Mouse Models of HIV-Associated Atherosclerosis
Abstract
1. Introduction
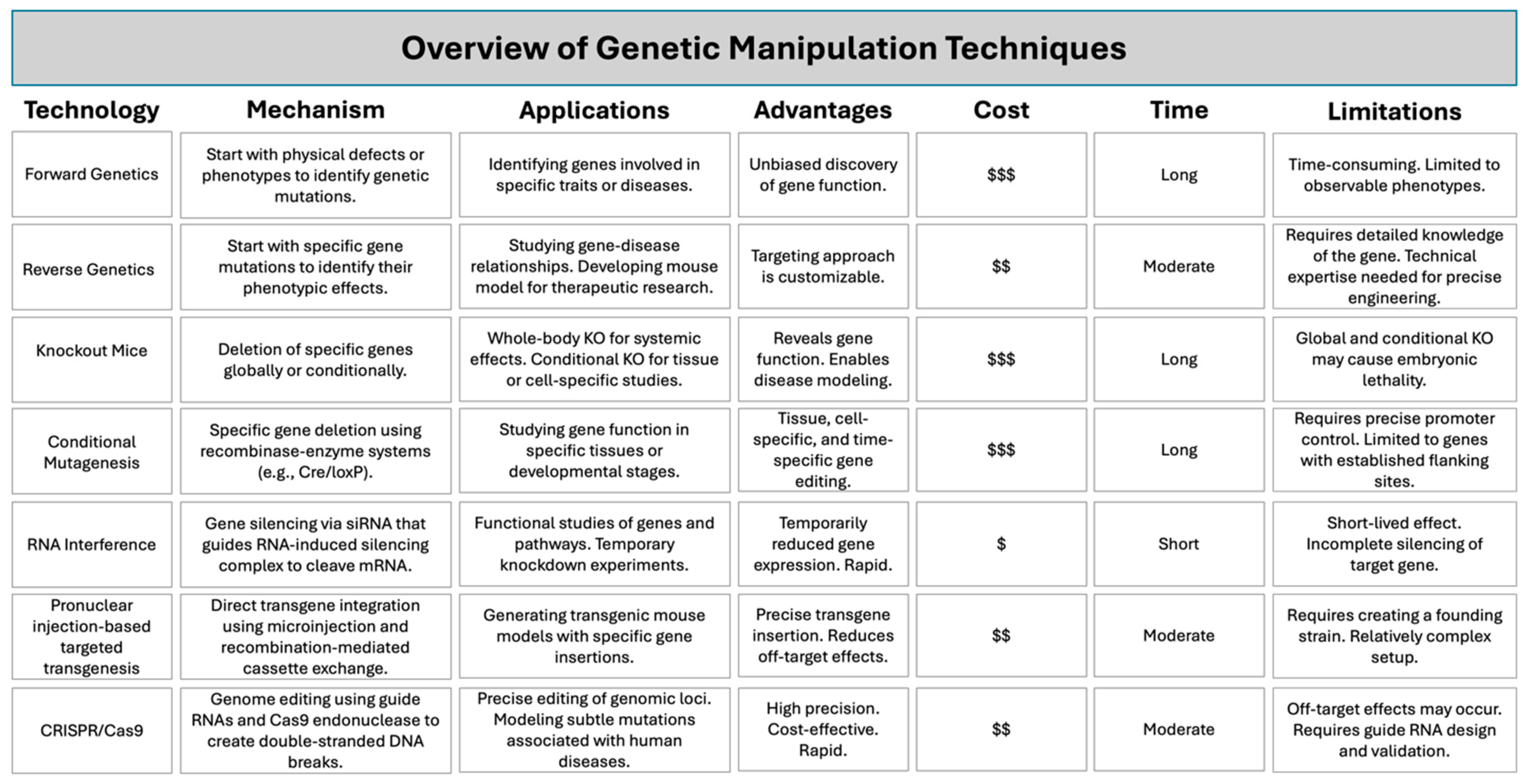
2. Advancements in Mouse Model Technology
3. Advantages of Using Mouse Models in Atherosclerotic Cardiovascular Disease Research
4. Mouse Models for Atherosclerosis
5. Mouse Models of HIV-Associated Atherosclerosis
6. Atherosclerotic Cardiovascular Disease in People Living with HIV
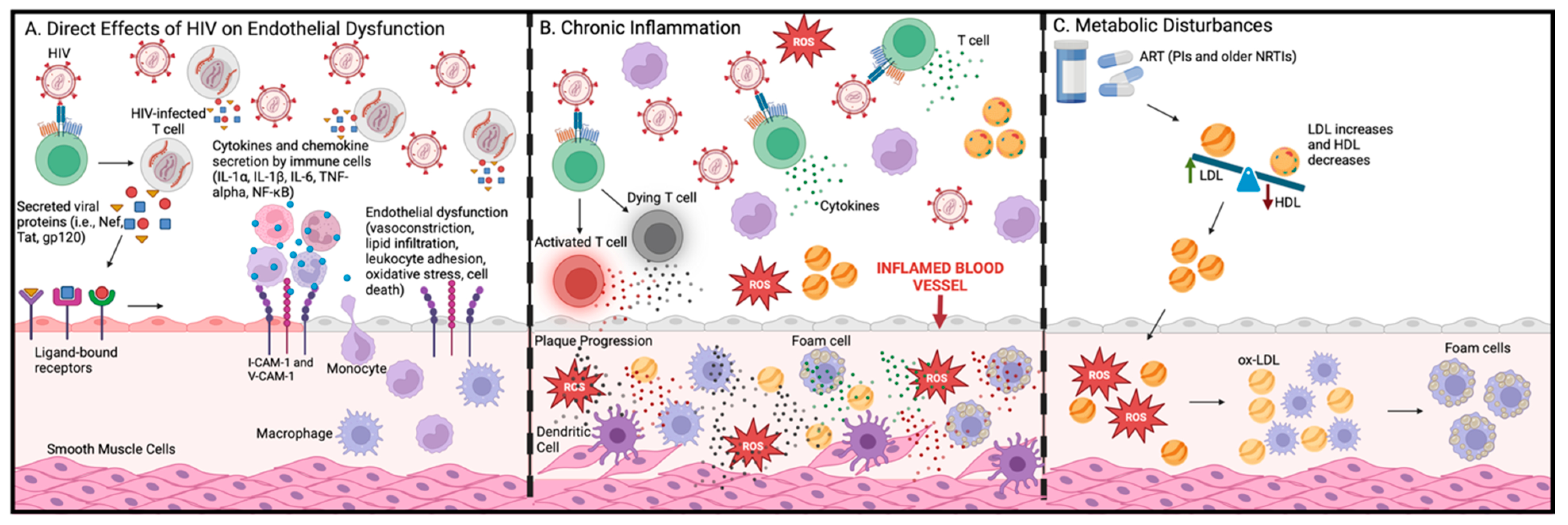
6.1. Direct Effects of HIV on Vascular Cells
6.2. HIV Glycoprotein (gp 120)
6.3. HIV Trans-Activator of Transcription (Tat)
6.4. HIV Negative Regulatory Factor (Nef)
6.5. Inflammation and Immune Activation
| PBMCs | Role in HIV | Pro-atherogenic Features and Mechanism of Atherogenesis | Ref. |
|---|---|---|---|
| MDMs | Monocytes and macrophages are reservoirs for HIV. HIV infection of these cells contributes to persistent inflammation. |
| [78,79] |
| DCs | DCs play an important role in viral transmission to CD4+ T cells. HIV also increases the activation of DCs, promoting inflammation. |
| [80,81,82] |
| CD4+ T cells | HIV targets CD4+ T cells and uses its machinery to replicate. This causes the cells to die, leading to low CD4+ T cell counts without ART. |
| [83,84] |
| B cells | HIV viral proteins and virions trigger the production of pro-inflammatory cytokines from B cells and increase apoptosis. HIV also alters their class switching capabilities. |
| [85] |
| NK cells | NK cells are crucial for the body’s defense against viruses. However, HIV can impair the antiviral properties of NK cells and inhibit their ability to interact with other immune cells, leading to increased production of inflammatory mediators. |
| [86,87] |
6.6. Insights into Pathogenic Mechanisms
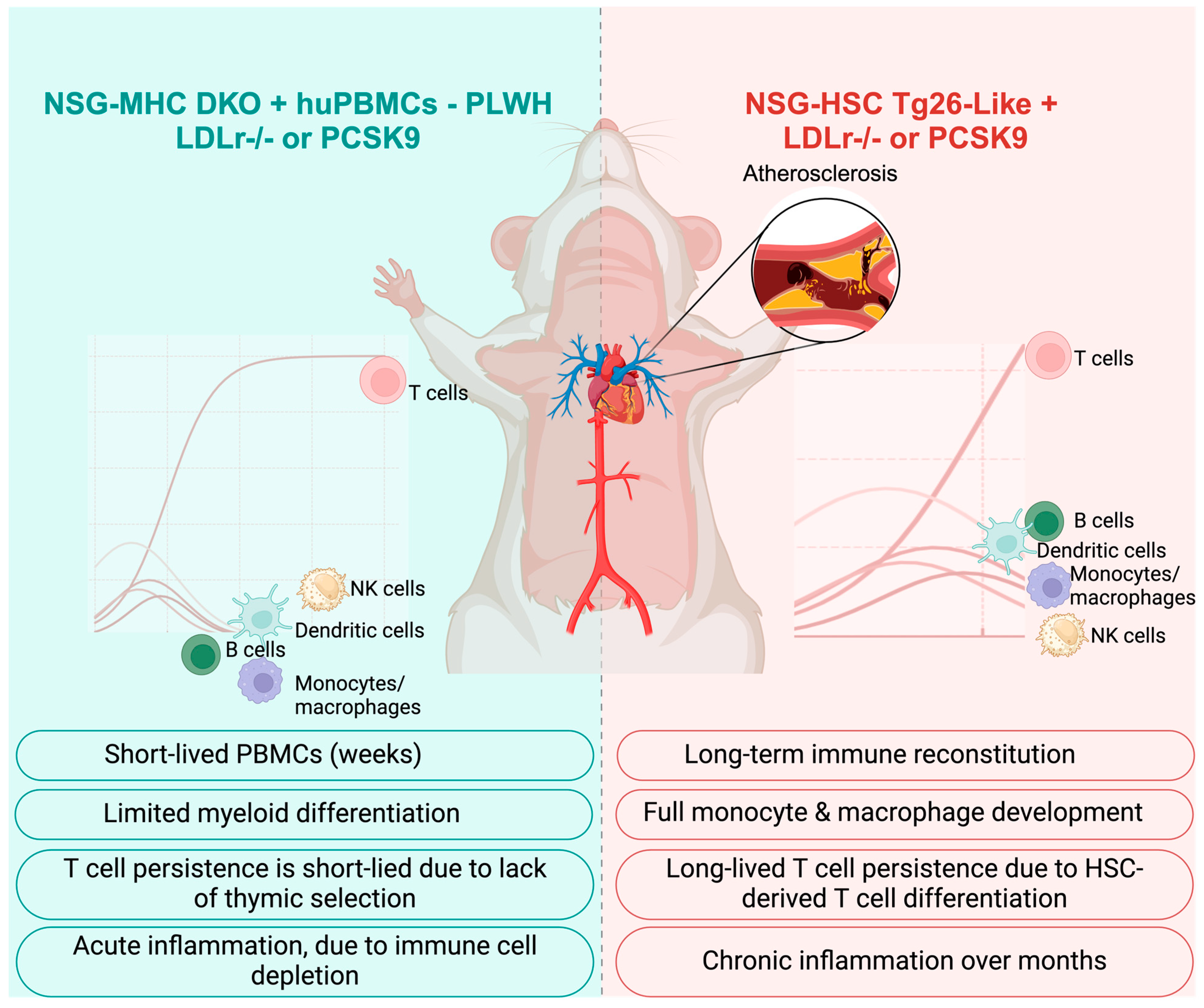
6.7. Optimizing Immune-Deficient Mouse Models for Atherosclerosis and HIV Research Using Human Immune Cells
6.8. Key Features of NSG-Based Mouse Models for the Study of Atherosclerosis Progression in HIV
7. Integration of Omics Approaches to Deepen Understanding
8. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| APOE | apolipoprotein E |
| ART | antiretroviral therapies |
| ASCVD | atherosclerotic cardiovascular disease |
| CAD | coronary artery disease |
| CAMs | cell adhesion molecules |
| cART | combination antiretroviral therapy |
| CETP | cholesteryl ester transfer protein |
| CMV | cytomegalovirus |
| CRISPR/Cas9 | clustered regularly interspaced short palindromic repeats/CRISPR-associated 9 |
| CVD | cardiovascular disease |
| DCs | dendritic cells |
| DKO | double knockout |
| DOI | DNA of interest |
| dsRNA | double-stranded RNA |
| ECM | extracellular matrix |
| ECs | endothelial cells |
| eNOS | endothelial nitric oxide synthase |
| eQTLs | expression quantitative trait loci |
| ER | endoplasmic reticulum |
| Fbn1 | fibrillin 1 |
| gp | glycoprotein |
| GWAS | genome-wide association studies |
| HDL | high-density lipoprotein |
| HEs | homing endonucleases |
| HIV | human immunodeficiency virus |
| HSC | hematopoietic stem cell |
| IFN | interferon |
| INSTI | integrase strand transfer inhibitors |
| KO | knockout |
| KTR | kynurenine to tryptophan ratio |
| LDL | low-density lipoproteins |
| LDLR | low-density lipoproteins receptor |
| MDM | monocyte-derived macrophage |
| MHC | major histocompatibility |
| M-tropic | macrophage-tropic |
| mRNA | messenger RNA |
| NADPH | nicotinamide adenine dinucleotide phosphate |
| NF-kB | nuclear factor kappa B |
| NHEJ | non-homologous end joining |
| NK | natural killer |
| NO | nitric oxide |
| NOX | nicotinamide adenine dinucleotide phosphate oxidase |
| NNRTI | non-nucleoside reverse transcriptase inhibitors |
| NRTI | nucleoside reverse transcriptase inhibitors |
| NSG | NOD scid gamma |
| ox-LDL | oxidized-LDL |
| PBMC | peripheral blood mononuclear cells |
| PI | protease inhibitors |
| PITT | pronuclear injection-based targeted transgenesis |
| PI3K | phosphoinositide 3-kinase |
| PLWH | people living with HIV |
| PWoH | people without HIV |
| RISC | RNA-induced silencing complex |
| RMCE | recombination-mediated cassette exchange |
| RNA | ribonucleic acid |
| RNAi | RNA interference |
| ROS | reactive oxygen species |
| siRNA | small interfering RNAs |
| TALENs | transcription activator-like effector nucleases |
| Tat | trans-activator of transcription |
| TREM | triggering receptor expressed on myeloid cells |
| UPR | unfolded protein response |
| VSMC | vascular smooth muscle cell |
| ZFNs | zinc-finger nucleases |
References
- Rydell-Törmänen, K.; Johnson, J.R. The Applicability of Mouse Models to the Study of Human Disease. Methods Mol. Biol. 2019, 1940, 3–22. [Google Scholar] [CrossRef] [PubMed]
- Bryda, E.C. The Mighty Mouse: The impact of rodents on advances in biomedical research. Mo. Med. 2013, 110, 207–211. [Google Scholar]
- Gurumurthy, C.B.; Lloyd, K.C.K. Generating mouse models for biomedical research: Technological advances. Dis. Model. Mech. 2019, 12, dmm029462. [Google Scholar] [CrossRef]
- McAlpine, W.; Russell, J.; Murray, A.R.; Beutler, B.; Turer, E. Research Techniques Made Simple: Forward Genetic Screening to Uncover Genes Involved in Skin Biology. J. Invest. Dermatol. 2019, 139, 1848–1853.e1. [Google Scholar] [CrossRef]
- Hall, B.; Limaye, A.; Kulkarni, A.B. Overview: Generation of gene knockout mice. Curr. Protoc. Cell Biol. 2009, 44, 19.12.1–19.12.17. [Google Scholar] [CrossRef] [PubMed]
- Park, E.C.; Finley, D.; Szostak, J.W. A strategy for the generation of conditional mutations by protein destabilization. Proc. Natl. Acad. Sci. USA 1992, 89, 1249–1252. [Google Scholar] [CrossRef]
- Kanasty, R.L.; Whitehead, K.A.; Vegas, A.J.; Anderson, D.G. Action and reaction: The biological response to siRNA and its delivery vehicles. Mol. Ther. 2012, 20, 513–524. [Google Scholar] [CrossRef] [PubMed]
- Ohtsuka, M.; Miura, H.; Sato, M.; Kimura, M.; Inoko, H.; Gurumurthy, C.B. PITT: Pronuclear injection-based targeted transgenesis, a reliable transgene expression method in mice. Exp. Anim. 2012, 61, 489–502. [Google Scholar] [CrossRef]
- Redman, M.; King, A.; Watson, C.; King, D. What is CRISPR/Cas9? Arch. Dis. Child. Educ. Pract. Ed. 2016, 101, 213–215. [Google Scholar] [CrossRef]
- Waterston, R.H.; Lindblad-Toh, K.; Birney, E.; Rogers, J.; Abril, J.F.; Agarwal, P.; Agarwala, R.; Ainscough, R.; Alexandersson, M.; An, P.; et al. Initial sequencing and comparative analysis of the mouse genome. Nature 2002, 420, 520–562. [Google Scholar] [CrossRef]
- Thompson, S.; Clarke, A.R.; Pow, A.M.; Hooper, M.L.; Melton, D.W. Germ line transmission and expression of a corrected HPRT gene produced by gene targeting in embryonic stem cells. Cell 1989, 56, 313–321. [Google Scholar] [CrossRef] [PubMed]
- Thomas, K.R.; Capecchi, M.R. Site-directed mutagenesis by gene targeting in mouse embryo-derived stem cells. Cell 1987, 51, 503–512. [Google Scholar] [CrossRef]
- Pulina, M.V.; Sahr, K.E.; Nowotschin, S.; Baron, M.H.; Hadjantonakis, A.K. A conditional mutant allele for analysis of Mixl1 function in the mouse. Genesis 2014, 52, 417–423. [Google Scholar] [CrossRef]
- Gassmann, M.; Casagranda, F.; Orioli, D.; Simon, H.; Lai, C.; Klein, R.; Lemke, G. Aberrant neural and cardiac development in mice lacking the ErbB4 neuregulin receptor. Nature 1995, 378, 390–394. [Google Scholar] [CrossRef] [PubMed]
- Gowen, L.C.; Johnson, B.L.; Latour, A.M.; Sulik, K.K.; Koller, B.H. Brca1 deficiency results in early embryonic lethality characterized by neuroepithelial abnormalities. Nat. Genet. 1996, 12, 191–194. [Google Scholar] [CrossRef]
- Ludwig, T.; Chapman, D.L.; Papaioannou, V.E.; Efstratiadis, A. Targeted mutations of breast cancer susceptibility gene homologs in mice: Lethal phenotypes of Brca1, Brca2, Brca1/Brca2, Brca1/p53, and Brca2/p53 nullizygous embryos. Genes. Dev. 1997, 11, 1226–1241. [Google Scholar] [CrossRef] [PubMed]
- Leung, R.K.; Whittaker, P.A. RNA interference: From gene silencing to gene-specific therapeutics. Pharmacol. Ther. 2005, 107, 222–239. [Google Scholar] [CrossRef]
- Tiscornia, G.; Singer, O.; Ikawa, M.; Verma, I.M. A general method for gene knockdown in mice by using lentiviral vectors expressing small interfering RNA. Proc. Natl. Acad. Sci. USA 2003, 100, 1844–1848. [Google Scholar] [CrossRef]
- Fire, A.; Xu, S.; Montgomery, M.K.; Kostas, S.A.; Driver, S.E.; Mello, C.C. Potent and specific genetic interference by double-stranded RNA in Caenorhabditis elegans. Nature 1998, 391, 806–811. [Google Scholar] [CrossRef]
- Ohtsuka, M.; Ogiwara, S.; Miura, H.; Mizutani, A.; Warita, T.; Sato, M.; Imai, K.; Hozumi, K.; Sato, T.; Tanaka, M.; et al. Pronuclear injection-based mouse targeted transgenesis for reproducible and highly efficient transgene expression. Nucleic Acids Res. 2010, 38, e198. [Google Scholar] [CrossRef]
- Hall, B.; Cho, A.; Limaye, A.; Cho, K.; Khillan, J.; Kulkarni, A.B. Genome Editing in Mice Using CRISPR/Cas9 Technology. Curr. Protoc. Cell Biol. 2018, 81, e57. [Google Scholar] [CrossRef]
- Jebari-Benslaiman, S.; Galicia-García, U.; Larrea-Sebal, A.; Olaetxea, J.R.; Alloza, I.; Vandenbroeck, K.; Benito-Vicente, A.; Martín, C. Pathophysiology of Atherosclerosis. Int. J. Mol. Sci. 2022, 23, 3346. [Google Scholar] [CrossRef]
- Oppi, S.; Lüscher, T.F.; Stein, S. Mouse Models for Atherosclerosis Research-Which Is My Line? Front. Cardiovasc. Med. 2019, 6, 46. [Google Scholar] [CrossRef]
- von Scheidt, M.; Zhao, Y.; Kurt, Z.; Pan, C.; Zeng, L.; Yang, X.; Schunkert, H.; Lusis, A.J. Applications and Limitations of Mouse Models for Understanding Human Atherosclerosis. Cell Metab. 2017, 25, 248–261. [Google Scholar] [CrossRef]
- Takahashi, S.; Fukami, T.; Masuo, Y.; Brocker, C.N.; Xie, C.; Krausz, K.W.; Wolf, C.R.; Henderson, C.J.; Gonzalez, F.J. Cyp2c70 is responsible for the species difference in bile acid metabolism between mice and humans. J. Lipid Res. 2016, 57, 2130–2137. [Google Scholar] [CrossRef]
- Xu, Y.; Li, F.; Zalzala, M.; Xu, J.; Gonzalez, F.J.; Adorini, L.; Lee, Y.K.; Yin, L.; Zhang, Y. Farnesoid X receptor activation increases reverse cholesterol transport by modulating bile acid composition and cholesterol absorption in mice. Hepatology 2016, 64, 1072–1085. [Google Scholar] [CrossRef] [PubMed]
- Vinué, Á.; Herrero-Cervera, A.; González-Navarro, H. Understanding the Impact of Dietary Cholesterol on Chronic Metabolic Diseases through Studies in Rodent Models. Nutrients 2018, 10, 939. [Google Scholar] [CrossRef] [PubMed]
- Paigen, B.; Holmes, P.A.; Mitchell, D.; Albee, D. Comparison of atherosclerotic lesions and HDL-lipid levels in male, female, and testosterone-treated female mice from strains C57BL/6, BALB/c, and C3H. Atherosclerosis 1987, 64, 215–221. [Google Scholar] [CrossRef]
- Paigen, B.; Morrow, A.; Brandon, C.; Mitchell, D.; Holmes, P. Variation in susceptibility to atherosclerosis among inbred strains of mice. Atherosclerosis 1985, 57, 65–73. [Google Scholar] [CrossRef]
- Schreyer, S.A.; Wilson, D.L.; LeBoeuf, R.C. C57BL/6 mice fed high fat diets as models for diabetes-accelerated atherosclerosis. Atherosclerosis 1998, 136, 17–24. [Google Scholar] [CrossRef]
- Watanabe, H.; Numata, K.; Ito, T.; Takagi, K.; Matsukawa, A. Innate immune response in Th1- and Th2-dominant mouse strains. Shock 2004, 22, 460–466. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, Y.; Plump, A.S.; Raines, E.W.; Breslow, J.L.; Ross, R. ApoE-deficient mice develop lesions of all phases of atherosclerosis throughout the arterial tree. Arterioscler. Thromb. 1994, 14, 133–140. [Google Scholar] [CrossRef] [PubMed]
- Tian, J.; Pei, H.; James, J.C.; Li, Y.; Matsumoto, A.H.; Helm, G.A.; Shi, W. Circulating adhesion molecules in apoE-deficient mouse strains with different atherosclerosis susceptibility. Biochem. Biophys. Res. Commun. 2005, 329, 1102–1107. [Google Scholar] [CrossRef] [PubMed]
- van den Maagdenberg, A.M.; Hofker, M.H.; Krimpenfort, P.J.; de Bruijn, I.; van Vlijmen, B.; van der Boom, H.; Havekes, L.M.; Frants, R.R. Transgenic mice carrying the apolipoprotein E3-Leiden gene exhibit hyperlipoproteinemia. J. Biol. Chem. 1993, 268, 10540–10545. [Google Scholar] [CrossRef]
- Westerterp, M.; van der Hoogt, C.C.; de Haan, W.; Offerman, E.H.; Dallinga-Thie, G.M.; Jukema, J.W.; Havekes, L.M.; Rensen, P.C. Cholesteryl ester transfer protein decreases high-density lipoprotein and severely aggravates atherosclerosis in APOE*3-Leiden mice. Arterioscler. Thromb. Vasc. Biol. 2006, 26, 2552–2559. [Google Scholar] [CrossRef]
- Ishibashi, S.; Goldstein, J.L.; Brown, M.S.; Herz, J.; Burns, D.K. Massive xanthomatosis and atherosclerosis in cholesterol-fed low density lipoprotein receptor-negative mice. J. Clin. Investig. 1994, 93, 1885–1893. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. Molecular medicine. The cholesterol quartet. Science 2001, 292, 1310–1312. [Google Scholar] [CrossRef]
- Van Herck, J.L.; De Meyer, G.R.; Martinet, W.; Van Hove, C.E.; Foubert, K.; Theunis, M.H.; Apers, S.; Bult, H.; Vrints, C.J.; Herman, A.G. Impaired fibrillin-1 function promotes features of plaque instability in apolipoprotein E-deficient mice. Circulation 2009, 120, 2478–2487. [Google Scholar] [CrossRef]
- Sato, K.; Nakano, K.; Katsuki, S.; Matoba, T.; Osada, K.; Sawamura, T.; Sunagawa, K.; Egashira, K. Dietary cholesterol oxidation products accelerate plaque destabilization and rupture associated with monocyte infiltration/activation via the MCP-1-CCR2 pathway in mouse brachiocephalic arteries: Therapeutic effects of ezetimibe. J. Atheroscler. Thromb. 2012, 19, 986–998. [Google Scholar] [CrossRef]
- Dickie, P.; Felser, J.; Eckhaus, M.; Bryant, J.; Silver, J.; Marinos, N.; Notkins, A.L. HIV-associated nephropathy in transgenic mice expressing HIV-1 genes. Virology 1991, 185, 109–119. [Google Scholar] [CrossRef]
- Kress, T.C.; Barris, C.T.; Kovacs, L.; Khakina, B.N.; Jordan, C.R.; Bruder-Nascimento, T.; Stepp, D.W.; MacArthur, R.; Patel, V.S.; Chen, J.; et al. CD4+ T Cells Expressing Viral Proteins Induce HIV-Associated Endothelial Dysfunction and Hypertension Through Interleukin 1α–Mediated Increases in Endothelial NADPH Oxidase 1. Circulation 2025. [Google Scholar] [CrossRef]
- Alam, M.A.; Caocci, M.; Ren, M.; Chen, Z.; Liu, F.; Khatun, M.S.; Kolls, J.K.; Qin, X.; Burdo, T.H. Deficiency of Caspase-1 Attenuates HIV-1-Associated Atherogenesis in Mice. Int. J. Mol. Sci. 2023, 24, 12871. [Google Scholar] [CrossRef] [PubMed]
- Bandera, A.; Masetti, M.; Fabbiani, M.; Biasin, M.; Muscatello, A.; Squillace, N.; Clerici, M.; Gori, A.; Trabattoni, D. The NLRP3 Inflammasome Is Upregulated in HIV-Infected Antiretroviral Therapy-Treated Individuals with Defective Immune Recovery. Front. Immunol. 2018, 9, 214. [Google Scholar] [CrossRef]
- Kearns, A.C.; Velasquez, S.; Liu, F.; Dai, S.; Chen, Y.; Lehmicke, G.; Gordon, J.; Rappaport, J.; Qin, X. Elevated indoleamine-2,3-dioxygenase enzyme activity in a novel mouse model of HIV-associated atherosclerosis. AIDS 2019, 33, 1557–1564. [Google Scholar] [CrossRef]
- Morimoto, K.; Shimada, N.; Naganawa, H.; Takita, T.; Umezawa, H.; Kambara, H. Minor congeners of antrimycin: Application of secondary ion mass spectrometry (SIMS) to structure determination. J. Antibiot. 1982, 35, 378–380. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cui, H.L.; Ditiatkovski, M.; Kesani, R.; Bobryshev, Y.V.; Liu, Y.; Geyer, M.; Mukhamedova, N.; Bukrinsky, M.; Sviridov, D. HIV protein Nef causes dyslipidemia and formation of foam cells in mouse models of atherosclerosis. FASEB J. 2014, 28, 2828–2839. [Google Scholar] [CrossRef] [PubMed]
- Pushkarsky, T.; Shilov, E.; Kruglova, N.; Naumann, R.; Brichacek, B.; Jennelle, L.; Sviridov, D.; Kruglov, A.; Nedospasov, S.A.; Bukrinsky, M. Short Communication: Accumulation of Neutral Lipids in Liver and Aorta of Nef-Transgenic Mice. AIDS Res. Hum. Retroviruses 2017, 33, 57–60. [Google Scholar] [CrossRef]
- Anand, A.R.; Rachel, G.; Parthasarathy, D. HIV Proteins and Endothelial Dysfunction: Implications in Cardiovascular Disease. Front. Cardiovasc. Med. 2018, 5, 185. [Google Scholar] [CrossRef]
- Yang, B.; Akhter, S.; Chaudhuri, A.; Kanmogne, G.D. HIV-1 gp120 induces cytokine expression, leukocyte adhesion, and transmigration across the blood-brain barrier: Modulatory effects of STAT1 signaling. Microvasc. Res. 2009, 77, 212–219. [Google Scholar] [CrossRef]
- Ren, Z.; Yao, Q.; Chen, C. HIV-1 envelope glycoprotein 120 increases intercellular adhesion molecule-1 expression by human endothelial cells. Lab. Invest. 2002, 82, 245–255. [Google Scholar] [CrossRef][Green Version]
- Kanmogne, G.D.; Primeaux, C.; Grammas, P. HIV-1 gp120 proteins alter tight junction protein expression and brain endothelial cell permeability: Implications for the pathogenesis of HIV-associated dementia. J. Neuropathol. Exp. Neurol. 2005, 64, 498–505. [Google Scholar] [CrossRef] [PubMed]
- Sufiawati, I.; Tugizov, S.M. HIV-induced matrix metalloproteinase-9 activation through mitogen-activated protein kinase signalling promotes HSV-1 cell-to-cell spread in oral epithelial cells. J. Gen. Virol. 2018, 99, 937–947. [Google Scholar] [CrossRef] [PubMed]
- Louboutin, J.P.; Reyes, B.A.; Agrawal, L.; Van Bockstaele, E.J.; Strayer, D.S. HIV-1 gp120 upregulates matrix metalloproteinases and their inhibitors in a rat model of HIV encephalopathy. Eur. J. Neurosci. 2011, 34, 2015–2023. [Google Scholar] [CrossRef]
- Annunziata, P.; Cioni, C.; Toneatto, S.; Paccagnini, E. HIV-1 gp120 increases the permeability of rat brain endothelium cultures by a mechanism involving substance P. AIDS 1998, 12, 2377–2385. [Google Scholar] [CrossRef]
- Urbinati, C.; Mitola, S.; Tanghetti, E.; Kumar, C.; Waltenberger, J.; Ribatti, D.; Presta, M.; Rusnati, M. Integrin alphavbeta3 as a target for blocking HIV-1 Tat-induced endothelial cell activation in vitro and angiogenesis in vivo. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 2315–2320. [Google Scholar] [CrossRef] [PubMed]
- Fanales-Belasio, E.; Moretti, S.; Nappi, F.; Barillari, G.; Micheletti, F.; Cafaro, A.; Ensoli, B. Native HIV-1 Tat protein targets monocyte-derived dendritic cells and enhances their maturation, function, and antigen-specific T cell responses. J. Immunol. 2002, 168, 197–206. [Google Scholar] [CrossRef]
- Park, I.W.; Ullrich, C.K.; Schoenberger, E.; Ganju, R.K.; Groopman, J.E. HIV-1 Tat induces microvascular endothelial apoptosis through caspase activation. J. Immunol. 2001, 167, 2766–2771. [Google Scholar] [CrossRef]
- Ben Haij, N.; Planès, R.; Leghmari, K.; Serrero, M.; Delobel, P.; Izopet, J.; BenMohamed, L.; Bahraoui, E. HIV-1 Tat Protein Induces Production of Proinflammatory Cytokines by Human Dendritic Cells and Monocytes/Macrophages through Engagement of TLR4-MD2-CD14 Complex and Activation of NF-κB Pathway. PLoS ONE 2015, 10, e0129425. [Google Scholar] [CrossRef]
- Pu, H.; Tian, J.; Flora, G.; Lee, Y.W.; Nath, A.; Hennig, B.; Toborek, M. HIV-1 Tat protein upregulates inflammatory mediators and induces monocyte invasion into the brain. Mol. Cell Neurosci. 2003, 24, 224–237. [Google Scholar] [CrossRef]
- Park, I.W.; Wang, J.F.; Groopman, J.E. HIV-1 Tat promotes monocyte chemoattractant protein-1 secretion followed by transmigration of monocytes. Blood 2001, 97, 352–358. [Google Scholar] [CrossRef]
- Wang, T.; Green, L.A.; Gupta, S.K.; Kim, C.; Wang, L.; Almodovar, S.; Flores, S.C.; Prudovsky, I.A.; Jolicoeur, P.; Liu, Z.; et al. Transfer of intracellular HIV Nef to endothelium causes endothelial dysfunction. PLoS ONE 2014, 9, e91063. [Google Scholar] [CrossRef]
- Duffy, P.; Wang, X.; Lin, P.H.; Yao, Q.; Chen, C. HIV Nef protein causes endothelial dysfunction in porcine pulmonary arteries and human pulmonary artery endothelial cells. J. Surg. Res. 2009, 156, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Acheampong, E.A.; Parveen, Z.; Muthoga, L.W.; Kalayeh, M.; Mukhtar, M.; Pomerantz, R.J. Human Immunodeficiency virus type 1 Nef potently induces apoptosis in primary human brain microvascular endothelial cells via the activation of caspases. J. Virol. 2005, 79, 4257–4269. [Google Scholar] [CrossRef]
- Lenassi, M.; Cagney, G.; Liao, M.; Vaupotic, T.; Bartholomeeusen, K.; Cheng, Y.; Krogan, N.J.; Plemenitas, A.; Peterlin, B.M. HIV Nef is secreted in exosomes and triggers apoptosis in bystander CD4+ T cells. Traffic 2010, 11, 110–122. [Google Scholar] [CrossRef] [PubMed]
- Lehmann, M.H.; Masanetz, S.; Kramer, S.; Erfle, V. HIV-1 Nef upregulates CCL2/MCP-1 expression in astrocytes in a myristoylation- and calmodulin-dependent manner. J. Cell Sci. 2006, 119, 4520–4530. [Google Scholar] [CrossRef] [PubMed]
- Aiello, A.; Giannessi, F.; Percario, Z.A.; Fecchi, K.; Arenaccio, C.; Leone, S.; Carollo, M.; D’Aversa, E.; Chaperot, L.; Gambari, R.; et al. HIV-1 Nef Protein Affects Cytokine and Extracellular Vesicles Production in the GEN2.2 Plasmacytoid Dendritic Cell Line. Viruses 2021, 14, 74. [Google Scholar] [CrossRef]
- Andrade, V.M.; Mavian, C.; Babic, D.; Cordeiro, T.; Sharkey, M.; Barrios, L.; Brander, C.; Martinez-Picado, J.; Dalmau, J.; Llano, A.; et al. A minor population of macrophage-tropic HIV-1 variants is identified in recrudescing viremia following analytic treatment interruption. Proc. Natl. Acad. Sci. USA 2020, 117, 9981–9990. [Google Scholar] [CrossRef]
- Giri, M.S.; Nebozyhn, M.; Raymond, A.; Gekonge, B.; Hancock, A.; Creer, S.; Nicols, C.; Yousef, M.; Foulkes, A.S.; Mounzer, K.; et al. Circulating monocytes in HIV-1-infected viremic subjects exhibit an antiapoptosis gene signature and virus- and host-mediated apoptosis resistance. J. Immunol. 2009, 182, 4459–4470. [Google Scholar] [CrossRef]
- Tyner, J.W.; Uchida, O.; Kajiwara, N.; Kim, E.Y.; Patel, A.C.; O’Sullivan, M.P.; Walter, M.J.; Schwendener, R.A.; Cook, D.N.; Danoff, T.M.; et al. CCL5-CCR5 interaction provides antiapoptotic signals for macrophage survival during viral infection. Nat. Med. 2005, 11, 1180–1187. [Google Scholar] [CrossRef]
- Kaul, M.; Ma, Q.; Medders, K.E.; Desai, M.K.; Lipton, S.A. HIV-1 coreceptors CCR5 and CXCR4 both mediate neuronal cell death but CCR5 paradoxically can also contribute to protection. Cell Death Differ. 2007, 14, 296–305. [Google Scholar] [CrossRef]
- Campbell, G.R.; To, R.K.; Spector, S.A. TREM-1 Protects HIV-1-Infected Macrophages from Apoptosis through Maintenance of Mitochondrial Function. mBio 2019, 10, 10–1128. [Google Scholar] [CrossRef]
- Castellano, P.; Prevedel, L.; Eugenin, E.A. HIV-infected macrophages and microglia that survive acute infection become viral reservoirs by a mechanism involving Bim. Sci. Rep. 2017, 7, 12866. [Google Scholar] [CrossRef]
- Voloshyna, I.; Littlefield, M.J.; Reiss, A.B. Atherosclerosis and interferon-γ: New insights and therapeutic targets. Trends Cardiovasc. Med. 2014, 24, 45–51. [Google Scholar] [CrossRef] [PubMed]
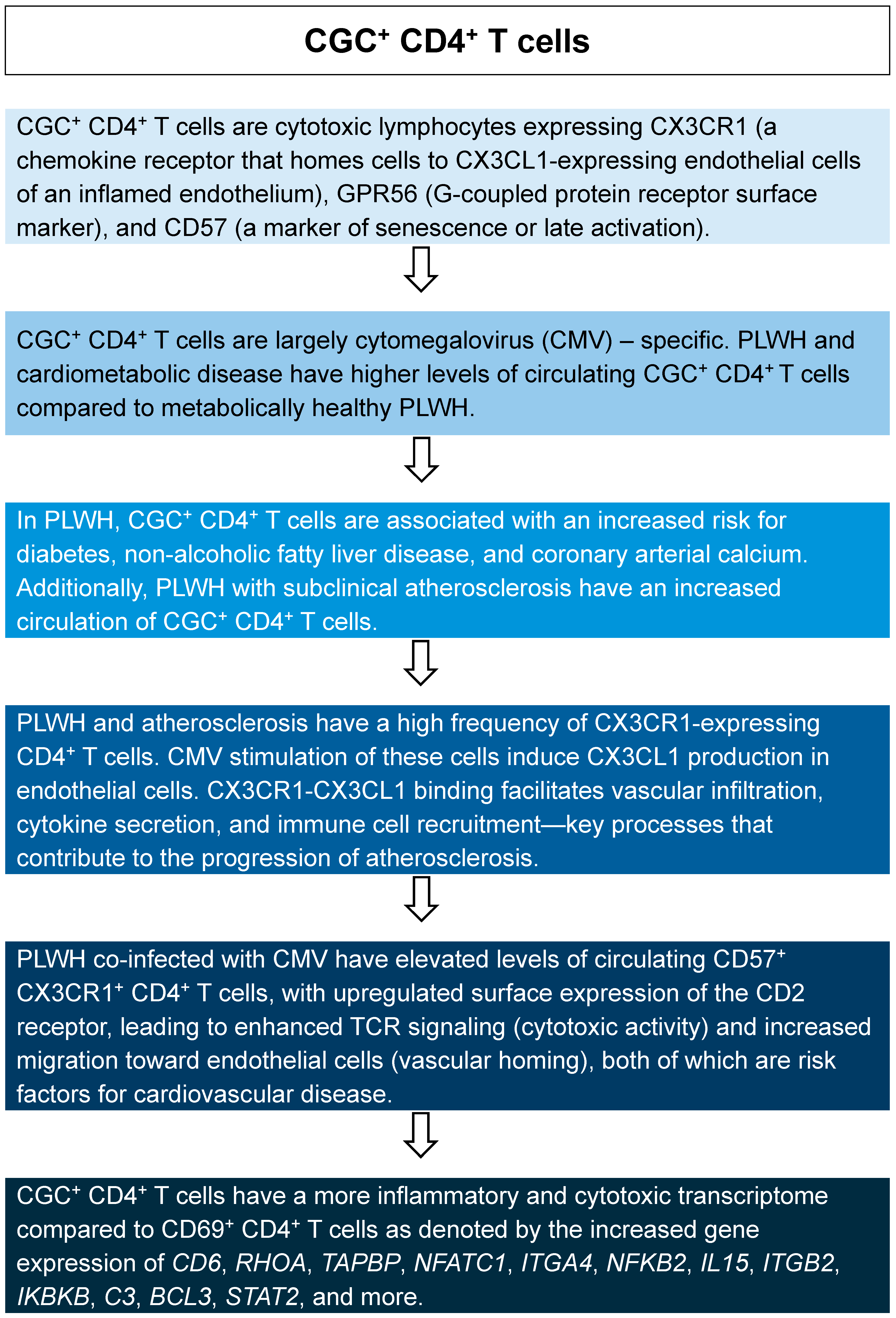
- Wanjalla, C.N.; Gabriel, C.L.; Fuseini, H.; Bailin, S.S.; Mashayekhi, M.; Simmons, J.; Warren, C.M.; Glass, D.R.; Oakes, J.; Gangula, R.; et al. CD4. Front. Immunol. 2023, 14, 1099356. [Google Scholar] [CrossRef]
- Wanjalla, C.N.; McDonnell, W.J.; Ram, R.; Chopra, A.; Gangula, R.; Leary, S.; Mashayekhi, M.; Simmons, J.D.; Warren, C.M.; Bailin, S.; et al. Single-cell analysis shows that adipose tissue of persons with both HIV and diabetes is enriched for clonal, cytotoxic, and CMV-specific CD4+ T cells. Cell Rep. Med. 2021, 2, 100205. [Google Scholar] [CrossRef] [PubMed]
- Wanjalla, C.N.; Mashayekhi, M.; Bailin, S.; Gabriel, C.L.; Meenderink, L.M.; Temu, T.; Fuller, D.T.; Guo, L.; Kawai, K.; Virmani, R.; et al. Anticytomegalovirus CD4. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1459–1473. [Google Scholar] [CrossRef]
- Wanjalla, C.N.; McDonnell, W.J.; Barnett, L.; Simmons, J.D.; Furch, B.D.; Lima, M.C.; Woodward, B.O.; Fan, R.; Fei, Y.; Baker, P.G.; et al. Adipose Tissue in Persons With HIV Is Enriched for CD4. Front. Immunol. 2019, 10, 408. [Google Scholar] [CrossRef]
- Cui, H.L.; Grant, A.; Mukhamedova, N.; Pushkarsky, T.; Jennelle, L.; Dubrovsky, L.; Gaus, K.; Fitzgerald, M.L.; Sviridov, D.; Bukrinsky, M. HIV-1 Nef mobilizes lipid rafts in macrophages through a pathway that competes with ABCA1-dependent cholesterol efflux. J. Lipid Res. 2012, 53, 696–708. [Google Scholar] [CrossRef]
- Veenhuis, R.T.; Abreu, C.M.; Costa, P.A.G.; Ferreira, E.A.; Ratliff, J.; Pohlenz, L.; Shirk, E.N.; Rubin, L.H.; Blankson, J.N.; Gama, L.; et al. Monocyte-derived macrophages contain persistent latent HIV reservoirs. Nat. Microbiol. 2023, 8, 833–844. [Google Scholar] [CrossRef]
- St Gelais, C.; Coleman, C.M.; Wang, J.H.; Wu, L. HIV-1 Nef enhances dendritic cell-mediated viral transmission to CD4+ T cells and promotes T-cell activation. PLoS ONE 2012, 7, e34521. [Google Scholar] [CrossRef][Green Version]
- Frostegård, J.; Zhang, Y.; Sun, J.; Yan, K.; Liu, A. Oxidized Low-Density Lipoprotein (OxLDL)-Treated Dendritic Cells Promote Activation of T Cells in Human Atherosclerotic Plaque and Blood, Which Is Repressed by Statins: microRNA let-7c Is Integral to the Effect. J. Am. Heart Assoc. 2016, 5, e003976. [Google Scholar] [CrossRef]
- Zhao, Y.; Zhang, J.; Zhang, W.; Xu, Y. A myriad of roles of dendritic cells in atherosclerosis. Clin. Exp. Immunol. 2021, 206, 12–27. [Google Scholar] [CrossRef]
- Vidya Vijayan, K.K.; Karthigeyan, K.P.; Tripathi, S.P.; Hanna, L.E. Pathophysiology of CD4+ T-Cell Depletion in HIV-1 and HIV-2 Infections. Front. Immunol. 2017, 8, 580. [Google Scholar] [CrossRef] [PubMed]
- Kaplan, R.C.; Kingsley, L.A.; Gange, S.J.; Benning, L.; Jacobson, L.P.; Lazar, J.; Anastos, K.; Tien, P.C.; Sharrett, A.R.; Hodis, H.N. Low CD4+ T-cell count as a major atherosclerosis risk factor in HIV-infected women and men. AIDS 2008, 22, 1615–1624. [Google Scholar] [CrossRef] [PubMed]
- Obare, L.M.; Bonami, R.H.; Doran, A.C.; Wanjalla, C.N. B cells and atherosclerosis: A HIV perspective. J. Cell Physiol. 2024, 239, e31270. [Google Scholar] [CrossRef]
- Selathurai, A.; Deswaerte, V.; Kanellakis, P.; Tipping, P.; Toh, B.H.; Bobik, A.; Kyaw, T. Natural killer (NK) cells augment atherosclerosis by cytotoxic-dependent mechanisms. Cardiovasc. Res. 2014, 102, 128–137. [Google Scholar] [CrossRef] [PubMed]
- Alles, M.; Gunasena, M.; Kettelhut, A.; Ailstock, K.; Musiime, V.; Kityo, C.; Richardson, B.; Mulhern, W.; Tamilselvan, B.; Rubsamen, M.; et al. Activated NK Cells with Pro-inflammatory Features are Associated with Atherogenesis in Perinatally HIV-Acquired Adolescents. medRxiv 2023. [Google Scholar] [CrossRef]
- Sacre, K.; Hunt, P.W.; Hsue, P.Y.; Maidji, E.; Martin, J.N.; Deeks, S.G.; Autran, B.; McCune, J.M. A role for cytomegalovirus-specific CD4+CX3CR1+ T cells and cytomegalovirus-induced T-cell immunopathology in HIV-associated atherosclerosis. AIDS 2012, 26, 805–814. [Google Scholar] [CrossRef]
- Chen, B.; Morris, S.R.; Panigrahi, S.; Michaelson, G.M.; Wyrick, J.M.; Komissarov, A.A.; Potashnikova, D.; Lebedeva, A.; Younes, S.A.; Harth, K.; et al. Cytomegalovirus Coinfection Is Associated with Increased Vascular-Homing CD57. J. Immunol. 2020, 204, 2722–2733. [Google Scholar] [CrossRef]
- Batty, M.; Bennett, M.R.; Yu, E. The Role of Oxidative Stress in Atherosclerosis. Cells 2022, 11, 3843. [Google Scholar] [CrossRef]
- Zidar, D.A.; Juchnowski, S.; Ferrari, B.; Clagett, B.; Pilch-Cooper, H.A.; Rose, S.; Rodriguez, B.; McComsey, G.A.; Sieg, S.F.; Mehta, N.N.; et al. Oxidized LDL Levels Are Increased in HIV Infection and May Drive Monocyte Activation. J. Acquir. Immune Defic. Syndr. 2015, 69, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Zhou, J.; Lhoták, S.; Hilditch, B.A.; Austin, R.C. Activation of the unfolded protein response occurs at all stages of atherosclerotic lesion development in apolipoprotein E-deficient mice. Circulation 2005, 111, 1814–1821. [Google Scholar] [CrossRef]
- Feng, B.; Yao, P.M.; Li, Y.; Devlin, C.M.; Zhang, D.; Harding, H.P.; Sweeney, M.; Rong, J.X.; Kuriakose, G.; Fisher, E.A.; et al. The endoplasmic reticulum is the site of cholesterol-induced cytotoxicity in macrophages. Nat. Cell Biol. 2003, 5, 781–792. [Google Scholar] [CrossRef]
- Schrijvers, D.M.; De Meyer, G.R.; Kockx, M.M.; Herman, A.G.; Martinet, W. Phagocytosis of apoptotic cells by macrophages is impaired in atherosclerosis. Arterioscler. Thromb. Vasc. Biol. 2005, 25, 1256–1261. [Google Scholar] [CrossRef] [PubMed]
- Clarke, M.C.; Talib, S.; Figg, N.L.; Bennett, M.R. Vascular smooth muscle cell apoptosis induces interleukin-1-directed inflammation: Effects of hyperlipidemia-mediated inhibition of phagocytosis. Circ. Res. 2010, 106, 363–372. [Google Scholar] [CrossRef] [PubMed]
- Duewell, P.; Kono, H.; Rayner, K.J.; Sirois, C.M.; Vladimer, G.; Bauernfeind, F.G.; Abela, G.S.; Franchi, L.; Nuñez, G.; Schnurr, M.; et al. NLRP3 inflammasomes are required for atherogenesis and activated by cholesterol crystals. Nature 2010, 464, 1357–1361. [Google Scholar] [CrossRef]
- Sheedy, F.J.; Grebe, A.; Rayner, K.J.; Kalantari, P.; Ramkhelawon, B.; Carpenter, S.B.; Becker, C.E.; Ediriweera, H.N.; Mullick, A.E.; Golenbock, D.T.; et al. CD36 coordinates NLRP3 inflammasome activation by facilitating intracellular nucleation of soluble ligands into particulate ligands in sterile inflammation. Nat. Immunol. 2013, 14, 812–820. [Google Scholar] [CrossRef]
- Jiang, Y.; Wang, M.; Huang, K.; Zhang, Z.; Shao, N.; Zhang, Y.; Wang, W.; Wang, S. Oxidized low-density lipoprotein induces secretion of interleukin-1β by macrophages via reactive oxygen species-dependent NLRP3 inflammasome activation. Biochem. Biophys. Res. Commun. 2012, 425, 121–126. [Google Scholar] [CrossRef]
- Amand, M.; Adams, P.; Schober, R.; Iserentant, G.; Servais, J.Y.; Moutschen, M.; Seguin-Devaux, C. The anti-caspase 1 inhibitor VX-765 reduces immune activation, CD4. eLife 2023, 12, e83207. [Google Scholar] [CrossRef]
- Rowinska, Z.; Koeppel, T.A.; Sanati, M.; Schelzig, H.; Jankowski, J.; Weber, C.; Zernecke, A.; Liehn, E.A. Role of the CX3C chemokine receptor CX3CR1 in the pathogenesis of atherosclerosis after aortic transplantation. PLoS ONE 2017, 12, e0170644. [Google Scholar] [CrossRef]
- Wan, W.; Murphy, P.M. Regulation of atherogenesis by chemokines and chemokine receptors. Arch. Immunol. Ther. Exp. 2013, 61, 1–14. [Google Scholar] [CrossRef]
- Dong, C.; Janas, A.M.; Wang, J.H.; Olson, W.J.; Wu, L. Characterization of human immunodeficiency virus type 1 replication in immature and mature dendritic cells reveals dissociable cis- and trans-infection. J. Virol. 2007, 81, 11352–11362. [Google Scholar] [CrossRef]
- Dinkins, C.; Pilli, M.; Kehrl, J.H. Roles of autophagy in HIV infection. Immunol. Cell Biol. 2015, 93, 11–17. [Google Scholar] [CrossRef]
- Blanchet, F.P.; Moris, A.; Nikolic, D.S.; Lehmann, M.; Cardinaud, S.; Stalder, R.; Garcia, E.; Dinkins, C.; Leuba, F.; Wu, L.; et al. Human immunodeficiency virus-1 inhibition of immunoamphisomes in dendritic cells impairs early innate and adaptive immune responses. Immunity 2010, 32, 654–669. [Google Scholar] [CrossRef]
- Proto, J.D.; Doran, A.C.; Subramanian, M.; Wang, H.; Zhang, M.; Sozen, E.; Rymond, C.C.; Kuriakose, G.; D’Agati, V.; Winchester, R.; et al. Hypercholesterolemia induces T cell expansion in humanized immune mice. J. Clin. Invest. 2018, 128, 2370–2375. [Google Scholar] [CrossRef] [PubMed]
- Wang, X.; Huang, R.; Zhang, L.; Li, S.; Luo, J.; Gu, Y.; Chen, Z.; Zheng, Q.; Chao, T.; Zheng, W.; et al. A severe atherosclerosis mouse model on the resistant NOD background. Dis. Model. Mech. 2018, 11, dmm033852. [Google Scholar] [CrossRef]
- Chuprin, J.; Buettner, H.; Seedhom, M.O.; Greiner, D.L.; Keck, J.G.; Ishikawa, F.; Shultz, L.D.; Brehm, M.A. Humanized mouse models for immuno-oncology research. Nat. Rev. Clin. Oncol. 2023, 20, 192–206. [Google Scholar] [CrossRef]
- Athieniti, E.; Spyrou, G.M. A guide to multi-omics data collection and integration for translational medicine. Comput. Struct. Biotechnol. J. 2023, 21, 134–149. [Google Scholar] [CrossRef] [PubMed]
- Kurt, Z.; Cheng, J.; McQuillen, C.N.; Saleem, Z.; Hsu, N.; Jiang, N.; Barrere-Cain, R.; Pan, C.; Franzen, O.; Koplev, S.; et al. Shared and distinct pathways and networks genetically linked to coronary artery disease between human and mouse. bioRxiv 2023. [Google Scholar] [CrossRef]
- Mikaeloff, F.; Gelpi, M.; Benfeitas, R.; Knudsen, A.D.; Vestad, B.; Høgh, J.; Hov, J.R.; Benfield, T.; Murray, D.; Giske, C.G.; et al. Network-based multi-omics integration reveals metabolic at-risk profile within treated HIV-infection. eLife 2023, 12, e82785. [Google Scholar] [CrossRef]
- Wong, G.; Trevillyan, J.M.; Fatou, B.; Cinel, M.; Weir, J.M.; Hoy, J.F.; Meikle, P.J. Plasma lipidomic profiling of treated HIV-positive individuals and the implications for cardiovascular risk prediction. PLoS ONE 2014, 9, e94810. [Google Scholar] [CrossRef] [PubMed]
- Peterson, T.E.; Hahn, V.S.; Moaddel, R.; Zhu, M.; Haberlen, S.A.; Palella, F.J.; Plankey, M.; Bader, J.S.; Lima, J.A.C.; Gerszten, R.E.; et al. Proteomic signature of HIV-associated subclinical left atrial remodeling and incident heart failure. Nat. Commun. 2025, 16, 610. [Google Scholar] [CrossRef] [PubMed]
- La Fazia, V.M.; Pierucci, N.; Mohanty, S.; Gianni, C.; Della Rocca, D.G.; Compagnucci, P.; MacDonald, B.; Mayedo, A.; Torlapati, P.G.; Bassiouny, M.; et al. Catheter ablation approach and outcome in HIV+ patients with recurrent atrial fibrillation. J. Cardiovasc. Electrophysiol. 2023, 34, 2527–2534. [Google Scholar] [CrossRef] [PubMed]


| Mouse Model | Research Focus |
|---|---|
| Apoe−/− mice |
Spontaneous atherosclerosis development Role of ApoE in atherosclerosis Inflammation and atherosclerosis |
| CETP.E3L mice | Role of age-related changes in cholesterol transport and lipid profiles in atherogenic processes |
| Ldlr−/− mice |
Diet-induced atherosclerosis Role of LDL receptor in cholesterol handling and atherosclerosis Interactions between diet and genetic differences in human LDL receptor Human-like lipid profile and atherosclerosis (for example to study the role of statins) Interaction between diet and inflammation |
| Apoe−/− Fbn1C1039G+/− mice | Arterial stiffening and plaque development |
| Tg26+/−/Apoe−/− mice | Role of HIV transcripts and proteins in atherosclerosis development and progression |
| Aspect | Mice | Humans |
|---|---|---|
| Cholesterol Transport | Transport most cholesterol in HDL particles (atheroprotective). | Transport most cholesterol in LDL particles (atherogenic). |
| Bile Acid Metabolism | Produce more hydrophilic bile acids, leading to reduced intestinal cholesterol uptake and increased fecal cholesterol excretion. | Produce less hydrophilic bile acids, leading to higher intestinal cholesterol absorption. |
| Atherosclerotic Plaque Location | Plaques develop in the brachiocephalic trunk, aortic arch, aortic sinus, and proximal aorta. | Plaques typically develop in the carotid and coronary arteries. |
| Plaque Development | Plaques develop but do not progress to advanced fibrous atheroma. | Plaques progress to advanced-stage fibrous atheroma. |
| Genetic Models | Common models include Apoe−/− and Ldlr−/− mice, manipulated for hypercholesterolemia. | Genetic mutations in Ldlr and Apoe are associated with familial hypercholesterolemia and ASCVD. |
| Plaque Rupture | Plaque rupture is rare except in specific models (e.g., Apoe−/− Fbn1C1039G+/− mice, angiotensin II-infused Apoe−/− mice). | Plaque rupture is common, leading to thrombosis and cardiovascular events. |
| Inflammatory Response | Th1-skewed immune response; IFN-γ plays a key role in lesion development. | Mixed Th1/Th2 immune response; inflammation plays a significant role in plaque rupture. |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Stephens, V.R.; Ameli, S.; Major, A.S.; Wanjalla, C.N. Mouse Models of HIV-Associated Atherosclerosis. Int. J. Mol. Sci. 2025, 26, 3417. https://doi.org/10.3390/ijms26073417
Stephens VR, Ameli S, Major AS, Wanjalla CN. Mouse Models of HIV-Associated Atherosclerosis. International Journal of Molecular Sciences. 2025; 26(7):3417. https://doi.org/10.3390/ijms26073417
Chicago/Turabian StyleStephens, Victoria R., Sharareh Ameli, Amy S. Major, and Celestine N. Wanjalla. 2025. "Mouse Models of HIV-Associated Atherosclerosis" International Journal of Molecular Sciences 26, no. 7: 3417. https://doi.org/10.3390/ijms26073417
APA StyleStephens, V. R., Ameli, S., Major, A. S., & Wanjalla, C. N. (2025). Mouse Models of HIV-Associated Atherosclerosis. International Journal of Molecular Sciences, 26(7), 3417. https://doi.org/10.3390/ijms26073417