Engineered ATP-Loaded Extracellular Vesicles Derived from Mesenchymal Stromal Cells: A Novel Strategy to Counteract Cell ATP Depletion in an In Vitro Model
Abstract
1. Introduction
2. Results
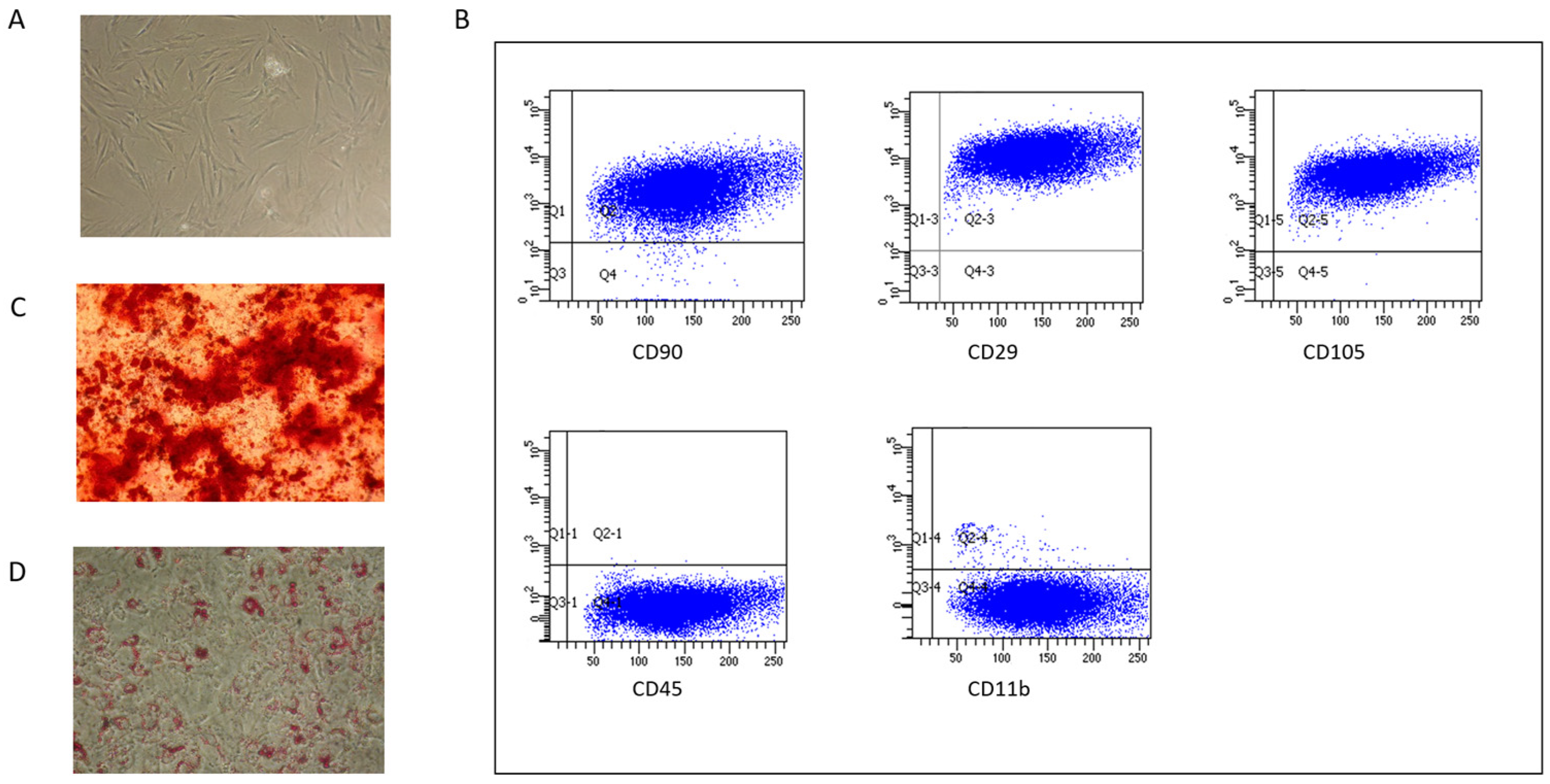
2.1. MSC Characterization
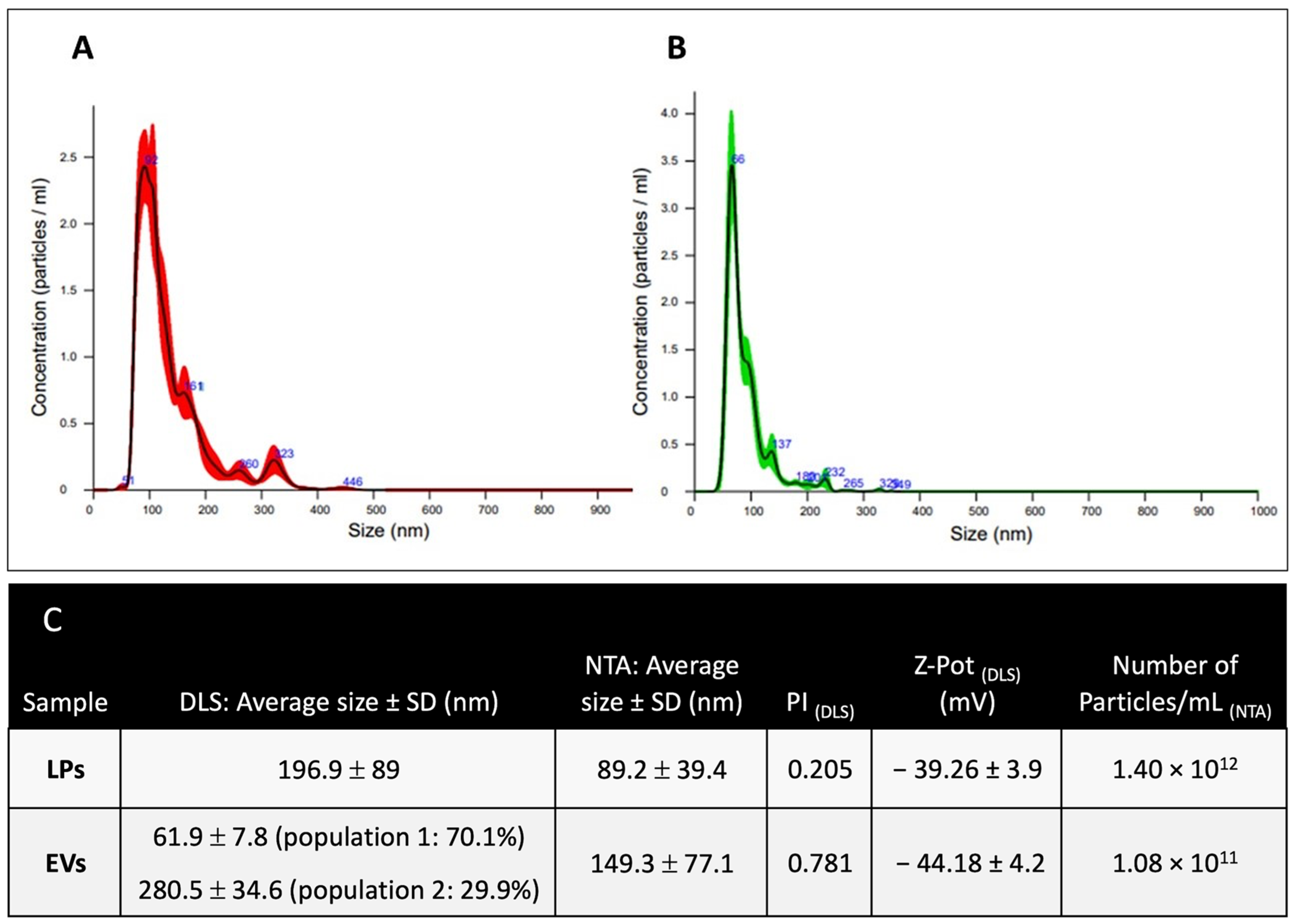
2.2. Liposomes and EVs Characterization
2.2.1. Dimensional Characterization
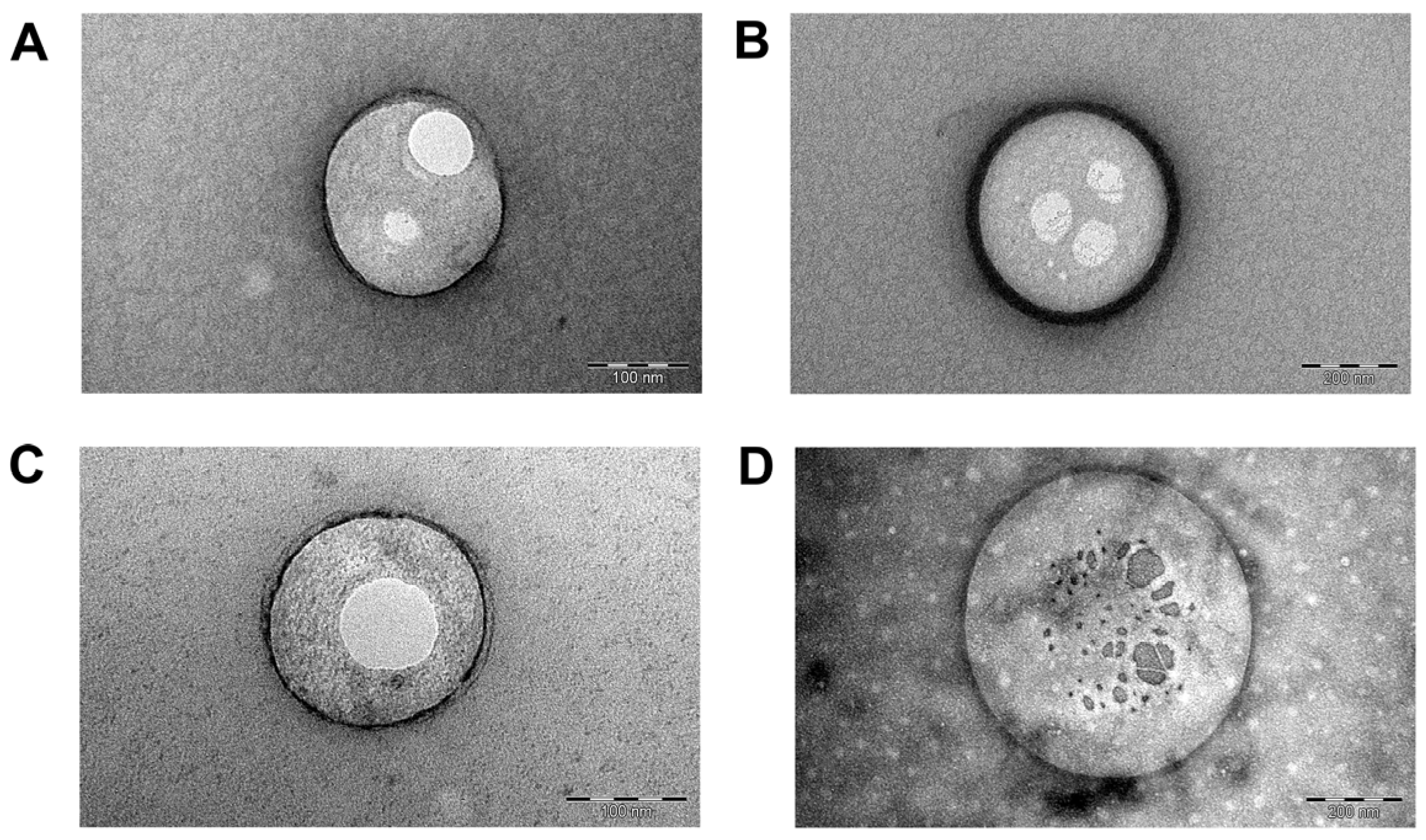
2.2.2. Morphometric Analysis
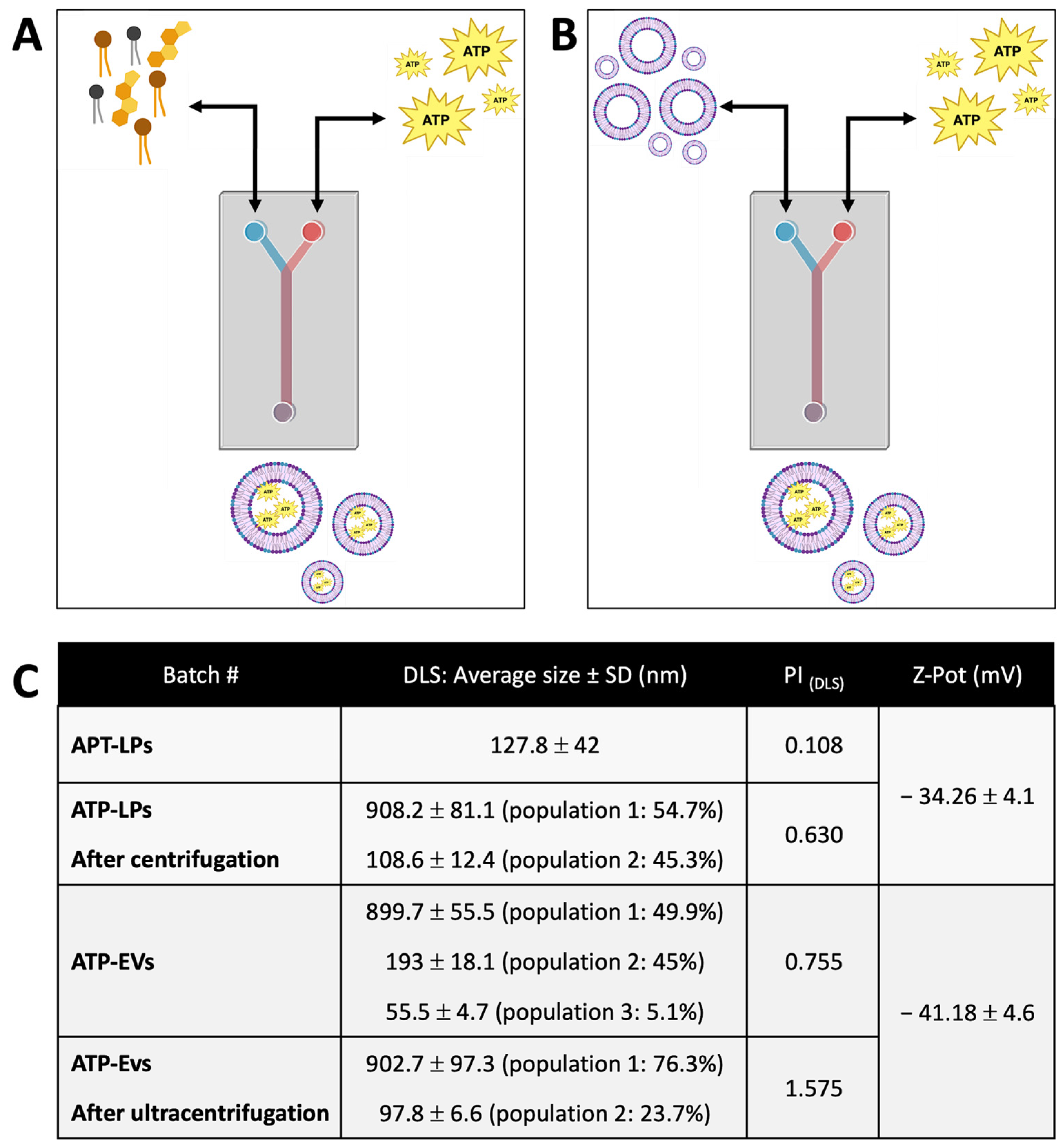
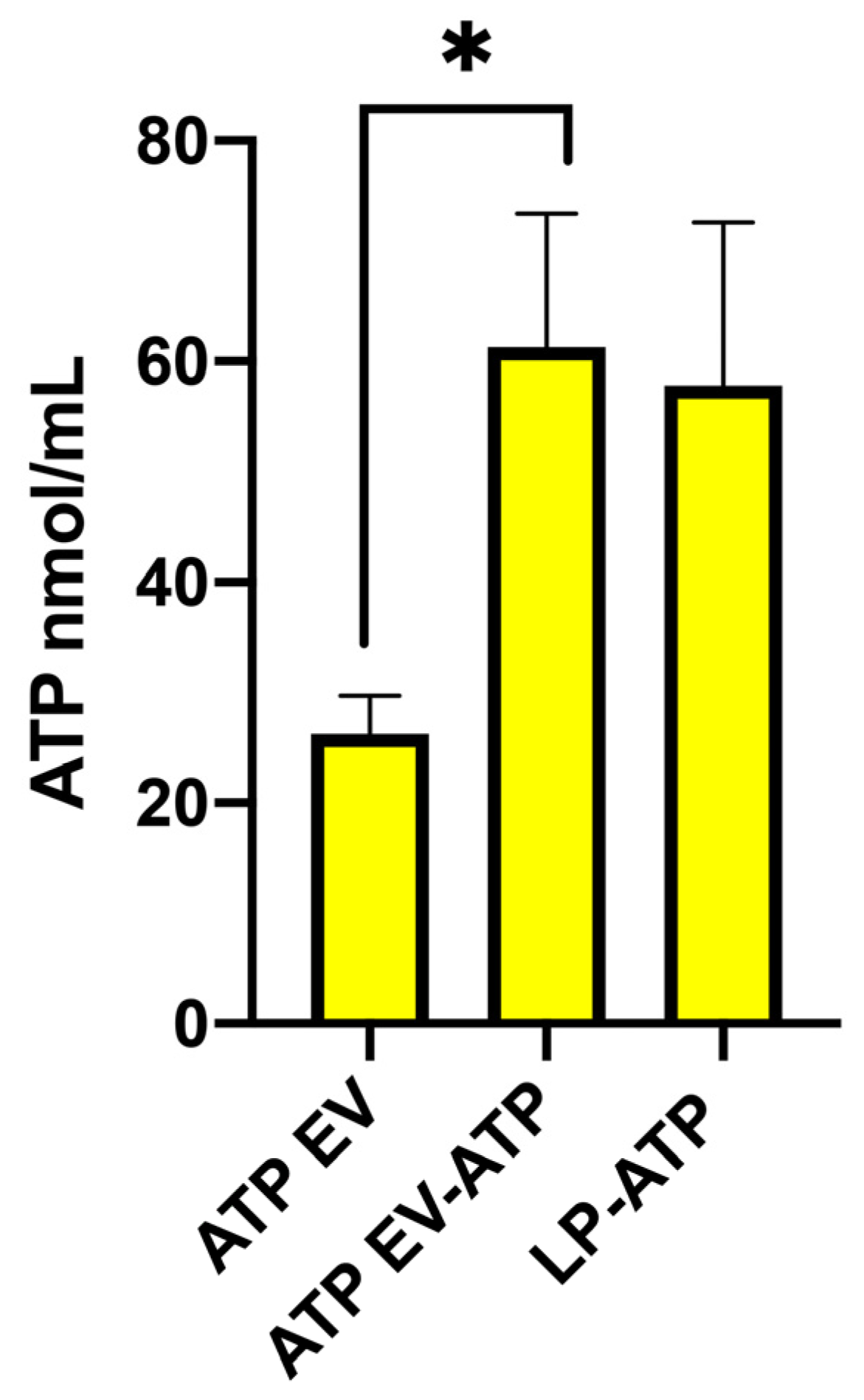
2.3. ATP Quantification in Liposomes and EVs
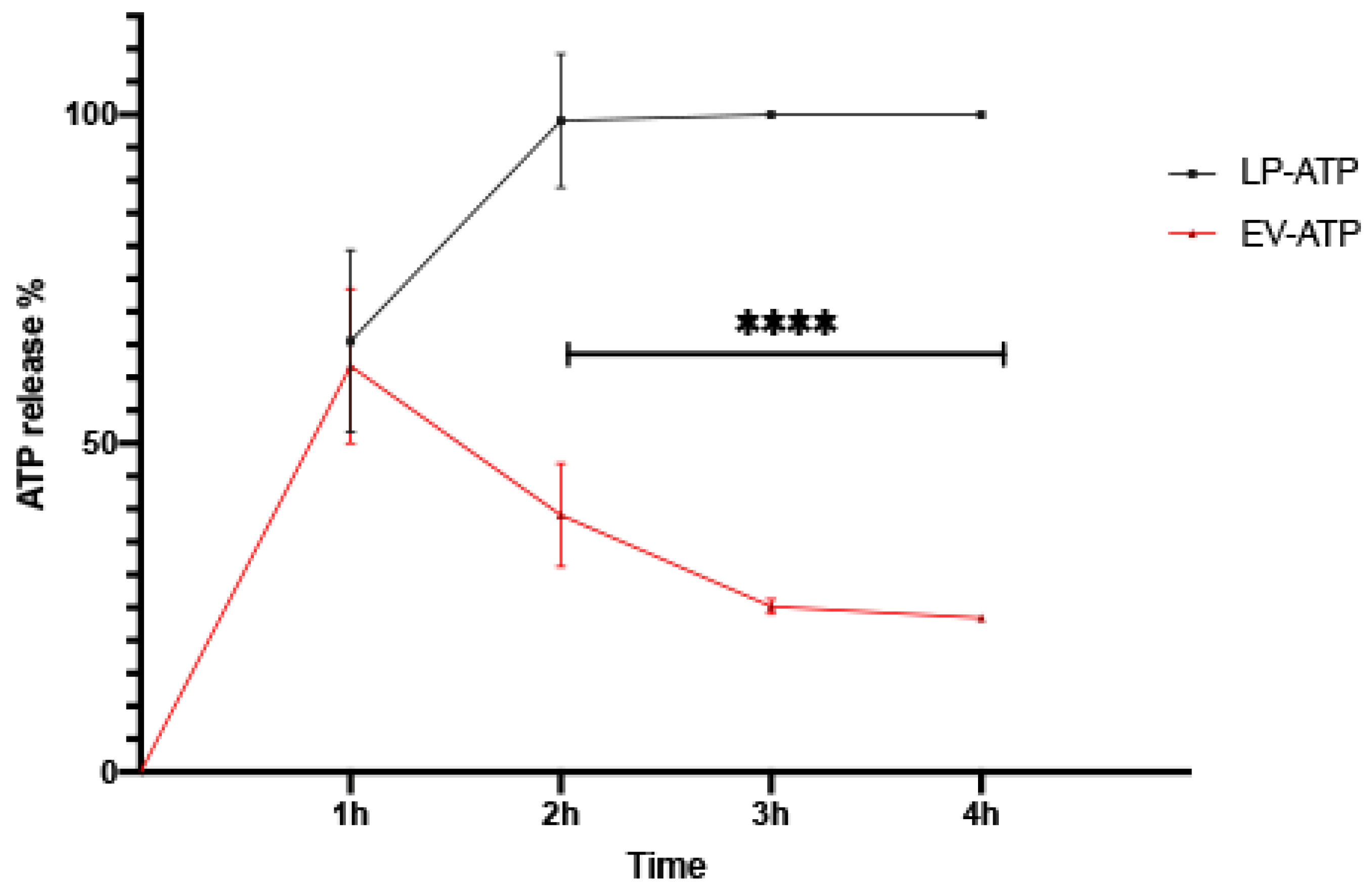
2.4. ATP In Vitro Release Study
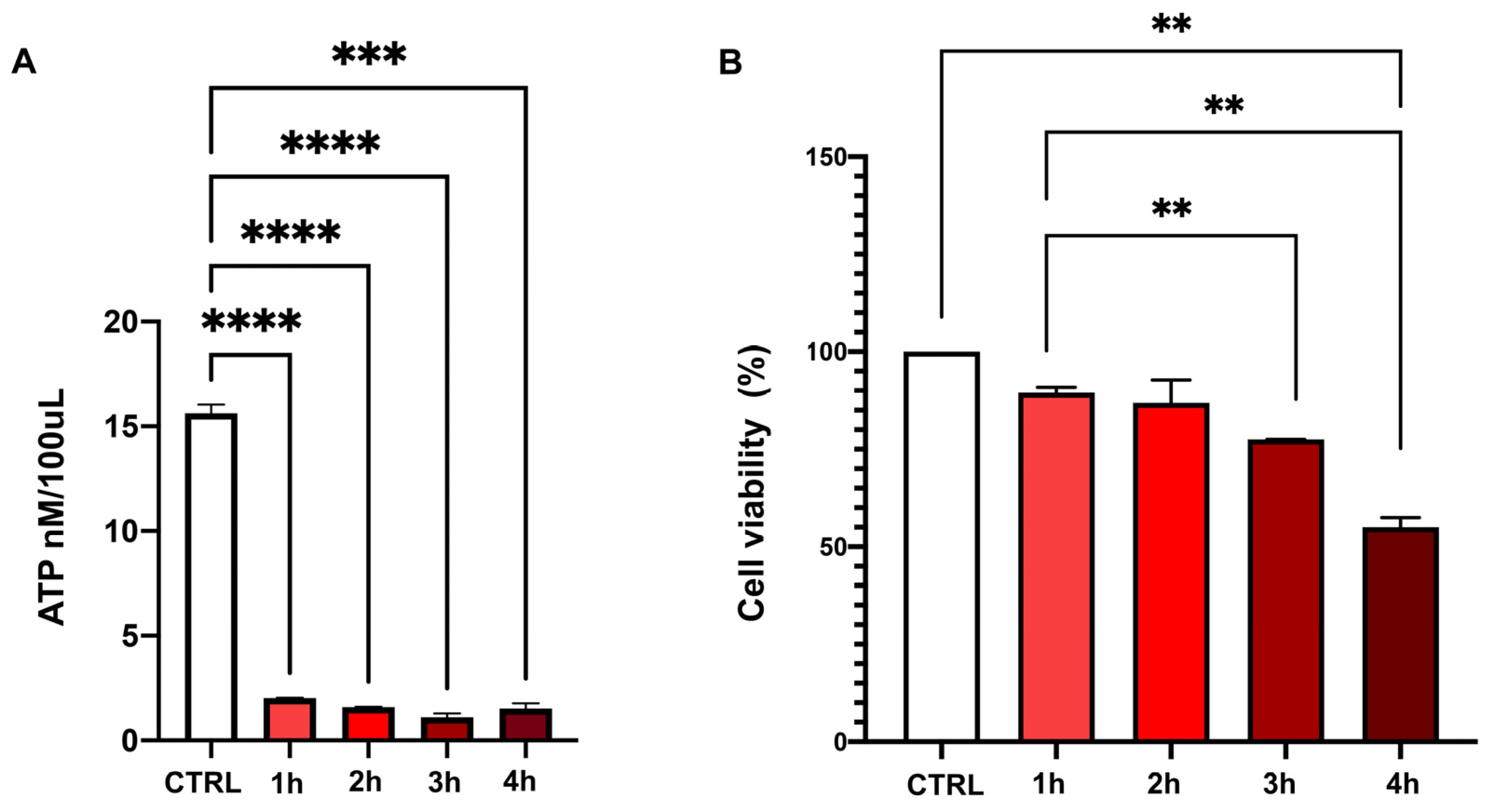
2.5. Cell ATP Concentration and Viability in Basal Conditions and After Chemical Ischemia
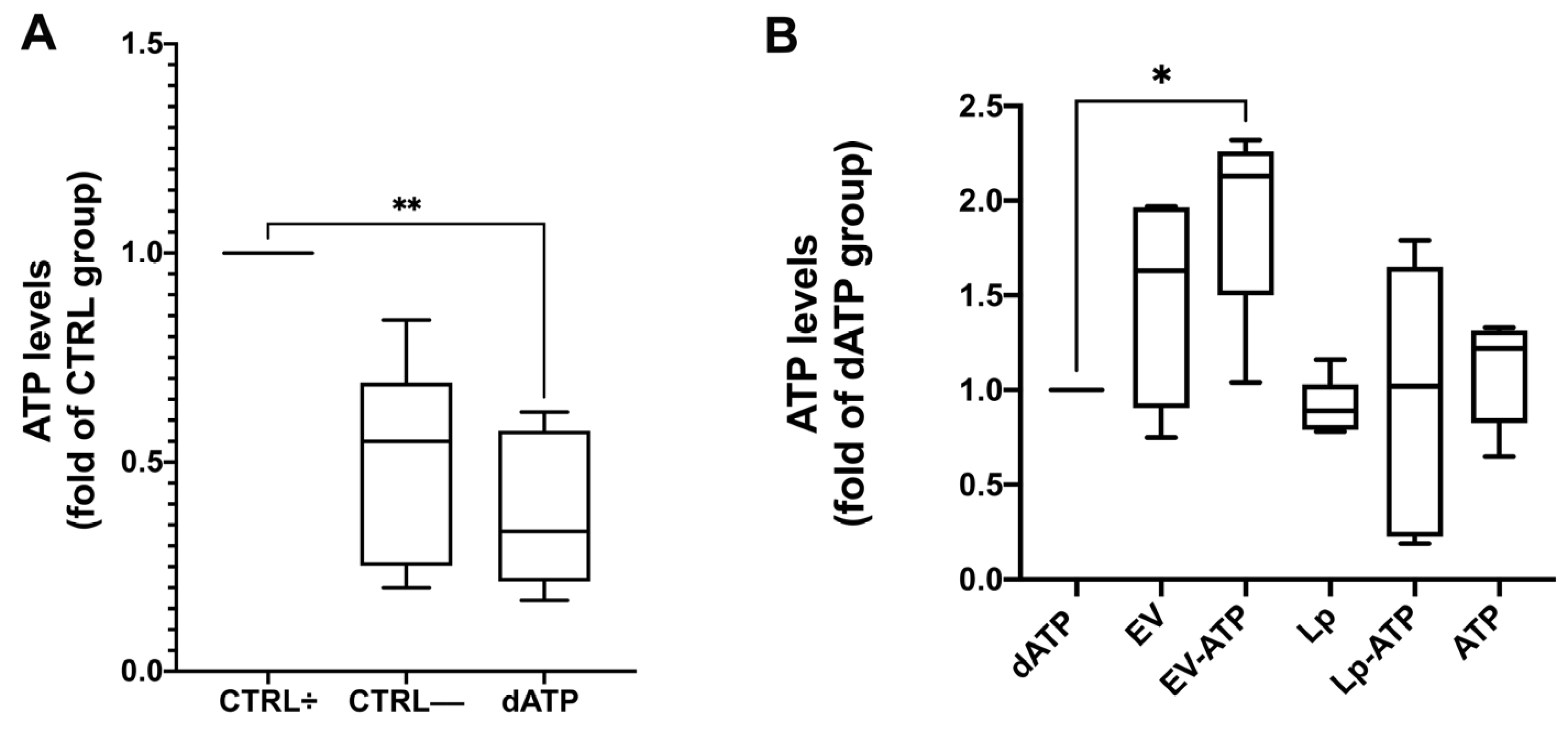
2.6. ATP Loading into HK2 Cells
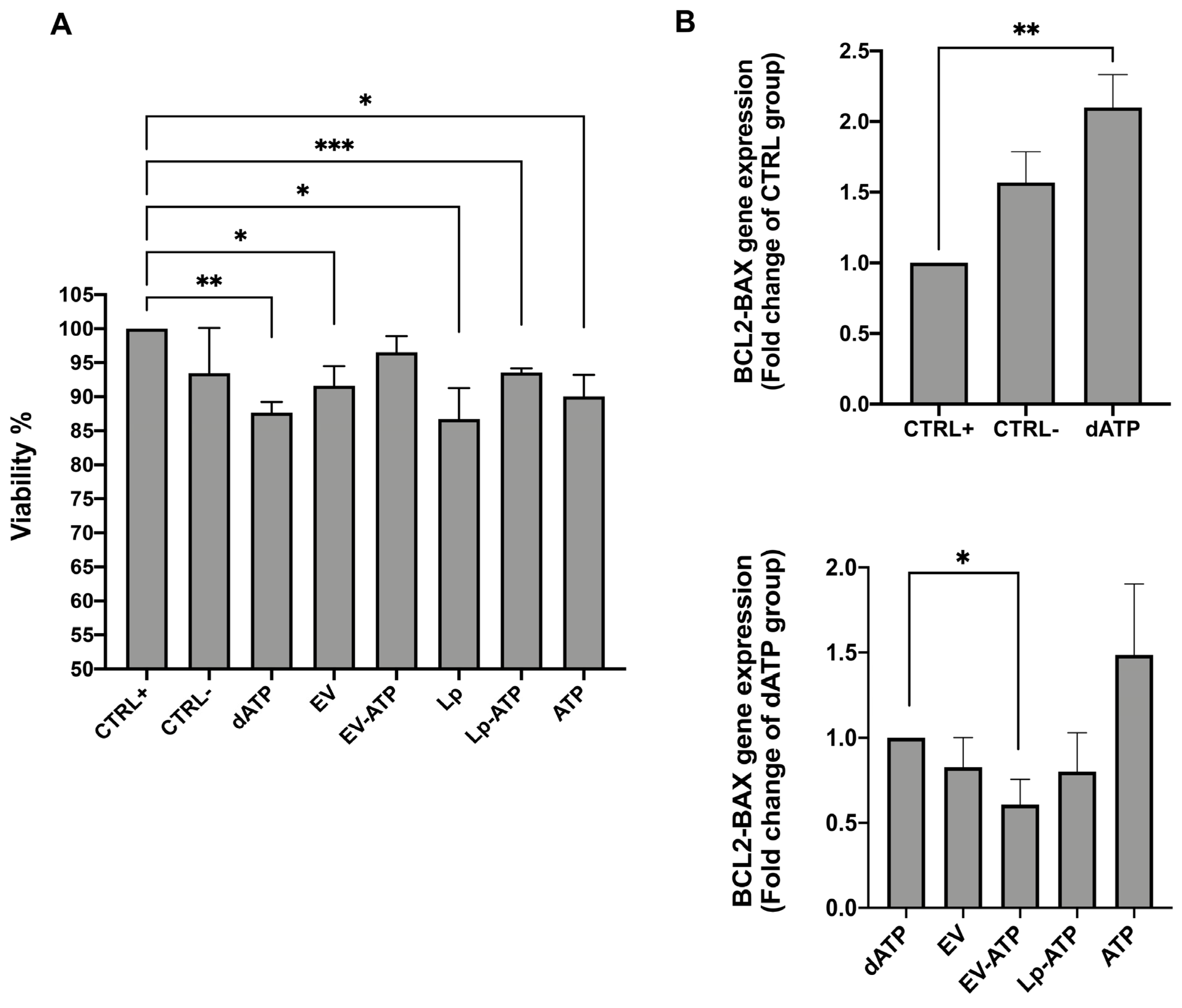
2.7. HK2 Viability
3. Discussion
4. Materials and Methods
4.1. BM-MSCs Expansion and Characterization
4.2. Isolation of MSC-EVs
4.3. Liposome Preparation
4.4. ATP Loading into Liposomes and EV
4.5. ATP Quantification in Liposomes and EVs
4.6. ATP In Vitro Release Study
4.7. Liposomes and EVs Characterization
4.7.1. Nanoparticle Tracking Analysis
4.7.2. Dynamic Light Scattering
4.7.3. Transmission Electron Microscopy
4.7.4. Cryo-Electron Microscopy
4.8. Renal Tubular Cell Culture
4.9. Cell ATP Depletion Injury Model
4.10. Experimental Design
4.11. Assay of Cell Viability
4.12. ATP Quantification in HK2
4.13. RNA Extraction and Quantitative Real-Time Polymerase Chain Reaction
4.14. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 2-DG | 2-Deoxyglucose |
| ANOVA | Analysis of Variance |
| ATP | Adenosine Triphosphate |
| ATP-EVs | ATP-loaded Extracellular Vesicles |
| ATP-LPs | ATP- loaded Liposomes |
| BAX | BCL2 Associated X Protein |
| BCL2 | B-Cell Lymphoma 2 |
| BM | Bone Marrow |
| BM-MSCs | Bone Marrow derived Mesenchymal Stem Cells |
| cDNA | Complementary DNA |
| CD | Cluster of Differentiation |
| CD11b | Cluster of Differentiation 11b |
| CD29 | Cluster of Differentiation 29 |
| CD45 | Cluster of Differentiation 45 |
| CD73 | Ecto-5′-Nucleotidase |
| CD90 | Cluster of Differentiation 90 |
| CD105 | Cluster of Differentiation 105 |
| cP | Centipoise |
| CTRL | Control Group |
| CTRL+ | human tubular cells in complete medium (Positive Control) |
| CTRL- | human tubular cells in medium without serum (Negative Control) |
| dATPs | Deoxyadenosine Triphosphate/ATP depleted human tubular cells |
| DLS | Dynamic Light Scattering |
| DMSO | Dimethyl Sulfoxide |
| DMEM | Dulbecco’s Modified Eagle Medium |
| F12 | Nutrient Mixture F-12 |
| DSPC | 1,2-Distearoyl-sn-glycero-3-phosphocholine |
| DOPS | 1,2-Dioleoyl-sn-glycero-3-phospho-L-serine |
| EDTA | Ethylenediaminetetraacetic Acid |
| ELISA | Enzyme-Linked Immunosorbent Assay |
| EM | Electron Microscopy |
| EV | ATP-depleted human tubular cells conditioned with extracellular vesicles for 4 h |
| EV-ATP | ATP-depleted human tubular cells conditioned with ATP-loaded Extracellular Vesicles for 4 h |
| EVs | Extracellular vesicles |
| FCS | Fetal Calf Serum |
| HK2 | Human Kidney 2 (cell line) |
| I/R | Ischemia/Reperfusion |
| IQR | Interquartile Range |
| LP | liposomes |
| Lp-ATP | ATP-depleted human tubular cells conditioned for ATP-loaded liposomes for 4 h |
| MNCs | Mononuclear Cells |
| MSCs | Mesenchymal Stem Cells |
| MSC-EVs | Mesenchymal Stromal Cell derived Extracellular Vesicles |
| NADH | Nicotinamide Adenine Dinucleotide (Reduced Form) |
| NTA | Nanoparticle Tracking Analysis |
| PBS | Phosphate-Buffered Saline |
| P4 | Passage 4 |
| PCR | Polymerase Chain Reaction |
| PGK | Glyceraldehyde 3-phosphate Dehydrogenase |
| RC | Regenerated Cellulose |
| RNA | Ribonucleic Acid |
| RT-PCR | Real-Time Polymerase Chain Reaction |
| SHM | Staggered Herringbone Micromixer |
| TEM | Transmission Electron Microscopy |
| TFR | Total Flow Rate |
| TRIzol | Tri Reagent for RNA Extraction |
| UV | Ultraviolet |
| Z-potential | Zeta Potential |
References
- Nørgård, M.Ø.; Svenningsen, P. Acute Kidney Injury by Ischemia/Reperfusion and Extracellular Vesicles. Int. J. Mol. Sci. 2023, 24, 15312. [Google Scholar] [CrossRef] [PubMed]
- Panconesi, R.; Widmer, J.; Carvalho, M.F.; Eden, J.; Dondossola, D.; Dutkowski, P.; Schlegel, A. Mitochondria and Ischemia Reperfusion Injury. Curr. Opin. Organ. Transplant. 2022, 27, 434–445. [Google Scholar] [CrossRef]
- Eltzschig, H.K.; Eckle, T. Ischemia and Reperfusion--from Mechanism to Translation. Nat. Med. 2011, 17, 1391–1401. [Google Scholar] [CrossRef] [PubMed]
- Sharfuddin, A.A.; Molitoris, B.A. Pathophysiology of Ischemic Acute Kidney Injury. Nat. Rev. Nephrol. 2011, 7, 189–200. [Google Scholar] [CrossRef]
- Kataria, A.; Magoon, S.; Makkar, B.; Gundroo, A. Machine Perfusion in Kidney Transplantation. Curr. Opin. Organ. Transplant. 2019, 24, 378–384. [Google Scholar] [CrossRef] [PubMed]
- Zulpaite, R.; Miknevicius, P.; Leber, B.; Strupas, K.; Stiegler, P.; Schemmer, P. Ex-Vivo Kidney Machine Perfusion: Therapeutic Potential. Front. Med. 2021, 8, 808719. [Google Scholar] [CrossRef]
- Gordon, J.L. Extracellular ATP: Effects, Sources and Fate. Biochem. J. 1986, 233, 309–319. [Google Scholar] [CrossRef]
- Puisieux, F.; Fattal, E.; Lahiani, M.; Auger, J.; Jouannet, P.; Couvreur, P.; Delattre, J. Liposomes, an Interesting Tool to Deliver a Bioenergetic Substrate (ATP). in Vitro and in Vivo Studies. J. Drug Target. 1994, 2, 443–448. [Google Scholar] [CrossRef]
- Li, P.; Ou, Q.; Shi, S.; Shao, C. Immunomodulatory Properties of Mesenchymal Stem Cells/Dental Stem Cells and Their Therapeutic Applications. Cell. Mol. Immunol. 2023, 20, 558–569. [Google Scholar] [CrossRef]
- Wang, Y.; Gao, T.; Wang, B. Application of Mesenchymal Stem Cells for Anti-Senescence and Clinical Challenges. Stem Cell Res. Ther. 2023, 14, 260. [Google Scholar] [CrossRef]
- Jung, S.-C.; Park, S. New Sources, Differentiation, and Therapeutic Uses of Mesenchymal Stem Cells 2.0. Int. J. Mol. Sci. 2023, 24, 3938. [Google Scholar] [CrossRef] [PubMed]
- Zaripova, L.N.; Midgley, A.; Christmas, S.E.; Beresford, M.W.; Pain, C.; Baildam, E.M.; Oldershaw, R.A. Mesenchymal Stem Cells in the Pathogenesis and Therapy of Autoimmune and Autoinflammatory Diseases. Int. J. Mol. Sci. 2023, 24, 16040. [Google Scholar] [CrossRef] [PubMed]
- Gao, P.; Kajiya, M.; Motoike, S.; Ikeya, M.; Yang, J. Application of Mesenchymal Stem/Stromal Cells in Periodontal Regeneration: Opportunities and Challenges. Jpn. Dent. Sci. Rev. 2024, 60, 95–108. [Google Scholar] [CrossRef]
- Gopalarethinam, J.; Nair, A.P.; Iyer, M.; Vellingiri, B.; Subramaniam, M.D. Advantages of Mesenchymal Stem Cell over the Other Stem Cells. Acta Histochem. 2023, 125, 152041. [Google Scholar] [CrossRef] [PubMed]
- Naji, A.; Eitoku, M.; Favier, B.; Deschaseaux, F.; Rouas-Freiss, N.; Suganuma, N. Biological Functions of Mesenchymal Stem Cells and Clinical Implications. Cell. Mol. Life Sci. 2019, 76, 3323–3348. [Google Scholar] [CrossRef]
- Rampino, T.; Gregorini, M.; Germinario, G.; Pattonieri, E.F.; Erasmi, F.; Grignano, M.A.; Bruno, S.; Alomari, E.; Bettati, S.; Asti, A.; et al. Extracellular Vesicles Derived from Mesenchymal Stromal Cells Delivered during Hypothermic Oxygenated Machine Perfusion Repair Ischemic/Reperfusion Damage of Kidneys from Extended Criteria Donors. Biology 2022, 11, 350. [Google Scholar] [CrossRef]
- Lotfy, A.; AboQuella, N.M.; Wang, H. Mesenchymal Stromal/Stem Cell (MSC)-Derived Exosomes in Clinical Trials. Stem Cell Res. Ther. 2023, 14, 66. [Google Scholar] [CrossRef]
- Jeong, J.-O.; Han, J.W.; Kim, J.-M.; Cho, H.-J.; Park, C.; Lee, N.; Kim, D.-W.; Yoon, Y.-S. Malignant Tumor Formation after Transplantation of Short-Term Cultured Bone Marrow Mesenchymal Stem Cells in Experimental Myocardial Infarction and Diabetic Neuropathy. Circ. Res. 2011, 108, 1340–1347. [Google Scholar] [CrossRef]
- Fennema, E.M.; Tchang, L.A.H.; Yuan, H.; van Blitterswijk, C.A.; Martin, I.; Scherberich, A.; de Boer, J. Ectopic Bone Formation by Aggregated Mesenchymal Stem Cells from Bone Marrow and Adipose Tissue: A Comparative Study. J. Tissue Eng. Regen. Med. 2018, 12, e150–e158. [Google Scholar] [CrossRef]
- Kusuma, G.D.; Menicanin, D.; Gronthos, S.; Manuelpillai, U.; Abumaree, M.H.; Pertile, M.D.; Brennecke, S.P.; Kalionis, B. Ectopic Bone Formation by Mesenchymal Stem Cells Derived from Human Term Placenta and the Decidua. PLoS ONE 2015, 10, e0141246. [Google Scholar] [CrossRef]
- Wang, S.; Guo, L.; Ge, J.; Yu, L.; Cai, T.; Tian, R.; Jiang, Y.; Zhao, R.C.; Wu, Y. Excess Integrins Cause Lung Entrapment of Mesenchymal Stem Cells. Stem Cells 2015, 33, 3315–3326. [Google Scholar] [CrossRef] [PubMed]
- Kim, H.J.; Park, J.-S. Usage of Human Mesenchymal Stem Cells in Cell-Based Therapy: Advantages and Disadvantages. Dev. Reprod. 2017, 21, 1–10. [Google Scholar] [CrossRef]
- Musiał-Wysocka, A.; Kot, M.; Majka, M. The Pros and Cons of Mesenchymal Stem Cell-Based Therapies. Cell Transplant. 2019, 28, 801–812. [Google Scholar] [CrossRef] [PubMed]
- Liang, X.; Ding, Y.; Zhang, Y.; Tse, H.-F.; Lian, Q. Paracrine Mechanisms of Mesenchymal Stem Cell-Based Therapy: Current Status and Perspectives. Cell Transplant. 2014, 23, 1045–1059. [Google Scholar] [CrossRef]
- Kosanović, M.; Milutinović, B.; Kutzner, T.J.; Mouloud, Y.; Bozic, M. Clinical Prospect of Mesenchymal Stromal/Stem Cell-Derived Extracellular Vesicles in Kidney Disease: Challenges and the Way Forward. Pharmaceutics 2023, 15, 1911. [Google Scholar] [CrossRef]
- Tan, F.; Li, X.; Wang, Z.; Li, J.; Shahzad, K.; Zheng, J. Clinical Applications of Stem Cell-Derived Exosomes. Signal Transduct. Target. Ther. 2024, 9, 17. [Google Scholar] [CrossRef]
- Ju, Y.; Hu, Y.; Yang, P.; Xie, X.; Fang, B. Extracellular Vesicle-Loaded Hydrogels for Tissue Repair and Regeneration. Mater. Today Bio 2023, 18, 100522. [Google Scholar] [CrossRef]
- Heldring, N.; Mäger, I.; Wood, M.J.A.; Le Blanc, K.; Andaloussi, S.E.L. Therapeutic Potential of Multipotent Mesenchymal Stromal Cells and Their Extracellular Vesicles. Hum. Gene Ther. 2015, 26, 506–517. [Google Scholar] [CrossRef]
- Börger, V.; Bremer, M.; Ferrer-Tur, R.; Gockeln, L.; Stambouli, O.; Becic, A.; Giebel, B. Mesenchymal Stem/Stromal Cell-Derived Extracellular Vesicles and Their Potential as Novel Immunomodulatory Therapeutic Agents. Int. J. Mol. Sci. 2017, 18, 1450. [Google Scholar] [CrossRef]
- Chen, T.S.; Lai, R.C.; Lee, M.M.; Choo, A.B.H.; Lee, C.N.; Lim, S.K. Mesenchymal Stem Cell Secretes Microparticles Enriched in Pre-microRNAs. Nucleic Acids Res. 2010, 38, 215–224. [Google Scholar] [CrossRef]
- Hendrix, A.; Westbroek, W.; Bracke, M.; De Wever, O. An Ex(o)Citing Machinery for Invasive Tumor Growth. Cancer Res. 2010, 70, 9533–9537. [Google Scholar] [CrossRef]
- Araldi, R.P.; Delvalle, D.A.; da Costa, V.R.; Alievi, A.L.; Teixeira, M.R.; Dias Pinto, J.R.; Kerkis, I. Exosomes as a Nano-Carrier for Chemotherapeutics: A New Era of Oncology. Cells 2023, 12, 2144. [Google Scholar] [CrossRef]
- Lou, G.; Chen, Z.; Zheng, M.; Liu, Y. Mesenchymal Stem Cell-Derived Exosomes as a New Therapeutic Strategy for Liver Diseases. Exp. Mol. Med. 2017, 49, e346. [Google Scholar] [CrossRef]
- Lu, X.; Guo, H.; Wei, X.; Lu, D.; Shu, W.; Song, Y.; Qiu, N.; Xu, X. Current Status and Prospect of Delivery Vehicle Based on Mesenchymal Stem Cell-Derived Exosomes in Liver Diseases. Int. J. Nanomed. 2023, 18, 2873–2890. [Google Scholar] [CrossRef]
- Rao, D.; Huang, D.; Sang, C.; Zhong, T.; Zhang, Z.; Tang, Z. Advances in Mesenchymal Stem Cell-Derived Exosomes as Drug Delivery Vehicles. Front. Bioeng. Biotechnol. 2021, 9, 797359. [Google Scholar] [CrossRef]
- Kim, M.S.; Haney, M.J.; Zhao, Y.; Mahajan, V.; Deygen, I.; Klyachko, N.L.; Inskoe, E.; Piroyan, A.; Sokolsky, M.; Okolie, O.; et al. Development of Exosome-Encapsulated Paclitaxel to Overcome MDR in Cancer Cells. Nanomedicine 2016, 12, 655–664. [Google Scholar] [CrossRef]
- Elsharkasy, O.M.; Nordin, J.Z.; Hagey, D.W.; de Jong, O.G.; Schiffelers, R.M.; Andaloussi, S.E.; Vader, P. Extracellular Vesicles as Drug Delivery Systems: Why and How? Adv. Drug Deliv. Rev. 2020, 159, 332–343. [Google Scholar] [CrossRef]
- Herrmann, I.K.; Wood, M.J.A.; Fuhrmann, G. Extracellular Vesicles as a Next-Generation Drug Delivery Platform. Nat. Nanotechnol. 2021, 16, 748–759. [Google Scholar] [CrossRef]
- Ulpiano, C.; da Silva, C.L.; Monteiro, G.A. Bioengineered Mesenchymal-Stromal-Cell-Derived Extracellular Vesicles as an Improved Drug Delivery System: Methods and Applications. Biomedicines 2023, 11, 1231. [Google Scholar] [CrossRef]
- Gregorini, M.; Corradetti, V.; Pattonieri, E.F.; Rocca, C.; Milanesi, S.; Peloso, A.; Canevari, S.; De Cecco, L.; Dugo, M.; Avanzini, M.A.; et al. Perfusion of Isolated Rat Kidney with Mesenchymal Stromal Cells/Extracellular Vesicles Prevents Ischaemic Injury. J. Cell. Mol. Med. 2017, 21, 3381–3393. [Google Scholar] [CrossRef]
- Grignano, M.A.; Bruno, S.; Viglio, S.; Avanzini, M.A.; Tapparo, M.; Ramus, M.; Croce, S.; Valsecchi, C.; Pattonieri, E.F.; Ceccarelli, G.; et al. CD73-Adenosinergic Axis Mediates the Protective Effect of Extracellular Vesicles Derived from Mesenchymal Stromal Cells on Ischemic Renal Damage in a Rat Model of Donation after Circulatory Death. Int. J. Mol. Sci. 2022, 23, 10681. [Google Scholar] [CrossRef]
- Korb, V.; Tep, K.; Escriou, V.; Richard, C.; Scherman, D.; Cynober, L.; Chaumeil, J.; Dumortier, G. Current Data on ATP-Containing Liposomes and Potential Prospects to Enhance Cellular Energy Status for Hepatic Applications. Crit. Rev. Ther. Drug Carrier Syst. 2008, 25, 305–345. [Google Scholar] [CrossRef]
- Atchison, N.; Swindlehurst, G.; Papas, K.K.; Tsapatsis, M.; Kokkoli, E. Maintenance of Ischemic β Cell Viability through Delivery of Lipids and ATP by Targeted Liposomes. Biomater. Sci. 2014, 2, 548–559. [Google Scholar] [CrossRef]
- Chiang, B.; Essick, E.; Ehringer, W.; Murphree, S.; Hauck, M.A.; Li, M.; Chien, S. Enhancing Skin Wound Healing by Direct Delivery of Intracellular Adenosine Triphosphate. Am. J. Surg. 2007, 193, 213–218. [Google Scholar] [CrossRef]
- Huang, C.-Y.; Kuo, W.-T.; Huang, C.-Y.; Lee, T.-C.; Chen, C.-T.; Peng, W.-H.; Lu, K.-S.; Yang, C.-Y.; Yu, L.C.-H. Distinct Cytoprotective Roles of Pyruvate and ATP by Glucose Metabolism on Epithelial Necroptosis and Crypt Proliferation in Ischaemic Gut. J. Physiol. 2017, 595, 505–521. [Google Scholar] [CrossRef]
- Dvoriantchikova, G.; Barakat, D.J.; Hernandez, E.; Shestopalov, V.I.; Ivanov, D. Liposome-Delivered ATP Effectively Protects the Retina against Ischemia-Reperfusion Injury. Mol. Vis. 2010, 16, 2882–2890. [Google Scholar]
- Choi, Y.S.; Cho, D.Y.; Lee, H.-K.; Cho, J.-K.; Lee, D.H.; Bae, Y.H.; Lee, J.-K.; Kang, H.C. Enhanced Cell Survival of pH-Sensitive Bioenergetic Nucleotide Nanoparticles in Energy/Oxygen-Depleted Cells and Their Intranasal Delivery for Reduced Brain Infarction. Acta Biomater. 2016, 41, 147–160. [Google Scholar] [CrossRef]
- Chapat, S.; Frey, V.; Claperon, N.; Bouchaud, C.; Puisieux, F.; Couvreur, P.; Rossignol, P.; Delattre, J. Efficiency of Liposomal ATP in Cerebral Ischemia: Bioavailability Features. Brain Res. Bull. 1991, 26, 339–342. [Google Scholar] [CrossRef]
- Laham, A.; Claperon, N.; Durussel, J.J.; Fattal, E.; Delattre, J.; Puisieux, F.; Couvreur, P.; Rossignol, P. Intracarotidal Administration of Liposomally-Entrapped ATP: Improved Efficiency against Experimental Brain Ischemia. Pharmacol. Res. Commun. 1988, 20, 699–705. [Google Scholar] [CrossRef]
- Laham, A.; Claperon, N.; Durussel, J.J.; Fattal, E.; Delattre, J.; Puisieux, F.; Couvreur, P.; Rossignol, P. Liposomally Entrapped Adenosine Triphosphate. Improved Efficiency against Experimental Brain Ischaemia in the Rat. J. Chromatogr. 1988, 440, 455–458. [Google Scholar] [CrossRef]
- Laham, A.; Claperon, N.; Durussel, J.J.; Fattal, E.; Delattre, J.; Puisieux, F.; Couvreur, P.; Rossignol, P. Liposomally-Entrapped ATP: Improved Efficiency against Experimental Brain Ischemia in the Rat. Life Sci. 1987, 40, 2011–2016. [Google Scholar] [CrossRef]
- Liu, S.; Zhen, G.; Li, R.-C.; Doré, S. Acute Bioenergetic Intervention or Pharmacological Preconditioning Protects Neuron against Ischemic Injury. J. Exp. Stroke Transl. Med. 2013, 6, 7–17. [Google Scholar] [CrossRef]
- Pi, F.; Tu, X.; Wu, Y. Preparation of ATP-2Na loaded liposome and its effect on tissues energy state in myocardial ischemic mice. Yao Xue Xue Bao 2010, 45, 1322–1326. [Google Scholar]
- Verma, D.D.; Levchenko, T.S.; Bernstein, E.A.; Torchilin, V.P. ATP-Loaded Liposomes Effectively Protect Mechanical Functions of the Myocardium from Global Ischemia in an Isolated Rat Heart Model. J. Control. Release 2005, 108, 460–471. [Google Scholar] [CrossRef]
- Hartner, W.C.; Verma, D.D.; Levchenko, T.S.; Bernstein, E.A.; Torchilin, V.P. ATP-Loaded Liposomes for Treatment of Myocardial Ischemia. Wiley Interdiscip. Rev. Nanomed. Nanobiotechnol. 2009, 1, 530–539. [Google Scholar] [CrossRef]
- Liang, W.; Levchenko, T.; Khaw, B.-A.; Torchilin, V. ATP-Containing Immunoliposomes Specific for Cardiac Myosin. Curr. Drug Deliv. 2004, 1, 1–7. [Google Scholar] [CrossRef]
- Verma, D.D.; Levchenko, T.S.; Bernstein, E.A.; Mongayt, D.; Torchilin, V.P. ATP-Loaded Immunoliposomes Specific for Cardiac Myosin Provide Improved Protection of the Mechanical Functions of Myocardium from Global Ischemia in an Isolated Rat Heart Model. J. Drug Target. 2006, 14, 273–280. [Google Scholar] [CrossRef]
- Pisani, S.; Chiesa, E.; Genta, I.; Dorati, R.; Gregorini, M.; Grignano, M.A.; Ramus, M.; Ceccarelli, G.; Croce, S.; Valsecchi, C.; et al. Liposome Formulation and In Vitro Testing in Non-Physiological Conditions Addressed to Ex Vivo Kidney Perfusion. Int. J. Mol. Sci. 2022, 23, 7999. [Google Scholar] [CrossRef]
- Huang, X.; Ma, Y.; Li, Y.; Han, F.; Lin, W. Targeted Drug Delivery Systems for Kidney Diseases. Front. Bioeng. Biotechnol. 2021, 9, 683247. [Google Scholar] [CrossRef]
- Tuffin, G.; Waelti, E.; Huwyler, J.; Hammer, C.; Marti, H.-P. Immunoliposome Targeting to Mesangial Cells: A Promising Strategy for Specific Drug Delivery to the Kidney. J. Am. Soc. Nephrol. 2005, 16, 3295–3305. [Google Scholar] [CrossRef]
- Grange, C.; Bussolati, B. Extracellular Vesicles in Kidney Disease. Nat. Rev. Nephrol. 2022, 18, 499–513. [Google Scholar] [CrossRef] [PubMed]
- Butreddy, A.; Kommineni, N.; Dudhipala, N. Exosomes as Naturally Occurring Vehicles for Delivery of Biopharmaceuticals: Insights from Drug Delivery to Clinical Perspectives. Nanomaterials 2021, 11, 1481. [Google Scholar] [CrossRef] [PubMed]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal Criteria for Defining Multipotent Mesenchymal Stromal Cells. The International Society for Cellular Therapy Position Statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Comite, P.; Cobianchi, L.; Avanzini, M.A.; Zonta, S.; Mantelli, M.; Achille, V.; De Martino, M.; Cansolino, L.; Ferrari, C.; Alessiani, M.; et al. Isolation and Ex Vivo Expansion of Bone Marrow-Derived Porcine Mesenchymal Stromal Cells: Potential for Application in an Experimental Model of Solid Organ Transplantation in Large Animals. Transplant. Proc. 2010, 42, 1341–1343. [Google Scholar] [CrossRef] [PubMed]
- Maeki, M.; Uno, S.; Niwa, A.; Okada, Y.; Tokeshi, M. Microfluidic Technologies and Devices for Lipid Nanoparticle-Based RNA Delivery. J. Control. Release 2022, 344, 80–96. [Google Scholar] [CrossRef]
- Bezelya, A.; Küçüktürkmen, B.; Bozkır, A. Microfluidic Devices for Precision Nanoparticle Production. Micro 2023, 3, 822–866. [Google Scholar] [CrossRef]
- Ceccotti, E.; Saccu, G.; Herrera Sanchez, M.B.; Bruno, S. Naïve or Engineered Extracellular Vesicles from Different Cell Sources: Therapeutic Tools for Kidney Diseases. Pharmaceutics 2023, 15, 1715. [Google Scholar] [CrossRef]
- Hood, R.R.; Vreeland, W.N.; DeVoe, D.L. Microfluidic Remote Loading for Rapid Single-Step Liposomal Drug Preparation. Lab Chip 2014, 14, 3359–3367. [Google Scholar] [CrossRef]
- Midekessa, G.; Godakumara, K.; Ord, J.; Viil, J.; Lättekivi, F.; Dissanayake, K.; Kopanchuk, S.; Rinken, A.; Andronowska, A.; Bhattacharjee, S.; et al. Zeta Potential of Extracellular Vesicles: Toward Understanding the Attributes That Determine Colloidal Stability. ACS Omega 2020, 5, 16701–16710. [Google Scholar] [CrossRef]
- Liu, J.; Nordin, J.Z.; McLachlan, A.J.; Chrzanowski, W. Extracellular Vesicles as the Next-Generation Modulators of Pharmacokinetics and Pharmacodynamics of Medications and Their Potential as Adjuvant Therapeutics. Clin. Transl. Med. 2024, 14, e70002. [Google Scholar] [CrossRef]
- Dosch, M.; Gerber, J.; Jebbawi, F.; Beldi, G. Mechanisms of ATP Release by Inflammatory Cells. Int. J. Mol. Sci. 2018, 19, 1222. [Google Scholar] [CrossRef]
- Lombardi, M.; Gabrielli, M.; Adinolfi, E.; Verderio, C. Role of ATP in Extracellular Vesicle Biogenesis and Dynamics. Front. Pharmacol. 2021, 12, 654023. [Google Scholar] [CrossRef]
- Bellini, L.; Vadori, M.; De Benedictis, G.M.; Busetto, R. Effects of Opioids on Proximal Renal Tubular Cells Undergoing ATP Depletion. J. Pharmacol. Sci. 2016, 131, 288–291. [Google Scholar] [CrossRef]
- Lee, H.T.; Emala, C.W. Preconditioning and Adenosine Protect Human Proximal Tubule Cells in an in Vitro Model of Ischemic Injury. J. Am. Soc. Nephrol. 2002, 13, 2753–2761. [Google Scholar] [CrossRef] [PubMed]
- Lindoso, R.S.; Collino, F.; Bruno, S.; Araujo, D.S.; Sant’Anna, J.F.; Tetta, C.; Provero, P.; Quesenberry, P.J.; Vieyra, A.; Einicker-Lamas, M.; et al. Extracellular Vesicles Released from Mesenchymal Stromal Cells Modulate miRNA in Renal Tubular Cells and Inhibit ATP Depletion Injury. Stem Cells Dev. 2014, 23, 1809–1819. [Google Scholar] [CrossRef] [PubMed]
- Piffoux, M.; Volatron, J.; Cherukula, K.; Aubertin, K.; Wilhelm, C.; Silva, A.K.A.; Gazeau, F. Engineering and Loading Therapeutic Extracellular Vesicles for Clinical Translation: A Data Reporting Frame for Comparability. Adv. Drug Deliv. Rev. 2021, 178, 113972. [Google Scholar] [CrossRef]
- Ma, Y.; Dong, S.; Grippin, A.J.; Teng, L.; Lee, A.S.; Kim, B.Y.S.; Jiang, W. Engineering Therapeutical Extracellular Vesicles for Clinical Translation. Trends Biotechnol. 2025, 43, 61–82. [Google Scholar] [CrossRef]
- Chiesa, E.; Dorati, R.; Pisani, S.; Conti, B.; Bergamini, G.; Modena, T.; Genta, I. The Microfluidic Technique and the Manufacturing of Polysaccharide Nanoparticles. Pharmaceutics 2018, 10, 267. [Google Scholar] [CrossRef]
- Pisani, S.; Di Martino, D.; Cerri, S.; Genta, I.; Dorati, R.; Bertino, G.; Benazzo, M.; Conti, B. Investigation and Comparison of Active and Passive Encapsulation Methods for Loading Proteins into Liposomes. Int. J. Mol. Sci. 2023, 24, 13542. [Google Scholar] [CrossRef]
| BCL-2 BAX | FW TGGAGCTGCAGAGGATGATTG |
| BCL-2 BAX | RW GGCCTTGAGCACCAGTTTG |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grignano, M.A.; Pisani, S.; Gregorini, M.; Rainaudo, G.; Avanzini, M.A.; Croce, S.; Valsecchi, C.; Ceccarelli, G.; Islami, T.; Margiotta, E.; et al. Engineered ATP-Loaded Extracellular Vesicles Derived from Mesenchymal Stromal Cells: A Novel Strategy to Counteract Cell ATP Depletion in an In Vitro Model. Int. J. Mol. Sci. 2025, 26, 3424. https://doi.org/10.3390/ijms26073424
Grignano MA, Pisani S, Gregorini M, Rainaudo G, Avanzini MA, Croce S, Valsecchi C, Ceccarelli G, Islami T, Margiotta E, et al. Engineered ATP-Loaded Extracellular Vesicles Derived from Mesenchymal Stromal Cells: A Novel Strategy to Counteract Cell ATP Depletion in an In Vitro Model. International Journal of Molecular Sciences. 2025; 26(7):3424. https://doi.org/10.3390/ijms26073424
Chicago/Turabian StyleGrignano, Maria Antonietta, Silvia Pisani, Marilena Gregorini, Giorgia Rainaudo, Maria Antonietta Avanzini, Stefania Croce, Chiara Valsecchi, Gabriele Ceccarelli, Tefik Islami, Elisabetta Margiotta, and et al. 2025. "Engineered ATP-Loaded Extracellular Vesicles Derived from Mesenchymal Stromal Cells: A Novel Strategy to Counteract Cell ATP Depletion in an In Vitro Model" International Journal of Molecular Sciences 26, no. 7: 3424. https://doi.org/10.3390/ijms26073424
APA StyleGrignano, M. A., Pisani, S., Gregorini, M., Rainaudo, G., Avanzini, M. A., Croce, S., Valsecchi, C., Ceccarelli, G., Islami, T., Margiotta, E., Portalupi, V., De Mauri, A., Stea, E. D., Pattonieri, E. F., Iadarola, P., Viglio, S., Conti, B., & Rampino, T. (2025). Engineered ATP-Loaded Extracellular Vesicles Derived from Mesenchymal Stromal Cells: A Novel Strategy to Counteract Cell ATP Depletion in an In Vitro Model. International Journal of Molecular Sciences, 26(7), 3424. https://doi.org/10.3390/ijms26073424










