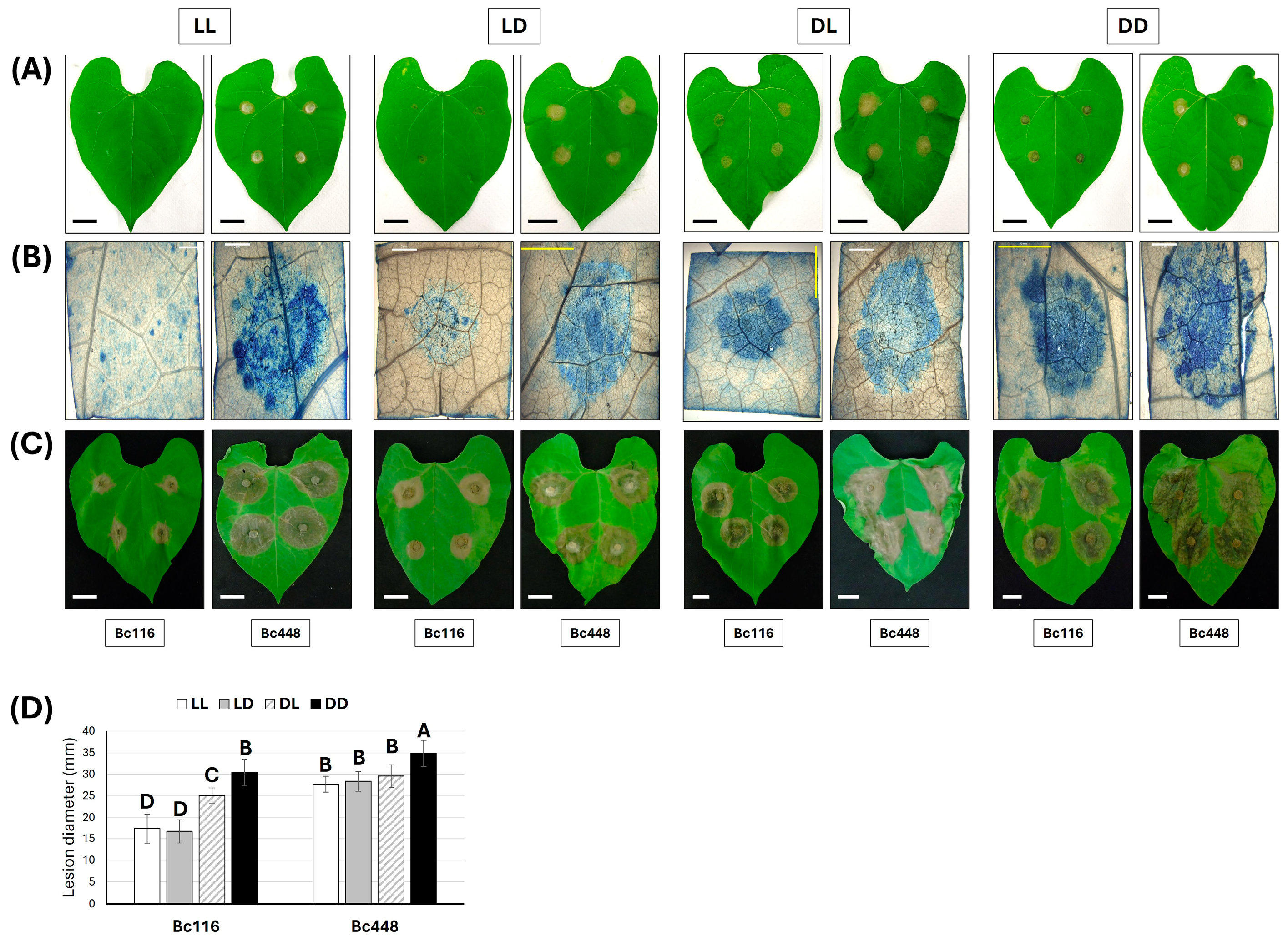
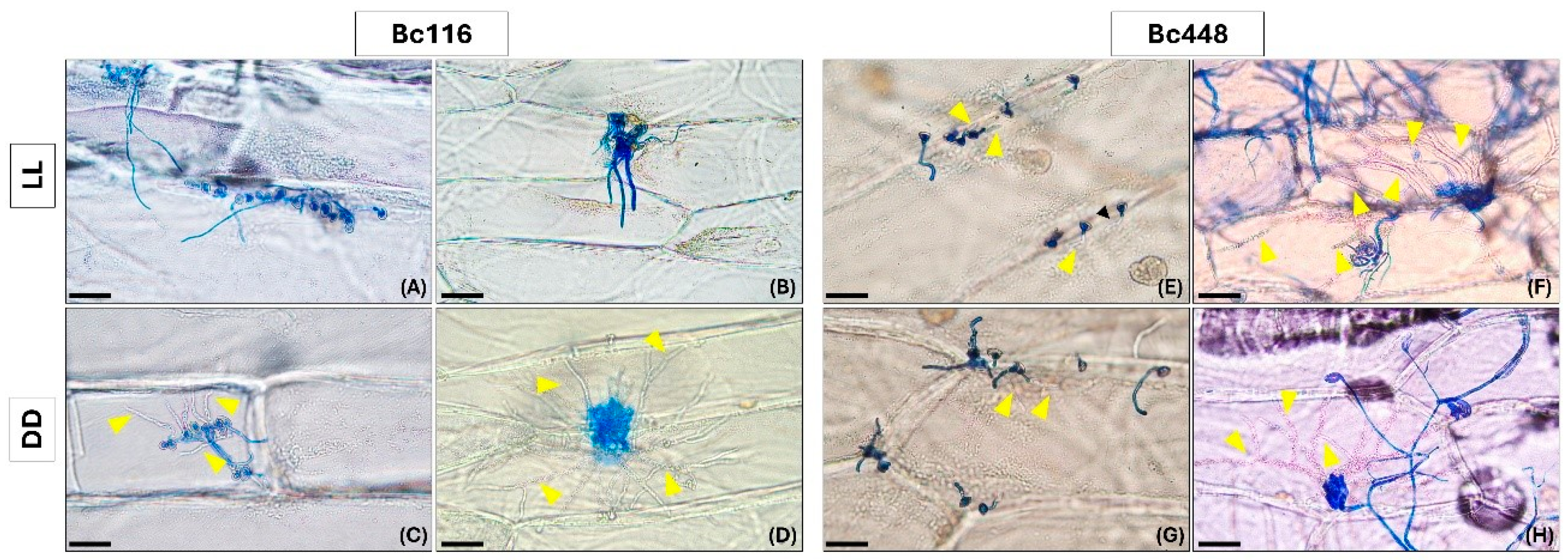
2.2. Light Limits Bc116 Ability to Penetrate Onion Epidermal Cells
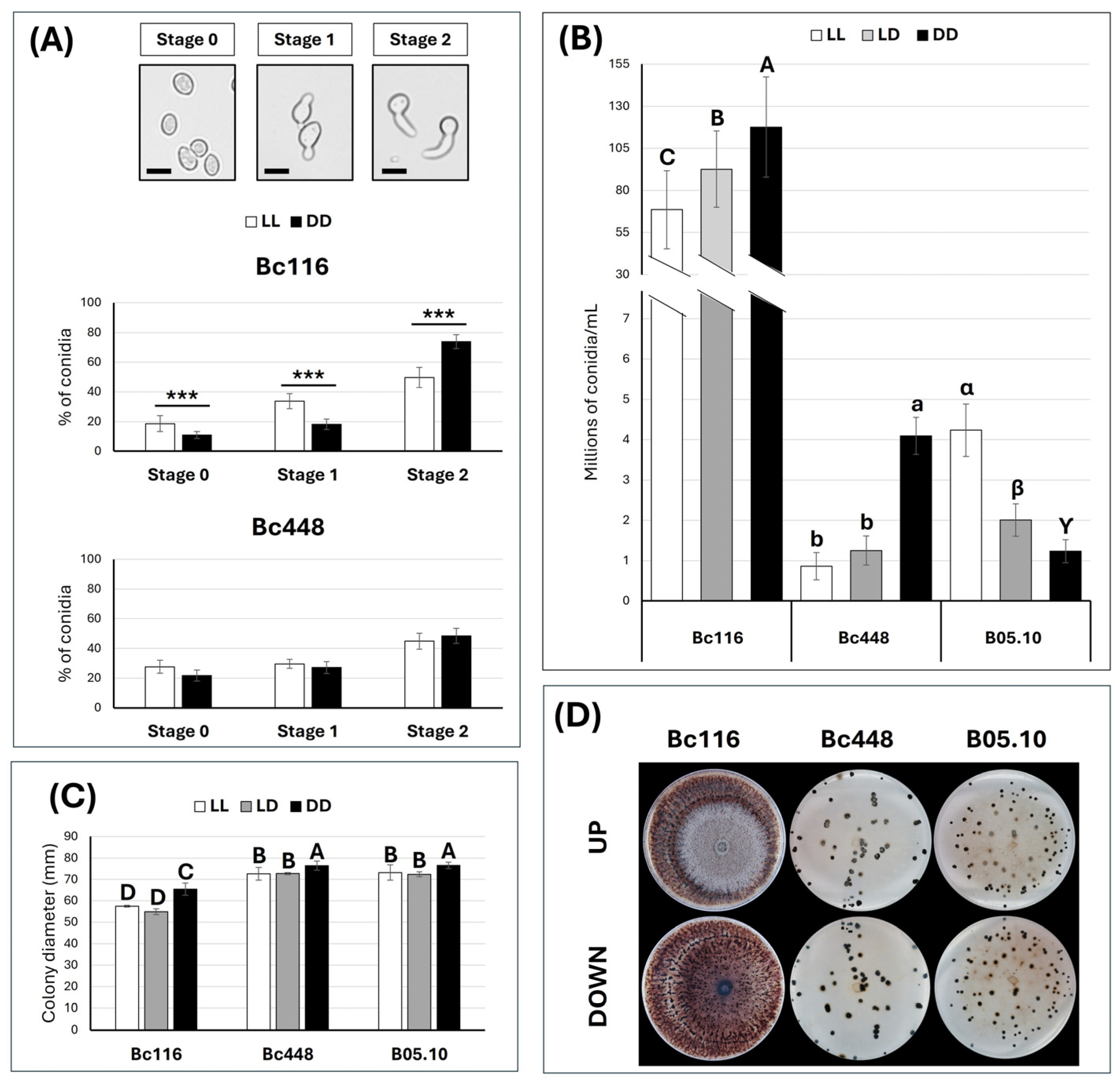
2.3. Light Affects Germination, Vegetative Growth Rate, and Conidiation in Bc116
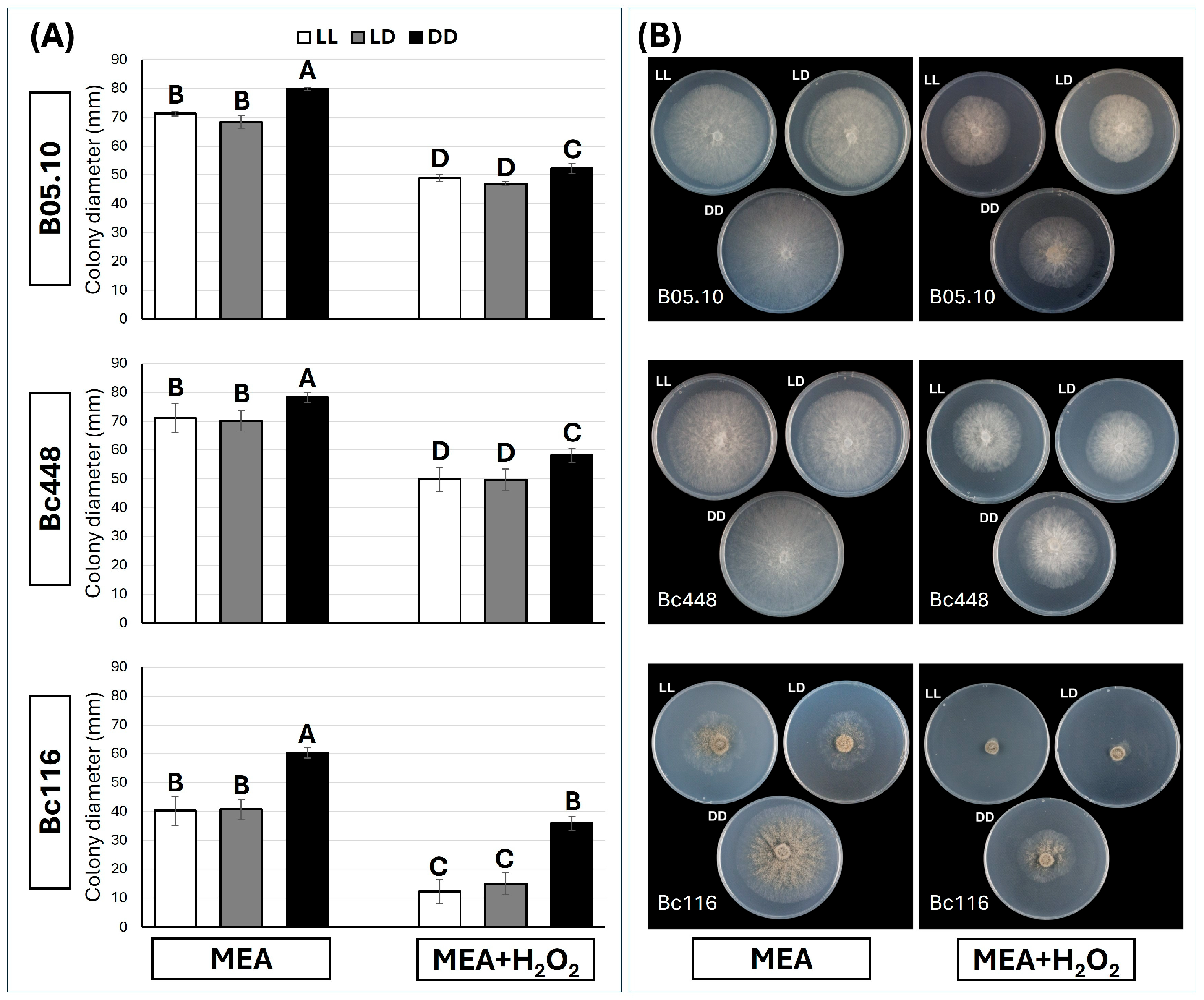
2.4. Bc116 Is Hypersensitive to Oxidative Stress Under LL Conditions
2.5. Genetic Analysis of the Bc116 Phenotype
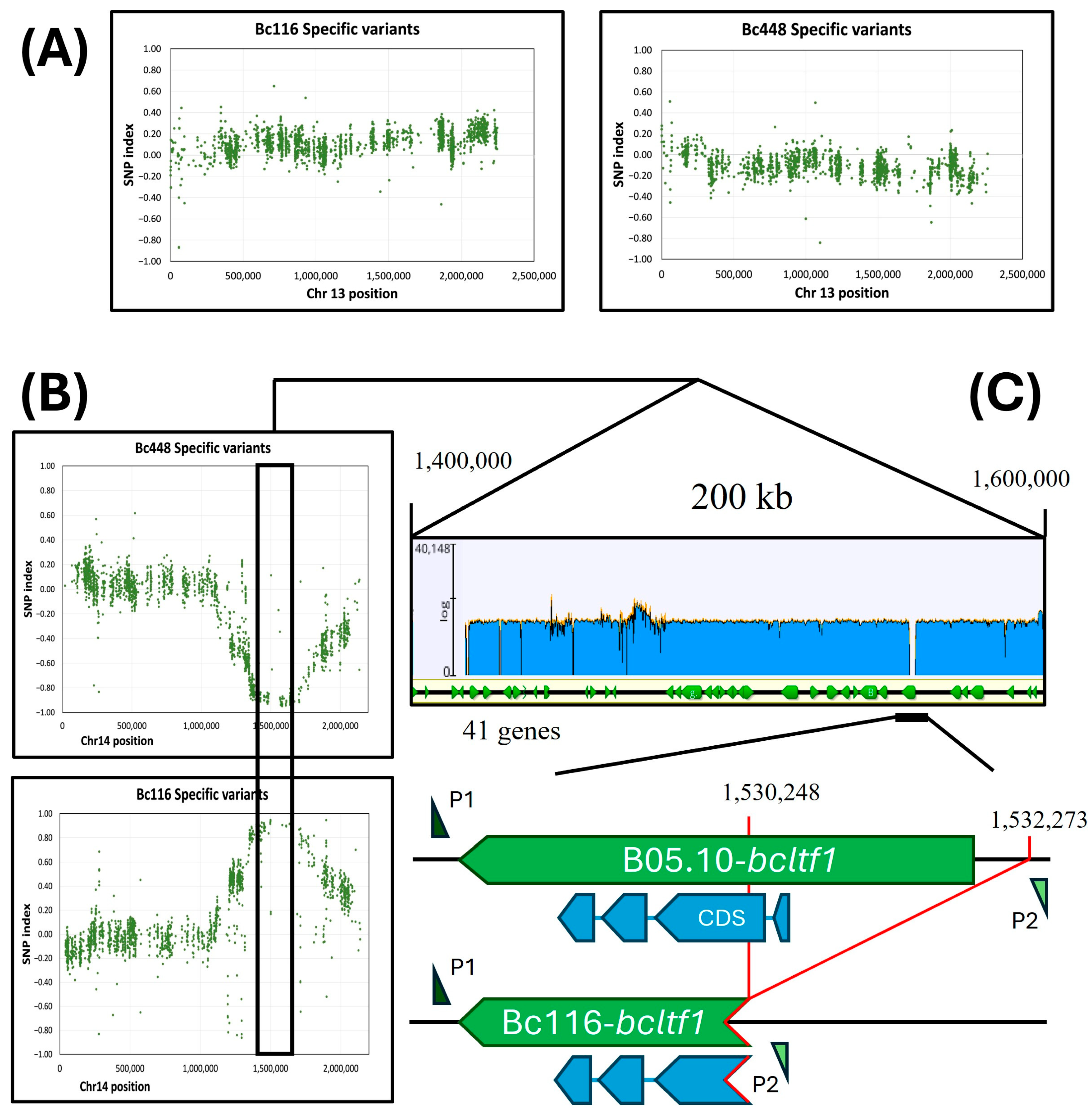
2.6. Mapping the Altered Gene in Bc116 by BSA
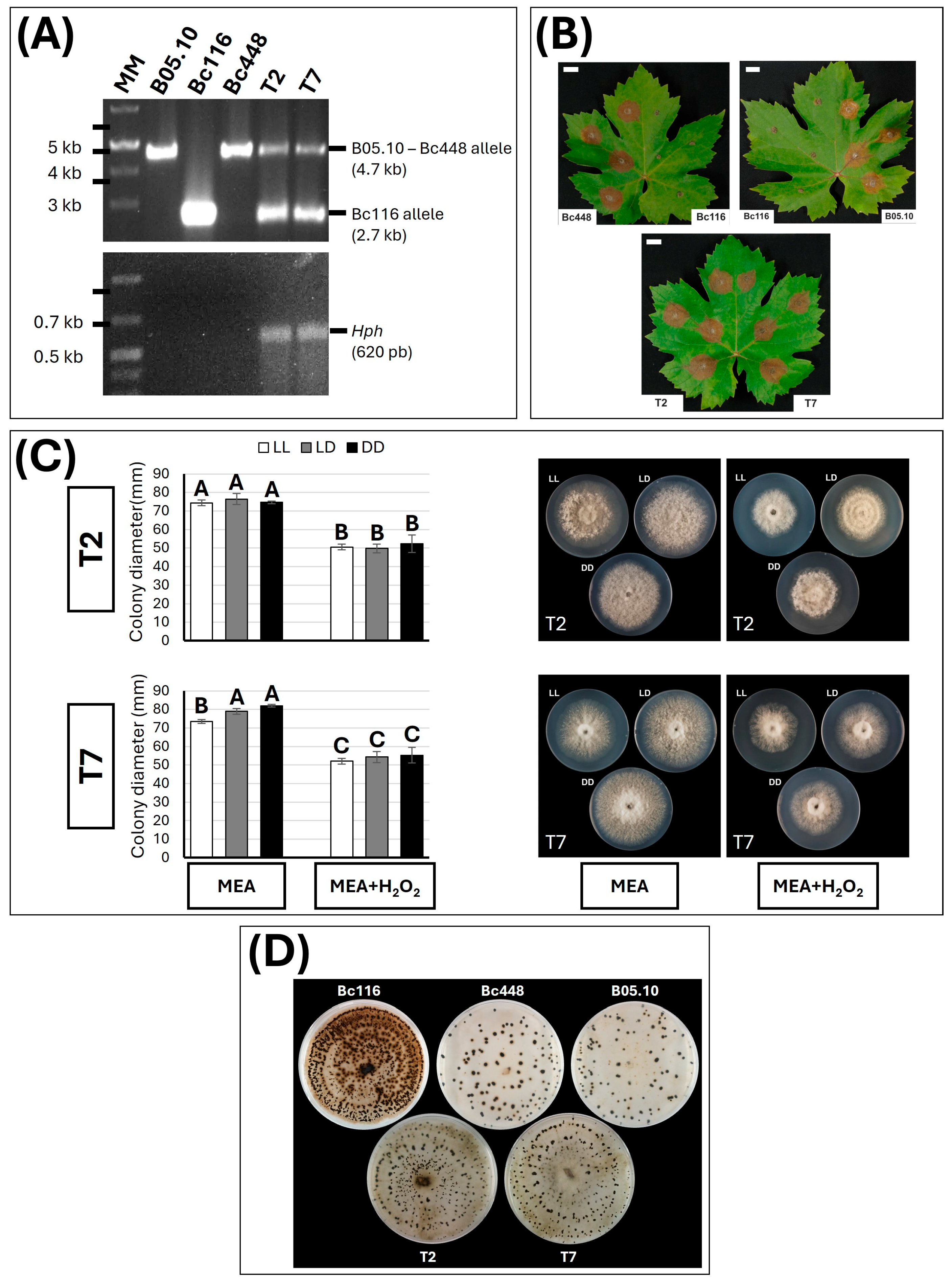
2.7. Bc116 Is Altered in the Light Responsive Transcription Factor BcLTF1
3. Discussion
3.1. Genetic and Genomic Tools Facilitate Association Mapping in B. cinerea and Identify a Natural Variant of bcltf1
3.2. Bc116 Resembles B05.10-Δbcltf1
3.3. Characterization of Bc116 Broadens the Knowledge of the BcLTF1 Functions
3.4. The bcltf1 Allele in the Field
4. Materials and Methods
4.1. Organisms and Growth Conditions
4.2. Germination, Conidiation, and Saprophytic Growth Experiments
4.3. Penetration Analysis
4.4. Inoculation Assays
4.5. Standard Molecular Techniques
4.6. B. cinerea Transformation
4.7. Construction of Bc116-bcltf1-Complemented Transformants
4.8. Crosses
4.9. Sequencing and Determination of Polymorphisms
4.10. BSA
4.11. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Elad, Y.; Pertot, I.; Cotes Prado, A.M.; Stewart, A. Plant hosts of Botrytis spp. In Botrytis—The Fungus, the Pathogen and Its Management in Agricultural Systems; Fillinger, S., Elad, Y., Eds.; Springer International Publishing: Cham, Switzerland, 2016; pp. 413–486. [Google Scholar] [CrossRef]
- van Kan, J.A.L. Licensed to kill: The lifestyle of a necrotrophic plant pathogen. Trends Plant Sci. 2006, 11, 247–253. [Google Scholar] [CrossRef] [PubMed]
- Williamson, B.; Tudzynski, B.; Tudzynski, P.; van Kan, J.A.L. Botrytis cinerea: The cause of grey mould disease. Mol. Plant Pathol. 2007, 8, 561–580. [Google Scholar] [CrossRef] [PubMed]
- Holz, G.; Coertze, S.; Williamson, B. The ecology of Botrytis on plant surfaces. In Botrytis: Biology, Pathology and Control; Elad, Y., Williamson, B., Tudzynski, P., Delen, N., Eds.; Springer: Dordrecht, The Netherlands, 2007; pp. 9–27. [Google Scholar] [CrossRef]
- Groves, J.W.; Loveland, C.A. The connection between Botryotinia fuckeliana and Botrytis cinerea. Mycologia 1953, 45, 415–425. Available online: https://www.jstor.org/stable/4547710 (accessed on 21 March 2025).
- Faretra, F.; Antonacci, E.; Pollastro, S. Sexual behaviour and mating system of Botryotinia fuckeliana, teleomorph of Botrytis cinerea. Microbiology 1988, 134, 2543–2550. [Google Scholar] [CrossRef]
- Lorenz, D.H.; Eichhorn, K.W. Untersuchungen an Botryotinia fuckeliana Whetz.; dem Perfektstadium von Botrytis cinerea Pers. Z. Pflanzenkrankh. Pflanzenschutz 1983, 90, 1–11. Available online: https://www.jstor.org/stable/43382905 (accessed on 21 March 2025).
- Beever, R.E.; Parkes, S.L. Mating behaviour and genetics of fungicide resistance of Botrytis cinerea in New Zealand. N. Z. J. Crop Hortic. Sci. 1993, 21, 303–310. [Google Scholar] [CrossRef]
- Faretra, F.; Antonacci, E.; Pollastro, S. Improvement of the technique used for obtaining apothecia of Botryotinia fuckeliana (Botrytis cinerea) under controlled conditions. Ann. Microbiol. 1988, 38, 29–40. [Google Scholar]
- Grindle, M. Phenotypic differences between natural and induced variants of Botrytis cinerea. J. Gen. Microbiol. 1979, 111, 109–120. [Google Scholar] [CrossRef]
- Martinez, F.; Blancard, D.; Lecomte, P.; Levis, C.; Dubos, B.; Fermaud, M. Phenotypic differences between vacuma and transposa subpopulations of Botrytis cinerea. Eur. J. Plant Pathol. 2003, 109, 479–488. [Google Scholar] [CrossRef]
- Leroch, M.; Kretschmer, M.; Hahn, M. Fungicide resistance phenotypes of Botrytis cinerea isolates from commercial vineyards in south west Germany. J. Phytopathol. 2011, 159, 63–65. [Google Scholar] [CrossRef]
- Acosta Morel, W.; Marques-Costa, T.M.; Santander-Gordón, D.; Fernández, F.A.; Zabalgogeazcoa, I.; de Aldana, B.R.V.; Sukno, S.A.; Díaz-Mínguez, J.M.; Benito, E.P. Physiological and population genetic analysis of Botrytis field isolates from vineyards in Castilla y León, Spain. Plant Pathol. 2019, 68, 523–536. [Google Scholar] [CrossRef]
- Canessa, P.; Schumacher, J.; Hevia, M.A.; Tudzynski, P.; Larrondo, L.F. Assessing the effects of light on differentiation and virulence of the plant pathogen Botrytis cinerea: Characterization of the White Collar Complex. PLoS ONE 2013, 8, e84223. [Google Scholar] [CrossRef] [PubMed]
- Kerssies, A.; Bosker-van Zessen, A.I.; Wagemakers, C.A.M.; van Kan, J.A.L. Variation in pathogenicity and DNA polymorphism among Botrytis cinerea isolates sampled inside and outside a glasshouse. Plant Dis. 1997, 81, 781–786. [Google Scholar] [CrossRef] [PubMed]
- Giraud, T.; Fortini, D.; Levis, C.; Leroux, P.; Brygoo, Y. RFLP markers show genetic recombination in Botryotinia fuckeliana (Botrytis cinerea) and transposable elements reveal two sympatric species. Mol. Biol. Evol. 1997, 14, 1177–1185. [Google Scholar] [CrossRef]
- Fournier, E.; Giraud, T.; Albertini, C.; Brygoo, Y. Partition of the Botrytis cinerea complex in France using multiple gene genealogies. Mycologia 2005, 97, 1251–1267. [Google Scholar] [CrossRef]
- Amselem, J.; Cuomo, C.A.; van Kan, J.A.L.; Viaud, M.; Benito, E.P.; Couloux, A.; Coutinho, P.M.; de Vries, R.P.; Dyer, P.S.; Fillinger, S.; et al. Genomic analysis of the necrotrophic fungal pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 7, e1002230. [Google Scholar] [CrossRef]
- van Kan, J.A.L.; Stassen, J.H.M.; Mosbach, A.; Van Der Lee, T.A.J.; Faino, L.; Farmer, A.D.; Papasotiriou, D.G.; Zhou, S.; Seidl, M.F.; Cottam, E.; et al. A gapless genome sequence of the fungus Botrytis cinerea. Mol. Plant Pathol. 2017, 18, 75–89. [Google Scholar] [CrossRef]
- Atwell, S.; Corwin, J.; Soltis, N.; Subedy, A.; Denby, K.; Kliebenstein, D.J. Whole genome resequencing of Botrytis cinerea isolates identifies high levels of standing diversity. Front. Microbiol. 2015, 6, 996. [Google Scholar] [CrossRef]
- Atwell, S.; Corwin, J.A.; Soltis, N.; Zhang, W.; Copeland, D.; Feusier, J.; Eshbaugh, R.; Kliebenstein, D.J. Resequencing and association mapping of the generalist pathogen Botrytis cinerea. bioRxiv 2018, 489799, Preprint. [Google Scholar] [CrossRef]
- Soltis, N.E.; Atwell, S.; Shi, G.; Fordyce, R.; Gwinner, R.; Gao, D.; Shafi, A.; Kliebenstein, D.J. Interactions of tomato and Botrytis cinerea genetic diversity: Parsing the contributions of host differentiation, domestication, and pathogen variation. Plant Cell 2019, 31, 502–519. [Google Scholar] [CrossRef]
- Michelmore, R.W.; Paran, I.; Kesseli, R.V. Identification of markers linked to disease-resistance genes by bulked segregant analysis: A rapid method to detect markers in specific genomic regions by using segregating populations. Proc. Natl. Acad. Sci. USA 1991, 88, 9828–9832. [Google Scholar] [CrossRef]
- Magwene, P.M.; Willis, J.H.; Kelly, J.K. The statistics of bulk segregant analysis using next generation sequencing. PLoS Comput. Biol. 2011, 7, e1002255. [Google Scholar] [CrossRef]
- Acosta Morel, W.; Anta Fernández, F.; Baroncelli, R.; Becerra, S.; Thon, M.R.; van Kan, J.A.L.; Díaz-Mínguez, J.M.; Benito, E.P. A major effect gene controlling development and pathogenicity in Botrytis cinerea identified through genetic analysis of natural mycelial non-pathogenic isolates. Front. Plant Sci. 2021, 12, 663870. [Google Scholar] [CrossRef]
- Soltis, N.E.; Caseys, C.; Zhang, W.; Corwin, J.A.; Atwell, S.; Kliebenstein, D.J. Pathogen genetic control of transcriptome variation in the Arabidopsis thaliana—Botrytis cinerea pathosystem. Genetics 2020, 215, 253–266. [Google Scholar] [CrossRef]
- Schumacher, J. How light affects the life of Botrytis. Fungal Genet. Biol. 2017, 106, 26–41. [Google Scholar] [CrossRef]
- Schumacher, J.; Gautier, A.; Morgant, G.; Studt, L.; Ducrot, P.H.; Le Pêcheur, P.; Azeddine, S.; Fillinger, S.; Leroux, P.; Tudzynski, B.; et al. A functional bikaverin biosynthesis gene cluster in rare strains of Botrytis cinerea is positively controlled by VELVET. PLoS ONE 2013, 8, e53729. [Google Scholar] [CrossRef]
- Schumacher, J.; Simon, A.; Cohrs, K.C.; Traeger, S.; Porquier, A.; Dalmais, B.; Viaud, M.; Tudzynski, B. The VELVET complex in the gray mold fungus Botrytis cinerea: Impact of BcLAE1 on differentiation, secondary metabolism, and virulence. Mol. Plant Microbe Interact. 2015, 28, 659–674. [Google Scholar] [CrossRef]
- Yang, Q.; Chen, Y.; Ma, Z. Involvement of BcVeA and BcVelB in regulating conidiation, pigmentation and virulence in Botrytis cinerea. Fungal Genet. Biol. 2013, 50, 63–71. [Google Scholar] [CrossRef]
- Schumacher, J.; Simon, A.; Cohrs, K.C.; Viaud, M.; Tudzynski, P. The transcription factor BcLTF1 regulates virulence and light responses in the necrotrophic plant pathogen Botrytis cinerea. PLoS Genet. 2014, 10, e1004040. [Google Scholar] [CrossRef]
- Chen, C.H.; Ringelberg, C.S.; Gross, R.H.; Dunlap, J.C.; Loros, J.J. Genome-wide analysis of light-inducible responses reveals hierarchical light signalling in Neurospora. EMBO J. 2009, 28, 1029–1042. [Google Scholar] [CrossRef]
- Han, K.H.; Han, K.Y.; Yu, J.H.; Chae, K.S.; Jahng, K.Y.; Han, D.M. The nsdD gene encodes a putative GATA-type transcription factor necessary for sexual development of Aspergillus nidulans. Mol. Microbiol. 2001, 41, 299–309. [Google Scholar] [CrossRef]
- Dean, R.; van Kan, J.A.L.; Pretorius, Z.A.; Hammond-Kosack, K.E.; Di Pietro, A.; Spanu, P.D.; Rudd, J.J.; Dickman, M.; Kahmann, R.; Ellis, J.; et al. The Top 10 fungal pathogens in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 414–430. [Google Scholar] [CrossRef]
- Veloso, J.; van Kan, J.A.L. Many shades of grey in Botrytis-host plant interactions. Trends Plant Sci. 2018, 23, 613–622. [Google Scholar] [CrossRef]
- Heller, J.; Tudzynski, P. Reactive oxygen species in phytopathogenic fungi: Signaling, development, and disease. Annu. Rev. Phytopathol. 2011, 49, 369–390. [Google Scholar] [CrossRef]
- Büttner, P.; Koch, F.; Voigt, K.; Quidde, T.; Risch, S.; Blaich, R.; Brückner, B.; Tudzynski, P. Variations in ploidy among isolates of Botrytis cinerea: Implications for genetic and molecular analyses. Curr. Genet. 1994, 25, 445–450. [Google Scholar] [CrossRef]
- Anta-Fernández, F.; Santander-Gordón, D.; Becerra, S.; Santamaría, R.; Díaz-Mínguez, J.M.; Benito, E.P. Nitric oxide metabolism affects germination in Botrytis cinerea and is connected to nitrate assimilation. J. Fungi 2022, 8, 699. [Google Scholar] [CrossRef]
- Raeder, U.; Broda, P. Rapid preparation of DNA from filamentous fungi. Lett. Appl. Microbiol. 1985, 1, 17–20. [Google Scholar] [CrossRef]
- ten Have, A.; Mulder, W.; Visser, J.; van Kan, J.A. The endopolygalacturonase gene Bcpg1 is required for full virulence of Botrytis cinerea. Mol. Plant Microbe Interact. 1998, 11, 1009–1016. [Google Scholar] [CrossRef]
- Reis, H.; Pfiffi, S.; Hahn, M. Molecular and functional characterization of a secreted lipase from Botrytis cinerea. Mol. Plant Pathol. 2005, 6, 257–267. [Google Scholar] [CrossRef]
- Leisen, T.; Bietz, F.; Werner, J.; Wegner, A.; Schaffrath, U.; Scheuring, D.; Willmund, F.; Mosbach, A.; Scalliet, G.; Hahn, M. CRISPR/Cas with ribonucleoprotein complexes and transiently selected telomere vectors allows highly efficient marker-free and multiple genome editing in Botrytis cinerea. PLoS Pathog. 2020, 16, e1008326. [Google Scholar] [CrossRef]
- Hilber, U.W.; Bodmer, M.; Smith, F.D.; Köller, W. Biolistic transformation of conidia of Botryotinia fuckeliana. Curr. Genet. 1994, 25, 124–127. [Google Scholar] [CrossRef]
| B05.10 Genome | Bc448 SNPs | Bc116 SNPs | |||
|---|---|---|---|---|---|
| Chr Size (kb) | Total Number | Exclusive | Total Number | Exclusive | |
| Chr 1 | 4109 | 23,260 | 9129 | 26,619 | 12,128 |
| Chr 2 | 3341 | 17,103 | 5335 | 19,466 | 7698 |
| Chr 3 | 3227 | 13,364 | 4379 | 15,161 | 6176 |
| Chr 4 | 2472 | 16,024 | 7069 | 18,731 | 9776 |
| Chr 5 | 2959 | 11,864 | 4260 | 12,701 | 5097 |
| Chr 6 | 2726 | 17,544 | 5529 | 19,339 | 7324 |
| Chr 7 | 2652 | 17,831 | 5715 | 18,805 | 6689 |
| Chr 8 | 2617 | 14,368 | 5583 | 15,433 | 6649 |
| Chr 9 | 2548 | 16,793 | 5866 | 18,543 | 7616 |
| Chr 10 | 2419 | 13,662 | 4484 | 15,798 | 6620 |
| Chr 11 | 2360 | 12,485 | 3928 | 15,300 | 6743 |
| Chr 12 | 2353 | 13,996 | 5298 | 14,802 | 6104 |
| Chr 13 | 2258 | 10,921 | 3949 | 13,106 | 6134 |
| Chr 14 | 2138 | 14,089 | 5718 | 15,952 | 7581 |
| Chr 15 | 2028 | 11,469 | 3246 | 14,335 | 6112 |
| Chr 16 | 1970 | 10,795 | 3556 | 13,634 | 6395 |
| Chr 17 | 247 | 107 | 74 | 54 | 51 |
| SUM | 42,424 | 235,675 | 83,118 | 267,779 | 114,893 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Casado-del Castillo, V.; Novac, V.P.M.; García, A.G.; Fernández, J.M.G.; Iriondo-Ocampo, P.; Díaz-Mínguez, J.M.; Benito, E.P. Genetic and Genomic Analysis Identifies bcltf1 as the Transcription Factor Coding Gene Mutated in Field Isolate Bc116, Deficient in Light Responses, Differentiation and Pathogenicity in Botrytis cinerea. Int. J. Mol. Sci. 2025, 26, 3481. https://doi.org/10.3390/ijms26083481
Casado-del Castillo V, Novac VPM, García AG, Fernández JMG, Iriondo-Ocampo P, Díaz-Mínguez JM, Benito EP. Genetic and Genomic Analysis Identifies bcltf1 as the Transcription Factor Coding Gene Mutated in Field Isolate Bc116, Deficient in Light Responses, Differentiation and Pathogenicity in Botrytis cinerea. International Journal of Molecular Sciences. 2025; 26(8):3481. https://doi.org/10.3390/ijms26083481
Chicago/Turabian StyleCasado-del Castillo, Virginia, Vlad Paul Mihaila Novac, Alessandro Gabrielli García, José María García Fernández, Paula Iriondo-Ocampo, José María Díaz-Mínguez, and Ernesto Pérez Benito. 2025. "Genetic and Genomic Analysis Identifies bcltf1 as the Transcription Factor Coding Gene Mutated in Field Isolate Bc116, Deficient in Light Responses, Differentiation and Pathogenicity in Botrytis cinerea" International Journal of Molecular Sciences 26, no. 8: 3481. https://doi.org/10.3390/ijms26083481
APA StyleCasado-del Castillo, V., Novac, V. P. M., García, A. G., Fernández, J. M. G., Iriondo-Ocampo, P., Díaz-Mínguez, J. M., & Benito, E. P. (2025). Genetic and Genomic Analysis Identifies bcltf1 as the Transcription Factor Coding Gene Mutated in Field Isolate Bc116, Deficient in Light Responses, Differentiation and Pathogenicity in Botrytis cinerea. International Journal of Molecular Sciences, 26(8), 3481. https://doi.org/10.3390/ijms26083481







