Recent Advances in Ex Situ Surface Treatments for Lithium Metal Negative Electrodes in Secondary Batteries
Abstract
1. Introduction
2. Ex Situ Surface Engineering Strategies for Lithium Metal Negative Electrodes
2.1. SEI Formation and Pretreatment Techniques
2.1.1. Metallic and Inorganic Coatings for SEI Stabilization
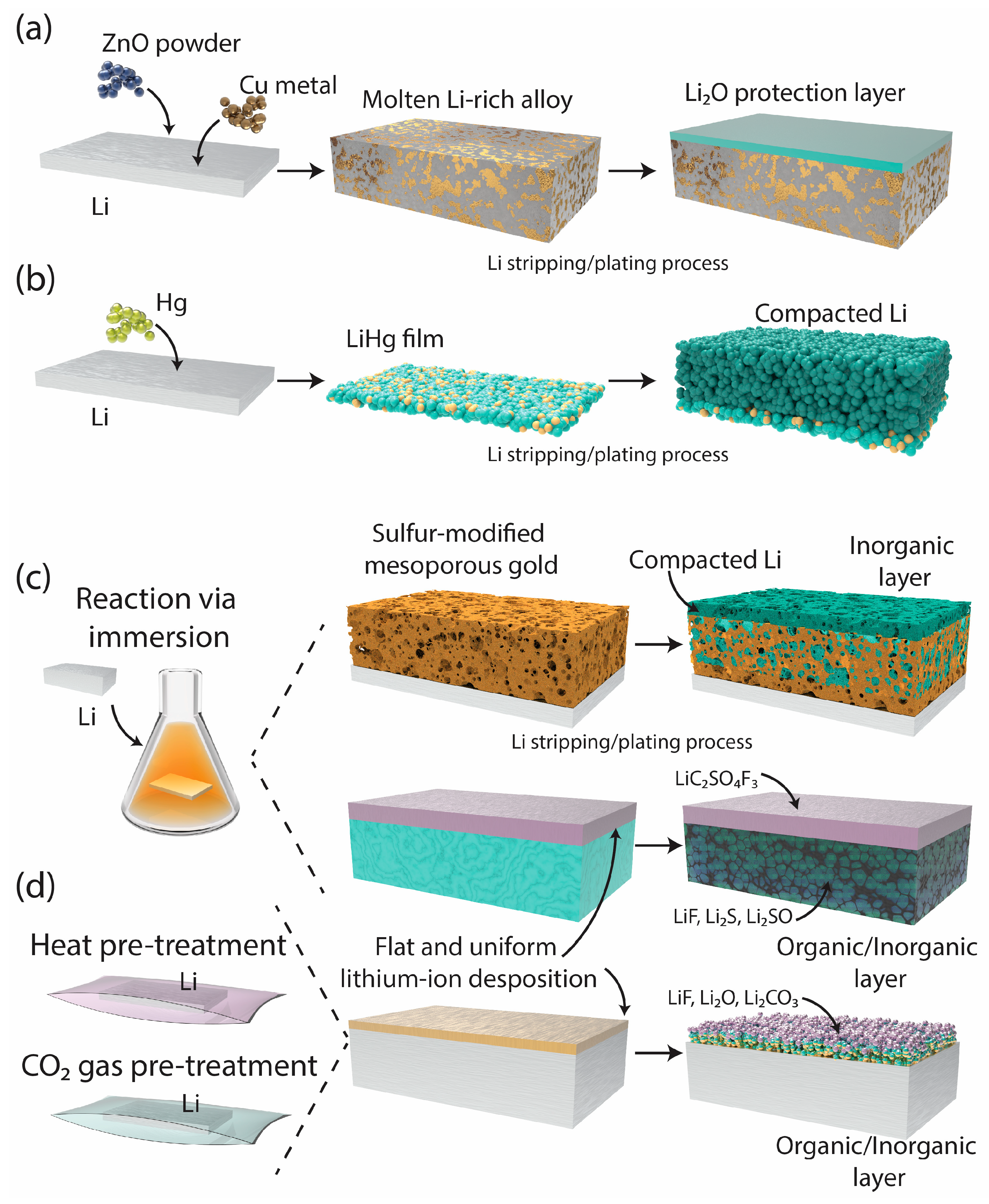
2.1.2. Polymer-Based Protective Layers

2.2. Direct Material Manipulation Methods for Surface Modification
2.2.1. Three-Dimensional Host Structures
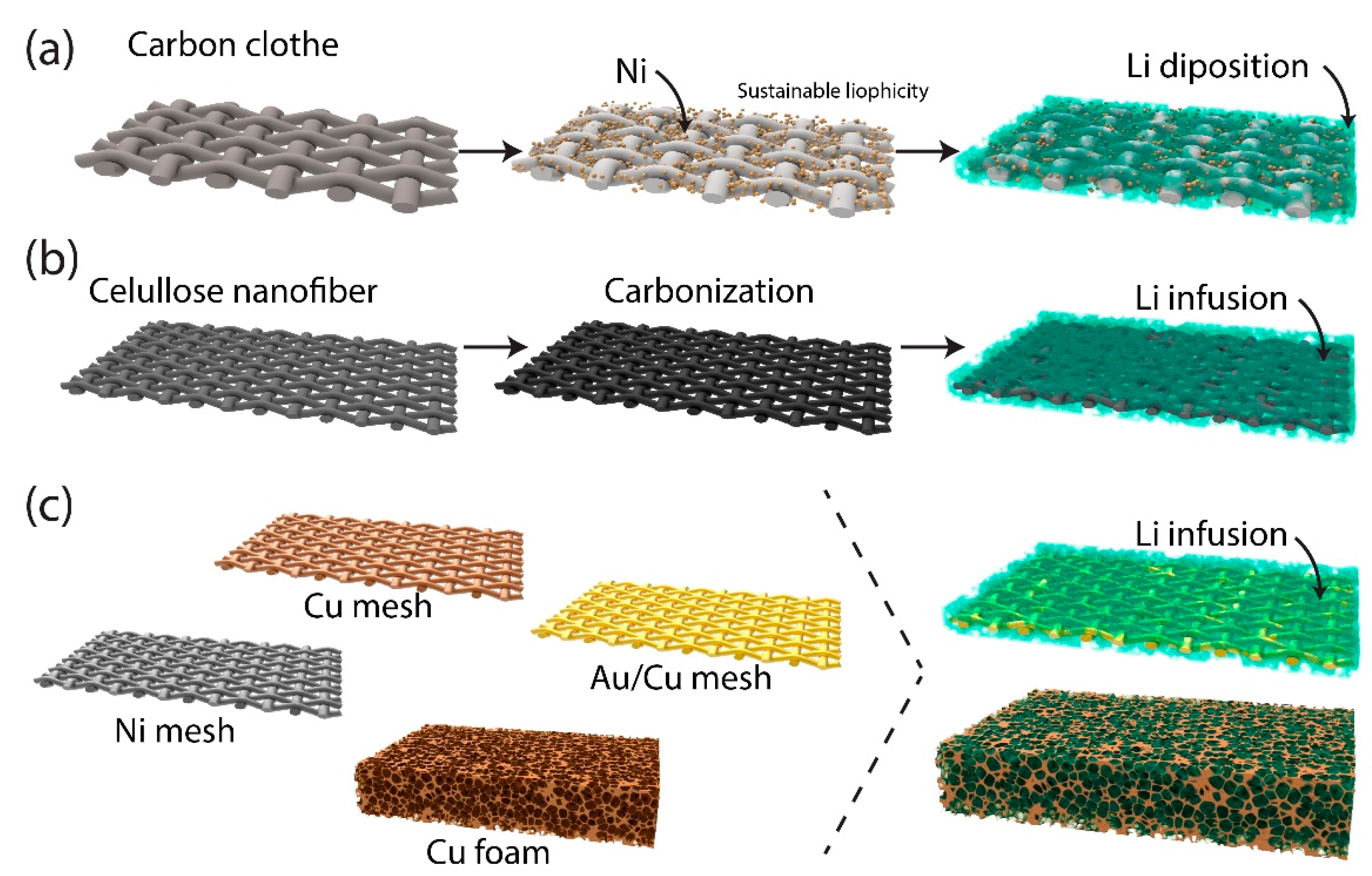
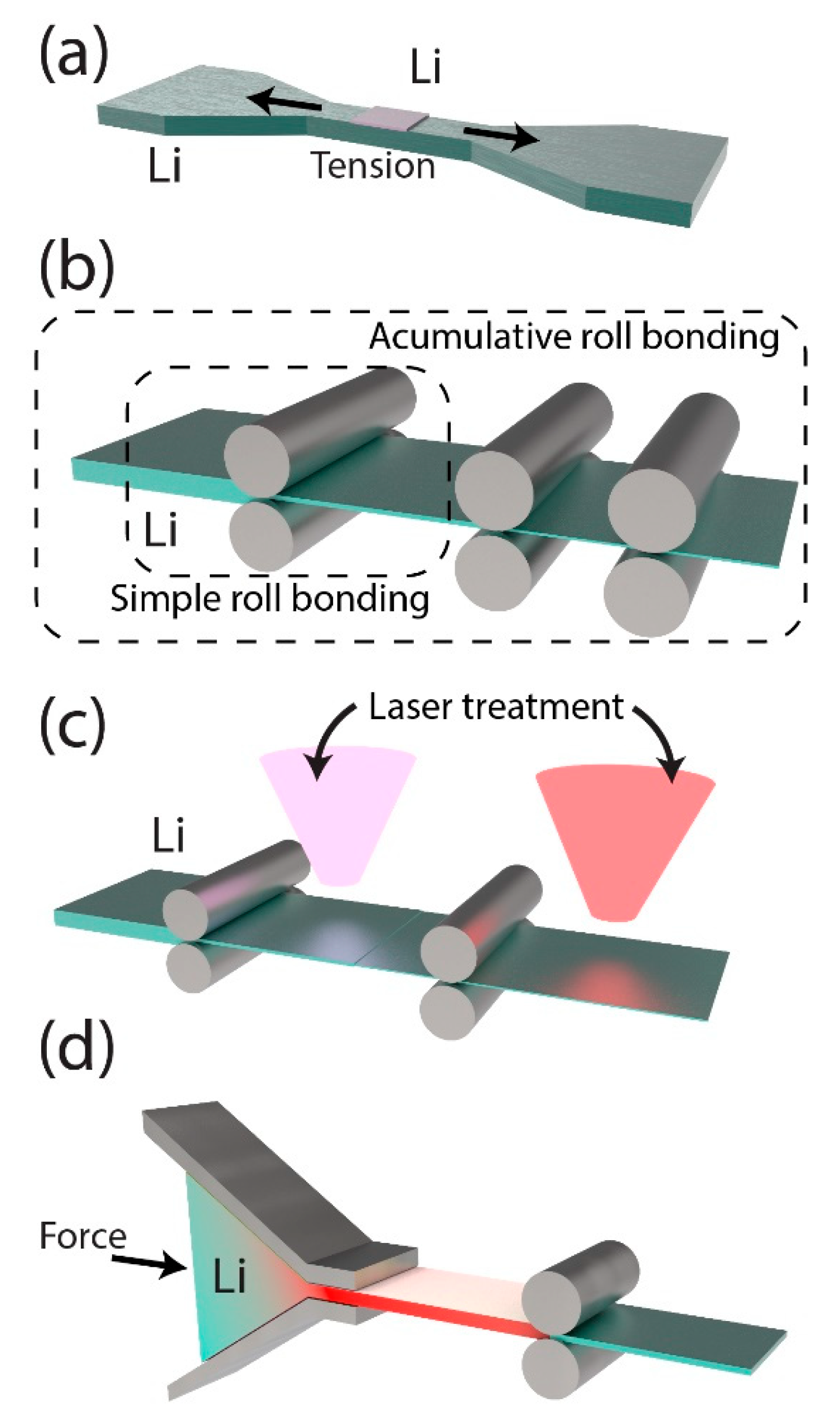
2.2.2. Mechanical Surface Modification Techniques
2.3. Chemical and Electrochemical Surface Modification
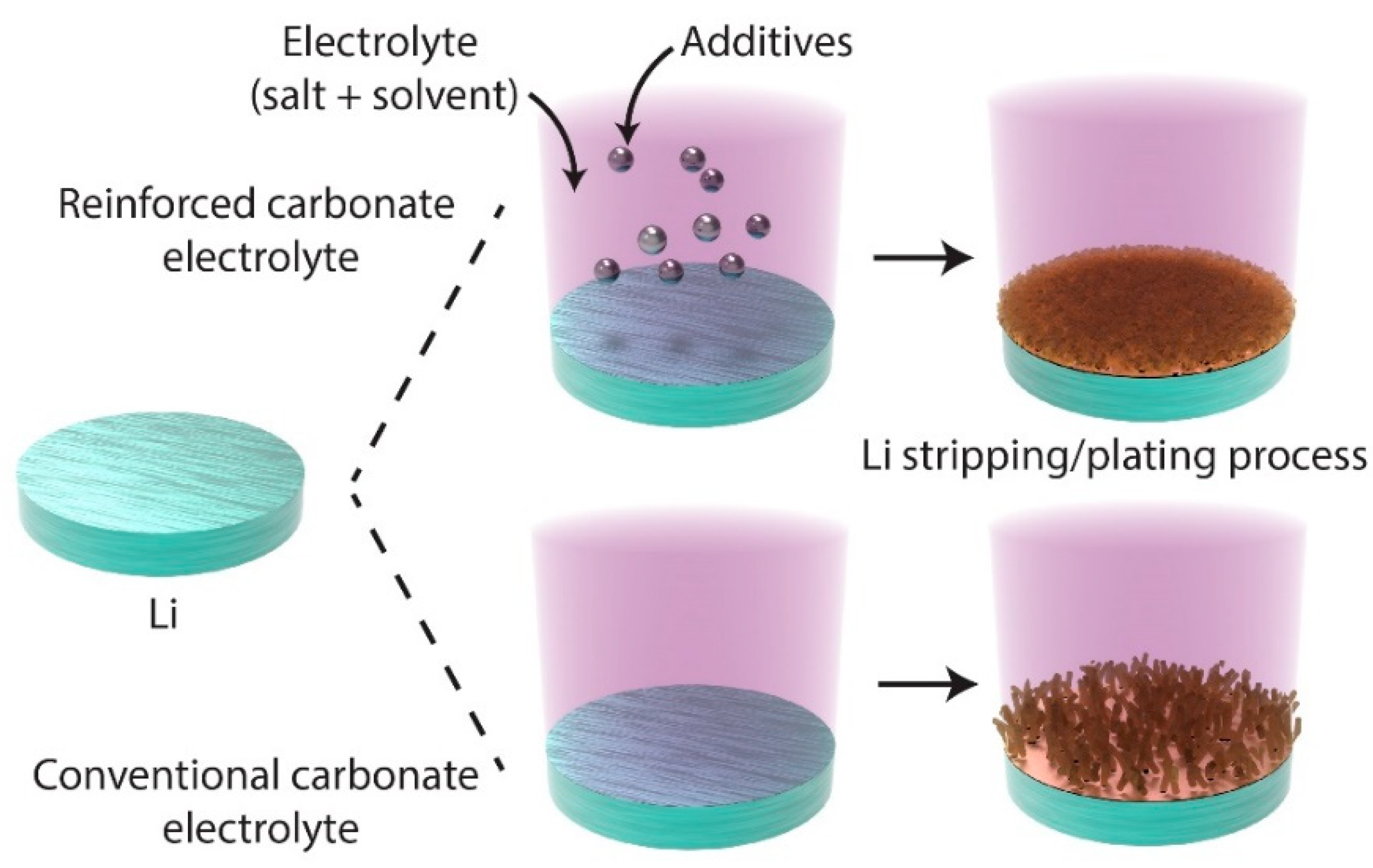
2.3.1. Liquid-Phase Chemical Reactions for SEI Formation
2.3.2. Solvent Engineering for SEI Optimization
3. Conclusions and Perspectives
3.1. Conclusions
3.2. Critical Perspectives
3.3. Future Directions
- Material Innovation: Developing cost-effective, abundant, and environmentally friendly materials is critical for advancing lithium-based technologies. Exploring alternatives such as aluminum- and silicon-based hosts and bio-derived polymers such as cellulose and chitosan can significantly reduce costs and mitigate environmental impacts. For example, bio-derived polymers exhibit compatibility with scalable processes and improved recyclability, making them promising candidates for future applications. Material innovation should also focus on enhancing compatibility with scalable manufacturing processes and improving recyclability, thereby enabling a more circular economy for battery materials.
- Scalable Manufacturing Processes: Building upon material innovation, scalable manufacturing processes will play a pivotal role in enabling commercial-scale production. Advances in fabrication technologies, including additive manufacturing and roll-to-roll processing, enable the large-scale production of complex structures and coatings. Techniques such as 3D host structures or polymeric protective layers may benefit from scalable processes such as roll-to-roll or continuous coating technologies for industrial production. Emphasis should be placed on reducing process costs and improving the throughput of high-precision techniques to meet commercial demands.
- Computational Modeling and Simulation: Leveraging computational tools to predict SEI formation mechanisms and optimize material properties can accelerate the design of effective surface treatments. Integrating machine learning with computational modeling may further enhance the efficiency and accuracy of experimental efforts and reduce development timelines. Computational models can also help design hybrid systems of surface treatments (such as polymer-based and metallic coatings) for optimal performance, thereby minimizing the trial-and-error phase in experimental setups. By combining these tools with experimental validation, researchers can establish a robust framework for designing next-generation surface treatments.
- Standardization and Long-Term Testing: Establishing standardized testing protocols is essential for enabling consistent comparisons across different technologies and providing insights into practical applications. Long-term cycling tests under realistic operating conditions, such as variable temperatures and current densities, are necessary to evaluate durability and ensure reliable performance. Adopting standardized protocols for capacity retention and Coulombic efficiency measurements, as shown in the comparative table, is important for reliably assessing the viability of various surface treatments across diverse lithium metal electrode configurations.
- Environmental Sustainability: Future research should incorporate comprehensive lifecycle assessments to evaluate and minimize the environmental footprint of battery production. For example, strategies using CO2-pretreated lithium or environmentally friendly polymer layers can significantly reduce the ecological impact. This approach not only reduces CO2 emissions but also enhances the reactivity and deposition uniformity of lithium. Such innovations will be essential for improving performance and sustainability, aligning with global sustainability goals such as net-zero carbon targets.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Xu, W.; Wang, J.; Ding, F.; Chen, X.; Nasybulin, E.; Zhang, Y.; Zhang, J.G. Lithium Metal Anodes for Rechargeable Batteries. Energy Environ. Sci. 2014, 7, 513–537. [Google Scholar] [CrossRef]
- Cheng, X.-B.; Zhang, R.; Zhao, C.-Z.; Zhang, Q. Toward Safe Lithium Metal Anode in Rechargeable Batteries: A Review. Chem. Rev. 2017, 117, 10403–10473. [Google Scholar] [CrossRef] [PubMed]
- Wu, H.; Wu, Q.; Chu, F.; Hu, J.; Cui, Y.; Yin, C.; Li, C. Sericin Protein as a Conformal Protective Layer to Enable Air-Endurable Li Metal Anodes and High-Rate Li-S Batteries. J. Power Sources 2019, 419, 72–81. [Google Scholar] [CrossRef]
- Li, C.; Wei, J.; Li, P.; Tang, W.; Feng, W.; Liu, J.; Wang, Y.; Xia, Y. A Dendrite-Free Li Plating Host towards High Utilization of Li Metal Anode in Li–O2 Battery. Sci. Bull. 2019, 64, 478–484. [Google Scholar] [CrossRef]
- Zu, C.; Azimi, N.; Zhang, Z.; Manthiram, A. Insight into Lithium–Metal Anodes in Lithium–Sulfur Batteries with a Fluorinated Ether Electrolyte. J. Mater. Chem. A 2015, 3, 14864–14870. [Google Scholar] [CrossRef]
- Li, T.; Zhang, X.Q.; Shi, P.; Zhang, Q. Fluorinated Solid-Electrolyte Interphase in High-Voltage Lithium Metal Batteries. Joule 2019, 3, 2647–2661. [Google Scholar] [CrossRef]
- Jagger, B.; Pasta, M. Solid Electrolyte Interphases in Lithium Metal Batteries. Joule 2023, 7, 2228–2244. [Google Scholar] [CrossRef]
- Liu, W.; Liu, P.; Mitlin, D. Review of Emerging Concepts in SEI Analysis and Artificial SEI Membranes for Lithium, Sodium, and Potassium Metal Battery Anodes. Adv. Energy Mater. 2020, 10, 2002297. [Google Scholar] [CrossRef]
- Ye, L.; Li, X. A Dynamic Stability Design Strategy for Lithium Metal Solid State Batteries. Nature 2021, 593, 218–222. [Google Scholar] [CrossRef]
- Cheng, X.B.; Zhao, C.Z.; Yao, Y.X.; Liu, H.; Zhang, Q. Recent Advances in Energy Chemistry between Solid-State Electrolyte and Safe Lithium-Metal Anodes. Chem 2019, 5, 74–96. [Google Scholar] [CrossRef]
- Chen, S.; Zheng, J.; Mei, D.; Han, K.S.; Engelhard, M.H.; Zhao, W.; Xu, W.; Liu, J.; Zhang, J.-G.; Chen, S.; et al. High-Voltage Lithium-Metal Batteries Enabled by Localized High-Concentration Electrolytes. Adv. Mater. 2018, 30, 1706102. [Google Scholar] [CrossRef]
- Yu, Z.; Cui, Y.; Bao, Z. Design Principles of Artificial Solid Electrolyte Interphases for Lithium-Metal Anodes. Cell. Rep. Phys. Sci. 2020, 1, 100119. [Google Scholar] [CrossRef]
- Li, N.-W.; Yin, Y.-X.; Yang, C.-P.; Guo, Y.-G. An Artificial Solid Electrolyte Interphase Layer for Stable Lithium Metal Anodes. Adv. Mater. 2016, 28, 1853–1858. [Google Scholar] [CrossRef] [PubMed]
- Zhang, R.; Li, N.W.; Cheng, X.B.; Yin, Y.X.; Zhang, Q.; Guo, Y.G. Advanced Micro/Nanostructures for Lithium Metal Anodes. Adv. Sci. 2017, 4, 1600445. [Google Scholar] [CrossRef]
- Chen, Y.; Ke, X.; Cheng, Y.; Fan, M.; Wu, W.; Huang, X.; Liang, Y.; Zhong, Y.; Ao, Z.; Lai, Y.; et al. Boosting the Electrochemical Performance of 3D Composite Lithium Metal Anodes through Synergistic Structure and Interface Engineering. Energy Storage Mater. 2020, 26, 56–64. [Google Scholar] [CrossRef]
- Tan, L.; Sun, Y.; Wei, C.; Tao, Y.; Tian, Y.; An, Y.; Zhang, Y.; Xiong, S.; Feng, J.; Tan, L.; et al. Design of Robust, Lithiophilic, and Flexible Inorganic-Polymer Protective Layer by Separator Engineering Enables Dendrite-Free Lithium Metal Batteries with LiNi0.8Mn0.1Co0.1O2 Cathode. Small 2021, 17, 2007717. [Google Scholar] [CrossRef]
- Shin, W.-K.; Kannan, A.G.; Kim, D.-W. Effective Suppression of Dendritic Lithium Growth Using an Ultrathin Coating of Nitrogen and Sulfur Codoped Graphene Nanosheets on Polymer Separator for Lithium Metal Batteries. ACS Appl. Mater. Interfaces 2015, 7, 23700–23707. [Google Scholar] [CrossRef]
- Xiang, J.; Yuan, L.; Shen, Y.; Cheng, Z.; Yuan, K.; Guo, Z.; Zhang, Y.; Chen, X.; Huang, Y.; Xiang, J.; et al. Improved Rechargeability of Lithium Metal Anode via Controlling Lithium-Ion Flux. Adv. Energy Mater. 2018, 8, 1802352. [Google Scholar] [CrossRef]
- Yi, L.; Wang, Z.; Chen, X.; Xing, J.; Huang, H.; Wei, C.; Zhao, Q.; Zhou, A.; Li, J. An Electron-Insulating Li2O Protection Layer Endowing a Li–Cu–Zn Ternary Alloy Composite Anode with High Performance. Chem. Commun. 2024, 60, 5832–5835. [Google Scholar] [CrossRef]
- Li, Q.; Liu, Y.; Zhang, Z.; Chen, J.; Yang, Z.; Deng, Q.; Mumyatov, A.V.; Troshin, P.A.; He, G.; Hu, N. Construction of Dynamic Alloy Interfaces for Uniform Li Deposition in Li-Metal Batteries. Energy Environ. Mater. 2024, 7, e12618. [Google Scholar] [CrossRef]
- Na, Z.; Li, W.; Wang, X.; Liu, D.; Sun, J.; Lang, M.; Liu, W.; Huang, G. Mesoporous Gold Film with Surface Sulfur Modification to Enable Dendrite-Free Lithium Plating/Stripping for Long-Life Lithium Metal Anodes. Small Methods 2023, 7, 2201218. [Google Scholar] [CrossRef] [PubMed]
- Pang, M.; Jiang, Z.; Luo, C.; Yao, Z.; Fu, T.; Pan, T.; Guo, Q.; Li, Y.; Xiong, S.; Zheng, C.; et al. A Surface Chemistry-Regulated Gradient Multi-Component Solid Electrolyte Interphase for a 460 W h Kg−1 Lithium Metal Pouch Cell. Energy Environ. Sci. 2024, 17, 7699–7711. [Google Scholar] [CrossRef]
- Wang, T.; Liu, X.; Huang, S.; Lu, J.; Li, J.; Ge, S.; Wang, C. Development of Polymer-Based Artificial Solid Electrolyte Interphase for Safer Li-Metal Batteries: Challenges, Strategies and Prospects. Nano Energy 2024, 129, 109970. [Google Scholar] [CrossRef]
- Nogales, P.M.; Lee, S.; Yang, S.; Jeong, S.K. Understanding the Impact of Li2CO3 Distribution within Solid Electrolyte Interphases on Lithium Metal via Thermal Conditioning. Electrochim. Acta 2024, 503, 144834. [Google Scholar] [CrossRef]
- Kim, Y.; Maldonado Nogales, P.; Lee, C.; Jeong, S.K. Enhancing Electrochemical Characteristics of Li-Metal Electrodes: The Impact of Pre-Treatment via CO2 Gas Reaction. ChemElectroChem 2024, 11, e202400211. [Google Scholar] [CrossRef]
- Schmitz, R.; Müller, R.; Krüger, S.; Schmitz, R.W.; Nowak, S.; Passerini, S.; Winter, M.; Schreiner, C. Investigation of Lithium Carbide Contamination in Battery Grade Lithium Metal. J. Power Sources 2012, 217, 98–101. [Google Scholar] [CrossRef]
- Golozar, M.; Hovington, P.; Paolella, A.; Bessette, S.; Lagacé, M.; Bouchard, P.; Demers, H.; Gauvin, R.; Zaghib, K. In Situ Scanning Electron Microscopy Detection of Carbide Nature of Dendrites in Li-Polymer Batteries. Nano Lett. 2018, 18, 7583–7589. [Google Scholar] [CrossRef]
- Lin, X.; Shen, Y.; Yu, Y.; Huang, Y. In Situ NMR Verification for Stacking Pressure-Induced Lithium Deposition and Dead Lithium in Anode-Free Lithium Metal Batteries. Adv. Energy Mater. 2024, 14, 2303918. [Google Scholar] [CrossRef]
- Ahmed, R.A.; Koirala, K.P.; Zhao, Q.; Kim, J.-M.; Anderson, C.; Wang, C.; Zhang, J.-G.; Xu, W. Surface-Treated Composite Polymer as a Stable Artificial Solid Electrolyte Interphase Layer for Lithium Metal Anode. ACS Appl. Energy Mater. 2024, 7, 12084–12091. [Google Scholar] [CrossRef]
- Chen, K.; Guo, X.; Chen, M. Controlled Radical Copolymerization toward Well-Defined Fluoropolymers. Angew. Chem.-Int. Ed. 2023, 135, e202310636. [Google Scholar] [CrossRef]
- Liu, Z.; Zhang, Q.; Song, X.; Shi, Y.; Zhu, X.; Liu, X.; Zhou, Y.; Chen, Z.; Feng, Y.; Chen, S.; et al. Construction of Inorganic/Polymer Tandem Layer on Li Metal with Long-Term Stability by LiNO3 Concentration Gradient Electrolyte. Small 2024, 20, 2312150. [Google Scholar] [CrossRef]
- Cao, S.; Ning, J.; He, X.; Wang, T.; Xu, C.; Chen, M.; Wang, K.; Zhou, M.; Jiang, K. In Situ Plasma Polymerization of Self-Stabilized Polythiophene Enables Dendrite-Free Lithium Metal Anodes with Ultra-Long Cycle Life. Small 2024, 20, 2311204. [Google Scholar] [CrossRef]
- Naren, T.; Kuang, G.C.; Jiang, R.; Qing, P.; Yang, H.; Lin, J.; Chen, Y.; Wei, W.; Ji, X.; Chen, L. Reactive Polymer as Artificial Solid Electrolyte Interface for Stable Lithium Metal Batteries. Angew. Chem.-Int. Ed. 2023, 62, e202305287. [Google Scholar] [CrossRef] [PubMed]
- Ma, C.; Zou, S.; Wu, Y.; Yue, K.; Cai, X.; Wang, Y.; Nai, J.; Guo, T.; Tao, X.; Liu, Y. A Triply-Periodic-Minimal-Surface Structured Interphase Based on Fluorinated Polymers Strengthening High-Energy Lithium Metal Batteries. Angew. Chem.-Int. Ed. 2024, 136, e202402910. [Google Scholar] [CrossRef]
- Song, H.; Lee, J.; Sagong, M.; Jeon, J.; Han, Y.; Kim, J.; Jung, H.G.; Yu, J.S.; Lee, J.; Kim, I.D. Overcoming Chemical and Mechanical Instabilities in Lithium Metal Anodes with Sustainable and Eco-Friendly Artificial SEI Layer. Adv. Mater. 2024, 36, 2407381. [Google Scholar] [CrossRef]
- Kwon, N.A.; Lee, J. Won Surface Modification of Lithium Metal Anode with Lithium Silicate-Lithium Phosphate Composite Layer for Enhanced Cycling Stability. Mater. Chem. Phys. 2023, 307, 128177. [Google Scholar] [CrossRef]
- Golozar, M.; Paolella, A.; Demers, H.; Bessette, S.; Lagacé, M.; Bouchard, P.; Guerfi, A.; Gauvin, R.; Zaghib, K. In Situ Observation of Solid Electrolyte Interphase Evolution in a Lithium Metal Battery. Commun. Chem. 2019, 2, 131. [Google Scholar] [CrossRef]
- Lang, S.; Colletta, M.; Krumov, M.R.; Seok, J.; Kourkoutis, L.F.; Wen, R.; Abruña, H.D. Multidimensional Visualization of the Dynamic Evolution of Li Metal via In Situ/Operando Methods. Proc. Natl. Acad. Sci. USA 2023, 120, e2220419120. [Google Scholar] [CrossRef]
- Zhang, X.S.; Wan, J.; Shen, Z.Z.; Lang, S.Y.; Xin, S.; Wen, R.; Guo, Y.G.; Wan, L.J. In Situ Analysis of Interfacial Morphological and Chemical Evolution in All-Solid-State Lithium-Metal Batteries. Angew. Chem. Int. Ed. 2024, 63, e202409435. [Google Scholar] [CrossRef]
- Huang, L.; Li, W.; Liang, L.; Zeng, B.; Lin, X.; Xie, Y.; Du, L.; Song, H.; Lu, Y.; Cui, Z. Anti-Perovskite Nitrides as Chemically Stable Lithiophilic Materials for Highly Reversible Li Plating/Stripping. Energy Storage Mater. 2024, 72, 103745. [Google Scholar] [CrossRef]
- Xiong, G.; Yu, J.; Xing, Y.; Yang, P.; Zhang, S. Intrinsic Lithiophilic Carbon Host Derived from Bacterial Cellulose Nanofiber for Dendrite-Free and Long-Life Lithium Metal Anode. Nano Res. 2024, 17, 4203–4210. [Google Scholar] [CrossRef]
- Kim, E.; Choi, W.; Ryu, S.; Yun, Y.; Jo, S.; Yoo, J. Effect of 3D Lithiophilic Current Collector for Anode-Free Li Ion Batteries. J. Alloys Compd. 2023, 966, 171393. [Google Scholar] [CrossRef]
- Zheng, H.; Cheng, X.; Zheng, Q.; Zhang, J.; Li, T.; Xie, E.; Xu, Y. The Thermodynamically Directed Dendrite-Free Lithium Metal Batteries on LiZn Alloy Surface. Nano Res. 2023, 16, 8354–8359. [Google Scholar] [CrossRef]
- Zeng, S.-Y.; Wang, W.-L.; Li, D.; Yang, C.; Zheng, Z.-J. Stable Ultrathin Lithium Metal Anode Enabled by Self-Adapting Electrochemical Regulating Strategy. Energy Mater. 2024, 4, 400029. [Google Scholar] [CrossRef]
- Hui, Y.; Wu, Y.; Sun, W.; Sun, X.; Huang, G.; Na, Z.; Hui, Y.; Wu, Y.; Sun, W.; Sun, X.; et al. Nanosecond Pulsed Laser-Assisted Deposition to Construct a 3D Quasi-Gradient Lithiophilic Skeleton for Stable Lithium Metal Anodes. Adv. Funct. Mater. 2023, 33, 2303319. [Google Scholar] [CrossRef]
- Li, C.; Yang, C.; Huang, T.; Wang, Y.; Yang, J.; Jiang, Y.; Mao, J.; Zheng, S.; Xia, S.; Li, C.; et al. In Situ Interphasial Engineering Enabling High-Rate and Long-Cycling Li Metal Batteries. Adv. Funct. Mater. 2024, 34, 2407149. [Google Scholar] [CrossRef]
- Bae, J.; Oh, S.; Lee, B.; Lee, C.H.; Chung, J.; Kim, J.; Jo, S.; Seo, S.; Lim, J.; Chung, S. High-Performance, Printable Quasi-Solid-State Electrolytes toward All 3D Direct Ink Writing of Shape-Versatile Li-Ion Batteries. Energy Storage Mater. 2023, 57, 277–288. [Google Scholar] [CrossRef]
- Tao, R.; Gu, Y.; Sharma, J.; Hong, K.; Li, J. A Conformal Heat-Drying Direct Ink Writing 3D Printing for High-Performance Lithium-Ion Batteries. Mater. Today Chem. 2023, 32, 101672. [Google Scholar] [CrossRef]
- Xu, D.; Zhou, N.; Wang, A.; Xu, Y.; Liu, X.; Tang, S.; Luo, J. Mechano-Electrochemically Promoting Lithium Atom Diffusion and Relieving Accumulative Stress for Deep-Cycling Lithium Metal Anodes. Adv. Mater. 2023, 35, 2302872. [Google Scholar] [CrossRef]
- Hu, X.; Gao, Y.; Sun, Y.; Hou, Z.; Luo, Y.; Wang, D.; Wang, J.; Zhang, B.; Zheng, Z.; Li, Q. Preserving the Li {110} Texture to Achieve Long Cycle Life in Li Metal Electrode at High Rates. Adv. Funct. Mater. 2024, 34, 2307404. [Google Scholar] [CrossRef]
- Tan, J.; Ma, L.; Yi, P.; Wang, Y.; Li, Z.; Fang, Z.; Li, X.; He, S.; Wang, X.; Ye, M.; et al. Scalable Customization of Crystallographic Plane Controllable Lithium Metal Anodes for Ultralong-Lasting Lithium Metal Batteries. Adv. Mater. 2024, 36, 2403570. [Google Scholar] [CrossRef]
- Kriegler, J.; Ballmes, H.; Dib, S.; Demir, A.G.; Hille, L.; Liang, Y.; Wach, L.; Weinmann, S.; Keilhofer, J.; Kim, K.J.; et al. Surface Reconditioning of Lithium Metal Electrodes by Laser Treatment for the Industrial Production of Enhanced Lithium Metal Batteries. Adv. Funct. Mater. 2024, 34, 2313766. [Google Scholar] [CrossRef]
- Nogales, P.M.; Song, H.Y.; Jo, M.H.; Jeong, S.K. Improvement in the Electrochemical Properties of Lithium Metal by Heat Treatment: Changes in the Chemical Composition of Native and Solid Electrolyte Interphase Films. Energies 2022, 15, 1419. [Google Scholar] [CrossRef]
- Liu, T.; Liu, Y.; Zhang, Y.; Zhang, L. Surface Modified by SnI2 Boosts Dendrite-Free All-Solid-State Lithium Metal Batteries. J. Electroanal. Chem. 2023, 949, 117826. [Google Scholar] [CrossRef]
- Zou, P.; Jiang, W.; Ma, L.; Ouyang, L. Highly Reversible Lithium Metal Anodes Enabled by a Lithium Sulfamate Layer with High Ionic Conductivity and a Low Surface Diffusion Barrier. J. Mater. Chem. A 2024, 12, 11960–11967. [Google Scholar] [CrossRef]
- Yang, M.; Zhang, R.; Shi, C.; Liu, E.; Zhao, N. Ultra-Long Cycle Life of Lithium Metal Anode Achieved by Fluoride Silane Artificial Layer. Mater. Lett. 2023, 350, 134951. [Google Scholar] [CrossRef]
- Tan, L.; Chen, Q.; Chen, P.; Huang, X.; Li, L.; Zou, K.; Liu, D. Lithium Chloride Protective Layer for Stable Lithium Metal Anode via a Facile Surface Chemistry. J. Electroanal. Chem. 2023, 928, 117063. [Google Scholar] [CrossRef]
- Krauskopf, B.; Otto, S.-K.; Moryson, Y.; Hoffmann, F.; Sann, J.; Janek, J. Thin and Homogenous Surface Functionalization of Lithium Metal Anodes by Defined Molecular Treatment. J. Electrochem. Soc. 2023, 170, 030537. [Google Scholar] [CrossRef]
- Xie, X.; Chen, J.; Chen, X.; Shi, Z. Exploring the Effect of Lithium Halide Artificial SEI on the Electrochemical Performance of Lithium Metal Batteries. J. Electroanal. Chem. 2023, 949, 117862. [Google Scholar] [CrossRef]
- Yao, X.; Wang, J.; Lin, S.; Tao, C.; Zhang, X.; Wang, W.; Zhao, C.; Wang, L.; Bao, J.L.; Wang, Y.; et al. Surface Bromination of Lithium-Metal Anode for High Cyclic Efficiency. Adv. Energy Mater. 2023, 13, 2203233. [Google Scholar] [CrossRef]
- Huang, D.; Zeng, C.; Liu, M.; Chen, X.; Li, Y.; Hu, S.; Pan, Q.; Zheng, F.; Li, Q.; Wang, H. Introducing KI as a Functional Electrolyte Additive to Stabilize Li Metal Anode. Chem. Eng. J. 2023, 454, 140395. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhou, T.; Jeurgens, L.P.H.; Kong, X.; Choi, J.W.; Coskun, A. Electrolyte Engineering for Highly Inorganic Solid Electrolyte Interphase in High-Performance Lithium Metal Batteries. Chem 2023, 9, 682–697. [Google Scholar] [CrossRef]
- Nogales, P.M.; Lee, S.; Yang, S.; Jeong, S.-K. Effects of Electrolyte Solvent Composition on Solid Electrolyte Interphase Properties in Lithium Metal Batteries: Focusing on Ethylene Carbonate to Ethyl Methyl Carbonate Ratios. Batteries 2024, 10, 210. [Google Scholar] [CrossRef]
- Fasulo, F.; Muñoz-García, A.B.; Massaro, A.; Crescenzi, O.; Huang, C.; Pavone, M. Vinylene Carbonate Reactivity at Lithium Metal Surface: First-Principles Insights into the Early Steps of SEI Formation. J. Mater. Chem. A 2023, 11, 5660–5669. [Google Scholar] [CrossRef]
- Tao, C.; Zheng, T.; Jia, P.; Gong, W.; Yila, G.; Wang, L.; Liu, T. Synergy of Weakly Solvated Electrolyte and LiF-Reinforced Interphase Enables Long-Term Operation of Li-Metal Batteries at Low Temperatures. ACS Appl. Mater. Interfaces 2024, 16, 23325–23333. [Google Scholar] [CrossRef]
- Wen, Z.; Fang, W.; Wang, F.; Kang, H.; Zhao, S.; Guo, S.; Chen, G. Dual-Salt Electrolyte Additive Enables High Moisture Tolerance and Favorable Electric Double Layer for Lithium Metal Battery. Angew. Chem.-Int. Ed. 2024, 63, e202314876. [Google Scholar] [CrossRef]
- Zhang, Y.; Gou, Z.; Zheng, K.; Dou, Y.; Zhou, Z. Enhancing the Stability of Metallic Li Anodes for Aprotic Li-O2 Batteries with Dual-Anion Electrolytes. J. Phys. Chem. Lett. 2024, 15, 6598–6604. [Google Scholar] [CrossRef]
- Park, M.; Ha, S.; Park, J.; Kang, D.H.; Hyun, J.C.; Yoon, J.; Jin, H.J.; Yun, Y.S. Multifunctional Surface-Engineering of 3D-Lithiophilic Nanocarbon Scaffold for High-Voltage Anode-Minimized Lithium Metal Batteries. Chem. Eng. J. 2023, 458, 141478. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, Q.; Wang, Z.; Chen, Y.; Wang, K.; Shen, F.; Guo, J.; Han, X. Guiding Lithium Growth Direction by Au Coated Separator for Improving Lithium Metal Anode. Energy Mater. 2024, 4, 400047. [Google Scholar] [CrossRef]
- Xiong, X.; Yan, W.; Zhu, Y.; Liu, L.; Fu, L.; Chen, Y.; Yu, N.; Wu, Y.; Wang, B.; Xiao, R.; et al. Li4Ti5O12 Coating on Copper Foil as Ion Redistributor Layer for Stable Lithium Metal Anode. Adv. Energy Mater. 2022, 12, 2103112. [Google Scholar] [CrossRef]
- Peng, B.; Liu, Z.; Zhou, Q.; Xiong, X.; Xia, S.; Yuan, X.; Wang, F.; Ozoemena, K.I.; Liu, L.; Fu, L.; et al. A Solid-State Electrolyte Based on Li0.95Na0.05FePO4 for Lithium Metal Batteries. Adv. Mater. 2024, 36, 2307142. [Google Scholar] [CrossRef]

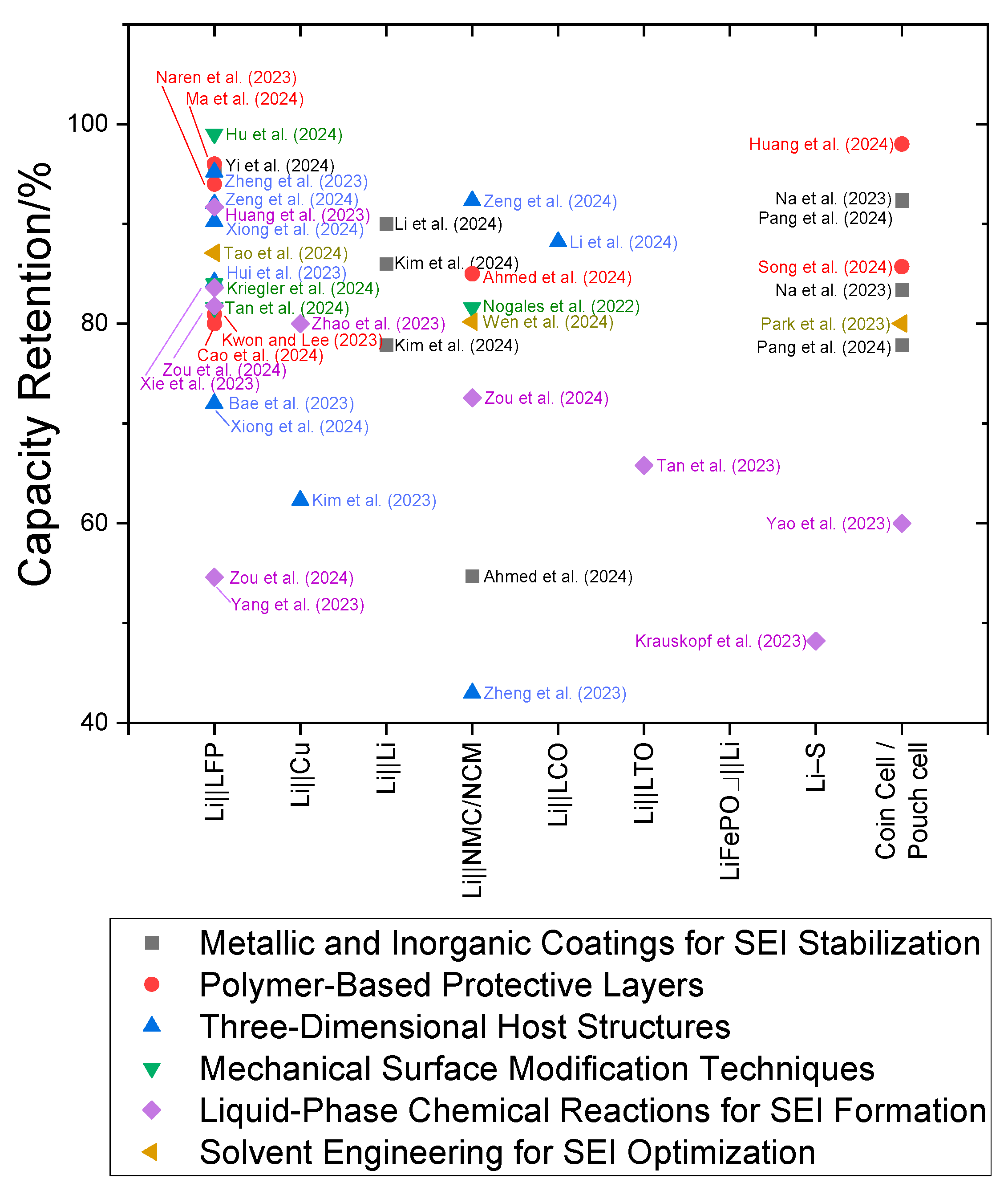
| Cell Type | Coulombic Efficiency | Capacity Retention | Ref. |
|---|---|---|---|
| Metallic and Inorganic Coatings for SEI Stabilization | |||
| Bare Li||LFP full cell | Not specified | 28% retention after 200 cycles (capacity decreased to 35 mAh g−1) | [19] |
| LCZO||LFP full cell | Not specified | 95.4% retention after 200 cycles (capacity of 124 mAh g−1) | [19] |
| Li||LiHg film cell | 100% over 160 cycles | Over 90% retention ~100 mAh g−1 after 100 cycles at 2 C rate | [20] |
| Li||LCO coin cell | 99.2% after 900 cycles | Theoretical capacity 145 mA g−1 125 mAh g−1 discharge capacity after 40 cycles at 120 °C heat treatment. 86% retention approximately | [24] |
| DFFSA-Li coin cell and pouch cell | Not specified | 77.85% retention after 1200 cycles in Li LCO coin cell at 1C discharging (from 153.98 mAh g−1 to 119.88 mAh g−1). 92.3% retention after 90 cycles in pouch cell with NCM811. 84.7% retention after 100 cycles in pouch cell with NCM811. 83.1% retention after 110 cycles in pouch cell with 100 µm DFFSA-Li. 86.67 mAh g−1 after 5 C charge/discharge. | [22] |
| Bare Li coin cell and pouch cell | Not specified | 28% retention after 530 cycles in Li LCO coin cell (from 145.7 mAh g−1 to 79.7 mAh g−1). 23 mAh g−1 after 65 cycles in pouch cell. | [22] |
| CO₂ pre-treated Li coin cell | 99.2% after 1200 cycles | 125 mAh g−1 initial discharge capacity 77.85% retention after 1200 cycles. | [25] |
| Bare Li coin cell | Not specified | 145.7 mAh g−1 initial discharge capacity 54.7% retention, 79.7 mAh g−1 after 530 cycles. | [25] |
| Li||SMGF@NF half cell | 99.2% Stable for 300 cycles at 0.5 C and 500 cycles at 1 C | 92.4% retention 113.4 mAh g−1 after 1000 cycles at 5 C. | [21] |
| Li||MGF@NF half cell | Not specified | Sharp decay after 246 cycles at 5 C. At 0.5 C, the capacity was 146.8 mAh g−1, | [21] |
| Li||NF half cell | Not specified | Sharp decay after 164 cycles at 5 C. At 0.5 C, the capacity retention was 83.4% of the initial capacity. | [21] |
| Polymer-Based Protective Layers | |||
| Li||LiFePO₄ (LFP) full cell | Not specified | Initial capacity was 143.2 mAh g−1, with 106.3 mAh g−1 remaining after 350 cycles. 80.94% retention after 800 cycles. | [31] |
| Li||LiFePO₄ (LFP) full cell with P(St-MaI)@Li anode | Not specified | Initial capacity: 155 mAh g−1 at 1 C 96% retention, After 930 cycles: (around 148 mAh g−1) | [33] |
| Li||LiFePO₄ (LFP) full cell | 99.8% during 250 cycles at a low N/P ratio (~3) | First cycle capacity: 148.4 mAh g−1 (at 1 C rate) 85.7% retention. after 500 cycles 145 mAh g−1 | [34] |
| LiFePO₄||Li (SP-lithium) | Not specified | Stable discharge capacity of 140 mAh g−1 after 600 cycles 98% retention after 300 cycles. | [36] |
| LiFePO₄ full cell with P-PTh-Li | Not specified | Initial reversible capacity: 146.8 mAh g−1 at 1 C. After 500 cycles, the capacity retention is 94.0%, with a capacity of 138.0 mAh g−1 | [32] |
| STCPL@Li||NMC811 | Not specified | 1st cycle is mentioned as 192.5 mAh g−1 85% retention, At C/2 charge/discharge rate, after 300 cycles | [29] |
| Li||NMC811 | Not specified | 1st cycle is mentioned as 193.6 mAh g−1 24% retention, After 300 cycles. | [29] |
| C-Li@P | Not specified | Initial capacity (774 mAh g−1) retaining > 80% capacity after 200 cycles. | [35] |
| Three-Dimensional Host Structures | |||
| CBC-Li||LFP | Not specified | Initial capacity 155 mAh g−1 90.2% retention after 700 cycles | [41] |
| Pure Li||LFP | Not specified | Initial capacity 155 mAh g−1 62.3% retention after 700 cycles | [41] |
| Li||LFP | Not specified | 151.74 mAh g−1 in the first cycle 95.2% retention after 400 cycles | [42] |
| Li/CC-Ag||LiFePO₄ full cell | Approaching 100% for 300 cycles at 1 C | 92% retention after 250 cycles (from 148 to 136 mAh g−1) | [44] |
| LFP||LAD-SSC@CF@Li full cell | Not specified | 149.2 mAh g−1 in the initial cycle 88.2% capacity retention after 300 cycles at 1 C | [45] |
| Li||NCM811 | Not specified | Cell type: Batteries deliver 179.07 mAh g−1 84.16% retention after 100 cycles. | [43] |
| Li/CC-Ag||NCM622 full cell | Not specified | 92.3% retention after 200 cycles (from 171.2 to 162.9 mAh g−1) | [44] |
| Li||LCO pouch cell with NSNF-hosted L | Not specified | 176.5 mAh g−1 after 8 cycles 72% retention, 127.5 mAh g−1 after 200 cycles | [46] |
| ZnNNi3@CC||Li | High efficiency (>99%) across long cycles | Initial capacity of 143.4 mAh g−1 72% retention after 1500 cycles. | [40] |
| 10Au@2D-Cu | Not specified | First discharge capacity is 159 mAh g−1 5% retention, 8 mAh g−1 in the 100th cycle. | [42] |
| 20Au@2D-Cu | Not specified | Exhibits 160 mAh g−1 in the 1st cycle 18.75% retention 30 mAh g−1 in the 100th cycle. | [42] |
| 20Au@3D-Cu: | Not specified | Exhibits 162 mAh g−1 in the 1st cycle 43% retention, 70 mAh g−1 in the 100th cycle. | [42] |
| Mechanical Surface Modification Techniques | |||
| Li||LFP full cell | Not specified | Initial discharge capacity over 150 mAh g−1 81.6% retention after 500 cycles at 5.0 C. | [51] |
| Li||LiFePO₄ (LFP) full cell | Near 100% | 80 mAh g−1 during 300 cycles at 5 C 99% retention | [50] |
| Li||NCM+LMO coin cell | Not specified | Initial discharge capacity over 112 mAh g−1 84% retention after 60 cycles and 53% capacity retention after 60 cycles in non-treated sample. | [52] |
| ELMA-based pouch cells | Energy density (375 Wh/kg) and maintained stability over 200 cycles | Not specified | [49] |
| Liquid-Phase Chemical Reactions for SEI Formation | |||
| Li–SA@Li||LFP full cell | Not specified | Initial discharge capacity of 157.7 mAh g−1 at 0.5 C 81.8% retention after 200 cycles | [55] |
| Bare Li||LFP full cell | Not specified | Initial capacity of 141.6 mAh g−1 54.6% retention | [55] |
| TFOS-Li||LFP full cell | 99.92% after 1650 cycles | Initial capacity ~250 mAh g−1 at 1.5 C Capacity retention: Not specified | [56] |
| Cu-mesh@Ag-Li||LFP | over > 99.7% | Initial discharge capacities 167.1 mAh g−1 91.7% retention of initial capacity after 800 cycles at 2 C. | [61] |
| Li||Cu cells (BTFM-based electrolytes) | 99.72% over 500 cycles | 80% retention over 600 cycles | [62] |
| Li–SA@Li||NCM full cell | Not specified | 72.6% retention after 200 cycles at 0.5 C | [55] |
| LiCl@Li||LTO full cell | Not specified | Initial capacity of 155 mAh g−1 65.8% retention after 1000 cycles | [57] |
| Li-S full cell | Not specified | 48.2% retention capacity retention after 120 cycles | [58] |
| LFP-Sn||LiI@Li | 99.8% after 1000 cycles | 120.6 mAh g−1 initial capacity Capacity retention: Not specified | [54] |
| LFP||LFCB811@Li full cell | 99.9% | Cell type with initial capacity 127.4 mAh g−1 83.6% retention after 1000 cycles | [59] |
| LiBr@Li full cell | 99.9% | Initial capacity ~155 mAh g−1 60% retention after 500 cycles | [60] |
| Solvent Engineering for SEI Optimization | |||
| Li||LFP cells (F/MA electrolyte) | 97% for 200 cycles | 87.1% retention after 400 cycles | [65] |
| Li||Cu cells with DAE1:1, LiNO3:LiFPFSI | 97.2% at the 160 cycle | Not specified | [67] |
| Li||NCM523 cells with LTFAN | Not specified | 80.2% retention capacity retention after 300 cycles at 1 C | [66] |
| 3D-CNS1600@LiNO3 | 95.3–98% | over 80% retention after 200 cycles | [68] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nogales, P.M.; Lee, S.; Yang, S.; Jeong, S.-K. Recent Advances in Ex Situ Surface Treatments for Lithium Metal Negative Electrodes in Secondary Batteries. Int. J. Mol. Sci. 2025, 26, 3446. https://doi.org/10.3390/ijms26073446
Nogales PM, Lee S, Yang S, Jeong S-K. Recent Advances in Ex Situ Surface Treatments for Lithium Metal Negative Electrodes in Secondary Batteries. International Journal of Molecular Sciences. 2025; 26(7):3446. https://doi.org/10.3390/ijms26073446
Chicago/Turabian StyleNogales, Paul Maldonado, Sangyup Lee, Seunga Yang, and Soon-Ki Jeong. 2025. "Recent Advances in Ex Situ Surface Treatments for Lithium Metal Negative Electrodes in Secondary Batteries" International Journal of Molecular Sciences 26, no. 7: 3446. https://doi.org/10.3390/ijms26073446
APA StyleNogales, P. M., Lee, S., Yang, S., & Jeong, S.-K. (2025). Recent Advances in Ex Situ Surface Treatments for Lithium Metal Negative Electrodes in Secondary Batteries. International Journal of Molecular Sciences, 26(7), 3446. https://doi.org/10.3390/ijms26073446








