Exploring the Role of Gut Microbiota and Probiotics in Acute Pancreatitis: A Comprehensive Review
Abstract
1. Introduction
2. Microbiota Modification During Acute Pancreatis (AP)
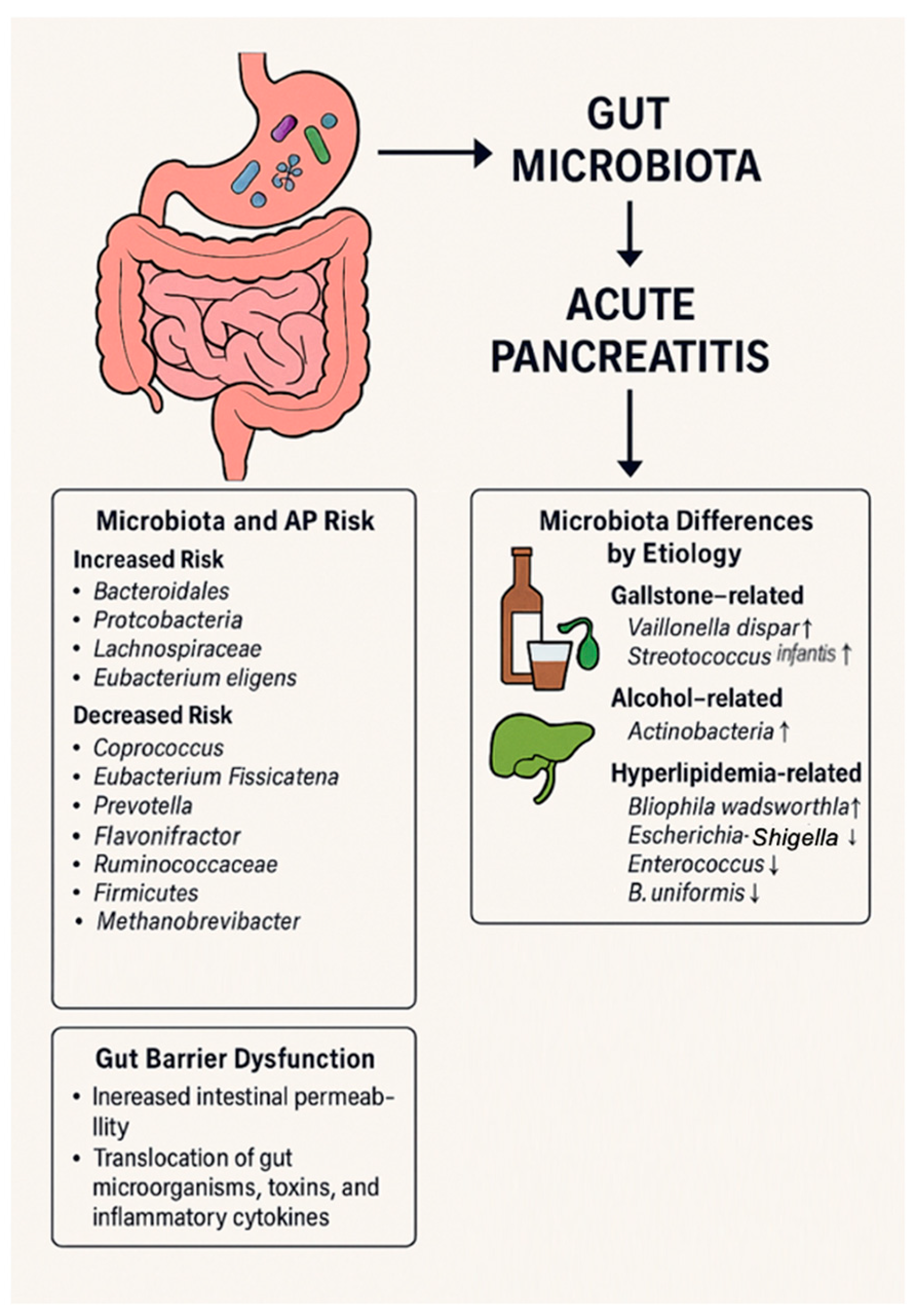
2.1. Gut Microbiota as a Risk Factor of Acute Pancreatitis
2.2. Microbiota Differences Based on AP Etiology
2.3. Altered Microbial Communities and Disease Severity
2.4. Gut Barrier Alteration
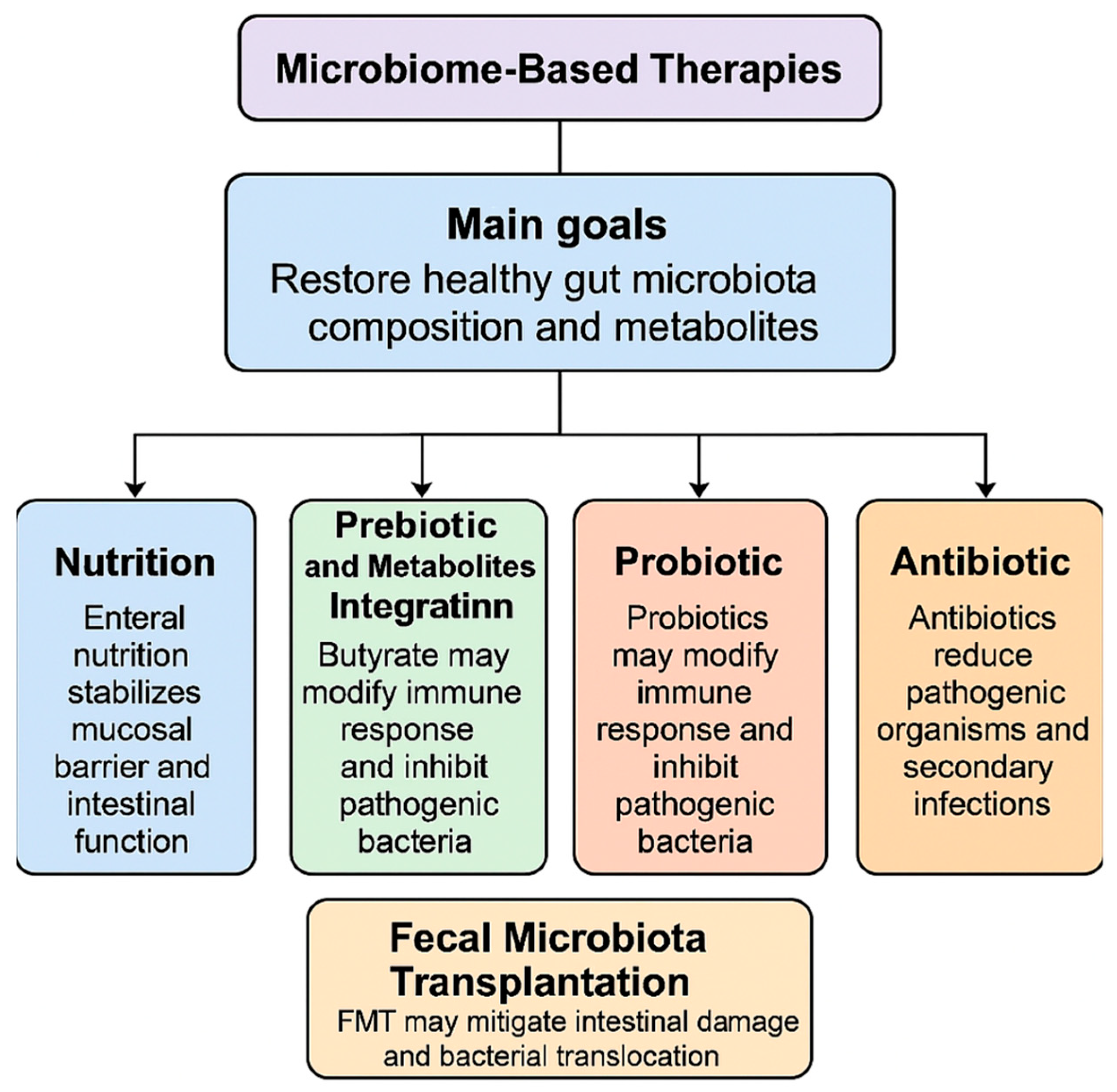
3. Potential Microbiota-Based Therapeutic Target for AP
3.1. Nutrition
3.2. Prebiotic and Metabolites Integration
3.3. Probiotic
3.4. Antibiotic
3.5. Role of Fecal Microbiota Transplantation in AP
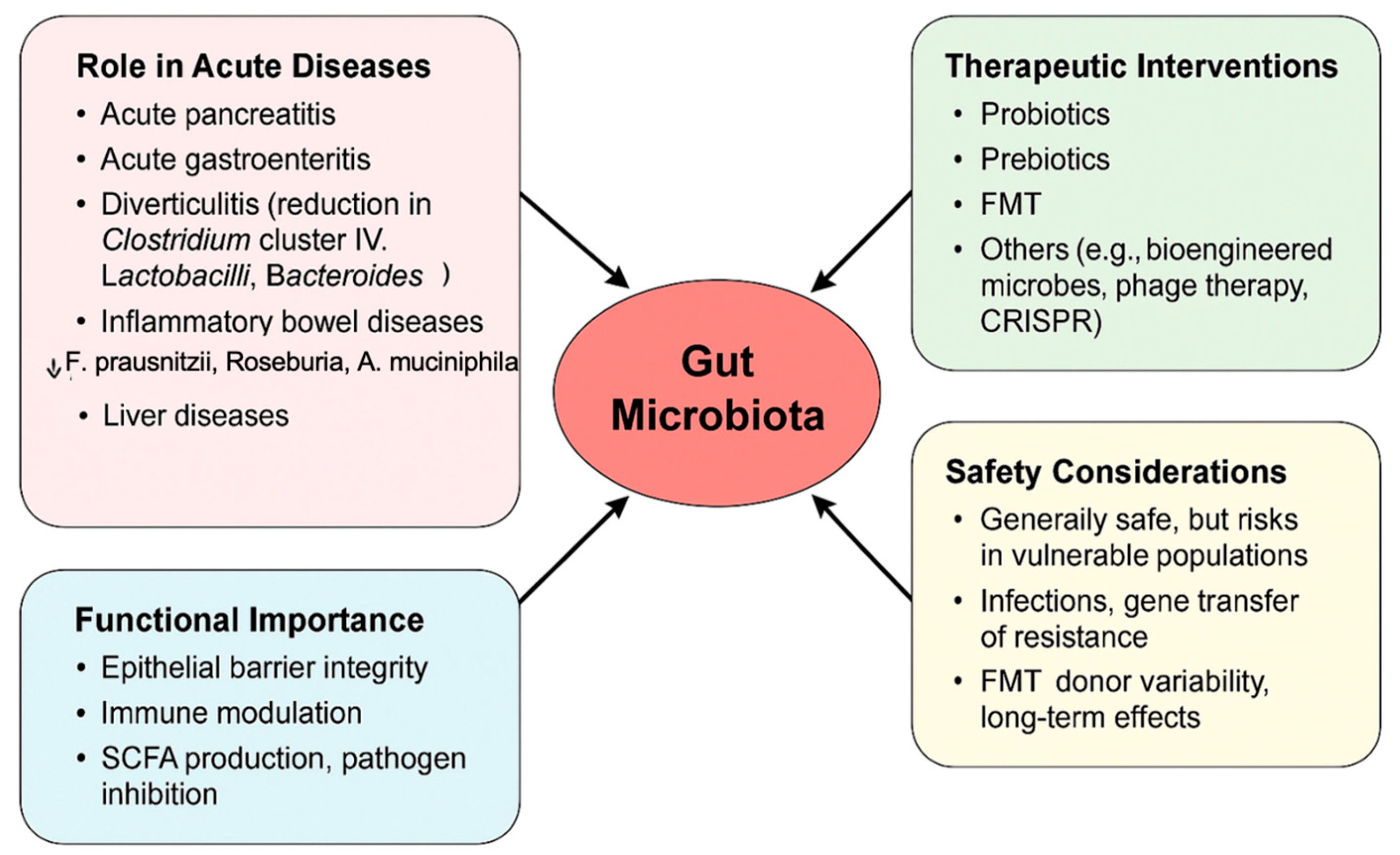
4. Experience of Microbiome Modulation in Other Acute Settings
4.1. Safety of Microbiota-Based Therapy
4.2. Standardization Issues
5. Challenges and Perspectives
6. Conclusions
Funding
Conflicts of Interest
References
- Ahmed, A.; Kothari, D.J.; Wardlaw, S.; Freedman, S.D.; Sheth, S.G. Reducing hospitalization in mild acute pancreatitis: Results of long-term follow-up. J. Clin. Gastroenterol. 2020, 55, 180–186. [Google Scholar] [CrossRef] [PubMed]
- Mayerle, J.; Sendler, M.; Hegyi, E.; Beyer, G.; Lerch, M.M.; Sahin-Tóth, M. Genetics, cell biology, and pathophysiology of pancreatitis. Gastroenterology 2019, 156, 1951–1968.e1. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Habtezion, A. Inflammation in acute and chronic pancreatitis. Curr. Opin. Gastroenterol. 2015, 31, 395–399. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Hong, W.; Pan, J.; Goyal, H.; Zippi, M. Editorial: Acute pancreatitis infection: Epidemiology, prevention, clinical characteristics, treatment, and prediction. Front. Cell. Infect. Microbiol. 2023, 13, 1175195. [Google Scholar] [CrossRef]
- Li, X.; He, C.; Li, N.; Ding, L.; Chen, H.; Wan, J.; Yang, X.; Xia, L.; He, W.; Xiong, H.; et al. The interplay between the gut microbiota and NLRP3 activation affects the severity of acute pancreatitis in mice. Gut Microbes 2020, 11, 1774–1789, Erratum in: Gut Microbes 2024, 16, 2409026. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- El-Sayed, A.; Aleya, L.; Kamel, M. Microbiota’s role in health and diseases. Environ. Sci. Pollut. Res. 2021, 28, 36967–36983. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Li, X.-Y.; He, C.; Zhu, Y.; Lu, N.-H. Role of gut microbiota on intestinal barrier function in acute pancreatitis. World J. Gastroenterol. 2020, 26, 2187–2193. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Wang, Z.; Liu, J.; Wang, Y.; Guo, H.; Li, F.; Cao, Y.; Zhao, L.; Chen, H. Identification of key biomarkers associated with immunogenic cell death and their regulatory mechanisms in severe acute pancreatitis based on wgcna and machine learning. Int. J. Mol. Sci. 2023, 24, 3033. [Google Scholar] [CrossRef]
- Wang, Z.; Liu, J.; Li, F.; Luo, Y.; Ge, P.; Zhang, Y.; Wen, H.; Yang, Q.; Ma, S.; Chen, H. The gut-lung axis in severe acute pancreatitis-associated lung injury: The protection by the gut microbiota through short-chain fatty acids. Pharmacol. Res. 2022, 182, 106321. [Google Scholar] [CrossRef] [PubMed]
- van Dijk, S.M.; Hallensleben, N.D.L.; van Santvoort, H.C.; Fockens, P.; van Goor, H.; Bruno, M.J.; Besselink, M.G. Dutch Pancreatitis Study Group. Acute pancreatitis: Recent advances through randomised trials. Gut 2017, 66, 2024–2032. [Google Scholar] [CrossRef] [PubMed]
- Gao, Z.; Yin, S.; Jin, K.; Nie, W.; Wang, L.; Cheng, L. Effectiveness and safety of probiotics on patients with severe acute pancreatitis: A systematic review and meta-analysis. Medicine 2023, 102, e36454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cao, F.; Jin, L.; Gao, Y.; Ding, Y.; Wen, H.; Qian, Z.; Zhang, C.; Hong, L.; Yang, H.; Zhang, J.; et al. Artificial-enzymes-armed Bifidobacterium longum probiotics for alleviating intestinal inflammation and microbiota dysbiosis. Nat. Nanotechnol. 2023, 18, 617–627. [Google Scholar] [CrossRef] [PubMed]
- Bongaerts, G.P.; Severijnen, R.S. A reassessment of the PROPATRIA study and its implications for probiotic therapy. Nat. Biotechnol. 2016, 34, 55–63. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Lih, T.M.; Lee, J.W.; Ohtsuka, T.; Hozaka, Y.; Mino-Kenudson, M.; Adsay, N.V.; Luchini, C.; Scarpa, A.; Maker, A.V.; et al. Multi-omic profiling of intraductal papillary neoplasms of the pancreas reveals distinct expression patterns and potential markers of progression. bioRxiv 2024. [CrossRef] [PubMed] [PubMed Central]
- Melzer, M.K.; Kleger, A. Acute pancreatitis: Murine model systems unravel disease-modifying genes with potential implications for diagnostics and patient stratification. United Eur. Gastroenterol. J. 2022, 10, 618–619. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Mallya, K.; Gautam, S.K.; Aithal, A.; Batra, S.K.; Jain, M. Modeling pancreatic cancer in mice for experimental therapeutics. Biochim. Biophys. Acta BBA Rev. Cancer 2021, 1876, 188554. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Cui, H.; Zhao, Y.; Xiong, S.; Feng, Y.; Li, P.; Lv, Y.; Chen, Q.; Wang, R.; Xie, P.; Luo, Z.; et al. Diagnosing solid lesions in the pancreas with multimodal artificial intelligence: A randomized crossover trial. JAMA Netw. Open 2024, 7, e2422454. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Korfiatis, P.; Suman, G.; Patnam, N.G.; Trivedi, K.H.; Karbhari, A.; Mukherjee, S.; Cook, C.; Klug, J.R.; Patra, A.; Khasawneh, H.; et al. Automated artificial intelligence model trained on a large data set can detect pancreas cancer on diagnostic computed tomography scans as well as visually occult preinvasive cancer on prediagnostic computed tomography scans. Gastroenterology 2023, 165, 1533–1546.e4. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Kumar, L.; Kumar, S.; Sandeep, K.; Patel, S.K.S. Therapeutic approaches in pancreatic cancer: Recent updates. Biomedicines 2023, 11, 1611. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Chen, Z.; Zhang, S.; Dong, S.; Xu, H.; Zhou, W. Association of the microbiota and pancreatic cancer: Opportunities and limitations. Front. Immunol. 2022, 13, 844401. [Google Scholar] [CrossRef]
- Memba, R.; Duggan, S.N.; Chonchubhair, H.M.N.; Griffin, O.M.; Bashir, Y.; O’Connor, D.B.; Murphy, A.; McMahon, J.; Volcov, Y.; Ryan, B.M. The potential role of gut microbiota in pancreatic disease: A systematic review. Pancreatology 2017, 17, 867–874. [Google Scholar] [CrossRef] [PubMed]
- Afify, S.M.; Hassan, G.; Seno, A.; Seno, M. Cancer-inducing niche: The force of chronic inflammation. Br. J. Cancer 2022, 127, 193–201. [Google Scholar] [CrossRef]
- Nista, E.C.; Del Gaudio, A.; Del Vecchio, L.E.; Mezza, T.; Pignataro, G.; Piccioni, A.; Gasbarrini, A.; Franceschi, F.; Candelli, M. Pancreatic cancer resistance to treatment: The role of microbiota. Biomedicines 2023, 11, 157. [Google Scholar] [CrossRef]
- Ammer-Herrmenau, C.; Antweiler, K.L.; Asendorf, T.; Beyer, G.; Buchholz, S.M.; Cameron, S.; Capurso, G.; Damm, M.; Dang, L.; Frost, F.; et al. Gut microbiota predicts severity and reveals novel metabolic signatures in acute pancreatitis. Gut 2023, 73, 485–495. [Google Scholar] [CrossRef]
- Wu, L.; Hu, J.; Yi, X.; Lv, J.; Yao, J.; Tang, W.; Zhang, S.; Wan, M. Gut microbiota interacts with inflammatory responses in acute pancreatitis. Ther. Adv. Gastroenterol. 2023, 16, 17562848231202133. [Google Scholar] [CrossRef] [PubMed]
- Medveczky, P.; Szmola, R.; Sahin-Tóth, M. Proteolytic activation of human pancreatitis-associated protein is required for peptidoglycan binding and bacterial aggregation. Biochem. J. 2009, 420, 335–344. [Google Scholar] [CrossRef]
- Doyle, C.J.; Yancey, K.; Pitt, H.A.; Wang, M.; Bemis, K.; Yip-Schneider, M.T.; Sherman, S.T.; Lillemoe, K.D.; Goggins, M.D.; Schmidt, C.M.M. The proteome of normal pancreatic juice. Pancreas 2012, 41, 186–194. [Google Scholar] [CrossRef] [PubMed]
- Stenwall, A.; Ingvast, S.; Skog, O.; Korsgren, O. Characterization of host defense molecules in the human pancreas. Islets 2019, 11, 89–101. [Google Scholar] [CrossRef]
- Hogan, P.G. The STIM1-ORAI1 microdomain. Cell Calcium 2015, 58, 357–367. [Google Scholar] [CrossRef]
- Sun, J.; Furio, L.; Mecheri, R.; van der Does, A.M.; Lundeberg, E.; Saveanu, L.; Chen, Y.; van Endert, P.; Agerberth, B.; Diana, J. Pancreatic β-cells limit autoimmune diabetes via an immunoregulatory antimicrobial peptide expressed under the influence of the gut microbiota. Immunity 2015, 43, 304–317. [Google Scholar] [CrossRef]
- Ahuja, M.; Schwartz, D.M.; Tandon, M.; Son, A.; Zeng, M.; Swaim, W.; Eckhaus, M.; Hoffman, V.; Cui, Y.; Xiao, B.; et al. Orai1-mediated antimicrobial secretion from pancreatic acini shapes the gut microbiome and regulates gut 1 innate immunity. Cell Metab. 2017, 25, 635–646. [Google Scholar] [CrossRef] [PubMed]
- Malmstrøm, M.L.; Hansen, M.B.M.; Andersen, A.M.; Ersbøll, A.K.M.; Nielsen, O.H.M.; Jørgensen, L.N.M.; Novovic, S. Cytokines and organ failure in acute pancreatitis: Inflammatory response in acute pancreatitis. Pancreas 2012, 41, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Kylänpää, M.; Repo, H.; Puolakkainen, P.A. Inflammation and immunosuppression in severe acute pancreatitis. World J. Gastroenterol. 2010, 16, 2867–2872. [Google Scholar] [CrossRef]
- Elfar, M.; Gaber, L.W.; Sabek, O.; Fischer, C.P.; Gaber, A.O. The inflammatory cascade in acute pancreatitis: Relevance to clinical disease. Surg. Clin. N. Am. 2007, 87, 1325–1340. [Google Scholar] [CrossRef] [PubMed]
- Tan, C.; Ling, Z.; Huang, Y.; Cao, Y.; Liu, Q.; Cai, T.; Yuan, H.; Liu, C.; Li, Y.; Xu, K. Dysbiosis of intestinal microbiota associated with inflammation involved in the progression of acute pancreatitis. Pancreas 2015, 44, 868–875. [Google Scholar] [CrossRef]
- Wahlström, A.; Sayin, S.I.; Marschall, H.; Bäckhed, F. Intestinal crosstalk between bile acids and microbiota and its impact on host metabolism. Cell Metab. 2016, 24, 41–50. [Google Scholar] [CrossRef]
- van den Berg, F.F.; van Dalen, D.; Hyoju, S.K.; van Santvoort, H.C.; Besselink, M.G.; Wiersinga, W.J.; Zaborina, O.; A Boermeester, M.; Alverdy, J. Western-type diet influences mortality from necrotising pancreatitis and demonstrates a central role for butyrate. Gut 2020, 70, 915–927. [Google Scholar] [CrossRef]
- Almeida, A.; Mitchell, A.L.; Boland, M.; Forster, S.C.; Gloor, G.B.; Tarkowska, A.; Lawley, T.D.; Finn, R.D. A new genomic blueprint of the human gut microbiota. Nature 2019, 568, 499–504. [Google Scholar] [CrossRef]
- Zhang, X.M.; Zhang, Z.Y.; Zhang, C.H.; Wu, J.; Wang, Y.X.; Zhang, G.X. Intestinal microbial community differs between acute pancreatitis patients and healthy volunteers. Biomed. Environ. Sci. 2018, 31, 81–86. [Google Scholar] [CrossRef]
- Chen, J.; Huang, C.; Wang, J.; Zhou, H.; Lu, Y.; Lou, L.; Zheng, J.; Tian, L.; Wang, X.; Cao, Z.; et al. Dysbiosis of intestinal microbiota and decrease in paneth cell antimicrobial peptide level during acute necrotizing 3 pancreatitis in rats. PLoS ONE 2017, 12, e0176583. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Q.; Li, S.; Jiao, J.; Hao, Y.; Zhang, G.; Zhang, Q.; Luo, F.; Zhang, Y.; Lv, Q.; et al. Integrative metagenomic and metabolomic analyses reveal the potential of gut microbiota to exacerbate acute pancreatitis. NPJ Biofilms Microbiomes 2024, 10, 29. [Google Scholar] [CrossRef] [PubMed]
- Roehrborn, A.; Thomas, L.; Potreck, O.; Ebener, C.; Ohmann, C.; Goretzki, P.E.; Röher, H.D. The microbiology of postoperative peritonitis. Clin. Infect. Dis. 2001, 33, 1513–1519. [Google Scholar] [CrossRef][Green Version]
- Sasaki, M.; Sitaraman, S.V.; Babbin, B.A.; Gerner-Smidt, P.; Ribot, E.M.; Garrett, N.; Alpern, J.A.; Akyildiz, A.; Theiss, A.L.; Nusrat, A.; et al. Invasive Escherichia coli are a feature of crohn’s disease. Lab. Investig. 2007, 87, 1042–1054. [Google Scholar] [CrossRef]
- Gevers, D.; Kugathasan, S.; Denson, L.A.; Vázquez-Baeza, Y.; Van Treuren, W.; Ren, B.; Schwager, E.; Knights, D.; Song, S.J.; Yassour, M.; et al. The treatment-naive microbiome in new-onset crohn’s disease. Cell Host Microbe 2014, 15, 382–392. [Google Scholar] [CrossRef] [PubMed]
- Jurić, I.; Primorac, D.; Žagar, Ž.; Biocǐć, M.; Pavïć, S.; Furlan, D.; Budimir, D.; Janković, S.; Hodžić, P.K.; Alfirević, D.; et al. Frequency of portal and systemic bacteremia in acute appendicitis. Pediatr. Int. 2001, 43, 152–156. [Google Scholar] [CrossRef]
- Liu, J.; Yan, Q.; Luo, F.; Shang, D.; Wu, D.; Zhang, H.; Shang, X.; Kang, X.; Abdo, M.; Liu, B.; et al. Acute cholecystitis associated with infection of enterobacteriaceae from gut microbiota. Clin. Microbiol. Infect. 2015, 21, 851.e1–851.e9. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Cui, M.; Jiang, Q.; Wang, J.; Fan, M.; Lu, Y. Characterization of duodenal microbiota in patients with acute pancreatitis and healthy controls. Dig. Dis. Sci. 2023, 68, 3341–3353. [Google Scholar] [CrossRef]
- Thomas, R.M.; Jobin, C. Microbiota in pancreatic health and disease: The next frontier in microbiome research. Nat. Rev. Gastroenterol. Hepatol. 2019, 17, 53–64. [Google Scholar] [CrossRef]
- Cook, T.M.; Mansuy-Aubert, V. Communication between the gut microbiota and peripheral nervous system in health and chronic disease. Gut Microbes 2022, 14, 2068365. [Google Scholar] [CrossRef]
- Guda, N.M.; Trikudanathan, G.; Freeman, M.L. Idiopathic recurrent acute pancreatitis. Lancet Gastroenterol. Hepatol. 2018, 3, 720–728. [Google Scholar] [CrossRef]
- Zhou, F.; Liu, Y.; Shi, Y.; Wu, N.; Xie, Y.; Zhou, X. Association between gut microbiota and acute pancreatitis: A bidirectional mendelian randomization study. J. Gastroenterol. Hepatol. 2024, 39, 1895–1902. [Google Scholar] [CrossRef] [PubMed]
- Qu, C.; Lu, J.; Chen, Y.; Li, J.; Xu, X.; Li, F. Unravelling the role of gut microbiota in acute pancreatitis: Integrating mendelian randomization with a nested case-control study. Front. Microbiol. 2024, 15, 1401056. [Google Scholar] [CrossRef] [PubMed]
- Zhu, S.J.; Ding, Z. Association between gut microbiota and seven gastrointestinal diseases: A mendelian randomized study. J. Gene Med. 2023, 26, e3623. [Google Scholar] [CrossRef]
- Nan, B.; Jin, L.; Wang, T.; Long, C.; Zhao, H.; Wang, C.; Zhang, W. Correlation between gut microbiota and pancreatitis: A bidirectional mendelian randomization. Eur. J. Gastroenterol. Hepatol. 2024, 37, 62–69. [Google Scholar] [CrossRef] [PubMed]
- Porcari, S.; Mullish, B.H.; Asnicar, F.; Ng, S.C.; Zhao, L.; Hansen, R.; O’Toole, P.W.; Raes, J.; Hold, G.; Putignani, L.; et al. International consensus statement on microbiome testing in clinical practice. Lancet Gastroenterol. Hepatol. 2024, 10, 154–167. [Google Scholar] [CrossRef]
- Feng, Z.; Long, W.; Hao, B.; Ding, D.; Ma, X.; Zhao, L.; Pang, X. A human stool-derived Bilophila wadsworthia strain caused systemic inflammation in specific-pathogen-free mice. Gut Pathog. 2017, 9, 59. [Google Scholar] [CrossRef]
- Weiner, S.; Gramatica, L.; Voegle, L.D.; Hauman, R.L.; Anderson, M.C. Role of the lymphatic system in the pathogenesis of inflammatory disease in the biliary tract and pancreas. Am. J. Surg. 1970, 119, 55–61. [Google Scholar] [CrossRef]
- Wang, D.; Doestzada, M.; Chen, L.; Andreu-Sánchez, S.; Munckhof, I.C.v.D.; Augustijn, H.E.; Koehorst, M.; Ruiz-Moreno, A.J.; Bloks, V.W.; Riksen, N.P.; et al. Characterization of gut microbial structural variations as determinants of human bile acid metabolism. Cell Host Microbe 2021, 29, 1802–1814.e5. [Google Scholar] [CrossRef]
- Yun, K.E.; Kim, J.; Kim, M.-H.; Park, E.; Kim, H.-L.; Chang, Y.; Ryu, S.; Kim, H.-N. Major lipids, apolipoproteins, and alterations of gut microbiota. J. Clin. Med. 2020, 9, 1589. [Google Scholar] [CrossRef]
- Hu, X.; Gong, L.; Zhou, R.; Han, Z.; Ji, L.; Zhang, Y.; Zhang, S.; Wu, D. Variations in gut microbiome are associated with prognosis of hypertriglyceridemia-associated acute pancreatitis. Biomolecules 2021, 11, 695. [Google Scholar] [CrossRef]
- Li, G.; Liu, L.; Lu, T.; Sui, Y.; Zhang, C.; Wang, Y.; Zhang, T.; Xie, Y.; Xiao, P.; Zhao, Z.; et al. Gut microbiota aggravates neutrophil extracellular traps-induced pancreatic injury in hypertriglyceridemic pancreatitis. Nat. Commun. 2023, 14, 6179. [Google Scholar] [CrossRef]
- Li, H.; Xu, H.; Li, Y.; Jiang, Y.; Hu, Y.; Liu, T.; Tian, X.; Zhao, X.; Zhu, Y.; Wang, S.; et al. Alterations of gut microbiota contribute to the progression of unruptured intracranial aneurysms. Nat. Commun. 2020, 11, 3218. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Zhang, Y.; Li, R.; Deng, S.; Qin, Q.; Ran, C.; Hao, Y.; Zhang, J.; Zhu, L. Mechanism of taurine reducing inflammation and organ injury in sepsis mice. Cell. Immunol. 2022, 375, 104503. [Google Scholar] [CrossRef]
- Stacy, A.; Andrade-Oliveira, V.; McCulloch, J.A.; Hild, B.; Oh, J.H.; Perez-Chaparro, P.J.; Sim, C.K.; Lim, A.I.; Link, V.M.; Enamorado, M.; et al. Infection trains the host for microbiota-enhanced resistance to pathogens. Cell 2021, 184, 615–627.e17. [Google Scholar] [CrossRef] [PubMed]
- Honda, M.; Kubes, P. Neutrophils and neutrophil extracellular traps in the liver and gastrointestinal system. Nat. Rev. Gastroenterol. Hepatol. 2018, 15, 206–221. [Google Scholar] [CrossRef] [PubMed]
- Leppkes, M.; Maueröder, C.; Hirth, S.; Nowecki, S.; Günther, C.; Billmeier, U.; Paulus, S.; Biermann, M.; Munoz, L.E.; Hoffmann, M.; et al. Externalized decondensed neutrophil chromatin occludes pancreatic ducts and drives pancreatitis. Nat. Commun. 2016, 7, 10973. [Google Scholar] [CrossRef]
- Tran, Q.T.; Sendler, M.; Wiese, M.L.; Doller, J.; Zierke, L.; Gischke, M.; Glaubitz, J.; Tran, V.H.; Lalk, M.; Bornscheuer, U.T.; et al. Systemic bile acids affect the severity of acute pancreatitis in mice depending on their hydrophobicity and the disease pathogenesis. Int. J. Mol. Sci. 2022, 23, 13592. [Google Scholar] [CrossRef]
- Philips, C.A.; Phadke, N.; Ganesan, K.; Rajesh, S.; Padsalgi, G.; Ahamed, R.; John, S.K.; Valiathan, G.C.; Augustine, P. Gut microbiota in alcoholic hepatitis is disparate from those in acute alcoholic pancreatitis and biliary disease. J. Clin. Exp. Hepatol. 2019, 9, 690–698. [Google Scholar] [CrossRef]
- Ciocan, D.; Rebours, V.; Voican, C.S.; Wrzosek, L.; Puchois, V.; Cassard, A.-M.; Perlemuter, G. Characterization of intestinal microbiota in alcoholic patients with and without alcoholic hepatitis or chronic alcoholic pancreatitis. Sci. Rep. 2018, 8, 4822. [Google Scholar] [CrossRef]
- Qin, X.; Deitch, E.A. Dissolution of lipids from mucus: A possible mechanism for prompt disruption of gut barrier function by alcohol. Toxicol. Lett. 2014, 232, 356–362. [Google Scholar] [CrossRef]
- Mutlu, E.A.; Gillevet, P.M.; Rangwala, H.; Sikaroodi, M.; Naqvi, A.; Engen, P.A.; Kwasny, M.; Lau, C.K.; Keshavarzian, A. Colonic microbiome is altered in alcoholism. Am. J. Physiol. Liver Physiol. 2012, 302, 966. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Han, Z.; Zhou, R.; Su, W.; Gong, L.; Yang, Z.; Song, X.; Zhang, S.; Shu, H.; Wu, D. Altered gut microbiota in the early stage of acute pancreatitis were related to the occurrence of acute respiratory distress syndrome. Front. Cell. Infect. Microbiol. 2023, 13, 1127369. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Y.; He, C.; Li, X.; Cai, Y.; Hu, J.; Liao, Y.; Zhao, J.; Xia, L.; He, W.; Liu, L.; et al. Gut microbiota dysbiosis worsens the severity of acute pancreatitis in patients and mice. J. Gastroenterol. 2018, 54, 347–358. [Google Scholar] [CrossRef] [PubMed]
- Zou, M.; Yang, Z.; Fan, Y.; Gong, L.; Han, Z.; Ji, L.; Hu, X.; Wu, D. Gut microbiota on admission as predictive biomarker for acute necrotizing pancreatitis. Front. Immunol. 2022, 13, 988326. [Google Scholar] [CrossRef]
- Li, Q.; Wang, C.; Tang, C.; He, Q.; Li, N.; Li, J. Bacteremia in patients with acute pancreatitis as revealed by 16S ribosomal RNA gene-based techniques. Crit. Care Med. 2013, 41, 1938–1950. [Google Scholar] [CrossRef]
- Fishman, J.E.; Levy, G.; Alli, V.; Zheng, X.; Mole, D.J.; Deitch, E.A. The intestinal mucus layer is a critical component of the gut barrier that is damaged during acute pancreatitis. Shock 2014, 42, 264–270. [Google Scholar] [CrossRef]
- Wang, H.; Chen, Y.; Han, Y.; Mu, S.; Wei, W.; Lan, L.; Li, X.; Xiang, H.; Tong, C.; Du, S. Comparative study of gut microbiota and metabolite variations between severe and mild acute pancreatitis patients at different stages. Microb. Pathog. 2024, 198, 107030. [Google Scholar] [CrossRef]
- Frossard, J.; Steer, M.L.; Pastor, C.M. Acute pancreatitis. Lancet 2008, 371, 143–152. [Google Scholar] [CrossRef]
- Rychter, J.W.; van Minnen, L.P.; Verheem, A.; Timmerman, H.M.; Rijkers, G.T.; Schipper, M.E.; Gooszen, H.G.; Akkermans, L.M.; Kroese, A.B. Pretreatment but not treatment with probiotics abolishes mouse intestinal barrier dysfunction in acute pancreatitis. Surgery 2009, 145, 157–167. [Google Scholar] [CrossRef]
- Capurso, G.; Zerboni, G.; Signoretti, M.; Valente, R.; Stigliano, S.; Piciucchi, M.; Fave, G.D. Role of the gut barrier in acute pancreatitis. J. Clin. Gastroenterol. 2012, 46, S46–S51. [Google Scholar] [CrossRef]
- Ammori, B.J. Role of the gut in the course of severe acute pancreatitis. Pancreas 2003, 26, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Marshall, J.C.; Christou, N.V.; Meakins, J.L. The gastrointestinal tract. the “undrained abscess” of multiple organ failure. Ann. Surg. 1993, 218, 111–119. [Google Scholar] [CrossRef]
- Swank, G.M.; Deitch, E.A. Role of the gut in multiple organ failure: Bacterial translocation and permeability changes. World J. Surg. 1996, 20, 411–417. [Google Scholar] [CrossRef]
- Geirnaert, A.; Calatayud, M.; Grootaert, C.; Laukens, D.; Devriese, S.; Smagghe, G.; De Vos, M.; Boon, N.; Van De Wiele, T. Butyrate-producing bacteria supplemented in vitro to crohn’s disease patient microbiota increased butyrate production and enhanced intestinal epithelial barrier integrity. Sci. Rep. 2017, 7, 11450. [Google Scholar] [CrossRef]
- Knudsen, K.E.B.; Lærke, H.N.; Hedemann, M.S.; Nielsen, T.S.; Ingerslev, A.K.; Nielsen, D.S.G.; Theil, P.K.; Purup, S.; Hald, S.; Schioldan, A.G.; et al. Impact of diet-modulated butyrate production on intestinal barrier function and inflammation. Nutrients 2018, 10, 1499. [Google Scholar] [CrossRef] [PubMed]
- Zhou, D.; Pan, Q.; Xin, F.-Z.; Zhang, R.-N.; He, C.-X.; Chen, G.-Y.; Liu, C.; Chen, Y.-W.; Fan, J.-G. Sodium butyrate attenuates high-fat diet-induced steatohepatitis in mice by improving gut microbiota and gastrointestinal barrier. World J. Gastroenterol. 2017, 23, 60–75. [Google Scholar] [CrossRef]
- Tian, L.; Zhou, X.-Q.; Jiang, W.-D.; Liu, Y.; Wu, P.; Jiang, J.; Kuang, S.-Y.; Tang, L.; Tang, W.-N.; Zhang, Y.-A.; et al. Sodium butyrate improved intestinal immune function associated with NF-κB and p38MAPK signalling pathways in young grass carp (Ctenopharyngodon idella). Fish Shellfish Immunol. 2017, 66, 548–563. [Google Scholar] [CrossRef]
- Wang, H.; Wang, P.; Wang, X.; Wan, Y.; Liu, Y. Butyrate enhances intestinal epithelial barrier function via up-regulation of tight junction protein claudin-1 transcription. Dig. Dis. Sci. 2012, 57, 3126–3135. [Google Scholar] [CrossRef]
- Wang, C.C.; Wu, H.; Lin, F.H.; Gong, R.; Xie, F.; Peng, Y.; Feng, J.; Hu, C.H. Sodium butyrate enhances intestinal integrity, inhibits mast cell activation, inflammatory mediator production and JNK signaling pathway in weaned pigs. J. Endotoxin Res. 2017, 24, 40–46. [Google Scholar] [CrossRef]
- Fukuda, S.; Toh, H.; Hase, K.; Oshima, K.; Nakanishi, Y.; Yoshimura, K.; Tobe, T.; Clarke, J.M.; Topping, D.L.; Suzuki, T.; et al. Bifidobacteria can protect from enteropathogenic infection through production of acetate. Nature 2011, 469, 543–547. [Google Scholar] [CrossRef]
- Balzan, S.; de Almeida Quadros, C.; de Cleva, R.; Zilberstein, B.; Cecconello, I. Bacterial translocation: Overview of mechanisms and clinical impact. J. Gastroenterol. Hepatol. 2007, 22, 464–471. [Google Scholar] [CrossRef] [PubMed]
- Ammori, B.J.; Leeder, P.C.; King, R.F.; Barclay, G.; Martin, I.G.; Larvin, M.; McMahon, M.J. Early increase in intestinal permeability in patients with severe acute pancreatitis: Correlation with endotoxemia, organ failure, and mortality. J. Gastrointest. Surg. 1999, 3, 252–262. [Google Scholar] [CrossRef]
- Juvonen, P.O.; Alhava, E.M.; Takala, J.A. Gut permeability in patients with acute pancreatitis. Scand. J. Gastroenterol. 2000, 35, 1314–1318. [Google Scholar] [CrossRef] [PubMed]
- Runkel, N.S.; Moody, F.G.; Smith, G.S.; Rodriguez, L.F.; LaRocco, M.T.; Miller, T.A. The role of the gut in the development of sepsis in acute pancreatitis. J. Surg. Res. 1991, 51, 18–23. [Google Scholar] [CrossRef]
- Tenner, S.; Vege, S.S.; Sheth, S.G.; Sauer, B.; Yang, A.; Conwell, D.L.; Yadlapati, R.H.; Gardner, T.B. American college of gastroenterology guidelines: Management of acute pancreatitis. Am. J. Gastroenterol. 2023, 119, 419–437. [Google Scholar] [CrossRef]
- Wereszczynska-Siemiatkowska, U.; Swidnicka-Siergiejko, A.; Siemiatkowski, A.; Dabrowski, A. Early enteral nutrition is superior to delayed enteral nutrition for the prevention of infected necrosis and mortality in acute pancreatitis. Pancreas 2013, 42, 640–646. [Google Scholar] [CrossRef]
- Petrov, M.S.; Pylypchuk, R.D.; Uchugina, A.F. A systematic review on the timing of artificial nutrition in acute pancreatitis. Br. J. Nutr. 2008, 101, 787–793. [Google Scholar] [CrossRef] [PubMed]
- De Lucia, S.S.; Candelli, M.; Polito, G.; Maresca, R.; Mezza, T.; Schepis, T.; Pellegrino, A.; Verme, L.Z.D.; Nicoletti, A.; Franceschi, F.; et al. Nutrition in acute pancreatitis: From the old paradigm to the new evidence. Nutrients 2023, 15, 1939. [Google Scholar] [CrossRef]
- Foitzik, T.; Kruschewski, M.; Kroesen, A.J.; Hotz, H.G.; Eibl, G.; Buhr, H.J. Does glutamine reduce bacterial translocation? Int. J. Color. Dis. 1999, 14, 143–149. [Google Scholar] [CrossRef]
- Huang, X.X.; Wang, X.P.; Ma, J.J.; Jing, D.D.; Wang, P.W.; Wu, K. Effects of enteral nutrition supplemented with glutamine and arginine on gut barrier in patients with severe acute pancreatitis: A prospective randomized controlled trial. Zhonghua Yi Xue Za Zhi 2008, 88, 2407–2409. [Google Scholar]
- Yong, L.; Lu, Q.; Liu, S.; Fan, H. Efficacy of glutamine-enriched nutrition support for patients with severe acute pancreatitis: A meta-analysis. J. Parenter. Enter. Nutr. 2016, 40, 83–94. [Google Scholar] [CrossRef]
- Kilian, M.; Gregor, J.I.; Heukamp, I.; Wagner, C.; Walz, M.K.; Schimke, I.; Kristiansen, G.; Wenger, F.A. Early inhibition of prostaglandin synthesis by n-3 fatty acids determinates histologic severity of necrotizing pancreatitis. Pancreas 2009, 38, 436–441. [Google Scholar] [CrossRef] [PubMed]
- Parada Venegas, D.; De La Fuente, M.K.; Landskron, G.; González, M.J.; Quera, R.; Dijkstra, G.; Harmsen, H.J.M.; Faber, K.N.; Hermoso, M.A. Short chain fatty acids (scfas)-mediated gut epithelial and immune regulation and its relevance for inflammatory bowel diseases. Front. Immunol. 2019, 10, 277. [Google Scholar] [CrossRef]
- Kasotakis, G.; Galvan, M.; King, E.; Sarkar, B.; Stucchi, A.; Mizgerd, J.P.S.; Burke, P.A.; Remick, D. Valproic acid mitigates the inflammatory response and prevents acute respiratory distress syndrome in a murine model of Escherichia coli pneumonia at the expense of bacterial clearance. J. Trauma Acute Care Surg. 2017, 82, 758–765. [Google Scholar] [CrossRef]
- Werawatganon, D.; Vivatvakin, S.; Somanawat, K.; Tumwasorn, S.; Klaikeaw, N.; Siriviriyakul, P.; Chayanupatkul, M. Effects of probiotics on pancreatic inflammation and intestinal integrity in mice with acute pancreatitis. BMC Complement. Med. Ther. 2023, 23, 166. [Google Scholar] [CrossRef]
- Mazziotta, C.; Tognon, M.; Martini, F.; Torreggiani, E.; Rotondo, J.C. Probiotics mechanism of action on immune cells and beneficial effects on human health. Cells 2023, 12, 184. [Google Scholar] [CrossRef]
- Żółkiewicz, J.; Marzec, A.; Ruszczyński, M.; Feleszko, W. Postbiotics—A step beyond pre- and probiotics. Nutrients 2020, 12, 2189. [Google Scholar] [CrossRef]
- Huang, F.C.; Lu, Y.T.; Liao, Y.H. Beneficial effect of probiotics on Pseudomonas aeruginosa–infected intestinal epithelial cells through inflammatory IL-8 and antimicrobial peptide human beta-defensin-2 modulation. J. Endotoxin Res. 2020, 26, 592–600. [Google Scholar] [CrossRef]
- Engevik, M.A.; Luk, B.; Chang-Graham, A.L.; Hall, A.; Herrmann, B.; Ruan, W.; Endres, B.T.; Shi, Z.; Garey, K.W.; Hyser, J.M.; et al. Bifidobacterium dentium Fortifies the Intestinal Mucus Layer via Autophagy and Calcium Signaling Pathways. mBio 2019, 10, e01087-19. [Google Scholar] [CrossRef]
- Servin, A.L. Antagonistic activities of lactobacilli and bifidobacteria against microbial pathogens. FEMS Microbiol. Rev. 2004, 28, 405–440. [Google Scholar] [CrossRef]
- Akyol, S.; Mas, M.R.; Comert, B.; Ateskan, Ü.; Yasar, M.; Aydogan, H.; Deveci, S.; Akay, C.; Mas, N.; Yener, N.; et al. The effect of antibiotic and probiotic combination therapy on secondary pancreatic infections and oxidative stress parameters in experimental acute necrotizing pancreatitis. Pancreas 2003, 26, 363–367. [Google Scholar] [CrossRef] [PubMed]
- Wan, Y.D.; Zhu, R.X.; Bian, Z.Z.; Sun, T.W. Effect of probiotics on length of hospitalization in mild acute pancreatitis: A randomized, double-blind, placebo-controlled trial. World J. Gastroenterol. 2021, 27, 224–232. [Google Scholar] [CrossRef] [PubMed]
- esselink, M.G.; Van Santvoort, H.C.; Buskens, E.; Boermeester, M.A.; Van Goor, H.; Timmerman, H.M.; Nieuwenhuijs, V.B.; Bollen, T.L.; van Ramshorst, B.; Witterman, B.J.; et al. Probiotic prophylaxis in predicted severe acute pancreatitis: A randomised, double-blind, placebo-controlled trial. Lancet 2008, 371, 651–659. [Google Scholar] [CrossRef]
- Morrow, L.E.; Gogineni, V.; Malesker, M.A. Probiotic, prebiotic, and synbiotic use in critically ill patients. Curr. Opin. Crit. Care 2012, 18, 186–191. [Google Scholar] [CrossRef]
- Soares, F.S.; Amaral, F.C.; Silva, N.L.C.; Valente, M.R.; Santos, L.K.R.; Yamashiro, L.H.; Scheffer, M.C.; Castanheira, F.V.E.S.; Ferreira, R.G.; Gehrke, L.; et al. Antibiotic-induced pathobiont dissemination accelerates mortality in severe experimental pancreatitis. Front. Immunol. 2017, 8, 1890. [Google Scholar] [CrossRef]
- Jia, L.; Chen, H.; Yang, J.; Fang, X.; Niu, W.; Zhang, M.; Li, J.; Pan, X.; Ren, Z.; Sun, J.; et al. Combinatory antibiotic treatment protects against experimental acute pancreatitis by suppressing gut bacterial translocation to pancreas and inhibiting NLRP3 inflammasome pathway. Innate Immun. 2020, 26, 48–61. [Google Scholar] [CrossRef]
- Sawa, H.; Ueda, T.; Takeyama, Y.; Yasuda, T.; Shinzeki, M.; Matsumura, N.; Nakajima, T.; Matsumoto, I.; Fujita, T.; Ajiki, T.; et al. Treatment outcome of selective digestive decontamination and enteral nutrition in patients with severe acute pancreatitis. J. Hepato Biliary Pancreat. Surg. 2007, 14, 503–508. [Google Scholar] [CrossRef] [PubMed]
- Bajaj, J.S.; Kassam, Z.; Fagan, A.; Gavis, E.A.; Liu, E.; Cox, I.J.; Kheradman, R.; Heuman, D.; Wang, J.; Gurry, T.; et al. Fecal microbiota transplant from a rational stool donor improves hepatic encephalopathy: A randomized clinical trial. Hepatology 2017, 66, 1727–1738. [Google Scholar] [CrossRef]
- Paramsothy, S.; Nielsen, S.; Kamm, M.A.; Deshpande, N.P.; Faith, J.J.; Clemente, J.C.; Paramsothy, R.; Walsh, A.J.; van den Bogaerde, J.; Samuel, D.; et al. Specific bacteria and metabolites associated with response to fecal microbiota transplantation in patients with ulcerative colitis. Gastroenterology 2019, 156, 1440–1454.e2. [Google Scholar] [CrossRef]
- Singh, T.; Bedi, P.; Bumrah, K.; Gandhi, D.; Arora, T.; Verma, N.; Schleicher, M.; Rai, M.P.; Garg, R.; Verma, B.; et al. Fecal microbiota transplantation and medical therapy for clostridium difficile infection: Meta-analysis of randomized controlled trials. J. Clin. Gastroenterol. 2021, 56, 881–888. [Google Scholar] [CrossRef]
- Yu, S.; Xiong, Y.; Xu, J.; Liang, X.; Fu, Y.; Liu, D.; Yu, X.; Wu, D. Identification of dysfunctional gut microbiota through rectal swab in patients with different severity of acute pancreatitis. Dig. Dis. Sci. 2020, 65, 3223–3237. [Google Scholar] [CrossRef] [PubMed]
- Ding, L.; He, C.; Li, X.; Huang, X.; Lei, Y.; Ke, H.; Chen, H.; Yang, Q.; Cai, Y.; Liao, Y.; et al. Efficacy and safety of faecal microbiota transplantation for acute pancreatitis: A randomised, controlled study. Front. Med. 2022, 8, 772454. [Google Scholar] [CrossRef] [PubMed]
- Mizutani, T.; Aboagye, S.Y.; Ishizaka, A.; Afum, T.; Mensah, G.I.; Asante-Poku, A.; Asandem, D.A.; Parbie, P.K.; Abana, C.Z.-Y.; Kushitor, D.; et al. Gut microbiota signature of pathogen-dependent dysbiosis in viral gastroenteritis. Sci. Rep. 2021, 11, 13945. [Google Scholar] [CrossRef]
- Piccioni, A.; Franza, L.; Brigida, M.; Zanza, C.; Torelli, E.; Petrucci, M.; Nicolò, R.; Covino, M.; Candelli, M.; Saviano, A.; et al. Gut microbiota and acute diverticulitis: Role of probiotics in management of this delicate pathophysiological balance. J. Pers. Med. 2021, 11, 298. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Santana, P.T.; Rosas, S.L.B.; Ribeiro, B.E.; Marinho, Y.; de Souza, H.S.P. Dysbiosis in inflammatory bowel disease: Pathogenic role and potential therapeutic targets. Int. J. Mol. Sci. 2022, 23, 3464. [Google Scholar] [CrossRef]
- Fang, J.; Yu, C.-H.; Li, X.-J.; Yao, J.-M.; Fang, Z.-Y.; Yoon, S.-H.; Yu, W.-Y. Gut dysbiosis in nonalcoholic fatty liver disease: Pathogenesis, diagnosis, and therapeutic implications. Front. Cell. Infect. Microbiol. 2022, 12, 997018. [Google Scholar] [CrossRef]
- Haneishi, Y.; Furuya, Y.; Hasegawa, M.; Picarelli, A.; Rossi, M.; Miyamoto, J. Inflammatory bowel diseases and gut microbiota. Int. J. Mol. Sci. 2023, 24, 3817. [Google Scholar] [CrossRef] [PubMed]
- Shen, Z.-H.; Zhu, C.-X.; Quan, Y.-S.; Yang, Z.-Y.; Wu, S.; Luo, W.-W.; Tan, B.; Wang, X.-Y. Relationship between intestinal microbiota and ulcerative colitis: Mechanisms and clinical application of probiotics and fecal microbiota transplantation. World J. Gastroenterol. 2018, 24, 5–14. [Google Scholar] [CrossRef]
- Fan, Y.; Pedersen, O. Gut microbiota in human metabolic health and disease. Nat. Rev. Microbiol. 2021, 19, 55–71. [Google Scholar] [CrossRef]
- Hou, K.; Wu, Z.-X.; Chen, X.-Y.; Wang, J.-Q.; Zhang, D.; Xiao, C.; Zhu, D.; Koya, J.B.; Wei, L.; Li, J.; et al. Microbiota in health and diseases. Signal Transduct. Target. Ther. 2022, 7, 135. [Google Scholar] [CrossRef]
- Gowen, R.; Gamal, A.; Di Martino, L.; McCormick, T.S.; Ghannoum, M.A. Modulating the microbiome for crohn’s disease treatment. Gastroenterology 2023, 164, 828–840. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, P.; Zhang, X. Probiotics regulate gut microbiota: An effective method to improve immunity. Molecules 2021, 26, 6076. [Google Scholar] [CrossRef]
- Soliman, M.G.; Mansour, H.A.; Hassan, W.A.; Shawky, E. Impact of oral probiotics in amelioration of immunological and inflammatory responses on experimentally induced acute diverticulitis. Probiotics Antimicrob. Proteins 2022, 15, 1113–1123. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello, C.; Marannino, M.; Migneco, A.; Brigida, M.; Saviano, A.; Piccioni, A.; Franceschi, F.; Ojetti, V. The efficacy of a mix of three probiotic strains in reducing abdominal pain and inflammatory biomarkers in acute uncomplicated diverticulitis. Eur. Rev. Med. Pharmacol. Sci. 2019, 23, 9126–9133. [Google Scholar] [CrossRef] [PubMed]
- Petruzziello, C.; Migneco, A.; Cardone, S.; Covino, M.; Saviano, A.; Franceschi, F.; Ojetti, V. Supplementation with Lactobacillus reuteri ATCC PTA 4659 in patients affected by acute uncomplicated diverticulitis: A randomized double-blind placebo controlled trial. Int. J. Color. Dis. 2019, 34, 1087–1094. [Google Scholar] [CrossRef]
- Ojetti, V.; Saviano, A.; Brigida, M.; Petruzziello, C.; Caronna, M.; Gayani, G.; Franceschi, F. Randomized control trial on the efficacy of Limosilactobacillus reuteri ATCC PTA 4659 in reducing inflammatory markers in acute uncomplicated diverticulitis. Eur. J. Gastroenterol. Hepatol. 2022, 34, 496–502. [Google Scholar] [CrossRef] [PubMed] [PubMed Central]
- Sheth, A.A.; Garcia-Tsao, G. Probiotics and liver disease. J. Clin. Gastroenterol. 2008, 42, S80–S84. [Google Scholar] [CrossRef]
- Ma, Y.; Yang, D.; Huang, J.; Liu, K.; Liu, H.; Wu, H.; Bao, C. Probiotics for inflammatory bowel disease: Is there sufficient evidence? Open Life Sci. 2024, 19, 20220821. [Google Scholar] [CrossRef]
- Doron, S.; Snydman, D.R. Risk and safety of probiotics. Clin. Infect. Dis. 2015, 60 (Suppl. 2), S129–S134. [Google Scholar] [CrossRef]
- Suez, J.; Zmora, N.; Segal, E.; Elinav, E. The pros, cons, and many unknowns of probiotics. Nat. Med. 2019, 25, 716–729. [Google Scholar] [CrossRef]
- Didari, T.; Solki, S.; Mozaffari, S.; Nikfar, S.; Abdollahi, M. A systematic review of the safety of probiotics. Expert Opin. Drug Saf. 2013, 13, 227–239. [Google Scholar] [CrossRef]
- Cohen, P.A. Probiotic Safety—No Guarantees. JAMA Intern. Med. 2018, 178, 1577–1578. [Google Scholar] [CrossRef] [PubMed]
- Merenstein, D.; Pot, B.; Leyer, G.; Ouwehand, A.C.; Preidis, G.A.; Elkins, C.A.; Hill, C.; Lewis, Z.T.; Shane, A.L.; Zmora, N.; et al. Emerging issues in probiotic safety: 2023 perspectives. Gut Microbes 2023, 15, 2185034. [Google Scholar] [CrossRef] [PubMed]
- Kelly, C.R.; Kahn, S.; Kashyap, P.; Laine, L.; Rubin, D.; Atreja, A.; Moore, T.; Wu, G. Update on Fecal Microbiota Transplantation 2015: Indications, Methodologies, Mechanisms, and Outlook. Gastroenterology 2015, 149, 223–237. [Google Scholar] [CrossRef] [PubMed]
- Terveer, E.; van Beurden, Y.; Goorhuis, A.; Seegers, J.; Bauer, M.; van Nood, E.; Dijkgraaf, M.; Mulder, C.; Vandenbroucke-Grauls, C.; Verspaget, H.W.; et al. How to: Establish and run a stool bank. Clin. Microbiol. Infect. 2017, 23, 924–930. [Google Scholar] [CrossRef]
- Mullish, B.H.; Quraishi, M.N.; Segal, J.P.; McCune, V.L.; Baxter, M.; Marsden, G.L.; Moore, D.; Colville, A.; Bhala, N.; Iqbal, T.H.; et al. The use of faecal microbiota transplant as treatment for recurrent or refractory Clostridium difficile infection and other potential indications: Joint British Society of Gastroenterology (BSG) and Healthcare Infection Society (HIS) guidelines. J. Hosp. Infect. 2018, 100 (Suppl. 2), S1–S31. [Google Scholar] [CrossRef]
- Woodworth, M.H.; Carpentieri, C.; Sitchenko, K.L.; Kraft, C.S. Challenges in fecal donor selection and screening for fecal microbiota transplantation: A review. Gut Microbes 2017, 8, 225–237. [Google Scholar] [CrossRef]
- Bakken, J.S.; Borody, T.; Brandt, L.J.; Brill, J.V.; Demarco, D.C.; Franzos, M.A.; Kelly, C.; Khoruts, A.; Louie, T.; Martinelli, L.P.; et al. Treating clostridium difficile infection with fecal microbiota transplantation. Clin. Gastroenterol. Hepatol. 2011, 9, 1044–1049. [Google Scholar] [CrossRef]
- Nieuwboer, M.v.D.; Claassen, E. Dealing with the remaining controversies of probiotic safety. Benef. Microbes 2019, 10, 605–616. [Google Scholar] [CrossRef]
- Cammarota, G.; Ianiro, G.; Tilg, H.; Rajilić-Stojanović, M.; Kump, P.; Satokari, R.; Sokol, H.; Arkkila, P.; Pintus, C.; Hart, A.; et al. European consensus conference on faecal microbiota transplantation in clinical practice. Gut 2017, 66, 569–580. [Google Scholar] [CrossRef]
- Jørgensen, S.M.; Hansen, M.M.; Erikstrup, C.; Dahlerup, J.F.; Hvas, C.L. Faecal microbiota transplantation: Establishment of a clinical application framework. Eur. J. Gastroenterol. Hepatol. 2017, 29, e36–e45. [Google Scholar] [CrossRef] [PubMed]
| Study | Type of Sample | Subject | Phylum Level | Family, Genus, or Species | Microbial Evaluation |
|---|---|---|---|---|---|
| Tan et al. (2015) [35] | Feces | AP patients vs. Healthy volunteers | - | ↓Bifidobacteria ↑Enterobacteriaceae ↑Enterococcus | PCR-DGGE |
| Wahlström et al. (2016) [36]; Van den berg (2021) [37]; Almeida et al. (2019) [38] | AP patients vs. Healthy volunteers | ↑Bacteroidetes ↑Proteobacteria ↓Firmicutes ↓Actinobacteria | ↑Escherichia-Shigella ↑Erysipelotrichaecease ↑Streptococcus ↑Enterococcus | 16S | |
| Zhang et al. (2018) [39] | Feces | AP patients vs. Healthy volunteers | ↑Bacteroidetes ↑Proteobacteria ↓Firmicutes ↓Actinobacteria | - | 16S |
| Chen et al. (2017) [40] | Feces | AP patients vs. Healthy volunteers | ↓Saccharibacteria ↓Tenericutes | ↑Escherichia-Shigella ↑Phascolarctobacterium ↓Candidatus Saccharimonas; ↓Prevotellaceae ↓Lachnospiraceae ↓Ruminiclostridium ↓Ruminococcaceae | 16S |
| Liu et al. (2024) [41] | Feces | AP patients vs. Healthy volunteers | - | ↑E. coli ↑Enterococcus ↑Parabacteroides ↑Clostridium ↑Veillonella | 16S |
| Zhao et al. (2023) [47] | Descending duodenum | MAP patients vs. Healthy individuals | - | ↑Streptococcus ↑Neisseria ↓Actinobacillus ↓Oribacterium | 16S |
| Study | Type of Sample | Subject | Phylum Level | Family, Genus, or Species | Microbial Evaluation |
|---|---|---|---|---|---|
| Liu et al. (2024) [41] | Feces | Biliary AP vs. others AP etiologies | - |
↑Bilophila wadsworthia ↑Streptococcus infantis ↓Veillonella dispar ↓Christensenella minuta | whole-metagenome shotgun sequencing |
| Liu et al. (2024) [41] | Feces | HTGP vs. others AP etiologies | - | ↑Bilophila wadsworthia | whole-metagenome shotgun sequencing |
| Li et al. (2023) [61] | Feces | HTGP vs. Healthy volunteers | ↑Firmicutes ↓Proteobacteria | ↑Enterococcaceae ↓Escherichia-shigella ↓Bacteroides ↓Faecalibacterium | 16S |
| Philips et al. (2019) [68] | Feces | Alcoholic AP vs. Healthy volunteers | ↑Actinobacteria ↓Bacteroidetes |
↑Moraxella ↑Acinetobacter | 16S |
| Ciocan et al. (2018) [69] | Feces | Alcoholic AP vs. Alcoholic | ↑Proteobacteria ↓Bacteroidetes | ↑Klebsiella pneumoniae ↑Lactobacillus ↑Enterococcus ↑Sphingomonas | 16S |
| Study | Type of Sample | Subject | Phylum Level | Family, Genus, or Species | Microbial Evaluation |
|---|---|---|---|---|---|
| Ammer-herrmenau et al. (2024) [24] | Buccal and rectas swab | SAP vs. non-SAP | - |
↑Leuconostoc citreum ↑Rothia nasimurium ↑Leuconostoc pseudomesenteroides ↑ Clavibacter michiganensis ↓Streptococcus pyogenes ↓Lawsonella clevalandensis ↓Aerococcus unnae ↓Finegoldia magna ↓Streptococcus dysgalactiae ↓Streptococcus pseudoporcinus ↓Peptoniphilus harei ↓Anaerococcus mediterraneensis ↓Peptoniphilus ivorii | |
| Tan et al. (2015) [35] | Feces | AP patients vs. Healthy volunteers | - | ↑Enterobacteriaceae ↑Enterococcus ↓Bifidobacterium ↔Lactobacillus | PCR-DGGE |
| Liu et al. (2024) [41] | Feces | SAP patients vs. MAP patients | - | ↑Eubacterium eligens | whole-metagenome shotgun sequencing |
| Li et al. (2023) [61] | Feces | HTGP vs. Healthy volunteers | - | ↓Faecalibacterium ↓Bacteroides uniformis | 16S |
| Hu et al. (2023) [72] | Feces | ARDS patients vs. non-ARDS patients | ↑Proteobacteria | ↑Escherichia-shigella ↑Enterobacteriaceae ↑Klebsiella pneumoniae ↓Bifidobacterium | 16S |
| Zhu et al. (2019) [73] | Feces | SAP patients vs. MAP patients | - | ↑Enterobacteriaceae ↑Enterococcus ↓Bifidobacteriaceae | 16S |
| Zou et al. (2022) [74] | Feces and rectal swabs | ANP patients vs. non-ANP patients | ↓Bacteroidetes | ↑Enterobacteriaceae ↑Enterococcus faecalis ↑Finegoldia magna ↓Clostridium | 16S |
| Wang et al. (2024) [77] | SAP patients vs. MAP patients | ↑Proteobacteria | ↑Stenotrophomonas ↑Enterobacter ↓ Blautia ↓Enterococcus ↓Faecalibacter contorta ↓Ruminococcaceae ↓Christensenella | 16S |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nista, E.C.; Parello, S.; Brigida, M.; Amadei, G.; Saviano, A.; De Lucia, S.S.; Petruzziello, C.; Migneco, A.; Ojetti, V. Exploring the Role of Gut Microbiota and Probiotics in Acute Pancreatitis: A Comprehensive Review. Int. J. Mol. Sci. 2025, 26, 3433. https://doi.org/10.3390/ijms26073433
Nista EC, Parello S, Brigida M, Amadei G, Saviano A, De Lucia SS, Petruzziello C, Migneco A, Ojetti V. Exploring the Role of Gut Microbiota and Probiotics in Acute Pancreatitis: A Comprehensive Review. International Journal of Molecular Sciences. 2025; 26(7):3433. https://doi.org/10.3390/ijms26073433
Chicago/Turabian StyleNista, Enrico Celestino, Simone Parello, Mattia Brigida, Giulio Amadei, Angela Saviano, Sara Sofia De Lucia, Carmine Petruzziello, Alessio Migneco, and Veronica Ojetti. 2025. "Exploring the Role of Gut Microbiota and Probiotics in Acute Pancreatitis: A Comprehensive Review" International Journal of Molecular Sciences 26, no. 7: 3433. https://doi.org/10.3390/ijms26073433
APA StyleNista, E. C., Parello, S., Brigida, M., Amadei, G., Saviano, A., De Lucia, S. S., Petruzziello, C., Migneco, A., & Ojetti, V. (2025). Exploring the Role of Gut Microbiota and Probiotics in Acute Pancreatitis: A Comprehensive Review. International Journal of Molecular Sciences, 26(7), 3433. https://doi.org/10.3390/ijms26073433








