Genome-Wide Identification and Expression Analysis of Thionin Family in Rice (Oryza sativa) and Functional Characterization of OsTHION15 in Drought Stress and ABA Stress
Abstract
1. Introduction
2. Results
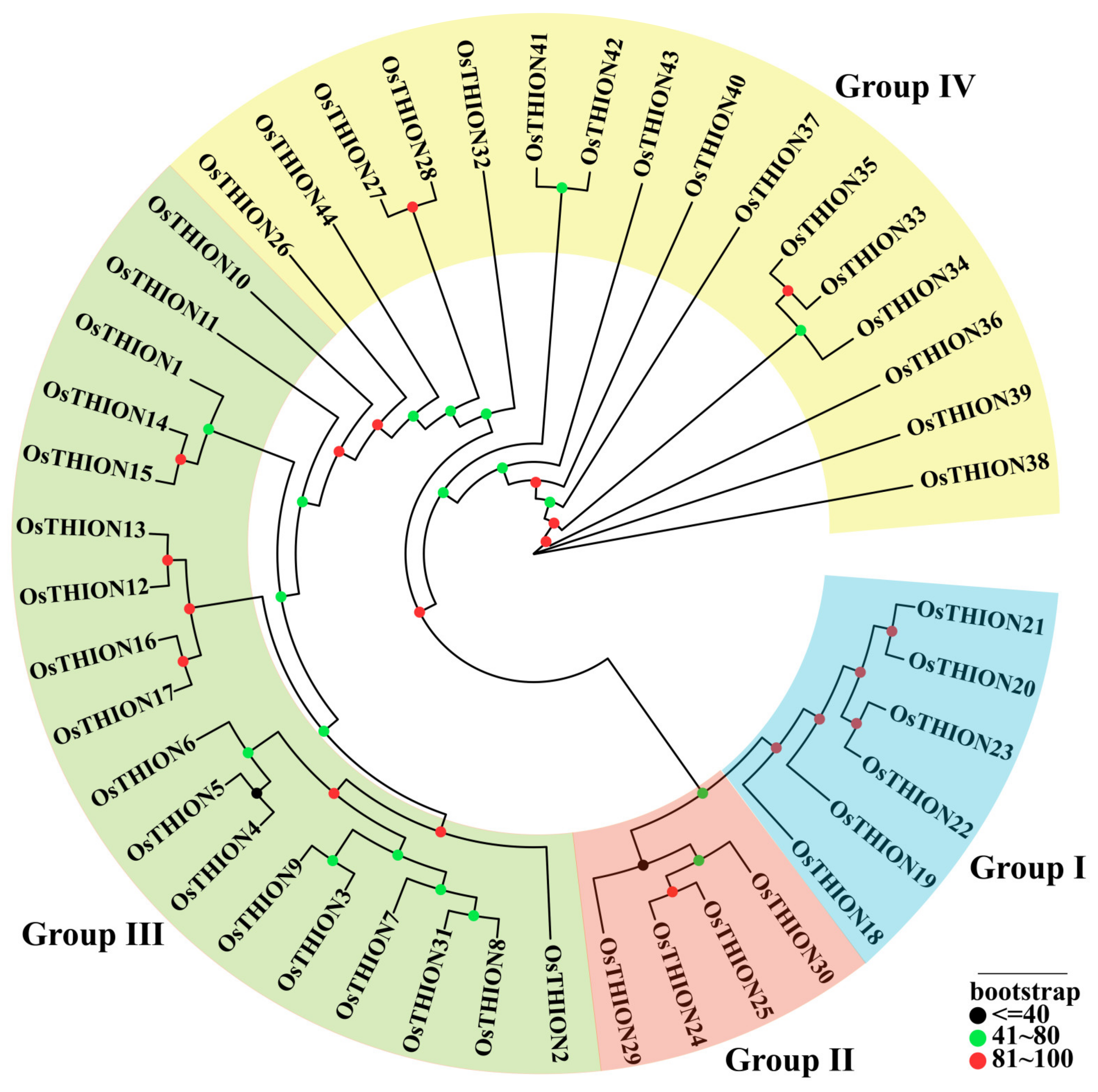
2.1. Evolutionary Analysis of OsTHION Genes
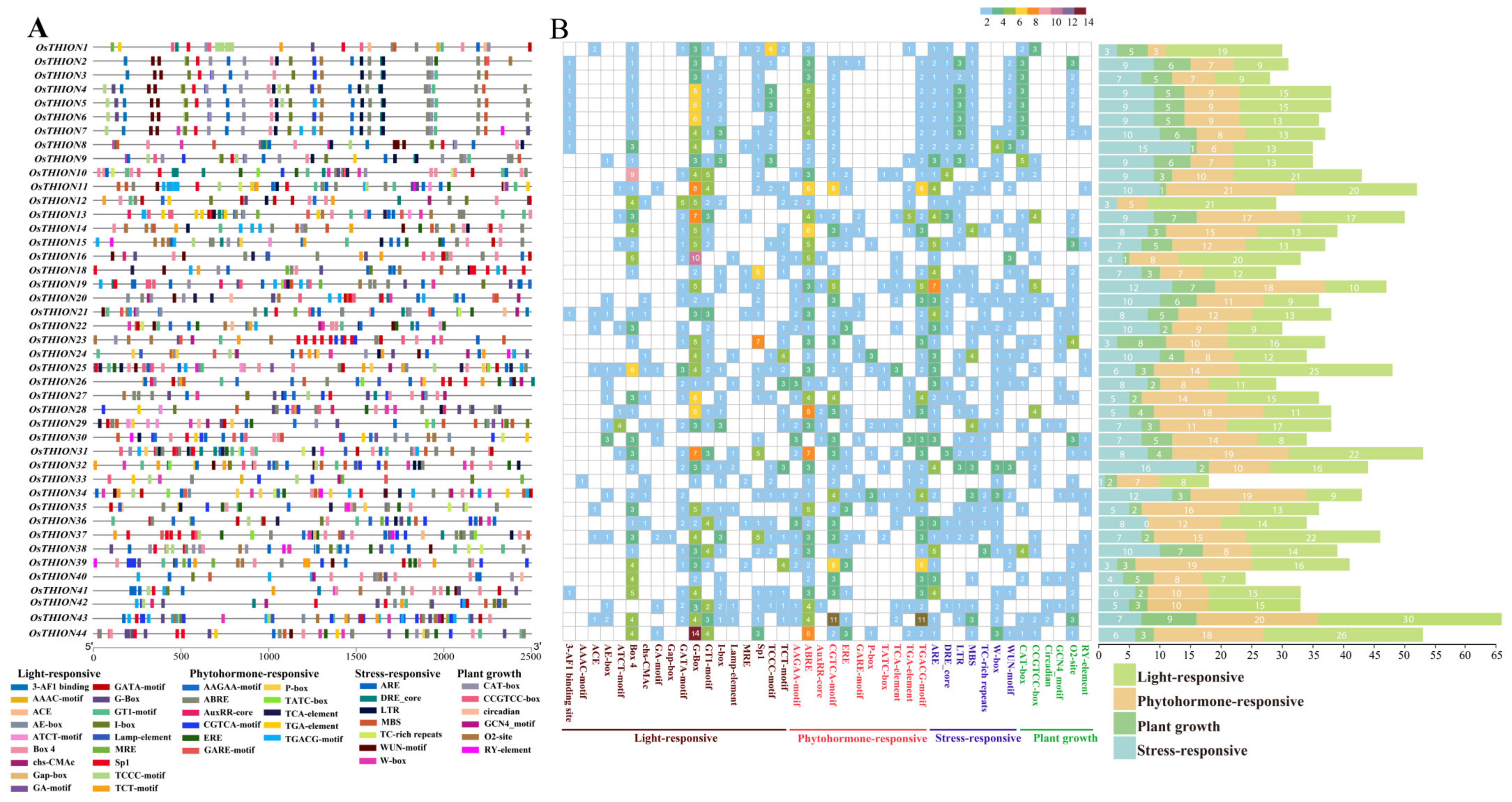
2.2. Cis-Acting Element Analysis of OsTHION Genes
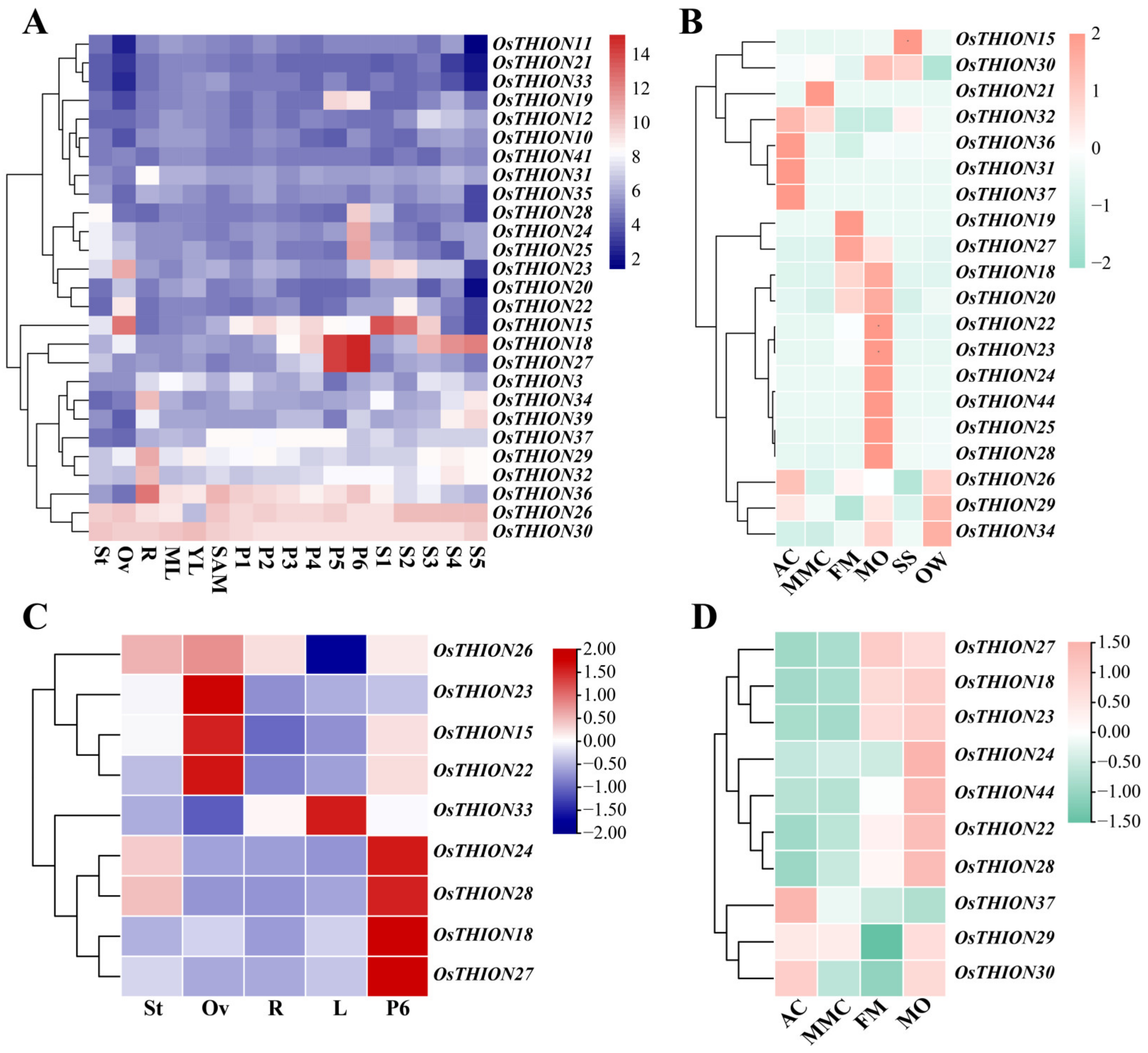
2.3. The Expression Pattern of OsTHION Family Genes in Rice Tissues
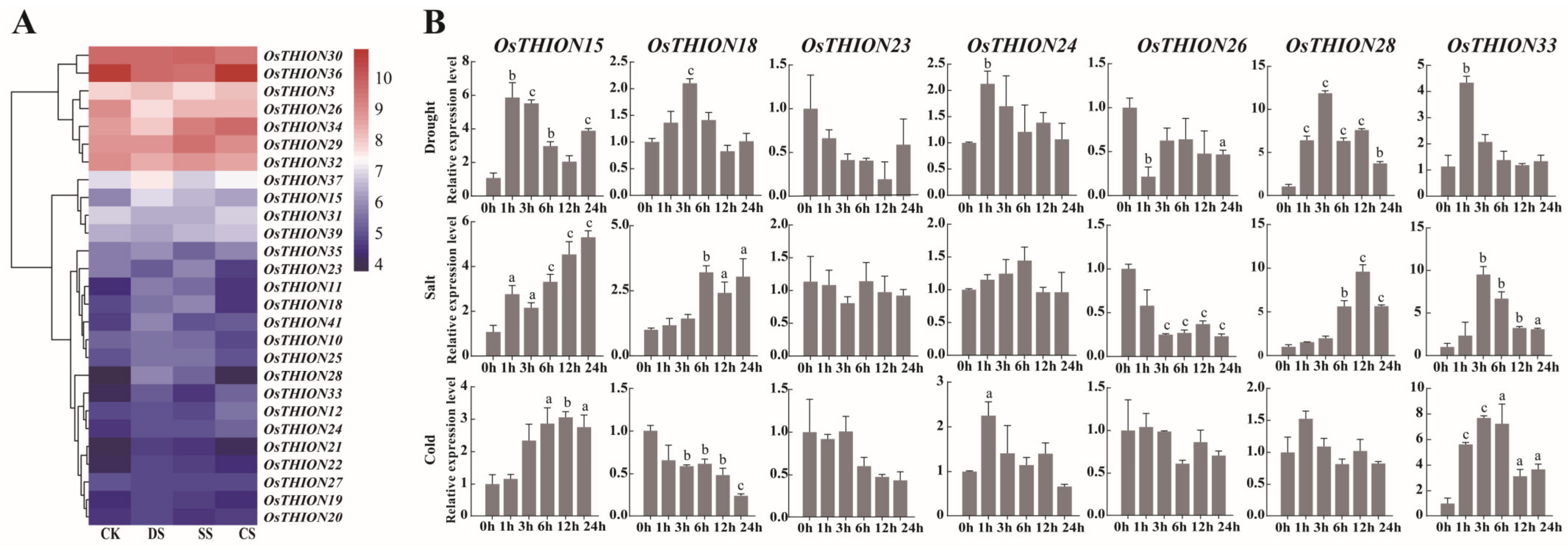
2.4. Expression Analysis of OsTHIONs Under Stress Conditions
2.5. The Expression Pattern and Subcellular Localization
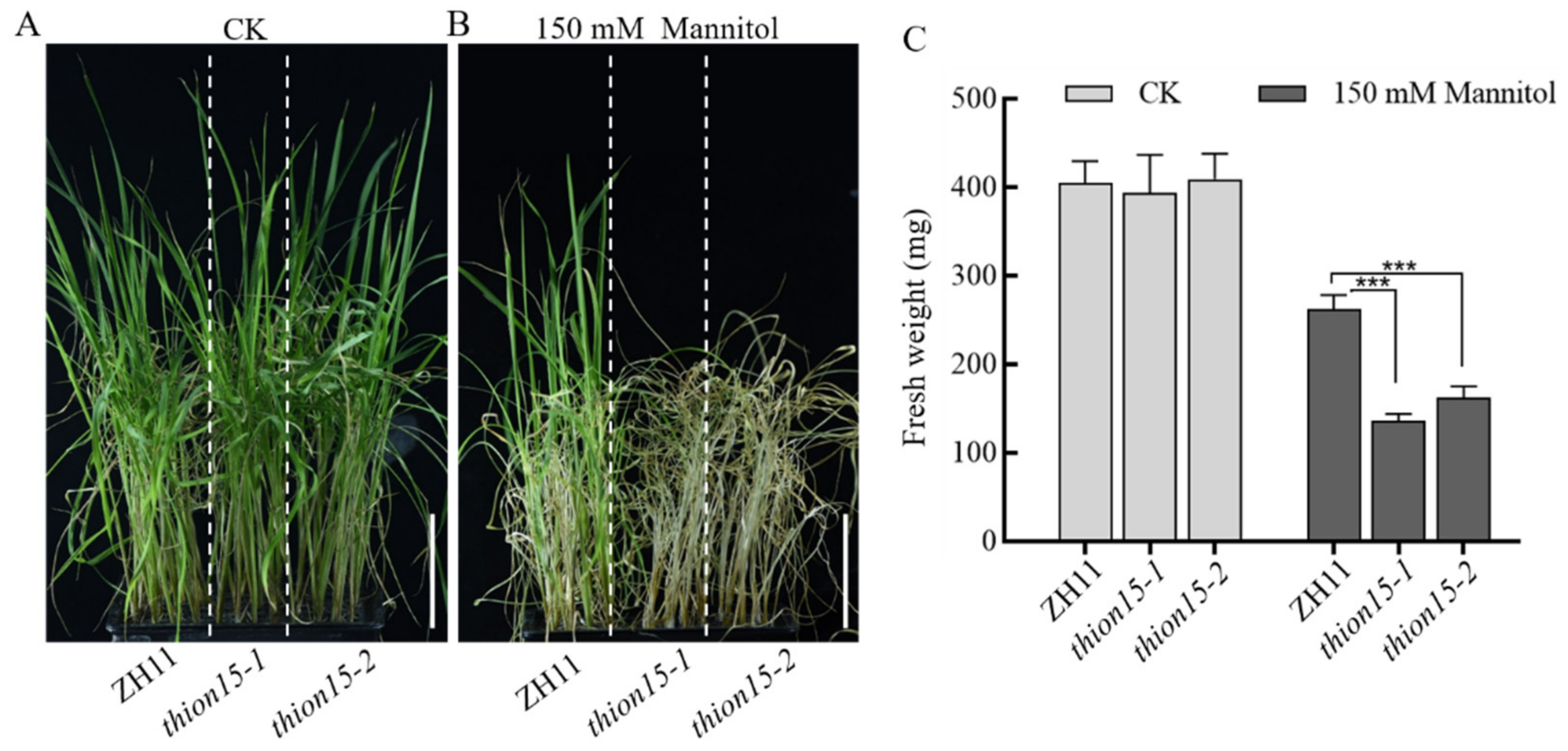
2.6. The Osthion15 Mutant Exhibits Increased Sensitivity to Drought Stress
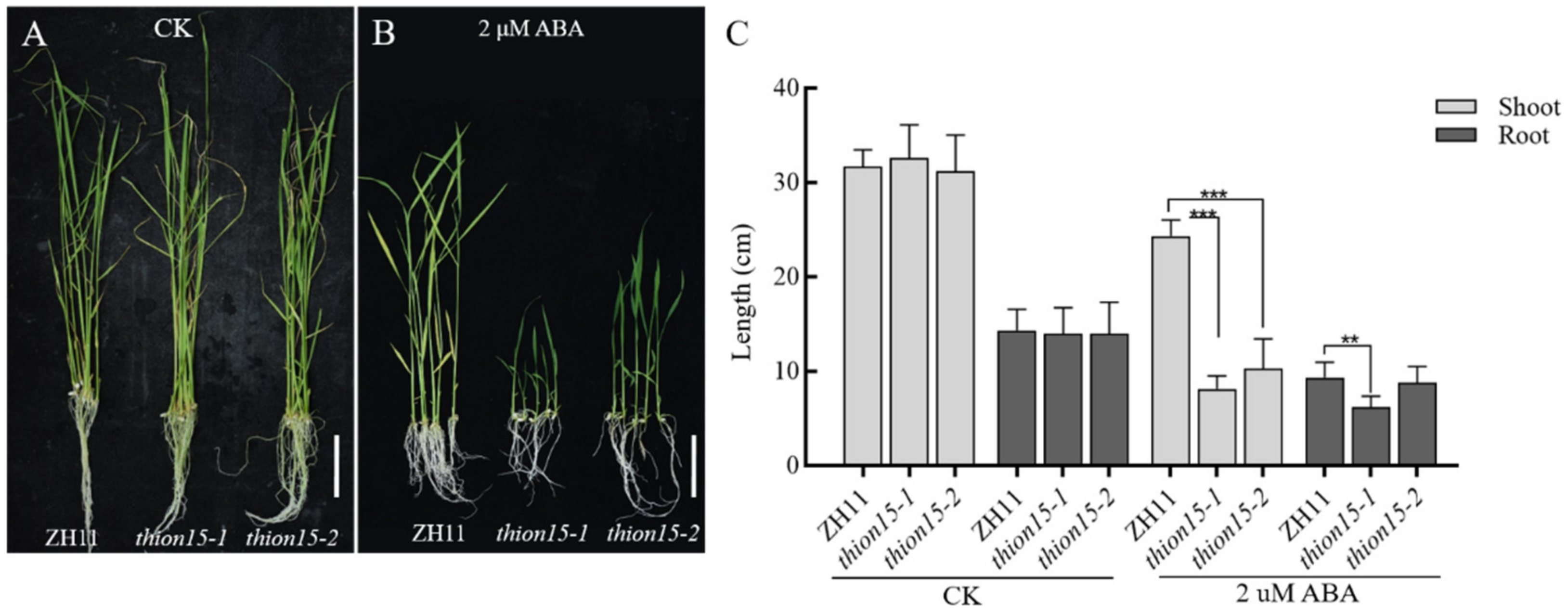
2.7. The Osthion15 Mutant Exhibits Increased Sensitivity to ABA Stress
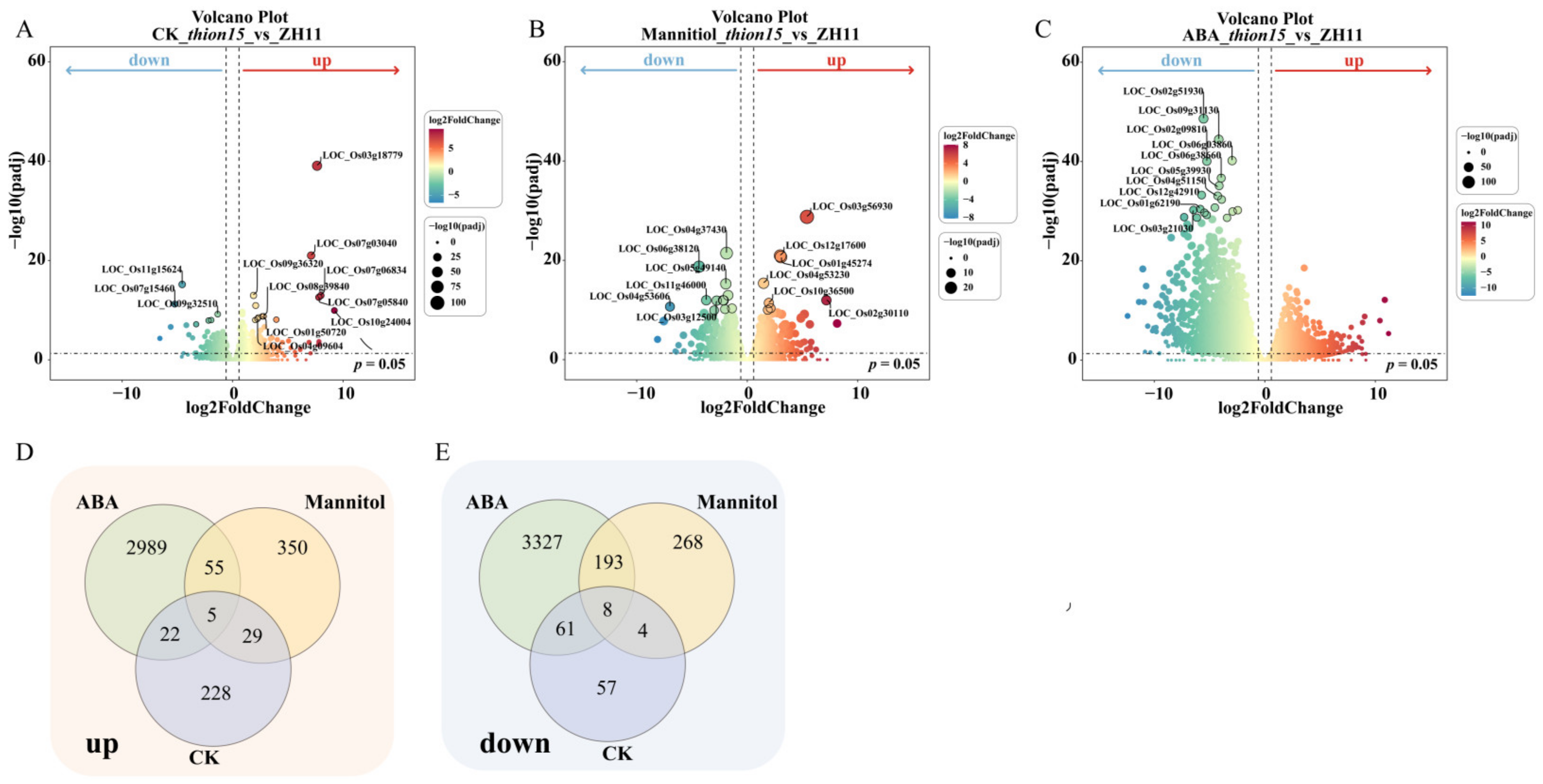
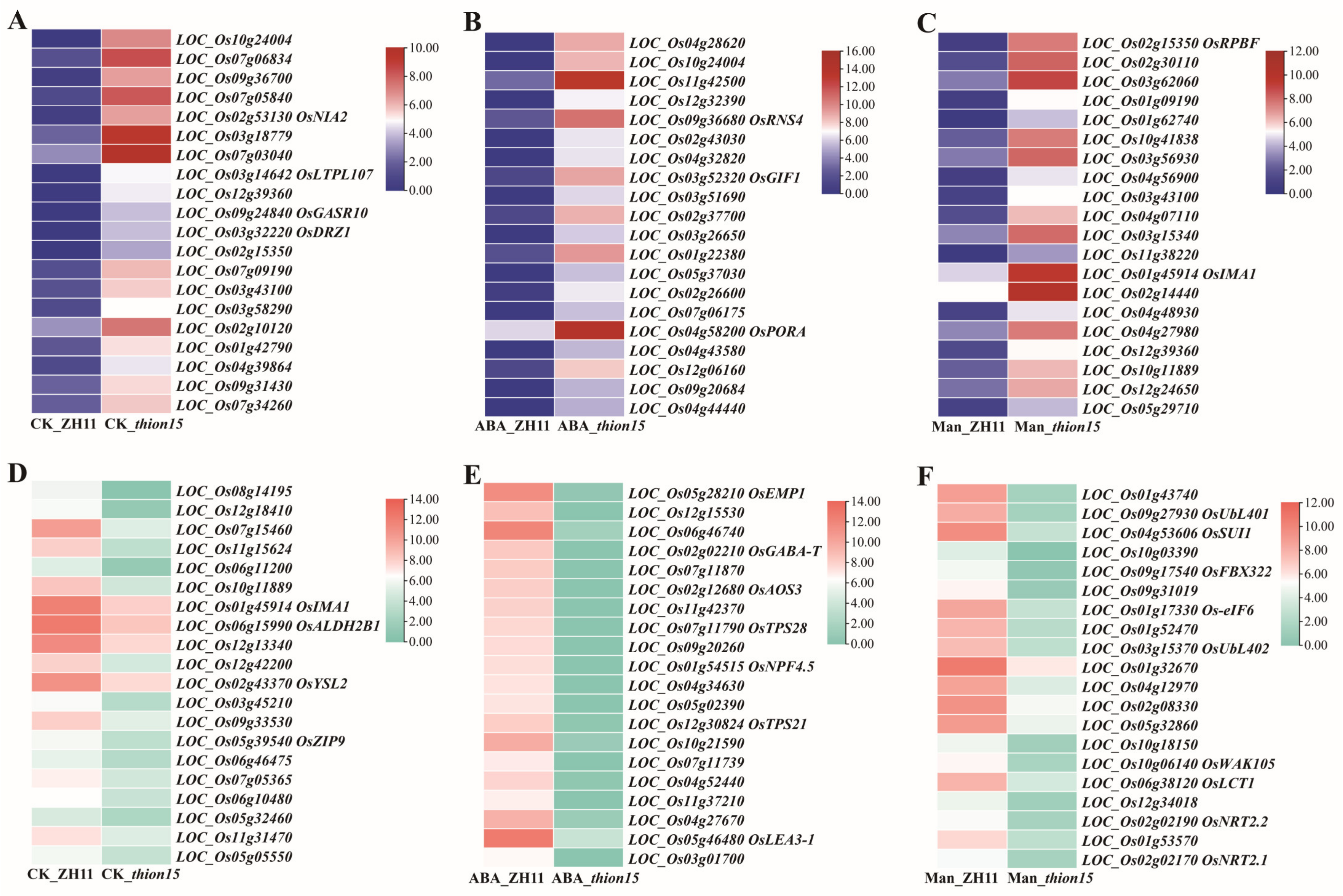
2.8. Differential Gene Expression Analysis of Osthion15 and ZH11 Under Drought and ABA Stress
2.9. Expression Profiles of Differentially Expressed Genes
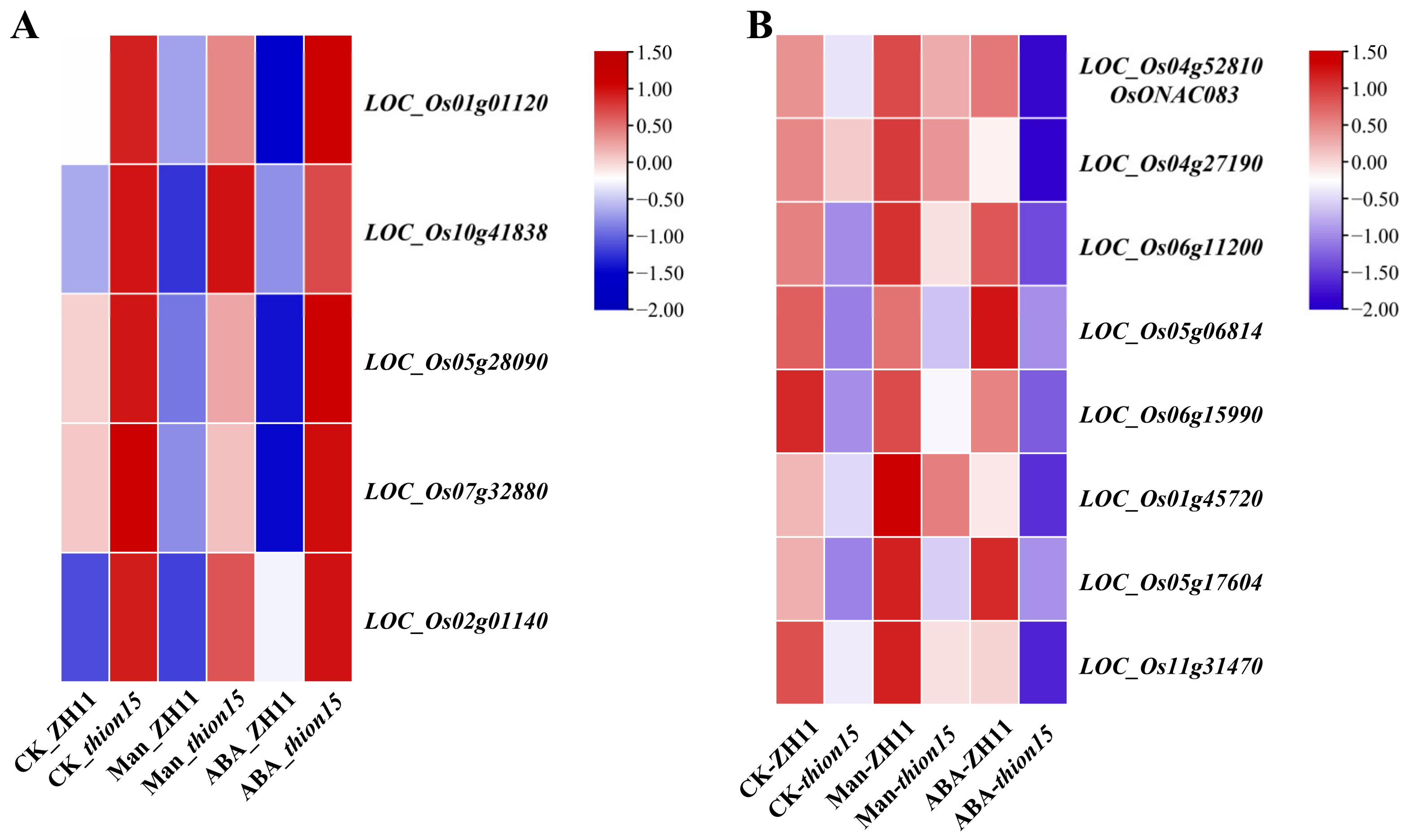
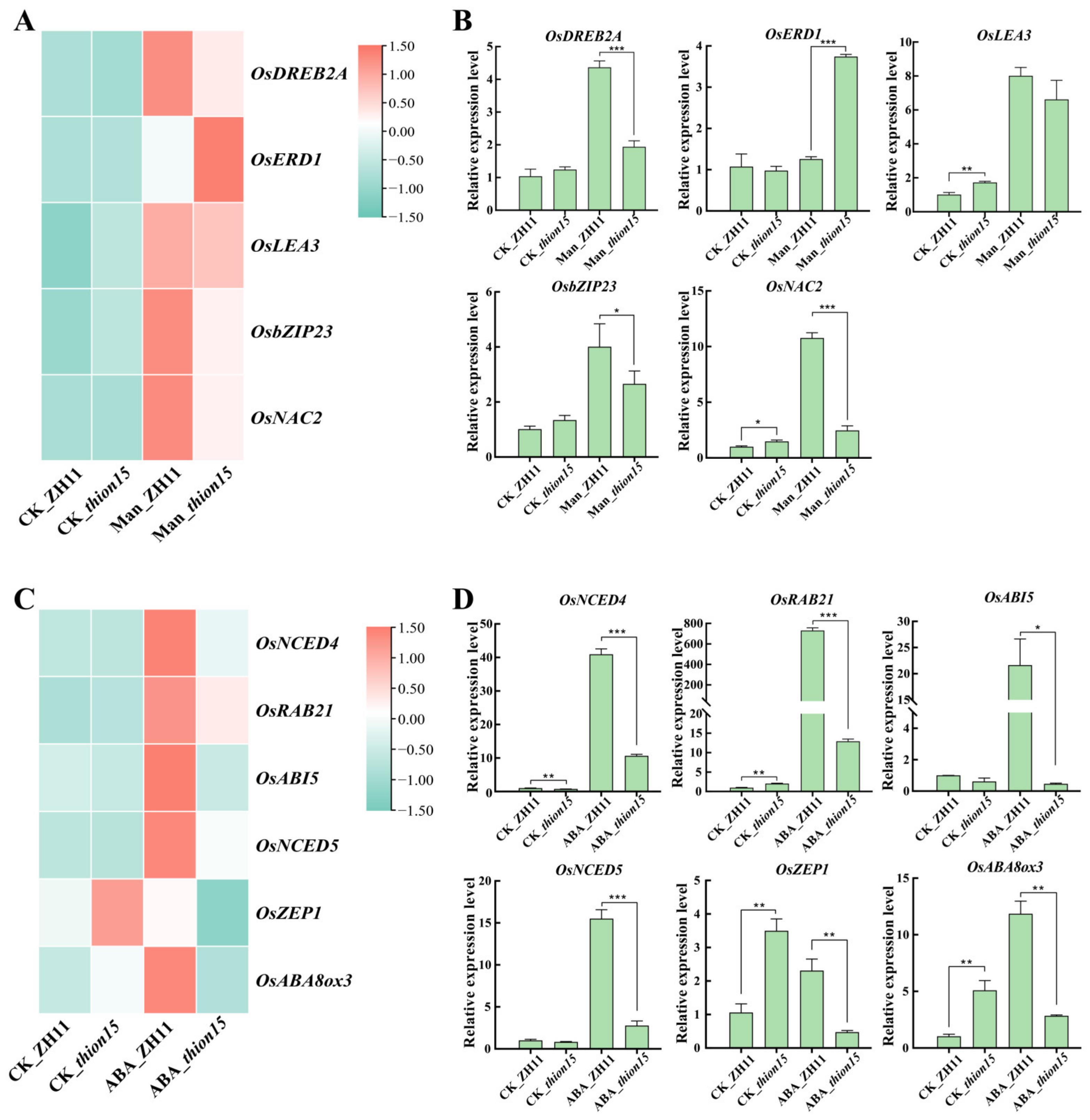
2.10. Expression Profiles of Stress-Responsive Genes
3. Discussion
4. Materials and Methods
4.1. Phylogenetic Analysis of OsTHION Gene Families
4.2. Analysis of Cis-Acting Regulatory Elements in OsTHION Gene Promoters
4.3. Expression Profiling of OsTHIONs in Different Tissues and Under Stress Conditions
4.4. Histochemical GUS Assay
4.5. Subcellular Localization of OsTHION15
4.6. Osthion15 Mutant Obtained by CRISPR/Cas 9
4.7. Stress Treatment Assays
4.8. RNA-Seq Data Analysis
4.9. RNA Extraction and Quantitative Real-Time PCR
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Singh, A.; Dilkes, B.; Sela, H.; Tzin, V. The Effectiveness of Physical and Chemical Defense Responses of Wild Emmer Wheat Against Aphids Depends on Leaf Position and Genotype. Front. Plant Sci. 2021, 12, 667820. [Google Scholar] [CrossRef]
- Gyan, N.M.; Yaakov, B.; Weinblum, N.; Singh, A.; Cna’ani, A.; Ben-Zeev, S.; Saranga, Y.; Tzin, V. Variation Between Three Eragrostis tef Accessions in Defense Responses to Rhopalosiphum padi Aphid Infestation. Front. Plant Sci. 2020, 11, 598483. [Google Scholar] [CrossRef]
- Durrant, W.E.; Dong, X. Systemic acquired resistance. Annu. Rev. Phytopathol. 2004, 42, 185–209. [Google Scholar] [CrossRef]
- Ali, S.; Ganai, B.A.; Kamili, A.N.; Bhat, A.A.; Mir, Z.A.; Bhat, J.A.; Tyagi, A.; Islam, S.T.; Mushtaq, M.; Yadav, P.; et al. Pathogenesis-related proteins and peptides as promising tools for engineering plants with multiple stress tolerance. Microbiol. Res. 2018, 212–213, 29–37. [Google Scholar] [CrossRef]
- Stec, B. Plant thionins--the structural perspective. Cell. Mol. Life Sci. 2006, 63, 1370–1385. [Google Scholar] [CrossRef]
- Schrader-Fischer, G.; Apel, K. cDNA-derived identification of novel thionin precursors in Viscum album that contain highly divergent thionin domains but conserved signal and acidic polypeptide domains. Plant Mol. Biol. 1993, 23, 1233–1242. [Google Scholar] [CrossRef]
- Samuelsson, G.; Pettersson, B. Toxic proteins from the mistletoe Dendrophtora clavata. II. The amino acid sequence of denclatoxin B. Acta Pharm. Suec. 1977, 14, 245–254. [Google Scholar]
- Pelegrini, P.B.; Franco, O.L. Plant gamma-thionins: Novel insights on the mechanism of action of a multi-functional class of defense proteins. Int. J. Biochem. Cell Biol. 2005, 37, 2239–2253. [Google Scholar] [CrossRef]
- Thevissen, K.; Terras, F.R.; Broekaert, W.F. Permeabilization of fungal membranes by plant defensins inhibits fungal growth. Appl. Environ. Microbiol. 1999, 65, 5451–5458. [Google Scholar] [CrossRef]
- Opata, M.M.; Izevbigie, E.B. Aqueous Vernomia amygdalina extracts alter MCF-7 cell membrane permeability and efflux. Int. J. Environ. Res. Public Health 2006, 3, 174–179. [Google Scholar] [CrossRef]
- Oard, S.V. Deciphering a mechanism of membrane permeabilization by α-hordothionin peptide. Biochim. Biophys. Acta 2011, 1808, 1737–1745. [Google Scholar] [CrossRef] [PubMed]
- Berrocal-Lobo, M.; Molina, A.; Rodriguez-Palenzuela, P.; Garcia-Olmedo, F.; Rivas, L. Leishmania donovani: Thionins, plant antimicrobial peptides with leishmanicidal activity. Exp. Parasitol. 2009, 122, 247–249. [Google Scholar] [CrossRef] [PubMed]
- Schmidt, M.; Arendt, E.K.; Thery, T.L.C. Isolation and characterisation of the antifungal activity of the cowpea defensin Cp-thionin II. Food Microbiol. 2019, 82, 504–514. [Google Scholar] [CrossRef] [PubMed]
- Taveira, G.B.; Mello, E.O.; Carvalho, A.O.; Regente, M.; Pinedo, M.; de La Canal, L.; Rodrigues, R.; Gomes, V.M. Antimicrobial activity and mechanism of action of a thionin-like peptide from Capsicum annuum fruits and combinatorial treatment with fluconazole against Fusarium solani. Biopolymers 2017, 108, e23008. [Google Scholar] [CrossRef]
- Iwai, T.; Kaku, H.; Honkura, R.; Nakamura, S.; Ochiai, H.; Sasaki, T.; Ohashi, Y. Enhanced resistance to seed-transmitted bacterial diseases in transgenic rice plants overproducing an oat cell-wall-bound thionin. Mol. Plant Microbe Interact. 2002, 15, 515–521. [Google Scholar] [CrossRef]
- Hao, G.; Stover, E.; Gupta, G. Overexpression of a Modified Plant Thionin Enhances Disease Resistance to Citrus Canker and Huanglongbing (HLB). Front. Plant Sci. 2016, 7, 1078. [Google Scholar] [CrossRef]
- Loeza-Angeles, H.; Sagrero-Cisneros, E.; Lara-Zárate, L.; Villagómez-Gómez, E.; López-Meza, J.E.; Ochoa-Zarzosa, A. Thionin Thi2.1 from Arabidopsis thaliana expressed in endothelial cells shows antibacterial, antifungal and cytotoxic activity. Biotechnol. Lett. 2008, 30, 1713–1719. [Google Scholar] [CrossRef]
- Epple, P.; Apel, K.; Bohlmann, H. Overexpression of an endogenous thionin enhances resistance of Arabidopsis against Fusarium oxysporum. Plant Cell 1997, 9, 509–520. [Google Scholar] [CrossRef]
- Holtorf, S.; Ludwig-Muller, J.; Apel, K.; Bohlmann, H. High-level expression of a viscotoxin in Arabidopsis thaliana gives enhanced resistance against Plasmodiophora brassicae. Plant Mol. Biol. 1998, 36, 673–680. [Google Scholar] [CrossRef]
- Oh, B.J.; Ko, M.K.; Kostenyuk, I.; Shin, B.; Kim, K.S. Coexpression of a defensin gene and a thionin-like via different signal transduction pathways in pepper and Colletotrichum gloeosporioides interactions. Plant Mol. Biol. 1999, 41, 313–319. [Google Scholar] [CrossRef]
- Escudero-Martinez, C.M.; Morris, J.A.; Hedley, P.E.; Bos, J.I.B. Barley transcriptome analyses upon interaction with different aphid species identify thionins contributing to resistance. Plant Cell Environ. 2017, 40, 2628–2643. [Google Scholar] [CrossRef]
- Leybourne, D.J.; Valentine, T.A.; Robertson, J.A.H.; Pérez-Fernández, E.; Main, A.M.; Karley, A.J.; Bos, J.I.B. Defence gene expression and phloem quality contribute to mesophyll and phloem resistance to aphids in wild barley. J. Exp. Bot. 2019, 70, 4011–4026. [Google Scholar] [CrossRef]
- Castillon, A.; Shen, H.; Huq, E. Phytochrome Interacting Factors: Central players in phytochrome-mediated light signaling networks. Trends Plant Sci. 2007, 12, 514–521. [Google Scholar] [CrossRef]
- Ji, H.; Gheysen, G.; Ullah, C.; Verbeek, R.; Shang, C.; De Vleesschauwer, D.; Höfte, M.; Kyndt, T. The role of thionins in rice defence against root pathogens. Mol. Plant Pathol. 2015, 16, 870–881. [Google Scholar] [CrossRef]
- Utriainen, M.; Kokko, H.; Auriola, S.; Sarrazin, O.; Kärenlampi, S. PR-10 protein is induced by copper stress in roots and leaves of a Cu/Zn tolerant clone of birch, Betula pendula. Plant Cell Environ. 2010, 21, 821–828. [Google Scholar]
- Wu, J.; Kim, S.G.; Kang, K.Y.; Kim, J.G.; Park, S.R.; Gupta, R.; Kim, Y.H.; Wang, Y.; Kim, S.T. Overexpression of a Pathogenesis-Related Protein 10 Enhances Biotic and Abiotic Stress Tolerance in Rice. Plant Pathol. J. 2016, 32, 552–562. [Google Scholar] [CrossRef]
- Desouky, A.F.; Ahmed, A.H.H.; Stutzel, H.; Jacobsen, H.J.; Pao, Y.C.; Hanafy, M.S. Enhanced Abiotic Stress Tolerance of Vicia faba L. Plants Heterologously Expressing the PR10a Gene from Potato. Plants 2021, 10, 173. [Google Scholar] [CrossRef]
- Seo, P.J.; Lee, A.K.; Xiang, F.; Park, C.M. Molecular and functional profiling of Arabidopsis pathogenesis-related genes: Insights into their roles in salt response of seed germination. Plant Cell Physiol. 2008, 49, 334–344. [Google Scholar] [CrossRef]
- Singh, N.K.; Kumar, K.R.; Kumar, D.; Shukla, P.; Kirti, P.B. Characterization of a pathogen induced thaumatin-like protein gene AdTLP from Arachis diogoi, a wild peanut. PLoS ONE 2013, 8, e83963. [Google Scholar] [CrossRef]
- Hong, J.K.; Hwang, B.K.J.P.P. Induction of enhanced disease resistance and oxidative stress tolerance by overexpression of pepper basic PR-1 gene in Arabidopsis. Physiol. Plant. 2005, 124, 1399–3054. [Google Scholar]
- Janska, A.A.; Marsík, P.; Zelenková, S.; Biology, J.O.J.P. Cold stress and acclimation—What is important for metabolic adjustment? Plant Biol. 2010, 12, 395–405. [Google Scholar] [PubMed]
- Archambault, A.; Strömvik, M.V. PR-10, defensin and cold dehydrin genes are among those over expressed in Oxytropis (Fabaceae) species adapted to the arctic. Funct. Integr. Genom. 2011, 11, 497–505. [Google Scholar] [CrossRef]
- Neyshabouri, F.A.; Ghotbi-Ravandi, A.A.; Shariatmadari, Z.; Tohidfar, M. Cadmium toxicity promotes hormonal imbalance and induces the expression of genes involved in systemic resistances in barley. Biometals 2024, 37, 1147–1160. [Google Scholar] [CrossRef] [PubMed]
- Boonpa, K.; Tantong, S.; Weerawanich, K.; Panpetch, P.; Pringsulaka, O.; Roytrakul, S.; Sirikantaramas, S. In Silico Analyses of Rice Thionin Genes and the Antimicrobial Activity of OsTHION15 Against Phytopathogens. Phytopathology 2019, 109, 27–35. [Google Scholar] [CrossRef]
- Toledo-Ortiz, G.; Johansson, H.; Lee, K.P.; Bou-Torrent, J.; Stewart, K.; Steel, G.; Rodríguez-Concepción, M.; Halliday, K.J. The HY5-PIF regulatory module coordinates light and temperature control of photosynthetic gene transcription. PLoS Genet. 2014, 10, e1004416. [Google Scholar] [CrossRef]
- Jain, M.; Nijhawan, A.; Arora, R.; Agarwal, P.; Ray, S.; Sharma, P.; Kapoor, S.; Tyagi, A.K.; Khurana, J.P. F-box proteins in rice. Genome-wide analysis, classification, temporal and spatial gene expression during panicle and seed development, and regulation by light and abiotic stress. Plant Physiol. 2007, 143, 1467–1483. [Google Scholar] [CrossRef]
- Zhao, H.; Guo, M.; Yan, M.; Cheng, H.; Liu, Y.; She, Z.; Lai, L.; Shi, C.; Zhang, M.; Li, Y.; et al. Comparative Expression Profiling Reveals Genes Involved in Megasporogenesis. Plant Physiol. 2020, 182, 2006–2024. [Google Scholar] [CrossRef]
- Gupta, A.; Rico-Medina, A.; Caño-Delgado, A.I. The physiology of plant responses to drought. Science 2020, 368, 266–269. [Google Scholar] [CrossRef]
- Silverstein, K.A.; Moskal, W.A., Jr.; Wu, H.C.; Underwood, B.A.; Graham, M.A.; Town, C.D.; VandenBosch, K.A. Small cysteine-rich peptides resembling antimicrobial peptides have been under-predicted in plants. Plant J. 2007, 51, 262–280. [Google Scholar] [CrossRef]
- Liu, X.; Gong, X.; Zhou, D.; Jiang, Q.; Liang, Y.; Ye, R.; Zhang, S.; Wang, Y.; Tang, X.; Li, F.; et al. Plant Defensin-Dissimilar Thionin OsThi9 Alleviates Cadmium Toxicity in Rice Plants and Reduces Cadmium Accumulation in Rice Grains. J. Agric. Food Chem. 2023, 71, 8367–8380. [Google Scholar] [CrossRef]
- Shiu, S.H.; Bleecker, A.B. Expansion of the receptor-like kinase/Pelle gene family and receptor-like proteins in Arabidopsis. Plant Physiol. 2003, 132, 530–543. [Google Scholar] [CrossRef] [PubMed]
- Yamaguchi-Shinozaki, K.; Shinozaki, K. Transcriptional regulatory networks in cellular responses and tolerance to dehydration and cold stresses. Annu. Rev. Plant Biol. 2006, 57, 781–803. [Google Scholar] [CrossRef] [PubMed]
- Jiao, Y.; Ma, L.; Strickland, E.; Deng, X.W. Conservation and divergence of light-regulated genome expression patterns during seedling development in rice and Arabidopsis. Plant Cell 2005, 17, 3239–3256. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Han, H.; Chen, M.; Yang, W.; Liu, L.; Li, N.; Ding, X.; Chu, Z. Overexpression of OsDT11, which encodes a novel cysteine-rich peptide, enhances drought tolerance and increases ABA concentration in rice. Plant Mol. Biol. 2017, 93, 21–34. [Google Scholar] [CrossRef]
- Meiyalaghan, S.; Thomson, S.J.; Fiers, M.W.; Barrell, P.J.; Latimer, J.M.; Mohan, S.; Jones, E.E.; Conner, A.J.; Jacobs, J.M. Structure and expression of GSL1 and GSL2 genes encoding gibberellin stimulated-like proteins in diploid and highly heterozygous tetraploid potato reveals their highly conserved and essential status. BMC Genom. 2014, 15, 2. [Google Scholar] [CrossRef]
- Sah, S.K.; Reddy, K.R.; Li, J. Abscisic Acid and Abiotic Stress Tolerance in Crop Plants. Front. Plant Sci. 2016, 7, 571. [Google Scholar] [CrossRef]
- Zhang, Q.; Liu, Y.; Jiang, Y.; Li, A.; Cheng, B.; Wu, J. OsASR6 Enhances Salt Stress Tolerance in Rice. Int. J. Mol. Sci. 2022, 23, 9340. [Google Scholar] [CrossRef]
- Hu, H.; You, J.; Fang, Y.; Zhu, X.; Qi, Z.; Xiong, L. Characterization of transcription factor gene SNAC2 conferring cold and salt tolerance in rice. Plant Mol. Biol. 2008, 67, 169–181. [Google Scholar] [CrossRef]
- Nakashima, K.; Tran, L.S.; Van Nguyen, D.; Fujita, M.; Maruyama, K.; Todaka, D.; Ito, Y.; Hayashi, N.; Shinozaki, K.; Yamaguchi-Shinozaki, K. Functional analysis of a NAC-type transcription factor OsNAC6 involved in abiotic and biotic stress-responsive gene expression in rice. Plant J. 2007, 51, 617–630. [Google Scholar] [CrossRef]
- Li, Y.; Han, S.; Sun, X.; Khan, N.U.; Zhong, Q.; Zhang, Z.; Zhang, H.; Ming, F.; Li, Z.; Li, J. Variations in OsSPL10 confer drought tolerance by directly regulating OsNAC2 expression and ROS production in rice. J. Integr. Plant Biol. 2023, 65, 918–933. [Google Scholar] [CrossRef]
- Chang, Y.; Liu, J.; Guo, M.; Ouyang, W.; Yan, J.; Xiong, L.; Li, X. Drought-responsive dynamics of H3K9ac-marked 3D chromatin interactions are integrated by OsbZIP23-associated super-enhancer-like promoter regions in rice. Genome Biol. 2024, 25, 262. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.X.; Tang, Y.J.; Ma, Q.B.; Yang, C.Y.; Mu, Y.H.; Suo, H.C.; Luo, L.H.; Nian, H. OsDREB2A, a rice transcription factor, significantly affects salt tolerance in transgenic soybean. PLoS ONE 2013, 8, e83011. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.G.; Lee, C.Y.; Tseng, C.S. Heterologous expression of rice 9-cis-epoxycarotenoid dioxygenase 4 (OsNCED4) in Arabidopsis confers sugar oversensitivity and drought tolerance. Bot. Stud. 2018, 59, 2. [Google Scholar] [CrossRef]
- Liu, Y.H.; Jiang, M.; Li, R.Q.; Huang, J.Z.; Shu, Q.Y. OsKEAP1 Interacts with OsABI5 and Its Downregulation Increases the Transcription of OsABI5 and the ABA Response Genes in Germinating Rice Seeds. Plants 2021, 10, 527. [Google Scholar] [CrossRef] [PubMed]
- Giolai, M.; Laine, A.L. A trade-off between investment in molecular defense repertoires and growth in plants. Science 2024, 386, 677–680. [Google Scholar] [CrossRef]
- Long, W.; He, Q.; Wang, Y.; Wang, Y.; Wang, J.; Yuan, Z.; Wang, M.; Chen, W.; Luo, L.; Luo, L.; et al. Genome evolution and diversity of wild and cultivated rice species. Nat. Commun. 2024, 15, 9994. [Google Scholar] [CrossRef]
- Goodstein, D.M.; Shu, S.; Howson, R.; Neupane, R.; Hayes, R.D.; Fazo, J.; Mitros, T.; Dirks, W.; Hellsten, U.; Putnam, N.; et al. Phytozome: A comparative platform for green plant genomics. Nucleic Acids Res. 2012, 40, D1178–D1186. [Google Scholar] [CrossRef]
- Ouyang, S.; Zhu, W.; Hamilton, J.; Lin, H.; Campbell, M.; Childs, K.; Thibaud-Nissen, F.; Malek, R.L.; Lee, Y.; Zheng, L.; et al. The TIGR Rice Genome Annotation Resource: Improvements and new features. Nucleic Acids Res. 2007, 35, D883–D887. [Google Scholar] [CrossRef]
- Liu, Y.; Aslam, M.; Yao, L.A.; Zhang, M.; Wang, L.; Chen, H.; Huang, Y.; Qin, Y.; Niu, X. Genomic analysis of SBP gene family in Saccharum spontaneum reveals their association with vegetative and reproductive development. BMC Genom. 2021, 22, 767. [Google Scholar] [CrossRef]
- Lescot, M.; Dehais, P.; Thijs, G.; Marchal, K.; Moreau, Y.; Van de Peer, Y.; Rouze, P.; Rombauts, S. PlantCARE, a database of plant cis-acting regulatory elements and a portal to tools for in silico analysis of promoter sequences. Nucleic Acids Res. 2002, 30, 325–327. [Google Scholar] [CrossRef]
- Chen, C.; Wu, Y.; Li, J.; Wang, X.; Zeng, Z.; Xu, J.; Liu, Y.; Feng, J.; Chen, H.; He, Y.; et al. TBtools-II: A “one for all, all for one” bioinformatics platform for biological big-data mining. Mol. Plant 2023, 16, 1733–1742. [Google Scholar] [CrossRef] [PubMed]
- Zeng, P.; Xie, T.; Shen, J.; Liang, T.; Yin, L.; Liu, K.; He, Y.; Chen, M.; Tang, H.; Chen, S.; et al. Potassium transporter OsHAK9 regulates seed germination under salt stress by preventing gibberellin degradation through mediating OsGA2ox7 in rice. J. Integr. Plant Biol. 2024, 66, 731–748. [Google Scholar] [CrossRef] [PubMed]
- Shen, Y.; Shen, L.; Shen, Z.; Jing, W.; Ge, H.; Zhao, J.; Zhang, W. The potassium transporter OsHAK21 functions in the maintenance of ion homeostasis and tolerance to salt stress in rice. Plant Cell Environ. 2015, 38, 2766–2779. [Google Scholar] [CrossRef] [PubMed]
- Wang, Q.; Guan, Y.; Wu, Y.; Chen, H.; Chen, F.; Chu, C. Overexpression of a rice OsDREB1F gene increases salt, drought, and low temperature tolerance in both Arabidopsis and rice. Plant Mol. Biol. 2008, 67, 589–602. [Google Scholar] [CrossRef]
- Ge, L.F.; Chao, D.Y.; Shi, M.; Zhu, M.Z.; Gao, J.P.; Lin, H.X. Overexpression of the trehalose-6-phosphate phosphatase gene OsTPP1 confers stress tolerance in rice and results in the activation of stress responsive genes. Planta 2008, 228, 191–201. [Google Scholar] [CrossRef]
- Do, V.G.; Kim, S.; Win, N.M.; Kwon, S.I.; Kweon, H.; Yang, S.; Park, J.; Do, G.; Lee, Y. Efficient Regeneration of Transgenic Rice from Embryogenic Callus via Agrobacterium-Mediated Transformation: A Case Study Using GFP and Apple MdFT1 Genes. Plants 2024, 13, 19. [Google Scholar] [CrossRef]
- Chen, S.; Zhou, Y.; Chen, Y.; Gu, J. fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, i884–i890. [Google Scholar] [CrossRef]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Kim, D.; Paggi, J.M.; Park, C.; Bennett, C.; Salzberg, S.L. Graph-based genome alignment and genotyping with HISAT2 and HISAT-genotype. Nat. Biotechnol. 2019, 37, 907–915. [Google Scholar] [CrossRef]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. clusterProfiler: An R package for comparing biological themes among gene clusters. Omics 2012, 16, 284–287. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yan, M.; Chai, M.; An, C.; Jiang, X.; Yang, F.; Fang, X.; Liu, T.; Ju, Y.; Tang, B.; Cai, H.; et al. Genome-Wide Identification and Expression Analysis of Thionin Family in Rice (Oryza sativa) and Functional Characterization of OsTHION15 in Drought Stress and ABA Stress. Int. J. Mol. Sci. 2025, 26, 3447. https://doi.org/10.3390/ijms26073447
Yan M, Chai M, An C, Jiang X, Yang F, Fang X, Liu T, Ju Y, Tang B, Cai H, et al. Genome-Wide Identification and Expression Analysis of Thionin Family in Rice (Oryza sativa) and Functional Characterization of OsTHION15 in Drought Stress and ABA Stress. International Journal of Molecular Sciences. 2025; 26(7):3447. https://doi.org/10.3390/ijms26073447
Chicago/Turabian StyleYan, Maokai, Mengnan Chai, Chang An, Xiaohu Jiang, Fan Yang, Xunlian Fang, Tingyu Liu, Yunfei Ju, Boping Tang, Hanyang Cai, and et al. 2025. "Genome-Wide Identification and Expression Analysis of Thionin Family in Rice (Oryza sativa) and Functional Characterization of OsTHION15 in Drought Stress and ABA Stress" International Journal of Molecular Sciences 26, no. 7: 3447. https://doi.org/10.3390/ijms26073447
APA StyleYan, M., Chai, M., An, C., Jiang, X., Yang, F., Fang, X., Liu, T., Ju, Y., Tang, B., Cai, H., & Qin, Y. (2025). Genome-Wide Identification and Expression Analysis of Thionin Family in Rice (Oryza sativa) and Functional Characterization of OsTHION15 in Drought Stress and ABA Stress. International Journal of Molecular Sciences, 26(7), 3447. https://doi.org/10.3390/ijms26073447







