What Can Fluorescence Tell Us About Wine?
Abstract
1. Introduction
2. Chemometric Approach to Wine Analysis
3. Fluorescent Components of Wines
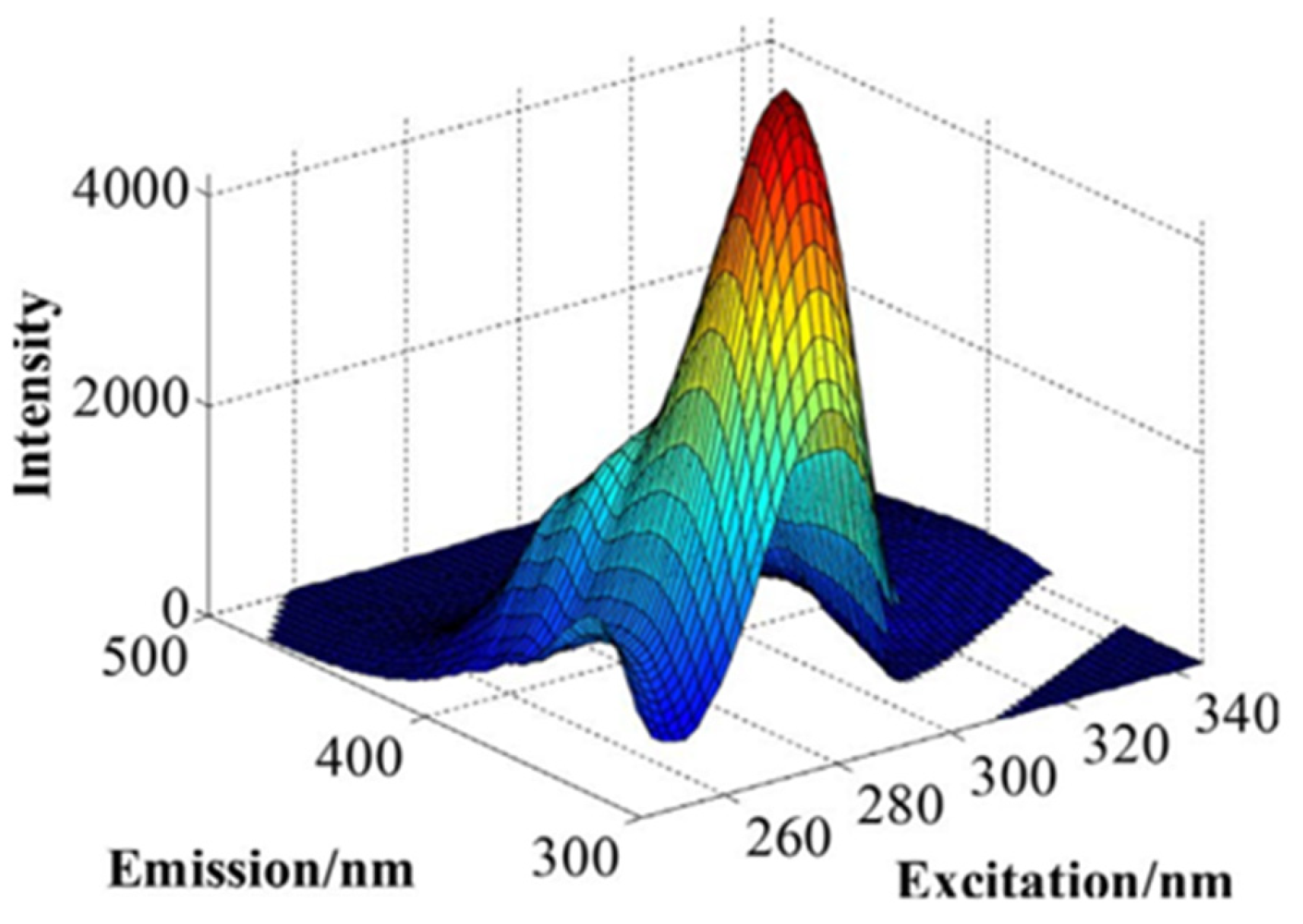
4. Analysis of Wine Autofluorescence
5. Application of Fluorescent Probes for the Analysis of Wine
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Harutyunyan, M.; Malfeito-Ferreira, M. The rise of wine among ancient civilizations across the Mediterranean basin. Heritage 2022, 5, 788–812. [Google Scholar] [CrossRef]
- Estreicher, S.K. Wine and France: A brief history. Eur. Rev. 2023, 31, 91–179. [Google Scholar]
- Mouret, M.; Monaco, G.L.; Urdapilleta, I.; Parr, W.V. Social representations of wine and culture: A comparison between France and New Zealand. Food Qual. Prefer. 2013, 30, 102–107. [Google Scholar]
- Swami, S.B.; Thakor, N.J.; Divate, A.D. Fruit wine production: A review. J. Food Res. Technol. 2014, 2, 93–100. [Google Scholar]
- Lanzmann-Petithory, D.; Professor Serge, C. Renaud (1927–2012): French Paradox and wine active compounds. Wine Stud. 2022, 1, 4477. [Google Scholar]
- de Gaetano, G.; Costanzo, S.; Di Castelnuovo, A. Wine consumption and cardiovascular health: The unresolved French paradox and the promise of objective biomarkers. Eur. Heart J. 2024, 46, 173–175. [Google Scholar]
- Buja, L.M. The history, science, and art of wine and the case for health benefits: Perspectives of an oenophilic cardiovascular pathologist. Cardiovasc. Pathol. 2022, 60, 107446. [Google Scholar]
- Perestrelo, R.; Silva, C.; Gonçalves, C.; Castillo, M.; Câmara, J.S. An approach of the madeira wine chemistry. Beverages 2020, 6, 12. [Google Scholar] [CrossRef]
- Valeur, B.; Berberan-Santos, M.N. A brief history of fluorescence and phosphorescence before the emergence of quantum theory. J. Chem. Educ. 2011, 88, 731–738. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006. [Google Scholar]
- Jameson, D.M. Introduction to Fluorescence, 2nd ed.; CRC Press: Boca Raton, FL, USA, 2025. [Google Scholar]
- Sádecká, J.; Tóthová, J. Fluorescence spectroscopy and chemometrics in the food classification—A Review. Czech J. Food Sci. 2007, 25, 159–173. [Google Scholar]
- Luykx, D.M.; Van Ruth, S.M. An overview of analytical methods for determining the geographical origin of food products. Food Chem. 2008, 107, 897–911. [Google Scholar] [CrossRef]
- Williams, R.T.; Bridges, J.W. Fluorescence of solutions: A review. J. Clin. Pathol. 1964, 17, 371. [Google Scholar] [CrossRef] [PubMed]
- Weiß, N.; Armbrecht, M.; Eppendorf, A.G. Absorbance or fluorescence: Which is the best way to quantify nucleic acids? Proteins 2018, 260, 280. [Google Scholar]
- Zacharioudaki, D.E.; Fitilis, I.; Kotti, M. Review of fluorescence spectroscopy in environmental quality applications. Molecules 2022, 27, 4801. [Google Scholar] [CrossRef]
- Contini, C.; Kuntz, J.; Massing, U.; Merfort, I.; Winkler, K.; Pütz, G. On the validity of fluorimetric intracellular calcium detection: Impact of lipid components. Biochem. Biophys. Commun. 2023, 643, 186–191. [Google Scholar] [CrossRef]
- Alosaimi, E.H. Recent developments in colorimetric and fluorimetric chemosensors for the detection of Mn2+ ions: A review (2010–2024). Crit. Rev. Anal. Chem. 2025, 19, 1–21. [Google Scholar] [CrossRef]
- Andina, D.; Leroux, J.C.; Luciani, P. Ratiometric fluorescent probes for the detection of reactive oxygen species. Chemistry 2017, 23, 13549–13573. [Google Scholar] [CrossRef]
- Fairless, R.; Beck, A.; Kravchenko, M.; Williams, S.K.; Wissenbach, U.; Diem, R.; Cavalié, A. Membrane potential measurements of isolated neurons using a voltage-sensitive dye. PLoS ONE 2013, 8, e58260. [Google Scholar] [CrossRef]
- Sauer, M.; Hofkens, J.; Enderlein, J. Handbook of Fluorescence Spectroscopy and Imaging: From Single Molecules to Ensembles; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2011. [Google Scholar]
- Radotić, K.; Stanković, M.; Bartolić, D.; Natić, M. Intrinsic fluorescence markers for food characteristics, shelf life, and safety estimation: Advanced analytical approach. Foods 2023, 12, 3023. [Google Scholar] [CrossRef]
- Freire, P.; Zamora, A.; Castillo, M. Synchronous front-face fluorescence spectra: A review of milk fluorophores. Foods 2024, 13, 812. [Google Scholar] [CrossRef]
- Karoui, R.; Dufour, E.; Bosset, J.O.; De Baerdemaeker, J. The use of front face fluorescence spectroscopy to classify the botanical origin of honey samples produced in Switzerland. Food Chem. 2007, 101, 314–323. [Google Scholar]
- Karoui, R.; Blecker, C. Fluorescence spectroscopy measurement for quality assessment of food systems—A review. Food Bioprocess Technol. 2011, 4, 364–386. [Google Scholar]
- Dufour, É.; Letort, A.; Laguet, A.; Lebecque, A.; Serra, J.N. Investigation of variety, typicality and vintage of French and German wines using front-face fluorescence spectroscopy. Anal. Chim. Acta 2006, 563, 292–299. [Google Scholar]
- Airado-Rodríguez, D.; Durán-Merás, I.; Galeano-Díaz, T.; Wold, J.P. Front-face fluorescence spectroscopy: A new tool for control in the wine industry. J. Food Compos. Anal. 2011, 24, 257–264. [Google Scholar] [CrossRef]
- Saad, R.; Bouveresse, D.J.R.; Locquet, N.; Rutledge, D.N. Using pH variations to improve the discrimination of wines by 3D front face fluorescence spectroscopy associated to Independent Components Analysis. Talanta 2016, 153, 278–284. [Google Scholar]
- Cabrera-Bañegil, M.; del Carmen Hurtado-Sánchez, M.; Galeano-Díaz, T.; Durán-Merás, I. Front-face fluorescence spectroscopy combined with second-order multivariate algorithms for the quantification of polyphenols in red wine samples. Food Chem. 2017, 220, 168–176. [Google Scholar]
- Schueuermann, C.; Silcock, P.; Bremer, P. Front-face fluorescence spectroscopy in combination with parallel factor analysis for profiling of clonal and vineyard site differences in commercially produced Pinot Noir grape juices and wines. J. Food Compos. Anal. 2018, 66, 30–38. [Google Scholar] [CrossRef]
- Geladi, P. Chemometrics in spectroscopy. Part I. Classical chemometrics. Spectrochim. Acta B 2003, 58, 767–782. [Google Scholar]
- Lenhardt Acković, L.; Zeković, I.; Dramićanin, T.; Bro, R.; Dramićanin, M.D. Modeling food fluorescence with PARAFAC. In Reviews in Fluorescence 2017; Geddes, C.D., Ed.; Springer: Cham, Switzerland, 2018; pp. 161–197. [Google Scholar]
- Dos Santos, I.; Bosman, G.; Aleixandre-Tudo, J.L.; du Toit, W. Direct quantification of red wine phenolics using fluorescence spectroscopy with chemometrics. Talanta 2022, 236, 122857. [Google Scholar]
- Christensen, J.; Nørgaard, L.; Bro, R.; Engelsen, S.B. Multivariate autofluorescence of intact food systems. Chem. Rev. 2006, 106, 1979–1994. [Google Scholar]
- Meena, D.; Chakraborty, S.; Mitra, J. Geographical origin identification of red chili powder using NIR spectroscopy combined with SIMCA and machine learning algorithms. Food Anal. Meth. 2024, 17, 1005–1023. [Google Scholar]
- Tufariello, M.; Pati, S.; Palombi, L.; Grieco, F.; Losito, I. Use of multivariate statistics in the processing of data on wine volatile compounds obtained by HS-SPME-GC-MS. Foods 2022, 11, 910. [Google Scholar] [CrossRef] [PubMed]
- Tsibulskaya, E.; Maslov, N. Decomposition of multi-component fluorescence spectra by narrow peak method based on principal component analysis. J. Chemom. 2021, 35, e3343. [Google Scholar]
- Kumar, K.; Giehl, A.; Patz, C.D. Chemometric assisted Fourier Transform Infrared (FTIR) Spectroscopic analysis of fruit wine samples: Optimizing the initialization and convergence criteria in the non-negative factor analysis algorithm for developing a robust classification model. Spectrochim. Acta A Mol. Biomol. Spectrosc. 2019, 209, 22–31. [Google Scholar]
- Monakhova, Y.B.; Godelmann, R.; Kuballa, T.; Mushtakova, S.P.; Rutledge, D.N. Independent components analysis to increase efficiency of discriminant analysis methods (FDA and LDA): Application to NMR fingerprinting of wine. Talanta 2015, 141, 60–65. [Google Scholar]
- Karoui, R.; Dufour, É. Dynamic testing rheology and fluorescence spectroscopy investigations of surface to centre differences in ripened soft cheeses. Int. Dairy J. 2003, 13, 973–985. [Google Scholar]
- dos Santos Freitas, M.M.; Barbosa, J.R.; dos Santos Martins, E.M.; da Silva Martins, L.H.; de Souza Farias, F.; Lourenço, L.D.F.H.; e Silva, N.D.S. KNN algorithm and multivariate analysis to select and classify starch films. Food Packag. Shelf Life 2022, 34, 100976. [Google Scholar]
- Han, F.L.; Zhang, W.N.; Pan, Q.H.; Zheng, C.R.; Chen, H.Y.; Duan, C.Q. Principal component regression analysis of the relation between CIELAB color and monomeric anthocyanins in young Cabernet Sauvignon wines. Molecules 2008, 13, 2859–2870. [Google Scholar] [CrossRef]
- Barth, J.; Katumullage, D.; Yang, C.; Cao, J. Classification of wines using principal component analysis. J. Wine Econ. 2021, 16, 56–67. [Google Scholar]
- Wang, N.; Zhou, Z.; Chen, S. Aging status characterization of Chinese rice wine based on key aging-marker profiles combined with principal components analysis and partial least-squares regression. Eur. Food Res. Technol. 2020, 246, 1283–1296. [Google Scholar]
- Garrido, J.; Borges, F. Wine and grape polyphenols—A chemical perspective. Food Res. Int. 2013, 54, 1844–1858. [Google Scholar] [CrossRef]
- Pereira, D.M.; Valentão, P.; Pereira, J.A.; Andrade, P.B. Phenolics: From chemistry to biology. Molecules 2009, 14, 2202–2211. [Google Scholar] [CrossRef]
- Gutiérrez-Escobar, R.; Aliaño-González, M.J.; Cantos-Villar, E. Wine polyphenol content and its influence on wine quality and properties: A review. Molecules 2021, 26, 718. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Bernal, Ó.A.; Vazquez-Flores, A.A.; de la Rosa, L.A.; Rodrigo-García, J.; Martínez-Ruiz, N.R.; Alvarez-Parrilla, E. Enriched red wine: Phenolic profile, sensory evaluation and in vitro bioaccessibility of phenolic compounds. Foods 2023, 12, 1194. [Google Scholar] [CrossRef]
- Makris, D.P.; Kallithraka, S.; Kefalas, P. Flavonols in grapes, grape products and wines: Burden, profile and influential parameters. J. Food Compos. Anal. 2006, 19, 396–404. [Google Scholar]
- Castillo-Muñoz, N.; Gómez-Alonso, S.; García-Romero, E.; Hermosín-Gutiérrez, I. Flavonol profiles of Vitis vinifera red grapes and their single-cultivar wines. J Agric. Food Chem. 2007, 55, 992–1002. [Google Scholar]
- Favre, G.; Gómez-Alonso, S.; Pérez-Navarro, J.; García-Romero, E.; Mena-Morales, A.; Piccardo, D.; González-Neves, G. Seed and skin-derived flavanols in red wine: A study of Syrah, Marselan, and Tannat cultivars. Eur. Food Res. Technol. 2024, 250, 845–857. [Google Scholar] [CrossRef]
- Kuhlman, B.A.; Aleixandre-Tudo, J.L.; Moore, J.P.; du Toit, W. Astringency perception in a red wine context—A review. OENO One 2024, 58, 1–17. [Google Scholar]
- de Pascual-Teresa, S.; Rivas-Gonzalo, J.C.; Santos-Buelga, C. Prodelphinidins and related flavanols in wine. Int. J. Food Sci. Technol. 2000, 35, 33–40. [Google Scholar]
- Darnal, A.; Poggesi, S.; Ceci, A.T.; Mimmo, T.; Boselli, E.; Longo, E. Effects of pre-and post-fermentative practices on oligomeric cyclic and non-cyclic condensed tannins in wine from Schiava grapes. Curr. Res. Food Sci. 2023, 6, 100513. [Google Scholar] [CrossRef]
- Fernandes, I.; Pérez-Gregorio, R.; Soares, S.; Mateus, N.; De Freitas, V. Wine flavonoids in health and disease prevention. Molecules 2017, 22, 292. [Google Scholar] [CrossRef]
- Garrido-Bañuelos, G.; Buica, A.; du Toit, W. Relationship between anthocyanins, proanthocyanidins, and cell wall polysaccharides in grapes and red wines. A current state-of-art review. Crit. Rev. Food Sci. Nutr. 2022, 62, 7743–7759. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Magariño, S.; González-San José, M.L. Evolution of flavanols, anthocyanins, and their derivatives during the aging of red wines elaborated from grapes harvested at different stages of ripening. J. Agric. Food Chem. 2004, 52, 1181–1189. [Google Scholar] [CrossRef]
- Mateus, N.; de Freitas, V. Evolution and stability of anthocyanin-derived pigments during port wine aging. J. Agric. Food Chem. 2001, 49, 5217–5222. [Google Scholar] [CrossRef]
- Fang, F.; Li, J.M.; Pan, Q.H.; Huang, W.D. Determination of red wine flavonoids by HPLC and effect of aging. Food Chem. 2007, 101, 428–433. [Google Scholar] [CrossRef]
- Li, X.; Teng, Z.; Luo, Z.; Yuan, Y.; Zeng, Y.; Hu, J.; Sun, J.; Bai, W. Pyruvic acid stress caused color attenuation by interfering with anthocyanins metabolism during alcoholic fermentation. Food Chem. 2022, 372, 131251. [Google Scholar] [CrossRef] [PubMed]
- Cheng, S.; Wu, T.; Gao, J.; Han, X.; Huang, W.; You, Y.; Zhan, J. Color myth: Anthocyanins reactions and enological approaches achieving their stabilization in the aging process of red wine. Food Innov. Adv. 2023, 2, 255–271. [Google Scholar] [CrossRef]
- Poulli, K.I.; Mousdis, G.A.; Georgiou, C.A. Classification of edible and lampante virgin olive oil based on synchronous fluorescence and total luminescence spectroscopy. Anal. Chim. Acta 2005, 542, 151–156. [Google Scholar] [CrossRef]
- Jakubíková, M.; Sádecká, J.; Hroboňová, K. Determination of total phenolic content and selected phenolic compounds in sweet wines by fluorescence spectroscopy and multivariate calibration. Microchem. J. 2022, 181, 107834. [Google Scholar] [CrossRef]
- Figueiredo, P.; Pina, F. Fluorescence spectra and decays of malvidin 3, 5-diglucoside in aqueous solutions. J. Photochem. Photobiol. A Chem. 1990, 52, 411–424. [Google Scholar] [CrossRef]
- Pina, F.; Melo, M.J.; Santos, H.; Lima, J.C.; Abreu, I.; Ballardini, R.; Maestri, M. Excited state proton transfer in synthetic flavylium salts: 4-methyl-7-hydroxyflavylium and 4′, 7-dihydroxyflavylium. Example of a four-level molecular device to invert the population of the excited state. New J. Chem. 1998, 22, 1093–1098. [Google Scholar] [CrossRef]
- Drabent, R.; Pliszka, B.; Huszcza-Ciołkowska, G.Y.; Smyk, B. Ultraviolet fluorescence of cyanidin and malvidin glycosides in aqueous environment. Spectrosc. Lett. 2007, 40, 165–182. [Google Scholar]
- Kennedy, J.A.; Saucier, C.; Glories, Y. Grape and wine phenolics: History and perspective. Am. J. Enol. Viticult. 2006, 57, 239–248. [Google Scholar]
- Clarke, S.; Bosman, G.; du Toit, W.; Aleixandre-Tudo, J.L. White wine phenolics: Current methods of analysis. J. Sci. Food Agric. 2023, 103, 7–25. [Google Scholar]
- Futterman, S.; Swanson, D.; Kalina, R.E. A new, rapid fluorometric determination of retinol in serum. Investig. Ophthalmol. 1975, 14, 125–130. [Google Scholar]
- Galbán, J.; Sanz-Vicente, I.; Navarro, J.; De Marcos, S. The intrinsic fluorescence of FAD and its application in analytical chemistry: A review. Methods Appl. Fluoresc. 2016, 4, 042005. [Google Scholar]
- Fernández-Cruz, E.; Álvarez-Fernández, M.A.; Valero, E.; Troncoso, A.M.; García-Parrilla, M.C. Melatonin and derived L-tryptophan metabolites produced during alcoholic fermentation by different wine yeast strains. Food Chem. 2017, 217, 431–437. [Google Scholar]
- Scutarașu, E.C.; Luchian, C.E.; Cioroiu, I.B.; Trincă, L.C.; Cotea, V.V. Increasing amino acids content of white wines with enzymes treatments. Agronomy 2022, 12, 1406. [Google Scholar] [CrossRef]
- Abarca-Rivas, C.; Martín-García, A.; Riu-Aumatell, M.; López-Tamames, E. Indole content profiling during biological ageing of cava sparkling wine. Foods 2025, 14, 722. [Google Scholar] [CrossRef]
- Zamora, F. Biochemistry of alcoholic fermentation. In Wine Chemistry and Biochemistry; Moreno-Arribas, M.V., Polo, M.C., Eds.; Springer: New York, NY, USA, 2009; pp. 3–26. [Google Scholar]
- Cannon, T.M.; Lagarto, J.L.; Dyer, B.T.; Garcia, E.; Kelly, D.J.; Peter, N.S.; Lyon, A.R.; French, P.M.W.; Dunsby, C. Characterization of NADH fluorescence properties under one-photon excitation with respect to temperature, pH, and binding to lactate dehydrogenase. OSA Contin. 2021, 4, 1610–1625. [Google Scholar]
- Elcoroaristizabal, S.; Callejón, R.M.; Amigo, J.M.; Ocaña-González, J.A.; Morales, M.L.; Ubeda, C. Fluorescence excitation-emission matrix spectroscopy as a tool for determining quality of sparkling wines. Food Chem. 2016, 206, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Risum, A.B.; Bevilacqua, M.; Li, C.; Engholm-Keller, K.; Poojary, M.M.; Rinnan, Å.; Lund, M.N. Resolving fluorescence spectra of Maillard reaction products formed on bovine serum albumin using parallel factor analysis. Food Res. Int. 2024, 178, 113950. [Google Scholar] [CrossRef] [PubMed]
- Airado-Rodŕiguez, D.; Durán-Merás, I.; Galeano-Díaz, T.; Wold, J. Usefulness of fluorescence excitation-emission matrices in combination with parafac, as fingerprints of red wines. J. Agric. Food Chem. 2009, 57, 1711–1720. [Google Scholar] [CrossRef] [PubMed]
- dos Santos, I.; Bosman, G.; du Toit, W.; Aleixandre-Tudo, J.L. The use of non-invasive fluorescence spectroscopy to quantify phenolic content under red wine real-time fermentation conditions. Food Control 2023, 147, 109616. [Google Scholar] [CrossRef]
- Ranaweera, R.K.; Gilmore, A.M.; Capone, D.L.; Bastian, S.E.; Jeffery, D.W. Authentication of the geographical origin of Australian Cabernet Sauvignon wines using spectrofluorometric and multi-element analyses with multivariate statistical modelling. Food Chem. 2021, 335, 127592. [Google Scholar] [CrossRef]
- Ranaweera, R.K.; Gilmore, A.M.; Capone, D.L.; Bastian, S.E.; Jeffery, D.W. Spectrofluorometric analysis combined with machine learning for geographical and varietal authentication, and prediction of phenolic compound concentrations in red wine. Food Chem. 2021, 361, 130149. [Google Scholar] [CrossRef]
- Sádecká, J.; Hroboňová, K.; Jakubíková, M. synchronous fluorescence spectroscopy as a green method for the prediction of antioxidant activity in wine brandy and sweet wine. Food Bioprocess Technol. 2025, 18, 2070–2082. [Google Scholar] [CrossRef]
- Hroboňová, K.; Sádecká, J. Coumarins content in wine: Application of HPLC, fluorescence spectrometry, and chemometric approach. J. Food Sci. Technol. 2020, 57, 200–209. [Google Scholar] [CrossRef]
- Agati, G.; Matteini, P.; Oliveira, J.; de Freitas, V.; Mateus, N. Fluorescence approach for measuring anthocyanins and derived pigments in red wine. J. Agric. Food Chem. 2013, 61, 10156–10162. [Google Scholar] [CrossRef]
- Berke, B.; Cheze, C.; Deffieux, G.; Vercauteren, J. Sulfur dioxide decolorization or resistance of anthocyanins: NMR structural elucidation of bisulfite-adducts. In Plant Polyphenols 2: Chemistry, Biology, Pharmacology, Ecology; Gross, G.G., Hemingway, R.W., Yoshida, T., Eds.; Kluwer Academic/Plenum Publishers: Dordrecht, The Netherlands, 2012; Volume 66. [Google Scholar]
- Nixdorf, S.L.; Hermosín-Gutiérrez, I. Brazilian red wines made from the hybrid grape cultivar Isabel: Phenolic composition and antioxidant capacity. Anal. Chim. Acta 2010, 659, 208–215. [Google Scholar] [CrossRef]
- Huang, K.; Hu, J.; Li, X.; Sun, J.; Bai, W. Advancements in the promotion of pyranoanthocyanins formation in wine: A review of current research. Food Chem. 2024, 438, 137990. [Google Scholar] [PubMed]
- Seya, K.; Kanemaru, K.; Sugimoto, C.; Suzuki, M.; Takeo, T.; Motomura, S.; Kitahara, H.; Niwa, M.; Oshima, Y.; Furukawa, K. Opposite effects of two resveratrol (trans-3,5,4′-trihydroxystilbene) tetramers, vitisin A and hopeaphenol, on apoptosis of myocytes isolated from adult rat heart. J. Pharmacol. Exp. Ther. 2009, 328, 90–98. [Google Scholar] [PubMed]
- Mi, J.S.; Davaatseren, M.; Kim, W.; Park, S.K.; Kim, S.H.; Hur, H.J.; Kim, M.S.; Kim, Y.S.; Kwon, D.Y. Vitisin A suppresses LPS-induced NO production by inhibiting ERK, p38, and NF-kappaB activation in RAW 264.7 cells. Int. Immunopharmacol. 2009, 9, 319–323. [Google Scholar]
- Gebala, S.; Przybylowski, P. Possibilities of using fluorescence in wine research. Polish J. Food Nutr. Sci. 2007, 57, 363–366. [Google Scholar]
- Alimelli, A.; Filippini, D.; Paolesse, R.; Moretti, S.; Ciolfi, G.; D’Amico, A.; Lundström, I.; Di Natale, C. Direct quantitative evaluation of complex substances using computer screen photo-assisted technology: The case of red wine. Anal. Chim. Acta 2007, 597, 103–112. [Google Scholar] [CrossRef]
- Trivellin, N.; Barbisan, D.; Badocco, D.; Pastore, P.; Meneghesso, G.; Meneghini, M.; Zanoni, E.; Belgioioso, G.; Cenedese, A. Study and development of a fluorescence based sensor system for monitoring oxygen in wine production: The WOW project. Sensors 2018, 18, 1130. [Google Scholar] [CrossRef]
- Catarino, A.; Alves, S.; Mira, H. Influence of technological operations in the dissolved oxygen content of wines. J. Chem. Chem. Eng. 2014, 8, 390–394. [Google Scholar]
- Morales, P.; Rojas, V.; Quirós, M.; Gonzalez, R. The impact of oxygen on the final alcohol content of wine fermented by a mixed starter culture. Appl. Microbiol. Biotechnol. 2015, 99, 3993–4003. [Google Scholar]
- Li, C.P.; Tan, S.; Ye, H.; Cao, J.; Zhao, H. A novel fluorescence assay for resveratrol determination in red wine based on competitive host-guest recognition. Food Chem. 2019, 283, 191–198. [Google Scholar]
- Ferreira, V.; Franco-Luesma, E.; Vela, E.; López, R.; Hernández-Orte, P. Elusive chemistry of hydrogen sulfide and mercaptans in wine. J. Agric. Food Chem. 2017, 66, 2237–2246. [Google Scholar]
- Bekker, M.Z.; Kreitman, G.Y.; Jeffery, D.W.; Danilewicz, J.C. Liberation of hydrogen sulfide from dicysteinyl polysulfanes in model wine. J. Agric. Food Chem. 2018, 66, 13483–13491. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Wang, H.; Yang, S.; Tian, H.; Liu, Y.; Sun, B. A novel fluorescent probe for detecting hydrogen sulfide in wine. Food Anal. Meth. 2018, 11, 1398–1404. [Google Scholar] [CrossRef]
- Duan, N.; Feng, J.; Deng, B.; Yang, S.; Tian, H.; Sun, B. A colourimetric fluorescent probe for the sensitive detection of total iron in wine. Food Chem. 2022, 383, 132594. [Google Scholar] [CrossRef]
- Wu, X.; Wang, H.; Yang, S.; Tian, H.; Liu, Y.; Sun, B. A novel coumarin-based fluorescent probe for sensitive detection of copper (II) in wine. Food Chem. 2019, 284, 23–27. [Google Scholar] [CrossRef] [PubMed]
- Doumani, N.; Bou-Maroun, E.; Maalouly, J.; Tueni, M.; Dubois, A.; Bernhard, C.; Denat, F.; Cayot, P.; Sok, N.A. New pH-dependent macrocyclic rhodamine b-based fluorescent probe for copper detection in white wine. Sensors 2019, 19, 4514. [Google Scholar] [CrossRef]
- Dai, X.; Liang, Z.; Li, Y.; Tao, J.; Qu, L.; Zhao, L.; Yang, R. Rapid self-calibrating fluorescent detection of copper (II) ions in wine with high accuracy. Food Chem. 2023, 405, 134984. [Google Scholar] [CrossRef]
- Zezza, F.; Longobardi, F.; Pascale, M.; Eremin, S.A.; Visconti, A. Fluorescence polarization immunoassay for rapid screening of ochratoxin A in red wine. Anal. Bioanalyt. Chem. 2009, 395, 1317–1323. [Google Scholar] [CrossRef]
- Van Sluyter, S.C.; McRae, J.M.; Falconer, R.J.; Smith, P.A.; Bacic, A.; Waters, E.J.; Marangon, M. Wine protein haze: Mechanisms of formation and advances in prevention. J. Agric. Food Chem. 2015, 63, 4020–4030. [Google Scholar] [CrossRef]
- Liu, Z.; Xu, L.; Wang, J.; Duan, C.; Sun, Y.; Kong, Q.; He, F. Research progress of protein haze in white wines. Food Sci. Hum. Wellness 2023, 12, 1427–1438. [Google Scholar] [CrossRef]
- Mierczynska-Vasilev, A.; Vasilev, A.; Reilly, T.; Bindon, K.; Vasilev, K. Fluorescence sensing technology for the rapid detection of haze-forming proteins in white wine. Food Chem. 2022, 374, 131770. [Google Scholar] [CrossRef]
- Fernández-Pérez, R.; Rodríguez, C.T.; Ruiz-Larrea, F. Fluorescence microscopy to monitor wine malolactic fermentation. Food Chem. 2019, 274, 228–233. [Google Scholar] [CrossRef] [PubMed]
- Azcarate, S.M.; de Araújo Gomes, A.; Alcaraz, M.R.; de Araújo, M.C.U.; Camiña, J.M.; Goicoechea, H.C. Modeling excitation-emission fluorescence matrices with pattern recognition algorithms for classification of Argentine white wines according grape variety. Food Chem. 2015, 184, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Sádecká, J.; Jakubíková, M. Varietal classification of white wines by fluorescence spectroscopy. J. Food Sci. Technol. 2020, 57, 2545–2553. [Google Scholar] [CrossRef] [PubMed]
- Suciu, R.C.; Zarbo, L.; Guyon, F.; Magdas, D.A. Application of fluorescence spectroscopy using classical right angle technique in white wines classification. Sci. Rep. 2019, 9, 18250. [Google Scholar] [CrossRef]
- Sádecká, J.; Jakubíková, M.; Májek, P. Fluorescence spectroscopy for discrimination of botrytized wines. Food Control 2018, 88, 75–84. [Google Scholar] [CrossRef]
- Cabrera-Bañegil, M.; Valdés-Sánchez, E.; Moreno, D.; Airado-Rodríguez, D.; Durán-Merás, I. Front-face fluorescence excitation-emission matrices in combination with three-way chemometrics for the discrimination and prediction of phenolic response to vineyard agronomic practices. Food Chem. 2019, 270, 162–172. [Google Scholar] [CrossRef]
- Coelho, C.; Aron, A.; Roullier-Gall, C.; Gonsior, M.; Schmitt-Kopplin, P.; Gougeon, R.G.D. Fluorescence fingerprinting of bottled white wines can reveal memories related to sulfur dioxide treatments of the must. Anal. Chem. 2015, 87, 8132–8137. [Google Scholar] [CrossRef]

| Component | λmax (exc) | λmax (em) | Tentatively Assigned Fluorophores |
|---|---|---|---|
| 1 | 278 | 315 | Monomeric catechins |
| 2 | 278 | 360 | Tryptophan, vanillic acid, syringic acid, gallic acid |
| 3 | 260 | (370)/ 390 | Caffeic acid |
| 4 | (278)/ 320 | 415 | Caffeic acid, p-coumaric acid, tyrosol |
| Component | λmax (exc) | λmax (em) | Tentatively Assigned Fluorophores |
|---|---|---|---|
| 1 | 395 | 485 | Unknown |
| 2 | 365 | 440 | Oxidation products, Maillard products, NADH |
| 3 | 465 | 530 | Vitamin B2 or riboflavin |
| 4 | 280, shoulder at 350 | 415 | Stilbenes such as trans-piceid and trans-resveratrol |
| Wine | Analysis | Results | Reference |
|---|---|---|---|
| French and German wines | Front phase, PCA | Differentiation between Gamay and Dornfelder wines, discrimination between typical and non-typical Beaujolais wines | [26] |
| Red wines | PARAFAC | Discrimination according to the country of origin and grape variety | [78] |
| Red wines | Front phase, PARAFAC | Separation between Rioja and Ribera del Guadiana wines, discrimination between Rioja and non-Rioja samples for Crianza and Reserva wines compared to young wines | [27] |
| White Argentinian wines | PCA, PARAFAC, other algorithms; best results with U-PLS-DA | Discrimination between the type of grape used for wine production | [108] |
| New Zealand Pinot Noir | Front phase, PARAFAC | Detection of differences in vineyard site, grape clone, winemaking process, and barrel properties | [30] |
| South African red wines | Front phase, PARAFAC, PCA, Bayesian optimization | Classification of South African red wine cultivars based on unique fluorescent fingerprints | [33] |
| White wines | PCA-LDA | Discrimination between Furmint, Lipovina, and Muscat Blanc wines | [109] |
| Pinot Gris and Riesling wines (Romania), Riesling (Romania) and Sauvignon (France) | Classical right-angle fluorimetry, PARAFAC, SIMCA | Classification based on the site of origin | [110] |
| Cabernet Sauvignon wines from 3 regions of Australia and Bordeaux | EEM of 200 times diluted wines analyzed by DA and SVMDA | Discrimination of wines according to location | [80] |
| Shiraz, Cabernet Sauvignon, and Merlot wines from 10 locations in Australia | EEM of 150-times diluted wines analyzed by XGB discriminant analysis and PLS | Discrimination of wine brand and geographical location | [81] |
| Four- to six-butt Tokaj wines | PCA followed by LDA | Distinguishing between botrytized wines of different quality (4-, 5- and 6- butt wines) and between unadulterated and adulterated wines | [111] |
| Cava sparkling wines | PARAFAC | Monitoring of browning in sparkling wines | [76] |
| Ribera del Guadiana and Rioja wines | Front phase, U-PLS/RBL | Good results for the quantification of caffeic and vanillic acids and resveratrol; acceptable results for epicatechin | [112] |
| Red wines (Cabernet Sauvignon) | Front-phase fluorescence; PCA, RMSE and MAE | Estimation of the content of total phenolics, total condensed tannins, and total anthocyanins following the course of fermentation | [79] |
| White Chardonnay wines | PARAFAC | Detection of the effect of SO2 treatment and/or vintage, even after several years of bottle aging | [113] |
| Porto wines and table red wines, Portugal | Diluted wines, standard fluorescence spectra | Fluorescence F700nm/F560nm ratio as a measure of monomeric/polymeric anthocyanins; excitation ratio Fex350nm/Fex 550 ratio as a measure of vitisin A/malvidin-3-O-glucoside ratio | [84] |
| Sweet Tokay wines | Synchronous emission spectra (260–290 nm), Δλ of 60 to 100 nm | Prediction of antioxidant capacity of wines based on estimation of the concentrations of phenolic compounds | [82] |
| Tokaj wines | Spectra at λex = 320 nm or synchronous fluorescence spectra | Determination of sum of concentrations of coumarins | [83] |
| Tokaj wines | Bulk and diluted (500 times), PLS | Estimation of concentrations of gallic, protocatechuic, caffeic, and p-coumaric acids and (+) catechin | [63] |
| White and red wines | Fluorescence sensor | Estimation of oxygen level in wine | [92] |
| Red wines | FRET-based fluorescence assay | Estimation of resveratrol concentration in wine | [95] |
| Red wines | Fluorescent probe 4-methyl-2-oxo-2H-chromen-7-yl-thiophene-2-carboxylate | Estimation of the level of H2S in wine | [98] |
| Red wine | BTPAP fluorescent probe | Estimation of Fe2+/Fe3+ concentration in wine | [99] |
| Red wine | Coumarin-based fluorescent probe | Estimation of Cu2+ concentration in wine | [100] |
| White wine | Macrocyclic Rhodamine B-based fluorescent probe | Estimation of Cu2+ concentration in wine | [101] |
| Red wines | Simultaneous use of two fluorescent probes, fluorescence intensity ratio at two wavelengths | Estimation of Cu2+ concentration in wine | [102] |
| White wines | New fluorescent probe | Estimation of concentration of haze-forming proteins | [106] |
| Red wines | Fluorescence polarization | Immunoassay for ochratoxin | [103] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sadowska-Bartosz, I.; Bartosz, G. What Can Fluorescence Tell Us About Wine? Int. J. Mol. Sci. 2025, 26, 3384. https://doi.org/10.3390/ijms26073384
Sadowska-Bartosz I, Bartosz G. What Can Fluorescence Tell Us About Wine? International Journal of Molecular Sciences. 2025; 26(7):3384. https://doi.org/10.3390/ijms26073384
Chicago/Turabian StyleSadowska-Bartosz, Izabela, and Grzegorz Bartosz. 2025. "What Can Fluorescence Tell Us About Wine?" International Journal of Molecular Sciences 26, no. 7: 3384. https://doi.org/10.3390/ijms26073384
APA StyleSadowska-Bartosz, I., & Bartosz, G. (2025). What Can Fluorescence Tell Us About Wine? International Journal of Molecular Sciences, 26(7), 3384. https://doi.org/10.3390/ijms26073384







