Molecular Mechanisms and Therapeutic Strategies to Overcome Resistance to Endocrine Therapy and CDK4/6 Inhibitors in Advanced ER+/HER2− Breast Cancer
Abstract
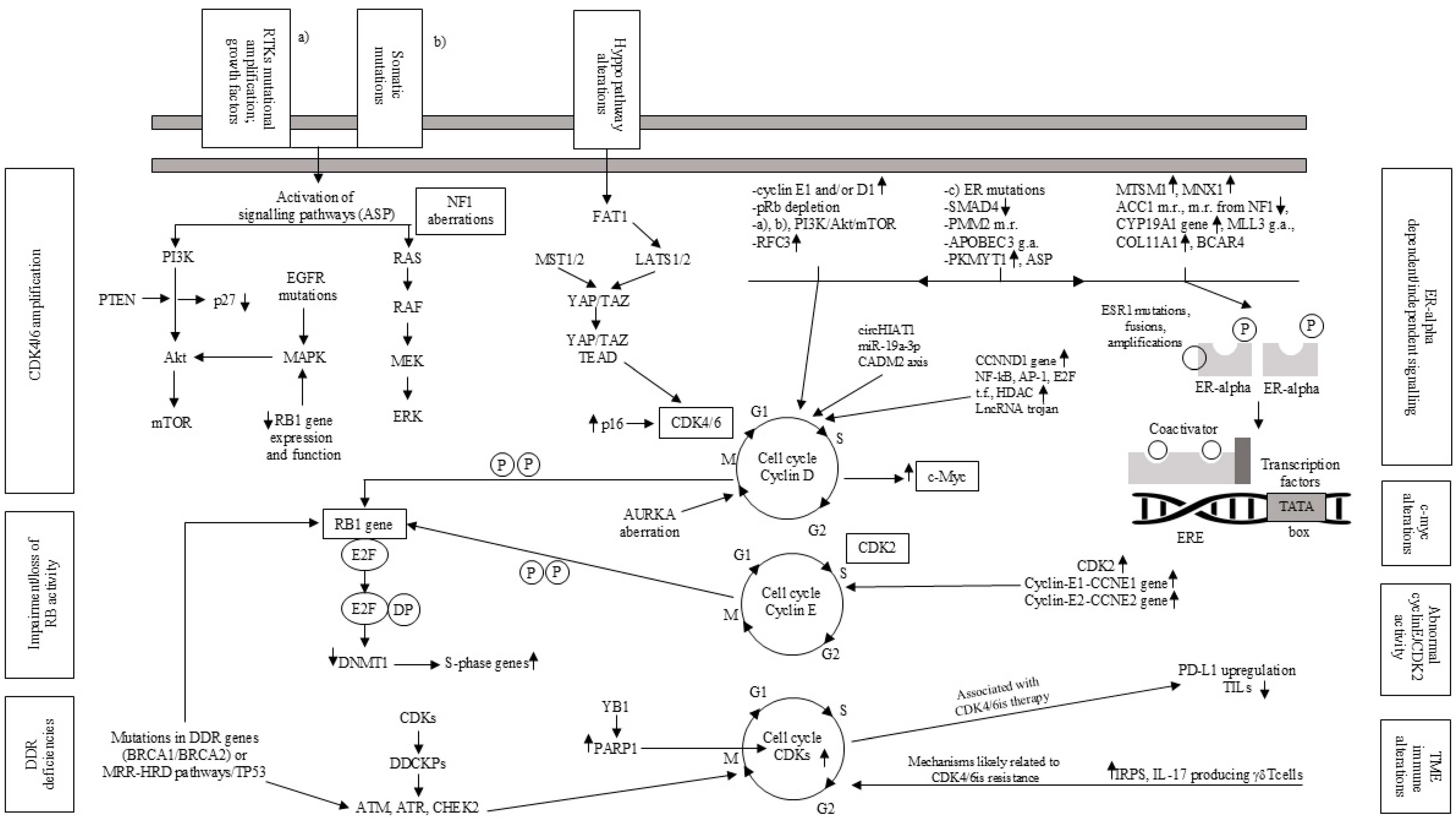
1. Introduction
2. Mechanisms of Resistance to ET
2.1. ESR1 Genetic Alterations
2.2. Regulators of the ERα Pathway
2.3. The PI3K/AKT/mTOR and Other Signaling Pathways
2.4. Metabolic Reprogramming
2.5. Further Mechanisms
3. CDK4/6is in Association with Endocrine Therapy
4. Resistance to ET and/or CDK4/6is
4.1. Genetic Alterations Involving Cell Cycle Regulation
4.2. Activation of Alternative Signaling Pathways
4.3. Modifications in Transcriptional and Epigenetic Regulators
4.4. Acquired CDK6 Amplification
4.5. Oncogene c-Myc Alteration
4.6. Immunological Alterations in Tumor Microenvironment (TME)
4.7. Proliferation Mechanisms Despite CDK Suppression
5. Common Therapeutic Strategies to Overcome Resistance to ET and/or CDK4/6is
5.1. Fulvestrant, a SERD and Novel Oral SERDs
5.2. PI3K/AKT/mTOR Pathway Inhibitors
5.3. Antibody–Drug Conjugates (ADCs)
6. Other Therapeutic Strategies
6.1. Continuing CDK4/6is
6.2. Next-Generation Endocrine Agents
6.2.1. CERANs
6.2.2. SSHs
6.2.3. SERCAs
6.2.4. ShERPAs
6.2.5. PROTACs
6.2.6. SARMs
6.3. Agents Targeting CDK7 and CDK2
6.4. Immune Therapy (IT)
6.5. Further Drugs and Targets
7. Discussion and Conclusions
Funding
Conflicts of Interest
References
- Allison, K.H.; Hammond, M.E.H.; Dowsett, M.; McKernin, S.E.; Carey, L.A.; Fitzgibbons, P.L.; Hayes, D.F.; Lakhani, S.R.; Chavez-MacGregor, M.; Perlmutter, J.; et al. Estrogen and Progesterone Receptor Testing in Breast Cancer: ASCO/CAP Guideline Update. J. Clin. Oncol. 2020, 38, 1346–1366. [Google Scholar] [CrossRef] [PubMed]
- Lumachi, F.; Santeufemia, D.A.; Basso, S.M. Current medical treatment of estrogen receptor-positive breast cancer. World J. Biol. Chem. 2015, 6, 231–239. [Google Scholar] [CrossRef] [PubMed]
- NCCN Guidelines, Breast Cancer. Available online: https://www.nccn.org/login?ReturnURL=https://www.nccn.org/professionals/physician_gls/pdf/breast.pdf (accessed on 15 March 2025).
- ESMO Clinical Practice Guidelines: Breast Cancer. Available online: https://www.esmo.org/guidelines/guidelines-by-topic/esmo-clinical-practice-guidelines-breast-cancer (accessed on 15 March 2025).
- Ferro, A.; Campora, M.; Caldara, A.; De Lisi, D.; Lorenzi, M.; Monteverdi, S.; Mihai, R.; Bisio, A.; Dipasquale, M.; Caffo, O.; et al. Novel Treatment Strategies for Hormone Receptor (HR)-Positive, HER2-Negative Metastatic Breast Cancer. J. Clin. Med. 2024, 13, 3611. [Google Scholar] [CrossRef] [PubMed]
- Cardoso, F.; Costa, A.; Cardoso, M.J.; Cufer, T.; El Saghir, N.; Fallowfield, L.; Fenech, D.; Francis, P.; Gelmon, K.; Giordano, S.H.; et al. European School of Oncology; European Society of Medical Oncology. ESO-ESMO 2nd international consensus guidelines for advanced breast cancer (ABC2). Breast 2014, 23, 489–502. [Google Scholar]
- Goel, S.; DeCristo, M.J.; McAllister, S.S.; Zhao, J.J. CDK4/6 Inhibition in Cancer: Beyond Cell Cycle Arrest. Trends Cell Biol. 2018, 28, 911–925. [Google Scholar]
- Hanker, A.B.; Sudhan, D.R.; Arteaga, C.L. Overcoming Endocrine Resistance in Breast Cancer. Cancer Cell 2020, 37, 496–513. [Google Scholar] [CrossRef]
- Razavi, P.; Chang, M.T.; Xu, G.; Bandlamudi, C.; Ross, D.S.; Vasan, N.; Cai, Y.; Bielski, C.M.; Donoghue, M.T.A.; Jonsson, P.; et al. The Genomic Landscape of Endocrine-Resistant Advanced Breast Cancers. Cancer Cell 2018, 34, 427–438.e6. [Google Scholar] [CrossRef]
- Cipolletti, M.; Acconcia, F. PMM2 controls ERα levels and cell proliferation in ESR1 Y537S variant expressing breast cancer cells. Mol. Cell Endocrinol. 2024, 584, 112160. [Google Scholar]
- De Marchi, T.; Lai, C.-F.; Simmons, G.M.; Goldsbrough, I.; Harrod, A.; Lam, T.; Buluwela, L.; Kjellström, S.; Brueffer, C.; Saal, L.H.; et al. Proteomic profiling reveals that ESR1 mutations enhance cyclin-dependent kinase signaling. Sci. Rep. 2024, 14, 6873. [Google Scholar]
- Veeraraghavan, J.; Tan, Y.; Cao, X.X.; Kim, J.A.; Wang, X.; Chamness, G.C.; Maiti, S.N.; Cooper, L.J.; Edwards, D.P.; Contreras, A.; et al. Recurrent ESR1-CCDC170 rearrangements in an aggressive subset of oestrogen receptor-positive breast cancers. Nat. Commun. 2014, 5, 4577. [Google Scholar] [CrossRef]
- Lei, J.T.; Shao, J.; Zhang, J.; Iglesia, M.; Chan, D.W.; Cao, J.; Anurag, M.; Singh, P.; He, X.; Kosaka, Y.; et al. Functional Annotation of ESR1 Gene Fusions in Estrogen Receptor-Positive Breast Cancer. Cell Rep. 2018, 24, 1434–1444.e7. [Google Scholar] [CrossRef] [PubMed]
- Holst, F.; Moelans, C.B.; Filipits, M.; Singer, C.F.; Simon, R.; van Diest, P.J. On the evidence for ESR1 amplification in breast cancer. Nat. Rev. Cancer 2012, 12, 149. [Google Scholar] [CrossRef] [PubMed]
- Moelans, C.B.; Holst, F.; Hellwinkel, O.; Simon, R.; van Diest, P.J. ESR1 amplification in breast cancer by optimized RNase FISH: Frequent but low-level and heterogeneous. PLoS ONE 2013, 8, e84189. [Google Scholar] [CrossRef][Green Version]
- Nielsen, K.V.; Ejlertsen, B.; Müller, S.; Møller, S.; Rasmussen, B.B.; Balslev, E.; Lænkholm, A.-V.; Christiansen, P.; Mouridsen, H.T. Amplification of ESR1 may predict resistance to adjuvant tamoxifen in postmenopausal patients with hormone receptor positive breast cancer. Breast Cancer Res. Treat. 2011, 127, 345–355. [Google Scholar] [CrossRef] [PubMed]
- Tomita, S.; Zhang, Z.; Nakano, M.; Ibusuki, M.; Kawazoe, T.; Yamamoto, Y.; Iwase, H. Estrogen receptor alpha gene ESR1 amplification may predict endocrine therapy responsiveness in breast cancer patients. Cancer Sci. 2009, 100, 1012–1017. [Google Scholar] [CrossRef]
- Reid, S.E.; Pantaleo, J.; Bolivar, P.; Bocci, M.; Sjölund, J.; Morsing, M.; Cordero, E.; Larsson, S.; Malmberg, M.; Seashore-Ludlow, B.; et al. Cancer-associated fibroblasts rewire the estrogen receptor response in luminal breast cancer, enabling estrogen independence. Oncogene 2024, 43, 1113–1126. [Google Scholar] [CrossRef]
- Luan, R.; He, M.; Li, H.; Bai, Y.; Wang, A.; Sun, G.; Zhou, B.; Wang, M.; Wang, C.; Wang, S.; et al. MYSM1 acts as a novel co-activator of ERα to confer antiestrogen resistance in breast cancer. EMBO Mol. Med. 2024, 16, 10–39. [Google Scholar] [CrossRef]
- Zhang, X.; Yang, F.; Huang, Z.; Liu, X.; Xia, G.; Huang, J.; Yang, Y.; Li, J.; Huang, J.; Liu, Y.; et al. Macrophages Promote Subtype Conversion and Endocrine Resistance in Breast Cancer. Cancers 2024, 16, 678. [Google Scholar] [CrossRef]
- Li, K.; Shu, D.; Li, H.; Lan, A.; Zhang, W.; Tan, Z.; Huang, M.; Tomasi, M.L.; Jin, A.; Yu, H.; et al. SMAD4 depletion contributes to endocrine resistance by integrating ER and ERBB signaling in HR + HER2− breast cancer. Cell Death Dis. 2024, 15, 444. [Google Scholar] [CrossRef]
- Nicolini, A.; Ferrari, P.; Duffy, M.J. Prognostic and predictive biomarkers in breast cancer: Past, present and future. Semin. Cancer Biol. 2018, 52 Pt 1, 56–73. [Google Scholar] [CrossRef]
- Millis, S.Z.; Ikeda, S.; Reddy, S.; Gatalica, Z.; Kurzrock, R. Landscape of Phosphatidylinositol-3-Kinase Pathway Alterations Across 19 784 Diverse Solid Tumors. JAMA Oncol. 2016, 2, 1565–1573. [Google Scholar] [PubMed]
- Kurokawa, H.; Lenferink, A.E.; Simpson, J.F.; Pisacane, P.I.; Sliwkowski, M.X.; Forbes, J.T.; Arteaga, C.L. Inhibition of HER2/neu (erbB-2) and mitogen-activated protein kinases enhances tamoxifen action against HER2-overexpressing, tamoxifen-resistant breast cancer cells. Cancer Res. 2000, 60, 5887–5894. [Google Scholar]
- Fribbens, C.; Murillas, I.G.; Beaney, M.; Hrebien, S.; O’leary, B.; Kilburn, L.; Howarth, K.; Epstein, M.; Green, E.; Rosenfeld, N.; et al. Tracking evolution of aromatase inhibitor resistance with circulating tumour DNA analysis in metastatic breast cancer. Ann. Oncol. 2018, 29, 145–153. [Google Scholar] [CrossRef]
- André, F.; Ciruelos, E.; Rubovszky, G.; Campone, M.; Loibl, S.; Rugo, H.S.; Iwata, H.; Conte, P.; Mayer, I.A.; Kaufman, B.; et al. SOLAR-1 Study Group. Alpelisib for PIK3CA-Mutated, Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2019, 380, 1929–1940. [Google Scholar] [CrossRef]
- Cancer Genome Atlas Network. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar]
- Angus, L.; Smid, M.; Wilting, S.M.; van Riet, J.; Van Hoeck, A.; Nguyen, L.; Nik-Zainal, S.; Steenbruggen, T.G.; Tjan-Heijnen, V.C.G.; Labots, M.; et al. The genomic landscape of metastatic breast cancer highlights changes in mutation and signature frequencies. Nat. Genet. 2019, 51, 1450–1458. [Google Scholar] [PubMed]
- Stoica, G.E.; Franke, T.F.; Moroni, M.; Mueller, S.; Morgan, E.; Iann, M.C.; Winder, A.D.; Reiter, R.; Wellstein, A.; Martin, M.B.; et al. Effect of estradiol on estrogen receptor-alpha gene expression and activity can be modulated by the ErbB2/PI 3-K/Akt pathway. Oncogene 2003, 22, 7998–8011. [Google Scholar] [PubMed]
- Sanchez, C.G.; Ma, C.X.; Crowder, R.J.; Guintoli, T.; Phommaly, C.; Gao, F.; Lin, L.; Ellis, M.J. Preclinical modeling of combined phosphatidylinositol-3-kinase inhibition with endocrine therapy for estrogen receptor-positive breast cancer. Breast Cancer Res. 2011, 13, R21. [Google Scholar]
- Miller, T.W.; Hennessy, B.T.; González-Angulo, A.M.; Fox, E.M.; Mills, G.B.; Chen, H.; Higham, C.; García-Echeverría, C.; Shyr, Y.; Arteaga, C.L. Hyperactivation of phosphatidylinositol-3 kinase promotes escape from hormone dependence in estrogen receptor-positive human breast cancer. J. Clin. Investig. 2010, 120, 2406–2413. [Google Scholar]
- Baselga, J.; Campone, M.; Piccart, M.; Burris, H.A., 3rd; Rugo, H.S.; Sahmoud, T.; Noguchi, S.; Gnant, M.; Pritchard, K.I.; Lebrun, F.; et al. Everolimus in postmenopausal hormone-receptor-positive advanced breast cancer. N. Engl. J. Med. 2012, 366, 520–529. [Google Scholar]
- Yamnik, R.L.; Holz, M.K. mTOR/S6K1 and MAPK/RSK signaling pathways coordinately regulate estrogen receptor alpha serine 167 phosphorylation. FEBS Lett. 2010, 584, 124–128. [Google Scholar] [CrossRef]
- Yamnik, R.L.; Digilova, A.; Davis, D.C.; Brodt, Z.; Murphy, C.J.; Holz, M.K. S6 kinase 1 regulates estrogen receptor alpha in control of breast cancer cell proliferation. J. Biol. Chem. 2009, 284, 6361–6369. [Google Scholar] [CrossRef] [PubMed]
- Efeyan, A.; Sabatini, D.M. mTOR and cancer: Many loops in one pathway. Curr. Opin. Cell Biol. 2010, 22, 169–176. [Google Scholar] [CrossRef]
- Brufsky, A.M.; Dickler, M.N. Estrogen Receptor-Positive Breast Cancer: Exploiting Signaling Pathways Implicated in Endocrine Resistance. Oncologist. 2018, 23, 528–539. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Zhao, D.; Li, Z.; Wang, Y.; Liu, J.; Du, T.; Zhou, L.; Chen, Y.; Yu, Q.; Chen, Q.; et al. Inactivated cGAS-STING Signaling Facilitates Endocrine Resistance by Forming a Positive Feedback Loop with AKT Kinase in ER+HER2− Breast Cancer. Adv. Sci. 2024, 11, e2403592. [Google Scholar] [CrossRef]
- Marin, A.; Morales, F.; Walbaum, B. Fibroblast growth factor receptor signaling in estrogen receptor-positive breast cancer: Mechanisms and role in endocrine resistance. Front. Oncol. 2024, 14, 1406951. [Google Scholar] [CrossRef] [PubMed]
- Pearson, A.; Proszek, P.Z.; Pascual, J.; Fribbens, C.; Shamsher, M.K.; Kingston, B.; O’Leary, B.; Herrera-Abreu, M.T.; Cutts, R.J.; Garcia-Murillas, I.; et al. Inactivating NF1 Mutations Are Enriched in Advanced Breast Cancer and Contribute to Endocrine Therapy Resistance. Clin. Cancer Res. 2020, 26, 608–622. [Google Scholar] [CrossRef]
- Garcia-Martinez, L.; Zhang, Y.; Nakata, Y.; Chan, H.L.; Morey, L. Epigenetic mechanisms in breast cancer therapy and resistance. Nat. Commun. 2021, 12, 1786. [Google Scholar] [CrossRef]
- Guo, Q.; Jin, Y.; Lin, M.; Zeng, C.; Zhang, J. NF-κB signaling in therapy resistance of breast cancer: Mechanisms, approaches, and challenges. Life Sci. 2024, 348, 122684. [Google Scholar]
- Bacci, M.; Lorito, N.; Smiriglia, A.; Subbiani, A.; Bonechi, F.; Comito, G.; Morriset, L.; El Botty, R.; Benelli, M.; López-Velazco, J.I.; et al. Acetyl-CoA carboxylase 1 controls a lipid droplet-peroxisome axis and is a vulnerability of endocrine-resistant ER+ breast cancer. Sci. Transl. Med. 2024, 16, eadf9874. [Google Scholar] [CrossRef]
- House, R.R.J.; Tovar, E.A.; Redlon, L.N.; Essenburg, C.J.; Dischinger, P.S.; Ellis, A.E.; Beddows, I.; Sheldon, R.D.; Lien, E.C.; Graveel, C.R.; et al. NF1 deficiency drives metabolic reprogramming in ER+ breast cancer. Mol. Metab. 2024, 80, 101876. [Google Scholar] [PubMed]
- Nguyen, V.T.M.; Barozzi, I.; Faronato, M.; Lombardo, Y.; Steel, J.H.; Patel, N.; Darbre, P.; Castellano, L.; Győrffy, B.; Woodley, L.; et al. Differential epigenetic reprogramming in response to specific endocrine therapies promotes cholesterol biosynthesis and cellular invasion. Nat. Commun. 2015, 6, 10044. [Google Scholar]
- Gala, K.; Li, Q.; Sinha, A.; Razavi, P.; Dorso, M.; Sanchez-Vega, F.; Chung, Y.R.; Hendrickson, R.; Hsieh, J.J.; Berger, M.; et al. KMT2C mediates the estrogen dependence of breast cancer through regulation of ERα enhancer function. Oncogene 2018, 37, 4692–4710. [Google Scholar] [PubMed]
- Saatci, O.; Alam, R.; Huynh-Dam, K.-T.; Isik, A.; Uner, M.; Belder, N.; Ersan, P.G.; Tokat, U.M.; Ulukan, B.; Cetin, M.; et al. Targeting LINC00152 activates cAMP/Ca2+/ferroptosis axis and overcomes tamoxifen resistance in ER+ breast cancer. Cell Death Dis. 2024, 15, 418. [Google Scholar] [CrossRef]
- Fu, C.; Duan, S.; Zhou, X.; Meng, Y.; Chen, X. Overexpression of COL11A1 confers tamoxifen resistance in breast cancer. NPJ Breast Cancer 2024, 10, 38. [Google Scholar] [CrossRef]
- Liao, M.; Webster, J.; Coonrod, E.M.; Weilbaecher, K.N.; Maher, C.A.; White, N.M. BCAR4 Expression as a Predictive Biomarker for Endocrine Therapy Resistance in Breast Cancer. Clin. Breast Cancer 2024, 24, 368–375.e2. [Google Scholar] [CrossRef] [PubMed]
- Nakagawa, S.; Miyashita, M.; Maeda, I.; Goda, A.; Tada, H.; Amari, M.; Kojima, Y.; Tsugawa, K.; Ohi, Y.; Sagara, Y.; et al. Potential role of Fbxo22 in resistance to endocrine therapy in breast cancer with invasive lobular carcinoma. Breast Cancer Res. Treat. 2024, 204, 453–463. [Google Scholar]
- Zhu, J.; Ye, L.; Sun, S.; Yuan, J.; Huang, J.; Zeng, Z. Involvement of RFC3 in tamoxifen resistance in ER-positive breast cancer through the cell cycle. Aging 2023, 15, 13738–13752. [Google Scholar] [CrossRef]
- Muise-Helmericks, R.C.; Grimes, H.L.; Bellacosa, A.; Malstrom, S.E.; Tsichlis, P.N.; Rosen, N. Cyclin D expression is controlled post-transcriptionally via a phosphatidylinositol 3-kinase/Akt-dependent pathway. J. Biol. Chem. 1998, 273, 29864–29872. [Google Scholar] [CrossRef]
- Kenny, F.S.; Hui, R.; Musgrove, E.A.; Gee, J.M.; Blamey, R.W.; Nicholson, R.I.; Sutherland, R.L.; Robertson, J.F. Overexpression of cyclin D1 messenger RNA predicts for poor prognosis in estrogen receptor-positive breast cancer. Clin. Cancer Res. 1999, 5, 2069–2076. [Google Scholar]
- Kilker, R.L.; Planas-Silva, M.D. Cyclin D1 is necessary for tamoxifen-induced cell cycle progression in human breast cancer cells. Cancer Res. 2006, 66, 11478–11484. [Google Scholar] [PubMed]
- Vora, S.R.; Juric, D.; Kim, N.; Mino-Kenudson, M.; Huynh, T.; Costa, C.; Lockerman, E.L.; Pollack, S.F.; Liu, M.; Li, X.; et al. CDK 4/6 inhibitors sensitize PIK3CA mutant breast cancer to PI3K inhibitors. Cancer Cell 2014, 26, 136–149. [Google Scholar] [PubMed]
- Finn, R.S.; Aleshin, A.; Slamon, D.J. Targeting the cyclin-dependent kinases (CDK) 4/6 in estrogen receptor-positive breast cancers. Breast Cancer Res. 2016, 18, 17. [Google Scholar]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Paluch-Shimon, S.; Campone, M.; Petrakova, K.; Blackwell, K.L.; Winer, E.P.; et al. Updated results from MONALEESA-2, a phase III trial of first-line ribociclib plus letrozole versus placebo plus letrozole in hormone receptor-positive, HER2-negative advanced breast cancer. Ann. Oncol. 2018, 29, 1541–1547, Erratum in Ann Oncol. 2019, 30, 1842. [Google Scholar]
- Im, S.-A.; Lu, Y.-S.; Bardia, A.; Harbeck, N.; Colleoni, M.; Franke, F.; Chow, L.; Sohn, J.; Lee, K.-S.; Campos-Gomez, S.; et al. Overall Survival with Ribociclib plus Endocrine Therapy in Breast Cancer. N. Engl. J. Med. 2019, 381, 307–316. [Google Scholar] [PubMed]
- Johnston, S.; Martin, M.; Di Leo, A.; Im, S.-A.; Awada, A.; Forrester, T.; Frenzel, M.; Hardebeck, M.C.; Cox, J.; Barriga, S.; et al. MONARCH 3 final PFS: A randomized study of abemaciclib as initial therapy for advanced breast cancer. NPJ Breast Cancer 2019, 5, 5. [Google Scholar]
- Rugo, H.S.; Finn, R.S.; Diéras, V.; Ettl, J.; Lipatov, O.; Joy, A.A.; Harbeck, N.; Castrellon, A.; Iyer, S.; Lu, D.R.; et al. Palbociclib plus letrozole as first-line therapy in estrogen receptor-positive/human epidermal growth factor receptor 2-negative advanced breast cancer with extended follow-up. Breast Cancer Res. Treat. 2019, 174, 719–729. [Google Scholar] [CrossRef]
- Hortobagyi, G.N.; Stemmer, S.M.; Burris, H.A.; Yap, Y.-S.; Sonke, G.S.; Hart, L.; Campone, M.; Petrakova, K.; Winer, E.P.; Janni, W.; et al. Overall Survival with Ribociclib plus Letrozole in Advanced Breast Cancer. N. Engl. J. Med. 2022, 386, 942–950. [Google Scholar]
- Lu, Y.S.; Im, S.A.; Colleoni, M.; Franke, F.; Bardia, A.; Cardoso, F.; Harbeck, N.; Hurvitz, S.; Chow, L.; Sohn, J.; et al. Updated Overall Survival of Ribociclib plus Endocrine Therapy versus Endocrine Therapy Alone in Pre- and Perimenopausal Patients with HR+/HER2− Advanced Breast Cancer in MONALEESA-7: A Phase III Randomized Clinical Trial. Clin. Cancer Res. 2022, 28, 851–859. [Google Scholar]
- Masurkar, P.P.; Prajapati, P.; Canedo, J.; Goswami, S.; Earl, S.; Bhattacharya, K. Cost-effectiveness of CDK4/6 inhibitors in HR+/HER2− metastatic breast cancer: A systematic review and meta-analysis. Curr. Med. Res. Opin. 2024, 40, 1753–1767. [Google Scholar] [CrossRef]
- Turner, N.C.; Slamon, D.J.; Ro, J.; Bondarenko, I.; Im, S.-A.; Masuda, N.; Colleoni, M.; DeMichele, A.; Loi, S.; Verma, S.; et al. Overall Survival with Palbociclib and Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2018, 379, 1926–1936. [Google Scholar] [CrossRef] [PubMed]
- Slamon, D.J.; Neven, P.; Chia, S.; Fasching, P.A.; De Laurentiis, M.; Im, S.-A.; Petrakova, K.; Bianchi, G.V.; Esteva, F.J.; Martín, M.; et al. Overall Survival with Ribociclib plus Fulvestrant in Advanced Breast Cancer. N. Engl. J. Med. 2020, 382, 514–524. [Google Scholar] [CrossRef]
- Neven, P.; Fasching, P.A.; Chia, S.; Jerusalem, G.; De Laurentiis, M.; Im, S.A.; Petrakova, K.; Bianchi, G.V.; Martín, M.; Nusch, A.; et al. Updated overall survival from the MONALEESA-3 trial in postmenopausal women with HR+/HER2− advanced breast cancer receiving first-line ribociclib plus fulvestrant. Breast Cancer Res. 2023, 25, 103. [Google Scholar] [CrossRef]
- Sledge, G.W., Jr.; Toi, M.; Neven, P.; Sohn, J.; Inoue, K.; Pivot, X.; Burdaeva, O.; Okera, M.; Masuda, N.; Kaufman, P.A.; et al. The Effect of Abemaciclib Plus Fulvestrant on Overall Survival in Hormone Receptor-Positive, ERBB2-Negative Breast Cancer That Progressed on Endocrine Therapy-MONARCH 2: A Randomized Clinical Trial. JAMA Oncol. 2020, 6, 116–124. [Google Scholar]
- Álvarez-Fernández, M.; Malumbres, M. Mechanisms of Sensitivity and Resistance to CDK4/6 Inhibition. Cancer Cell 2020, 37, 514–529. [Google Scholar]
- Lee, J.S.; Hackbart, H.; Cui, X.; Yuan, Y. CDK4/6 Inhibitor Resistance in Hormone Receptor-Positive Metastatic Breast Cancer: Translational Research, Clinical Trials, and Future Directions. Int. J. Mol. Sci. 2023, 24, 11791. [Google Scholar] [CrossRef]
- Gupta, A.; Gazzo, A.; Selenica, P.; Safonov, A.; Pareja, F.; da Silva, E.M.; Brown, D.N.; Zhu, Y.; Patel, J.; Blanco-Heredia, J.; et al. APOBEC3 mutagenesis drives therapy resistance in breast cancer. bioRxiv 2024, 591453. [Google Scholar]
- Lloyd, M.R.; Brett, J.O.; Carmeli, A.; Weipert, C.M.; Zhang, N.; Yu, J.; Bucheit, L.; Medford, A.J.; Wagle, N.; Bardia, A.; et al. CDK4/6 Inhibitor Efficacy in ESR1-Mutant Metastatic Breast Cancer. NEJM Evid. 2024, 3, EVIDoa2300231. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Litchfield, L.M.; Webster, Y.; Chio, L.-C.; Wong, S.S.; Stewart, T.R.; Dowless, M.; Dempsey, J.; Zeng, Y.; Torres, R.; et al. Genomic Aberrations that Activate D-type Cyclins Are Associated with Enhanced Sensitivity to the CDK4 and CDK6 Inhibitor Abemaciclib. Cancer Cell 2017, 32, 761–776.e6. [Google Scholar] [CrossRef]
- Turner, N.C.; Liu, Y.; Zhu, Z.; Loi, S.; Colleoni, M.; Loibl, S.; DeMichele, A.; Harbeck, N.; André, F.; Bayar, M.A.; et al. Cyclin E1 Expression and Palbociclib Efficacy in Previously Treated Hormone Receptor-Positive Metastatic Breast Cancer. J. Clin. Oncol. 2019, 37, 1169–1178. [Google Scholar] [CrossRef]
- Wander, S.A.; Cohen, O.; Gong, X.; Johnson, G.N.; Buendia-Buendia, J.E.; Lloyd, M.R.; Kim, D.; Luo, F.; Mao, P.; Helvie, K.; et al. The Genomic Landscape of Intrinsic and Acquired Resistance to Cyclin-Dependent Kinase 4/6 Inhibitors in Patients with Hormone Receptor-Positive Metastatic Breast Cancer. Cancer Discov. 2020, 10, 1174–1193. [Google Scholar] [CrossRef] [PubMed]
- Palafox, M.; Monserrat, L.; Bellet, M.; Villacampa, G.; Gonzalez-Perez, A.; Oliveira, M.; Brasó-Maristany, F.; Ibrahimi, N.; Kannan, S.; Mina, L.; et al. High p16 expression and heterozygous RB1 loss are biomarkers for CDK4/6 inhibitor resistance in ER+ breast cancer. Nat. Commun. 2022, 13, 5258. [Google Scholar] [CrossRef]
- Safonov, A.; Marra, A.; Bandlamudi, C.; O’Leary, B.; Wubbenhorst, B.; Ferraro, E.; Moiso, E.; Lee, M.; An, J.; Donoghue, M.T.A.; et al. Tumor suppressor heterozygosity and homologous recombination deficiency mediate resistance to front-line therapy in breast cancer. bioRxiv 2024. [Google Scholar] [CrossRef]
- Sablin, M.P.; Gestraud, P.; Jonas, S.F.; Lamy, C.; Lacroix-Triki, M.; Bachelot, T.; Filleron, T.; Lacroix, L.; Tran-Dien, A.; Jézéquel, P.; et al. Copy number alterations in metastatic and early breast tumours: Prognostic and acquired biomarkers of resistance to CDK4/6 inhibitors. Br. J. Cancer 2024, 131, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Mouery, B.L.; Baker, E.M.; Mei, L.; Wolff, S.C.; Mills, C.A.; Fleifel, D.; Mulugeta, N.; Herring, L.E.; Cook, J.G. APC/C prevents a noncanonical order of cyclin/CDK activity to maintain CDK4/6 inhibitor-induced arrest. Proc. Natl. Acad. Sci. USA 2024, 121, e2319574121. [Google Scholar] [CrossRef] [PubMed]
- Torrisi, R.; Vaira, V.; Giordano, L.; Fernandes, B.; Saltalamacchia, G.; Palumbo, R.; Carnaghi, C.; Basilico, V.; Gentile, F.; Masci, G.; et al. Identification of a Panel of miRNAs Associated with Resistance to Palbociclib and Endocrine Therapy. Int. J. Mol. Sci. 2024, 25, 1498. [Google Scholar] [CrossRef]
- Formisano, L.; Lu, Y.; Servetto, A.; Hanker, A.B.; Jansen, V.M.; Bauer, J.A.; Sudhan, D.R.; Guerrero-Zotano, A.L.; Croessmann, S.; Guo, Y.; et al. Aberrant FGFR signaling mediates resistance to CDK4/6 inhibitors in ER+ breast cancer. Nat. Commun. 2019, 10, 1373. [Google Scholar] [CrossRef]
- Li, Z.; Razavi, P.; Li, Q.; Toy, W.; Liu, B.; Ping, C.; Hsieh, W.; Sanchez-Vega, F.; Brown, D.N.; Da Cruz Paula, A.F.; et al. Loss of the FAT1 Tumor Suppressor Promotes Resistance to CDK4/6 Inhibitors via the Hippo Pathway. Cancer Cell 2018, 34, 893–905.e8. [Google Scholar] [CrossRef]
- Talia, M.; Cirillo, F.; Scordamaglia, D.; Di Dio, M.; Zicarelli, A.; De Rosis, S.; Miglietta, A.M.; Capalbo, C.; De Francesco, E.M.; Belfiore, A.; et al. The G Protein Estrogen Receptor (GPER) is involved in the resistance to the CDK4/6 inhibitor palbociclib in breast cancer. J. Exp. Clin. Cancer Res. 2024, 43, 171. [Google Scholar] [CrossRef]
- Belli, S.; Esposito, D.; Ascione, C.M.; Messina, F.; Napolitano, F.; Servetto, A.; De Angelis, C.; Bianco, R.; Formisano, L. EGFR and HER2 hyper-activation mediates resistance to endocrine therapy and CDK4/6 inhibitors in ER+ breast cancer. Cancer Lett. 2024, 593, 216968. [Google Scholar] [CrossRef]
- Chen, A.; Kim, B.J.; Mitra, A.; Vollert, C.T.; Lei, J.T.; Fandino, D.; Anurag, M.; Holt, M.V.; Gou, X.; Pilcher, J.B.; et al. PKMYT1 Is a Marker of Treatment Response and a Therapeutic Target for CDK4/6 Inhibitor-Resistance in ER+ Breast Cancer. Mol. Cancer Ther. 2024, 23, 1494–1510. [Google Scholar] [PubMed]
- Kindt, C.K.; Alves, C.L.; Ehmsen, S.; Kragh, A.; Reinert, T.; Vogsen, M.; Kodahl, A.R.; Rønlev, J.D.; Ardik, D.; Sørensen, A.L.; et al. Genomic alterations associated with resistance and circulating tumor DNA dynamics for early detection of progression on CDK4/6 inhibitor in advanced breast cancer. Int. J. Cancer 2024, 155, 2211–2222. [Google Scholar]
- Li, X.; Niu, C.; Yi, G.; Zhang, Y.; Jin, W.; Zhang, Z.; Zhang, W.; Li, B. Quercetin inhibits the epithelial-mesenchymal transition and reverses CDK4/6 inhibitor resistance in breast cancer by regulating circHIAT1/miR-19a-3p/CADM2 axis. PLoS ONE 2024, 19, e0305612. [Google Scholar] [CrossRef]
- Cui, Y.; Li, Y.; Xu, Y.; Liu, X.; Kang, X.; Zhu, J.; Long, S.; Han, Y.; Xue, C.; Sun, Z.; et al. SLC7A11 protects luminal A breast cancer cells against ferroptosis induced by CDK4/6 inhibitors. Redox Biol. 2024, 76, 103304. [Google Scholar]
- Zhang, Y.; Zhou, S.; Kai, Y.; Zhang, Y.Q.; Peng, C.; Li, Z.; Mughal, M.J.; Julie, B.; Zheng, X.; Ma, J.; et al. O-GlcNAcylation of MITF regulates its activity and CDK4/6 inhibitor resistance in breast cancer. Nat. Commun. 2024, 15, 5597. [Google Scholar] [CrossRef]
- Lee, J.; Lim, B.; Pearson, T.; Tripathy, D.; Ordentlich, P.; Ueno, N. The synergistic antitumor activity of entinostat (MS-275) in combination with palbociclib (PD 0332991) in estrogen receptor-positive and triple-negative breast cancer. Cancer Res. 2018, 78 (Suppl. S4), P5-21-15. [Google Scholar]
- Papadimitriou, M.C.; Pazaiti, A.; Iliakopoulos, K.; Markouli, M.; Michalaki, V.; Papadimitriou, C.A. Resistance to CDK4/6 inhibition: Mechanisms and strategies to overcome a therapeutic problem in the treatment of hormone receptor-positive metastatic breast cancer. Biochim. Biophys. Acta Mol. Cell Res. 2022, 1869, 119346. [Google Scholar] [CrossRef] [PubMed]
- Cornell, L.; Wander, S.A.; Visal, T.; Wagle, N.; Shapiro, G.I. MicroRNA-Mediated Suppression of the TGF-β Pathway Confers Transmissible and Reversible CDK4/6 Inhibitor Resistance. Cell Rep. 2019, 26, 2667–2680.e7. [Google Scholar]
- Jin, X.; Ge, L.P.; Li, D.Q.; Shao, Z.M.; Di, G.H.; Xu, X.E.; Jiang, Y.Z. LncRNA TROJAN promotes proliferation and resistance to CDK4/6 inhibitor via CDK2 transcriptional activation in ER+ breast cancer. Mol. Cancer 2020, 19, 87. [Google Scholar] [PubMed]
- Yang, C.; Li, Z.; Bhatt, T.; Dickler, M.; Giri, D.; Scaltriti, M.; Baselga, J.; Rosen, N.; Chandarlapaty, S. Acquired CDK6 amplification promotes breast cancer resistance to CDK4/6 inhibitors and loss of ER signaling and dependence. Oncogene 2017, 36, 2255–2264. [Google Scholar]
- Wu, X.; Yang, X.; Xiong, Y.; Li, R.; Ito, T.; Ahmed, T.A.; Karoulia, Z.; Adamopoulos, C.; Wang, H.; Wang, L.; et al. Distinct CDK6 complexes determine tumor cell response to CDK4/6 inhibitors and degraders. Nat. Cancer 2021, 2, 429–443. [Google Scholar]
- Al-Qasem, A.J.; Alves, C.L.; Ehmsen, S.; Tuttolomondo, M.; Terp, M.G.; Johansen, L.E.; Vever, H.; Hoeg, L.V.A.; Elias, D.; Bak, M.; et al. Co-targeting CDK2 and CDK4/6 overcomes resistance to aromatase and CDK4/6 inhibitors in ER+ breast cancer. NPJ Precis. Oncol. 2022, 6, 68. [Google Scholar] [CrossRef] [PubMed]
- Chen, Z.G.; Saba, N.F.; Teng, Y. The diverse functions of FAT1 in cancer progression: Good, bad, or ugly? J. Exp. Clin. Cancer Res. 2022, 41, 248. [Google Scholar] [CrossRef]
- Martin, D.; Degese, M.S.; Vitale-Cross, L.; Iglesias-Bartolome, R.; Valera, J.L.C.; Wang, Z.; Feng, X.; Yeerna, H.; Vadmal, V.; Moroishi, T.; et al. Assembly and activation of the Hippo signalome by FAT1 tumor suppressor. Nat. Commun. 2018, 9, 2372. [Google Scholar] [CrossRef]
- Steinhardt, A.A.; Gayyed, M.F.; Klein, A.P.; Dong, J.; Maitra, A.; Pan, D.; Montgomery, E.A.; Anders, R.A. Expression of Yes-associated protein in common solid tumors. Hum. Pathol. 2008, 39, 1582–1589. [Google Scholar] [CrossRef]
- Gao, F.Y.; Li, X.T.; Xu, K.; Wang, R.T.; Guan, X.X. c-MYC mediates the crosstalk between breast cancer cells and tumor microenvironment. Cell Commun. Signal. 2023, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Pandey, K.; Park, N.; Park, K.S.; Hur, J.; Cho, Y.B.; Kang, M.; An, H.J.; Kim, S.; Hwang, S.; Moon, Y.W. Combined CDK2 and CDK4/6 Inhibition Overcomes Palbociclib Resistance in Breast Cancer by Enhancing Senescence. Cancers 2020, 12, 3566. [Google Scholar] [CrossRef] [PubMed]
- Goetz, M.P.; Hamilton, E.P.; Campone, M.; Hurvitz, S.A.; Cortes, J.; Johnston, S.R.D.; Jerusalem, G.H.M.; Graham, H.; Wang, H.; Litchfield, L.; et al. Acquired genomic alterations in circulating tumor DNA from patients receiving abemaciclib alone or in combination with endocrine therapy. J. Clin. Oncol. 2020, 38 (Suppl. S15), 3519. [Google Scholar] [CrossRef]
- Mo, H.; Liu, X.; Xue, Y.; Chen, H.; Guo, S.; Li, Z.; Wang, S.; Li, C.; Han, J.; Fu, M.; et al. S6K1 amplification confers innate resistance to CDK4/6 inhibitors through activating c-Myc pathway in patients with estrogen receptor-positive breast cancer. Mol. Cancer 2022, 21, 171. [Google Scholar] [CrossRef]
- Petroni, G.; Formenti, S.C.; Chen-Kiang, S.; Galluzzi, L. Immunomodulation by anticancer cell cycle inhibitors. Nat. Rev. Immunol. 2020, 20, 669–679. [Google Scholar] [CrossRef]
- Zhang, J.; Bu, X.; Wang, H.; Zhu, Y.; Geng, Y.; Nihira, N.T.; Tan, Y.; Ci, Y.; Wu, F.; Dai, X.; et al. Cyclin D-CDK4 kinase destabilizes PD-L1 via cullin 3-SPOP to control cancer immune surveillance. Nature 2018, 553, 91–95. [Google Scholar] [CrossRef]
- The, J.L.F. Arrested developments: CDK4/6 inhibitor resistance and alterations in the tumor immune microenvironment. Clin. Cancer Res. 2019, 25, 921–927. [Google Scholar]
- De Angelis, C. Activation of the IFN signaling pathway is associated with resistance to CDK4/6 inhibitors and immune checkpoint activation in ER-positive breast cancer. Clin. Cancer Res. 2021, 27, 4870–4882. [Google Scholar]
- Petroni, G.; Gouin, K.; Galassi, C.; Buqué, A.; Bloy, N.; Yamazaki, T.; Sato, A.; Jiménez-Cortegana, C.; Kirchmair, A.; Massa, C.; et al. IL17-Producing γδ T Cells Promote Resistance to CDK4/6 Inhibitors in HR+HER2− Breast cancer. BMJ Spec. J. 2022, 10, A1603. [Google Scholar]
- Santamaría, D.; Barrière, C.; Cerqueira, A.; Hunt, S.; Tardy, C.; Newton, K.; Cáceres, J.F.; Dubus, P.; Malumbres, M.; Barbacid, M. Cdk1 is sufficient to drive the mammalian cell cycle. Nature 2007, 448, 811–815. [Google Scholar] [PubMed]
- Freeman-Cook, K.D.; Hoffman, R.L.; Behenna, D.C.; Boras, B.; Carelli, J.; Diehl, W.; Ferre, R.A.; He, Y.-A.; Hui, A.; Huang, B.; et al. Discovery of PF-06873600, a CDK2/4/6 Inhibitor for the Treatment of Cancer. J. Med. Chem. 2021, 64, 9056–9077. [Google Scholar] [CrossRef]
- Arora, M.; Moser, J.; Hoffman, T.E.; Watts, L.P.; Min, M.; Musteanu, M.; Rong, Y.; Ryland, C., III; Nangia, V.; Schneider, J.; et al. Rapid adaptation to CDK2 inhibition exposes intrinsic cell-cycle plasticity. Cell 2023, 186, 2628–2643.e21. [Google Scholar] [PubMed]
- Glover-Cutter, K.; Larochelle, S.; Erickson, B.; Zhang, C.; Shokat, K.; Fisher, R.P.; Bentley, D.L. TFIIH-associated Cdk7 kinase functions in phosphorylation of C-terminal domain Ser7 residues, promoter-proximal pausing, and termination by RNA polymerase II. Mol. Cell Biol. 2009, 29, 5455–5464. [Google Scholar] [CrossRef]
- Coombes, R.C.; Howell, S.; Lord, S.R.; Kenny, L.; Mansi, J.; Mitri, Z.; Palmieri, C.; Chap, L.I.; Richards, P.; Gradishar, W.; et al. Dose escalation and expansion cohorts in patients with advanced breast cancer in a Phase I study of the CDK7-inhibitor samuraciclib. Nat. Commun. 2023, 14, 4444, Erratum in Nat. Commun. 2023, 14, 4741. [Google Scholar]
- Wilson, G.A.; Vuina, K.; Sava, G.; Huard, C.; Meneguello, L.; Coulombe-Huntington, J.; Bertomeu, T.; Maizels, R.J.; Lauring, J.; Kriston-Vizi, J.; et al. Active growth signaling promotes senescence and cancer cell sensitivity to CDK7 inhibition. Mol. Cell. 2023, 83, 4078–4092.e6. [Google Scholar] [CrossRef]
- Munzone, E.; Pagan, E.; Bagnardi, V.; Montagna, E.; Cancello, G.; Dellapasqua, S.; Iorfida, M.; Mazza, M.; Colleoni, M. Systematic review and meta-analysis of post-progression outcomes in ER+/HER2− metastatic breast cancer after CDK4/6 inhibitors within randomized clinical trials. ESMO Open 2021, 6, 100332. [Google Scholar] [CrossRef]
- Gennari, A. ESMO Clinical Practice Guideline for the diagnosis, staging and treatment of patients with metastatic breast cancer. Ann. Oncol. 2021, 32, 1475–1495. [Google Scholar]
- Leo, A.D.; Jerusalem, G.; Petruzelka, L.; Torres, R.; Bondarenko, I.N.; Khasanov, R.; Verhoeven, D.; Pedrini, J.L.; Smirnova, I.; Lichinitser, M.R.; et al. Final overall survival: Fulvestrant 500 mg vs. 250 mg in the randomized CONFIRM trial. J. Natl. Cancer Inst. 2014, 106, djt337. [Google Scholar] [PubMed]
- Kornblum, N.; Zhao, F.; Manola, J.; Klein, P.; Ramaswamy, B.; Brufsky, A.; Stella, P.J.; Burnette, B.; Telli, M.; Makower, D.F.; et al. Randomized Phase II Trial of Fulvestrant Plus Everolimus or Placebo in Postmenopausal Women with Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer Resistant to Aromatase Inhibitor Therapy: Results of PrE0102. J. Clin. Oncol. 2018, 36, 1556–1563. [Google Scholar] [PubMed]
- Schmid, P.; Zaiss, M.; Harper-Wynne, C.; Ferreira, M.; Dubey, S.; Chan, S.; Makris, A.; Nemsadze, G.; Brunt, A.M.; Kuemmel, S.; et al. Fulvestrant Plus Vistusertib vs Fulvestrant Plus Everolimus vs Fulvestrant Alone for Women with Hormone Receptor-Positive Metastatic Breast Cancer: The MANTA Phase 2 Randomized Clinical Trial. JAMA Oncol. 2019, 5, 1556–1564. [Google Scholar] [PubMed]
- Kalinsky, K.; Accordino, M.K.; Chiuzan, C.; Mundi, P.S.; Sakach, E.; Sathe, C.; Ahn, H.; Trivedi, M.S.; Novik, Y.; Tiersten, A.; et al. Randomized Phase II Trial of Endocrine Therapy with or Without Ribociclib After Progression on Cyclin-Dependent Kinase 4/6 Inhibition in Hormone Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer: MAINTAIN Trial. J. Clin. Oncol. 2023, 41, 4004–4013. [Google Scholar]
- Lindeman, G.J.; Fernando, T.M.; Bowen, R.; Jerzak, K.J.; Song, X.; Decker, T.; Boyle, F.; McCune, S.; Armstrong, A.; Shannon, C.; et al. VERONICA: Randomized Phase II Study of Fulvestrant and Venetoclax in ER-Positive Metastatic Breast Cancer Post-CDK4/6 Inhibitors—Efficacy, Safety, and Biomarker Results. Clin. Cancer Res. 2022, 28, 3256–3267. [Google Scholar]
- Hardy-Bessard, A.-C.; Dalenc, F.; Bachelot, T.; Pierga, J.-Y.; Sabatier, R.; Dubot, C.; Frenel, J.-S.; Ferrero, J.M.; Ladoire, S.; Levy, C.; et al. PADA-1 investigators. Switch to fulvestrant and palbociclib versus no switch in advanced breast cancer with rising ESR1 mutation during aromatase inhibitor and palbociclib therapy (PADA-1): A randomised, open-label, multicentre, phase 3 trial. Lancet Oncol. 2022, 23, 1367–1377. [Google Scholar]
- Bidard, F.C.; Kaklamani, V.G.; Neven, P.; Streich, G.; Montero, A.J.; Forget, F.; Mouret-Reynier, M.A.; Sohn, J.H.; Taylor, D.; Harnden, K.K.; et al. Elacestrant (oral selective estrogen receptor degrader) Versus Standard Endocrine Therapy for Estrogen Receptor-Positive, Human Epidermal Growth Factor Receptor 2-Negative Advanced Breast Cancer: Results from the Randomized Phase III EMERALD Trial. J. Clin. Oncol. 2022, 40, 3246–3256, Erratum in J. Clin. Oncol. 2023, 41, 3962. [Google Scholar]
- Bardia, A.; Cortés, J.; Bidard, F.C.; Neven, P.; Garcia-Sáenz, J.; Aftimos, P.; O’Shaughnessy, J.; Lu, J.; Tonini, G.; Scartoni, S.; et al. Elacestrant in ER+, HER2− Metastatic Breast Cancer with ESR1-Mutated Tumors: Subgroup Analyses from the Phase III EMERALD Trial by Prior Duration of Endocrine Therapy plus CDK4/6 Inhibitor and in Clinical Subgroups. Clin. Cancer Res. 2024, 30, 4299–4309. [Google Scholar]
- Rugo, H.S.; O’Shaughnessy, J.; Cortes, J.; Bardia, A.; Hamilton, E.P.; Hurvitz, S.A.; Kaklamani, V.G.; Romero, P.M.; Vallespir, B.P.; Wasserman, T.; et al. Elacestrant in various combinations in patients (pts) with estrogen receptor-positive (ER+), HER2-negative (HER2−) locally advanced or metastatic breast cancer (adv/mBC): Preliminary data from ELEVATE, a phase 1b/2, open-label, umbrella study. J. Clin. Oncol. 2024, 42 (Suppl. S16), 1069. [Google Scholar]
- Oliveira, M.; Pominchuk, D.; Nowecki, Z.; Hamilton, E.; Kulyaba, Y.; Andabekov, T.; Hotko, Y.; Melkadze, T.; Nemsadze, G.; Neven, P.; et al. Camizestrant, a next-generation oral SERD, versus fulvestrant in post-menopausal women with oestrogen receptor-positive, HER2-negative advanced breast cancer (SERENA-2): A multi-dose, open-label, randomised, phase 2 trial. Lancet Oncol. 2024, 25, 1424–1439. [Google Scholar]
- Turner, N.; Huang-Bartlett, C.; Kalinsky, K.; Cristofanilli, M.; Bianchini, G.; Chia, S.; Iwata, H.; Janni, W.; Ma, C.X.; Mayer, E.L.; et al. Design of SERENA-6, a phase III switching trial of camizestrant in ESR1-mutant breast cancer during first-line treatment. Future Oncol. 2023, 19, 559–573. [Google Scholar] [PubMed]
- Jhaveri, K.L.; Neven, P.; Casalnuovo, M.L.; Kim, S.-B.; Tokunaga, E.; Aftimos, P.; Saura, C.; O’shaughnessy, J.; Harbeck, N.; Carey, L.A.; et al. EMBER-3 Study Group. Imlunestrant with or without Abemaciclib in Advanced Breast Cancer. N. Engl. J. Med. 2024, 392, 1189–1202. [Google Scholar] [PubMed]
- Liang, J.; Zbieg, J.R.; Blake, R.A.; Chang, J.H.; Daly, S.; DiPasquale, A.G.; Friedman, L.S.; Gelzleichter, T.; Gill, M.; Giltnane, J.M.; et al. GDC-9545 (Giredestrant): A Potent and Orally Bioavailable Selective Estrogen Receptor Antagonist and Degrader with an Exceptional Preclinical Profile for ER+ Breast Cancer. J. Med. Chem. 2021, 64, 11841–11856. [Google Scholar]
- Martín, M.; Lim, E.; Chavez-MacGregor, M.; Bardia, A.; Wu, J.; Zhang, Q.; Nowecki, Z.; Cruz, F.M.; Safin, R.; Kim, S.-B.; et al. Giredestrant for Estrogen Receptor-Positive, HER2-Negative, Previously Treated Advanced Breast Cancer: Results from the Randomized, Phase II acelERA Breast Cancer Study. J. Clin. Oncol. 2024, 42, 2149–2160. [Google Scholar] [PubMed]
- Miron, A.; Varadi, M.; Carrasco, D.; Li, H.; Luongo, L.; Kim, H.J.; Park, S.Y.; Cho, E.Y.; Lewis, G.; Kehoe, S.; et al. PIK3CA mutations in in situ and invasive breast carcinomas. Cancer Res. 2010, 70, 5674–5678. [Google Scholar]
- André, F.; Ciruelos, E.M.; Juric, D.; Loibl, S.; Campone, M.; Mayer, I.A.; Rubovszky, G.; Yamashita, T.; Kaufman, B.; Lu, Y.S.; et al. Alpelisib plus fulvestrant for PIK3CA-mutated, hormone receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Final overall survival results from SOLAR-1. Ann. Oncol. 2021, 32, 208–217. [Google Scholar]
- Rugo, H.S.; Lerebours, F.; Ciruelos, E.; Drullinsky, P.; Ruiz-Borrego, M.; Neven, P.; Park, Y.H.; Prat, A.; Bachelot, T.; Juric, D.; et al. Alpelisib plus fulvestrant in PIK3CA-mutated, hormone receptor-positive advanced breast cancer after a CDK4/6 inhibitor (BYLieve): One cohort of a phase 2, multicentre, open-label, non-comparative study. Lancet Oncol. 2021, 22, 489–498, Erratum in Lancet Oncol. 2021, 22, e184. [Google Scholar]
- Howell, S.J.; Casbard, A.; Carucci, M.; Ingarfield, K.; Butler, R.; Morgan, S.; Meissner, M.; Bale, C.; Bezecny, P.; Moon, S.; et al. Fulvestrant plus capivasertib versus placebo after relapse or progression on an aromatase inhibitor in metastatic, oestrogen receptor-positive, HER2-negative breast cancer (FAKTION): Overall survival, updated progression-free survival, and expanded biomarker analysis from a randomised, phase 2 trial. Lancet Oncol. 2022, 23, 851–864. [Google Scholar]
- Turner, N.C.; Oliveira, M.; Howell, S.J.; Dalenc, F.; Cortes, J.; Moreno, H.L.G.; Hu, X.; Jhaveri, K.; Krivorotko, P.; Loibl, S.; et al. Capivasertib in Hormone Receptor-Positive Advanced Breast Cancer. N. Engl. J. Med. 2023, 388, 2058–2070. [Google Scholar]
- Turner, N.C.; Im, S.-A.; Saura, C.; Juric, D.; Loibl, S.; Kalinsky, K.; Schmid, P.; Loi, S.; Sunpaweravong, P.; Musolino, A.; et al. Inavolisib-Based Therapy in PIK3CA-Mutated Advanced Breast Cancer. N. Engl. J. Med. 2024, 391, 1584–1596. [Google Scholar] [PubMed]
- Oliveira, M.; Bardia, A.; Kim, S.B.; Niikura, N.; Hernando, C.; Werutsky, G.; Antill, Y.; Liedke, P.; Oakman, C.; Tokunaga, E.; et al. Ipatasertib in combination with palbociclib (palbo) and fulvestrant (fulv) in patients with hormone receptor-positive HER2-negative advanced breast cancer. Cancer Res. 2022, 82 (Suppl. S4), P5-16-11. [Google Scholar]
- Piccart, M.; Hortobagyi, G.N.; Campone, M.; Pritchard, K.I.; Lebrun, F.; Ito, Y.; Noguchi, S.; Perez, A.; Rugo, H.S.; Deleu, I.; et al. Everolimus plus exemestane for hormone-receptor-positive, human epidermal growth factor receptor-2-negative advanced breast cancer: Overall survival results from BOLERO-2. Ann. Oncol. 2014, 25, 2357–2362. [Google Scholar] [CrossRef]
- Dhakal, A.; Thomas, R.A.; Levine, E.G.; Brufsky, A.; Takabe, K.; Hanna, M.G.; Attwood, K.; Miller, A.; Khoury, T.; Early, A.P.; et al. Outcome of Everolimus-Based Therapy in Hormone-Receptor-Positive Metastatic Breast Cancer Patients After Progression on Palbociclib. Breast Cancer 2020, 14, 1178223420944864. [Google Scholar] [CrossRef]
- Cook, M.M.; Al Rabadi, L.; Kaempf, A.J.; Saraceni, M.M.; Savin, M.A.; Mitri, Z.I. Everolimus Plus Exemestane Treatment in Patients with Metastatic Hormone Receptor-Positive Breast Cancer Previously Treated with CDK4/6 Inhibitor Therapy. Oncologist 2021, 26, 101–106. [Google Scholar] [CrossRef] [PubMed]
- Layman, R.M.; Han, H.S.; Rugo, H.S.; Stringer-Reasor, E.M.; Specht, J.M.; Dees, E.C.; Kabos, P.; Suzuki, S.; Mutka, S.C.; Sullivan, B.F.; et al. Gedatolisib in combination with palbociclib and endocrine therapy in women with hormone receptor-positive, HER2-negative advanced breast cancer: Results from the dose expansion groups of an open-label, phase 1b study. Lancet Oncol. 2024, 25, 474–487. [Google Scholar] [CrossRef]
- Dumontet, C.; Reichert, J.M.; Senter, P.D.; Lambert, J.M.; Beck, A. Antibody-drug conjugates come of age in oncology. Nat. Rev. Drug Discov. 2023, 22, 641–661. [Google Scholar]
- Staudacher, A.H.; Brown, M.P. Antibody drug conjugates and bystander killing: Is antigen-dependent internalisation required? Br. J. Cancer 2017, 117, 1736–1742. [Google Scholar] [PubMed]
- Modi, S.; Jacot, W.; Yamashita, T.; Sohn, J.; Vidal, M.; Tokunaga, E.; Tsurutani, J.; Ueno, N.T.; Prat, A.; Chae, Y.S.; et al. Trastuzumab Deruxtecan in Previously Treated HER2-Low Advanced Breast Cancer. N. Engl. J. Med. 2022, 387, 9–20. [Google Scholar] [CrossRef]
- Bardia, A.; Hu, X.; Dent, R.; Yonemori, K.; Barrios, C.H.; O’shaughnessy, J.A.; Wildiers, H.; Pierga, J.-Y.; Zhang, Q.; Saura, C.; et al. DESTINY-Breast06 Trial Investigators. Trastuzumab Deruxtecan after Endocrine Therapy in Metastatic Breast Cancer. N. Engl. J. Med. 2024, 391, 2110–2122. [Google Scholar] [CrossRef]
- Curigliano, G.; Hu, X.; Dent, R.A.; Yonemori, K.; Barrios, C.H.; O’Shaughnessy, J.; Wildiers, H.; Zhang, Q.; Im, S.-A.; Saura, C.; et al. Trastuzumab deruxtecan (T-DXd) vs. physician’s choice of chemotherapy (TPC) in patients (pts) with hormone receptor-positive (HR+), human epidermal growth factor receptor 2 (HER2)-low or HER2-ultralow metastatic breast cancer (mBC) with prior endocrine therapy (ET): Primary results from DESTINY-Breast06 (DB-06). J. Clin. Oncol. 2024, 42 (Suppl. S17), LBA1000. [Google Scholar]
- Syed, Y.Y. Sacituzumab Govitecan: First Approval. Drugs 2020, 80, 1019–1025. [Google Scholar] [CrossRef] [PubMed]
- Vidula, N.; Yau, C.; Rugo, H. Trophoblast Cell Surface Antigen 2 gene (TACSTD2) expression in primary breast cancer. Breast Cancer Res. Treat. 2022, 194, 569–575. [Google Scholar]
- Rugo, H.S.; Bardia, A.; Marmé, F.; Cortes, J.; Schmid, P.; Loirat, D.; Trédan, O.; Ciruelos, E.; Dalenc, F.; Pardo, P.G.; et al. Sacituzumab Govitecan in Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor 2-Negative Metastatic Breast Cancer. J. Clin. Oncol. 2022, 40, 3365–3376. [Google Scholar] [CrossRef]
- Rugo, H.; Bardia, A.; Marmé, F.; Cortés, J.; Schmid, P.; Loirat, D. Sacituzumab Govitecan (SG) vs Treatment of Physician’s Choice (TPC): Efficacy by Trop-2 Expression in the TROPiCS-02 Study of Patients (Pts) With HR+/HER2– Metastatic Breast Cancer (mBC). Cancer Res. 2023, 83 (Suppl. S5), GS1-11. [Google Scholar]
- Meric-Bernstam, F.; Krop, I.; Juric, D.; Kogawa, T.; Hamilton, E.; Spira, A.I.; Mukohara, T.; Tsunoda, T.; Damodaran, S.; Greenberg, J.; et al. Phase 1 TROPION-PanTumor01 Study Evaluating Datopotamab Deruxtecan (Dato-DXd) in Unresectable or Metastatic Hormone Receptor–Positive/HER2–Negative Breast Cancer (BC). Cancer Res. 2023, 83 (Suppl. S5), PD13-08. [Google Scholar]
- Bardia, A.; Jhaveri, K.; Im, S.-A.; De Laurentiis, M.; Xu, B.; Pernas, S.; Borges, G.; Cescon, D.; Hattori, M.; Lu, Y.-S.; et al. Randomized phase 3 study of datopotamab deruxtecan vs. chemotherapy for patients with previously-treated inoperable or metastatic hormone receptor-positive, HER2-negative breast cancer: Results from TROPION-Breast01. Cancer Res. 2024, 84 (Suppl. S9), GS02-01. [Google Scholar]
- Bardia, A.; Jhaveri, K.; Im, S.A.; Pernas, S.; De Laurentiis, M.; Wang, S.; Martínez Jañez, N.; Borges, G.; Cescon, D.W.; Hattori, M.; et al. TROPION-Breast01 Investigators. Datopotamab Deruxtecan Versus Chemotherapy in Previously Treated Inoperable/Metastatic Hormone Receptor-Positive Human Epidermal Growth Factor Receptor 2-Negative Breast Cancer: Primary Results From TROPION-Breast01. J. Clin. Oncol. 2024, 43, JCO2400920. [Google Scholar]
- Mayer, E.L.; Ren, Y.; Wagle, N.; Mahtani, R.; Ma, C.; DeMichele, A.; Cristofanilli, M.; Meisel, J.; Miller, K.D.; Abdou, Y.; et al. PACE: A Randomized Phase II Study of Fulvestrant, Palbociclib, and Avelumab After Progression on Cyclin-Dependent Kinase 4/6 Inhibitor and Aromatase Inhibitor for Hormone Receptor-Positive/Human Epidermal Growth Factor Receptor-Negative Metastatic Breast Cancer. J. Clin. Oncol. 2024, 42, 2050–2060. [Google Scholar] [PubMed]
- Cussac, A.L.; Harper-Wynne, C.; Perello, A.; Hennequin, A.; Fernandez, A.; Colleoni, M.; Carañana, V.; Quiroga, V.; Medioni, J.; Iranzo, V.; et al. Second-line endocrine therapy (ET) with or without palbociclib (P) maintenance in patients (pts) with hormone receptor-positive (HR[+])/human epidermal growth factor receptor 2-negative (HER2[−]) advanced breast cancer (ABC): PALMIRA trial. J. Clin. Oncol. 2023, 41 (Suppl. S16), 1001. [Google Scholar]
- Kalinski, K.; Bianchini, G.; Hamilton, E.P.; Graff, S.L.; Park, K.H.; Jeselsohn, R.; Demirci, U.; Martin, M.; Layman, R.M.; Hurvitz, S.A.; et al. Abemaciclib plus fulvestrant vs fulvestrant alone for HR+, HER2− advanced breast cancer following progression on a prior CDK4/6 inhibitor plus endocrine therapy: Primary outcome of the phase 3 postMONARCH trial. J. Clin. Oncol. 2024, 42 (Suppl. S17), LBA1001. [Google Scholar]
- Raheem, F.; Karikalan, S.A.; Batalini, F.; El Masry, A.; Mina, L. Metastatic ER+ Breast Cancer: Mechanisms of Resistance and Future Therapeutic Approaches. Int. J. Mol. Sci. 2023, 24, 16198. [Google Scholar] [CrossRef] [PubMed]
- Patel, R.; Klein, P.; Tiersten, A.; Sparano, J.A. An emerging generation of endocrine therapies in breast cancer: A clinical perspective. NPJ Breast Cancer 2023, 9, 20. [Google Scholar]
- Parisian, A.D.; Barratt, S.A.; Hodges-Gallagher, L.; Ortega, F.E.; Peña, G.; Sapugay, J.; Robello, B.; Sun, R.; Kulp, D.; Palanisamy, G.S.; et al. Palazestrant (OP-1250), A Complete Estrogen Receptor Antagonist, Inhibits Wild-type and Mutant ER-positive Breast Cancer Models as Monotherapy and in Combination. Mol. Cancer Ther. 2024, 23, 285–300. [Google Scholar] [PubMed]
- Lainé, M.; E Greene, M.; Kurleto, J.D.; Bozek, G.; Leng, T.; Huggins, R.J.; Komm, B.S.; Greene, G.L. Lasofoxifene as a potential treatment for aromatase inhibitor-resistant ER-positive breast cancer. Breast Cancer Res. 2024, 26, 95. [Google Scholar]
- Goetz, M.P.; Bagegni, N.A.; Batist, G.; Brufsky, A.; Cristofanilli, M.A.; Damodaran, S.; Daniel, B.R.; Fleming, G.F.; Gradishar, W.J.; Graff, S.L.; et al. Lasofoxifene versus fulvestrant for ER+/HER2− metastatic breast cancer with an ESR1 mutation: Results from the randomized, phase II ELAINE 1 trial. Ann. Oncol. 2023, 34, 1141–1151. [Google Scholar] [PubMed]
- Damodaran, S.; O’Sullivan, C.C.; Elkhanany, A.; Anderson, I.C.; Barve, M.; Blau, S.; Cherian, M.A.; Peguero, J.A.; Goetz, M.P.; Plourde, P.V.; et al. Open-label, phase II, multicenter study of lasofoxifene plus abemaciclib for treating women with metastatic ER+/HER2− breast cancer and an ESR1 mutation after disease progression on prior therapies: ELAINE 2. Ann. Oncol. 2023, 34, 1131–1140. [Google Scholar] [CrossRef]
- Goetz, M.P.; Wander, S.A.; Bachelot, T.; Batist, G.; Cortes, J.; Cristofanilli, M.; Curigliano, G.; de Nonneville, A.; Gal-Yam, E.N.; Jhaveri, K.L.; et al. Open-label, randomized, multicenter, phase 3, ELAINE 3 study of the efficacy and safety of lasofoxifene plus abemaciclib for treating ER+/HER2−, locally advanced or metastatic breast cancer with an ESR1 mutation. J. Clin. Oncol. 2024, 42 (Suppl. S16), TPS1127. [Google Scholar]
- Fanning, S.W.; Jeselsohn, R.; Dharmarajan, V.; Mayne, C.G.; Karimi, M.; Buchwalter, G.; Houtman, R.; Toy, W.; Fowler, C.E.; Han, R.; et al. The SERM/SERD bazedoxifene disrupts ESR1 helix 12 to overcome acquired hormone resistance in breast cancer cells. eLife 2018, 7, e37161. [Google Scholar] [CrossRef]
- Tsuji, J.; Li, T.; Grinshpun, A.; Coorens, T.; Russo, D.; Anderson, L.; Rees, R.; Nardone, A.; Patterson, C.; Lennon, N.J.; et al. Clinical Efficacy and Whole-Exome Sequencing of Liquid Biopsies in a Phase IB/II Study of Bazedoxifene and Palbociclib in Advanced Hormone Receptor-Positive Breast Cancer. Clin. Cancer Res. 2022, 28, 5066–5078. [Google Scholar]
- Furman, C.; Puyang, X.; Zhang, Z.; Wu, Z.J.; Banka, D.; Aithal, K.B.; Albacker, L.A.; Hao, M.-H.; Irwin, S.; Kim, A.; et al. Covalent ERα Antagonist H3B-6545 Demonstrates Encouraging Preclinical Activity in Therapy-Resistant Breast Cancer. Mol. Cancer Ther. 2022, 21, 890–902. [Google Scholar]
- Hamilton, E.P.; Wang, J.S.; Pluard, T.; Johnston, S.R.D.; Morikawa, A.; Dees, E.C.; Jones, R.H.; Haley, B.B.; Armstrong, A.C.; Cohen, A.L.; et al. H3B-6545, a novel selective estrogen receptor covalent antagonist (SERCA), in estrogen receptor positive (ER+), human epidermal growth factor receptor 2 negative (HER2−) advanced breast cancer—A phase II study. Cancer Res. 2022, 82, P1-17-10. [Google Scholar] [CrossRef]
- Xiong, R.; Patel, H.K.; Gutgesell, L.M.; Zhao, J.; Delgado-Rivera, L.; Pham, T.N.D.; Zhao, H.; Carlson, K.; Martin, T.; Katzenellenbogen, J.A.; et al. Selective Human Estrogen Receptor Partial Agonists (ShERPAs) for Tamoxifen-Resistant Breast Cancer. J. Med. Chem. 2016, 59, 219–237. [Google Scholar] [CrossRef]
- Dudek, A.Z.; Liu, L.C.; Fischer, J.H.; Wiley, E.L.; Sachdev, J.C.; Bleeker, J.; Hurley, R.W.; Tonetti, D.A.; Thatcher, G.R.J.; Venuti, R.P.; et al. Phase 1 study of TTC-352 in patients with metastatic breast cancer progressing on endocrine and CDK4/6 inhibitor therapy. Breast Cancer Res. Treat. 2020, 183, 617–627. [Google Scholar] [CrossRef]
- Bondeson, D.P.; Mares, A.; Smith, I.E.D.; Ko, E.; Campos, S.; Miah, A.H.; Mulholland, K.E.; Routly, N.; Buckley, D.L.; Gustafson, J.L.; et al. Catalytic in vivo protein knockdown by small-molecule PROTACs. Nat. Chem. Biol. 2015, 11, 611–617. [Google Scholar] [CrossRef] [PubMed]
- Snyder, L.B.; Flanagan, J.J.; Qian, Y.; Gough, S.M.; Andreoli, M.; Bookbinder, M.; Cadelina, G.; Bradley, J.; Rousseau, E.; Chandler, J.; et al. The discovery of ARV-471, an orally bioavailable estrogen receptor degrading PROTAC for the treatment of patients with breast cancer. AACR Meeting 2021. Cancer Res. 2021, 81, 44. [Google Scholar] [CrossRef]
- Hamilton, E.; Vahdat, L.; Han, H.; Ranciato, J.; Gedrich, R.; Keung, C.F.; Chirnomas, D.; Hurvitz, S. First-in-human safety and activity of ARV-471, a novel PROTAC® estrogen receptor degrader, in ER+/HER2− locally advanced or metastatic breast cancer. Cancer Res. 2022, 82, PD13-08. [Google Scholar] [CrossRef]
- Schott, A.F.; Hurvitz, S.; Ma, C.; Hamilton, A.P.; Nanda, R.; Zahrah, G.; Hunter, N.; Tan, A.R.; Telli, M.L.; Mesias, J.A.; et al. GS3-03 ARV-471, a PROTAC® estrogen receptor (ER) degrader in advanced ER-positive/human epidermal growth factor receptor 2 (HER2)-negative breast cancer: Phase 2 expansion (VERITAC) of a phase 1/2 study. Cancer Res. 2023, 83, GS3-03. [Google Scholar] [CrossRef]
- Gough, S.M.; Flanagan, J.J.; Teh, J.; Andreoli, M.; Rousseau, E.; Pannone, M.; Bookbinder, M.; Willard, R.; Davenport, K.; Bortolon, E.; et al. Oral Estrogen Receptor PROTAC Vepdegestrant (ARV-471) Is Highly Efficacious as Monotherapy and in Combination with CDK4/6 or PI3K/mTOR Pathway Inhibitors in Preclinical ER+ Breast Cancer Models. Clin. Cancer Res. 2024, 30, 3549–3563. [Google Scholar] [CrossRef]
- Ricciardelli, C.; Bianco-Miotto, T.; Jindal, S.; Butler, L.M.; Leung, S.; McNeil, C.M.; O’Toole, S.A.; Ebrahimie, E.; Millar, E.K.A.; Sakko, A.J.; et al. The Magnitude of Androgen Receptor Positivity in Breast Cancer Is Critical for Reliable Prediction of Disease Outcome. Clin. Cancer Res. 2018, 24, 2328–2341. [Google Scholar] [CrossRef]
- Khan, A.F.; Karami, S.; Peidl, A.S.; Waiters, K.D.; Babajide, M.F.; Bawa-Khalfe, T. Androgen Receptor in Hormone Receptor-Positive Breast Cancer. Int. J. Mol. Sci. 2023, 25, 476. [Google Scholar] [CrossRef]
- Krop, I.; Abramson, V.G.; Colleoni, M.; Traina, T.A.; Holmes, F.; Garcia-Estevez, L.; Hart, L.; Awada, A.; Zamagni, C.; Morris, P.G.; et al. A Randomized Placebo Controlled Phase II Trial Evaluating Exemestane with or without Enzalutamide in Patients with Hormone Receptor-Positive Breast Cancer. Clin. Cancer Res. 2020, 26, 6149–6157. [Google Scholar] [CrossRef]
- Wei, L.; Gao, H.; Yu, J.; Zhang, H.; Nguyen, T.T.L.; Gu, Y.; Passow, M.R.; Carter, J.M.; Qin, B.; Boughey, J.C.; et al. Pharmacological Targeting of Androgen Receptor Elicits Context-Specific Effects in Estrogen Receptor-Positive Breast Cancer. Cancer Res. 2023, 83, 456–470. [Google Scholar] [CrossRef]
- Palmieri, C.; Linden, H.M.; Birrell, S.; Lim, E.; Schwartzberg, L.S.; Rugo, H.S.; Cobb, P.W.; Jain, K.; Vogel, C.L.; O’Shaughnessy, J.; et al. Efficacy of enobosarm, a selective androgen receptor (AR) targeting agent, correlates with the degree of AR positivity in advanced AR+/estrogen receptor (ER)+ breast cancer in an international phase 2 clinical study. J. Clin. Oncol. 2021, 39, 1020. [Google Scholar] [CrossRef]
- Rinn, K.; Linden, H.; Schwartzberg, L.; Barnette, G.; Rodriguez, D.; Shalev, I.; Steiner, M.; Brufsky, A.; O’Shaughnessy, J. Design of Active Phase 3 ENABLAR-2 Study Evaluating Enobosarm +/− Abemaciclib in Patients with AR+ER+HER2− 2nd-Line Metastatic Breast Cancer Following Tumor Progression on an Estrogen Blocking Agent Plus Palbociclib or Ribociclib. Cancer Res. 2024, 84 (Suppl. S9), PO4-27-06. [Google Scholar]
- Song, X.; Fang, C.; Dai, Y.; Sun, Y.; Qiu, C.; Lin, X.; Xu, R. Cyclin-dependent kinase 7 (CDK7) inhibitors as a novel therapeutic strategy for different molecular types of breast cancer. Br. J. Cancer 2024, 130, 1239–1248. [Google Scholar] [CrossRef]
- Guarducci, C.; Nardone, A.; Russo, D.; Nagy, Z.; Heraud, C.; Grinshpun, A.; Zhang, Q.; Freelander, A.; Leventhal, M.J.; Feit, A.; et al. Selective CDK7 Inhibition Suppresses Cell Cycle Progression and MYC Signaling While Enhancing Apoptosis in Therapy-resistant Estrogen Receptor-positive Breast Cancer. Clin. Cancer Res. 2024, 30, 1889–1905. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhang, T.; Kwiatkowski, N.; Abraham, B.J.; Lee, T.I.; Xie, S.; Yuzugullu, H.; Von, T.; Li, H.; Lin, Z.; et al. CDK7-dependent transcriptional addiction in triple-negative breast cancer. Cell. 2015, 163, 174–186. [Google Scholar] [CrossRef] [PubMed]
- Patel, H.; Periyasamy, M.; Sava, G.P.; Bondke, A.; Slafer, B.W.; Kroll, S.H.B.; Barbazanges, M.; Starkey, R.; Ottaviani, S.; Harrod, A.; et al. ICEC0942, an Orally Bioavailable Selective Inhibitor of CDK7 for Cancer Treatment. Mol. Cancer Ther. 2018, 17, 1156–1166. [Google Scholar] [CrossRef] [PubMed]
- Patel, M.R.; Juric, D.; Henick, B.S.; Duska, L.R.; Wu, R.; Guo, J.; Zhang, H.; Newberry, K.; Rinne, M.; Yap, T.A. BLU-222, an oral, potent, and selective CDK2 inhibitor, in patients with advanced solid tumors: Phase 1 monotherapy dose escalation. J. Clin. Oncol. 2023, 41 (Suppl. S16), 3095. [Google Scholar] [CrossRef]
- Yap, T.A.; Elhaddad, A.M.; Grisham, R.N.; Hamm, J.T.; Marks, D.K.; Shapiro, G.; Le Corre, C.; Li, J.; Lin, T.T.; Liu, F.; et al. First-in-human phase 1/2a study of a potent and novel CDK2-selective inhibitor PF-07104091 in patients (pts) with advanced solid tumors, enriched for CDK4/6 inhibitor resistant HR+/HER2− breast cancer. J. Clin. Oncol. 2023, 41 (Suppl. S16), 3010. [Google Scholar] [CrossRef]
- Goldberg, J.; Qiao, N.; Guerriero, J.L.; Gross, B.; Meneksedag, Y.; Lu, Y.F.; Philips, A.V.; Rahman, T.; Meric-Bernstam, F.; Roszik, J.; et al. Estrogen Receptor Mutations as Novel Targets for Immunotherapy in Metastatic Estrogen Receptor-positive Breast Cancer. Cancer Res. Commun. 2024, 4, 496–504. [Google Scholar] [CrossRef]
- Dailey, G.P.; Rabiola, C.A.; Lei, G.; Wei, J.; Yang, X.-Y.; Wang, T.; Liu, C.-X.; Gajda, M.; Hobeika, A.C.; Summers, A.; et al. Vaccines targeting ESR1 activating mutations elicit anti-tumor immune responses and suppress estrogen signaling in therapy resistant ER+ breast cancer. Hum. Vaccin. Immunother. 2024, 20, 2309693. [Google Scholar] [PubMed]
- Nicolini, A.; Carpi, A. Beta-interferon and interleukin-2 prolong more than three times the survival of 26 consecutive endocrine dependent breast cancer patients with distant metastases: An exploratory trial. Biomed. Pharmacother. 2005, 59, 253–263. [Google Scholar] [PubMed]
- Nicolini, A.; Carpi, A.; Ferrari, P.; Biava, P.M.; Rossi, G. Immunotherapy and Hormone-therapy in Metastatic Breast Cancer: A Review and an Update. Curr. Drug Targets 2016, 17, 1127–1139. [Google Scholar]
- Nicolini, A.; Rossi, G.; Ferrari, P.; Morganti, R.; Carpi, A. A new immunotherapy schedule in addition to first-line hormone therapy for metastatic breast cancer patients in a state of clinical benefit during hormone therapy. J. Mol. Med. 2020, 98, 375–382. [Google Scholar] [PubMed]
- Nicolini, A.; Rossi, G.; Ferrari, P.; Morganti, R.; Carpi, A. Final results of a 2:1 control–case observational study using interferon beta and interleukin-2, in addition to first-line hormone therapy, in estrogen receptor-positive, endocrine-responsive metastatic breast cancer patients. J. Cancer Metastasis Treat. 2022, 8, 13. [Google Scholar]
- Sultan, R.; Ahmed, A.; Wei, L.; Saeed, H.; Ishaq, M. The anticancer potential of chemical constituents of Moringa oleifera targeting CDK-2 inhibition in estrogen receptor positive breast cancer using in-silico and in vitro approches. BMC Complement. Med. Ther. 2023, 23, 396. [Google Scholar] [CrossRef]
- Pandithar, S.; Galke, D.; Akume, A.; Belyakov, A.; Lomonaco, D.; Guerra, A.A.; Park, J.; Reff, O.; Jin, K. The role of CXCL1 in crosstalk between endocrine resistant breast cancer and fibroblast. Mol. Biol. Rep. 2024, 51, 331. [Google Scholar]
- Pastò, B.; Vida, R.; Dri, A.; Foffano, L.; Della Rossa, S.; Gerratana, L.; Puglisi, F. Beyond Hormone Receptors: Liquid biopsy tools to unveil new clinical meanings and empower therapeutic decision-making in Luminal-like metastatic breast cancer. Breast 2024, 79, 103859. [Google Scholar]
- Foffano, L.; Cucciniello, L.; Nicolò, E.; Migliaccio, I.; Noto, C.; Reduzzi, C.; Malorni, L.; Cristofanilli, M.; Gerratana, L.; Puglisi, F. Cyclin-dependent kinase 4 and 6 inhibitors (CDK4/6i): Mechanisms of resistance and where to find them. Breast 2024, 79, 103863. [Google Scholar]
- Fusco, N.; Malapelle, U. Next-generation sequencing for PTEN testing in HR+/HER2− metastatic breast cancer. Crit. Rev. Oncol. Hematol. 2025, 207, 104626. [Google Scholar]


| (A) | ||||
| Study (Phase) | Therapy | Setting | Main Endpoints | Reference |
| CONFIRM (III) | Fulvestrant 500 mg vs. 250 mg | Postmenopausal MBC women progressing on ET | mPFS 6.5 months mOS 26.4 months | [115] |
| MAINTAIN (Control arm) | Fulvestrant 500 mg | ABC patients progressed on ET plus a CDK4/6i | mPFS 2.76 months | [118] |
| VERONICA (Control arm) | Fulvestrant 500 mg | MBC patients, post CDK4/6i progression | m PFS 1.94 months | [119] |
| EMERALD (III) | Elacestrant vs. standard ET | ABC patients after 1–2 lines of ET, including a CDK4/6i and ≤1 chemotherapy | 6-month PFS rate: 34.3% vs. 20.4% in all patients; 40.8% vs. 19.1% in patients with ESR1 mutation 12-month PFS rate: 22.3% vs. 9.4% in all patients; 26.8% and 8.2% in patients with ESR1 mutation | [121] |
| ELEVATE (Ib/II) | Elacestrant in combination with alpelisib, or capivasertib, or everolimus, or palbociclib, or abemaciclib, or ribociclib | ABC/MBC, progressing on one or up to two prior lines of ET (inclusion criteria differ in every study arm) | Primary: PFS, safety Secondary: ORR, DOR, CBR, OS | [123] |
| ADELA (III) | Elacestrant plus everolimus vs. elacestrant alone | ABC patients with ESR1-mutated tumors progressing on ET plus CDK4/6i | Primary: PFS Secondary: OS, ORR, CBR, DOR, safety and quality of life | NCT06382948 |
| SERENA-2 (II) | Camizestrant once daily at 75 mg, 150 mg, or 300 mg vs. fulvestrant at 500 mg | ABC patients progressing on at least one line of ET and no more than one previous ET in the advanced setting | mPFS 7.2 months with camizestrant 75 mg, 7.7 with 150 mg, vs. 3.7 months with fulvestrant | [124] |
| SERENA-6 (III) | Camizestrant plus CDK4/6i maintaining | ABC patients progressing on AI plus CDK4/6i upon detection of ESR1m in ctDNA before clinical disease progression | Primary endpoint: PFS; secondary: CT-free survival, PFS2, OS, safety | NCT04964934 |
| EMBER-3 (III) | Imlunestrant vs. monoET (88% fulvestrant) vs. imlunestrant plus abemaciclib | ABC patients recurring or progressing during or after AI alone or AI plus a CDK4/6i (59.8%) | Patients with ESR1 mutations: mPFS 5.5 months with imlunestrant vs. 3.8 with monoET Overall population: mPFS 5.6 months with imlunestrant vs. 5.5 with monoET mPFS 9.4 months with imlunestrant plus abemaciclib vs. 5.5 with imlunestrant regardless of ESR1-mutation status | [126] |
| acelERA Breast Cancer Study (II) | Giredestrant vs. physician’s choice of endocrine monotherapy (PCET) | MBC patients progressing after 1–2 lines of systemic therapy; 1 previous targeted agent, 1 line of chemotherapy, and prior fulvestrant were allowed | Overall population: mPFS 5.6 months in the giredestrant arm vs. 5.4 months in the PCET arm. ESR1 mutated patients: mPFS 5.3 months vs. 3.5 months. | [128] |
| MORPHEUS-Breast cancer (I-IIb umbrella study) Cohort 1 | Giredestrant alone vs. giredestrant in combination with abemaciclib, or ipatasertib, or inavolisib, or ribociclib, or everolimus, or samuraciclib, or atezolizumab | ABC/MBC patients progressing on AI + CDK4/6i as first or second line | Primary: ORR, safety Secondary: PFS, DCR, CBR, OS, DOR | NCT04802759 |
| (B) | ||||
| Study (Phase) | Therapy | Setting | Main Endpoints | Reference |
| SOLAR-1 (III) | Alpelisib plus fulvestrant vs. placebo plus fulvestrant | ABC patients progressing on or after AI | mOS 39.3 vs. 31.4 months in the PIK3CA-mutated cohort | [130] |
| BYLieve (II) | Alpelisib plus fulvestrant | ABC patients with tumor PIK3CA mutation, progressing on or after previous ET, including CDK4/6i | 53.8% of patients were alive without disease progression at 6 months, after a median follow-up of 21.8 months | [131] |
| CAPItello-291 (III) | Capivasertib plus fulvestrant vs. placebo plus fulvestrant | ABC patients relapsed or progressed during or after treatment with an AI, with or without CDK4/6i | mPFS 7.2 vs. 3.6 months in the overall population mPFS 7.3 vs. 3.1 months in patients with AKT pathway alterations | [133] |
| INAVO-120 (III) | Inavolisib plus palbociclib-fulvestrant vs. placebo plus palbociclib-fulvestrant | PIK3CA-mutated ABC/MBC patients relapsed during or within 12 months after the completion of adjuvant ET | mPFS 15.0 months vs. 7.3 months ORR 58.4% vs. 25.0% | [134] |
| INAVO-121 (III) | Inavolisib plus fulvestrant vs. alpelisib plus fulvestrant | PIK3CA-mutated ABC/MBC patients progressing after ET plus CDK4/6i | Primary: PFS Secondary: ORR, CBR, DOR, OS | NCT05646862 |
| BOLERO-2 (III) | Everolimus plus exemestane vs. placebo plus exemestane | Patients who had recurrence or progression on NSAI | mPFS 6.9 months vs. 2.8 months | [32] |
| Retrospective | Everolimus plus ET | MBC patients progressing on palbociclib | mPFS 4.2 months, mOS 18.7 months | [137] |
| Retrospective | Everolimus plus exemestane | MBC progressing on an NSAI alone or in association with a CDK4/6i (40%) | No significant difference in mPFS (3.6 vs. 4.2 months) or mOS (15.6 vs. 11.3 months) between patients who had received prior CDK4/6is and those who had not | [138] |
| IPATunity-150 (Ib) | Ipatasertib plus fulvestrant plus palbociclib | Patients relapsed during adjuvant ET who had not previously received a CDK4/6i | ORR 45%, mDOR 9.6 months | [135] |
| FINER (III) | Ipatasertib plus fulvestrant vs. placebo plus fulvestrant | ABC patients progressing on AI plus CDK4/6i | Primary: PFS Secondary: PFS in PIK3CA/AKT1/PTEN altered and non-altered cohorts, RR, DOR, CBR, OS, TSST, safety | NCT04650581 |
| VIKTORIA-1 (III) | Gedatolisib plus fulvestrant with or without palbociclib vs. standard-of-care ET | ABC/MBC patients progressing on AI plus CDK4/6i | Primary: PFS Secondary: OS, ORR, DOR, TTR, CBR | NCT05501886 |
| (C) | ||||
| Study (Phase) | Therapy | Setting | Main Endpoints | Reference |
| Destiny-Breast-04 (III) | TDX-d vs. physician’s choice of CT. | HR+/HER2low and HR-/HER2low MBC patients progressing after CT for metastatic disease or within 6 months after completing adjuvant CT; HR+ patients must have received at least 1 line of ET | mPFS in HR+ cohort 10.1 vs. 5.4 months | [142] |
| Destiny-Breast-06 (III) | TDX-d vs. physician’s choice of CT | ER+/HER2 low or ultralow MBC patients progressing after one or more lines of ET and no previous CT for MBC | HER2-low disease: mPFS 13.2 months vs. 8.1 HER2-ultralow disease: mPFS 13.2 months vs. 8.3 | [143,144] |
| TROPiCS-02 (III) | Sacituzumab-Govitecan vs. physician’s CT | MBC patients progressed after at least two prior systemic CT regimens for metastatic disease. Patients must have received at least one taxane, at least one ET, and at least one CDK4/6i | mPFS 5.5 vs. 4.0 months | [147,148] |
| TROPION-Breast-01 (III) | Dato-DXd vs. ICCT (eribulin or vinorelbine or capecitabine or gemcitabine) | ABC patients progressing on ET and after having received 1–2 prior lines of CT | mPFS 6.9 vs. 4.9 months At 12 months, 25.5% of patients in the Dato-DXd arm versus 14.6% in the ICCT arm were progression-free OS data immature | [150,151] |
| (D) | ||||
| Study (Phase) | Therapy | Setting | Main Endpoints | Reference |
| MAINTAIN (II) | Stop CDK4/6i, switch ET (fulvestrant or exemestane), and randomize to receive ribociclib or placebo | MBC patients progressing during ET and CDK4/6i (86.5% palbociclib and 11.7% ribociclib) | mPFS 5.29 months in patients assigned to switched ET plus ribociclib, 2.76 in patients switched ET plus placebo | [118] |
| PACE (II) | Fulvestrant (F) vs. fulvestrant plus palbociclib (F + P) vs. fulvestrant plus palbociclib and avelumab (F + P + A) | MBC patients progressing on previous AI plus CDK4/6i (90.9% palbociclib) | mPFS: 4.8 months on F, 4.6 months on F + P, 8.1 on F + P + A The difference in PFS with F + P and F + P + A versus F was greater among patients with baseline ESR1 and PIK3CA alteration | [152] |
| PALMIRA (II) | Palbociclib (P) maintenance plus second-line ET | ABC patients progressing on first-line palbociclib plus ET (AI or fulvestrant) | PFS was 4.2 months (95% CI 3.5–5.8) in the P + ET vs. 3.6 months (95% CI 2.7–4.2) in the ET arm (hazard ratio 0.8, 95% CI 0.6–1.1, p = 0.206). The 6-month PFS rate was 40.9% and 28.6% for P + ET and ET, respectively. Among 138 pts with measurable disease, no significant differences were observed in ORR (6.4% vs. 2.3%) or CBR (33.0% vs. 29.5%) for P + ET and ET, respectively | [153] |
| Post-MONARCH (III) | Abemaciclib + fulvestrant vs. placebo + fulvestrant | MBC patients progressing on prior CDK4/6i plus AI or recurred on/after adjuvant CDK4/6i + ET | mPFS 6.0 versus 5.3 months, regardless of ESR1 or PIK3CA mutations | [154] |
| (E) | ||||
| Study (Phase) | Therapy | Setting | Main Endpoints | Reference |
| Opera-01 (III) | Palazestrant vs. standard ET (fulvestrant or an AI) | ABC patients progressing after 1 or 2 prior lines of standard ET, including a CDK 4/6i | Primary: dose selection, PFS Secondary: OS, ORR, CBR, DOR, safety in patients with and without ESR1 mutation | NCT06016738 |
| ELAINE-2 (II) | Lasofoxifene plus abemaciclib | ESR1-mutated MBC patients progressing on prior ET, including CDK4/6i | mPFS 56.0 weeks PFS rates at 6, 12, and 18 months: 76.1%, 56.1%, and 38.8%, respectively. CBR at 24 weeks: 65.5% | [160] |
| ELAINE-3 | Lasofoxifene plus abemaciclib vs. fulvestrant plus abemaciclib | ABC patients with ≥1 acquired ESR1 mutation, progressing on AI plus palbociclib or ribociclib; ≤1 line of chemotherapy in the advanced/metastatic setting | Primary: PFS Secondary: ORR, OS, CBR, DOR, TTR, safety | NCT05696626 |
| VERITAC (I–II) | Vepdegestrant | MBC patients pretreated with anti-estrogens plus CDK 4/6is | CBR up to 38.9% and 54.5% in the overall population and in patients with ESR1-mutated tumors, respectively | [170] |
| I−II (NCT04072952) | Vepdegestrant plus palbociclib | Pretreated MBC patients | CBR 63% in ITT population, 72.4% in ESR1 mutant patients | [171] |
| VERITAC-2 (III) | Vepdegestrant vs. fulvestrant | Patients progressing after 1st line ET plus CDK4/6i | Primary: PFS in the ITT population and ESR1 mutation-positive subpopulation. Secondary: OS, RR, safety | NCT05654623 |
| II (NCT02463032) | Enobosarm 9 mg or 18 mg daily | Pretreated MBC patients | CBR of 32% and 29%, depending on the dose used. ORR 48% and 0% in patients with more or less than 40% AR staining, respectively | 177 |
| ENABLAR-2 (2-staged, phase III) | Enobosarm +/− Abemaciclib | AR+ER+HER2− MBC patients progressing after ET + Palbociclib or Ribociclib | PFS, OS, ORR, CBR | [178] |
| Multimodular I–II (NCT03363893) | Samuraciclib (dose escalation) plus fulvestrant | MBC patients progressing after an AI plus a CDK4/6i | CBR 36% | [111] |
| SUMIT-BS (II) | Samuraciclib plus fulvestrant versus fulvestrant alone | ABC/MBC patients progressing after AI plus CDK4/6i | Primary: CBR Secondary: ORR, DOR, PFS, safety | NCT05963984 |
| SUMIT-ELA (Ib-II) | Samuraciclib plus elacestrant | ABC/MBC patients progressing after AI plus CDK4/6i | Primary: dose, PFS Secondary: ORR, CBR, DOR, safety | NCT05963997 |
| Retrospective observational study | ET in combination with cyclic interferon beta and interleukin-2 | Endocrine-dependent MBC patients (also see text) | mPFS 33 months mOS 81 months | [190] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ferrari, P.; Schiavone, M.L.; Scatena, C.; Nicolini, A. Molecular Mechanisms and Therapeutic Strategies to Overcome Resistance to Endocrine Therapy and CDK4/6 Inhibitors in Advanced ER+/HER2− Breast Cancer. Int. J. Mol. Sci. 2025, 26, 3438. https://doi.org/10.3390/ijms26073438
Ferrari P, Schiavone ML, Scatena C, Nicolini A. Molecular Mechanisms and Therapeutic Strategies to Overcome Resistance to Endocrine Therapy and CDK4/6 Inhibitors in Advanced ER+/HER2− Breast Cancer. International Journal of Molecular Sciences. 2025; 26(7):3438. https://doi.org/10.3390/ijms26073438
Chicago/Turabian StyleFerrari, Paola, Maria Luisa Schiavone, Cristian Scatena, and Andrea Nicolini. 2025. "Molecular Mechanisms and Therapeutic Strategies to Overcome Resistance to Endocrine Therapy and CDK4/6 Inhibitors in Advanced ER+/HER2− Breast Cancer" International Journal of Molecular Sciences 26, no. 7: 3438. https://doi.org/10.3390/ijms26073438
APA StyleFerrari, P., Schiavone, M. L., Scatena, C., & Nicolini, A. (2025). Molecular Mechanisms and Therapeutic Strategies to Overcome Resistance to Endocrine Therapy and CDK4/6 Inhibitors in Advanced ER+/HER2− Breast Cancer. International Journal of Molecular Sciences, 26(7), 3438. https://doi.org/10.3390/ijms26073438







