Abstract
Oxidative stress (OS) is caused by an imbalance between the production of reactive oxygen species (ROS) in cells and tissues and the ability of the biological system to detoxify these products. In chronic kidney disease (CKD), OS contributes to deterioration of kidney function and disease progression. In patients with end-stage kidney disease undergoing hemodialysis or peritoneal dialysis, OS is further increased and associated with adverse clinical outcomes, including deterioration and subsequent loss of residual renal function, atherosclerosis, hypertension, cardiovascular disease and death. However, currently, there is no consensus or guidelines for the diagnosis and treatment of OS in these patients. Herein, we aim to present the existing data regarding biomarkers of OS, pro-oxidants (oxidized albumin, advanced oxidation protein products, xanthine oxidase/dehydrogenase, nitrite/nitrate, malondialdehyde) and antioxidants (superoxide dismutase, catalase, vitamin E, total antioxidant capacity, N-acetylcysteine) that are most clinically relevant and have been more extensively studied in patients with chronic kidney disease, aiming to provide a clearer understanding of this complex area.
1. Introduction
ROS are highly reactive molecules and free radicals, containing at least one atom of oxygen, that are produced during the normal metabolism of oxygen inside the mitochondrial matrix. Within normal ranges, ROS are intrinsic to cell homeostasis and signaling functions, including redox signaling, cell metabolism, survival, proliferation and differentiation through the modulation of transcription factors and epigenetic pathways [1]. Moreover, ROS are important regulators of inflammation and protective immune response [2]. Increased accumulation of ROS, resulting from endogenous overproduction or exogenous administration, might disrupt the naturally occurring antioxidant defense mechanisms, and lead to impaired redox homeostasis, defined as OS [3]. In this setting, excessive ROS trigger pathological redox signaling, causing cellular damage and subsequent oxidative modifications to cellular macromolecules (DNA, lipids, proteins, etc.) [4]. These deleterious oxidative effects to biomolecules cause severe damage to both structure and function (peroxidation of lipids, deactivation of enzymes, fragmentation of proteins, mutations of DNA and modifications of carbohydrates), through mechanisms that cause an accelerated aging process [5]. To counteract these deleterious effects of ROS, naturally occurring antioxidant defense mechanisms exist, which, along with exogenously administered antioxidants, work together to attack and deactivate free radicals and repair oxidative biomolecules [6]. Although the pathogenesis of OS has been known for over two decades, several issues still remain to be fully elucidated, including the underlying mechanisms, clinical effects and treatment options, in various settings [7].
In CKD and end-stage kidney disease (ESKD), research has focused on OS during the past two decades. OS occurs in early CKD stages, is gradually aggravated in parallel to the deterioration of kidney function and is an independent risk factor for cardiovascular disease (CVD), death and progression to ESKD [8,9]. Notably, oxidation of lipids might also play a regulatory role and magnify the risk of CKD progression that is conferred by proteinuria [10]. When reaching ESKD, OS is further increased and associated with adverse clinical outcomes, including loss of residual renal function, atherosclerosis, hypertension (HT), CVD and death [11]. This might be explained by the fact that cellular OS induces apoptosis, senescence and reduced regenerative capability, and triggers inflammation via the formation of pro-inflammatory oxidized lipids or advanced oxidation protein products (AOPPs), while stimulation of nuclear factor κB (NFκB) in the pro-oxidant environment promotes the expression of pro-inflammatory cytokines and recruitment of pro-inflammatory cells [7]. Moreover, the effect of OS on renal pathology lies in the accumulation of extracellular matrix proteins, podocyte damage, mesangial expansion, renal hypertrophy, endothelial dysfunction, tubulointerstitial fibrosis and glomerulosclerosis [12]. A pro-oxidant state can occur as early as in CKD stage 3, as evidenced by reported increases in circulating OS biomarkers while antioxidant systems are compromised in CKD patients and deteriorate gradually with the degree of renal function [11,13]. In ESKD, the dialysis modality plays a crucial role in the pathogenesis of OS. In hemodialysis (HD), the main culprit of OS is the bioincompatibility of dialysate, membrane and plastic tubes and connections. At the initiation of a HD session, the patient’s blood comes into contact with the synthetic, bioincompatible dialysis filter and dialysate, and the white blood cells are stimulated to produce ROS. This is why within the first 15 min, there is a 14-fold increase in ROS levels. Other factors promoting the overproduction of free radicals in HD include the use of central venous catheters, renal anemia, intravenous administration of iron and the presence of microinflammation and microorganisms in the dialysate [14,15]. Moreover, HD is also a state of impaired antioxidant defense mechanisms, due to losses of hydrophilic unbound small-molecular-weight substances, such as vitamin C, trace elements and enzyme-regulatory compounds, and dietary restrictions leading to limited dietary intake of vitamins and antioxidants [16]. On the other hand, in peritoneal dialysis (PD), the membrane used is the natural peritoneal membrane, there are no central venous catheters, iron is rarely needed and there is no blood contact; still, PD patients exhibit significantly higher OS compared to pre-dialysis CKD stage 3–4 patients, although this is lower than in HD patients. In this setting, OS is triggered mainly by the non-physiologic composition of conventional PD solutions, including their high glucose concentration, increased osmolarity and acidic pH. When the peritoneal mesothelial cells (PMCc) are exposed to the bioincompatible PD fluid, structural alterations occur, leading to peritoneal fibrosis, vasculopathy, neoangiogenesis and progressive degradation of the peritoneal membrane [17,18].
In CKD patients, the leading cause of mortality is CVD; however, this heavy CVD risk cannot be solely explained by traditional Framingham risk factors (diabetes (DM), HT, dyslipidemia) [19]. During the past two decades, accumulating evidence has suggested that OS might play a central role in the pathogenesis and progress of atherosclerosis in CKD, and should therefore be considered as a novel uremia-related risk factor for CVD [20]. The oxidative transformation of molecules such as lipids, proteins, carbohydrates and nucleic acids leads to cell apoptosis and death, triggering inflammation and the development of endothelial dysfunction, which is considered the hallmark of atherosclerosis [21]. Furthermore, OS induces the osteoblastic transformation of vascular smooth muscle cells, which is the first step towards vascular calcification (VC) [22]. Although during recent years, it has become clear that OS has a clinical impact in CKD and ESKD (Figure 1), there is still no consensus or guidelines suggesting how to measure, quantify or treat OS in these patients. This is mainly attributed to the fact that direct measurement of ROS is practically impossible, due to their low concentration, highly reactive nature and short half-life (which is usually less than a second). Thus, we measure the oxidative by-products of ROS reactions and antioxidant molecules [20]. The high heterogeneity of many OS biomarkers causes confusion and a lack of uniformity [23]. Among the most important OS biomarkers are the products of oxidative modifications of lipids, proteins and nucleic acids, as well as the measurement of the total antioxidant capacity (TAC) of the biological fluids. This review article will focus on the biomarkers of OS and the antioxidant defense mechanisms that are most clinically relevant and have been more extensively studied in patients with CKD, aiming to provide a clearer understanding of this complex issue.
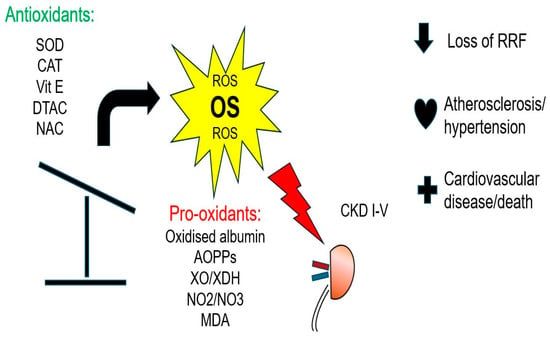
Figure 1.
Clinical impact of oxidative stress in CKD and ESKD.
2. Biomarkers of OS: Pro-Oxidants
Figure 2 shows the molecular mechanisms underlying the effects of pro-oxidants.
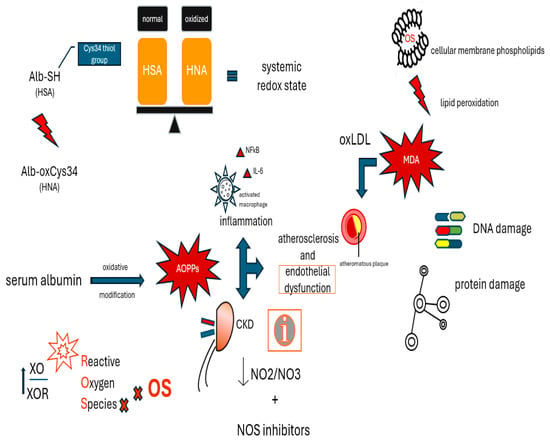
Figure 2.
Molecular mechanisms underlying the effects of pro-oxidants.
2.1. Oxidized Albumin
Human serum albumin (HSA) is a multifunctional protein that transports numerous endogenous and exogenous substances, regulates osmotic pressure and blood pH and supports antioxidative defense. The antioxidant effects of HSA result from the fact that HSA is a predominant source of free thiols (Cys34 thiol group in particular) and exhibits a high copper-binding affinity [24]. Since post-translational oxidation and glycation of HSA directly affect the Cys34 thiol group, it was hypothesized that oxidized albumin might serve as a marker of OS. Albumin containing a reduced Cys34 thiol group is called “human mercaptoalbumin” (HMA), while albumin containing a Cys34 thiol group that exists in an oxidized state is called “human non-mercaptoalbumin” (HNA) [25]. The oxidation of albumin in Cys34 has been associated with various adverse clinical effects in both the liver and kidneys [26], and levels of oxidized albumin increase in parallel with CKD progression. The ratio between the oxidized and normal forms of HSA, represented in the redox states of Cys34, serves as a marker of systemic redox state, and has been closely associated with early diagnosis and prediction of the prognosis of kidney disease [25,27]. Liu et al. [28] established a rapid and quite accurate method to quantify oxidized albumin by high-performance liquid chromatography (HPLC), and in order to validate their method, they conducted an experimental study in a rat model of albuminuria and HT. In this setting, the antioxidant Tempol was evaluated as a potential therapeutic agent to halt CKD progression, and the measurement of oxidized albumin was proposed as a practical and sufficiently validated biomarker of OS. In 5/6 nephrectomized rats with high salt intake, oxidized albumin was significantly higher in comparison to in the normal diet group, and was positively correlated with kidney function. After Tempol administration, the impact of high salt loading was reversed, leading to normal levels of oxidized albumin and inhibition of inflammatory and pro-fibrotic signaling pathways [29]. Terawaki et al. used the redox state of HSA as a marker to quantify the status of OS in 55 pre-dialysis patients, and demonstrated that OS correlates with the degree of kidney function, and is increased even in pre-dialysis CKD [30]. The HMA fraction of albumin was significantly reduced in HD patients compared to normal age-matched control subjects. Moreover, this percentage increased 3–5 h after the initiation of a single HD session, and then was reduced to subnormal levels, thus indicating that serum albumin might serve as a crucial extracellular antioxidant in HD patients, which is heavily affected by the HD procedure per se [31]. Reduced serum antioxidant activity in HD patients, assessed by an impaired HSA-redox state, contributes to the high oxidative damage seen in these patients, and is tightly associated with accelerated atherosclerotic changes and subsequent cardiovascular (CV) mortality [32]. In a prospective observational study with a long follow-up, Lim et al. showed that high levels of HNA in 249 normoalbuminemic HD patients were associated with a 2.2-fold increase in CV death risk and a hazard ratio of 1.57 for all-cause mortality, after adjusting for traditional CV risk factors [33]. Experimental data from HD patients suggest that a low albumin index due to oxidative modifications triggers an inflammatory response in endothelial cells, increasing mRNA expression of inflammatory cytokines (interleukins IL-6, IL-8 and IL-1β), and oxidized HD albumin upregulates the vascular cell adhesion molecule (VCAM) in endothelial cells [34], causing inflammation and endothelial dysfunction. Since these two entities are interrelated and both promote the development and progression of kidney disease, it might be useful to evaluate not only albumin levels, but also the albumin’s redox state, for the prognosis of kidney disease [25]. Bar-Or et al. observed a phenomenon of reduced in vitro binding of exogenous cobalt [Co(II)] to the N-terminus of HSA in ischemic conditions [35]. Ischemia-modified albumin (IMA) is a marker of ischemic OS, and has been used as an early marker in the evaluation of patients with acute coronary syndrome, but has also been evaluated in various clinical conditions, including CKD [36]. Kıyıcı et al. measured albumin and IMA levels before and after a HD session in 30 stable, maintenance HD patients, and showed that there was a significant post-dialysis increase in IMA [37]. Moreover, compared to age- and gender- healthy controls, children with CKD exhibited significantly higher IMA levels in the saliva (but not in serum or urine), and, most interestingly, the saliva concentration of IMA was correlated with standard markers of kidney function, including eGFR, stage of CKD, and serum creatinine and urea levels [38]. Circulating IMA levels were significantly increased in pediatric steroid-sensitive nephrotic syndrome (SSNS) patients compared to healthy controls, and these alterations in IMA levels were more prominent in the relapse group than in the remission group, thus suggesting that elevated IMA could indirectly reflect the degree of oxidative damage in the glomeruli [39].
Overall, oxidized albumin is a marker of OS and a kidney function index, but also a nephrotoxic molecule; therefore, the assessment of its levels might serve as a novel and exciting marker for the diagnosis and prognosis of CKD complications, including atherosclerosis, CVD and CKD progression to ESKD.

Table 1.
Associations of OS markers with CVD indices in CKD and ESKD.

Table 2.
Associations of OS markers with CKD indices in CKD and ESKD.
2.2. Advanced Oxidation Protein Products (AOPPs)
Proteins are molecular targets for oxidation reactions, because of their rapid reaction rates with oxidants and their high abundance in cells, extracellular tissues and body fluids. OS degrades lipids and carbohydrates to highly reactive intermediates, which eventually attack proteins at various functional sites. Consequently, protein oxidation, glycoxidation and lipoxidation cause a wide variety of distinct post-translational protein modifications [55]. Reversible modifications constitute signaling mechanisms under appropriate conditions (“redox signaling”), while non-reversible modifications may contribute to pathological conditions and several diseases [56]. AOPPs were identified in the plasma of uremic patients by Witko-Sarsat et al., who first described a spectrophotometric assay to quantify AOPPs in μmol/L of chloramine-T equivalents [57]. AOPPs are products generated by the attack of free radicals on serum albumin, and serve as OS biomarkers [20]. In human embryonic kidney cells, AOPPs induced the transcription of inflammatory genes (ex., NF-kB, IL-6) [58]. In streptozotocin-induced diabetic rats and in 5/6 nephrectomized rats, i.v. administration of AOPPs-RSA (AOPP-modified rat serum albumin) led to renal macrophage infiltration and overexpression of chemoattractants and pro-fibrotic agents, and led to structural and functional abnormalities, such as glomerular hypertrophy, fibronectin accumulation, accelerated fibrosis and albuminuria, probably through activation of renal NADPH (nicotinamide adenine dinucleotide phosphate hydrogen) oxidase [59,60]. The same enzymatic complex is implicated in the pathogenesis of muscle atrophy-sarcopenia. In 5/6 nephrectomized mice, AOPP overload was associated with decreased running and hanging time, a finding that was also validated in HD patients, where higher serum AOPP levels co-existed with sarcopenia [61]. Arimura et al. investigated the relationship between adipose tissue and AOPPs by conducting both in vitro and in vivo studies on adenine-induced CKD mice, and showed that AOPPs contribute to macrophage-mediated adipose inflammation, and, interestingly, the activation of inflammation could be suppressed at the gene expression level by the presence of NADPH oxidase inhibitors [62].
Declining renal function has been associated with an increase in AOPP levels, thus making them candidate markers of CKD progression [63]. Conti et al. evaluated serum levels of AOPPs in a cohort of 62 patients with DM and 56 with HT, with or without renal complications, and showed that AOPPs were increased in diabetic and hypertensive subjects compared to healthy controls, and were increased in parallel with deterioration of kidney function. The authors concluded that assessment of circulating AOPPs might predict the onset and progression of kidney disease [49]. In patients with type 1 DM, specific SOD2 allelic variations were associated with higher plasma AOPPs and progression to severe diabetic nephropathy (DN) [64]. A longitudinal study in 205 patients with CKD stages 2–5 and 40 healthy controls showed that AOPP levels increased with the decline of kidney function, and were positively correlated with the intima media thickness (IMT) of the common carotid artery. Furthermore, AOPP mean values were higher in patients with atherosclerotic plaques compared to those without plaques. The authors concluded that AOPPs might serve not only as an OS marker, but also as a surrogate biomarker for CKD progression and vascular calcification [40]. The exact mechanisms underlying these associations might not be limited only to OS, but might also include inflammation and changes in the adipose tissue. In agreement with these findings, Vinereanu et al. showed that in pre-dialysis CKD stages G3–G5, AOPPs were significantly associated with C-reactive protein (CRP), high-density lipoprotein (HDL) levels, glycated hemoglobin (HbA1c) and pulse wave velocity (PWV) values, thus suggesting that the measurement of AOPPs could be of use for CV risk assessment in pre-dialysis CKD [41]. The prognostic value of AOPP measurement has also been tested after cardiac interventions; serum AOPPs measured 1 h after cardiopulmonary bypass had a positive predictive value of 68% for predicting acute kidney injury (AKI) [65], and in a prospective study after coronary artery bypass grafting, increased AOPP levels indicated poor recovery from AKI, deteriorating overall long-term prognosis [66]. In the setting of an intensive care unit, Lentini et al. recruited 86 medical and surgical patients, and found that AOPP levels were higher in AKI compared to non-AKI critically ill patients, but their measurement could not identify those patients at risk of developing AKI within a four-day observation window [67].
The role of AOPPs in inflammation and OS in patients undergoing HD has been extensively studied. AOPPs are active inflammatory mediators through their ability to trigger oxidative burst and the synthesis of pro-inflammatory cytokines in phagocytes, and might be regarded as uremic toxins [68]. Colombo et al. reported a tight association between AOPPs and uremia in HD [69], whereas AOPPs seem to be more accurate and reliable markers of inflammation than AGEs, and correlate significantly with prothrombotic fibrinogen, thus suggesting an additional role in accelerated atherosclerosis [70]. In a cohort of 60 HD patients, AOPP concentration was significantly associated with common carotid artery IMT and wall-to-lumen ratio [42]. A large cross-sectional study on dialysis patients showed that the accumulation of AOPPs was an independent risk factor for the presence of ischemic heart disease only in the HD group, but not in the PD group [71], and an 8-year follow-up prospective study on 199 Chinese HD patients concluded that elevated serum AOPP levels were associated with a higher risk of all-cause mortality [72]. Contrary to previous findings, in another prospective study on 347 HD patients, Zuo et al. conducted a head-to-head comparison of the predictive value of four different OS biomarkers, and concluded that both AOPPs and oxidized low-density lipoprotein (oxLDL) had no impact on all-cause mortality. The authors attributed this difference to the longer follow-up period and different demographics of the aforementioned study [73].
In patients undergoing PD, AOPP levels tend to increase in parallel with the duration of the treatment. In a prospective sequential study, 22 PD patients were recruited, and serum AGEs and AOPP levels were measured prior to, and 6 and 12 months after, the initiation of the method. The rise in AGEs was attributed to the patients’ exposure to glucose-containing dialysates, whereas concerning AOPP levels, a decrease was noted [74]. Later, Gonzalez et al. measured plasma AOPP levels in 48 patients 6 months and 1 year after starting PD. The vast majority of patients showed an increase in plasma AOPP level at 1 year, and interestingly, those patients in whom the AOPP levels increased more than 50% above the baseline value had a 4.7 times greater risk of suffering a CV event compared to those with a smaller increase, even after adjusting for previous CV history [43]. In a longitudinal study, loss of residual renal function (decreased urine output and renal creatinine clearance) was associated with increased plasma AOPP levels [50]. Additionally, in a cross-sectional study of 75 PD patients, increased levels of AOPPs were independently associated with central systolic and diastolic blood pressure (cSBP, cDBP), even after adjusting for markers of fluid overload (extracellular water by multi-frequency bioimpedance, N-terminal pro-B-type natriuretic peptide-NT-proBNP), inculpating OS in the pathogenesis of HT [44]. Lastly, Furuya et al. conducted an interventional study to evaluate the effect of candesartan, an angiotensin II receptor blocker, on plasma levels of adiponectin and AOPPs in eight non-diabetic PD patients. They concluded that it decreased AOPP levels in a treatment-dependent manner, and that the increase in adiponectin levels could signify mitigation of OS and atherogenesis [75].
In CKD and ESKD, circulating AOPPs are implicated in several molecular pathways, including OS, inflammation, atherosclerosis, sarcopenia and kidney function. Therefore, the assessment of AOPPs might be applicable for the diagnosis and prognosis of CKD, AKI, CVD and mortality. In the future, large, prospective cohort studies are needed, with long follow-ups and large sample sizes, in order to fully elucidate the possible role of AOPPs in these aforementioned settings.
2.3. Xanthine Oxidase/Dehydrogenase
Xanthine oxidase (XO) is the oxidative radical-producing isoform of xanthine oxidoreductase (XOR), also known as urate-producing enzyme. XO is formed by reversible or irreversible oxidation of another isoform, xanthine dehydrogenase (XDH). The XO enzyme catalyzes oxidation of hypoxanthine to xanthine, and then xanthine to uric acid [76]. The XOR and XO activities both show positive correlations with eGFR; however, the ratio of XO to total XOR, known as plasma XOR redox, is inversely correlated with eGFR. A high XO/XOR ratio contributes to the elevation of OS via ROS generation, and a ratio value >1 indicates severely impaired renal function in the setting of accelerated plasma XOR conversion to XO [77].
Terawaki et al. included in their study 13 non-dialysis patients with varying degrees of kidney function, and showed that plasma XOR redox is closely related to HSA redox [78]. In a prospective study, Gondouin et al. included 51 non-diabetic patients with CKD stages 3–5 and 50 chronic HD and 38 matched healthy controls. After a 3-year follow-up time, they found that XO activity was an independent predictor of CV events in CKD and HD patients, regardless of uric acid levels, thus suggesting that the beneficial effects observed with XO inhibitors on CVD in CKD may be due to the reduction of OS [79]. Focusing on the effect of allopurinol treatment on vascular function, Sun et al. found no association between serum XO activity or endothelial XO expression and biomarkers of vascular inflammation or OS in patients with stage 3 CKD and asymptomatic hyperuricemia [80]. Additionally, in vivo studies in uninephrectomized mice and in vitro studies in human kidney proximal tubule epithelial (HK-2) cells demonstrated that XO could be a novel therapeutic target for hypercholesterolemia-associated kidney injury in uninephrectomized patients [81].
The data regarding the possible role of XO in kidney diseases are extremely limited, and are mainly derived from experimental studies. However, the idea that this OS biomarker might reflect kidney function is attractive, and warrants more research.
2.4. NO Synthase-Nitrite and Nitrate (NO2 and NO3)
Nitric oxide (NO) is a gaseous signaling molecule, produced by endothelial cells through the action of endothelial NO synthase (eNOS), that plays a major role in controlling vascular tone. NO production is stimulated by circulating mediators, hormonal modulators and physical factors (e.g., shear stress), while experiments in isolated blood vessels and endothelial cell cultures have shown that there is a basal release of NO, even in the absence of obvious external stimuli. When NO reaches vascular smooth muscle, it causes relaxation and vasodilation, but also inhibits platelet aggregation and white blood cells’ adhesion at the surface of the endothelial cells [82].
NO is a highly reactive molecule and has a very short half-life, so the inorganic anions formed by oxidization of NOS-derived NO-nitrate and -nitrite are measured instead. These anions are endogenously formed, but can also be found in high amounts in our daily diet. Still, they are useful indirect markers for evaluating NO production, eNOS activity and the status of endothelial function, making them important tools in clinical research related to CVD [83]. Preclinical and clinical studies have shown that reduced NO generation due to compromised eNOS (primarily found in the endothelial cells of the afferent arteriole) and/or neuronal nNOS (primarily expressed in the macula densa cells) activity can increase preglomerular resistance, reduce glomerular perfusion and filtration and increase tubular reabsorption of sodium, which subsequently lead to the development or progression of HT and kidney damage [84,85]. Using a rodent model, Prabhakar et al. found that in DN, both renal NO production and expression of renal NOS were decreased, while markers of mitochondrial OS (8-hydroxydeoxyguanosine) were upregulated. Interestingly, the administration of the antioxidant alpha-lipoic acid could reduce proteinuria and OS, and treatment of hyperglycemia could delay the progression of CKD [86]. Reduced eNOS immunoreactivity and endothelial dysfunction preceded capillary loss and led to worsening of renal disease in experimental polycystic kidney disease [87]. The enhancement of NO bioavailability in CKD rats has been tested with the use of antioxidant mixtures (L-carnitine, Catechin, vitamins E and C) [88] and sodium hydrosulfide [89], providing evidence that certain interventions could exert renoprotective effects by attenuating inflammation and OS, and could restore kidney function.
In a large cohort of 375 participants, Kleinbongard et al. showed that plasma nitrite levels were tightly correlated with surrogate markers of endothelial dysfunction (flow mediated dilation (FMD), carotid IMT) and with traditional, Framingham CV risk factors (HT, dyslipidemia, smoking, advanced age). Furthermore, there was a progressive reduction in plasma nitrate with an increasing score of the aforementioned CV risk factors [45]. In patients with isolated essential HT, the presence of constitutive NOS (cNOS) inhibitors and lipid peroxidation products was found to worsen blood pressure control and lead to microvascular endothelial dysfunction [90]. Therapeutic strategies that correct the imbalance between the production of ROS and NO may have clinical implications. Oral nitrate ingestion was found to have a consistent BP-lowering effect in both healthy volunteers and hypertensive patients [91], possibly through antioxidative effects via different targets and cellular mechanisms (modulation of mitochondrial function, reduction in NOX- and XO-derived ROS production, restoration of eNOS function). Through the improvement of endothelial dysfunction and OS, nitrate treatment might delay the progression of cardiorenal complications [92].
In CKD, inflammation, OS, mineral bone disease (low vitamin D, hyperphosphatemia, high fibroblast growth factor 23 (FGF23) and low Klotho) and the accumulation of NOS inhibitors all contribute to reduced NO bioavailability and endothelial dysfunction [21,93], resulting in the development and progression of CVD [92]. In a small observational study on 27 ESKD patients, reduced NO bioavailability post-dialysis, confirmed by measuring plasma levels of nitrates and nitrites, was associated with increased CV mortality [94]. In PD patients, NO levels were decreased compared to healthy controls, irrespective of peritoneal transport status, but there was an interesting negative correlation between NO and the peritoneal equilibration test (PET) that could suggest alterations in the bioavailability of NO in the vascular endothelium in this setting [95].
In living kidney donors, NO biomarker improvement begins on the first day of kidney transplantation, and NO levels on day six could be a predictive marker of eGFR at six months [51]. Nitrite therapy might be beneficial during the procedure of kidney transplantation, as suggested by experimental evidence that topically administered sodium nitrite protects the kidney from ischemia/reperfusion injury via the generation of XOR-catalyzed NO [96].
NO plays a pivotal role within the endothelium, and therefore reflects endothelial dysfunction, the hallmark of atherosclerosis. The clinical plausibility of this OS marker relies on the fact that besides diagnosis and prognosis, NO might also serve as a therapeutic target in kidney and CVD.
2.5. Malondialdehyde (MDA)
MDA is a highly reactive and toxic aldehyde, generated by polyunsaturated fatty acid peroxidation or prostaglandin breakdown by cyclooxygenase activity, or via various non-lipid precursors, involving amino acids and carbohydrates [97]. MDA is an established marker of lipid peroxidation status, thus indicating OS and early atherogenesis in both clinical research and epidemiological studies [98]. On a molecular level, lipid oxidation of cell membranes releases MDA and causes impaired elasticity and limited membrane function. MDA forms covalent bonds with biomolecules, especially proteins and nucleic acids. The assay to determine MDA in tissues or blood involves the reaction of MDA with Thiobarbituric acid to form TBARSs (thiobarbituric acid reactive substances) in an acidic medium [99]. MDA, like other reactive aldehyde species (4-hydroxy-2-nonenal (HNE), acrolein), is generated within enzymatic pathways that lead to post-transcriptional alterations in DNA and proteins, eventually resulting in genotoxicity, inhibition of gene expression, cytotoxicity and cellular death [97]. In vitro and in vivo studies have coherently showed that MDA is toxic to cells and tissues [100], promotes atherogenesis [101] and is heavily implicated in the pathogenesis of chronic inflammation [102].
In human studies, higher serum MDA levels were found in CKD patients compared to healthy controls, while MDA was negatively correlated with eGFR. Tomás-Simó et al. conducted a prospective study in 155 non-dialysis patients at different stages of CKD and 45 healthy controls, and found a progressive increase in circulating levels between controls and CKD stage 1, and among CKD stages (2 versus 1, 3 versus 2, 4 versus 3) [52]. In agreement with these findings, in 402 patients with DN, serum MDA-modified LDL (MDA-LDL) levels were significantly increased in severe albuminuria, and an increased MDA-LDL/LDL ratio was significantly associated with both increased albuminuria and reduced eGFR, thus suggesting that this marker is implicated in the progression of DN [53]. Moreover, among 120 CKD pre-dialysis patients, the presence of DM type 2 was associated with significantly increased plasma MDA levels [103]. Table 3 shows the differential expression of MDA and other OS markers in CKD/ESKD patients and healthy controls.

Table 3.
Differential expression of OS markers in CKD patients and healthy controls.
In HD patients, MDA levels are higher than in healthy controls matched for age, gender and body mass index (BMI) and pre-dialysis CKD patients [106,107]. This might not be solely attributed to disease progression; it has been shown that MDA levels do not differ significantly between CKD stages 4 and 5 [52]. Therefore, the effect of a single HD session on MDA levels has been evaluated in several small single-center studies. MDA is a water-soluble compound with a low molecular weight, and thus is expected to diffuse across HD membranes. On the other hand, each HD session triggers acute autoimmune activation reactions and causes abrupt generation of free radicals, which, in turn, might interact with cell membrane lipids to produce MDA. After accounting for the clearance of MDA through the membrane, Lakshmi et al. found a significant increase in circulating MDA from pre to post in a single HD session, thus indicating that HD modality, overall, triggers lipid peroxidation [108]. In agreement with this finding, a study that included 20 patients on HD, 16 patients on CAPD and 20 healthy controls showed that MDA was elevated in post-HD and CAPD patients in comparison to pre-HD and control groups, and the increased lipid peroxidation was accompanied by low levels of the antioxidant glutathione [104]. However, a small observational study that investigated the effect of plasma vitamin concentrations on markers of OS during HD showed that the plasma MDA concentration did not change significantly, while vitamin C levels decreased by 40% after a single HD session [109]. Steghens et al. provided a methodological explanation by measuring true total and free plasma MDA in HD patients, before and after the sessions, by the HPLC method, concluding that free MDA is bound to low-molecular-weight compounds, and total MDA is mainly produced during HD sessions [110].
Besides kidney function and OS, MDA might serve as a surrogate marker of endothelial dysfunction and sarcopenia. In HD, MDA was inversely associated with performance on 6 min walk test (a measure of functional capacity) [111], and was positively associated with carotid IMT, left ventricular hypertrophy [112], PWV and vascular stiffness [46,47,105]. Specifically, serum MDA-modified LDL, along with DM and HT, were independent predictors of arterial stiffness (AS) (defined by carotid-femoral cfPWV) in HD patients [46], whereas ROC (receiver operating characteristic) curve analysis showed that the critical cut-off value of 80.91 mg/dL for MDA-LDL predicted peripheral arterial stiffness (PAS) with accuracy [47]. The authors concluded that not only does MDA plays a role in the pathogenesis of arterial stiffness, but it could also be considered as a therapeutic target in this condition. Interestingly, the presence of antibodies to MDA-LDL had no correlation with thromboembolic events [113]. In 39 HD patients, pre-dialysis serum MDA concentrations were correlated with coronary artery calcification (CAC—calculated by multirow spiral computed tomography), and those patients with the highest MDA levels were four times more likely to have severe CAC [48]. In a cross-sectional study, Boaz et al. concluded that serum MDA levels, both before and after the midweek session, were the strongest predictor of CVD prevalence, while other known risk factors, such as serum lipids and plasma fibrinogen, failed to show any association [114,115].
Patients on PD have higher MDA levels compared to pre-dialysis CKD patients, but lower MDA levels compared to HD, which can be attributed to the fact that PD is a more biocompatible dialysis modality than HD [116]. In contrast to HD, the presence of DM in PD patients did not significantly affect MDA levels [117]. However, PD efficiency, assessed by total weekly Kt/V, has been negatively associated with circulating MDA [118]. The culprit for this might be the conventional peritoneal dialysates that have low pH, high osmolarity, and high glucose, GDPs and AGEs. Chronic exposure of the peritoneal membrane to glucose gradually leads to neo-angiogenesis, thickening of the membrane, inflammation and OS and apoptosis of the mesothelial cells. This hypothesis is also supported by the fact that 6 months of use of icodextrin-PD solutions (which is a non-glucose, osmotic colloid agent) caused a significant decrease in MDA levels [119].
Kidney transplantation partially restores kidney function, and this seems to cause a significant and time-dependent decrease in MDA levels, which leads to normalization within the first 3 years post-transplantation [120]. A prospective study in 40 kidney transplant recipients showed that increased MDA levels on the first day postoperatively might be an early prognostic indicator of delayed graft function (DGF), and if increased MDA persists after 7 days, this might be a negative predictor of 1-year graft function [54].
Various pharmacological and non-pharmacological approaches, including antioxidant supplementation, lifestyle modifications and drug therapies, have shown potential in mitigating oxidative damage by reducing MDA levels in patients with kidney disease. In a meta-analysis of seven randomized controlled trials (RCTs), including 392 HD patients, probiotic, prebiotic and synbiotic supplementation effectively reduced the levels of circulating uremic toxins and inflammatory biomarkers, while restoring the balance between antioxidant and pro-oxidant markers, with a significant reduction in MDA levels in the intervention group when compared with the placebo group [121]. Also, aerobic exercise managed to successfully suppress circulating MDA in CKD patients [122], thus highlighting the beneficial antioxidant effects of chronic exercise in these patients.
MDA plays a central role in the lipid peroxidation of biological membranes. However, multiple other roles in the pathogenesis and progression of various conditions have been proposed for this oxidant, including CVD, arterial stiffness, CKD, inflammation and sarcopenia. Besides serving as a marker of diagnosis and prognosis, MDA might also serve as a therapeutic target in dialysis populations.
3. Biomarkers of OS: Antioxidants
To counteract the deleterious effects of free radicals and pro-oxidants on biomolecules, there are both naturally occurring and endogenously and exogenously administered antioxidants. The main mechanisms underlying the effects of antioxidants are shown in Figure 3.
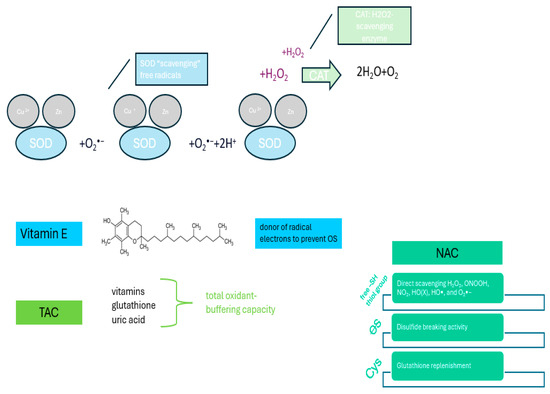
Figure 3.
Molecular mechanisms underlying the effects of antioxidants.
3.1. Superoxide Dismutase (SOD)
There are three mammalian SODs: copper–zinc SOD (SOD1) and manganese SOD (SOD2), which exist exclusively in the intracellular environment, and extracellular SOD (EC-SOD), primarily localized in the extracellular space. EC-SOD is an antioxidant enzyme expressed in abundance in the kidney, and has a special role in the homeostasis of the kidney and the vasculature, mainly by regulating the free radical superoxide anion, which is generated in renal disease through upregulation of NADPH oxidases or a leak from the mitochondrial electron transport chain [123]. All SODs catalyze the dismutation reaction of the superoxide anion free radical to hydrogen peroxide and normal oxygen [124]. Therefore, the strong antioxidant effects of SOD lie in its ability to directly scavenge free radicals. SOD has been associated with local kidney oxidation in CKD, whereas in dialysis, the uremic environment is hypothesized to significantly downregulate both the function and concentration of SOD.
The first reports regarding the association of SOD with kidney function date from three decades ago. Kashem et al. suggested that low levels of SOD activity (which might be either the cause or the result of glomerulonephritis) lead to reduced local antioxidant defense, oxidation of the kidney cells and deterioration of kidney function [125]. Since then, the significance of SOD has been studied in various settings. SOD2 dysfunction has been reported to aggravate renal damage and apoptosis in the kidney at the cellular level due to mitochondrial OS [126].
In animal models, EC-SOD knockout mice showed attenuated renal blood flow and decreased SOD activity after kidney ischemia/reperfusion [127], while Rodríguez-Iturbe et al. showed that SOD2 deficiency was associated with renal interstitial inflammation and accelerated glomerulosclerosis, tubulointerstitial damage and salt-sensitive HT, especially in aged mice [128]. SOD has also been associated with albuminuria; downregulation of renal SOD1 and SOD3 was observed in progressive DN [129], and finerenone, a novel non-steroidal mineralocorticoid receptor antagonist, achieved an increase in renal SOD activity and a reduction in albuminuria in CKD animal models [130].
In a retrospective study of 134 hypertensive patients with microalbuminuria, Yu et al. found a negative correlation between SOD and urine albumin–creatinine ratio (UACR), and thus, SOD was proposed as an independent protective factor against early renal damage in this group of patients [131]. There is a J-shaped pattern of circulating SOD across CKD stages: SOD is progressively reduced in stages 1–4, and then increases in ESKD [132]. Moreover, the expression of the SOD2 gene was similar between groups, whereas increased SOD2 protein content was associated with an increased risk of all-cause mortality in the HD group. The gradual decline in SOD2 protein content across CKD stages 1–4 might be explained by the fact that OS, inflammation and uremia all contribute to excessive protein degradation. OS and inflammation act synergically to release free radicals in CKD, which, in turn, downregulate SOD2 protein content. Additionally, through activation of the ubiquitin-proteasomal pathway, the uremic environment triggers further degradation of SOD2 protein content. The exact mechanisms underlying the abrupt increase in SOD2 in ESKD have not yet been fully elucidated. In uremic animals, supplementation of vitamin D receptor antagonists (VDRAs) successfully reversed SOD2 protein upregulation. Therefore, the authors hypothesized that since ESKD is characterized by poor vitamin D status, the use of VDRAs might partially explain the J-shaped pattern of SOD2 protein content. When compared with controls, HD patients present decreased SOD activity, which is probably influenced by zinc nutritional status [133], and this reduced antioxidant activity is negatively correlated with plasma MDA levels [134]. In a survival analysis, HD patients with higher SOD activity had a survival benefit, suggesting that SOD can serve as a predictor of all-cause and CV mortality [135]. A genotype–phenotype association study involving 178 HD patients showed that a specific EC-SOD polymorphism (EC-SOD Arg213Gly) could accelerate OS and atherosclerosis in its carriers, demonstrated by higher IMT and plasma OxLDL values [136].
Studies on PD patients showed increased SOD activity, irrespective of the peritoneal membrane transport status, when compared with healthy controls, possibly in an attempt to balance OS, while the levels of the DNA repair enzyme hOGG1 (human 8-oxoguanine DNA glycosylase 1) and TAC were both significantly decreased [95]. When measuring SOD activity in the erythrocytes of ESKD patients, a significant decrease was observed in HD patients using polyacrylonitrile or cuprophane membranes, whereas enzymatic activity increased in patients undergoing CAPD [137]. Inhibition of SOD activity in the red blood cells (RBCs) of dialysis patients might also contribute to renal anemia [138]. Therapeutic approaches that involve enhancing SOD activity are under investigation. SOD-gliadin supplementation in 28 patients undergoing HD was found to significantly decrease serum tumor necrosis factor α (TNF-α) and tumor growth factor β (TGF-β) levels [139]. The marked increase in Cu/Zn-SOD production in HD patients, as a response to OS and inflammation, suggest that SOD might also serve as a potential therapeutic target. In a small interventional study, Washio et al. studied the effect of orally administered vitamin C on Cu/Zn-SOD levels in 16 HD patients, but this vitamin supplementation did not effectively suppress expression enhancement [140]. In disagreement with these, vitamin E supplementation and the use of vitamin E-coated membranes (VECMs) for 6 months both resulted in a progressive decrease in Cu/Zn-SOD mRNA in the leukocytes of HD patients to the level of non-dialyzed CKD patients [141].
3.2. Catalase (CAT)
Among ROS, hydrogen peroxide (H2O2) is an effective secondary messenger that can diffuse to different cell compartments and the extracellular space, and catalase (CAT) is probably the most effective H2O2-scavenging enzyme [142]. Polymorphisms in genes encoding CAT have been associated with a decreased risk of CKD [143], while in vitro studies suggest a protective effect of CAT on VC induced by calcium and phosphorous [144]. Experimental CKD (5/6 nephrectomy) is accompanied by a reduction in CAT activity [145], which promotes the accumulation of collagen, fibrosis, inflammation, reduced kidney function and the onset of albuminuria [146].
Clinical data also suggest a close association of CAT with kidney function [147] and CVD [148]. In HD patients, the bioincompatibility of the modality aggravates OS by generating pro-oxidants and reducing antioxidants. Small interventional studies have focused on the effect of dialysis membranes on erythrocyte CAT activity. When HD patients were dialyzed with the newest, more biocompatible polysulfone membrane, a significant increase in CAT levels was observed [149]. CAT activity was higher in all ESKD patients (undergoing HD or CAPD) when compared to healthy controls, and an enhancement in CAT activity was observed over the course of several months in renal replacement therapy, indicating the significant exposure of patients’ erythrocytes to OS [137]. In PD patients, Stępniewska et al. investigated platelet antioxidant activity, and found that CAT activity was significantly higher in PD compared to HD patients [150]; however data on PD patients are extremely limited.
In recent years, nanobiotechnology has attempted to create nanozymes (nanomaterials exhibiting natural enzyme-like activity) in order to effectively supplement antioxidants. In an experimental study, Choi et al. developed an inflammation-sensing, ROS-scavenging versatile nanoplatform by stably loading CAT-mimicking dMn3O4 nanoparticles inside ROS-sensitive nanomicelles (PTC), resulting in an ROS-sensitive nanozyme. After i.v. administration of the nanozyme to mice with ischemia/reperfusion AKI, they observed an attenuation of inflammation and apoptosis in kidney cells [151], thus suggesting that high-performance ROS depletion strategies are yet to be developed.
3.3. Vitamin E
Vitamin E (or alpha-tocopherol) is a fat-soluble vitamin known for its antioxidant properties. It plays a role in the detoxification chain of fat-soluble to water-soluble toxic radicals, thus protecting various tissues from lipid peroxidation. Through its protein-dependent functions and gene modulation effects, it inhibits the production of pro-inflammatory cytokines, activates protective pathways like Nrf2 and modulates the activity of immune cells (ex., T-cells, macrophages) [152]. Specifically, vitamin E blocks monocyte O2 discharge, IL-1β and TNF-α synthesis, neutrophil chemotaxis and their adhesion to the endothelium, which all contribute to atherogenesis [153], and it preserves the integrity of the glomerular basement membrane [154].
The renoprotective effect of vitamin E supplementation was studied in a double-blind, randomized cross-over trial, where 30 type 2 diabetic patients with microalbuminuria were treated with combined oral supplementation of vitamin C and E. UACR was reduced by 19% after 4 weeks, even though the participants had stopped treatment with ACE inhibitors before randomization [155]. However, in a large observational study on 127,081 Korean adults with preserved renal function, dietary intake of vitamins C, E and A was not significantly associated with the risk of CKD progression [156].
CKD is a state of deficiency and impaired metabolism of vitamin E. Defects in the intake, metabolism and antioxidant function of vitamin E contribute to the premature aging of CKD patients, increasing the uremic tissues’ susceptibility to OS (a phenomenon called “inflammaging”) and abnormal lipid peroxidation [157,158]. The potential benefits of vitamin E supplementation in CKD patients have been studied in various clinical trials, especially after Rasool et al. found an association between low serum vitamin E levels and CVD in ESKD patients [148]. In a post hoc analysis of the HOPE study (Heart Outcomes Prevention Evaluation), the 993 participants with mild–moderate CKD at high CV risk saw no apparent benefit on CV outcomes with daily vitamin E supplementation [159], and a large meta-analysis of 19 clinical trials with different study populations showed that high-dosage (≥400 IU/day) vitamin E supplementation may increase all-cause mortality, and therefore, its supplementation in chronic conditions should be considered with caution [160].
The SPACE study showed that high-dose vitamin E supplementation (800 IU/day vitamin E) in 196 HD patients with pre-existing CVD significantly reduced the risk of CVD events (especially myocardial infarction) compared to a placebo group [161]. Based on these findings, and to counteract the oxidative burst that is triggered from the blood coming into contact with artificial plastic materials during HD sessions, VECMs were developed. These membranes consist of a multilayer membrane bearing a hydrophilic polymer that strongly binds liposoluble vitamin E on the blood surface. In this way, the polymer acts as a powerful radical scavenger, protecting plasma lipids and cell membranes from peroxidative events. Furthermore, VECMs are more biocompatible and exert an in situ effect on blood cells, leading to decreased activation of circulating leukocytes, decreased endothelial cell activation and decreased OS [162].
Compared to conventional dialyzers, HD with VECMs significantly decreased inflammation (CRP), OS (oxLDL) and endothelial activation (soluble intercellular adhesion molecule, sICAM-1) [163], whereas a meta-analysis of 60 RCTs showed that VECMs improved the Erythropoetin (EPO) Resistance Index and reduced inflammation and OS, as assessed by a significant decrease in IL-6 levels, TBARS, plasma and RBC MDA, while providing similar dialysis adequacy, in comparison with conventional HD membranes [164]. However, the use of VECMs failed to show any beneficial effects on lipid profile parameters or carotid IMT in HD patients [165]. More recently, a novel idea of developing nanoparticles for targeted delivery of vitamin E to renal injury cells after ischemia/reperfusion [166] has emerged, suggesting that the role of vitamin E in kidney disease remains scientifically relevant and holds potential for future clinical applications.
Vitamin E is one of the most powerful antioxidants and a promising therapeutic target in HD. However, it should be noted that the results regarding the potential beneficial effects of its supplementation in HD are contradictory, and high-dosage (≥400 IU/day) vitamin E supplementation may increase all-cause mortality. The exact mechanisms underlying this association are not yet known, but its supplementation in chronic conditions should be considered with caution. This is why, currently, the KDIGO guidelines do not recommend vitamin E supplementation in ESKD patients. The use of VECMs is attractive because they significantly improve the biocompatibility of HD and might be clinically beneficial for HD patients. However, we need future RCTs with hard clinical endpoints (such as mortality and CVD) to evaluate the potential benefits and safety of this therapeutic approach.
3.4. Total Antioxidant Capacity (TAC)
TAC, also known as non-enzymatic total antioxidant capacity (vitamins, glutathione and uric acid), encompasses the synergistic interaction effects of all antioxidants in a given matrix (diet/foods or body fluids), and its measurement remains one of the most widely used methods to quantify the possible oxidant-buffering capacity of an organism [167]. The overall antioxidant capacity of the diet, which can be computed by a new tool called the dietary TAC (DTAC), defines the synergistic actions of all antioxidants, and has been associated with various clinical outcomes [168,169].
Abbasi et al. studied 210 (102 cases and 108 controls) patients with type 2 DM, classified based on their CKD status, and estimated DTAC based on the ferric-reducing antioxidant power of selected foods. No significant association was found between DTAC and CKD, thus suggesting that the effects of DTAC on the risk of CKD require further validation [170]. However, a larger study on 1747 subjects with CKD found that participants with higher DTAC scores had a higher GFR, while no association was revealed between higher DTAC and kidney stone formation [169]. Moreover, diets high in TAC were associated with a lower risk of incident CKD among subjects with hyperglycemia after 3 years of follow-up [171], but this association might be weakened in the elderly, suggesting that certain molecular mechanisms, such as OS, are heavily degraded with advanced age [172].
DTAC was associated with lower all-cause mortality among patients with CKD stages 1–2, but there was no apparent benefit for patients with stages 3–5 CKD or albuminuria, implying that as uremia progresses, more contraindicatory mechanisms might be activated [173]. Every HD session is accompanied by a significant decrease in TAC [174], which can be significantly increased via engagement in chronic intradialytic CV exercise [175].
3.5. N-Acetylcysteine (NAC)
NAC is one of the most widely studied antioxidants in clinical studies, animal experiments and cell culture experiments. NAC is a thiol-containing agent that acts as an antioxidant through various mechanisms; it scavenges oxidants directly, acts as a source of cysteine (Cys) for increased glutathione (GSH) biosynthesis and increases sulfane sulfur production [176]. NAC has been widely used as a mycolytic and for the prevention of contrast-induced nephropathy (CIN) [177]. Moreover, NAC is the mainstay of treatment for paracetamol overdose/poisoning. By restoring hepatic glutathione stores, NAC is a safe and quite effective antidote for paracetamol-induced hepatotoxicity. The antioxidant and anti-inflammatory effects of NAC have been studied mainly in experimental models. Medipally et al. found that NAC treatment could mitigate the degree of interstitial fibrosis/tubular atrophy in 5/6 nephrectomized mice with chronic hematuria by reducing OS in the kidney [178]. In an experimental DM model, NAC exhibited protective effects against DN [179], but in an attempt to associate OS parameters with mineral bone disorder in a rat model of progressive CKD, NAC failed to significantly improve bone architecture/geometry/mechanical properties [180].
Although the clinical data regarding the effect of NAC administration in CKD patients are limited, NAC has been studied as a renoprotective agent. Chiu et al., in a retrospective study, divided 554 patients with CKD stages 3–5, 1:1, into 2 groups: NAC users/NAC non-users. After 3 years, they reported that the incidence rate of reaching HD was significantly lower in NAC users than in non-NAC users, due to the modulation of serum creatinine and eGFR levels [181]. In a small placebo-controlled study with 20 non-diabetic patients with proteinuria and mildly decreased kidney function (up to stage III), oral supplementation of NAC—as an add-on therapy to renin–angiotensin–aldosterone system (RAAS) blockade—had no effect on proteinuria, surrogate markers of tubular injury or renal fibrosis [182]. A meta-analysis by Ye et al. showed that NAC supplementation is safe for CKD patients, and there was evidence of benefits on kidney function, inflammation markers and CV risk reduction [183].
In HD patients, oral NAC managed to reduce HSA-AOPP, the subsequent phagocyte oxidative responses [184], serum MDA [185] and plasma ADMA levels [186]. A systematic review by Coombes et al. concluded that NAC was the most efficacious supplement for ameliorating OS in patients undergoing HD [187]. Apart from its antioxidant properties, NAC has shown a favorable effect on uremic anemia. With a stable EPO dosage, NAC users on stable HD had a significant increase in hematocrit, accompanied by a decrease in plasma levels of 8-isoprostane and oxLDL, after 3 months of thrice-daily supplementation [188]. Finally, a prospective, randomized, placebo-controlled trial that included 134 maintenance HD patients showed that those in the NAC group (oral administration of NAC 600 mg/day) had a 40% reduction in the risk of reaching the primary CV endpoint (a composite variable consisting of cardiac events including fatal and nonfatal myocardial infarction, CVD death, need for coronary angioplasty or coronary bypass surgery, ischemic stroke, peripheral vascular disease with amputation or need for angioplasty), for a median follow-up period of 14.5 months [189].
In the setting of PD, experimental studies have provided some exciting results. The addition of NAC into the high-glucose compartment of neutral-pH-type PD fluids has been associated with a reduction in glucose degradation products, the main culprit of peritoneal membrane failure [190]. When human PMCs are exposed to high-dialysate glucose, there is a reduction in intracellular glutathione concentration, leading to cell dysfunction and apoptosis through mitochondrial damage, a process that can be avoided if NAC is added to the cell culture [191,192]. In patients undergoing PD, oral NAC supplementation was associated with a decrease in inflammatory biomarkers [193,194] and a positive effect on residual renal function [195]. However, in a placebo-controlled study that included 30 patients on regular PD, NAC treatment did not significantly affect OS markers (AOPPs, ADMA, etc.) [196], calling into question the antioxidant benefit of NAC use in PD patients.
Among all the available methods, NAC is probably the most promising exogenously administered antioxidant in HD and PD. Through its antioxidant, anti-inflammatory and anti-atherosclerotic effects, NAC has been associated with improved outcomes in these populations and no serious side effects. However, the existing data are of low quality, derived from either experimental or clinical studies with small sample sizes, short follow-up periods, surrogate endpoints and large heterogeneity in design. Currently, there is no consensus or guidelines recommending the supplementation of NAC in ESKD. However, this is an area of research of scientific interest. In the future, large RCTs examining clinical hard endpoints are expected to shed some light on this area.
4. Conclusions
Although OS has been at the center of research efforts for more than two decades, there are still more questions than answers in the case of CKD and ESKD. Free radicals and ROS are highly reactive and have a half-life of milliseconds, making them practically impossible to quantify. Alternatively, pro- and antioxidants are measured as surrogate markers of OS. However, there is no consensus on which markers to measure and how often in CKD and dialysis patients. Moreover, there is no universally accepted standard for measuring OS. Different laboratories may use different techniques, reagents or protocols to assess the same marker, leading to inconsistencies in results. Additionally, OS markers can vary widely between individuals, due to factors such as age, diet, lifestyle and underlying health conditions, which can further complicate comparisons across studies. The main drawback is that the reported correlations of various OS markers with diseases and conditions such as CVD and CKD are derived from small, low-quality observational studies, and thus, no causality can be established. Moreover, it is not clear whether OS is the cause or the consequence of CKD progression. Several conditions that predispose individuals to CKD onset and progression, such as advanced age, hypertension, obesity and diabetes, also trigger OS. On the other hand, OS, along with inflammation, has been shown to promote endothelial dysfunction, vascular calcification and CKD. Therefore, it could be hypothesized that the association between CKD and OS might be a chicken and egg paradigm.
Another unresolved issue is the fact that the clinical importance and relevance of OS in CKD settings have not yet been fully elucidated. In the future, larger RCTs examining the effect of exogenously administered antioxidants on hard clinical endpoints might shed some light on this area. In order to draw definitive conclusions for the possible clinical utility of OS markers, these trials should have a large sample size, long follow-up and homogeneity regarding the populations and the markers that are examined. Another issue that remains to be examined in future studies is the safety and adverse effects of antioxidant supplements in both CKD and ESKD populations. Finally, since in HD and PD, the main culprits of increased OS are the biocompatibility of the modalities, we desperately need the development of new, more biocompatible PD fluids and HD membranes, which will be examined in large, multicenter prospective studies. The data from these future studies will help us to understand the pathophysiology of OS, the clinical impact on hard clinical endpoints and the potential benefit from antioxidant supplementation in uremic patients.
Author Contributions
Conceptualization, S.R. and V.L.; methodology, A.S., A.V. and K.L.; writing—original draft preparation, S.R., A.T., I.E.N., A.S. and G.V.; writing—review and editing, A.V. and V.L.; supervision, K.L. and V.L. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Conflicts of Interest
The authors declare no conflicts of interest.
Abbreviations
| ACE | Angiotensin-converting enzyme |
| ADMA | Asymmetric dimethylarginine |
| AGEs | Advanced glycation end products |
| AKI | Acute kidney injury |
| AOPPs | Advanced oxidation protein products |
| AOPPs-RSA | Advanced oxidation protein product-modified rat serum albumin |
| AS | Arterial stiffness |
| baPWV | Brachial–ankle pulse wave velocity |
| BMI | Body mass index |
| BP | Blood pressure |
| CAC | Coronary artery calcification |
| CAPD | Continuous ambulatory peritoneal dialysis |
| CAT | Catalase |
| cDBP | Central diastolic blood pressure |
| cfPWV | Carotid–femoral pulse wave velocity |
| CIN | Contrast-induced nephropathy |
| CKD | Chronic kidney disease |
| cNOS | Constitutive nitric oxide synthase |
| CRP | C-reactive protein |
| cSBP | Central systolic blood pressure |
| CV | Cardiovascular |
| CVD | Cardiovascular disease |
| DGF | Delayed graft function |
| DM | Diabetes mellitus |
| DN | Diabetic nephropathy |
| DTAC | Dietary total antioxidant capacity |
| EC-SOD | Extracellular superoxide dismutase |
| eGFR | Estimated glomerular filtration rate |
| eNOS | Endothelial nitric oxide synthase |
| EPO | Erythropoietin |
| ESKD | End-stage kidney disease |
| FGF23 | Fibroblast growth factor 23 |
| FMD | Flow-mediated dilation |
| HbA1c | Glycated hemoglobin |
| HD | Hemodialysis |
| HDL | High-density lipoprotein |
| HMA | Human mercaptoalbumin |
| HNA | Human non-mercaptoalbumin |
| HNE | 4-hydroxy-2-nonenal |
| hOGG1 | Human 8-oxoguanine DNA glycosylase 1 |
| HPLC | High-performance liquid chromatography |
| HPMCs | Human peritoneal mesothelial cells |
| HSA | Human serum albumin |
| HT | Hypertension |
| ICAM | Intercellular adhesion molecule |
| IL- | Interleukin |
| IMA | Ischemia-modified albumin |
| IMT | Intima media thickness |
| LDL | Low-density lipoprotein |
| MDA | Malondialdehyde |
| MNC | Mononuclear cells |
| NAC | N-acetylcysteine |
| NADPH | Nicotinamide adenine dinucleotide phosphate hydrogen |
| NFκB | Nuclear factor κΒ |
| nNOS | Neuronal nitric oxide synthase |
| NO | Nitric oxide |
| NOS | Nitric oxide synthase |
| NT-proBNP | N-terminal pro-B-type natriuretic peptide |
| OS | Oxidative stress |
| Ox-LDL | Oxidized low-density lipoprotein |
| PAS | Peripheral arterial stiffness |
| PD | Peritoneal dialysis |
| pDBP | Peripheral diastolic blood pressure |
| PET | Peritoneal equilibration test |
| PMCs | Peritoneal mesothelial cells |
| Post-Tx | Post-transplantation |
| pSBP | Peripheral systolic blood pressure |
| PWV | Pulse wave velocity |
| RAAS | Renin–angiotensin–aldosterone system |
| RBC | Red blood cell |
| RCT | Randomized controlled trial |
| ROC | Receiver operating characteristic |
| ROS | Reactive oxygen species |
| sICAM | Soluble intercellular adhesion molecule |
| SOD | Superoxide dismutase |
| SSNS | Teroid-sensitive nephrotic syndrome |
| TAC | Total antioxidant capacity |
| TBARS | Thiobarbituric acid-reactive substances |
| TGF-β | Tumor growth factor β |
| TNF-α | Tumor necrosis factor α |
| UACR | Urine albumin–creatinine ratio |
| UAER | Urinary albumin excretion rate |
| VC | Vascular calcification |
| VCAM | Vascular cell adhesion molecule |
| VECM | Vitamin E-coated membrane |
| XDH | Xanthine dehydrogenase |
| XO | Xanthine oxidase |
| XOR | Xanthine oxidoreductase |
References
- Sinenko, S.A.; Starkova, T.Y.; Kuzmin, A.A.; Tomilin, A.N. Physiological Signaling Functions of Reactive Oxygen Species in Stem Cells: From Flies to Man. Front. Cell Dev. Biol. 2021, 9, 714370. [Google Scholar]
- Forrester, S.J.; Kikuchi, D.S.; Hernandes, M.S.; Xu, Q.; Griendling, K.K. Reactive Oxygen Species in Metabolic and Inflammatory Signaling. Circ. Res. 2018, 122, 877–902. [Google Scholar] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxidative Med. Cell. Longev. 2017, 2017, 8416763. [Google Scholar]
- Chen, Z.; Tian, R.; She, Z.; Cai, J.; Li, H. Role of oxidative stress in the pathogenesis of nonalcoholic fatty liver disease. Free Radic. Biol. Med. 2020, 152, 116–141. [Google Scholar]
- Haigis, M.C.; Yankner, B.A. The Aging Stress Response. Mol. Cell 2010, 40, 333–344. [Google Scholar]
- Sies, H. Strategies of antioxidant defense. Eur. J. Biochem. 1993, 215, 213–219. [Google Scholar]
- Hajam, Y.A.; Rani, R.; Ganie, S.Y.; Sheikh, T.A.; Javaid, D.; Qadri, S.S.; Pramodh, S.; Alsulimani, A.; Alkhanani, M.F.; Harakeh, S.; et al. Oxidative Stress in Human Pathology and Aging: Molecular Mechanisms and Perspectives. Cells 2022, 11, 552. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Roumeliotis, A.; Georgianos, P.I.; Stamou, A.; Manolopoulos, V.G.; Panagoutsos, S.; Liakopoulos, V. Oxidized LDL Is Associated with eGFR Decline in Proteinuric Diabetic Kidney Disease: A Cohort Study. Oxidative Med. Cell. Longev. 2021, 2021, 2968869. [Google Scholar]
- Roumeliotis, A.; Roumeliotis, S.; Tsetsos, F.; Georgitsi, M.; Georgianos, P.I.; Stamou, A.; Vasilakou, A.; Kotsa, K.; Tsekmekidou, X.; Paschou, P.; et al. Oxidative Stress Genes in Diabetes Mellitus Type 2: Association with Diabetic Kidney Disease. Oxidative Med. Cell. Longev. 2021, 2021, 2531062. [Google Scholar]
- Roumeliotis, S.; Georgianos, P.I.; Roumeliotis, A.; Eleftheriadis, T.; Stamou, A.; Manolopoulos, V.G.; Panagoutsos, S.; Liakopoulos, V. Oxidized LDL Modifies the Association between Proteinuria and Deterioration of Kidney Function in Proteinuric Diabetic Kidney Disease. Life 2021, 11, 504. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Roumeliotis, S.; Peschos, D.; Dounousi, E. Oxidative Stress in the Pathogenesis and Evolution of Chronic Kidney Disease: Untangling Ariadne’s Thread. Int. J. Mol. Sci. 2019, 20, 3711. [Google Scholar] [CrossRef]
- Jha, J.C.; Banal, C.; Chow, B.S.M.; Cooper, M.E.; Jandeleit-Dahm, K. Diabetes and Kidney Disease: Role of Oxidative Stress. Antioxid. Redox Signal. 2016, 25, 657–684. [Google Scholar] [PubMed]
- Oberg, B.P.; McMenamin, E.; Lucas, F.L.; McMonagle, E.; Morrow, J.; Ikizler, T.A.; Himmelfarb, J. Increased prevalence of oxidant stress and inflammation in patients with moderate to severe chronic kidney disease. Kidney Int. 2004, 65, 1009–1016. [Google Scholar] [PubMed]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Dounousi, E.; Mertens, P.R. Oxidative Stress in Hemodialysis Patients: A Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 3081856. [Google Scholar]
- Liakopoulos, V.; Roumeliotis, S.; Zarogiannis, S.; Eleftheriadis, T.; Mertens, P.R. Oxidative stress in hemodialysis: Causative mecha-nisms, clinical implications, and possible therapeutic interventions. Semin. Dial. 2019, 32, 58–71. [Google Scholar] [CrossRef]
- Morena, M.; Cristol, J.P.; Canaud, B. Why Hemodialysis Patients Are in a Prooxidant State? What Could Be Done to Correct the Pro/Antioxidant Imbalance. Blood Purif. 2000, 18, 191–199. [Google Scholar]
- Liakopoulos, V.; Roumeliotis, S.; Gorny, X.; Eleftheriadis, T.; Mertens, P.R. Oxidative Stress in Patients Undergoing Peritoneal Dialysis: A Current Review of the Literature. Oxidative Med. Cell. Longev. 2017, 2017, 3494867. [Google Scholar]
- Roumeliotis, S.; Dounousi, E.; Salmas, M.; Eleftheriadis, T.; Liakopoulos, V. Unfavorable Effects of Peritoneal Dialysis Solutions on the Peritoneal Membrane: The Role of Oxidative Stress. Biomolecules 2020, 10, 768. [Google Scholar] [CrossRef]
- Duni, A.; Liakopoulos, V.; Rapsomanikis, K.P.; Dounousi, E. Chronic Kidney Disease and Disproportionally Increased Cardiovas-cular Damage: Does Oxidative Stress Explain the Burden? Oxidative Med. Cell Longev. 2017, 2017, 9036450. [Google Scholar]
- Ling, X.C.; Kuo, K.L. Oxidative stress in chronic kidney disease. Ren. Replace. Ther. 2018, 4, 53. [Google Scholar] [CrossRef]
- Roumeliotis, S.; Mallamaci, F.; Zoccali, C. Endothelial Dysfunction in Chronic Kidney Disease, from Biology to Clinical Outcomes: A 2020 Update. J. Clin. Med. 2020, 9, 2359. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.; Zheng, L.; Xu, H.; Tang, D.; Lin, L.; Zhang, J.; Li, C.; Wang, W.; Yuan, Q.; Tao, L.; et al. Oxidative stress contributes to vascular calcification in patients with chronic kidney disease. J. Mol. Cell. Cardiol. 2020, 138, 256–268. [Google Scholar] [CrossRef]
- Tejchman, K.; Kotfis, K.; Sieńko, J. Biomarkers and Mechanisms of Oxidative Stress—Last 20 Years of Research with an Emphasis on Kidney Damage and Renal Transplantation. Int. J. Mol. Sci. 2021, 22, 8010. [Google Scholar] [CrossRef] [PubMed]
- Baralić, M.; Spasojević, I.; Miljuš, G.; Šunderić, M.; Robajac, D.; Dobrijević, Z.; Gligorijević, N.; Nedić, O.; Penezić, A. Albumin at the intersection between antioxidant and pro-oxidant in patients on peritoneal dialysis. Free Radic. Biol. Med. 2022, 187, 105–112. [Google Scholar]
- Figueroa, S.M.; Araos, P.; Reyes, J.; Gravez, B.; Barrera-Chimal, J.; Amador, C.A. Oxidized Albumin as a Mediator of Kidney Disease. Antioxidants 2021, 10, 404. [Google Scholar] [CrossRef] [PubMed]
- Jovanović, V.B.; Penezić-Romanjuk, A.Z.; Pavićević, I.D.; Aćimović, J.M.; Mandić, L.M. Improving the reliability of human serum al-bumin-thiol group determination. Anal. Biochem. 2013, 439, 17–22. [Google Scholar] [CrossRef]
- Watanabe, H. Oxidized Albumin: Evaluation of Oxidative Stress as a Marker for the Progression of Kidney Disease. Biol. Pharm. Bull. 2022, 45, 1728–1732. [Google Scholar] [CrossRef]
- Liu, B.; Yasukawa, K.; Koid, S.S.; Yeerbolati, A.; Reheman, L.; Wang, C.; Yatomi, Y.; Shimosawa, T. A rapid method for measuring serum oxidized albumin in a rat model of proteinuria and hypertension. Sci. Rep. 2019, 9, 8620. [Google Scholar]
- Liu, B.; Hu, Y.; Tian, D.; Dong, J.; Li, B.F. Assessing the effects of tempol on renal fibrosis, inflammation, and oxidative stress in a high-salt diet combined with 5/6 nephrectomy rat model: Utilizing oxidized albumin as a biomarker. BMC Nephrol. 2024, 25, 64. [Google Scholar]
- Terawaki, H.; Yoshimura, K.; Hasegawa, T.; Matsuyama, Y.; Negawa, T.; Yamada, K.; Matsushima, M.; Nakayama, M.; Hosoya, T.; Era, S. Oxidative stress is enhanced in correlation with renal dysfunction: Examination with the redox state of albumin. Kidney Int. 2004, 66, 1988–1993. [Google Scholar]
- Soejima, A.; Matsuzawa, N.; Hayashi, T.; Kimura, R.; Ootsuka, T.; Fukuoka, K.; Yamada, A.; Nagasawa, T.; Era, S. Alteration of Redox State of Human Serum Albumin before and after Hemodialysis. Blood Purif. 2004, 22, 525–529. [Google Scholar] [PubMed]
- Terawaki, H.; Takada, Y.; Era, S.; Funakoshi, Y.; Nakayama, K.; Nakayama, M.; Ogura, M.; Ito, S.; Hosoya, T. The Redox State of Albumin and Serious Car-diovascular Incidence in Hemodialysis Patients. Ther. Apher. Dial. 2010, 14, 465–471. [Google Scholar] [CrossRef]
- Lim, P.S.; Jeng, Y.; Wu, M.Y.; Pai, M.A.; Wu, T.K.; Liu, C.S.; Chen, C.H.; Kuo, Y.C.; Chien, S.W.; Chen, H.P. Serum oxidized albumin and cardiovascular mortality in normoalbuminemic hemodialysis patients: A cohort study. PLoS ONE 2013, 8, e70822. [Google Scholar] [CrossRef]
- Magzal, F.; Sela, S.; Szuchman-Sapir, A.; Tamir, S.; Michelis, R.; Kristal, B. In-vivo oxidized albumin– a pro-inflammatory agent in hypoalbuminemia. PLoS ONE 2017, 12, e0177799. [Google Scholar] [CrossRef]
- Bar–Or, D.; Lau, E.; Winkler, J.V. A novel assay for cobalt-albumin binding and its potential as a marker for myocardial ischemia—A preliminary report. J. Emerg. Med. 2000, 19, 311–315. [Google Scholar]
- Yücel, D. Ischemia—Modified albumin by albumin cobalt binding test: A false myth or reality. Turk. J. Biochem. 2023, 48, 1–4. [Google Scholar]
- Kıyıcı, A.; Mehmetoğlu, İ.; Karaoğlan, H.; Atalay, H.; Solak, Y.; Türk, S. Ischemia-Modified albumin levels in patients with end-stage renal disease patients on hemodialysis: Does albumin analysis method affect albumin-adjusted Ischemia-Modified albumin levels? J. Clin. Lab. Anal. 2010, 24, 273–277. [Google Scholar] [PubMed]
- Szulimowska, J.; Zalewska, A.; Taranta-Janusz, K.; Trocka, D.; Żendzian-Piotrowska, M.; Tomasiuk, R.; Maciejczyk, M. Association of Ischemia-Modified Albumin (IMA) in Saliva, Serum, and Urine with Diagnosis of Chronic Kidney Disease (CKD) in Children: A Case-Control Study. Med. Sci. Monit. 2023, 29, e942230. [Google Scholar] [CrossRef]
- Cakirca, G.; Guzelcicek, A.; Yilmaz, K.; Nas, C. Increased ischemia-modified albumin levels in children with steroid-sensitive nephrotic syndrome. Pak. J. Med. Sci. 2020, 36, 1490–1494. [Google Scholar] [CrossRef]
- Azouaou Toualbi, L.; Mounir, A.; Wafa, B.; Medina, A.; Abderrezak, K.; Chahine, T.; Henni, C.; Abdelghani, B.; Atmane, S. Implications of advanced oxidation protein products and vitamin E in atherosclerosis progression. Arch. Med. Sci.-Atheroscler. Dis. 2021, 6, 135–144. [Google Scholar] [CrossRef]
- Vinereanu, I.V.; Peride, I.; Niculae, A.; Tiron, A.T.; Caragheorgheopol, A.; Manda, D.; Checherita, I.A. The Relationship between Advanced Oxi-dation Protein Products, Vascular Calcifications and Arterial Stiffness in Predialysis Chronic Kidney Disease Patients. Medicina 2021, 57, 452. [Google Scholar] [CrossRef] [PubMed]
- Drüeke, T.; Witko-Sarsat, V.; Massy, Z.; Descamps-Latscha, B.; Guerin, A.P.; Marchais, S.J.; Gausson, V.; London, G.M. Iron therapy, advanced oxidation protein products, and carotid artery intima-media thickness in end-stage renal disease. Circulation 2002, 106, 2212–2217. [Google Scholar] [PubMed]
- Gonzalez, E.; Bajo, M.A.; Carrero, J.J.; Lindholm, B.; Grande, C.; Sánchez-Villanueva, R.; Del Peso, G.; Díaz-Almirón, M.; Iglesias, P.; Díez, J.J.; et al. An Increase of Plasma Advanced Oxidation Protein Products Levels Is Associated with Cardiovascular Risk in Incident Peritoneal Dialysis Patients: A Pilot Study. Oxidative Med. Cell. Longev. 2015, 2015, 219569. [Google Scholar]
- Xu, H.; Cabezas-Rodriguez, I.; Qureshi, A.R.; Heimburger, O.; Barany, P.; Snaedal, S.; Anderstam, B.; Helin, A.C.B.; Carrero, J.J.; Stenvinkel, P. Increased Levels of Modified Advanced Oxidation Protein Products Are Associated with Central and Peripheral Blood Pressure in Peritoneal Dialysis Patients. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2015, 35, 460–470. [Google Scholar]
- Kleinbongard, P.; Dejam, A.; Lauer, T.; Jax, T.; Kerber, S.; Gharini, P.; Balzer, J.; Zotz, R.B.; Scharf, R.E.; Willers, R.; et al. Plasma nitrite concentrations reflect the degree of endo-thelial dysfunction in humans. Free Radic. Biol. Med. 2006, 40, 295–302. [Google Scholar] [CrossRef]
- Hou, J.S.; Wang, C.H.; Lai, Y.H.; Kuo, C.H.; Lin, Y.L.; Hsu, B.G.; Tsai, J.P. Serum Malondialdehyde-Modified Low-Density Lipoprotein Is a Risk Factor for Central Arterial Stiffness in Maintenance Hemodialysis Patients. Nutrients 2020, 12, 2160. [Google Scholar] [CrossRef]
- Liu, W.N.; Hsu, Y.C.; Lu, C.W.; Lin, S.C.; Wu, T.J.; Lin, G.M. Serum Malondialdehyde-Modified Low-Density Lipoprotein as a Risk Marker for Peripheral Arterial Stiffness in Maintenance Hemodialysis Patients. Medicina 2024, 60, 697. [Google Scholar] [CrossRef]
- Jung, H.H.; Choi, D.H.; Lee, S.H. Serum malondialdehyde and coronary artery disease in hemodialysis patients. Am. J. Nephrol. 2004, 24, 537–542. [Google Scholar] [CrossRef]
- Conti, G.; Caccamo, D.; Siligato, R.; Gembillo, G.; Satta, E.; Pazzano, D.; Carucci, N.; Carella, A.; Del Campo, G.; Salvo, A.; et al. Association of Higher Advanced Oxidation Protein Products (AOPPs) Levels in Patients with Diabetic and Hypertensive Nephropathy. Medicina 2019, 55, 675. [Google Scholar] [CrossRef]
- Furuya, R.; Kumagai, H.; Odamaki, M.; Takahashi, M.; Miyaki, A.; Hishida, A. Impact of residual renal function on plasma levels of advanced oxidation protein products and pentosidine in peritoneal dialysis patients. Nephron Clin. Pract. 2009, 112, c255–c261. [Google Scholar] [CrossRef]
- Izemrane, D.; Benziane, A.; Makrelouf, M.; Hamdis, N.; Rabia, S.H.; Boudjellaba, S.; Baz, A.; Benaziza, D. Living donors kidney transplantation and oxidative stress: Nitric oxide as a predictive marker of graft function. PLoS ONE 2024, 19, e0307824. [Google Scholar]
- Tomás-Simó, P.; D’marco, L.; Romero-Parra, M.; Tormos-Muñoz, M.C.; Sáez, G.; Torregrosa, I.; Estañ-Capell, N.; Miguel, A.; Gorriz, J.L.; Puchades, M.J. Oxidative Stress in Non-Dialysis-Dependent Chronic Kidney Disease Patients. Int. J. Environ. Res. Public Health 2021, 18, 7806. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, S.; Suzuki, H.; Fujihara, K.; Kobayashi, K.; Iwasaki, H.; Sugano, Y.; Yatoh, S.; Sekiya, M.; Yahagi, N.; Shimano, H. Malondialdehyde-modified LDL-related variables are associated with diabetic kidney disease in type 2 diabetes. Diabetes Res. Clin. Pract. 2018, 141, 237–243. [Google Scholar] [PubMed]
- Fonseca, I.; Reguengo, H.; Almeida, M.; Dias, L.; Martins, L.S.; Pedroso, S.; Santos, J.; Lobato, L.; Henriques, A.C.; Mendonça, D. Oxidative Stress in Kidney Transplantation: Malondialdehyde Is an Early Predictive Marker of Graft Dysfunction. Transplantation 2014, 97, 1058–1065. [Google Scholar] [CrossRef]
- Kehm, R.; Baldensperger, T.; Raupbach, J.; Höhn, A. Protein oxidation—Formation mechanisms, detection and relevance as bi-omarkers in human diseases. Redox Biol. 2021, 42, 101901. [Google Scholar] [CrossRef]
- Spickett, C.M.; Pitt, A.R. Protein oxidation: Role in signalling and detection by mass spectrometry. Amino Acids 2012, 42, 5–21. [Google Scholar] [CrossRef]
- Witko-Sarsat, V.; Friedlander, M.; Capeillère-Blandin, C.; Nguyen-Khoa, T.; Nguyen, A.T.; Zingraff, J.; Jungers, P.; Descamps-Latscha, B. Advanced oxidation protein products as a novel marker of oxidative stress in uremia. Kidney Int. 1996, 49, 1304–1313. [Google Scholar]
- Bochi, G.V.; Torbitz, V.D.; Santos, R.C.V.; Cubillos-Rojas, M.; López, J.L.R.; Siebel, A.M.; Gomes, P.; de Oliveira, J.R.; Moresco, R.N. Fenton Reaction-Generated Advanced Oxidation Protein Products Induces Inflammation in Human Embryonic Kidney Cells. Inflammation 2016, 39, 1285–1290. [Google Scholar]
- Li, H.Y.; Hou, F.F.; Zhang, X.; Chen, P.Y.; Liu, S.X.; Feng, J.X.; Liu, Z.Q.; Shan, Y.X.; Wang, G.B.; Zhou, Z.M.; et al. Advanced oxidation protein products accelerate renal fibrosis in a remnant kidney model. J. Am. Soc. Nephrol. 2007, 18, 528–538. [Google Scholar] [CrossRef]
- Shi, X.Y.; Hou, F.F.; Niu, H.X.; Wang, G.B.; Xie, D.; Guo, Z.J.; Zhou, Z.M.; Yang, F.; Tian, J.W.; Zhang, X. Advanced oxidation protein products promote inflammation in diabetic kidney through activation of renal nicotinamide adenine dinucleotide phosphate oxidase. Endocrinology 2008, 149, 1829–1839. [Google Scholar]
- Kato, H.; Watanabe, H.; Imafuku, T.; Arimura, N.; Fujita, I.; Noguchi, I.; Tanaka, S.; Nakano, T.; Tokumaru, K.; Enoki, Y.; et al. Advanced oxidation protein products contribute to chronic kidney disease-induced muscle atrophy by inducing oxidative stress via CD36/NADPH oxidase pathway. J. Cachex-Sarcopenia Muscle 2021, 12, 1832–1847. [Google Scholar]
- Arimura, N.; Watanabe, H.; Kato, H.; Imafuku, T.; Nakano, T.; Sueyoshi, M.; Chikamatsu, M.; Tokumaru, K.; Nagasaki, T.; Maeda, H.; et al. Advanced Oxidation Protein Products Contribute to Chronic-Kidney-Disease-Induced Adipose Inflammation through Macrophage Activation. Toxins 2023, 15, 179. [Google Scholar] [CrossRef] [PubMed]
- Sung, C.C.; Hsu, Y.C.; Chen, C.C.; Lin, Y.F.; Wu, C.C. Oxidative Stress and Nucleic Acid Oxidation in Patients with Chronic Kidney Disease. Oxidative Med. Cell. Longev. 2013, 2013, 301982. [Google Scholar]
- Mohammedi, K.; Bellili-Muñoz, N.; Driss, F.; Roussel, R.; Seta, N.; Fumeron, F.; Hadjadj, S.; Marre, M.; Velho, G. Manganese superoxide dismutase (SOD2) polymorphisms, plasma advanced oxidation protein products (AOPP) concentration and risk of kidney complications in subjects with type 1 diabetes. PLoS ONE 2014, 9, e96916. [Google Scholar]
- Du, S.L.; Zeng, X.Z.; Tian, J.W.; Ai, J.; Wan, J.; He, J.X. Advanced oxidation protein products in predicting acute kidney injury following cardiac surgery. Biomarkers 2015, 20, 206–211. [Google Scholar] [CrossRef]
- Liang, X.; Chen, Y.; Zhuang, J.; Zhang, M.; Xiong, W.; Guo, H.; Jiang, F.; Hu, P.; Guo, D.; Shi, W. Advanced oxidation protein products as prognostic biomarkers for recovery from acute kidney injury after coronary artery bypass grafting. Biomarkers 2012, 17, 507–512. [Google Scholar]
- Lentini, P.; de Cal, M.; Cruz, D.; Chronopoulos, A.; Soni, S.; Nalesso, F.; Zanella, M.; Garzotto, F.; Brendolan, A.; Piccinni, P.; et al. The role of advanced oxidation protein products in intensive care unit patients with acute kidney injury. J. Crit. Care 2010, 25, 605–609. [Google Scholar]
- Witko-Sarsat, V.; Gausson, V.; Descamps-Latscha, B. Are advanced oxidation protein products potential uremic toxins? Kidney Int. 2003, 63, S11–S14. [Google Scholar]
- Colombo, G.; Reggiani, F.; Astori, E.; Altomare, A.; Finazzi, S.; Garavaglia, M.L.; Angelini, C.; Milzani, A.; Badalamenti, S.; Dalle-Donne, I. Advanced oxidation protein products in nondiabetic end stage renal disease patients on maintenance haemodialysis. Free Radic. Res. 2019, 53, 1114–1124. [Google Scholar]
- Kalousová, M.; Zima, T.; Tesař, V.; Sulková, S.; Fialová, L. Relationship between advanced glycoxidation end products, inflammatory markers/acute-phase reactants, and some autoantibodies in chronic hemodialysis patients. Kidney Int. 2003, 63, S62–S64. [Google Scholar]
- Zhou, Q.; Wu, S.; Jiang, J.; Tian, J.; Chen, J.; Yu, X.; Chen, P.; Mei, C.; Xiong, F.; Shi, W.; et al. Accumulation of circulating advanced oxidation protein products is an independent risk factor for ischaemic heart disease in maintenance haemodialysis patients. Nephrology 2012, 17, 642–649. [Google Scholar] [CrossRef] [PubMed]
- Zhou, C.; Zhang, Y.; Chen, J.; Mei, C.; Xiong, F.; Shi, W.; Zhou, W.; Liu, X.; Sun, S.; Tian, J.; et al. Association between serum advanced oxidation protein products and mortality risk in maintenance hemodialysis patients. J. Transl. Med. 2021, 19, 284. [Google Scholar] [CrossRef]
- Zuo, J.; Chaykovska, L.; Chu, C.; Chen, X.; Hasan, A.A.; Krämer, B.K.; Tepel, M.; Hocher, B. Head-to-Head Comparison of Oxidative Stress Biomarkers for All-Cause Mortality in Hemodialysis Patients. Antioxidants 2022, 11, 1975. [Google Scholar] [CrossRef] [PubMed]
- van Ypersele de Strihou, C.; Miyata, T. Advanced glycation and advanced oxidation protein products: The effect of peritoneal dialysis. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2006, 26, 185–187. [Google Scholar] [CrossRef]
- Furuya, R.; Odamaki, M.; Kumagai, H.; Hishida, A. Impact of angiotensin II receptor blocker on plasma levels of adiponectin and advanced oxidation protein products in peritoneal dialysis patients. Blood Purif. 2006, 24, 445–450. [Google Scholar] [CrossRef]
- Verma, S.; Singh, P.; Khurana, S.; Ganguly, N.K.; Kukreti, R.; Saso, L.; Rana, D.S.; Taneja, V.; Bhargava, V. Implications of oxidative stress in chronic kidney disease: A review on current concepts and therapies. Kidney Res. Clin. Pract. 2021, 40, 183–193. [Google Scholar] [CrossRef]
- Terawaki, H.; Murase, T.; Nakajima, A.; Aoyagi, K.; Fukushima, N.; Tani, Y.; Nakamura, T.; Kazama, J.J. The Relationship between Xanthine Oxidoreductase and Xanthine Oxidase Activities in Plasma and Kidney Dysfunction. J. Clin. Exp. Nephrol. 2017, 2, 31. [Google Scholar] [CrossRef]
- Terawaki, H.; Hayashi, T.; Murase, T.; Iijima, R.; Waki, K.; Tani, Y.; Nakamura, T.; Yoshimura, K.; Uchida, S.; Kazama, J.J. Relationship between Xanthine Oxidoreductase Redox and Oxidative Stress among Chronic Kidney Disease Patients. Oxidative Med. Cell. Longev. 2018, 2018, 9714710. [Google Scholar] [CrossRef]
- Gondouin, B.; Jourde-Chiche, N.; Sallee, M.; Dou, L.; Cerini, C.; Loundou, A.; Morange, S.; Berland, Y.; Burtey, S.; Brunet, P.; et al. Plasma Xanthine Oxidase Activity Is Predictive of Cardiovascular Disease in Patients with Chronic Kidney Disease, Independently of Uric Acid Levels. Nephron 2015, 131, 167–174. [Google Scholar] [CrossRef]
- Sun, M.; Hines, N.; Scerbo, D.; Buchanan, J.; Wu, C.; Ten Eyck, P.; Zepeda-Orozco, D.; Taylor, E.B.; Jalal, D.I. Allopurinol Lowers Serum Urate but Does Not Reduce Oxi-dative Stress in CKD. Antioxidants. 2022, 11, 1297. [Google Scholar] [CrossRef]
- Kim, Y.J.; Oh, S.H.; Ahn, J.S.; Yook, J.M.; Kim, C.D.; Park, S.H.; Cho, J.H.; Kim, Y.L. The Crucial Role of Xanthine Oxidase in CKD Progression Associated with Hypercholesterolemia. Int. J. Mol. Sci. 2020, 21, 7444. [Google Scholar] [CrossRef] [PubMed]
- Vanhoutte, P.M.; Zhao, Y.; Xu, A.; Leung, S.W.S. Thirty Years of Saying NO: Sources, Fate, Actions, and Misfortunes of the Endo-thelium-Derived Vasodilator Mediator. Circ. Res. 2016, 119, 375–396. [Google Scholar] [PubMed]
- Park, K.H.; Park, W.J. Endothelial Dysfunction: Clinical Implications in Cardiovascular Disease and Therapeutic Approaches. J. Korean Med. Sci. 2015, 30, 1213. [Google Scholar] [PubMed]
- Ollerstam, A.; Pittner, J.; E Persson, A.; Thorup, C. Increased blood pressure in rats after long-term inhibition of the neuronal isoform of nitric oxide synthase. J. Clin. Investig. 1997, 99, 2212–2218. [Google Scholar] [CrossRef]
- Klahr, S. The role of nitric oxide in hypertension and renal disease progression. Nephrol. Dial. Transplant. 2001, 16 (Suppl. S1), 60–62. [Google Scholar] [CrossRef]
- Prabhakar, S.; Starnes, J.; Shi, S.; Lonis, B.; Tran, R. Diabetic Nephropathy Is Associated with Oxidative Stress and Decreased Renal Nitric Oxide Production. J. Am. Soc. Nephrol. 2007, 18, 2945–2952. [Google Scholar] [PubMed]
- Kahveci, A.S.; Barnatan, T.T.; Kahveci, A.; Adrian, A.E.; Arroyo, J.; Eirin, A.; Harris, P.C.; Lerman, A.; Lerman, L.O.; Torres, V.E.; et al. Oxidative Stress and Mitochondrial Abnormalities Contribute to Decreased Endothelial Nitric Oxide Synthase Expression and Renal Disease Progression in Early Experimental Polycystic Kidney Disease. Int. J. Mol. Sci. 2020, 21, 1994. [Google Scholar] [CrossRef]
- Korish, A.A. Oxidative stress and nitric oxide deficiency in inflammation of chronic renal failure. Possible preventive role of L-arginine and multiple antioxidants. Saudi Med. J. 2009, 30, 1150–1157. [Google Scholar]
- Shirazi, M.K.; Azarnezhad, A.; Abazari, M.F.; Poorebrahim, M.; Ghoraeian, P.; Sanadgol, N.; Bokharaie, H.; Heydari, S.; Abbasi, A.; Kabiri, S.; et al. The role of nitric oxide signaling in renoprotective effects of hydrogen sulfide against chronic kidney disease in rats: Involvement of oxidative stress, autophagy and apoptosis. J. Cell. Physiol. 2019, 234, 11411–11423. [Google Scholar]
- Wang, D.; Strandgaard, S.; Iversen, J.; Wilcox, C.S. Asymmetric dimethylarginine, oxidative stress, and vascular nitric oxide synthase in essential hypertension. Am. J. Physiol.-Regul. Integr. Comp. Physiol. 2009, 296, R195–R200. [Google Scholar]
- Ahluwalia, A.; Gladwin, M.; Coleman, G.D.; Hord, N.; Howard, G.; Kim-Shapiro, D.B.; Lajous, M.; Larsen, F.J.; Lefer, D.J.; McClure, L.A.; et al. Dietary Nitrate and the Epidemiology of Cardiovascular Disease: Report From a National Heart, Lung, and Blood Institute Workshop. J. Am. Heart Assoc. 2016, 5, e003402. [Google Scholar] [PubMed]
- Carlstrom, M.; Montenegro, M.F. Therapeutic value of stimulating the nitrate-nitrite-nitric oxide pathway to attenuate oxidative stress and restore nitric oxide bioavailability in cardiorenal disease. J. Intern. Med. 2019, 285, 2–18. [Google Scholar] [CrossRef] [PubMed]
- Araujo, M.; Welch, W.J. Oxidative stress and nitric oxide in kidney function. Curr. Opin. Nephrol. Hypertens. 2006, 15, 72–77. [Google Scholar] [CrossRef]
- Bryan, N.S.; Torregrossa, A.C.; Mian, A.I.; Lindsey Berkson, D.; Westby, C.M.; Moncrief, J.W. Acute effects of hemodialysis on nitrite and nitrate: Potential cardiovascular implications in dialysis patients. Free Radic. Biol. Med. 2013, 58, 46–51. [Google Scholar]
- Gutiérrez-Prieto, J.A.; Soto-Vargas, J.; Parra-Michel, R.; Pazarín-Villaseñor, H.L.; García-Sánchez, A.; Miranda-Díaz, A.G. The Behavior of the Type of Peritoneal Transport in the Inflammatory and Oxidative Status in Adults Under Peritoneal Dialysis. Front. Med. 2019, 6, 210. [Google Scholar]
- Tripatara, P.; Patel, N.S.A.; Webb, A.; Rathod, K.; Lecomte, F.M.J.; Mazzon, E.; Cuzzocrea, S.; Yaqoob, M.M.; Ahluwalia, A.; Thiemermann, C. Nitrite-Derived Nitric Oxide Protects the Rat Kidney against Ischemia/Reperfusion Injury In Vivo: Role for Xanthine Oxidoreductase. J. Am. Soc. Nephrol. 2007, 18, 570–580. [Google Scholar] [CrossRef]
- Taso, O.V.; Philippou, A.; Moustogiannis, A.; Zevolis, E.; Koutsilieris, M. Lipid peroxidation products and their role in neurodegenerative diseases. Ann. Res. Hosp. 2019, 3, 2. [Google Scholar] [CrossRef]
- Cui, X.; Gong, J.; Han, H.; He, L.; Teng, Y.; Tetley, T.; Sinharay, R.; Chung, K.F.; Islam, T.; Gilliland, F.; et al. Relationship between free and total malondialdehyde, a well-established marker of oxidative stress, in various types of human biospecimens. J. Thorac. Dis. 2018, 10, 3088–3197. [Google Scholar]
- Tsikas, D. Assessment of lipid peroxidation by measuring malondialdehyde (MDA) and relatives in biological samples: Analytical and biological challenges. Anal. Biochem. 2017, 524, 13–30. [Google Scholar] [CrossRef]
- Niedernhofer, L.J.; Daniels, J.S.; Rouzer, C.A.; Greene, R.E.; Marnett, L.J. Malondialdehyde, a Product of Lipid Peroxidation, Is Muta-genic in Human Cells. J. Biol. Chem. 2003, 278, 31426–31433. [Google Scholar] [CrossRef]
- Lankin, V.Z.; Tikhaze, A.K.; Melkumyants, A.M. Malondialdehyde as an Important Key Factor of Molecular Mechanisms of Vascular Wall Damage under Heart Diseases Development. Int. J. Mol. Sci. 2022, 24, 128. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef] [PubMed]
- Koppara, N.; Medooru, K.; Yadagiri, L.; Vishnubotla, S.; Rapur, R.; Bitla, A. A study of oxidative stress, inflammation, and endothelial dysfunction in diabetic and nondiabetic chronic kidney disease pre-dialysis patients. Indian J. Nephrol. 2023, 33, 420. [Google Scholar] [PubMed]
- Ozden, M.; Maral, H.; Akaydin, D.; Cetinalp, P.; Kalender, B. Erythrocyte glutathione peroxidase activity, plasma malondialdehyde and erythrocyte glutathione levels in hemodialysis and CAPD patients. Clin. Biochem. 2002, 35, 269–273. [Google Scholar] [CrossRef]
- Papadea, P.; Kalaitzopoulou, E.; Skipitari, M.; Varemmenou, A.; Papasotiriou, M.; Papachristou, E.; Goumenos, D.; Grune, T.; Georgiou, C.D. Novel oxidized LDL-based clinical markers in peritoneal dialysis patients for atherosclerosis risk assessment. Redox Biol. 2023, 64, 102762. [Google Scholar] [CrossRef]
- Ben Omrane Sioud, O.; El Ati, Z.; Bouzidi, H.; Kerkeni, M.; Hammami, M. Lipid and oxidative profile in hemodialysis patients: Clinical follow-up for three years. Tunis. Médicale 2019, 97, 551–555. [Google Scholar]
- Silveira-Silva, P.C.; Silva, R.E.; Santos, E.C.; Justino, P.B.I.; Santos, M.P.; Gonçalves, R.V.; Novaes, R.D. Advanced glycosylation end products as metabolic predictors of systemic pro-inflammatory and prooxidant status in patients with end-stage renal disease. Cytokine 2023, 166, 156189. [Google Scholar] [CrossRef]
- Sangeetha Lakshmi, B.; Harini Devi, N.; Suchitra, M.M.; Srinivasa Rao, P.V.L.N.; Siva Kumar, V. Changes in the inflammatory and oxidative stress markers during a single hemodialysis session in patients with chronic kidney disease. Ren. Fail. 2018, 40, 534–540. [Google Scholar] [CrossRef]
- Hultqvist, M.; Hegbrant, J.; Nilsson-Thorell, C.; Lindholm, T.; Nilsson, P.; Lindén, T.; Hultqvist-Bengtsson, U. Plasma concentrations of vitamin C, vitamin E and/or malondialdehyde as markers of oxygen free radical production during hemodialysis. Clin. Nephrol. 1997, 47, 37–46. [Google Scholar]
- Steghens, J.P.; Combarnous, F.; Arkouche, W.; Flourie, F.; Hadj-Aissa, A. Influence of hemodialysis on total and free malondialde-hyde measured by a new HPLC method. Nephrol. Ther. 2005, 1, 121–125. [Google Scholar] [CrossRef]
- Silva, Í.C.; Marizeiro, D.F.; De Francesco Daher, E.; Veras De Sandes-Freitas, T.; Meneses, G.C.; Bezerra, G.F.; Libório, A.B.; Martins, A.M.C.; Campos, N.G. Correlation between functional capacity and oxidative stress and inflammation in hemodialysis patients. J. Bodyw. Mov. Ther. 2021, 27, 339–343. [Google Scholar] [CrossRef] [PubMed]
- Azouaou, L.T.; Adnane, M.; Khelfi, A.; Ballouti, W.; Arab, M.; Toualbi, C.; Chader, H.; Tahae, R.; Seba, A. Oxidative stress accelerates the carotid atherosclerosis process in patients with chronic kidney disease. Arch. Med. Sci.-Atheroscler. Dis. 2020, 5, 245–254. [Google Scholar]
- George, J.; Aron, A.; Levy, Y.; Gilburd, B.; Ben-David, A.; Renaudineau, Y.; Zonana-Nachach, A.; Youinou, P.; Harats, D.; Shoenfeld, Y. Anti-cardiolipin, anti-endothelial-cell and anti-malondialdehyde-LDL antibodies in uremic patients undergoing hemodialysis: Relationship with vascular access thrombosis and thromboembolic events. Hum. Antibodies 1999, 9, 125–131. [Google Scholar] [CrossRef]
- Boaz, M.; Matas, Z.; Biro, A.; Katzir, Z.; Green, M.; Fainaru, M.; Smetana, S. Serum malondialdehyde and prevalent cardiovascular disease in hemodialysis. Kidney Int. 1999, 56, 1078–1083. [Google Scholar]
- Boaz, M.; Matas, Z.; Biro, A.; Katzir, Z.; Green, M.; Fainaru, M.; Smetana, S. Comparison of hemostatic factors and serum malondialdehyde as predictive factors for cardiovascular disease in hemodialysis patients. Am. J. Kidney Dis. Off. J. Natl. Kidney Found. 1999, 34, 438–444. [Google Scholar]
- Vida, C.; Oliva, C.; Yuste, C.; Ceprián, N.; Caro, P.J.; Valera, G.; de Pablos, I.G.; Morales, E.; Carracedo, J. Oxidative Stress in Patients with Advanced CKD and Renal Replacement Therapy: The Key Role of Peripheral Blood Leukocytes. Antioxidants 2021, 10, 1155. [Google Scholar] [CrossRef] [PubMed]
- Nannapaneni, S.S.; Nimmanapalli, H.D.; Lakshmi, A.Y.; Vishnubotla, S.K. Markers of Oxidative Stress, Inflammation, and Endothelial Dysfunction in Diabetic and Nondiabetic Patients with Chronic Kidney Disease on Peritoneal Dialysis. Saudi J. Kidney Dis. Transplant. 2022, 33, 361–372. [Google Scholar] [CrossRef]
- Stepanova, N.; Korol, L.; Burdeyna, O. Oxidative stress in peritoneal dialysis patients: Association with the dialysis adequacy and technique survival. Indian J. Nephrol. 2019, 29, 309. [Google Scholar] [CrossRef]
- Basso, A.; Baldini, P.; Bertoldi, G.; Driussi, G.; Caputo, I.; Bettin, E.; Cacciapuoti, M.; Calò, L.A. Oxidative stress reduction by icodextrin-based glucose-free solutions in peritoneal dialysis: Support for new promising approaches. Artif. Organs 2024, 48, 1031–1037. [Google Scholar] [CrossRef]
- Kamijo, Y.; Wang, L.; Matsumoto, A.; Nakajima, T.; Hashimoto, K.; Higuchi, M.; Kyogashima, M.; Aoyama, T.; Hara, A. Long-term improvement of oxidative stress via kidney transplantation ameliorates serum sulfatide levels. Clin. Exp. Nephrol. 2012, 16, 959–967. [Google Scholar] [CrossRef]
- Nguyen, T.T.U.; Kim, H.W.; Kim, W. Effects of Probiotics, Prebiotics, and Synbiotics on Uremic Toxins, Inflammation, and Oxidative Stress in Hemodialysis Patients: A Systematic Review and Meta-Analysis of Randomized Controlled Trials. J. Clin. Med. 2021, 10, 4456. [Google Scholar] [CrossRef] [PubMed]
- Zhao, M.; Xiao, M.; Tan, Q.; Lyu, J.; Lu, F. The effect of aerobic exercise on oxidative stress in patients with chronic kidney disease: A systematic review and meta-analysis with trial sequential analysis. Ren. Fail. 2023, 45, 2252093. [Google Scholar] [CrossRef]
- Fattman, C.L.; Schaefer, L.M.; Oury, T.D. Extracellular superoxide dismutase in biology and medicine. Free Radic. Biol. Med. 2003, 35, 236–256. [Google Scholar] [CrossRef]
- Brownlee, M. Biochemistry and molecular cell biology of diabetic complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Kashem, A.; Endoh, M.; Yamauchi, F.; Yano, N.; Nomoto, Y.; Sakai, H.; Pronai, L.; Tanaka, M.; Nakazawa, H. Superoxide dismutase activity in human glomerulonephritis. Am. J. Kidney Dis. 1996, 28, 14–22. [Google Scholar] [CrossRef] [PubMed]
- Kitada, M.; Xu, J.; Ogura, Y.; Monno, I.; Koya, D. Manganese Superoxide Dismutase Dysfunction and the Pathogenesis of Kidney Disease. Front. Physiol. 2020, 11, 755. [Google Scholar] [CrossRef]
- Schneider, M.P.; Sullivan, J.C.; Wach, P.F.; Boesen, E.I.; Yamamoto, T.; Fukai, T.; Harrison, D.G.; Pollock, D.M.; Pollock, J.S. Protective role of extracellular superoxide dismutase in renal ischemia/reperfusion injury. Kidney Int. 2010, 78, 374–381. [Google Scholar] [CrossRef]
- Rodriguez-Iturbe, B.; Sepassi, L.; Quiroz, Y.; Ni, Z.; Vaziri, N.D. Association of mitochondrial SOD deficiency with salt-sensitive hypertension and accelerated renal senescence. J. Appl. Physiol. 2007, 102, 255–260. [Google Scholar] [CrossRef]
- Fujita, H.; Fujishima, H.; Chida, S.; Takahashi, K.; Qi, Z.; Kanetsuna, Y.; Breyer, M.D.; Harris, R.C.; Yamada, Y.; Takahashi, T. Reduction of Renal Superoxide Dismutase in Progressive Diabetic Nephropathy. J. Am. Soc. Nephrol. 2009, 20, 1303–1313. [Google Scholar] [CrossRef]
- González-Blázquez, R.; Somoza, B.; Gil-Ortega, M.; Martín Ramos, M.; Ramiro-Cortijo, D.; Vega-Martín, E.; Schulz, A.; Ruilope, L.M.; Kolkhof, P.; Kreutz, R.; et al. Finerenone Attenuates Endothelial Dysfunction and Albuminuria in a Chronic Kidney Disease Model by a Reduction in Oxidative Stress. Front. Pharmacol. 2018, 9, 1131. [Google Scholar] [CrossRef]
- Yu, X.; Xu, R.; Huang, W.; Lin, L.; Zheng, F.; Wu, X. Superoxide dismutase as a protective factor for microalbuminuria in hypertensive patients. Sci. Rep. 2022, 12, 20432. [Google Scholar]
- Krueger, K.; Shen, J.; Maier, A.; Tepel, M.; Scholze, A. Lower Superoxide Dismutase 2 (SOD2) Protein Content in Mononuclear Cells Is Associated with Better Survival in Patients with Hemodialysis Therapy. Oxidative Med. Cell. Longev. 2016, 2016, 7423249. [Google Scholar]
- Noleto Magalhães, R.C.; Guedes Borges de Araujo, C.; Batista de Sousa Lima, V.; Machado Moita Neto, J.; do Nascimento Nogueira, N.; do Nascimento Marreiro, D. Nutritional status of zinc and activity superoxide dismutase in chronic renal patients undergoing hemodialysis. Nutr. Hosp. 2011, 26, 1456–1461. [Google Scholar]
- Montazerifar, F.; Hashemi, M.; Karajibani, M.; Sanadgol, H.; Dikshit, M. Evaluation of lipid peroxidation and erythrocyte glutathione peroxidase and superoxide dismutase in hemodialysis patients. Saudi J. Kidney Dis. Transpl. Off. Publ. Saudi Cent. Organ. Transpl. Saudi Arab. 2012, 23, 274–279. [Google Scholar]
- Antunovic, T.; Stefanovic, A.; Ratkovic, M.; Gledovic, B.; Gligorovic-Barhanovic, N.; Bozovic, D.; Ivanisevic, J.; Prostran, M.; Stojanov, M. High uric acid and low superoxide dismutase as possible predictors of all-cause and cardiovascular mortality in hemodialysis patients. Int. Urol. Nephrol. 2013, 45, 1111–1119. [Google Scholar] [CrossRef] [PubMed]
- Nakamura, M.; Ando, Y.; Sasada, K.; Haraoka, K.; Ueda, M.; Okabe, H.; Motomiya, Y. Role of extracellular superoxide dismutase in patients under maintenance hemodialysis. Nephron Clin. Pract. 2005, 101, c109–c115. [Google Scholar]
- De Rojas, A.H.; Mateo, M.C.M. Superoxide Dismutase and Catalase Activities in Patients Undergoing Hemodialysis and Contin-uous Ambulatory Peritoneal Dialysis. Ren. Fail. 1996, 18, 937–946. [Google Scholar] [CrossRef]
- Shainkin-Kestenbaum, R.; Caruso, C.; Berlyne, G.M. Reduced Superoxide Dismutase Activity in Erythrocytes of Dialysis Patients: A Possible Factor in the Etiology of Uremic Anemia. Nephron 1990, 55, 251–253. [Google Scholar]
- Supriyadi, R.; Rakhmawati Kurniaatmaja, E.; Huang, I.; Sukesi, L.; Makmun, A. The effect of superoxide dismutase supplementation on TNF-α and TGF-β levels in patients undergoing hemodialysis. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 893–898. [Google Scholar]
- Washio, K.; Inagaki, M.; Tsuji, M.; Morio, Y.; Akiyama, S.; Gotoh, H.; Gotoh, T.; Gotoh, Y.; Oguchi, K. Oral vitamin C supplementation in hemodialysis patients and its effect on the plasma level of oxidized ascorbic acid and Cu/Zn superoxide dismutase, an oxidative stress marker. Nephron Clin. Pract. 2008, 109, c49–c54. [Google Scholar]
- Akiyama, S.; Inagaki, M.; Tsuji, M.; Gotoh, H.; Gotoh, T.; Washio, K.; Gotoh, Y.; Oguchi, K. Comparison of effect of vitamin E-coated dialyzer and oral vitamin E on hemodialysis-induced Cu/Zn-superoxide dismutase. Am. J. Nephrol. 2005, 25, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Carrillo-López, N.; Panizo, S.; Martín-Carro, B.; Barrallo, J.C.M.; Román-García, P.; García-Castro, R.; Fernández-Gómez, J.M.; Hevia-Suárez, M.Á.; Martín-Vírgala, J.; Fernández-Villabrille, S.; et al. Redox Metabolism and Vascular Calcification in Chronic Kidney Disease. Biomolecules 2023, 13, 1419. [Google Scholar] [CrossRef] [PubMed]
- Hishida, A.; Okada, R.; Naito, M.; Morita, E.; Wakai, K.; Hamajima, N.; Hosono, S.; Nanri, H.; Turin, T.C.; Suzuki, S.; et al. Polymorphisms in genes encoding antioxidant enzymes (SOD2, CAT, GPx, TXNRD, SEPP1, SEP15 and SELS) and risk of chronic kidney disease in Japanese-cross-sectional data from the J-MICC study. J. Clin. Biochem. Nutr. 2013, 53, 15–20. [Google Scholar] [CrossRef] [PubMed]
- Martínez Arias, L.; Panizo García, S.; Carrillo López, N.; Barrio Vázquez, S.; Quirós González, I.; Román García, P.; Mora Valenciano, I.; Miguel Fernández, D.; Añón Álvarez, E.; Fernández Martín, J.L.; et al. Efecto de la enzima antioxidante catalasa en la calcificación vascular y desmineralización ósea. Rev. Osteoporos. Metab. Miner. 2017, 9, 13–19. [Google Scholar] [CrossRef]
- Nogueira, F.N.; Romero, A.C.; Pedrosa, M.D.S.; Ibuki, F.K.; Bergamaschi, C.T. Oxidative stress and the antioxidant system in salivary glands of rats with experimental chronic kidney disease. Arch. Oral. Biol. 2020, 113, 104709. [Google Scholar] [CrossRef]
- Kobayashi, M.; Sugiyama, H.; Wang, D.H.; Toda, N.; Maeshima, Y.; Yamasaki, Y.; Masuoka, N.; Yamada, M.; Kira, S.; Makino, H. Catalase deficiency renders remnant kidneys more susceptible to oxidant tissue injury and renal fibrosis in mice. Kidney Int. 2005, 68, 1018–1031. [Google Scholar] [CrossRef]
- Chen, J.X.; Zhou, J.F.; Shen, H.C. Oxidative stress and damage induced by abnormal free radical reactions and IgA nephropathy. J. Zhejiang Univ.-Sci. B 2005, 6, 61–68. [Google Scholar] [CrossRef]
- Rasool, M.; Ashraf, M.A.B.; Malik, A.; Waquar, S.; Khan, S.A.; Qazi, M.H.; Ahmad, W.; Asif, M.; Khan, S.U.; Zaheer, A.; et al. Comparative study of extrapolative factors linked with oxidative injury and anti-inflammatory status in chronic kidney disease patients experiencing cardiovascular distress. PLoS ONE 2017, 12, e0171561. [Google Scholar] [CrossRef]
- Dursun, B.; Varan, H.I.; Dursun, E.; Ozben, T.; Suleymanlar, G. Acute effects of hemodialysis on oxidative stress parameters in chronic uremic patients: Comparison of two dialysis membranes. Int. J. Nephrol. Renov. Dis. 2010, 3, 39–45. [Google Scholar] [CrossRef]
- Stępniewska, J.; Dołęgowska, B.; Cecerska-Heryć, E.; Gołembiewska, E.; Malinowska-Jędraszczyk, A.; Marchelek-Myśliwiec, M.; Ciechanowski, K. The activity of antioxidant enzymes in blood platelets in different types of renal replacement therapy: A cross-sectional study. Int. Urol. Nephrol. 2016, 48, 593–599. [Google Scholar] [CrossRef]
- Choi, H.S.; Mathew, A.P.; Uthaman, S.; Vasukutty, A.; Kim, I.J.; Suh, S.H.; Kim, C.S.; Ma, S.K.; Graham, S.A.; Kim, S.W.; et al. Inflammation-sensing catalase-mimicking nanozymes alleviate acute kidney injury via reversing local oxidative stress. J. Nanobiotechnol. 2022, 20, 205. [Google Scholar]
- Baltusnikiene, A.; Staneviciene, I.; Jansen, E. Beneficial and adverse effects of vitamin E on the kidney. Front. Physiol. 2023, 14, 1145216. [Google Scholar]
- Karamouzis, I.; Sarafidis, P.A.; Karamouzis, M.; Iliadis, S.; Haidich, A.B.; Sioulis, A.; Triantos, A.; Vavatsi-Christaki, N.; Grekas, D.M. Increase in Oxidative Stress but Not in Antioxidant Capacity with Advancing Stages of Chronic Kidney Disease. Am. J. Nephrol. 2008, 28, 397–404. [Google Scholar] [CrossRef] [PubMed]
- Tain, Y.L.; Freshour, G.; Dikalova, A.; Griendling, K.; Baylis, C. Vitamin E reduces glomerulosclerosis, restores renal neuronal NOS, and suppresses oxidative stress in the 5/6 nephrectomized rat. Am. J. Physiol.-Ren. Physiol. 2007, 292, F1404–F1410. [Google Scholar]
- Gæde, P.; Poulsen, H.E.; Parving, H.-H.; Pedersen, O. Double-blind, randomised study of the effect of combined treatment with vitamin C and E on albuminuria in Type 2 diabetic patients. Diabet. Med. 2001, 18, 756–760. [Google Scholar]
- Park, S.K.; Oh, C.M.; Kim, E.; Jung, J.Y. Dietary Intake of Antioxidant Vitamins and Its Relation to the Progression of Chronic Kidney Disease in Adults With Preserved Renal Function. J. Ren. Nutr. 2024, 34, 438–446. [Google Scholar]
- Galli, F.; Bonomini, M.; Bartolini, D.; Zatini, L.; Reboldi, G.; Marcantonini, G.; Gentile, G.; Sirolli, V.; Di Pietro, N. Vitamin E (Alpha-Tocopherol) Metabolism and Nutrition in Chronic Kidney Disease. Antioxidants 2022, 11, 989. [Google Scholar] [CrossRef]
- Rojo-Trejo, M.H.; Robles-Osorio, M.L.; Sabath, E. Liposoluble vitamins A and E in kidney disease. World J. Nephrol. 2022, 11, 96–104. [Google Scholar] [CrossRef]
- Mann, J.F.E.; Lonn, E.M.; Yi, Q.; Gerstein, H.C.; Hoogwerf, B.J.; Pogue, J.; Bosch, J.; Dagenais, G.R.; Yusuf, S. Effects of vitamin E on cardiovascular outcomes in people with mild-to-moderate renal insufficiency: Results of the HOPE Study. Kidney Int. 2004, 65, 1375–1380. [Google Scholar]
- Miller, E.R.; Pastor-Barriuso, R.; Dalal, D.; Riemersma, R.A.; Appel, L.J.; Guallar, E. Meta-Analysis: High-Dosage Vitamin E Supplementation May Increase All-Cause Mortality. Ann. Intern. Med. 2005, 142, 37. [Google Scholar]
- Boaz, M.; Smetana, S.; Weinstein, T.; Matas, Z.; Gafter, U.; Iaina, A.; Knecht, A.; Weissgarten, Y.; Brunner, D.; Fainaru, M.; et al. Secondary prevention with antioxidants of cardiovascular disease in endstage renal disease (SPACE): Randomised placebo-controlled trial. Lancet 2000, 356, 1213–1218. [Google Scholar] [CrossRef]
- Panagiotou, A.; Nalesso, F.; Zanella, M.; Brendolan, A.; de Cal, M.; Cruz, D.; Basso, F.; Floris, M.; Clementi, A.; Ronco, C. Antioxidant Dialytic Approach with Vitamin E-Coated Membranes. Contrib. Nephrol. 2011, 171, 101–106. [Google Scholar] [PubMed]
- Kirmizis, D.; Papagianni, A.; Belechri, A.M.; Memmos, D. Effects of vitamin E-coated membrane dialyser on markers of oxidative stress and inflammation in patients on chronic haemodialysis. Nephrol. Dial. Transplant. 2011, 26, 2296–2301. [Google Scholar] [CrossRef]
- D’Arrigo, G.; Baggetta, R.; Tripepi, G.; Galli, F.; Bolignano, D. Effects of Vitamin E-Coated versus Conventional Membranes in Chronic Hemodialysis Patients: A Systematic Review and Meta-Analysis. Blood Purif. 2017, 43, 101–122. [Google Scholar] [CrossRef] [PubMed]
- Huang, J.; Yi, B.; Li, A.M.; Zhang, H. Effects of vitamin E-coated dialysis membranes on anemia, nutrition and dyslipidemia status in hemodialysis patients: A meta-analysis. Ren. Fail. 2015, 37, 398–407. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Ren, X.; Nie, Z.; You, Y.; Zhu, Y.; Chen, H.; Yu, H.; Mo, G.P.; Su, L.; Peng, Z.; et al. Dual-responsive renal injury cells targeting nanoparticles for vitamin E delivery to treat ischemia reperfusion-induced acute kidney injury. J. Nanobiotechnol. 2024, 22, 626. [Google Scholar]
- Silvestrini, A.; Meucci, E.; Ricerca, B.M.; Mancini, A. Total Antioxidant Capacity: Biochemical Aspects and Clinical Significance. Int. J. Mol. Sci. 2023, 24, 10978. [Google Scholar] [CrossRef]
- Mozaffari, H.; Daneshzad, E.; Surkan, P.J.; Azadbakht, L. Dietary Total Antioxidant Capacity and Cardiovascular Disease Risk Factors: A Systematic Review of Observational Studies. J. Am. Coll. Nutr. 2018, 37, 533–545. [Google Scholar] [CrossRef]
- Moludi, J.; Tandorost, A.; Kamari, N.; Abdollahzad, H.; Pakzad, R.; Najafi, F.; Pasdar, Y. Dietary total antioxidant capacity and its association with renal function and kidney stones: Results of a RaNCD cohort study. Food Sci. Nutr. 2022, 10, 1442–1450. [Google Scholar] [CrossRef]
- Abbasi, M.; Daneshpour, M.S.; Hedayati, M.; Mottaghi, A.; Pourvali, K.; Azizi, F. Dietary Total Antioxidant Capacity and the Risk of Chronic Kidney Disease in Patients With Type 2 Diabetes: A Nested Case-Control Study in the Tehran Lipid Glucose Study. J. Ren. Nutr. 2019, 29, 394–398. [Google Scholar] [CrossRef]
- Asghari, G.; Yuzbashian, E.; Shahemi, S.; Gaeini, Z.; Mirmiran, P.; Azizi, F. Dietary total antioxidant capacity and incidence of chronic kidney disease in subjects with dysglycemia: Tehran Lipid and Glucose Study. Eur. J. Nutr. 2018, 57, 2377–2385. [Google Scholar] [PubMed]
- Ghorbaninejad, P.; Mohammadpour, S.; Djafari, F.; Tajik, S.; Shab-Bidar, S. Dietary Total Antioxidant Capacity and Its Association with Renal Function and Progression of Chronic Kidney Disease in Older Adults: A Report from a Developing Country. Clin. Nutr. Res. 2020, 9, 296. [Google Scholar] [PubMed]
- Li, Y.; Ling, G.C.; Ni, R.B.; Ni, S.H.; Sun, S.N.; Liu, X.; Deng, J.P.; Ou-Yang, X.L.; Li, J.; Xian, S.X.; et al. Association of dietary total antioxidant capacity with all-cause and cardiovascular mortality in patients with chronic kidney disease: Based on two retrospective cohort studies of NHANES. Ren. Fail. 2023, 45, 2205950. [Google Scholar] [CrossRef]
- Clermont, G.; Lecour, S.; Lahet, J.J.; Siohan, P.; Vergely, C.; Chevet, D.; Rifle, G.; Rochette, L. Alteration in plasma antioxidant capacities in chronic renal failure and hemodialysis patients: A possible explanation for the increased cardiovascular risk in these patients. Cardiovasc. Res. 2000, 47, 618–623. [Google Scholar] [PubMed]
- Sovatzidis, A.; Chatzinikolaou, A.; Fatouros, I.G.; Panagoutsos, S.; Draganidis, D.; Nikolaidou, E.; Avloniti, A.; Michailidis, Y.; Mantzouridis, I.; Batrakoulis, A.; et al. Intradialytic Cardiovascular Exercise Training Alters Redox Status, Reduces Inflammation and Improves Physical Performance in Patients with Chronic Kidney Disease. Antioxidants 2020, 9, 868. [Google Scholar] [CrossRef]
- Ezeriņa, D.; Takano, Y.; Hanaoka, K.; Urano, Y.; Dick, T.P. N-Acetyl Cysteine Functions as a Fast-Acting Antioxidant by Triggering Intracellular H2S and Sulfane Sulfur Production. Cell Chem. Biol. 2018, 25, 447–459.e4. [Google Scholar] [CrossRef]
- Sharp, A.J.; Patel, N.; Reeves, B.C.; Angelini, G.D.; Fiorentino, F. Pharmacological interventions for the prevention of contrast-induced acute kidney injury in high-risk adult patients undergoing coronary angiography: A systematic review and meta-analysis of randomised controlled trials. Open Heart 2019, 6, e000864. [Google Scholar]
- Medipally, A.; Xiao, M.; Satoskar, A.A.; Biederman, L.; Dasgupta, A.; Ivanov, I.; Mikhalina, G.; Rovin, B.; Brodsky, S.V. N-acetylcysteine ameliorates hematuria-associated tubulointerstitial injury in 5/6 nephrectomy mice. Physiol. Rep. 2023, 11, e15767. [Google Scholar]
- Yalçın, A.; Gürel, A. Effects of N-acetylcysteine on kidney tissue, matrix metalloproteinase-2, irisin and oxidative stress in a dia-betes mellitus model. Biotech. Histochem. 2021, 96, 616–622. [Google Scholar]
- Allen, M.R.; Wallace, J.; McNerney, E.; Nyman, J.; Avin, K.; Chen, N.; Moe, S. N-acetylcysteine (NAC), an anti-oxidant, does not improve bone mechanical properties in a rat model of progressive chronic kidney disease-mineral bone disorder. PLoS ONE 2020, 15, e0230379. [Google Scholar]
- Chiu, A.H.; Wang, C.J.; Lin, Y.L.; Wang, C.L.; Chiang, T.I. N-Acetylcysteine Alleviates the Progression of Chronic Kidney Disease: A Three-Year Cohort Study. Medicina 2023, 59, 1983. [Google Scholar] [CrossRef]
- Renke, M.; Tylicki, L.; Rutkowski, P.; Larczyński, W.; Aleksandrowicz, E.; Łysiak-Szydłowska, W.; Rutkowski, B. The effect of N-acetylcysteine on proteinuria and markers of tubular injury in non-diabetic patients with chronic kidney disease. A placebo-controlled, randomized, open, cross-over study. Kidney Blood Press. Res. 2008, 31, 404–410. [Google Scholar] [CrossRef] [PubMed]
- Ye, M.; Lin, W.; Zheng, J.; Lin, S. N-acetylcysteine for chronic kidney disease: A systematic review and meta-analysis. Am. J. Transl. Res. 2021, 13, 2472–2485. [Google Scholar] [PubMed]
- Witko-Sarsat, V.; Gausson, V.; Nguyen, A.T.; Touam, M.; Drüeke, T.; Santangelo, F.; Descamps-Latscha, B. AOPP-induced activation of human neu-trophil and monocyte oxidative metabolism: A potential target for N-acetylcysteine treatment in dialysis patients. Kidney Int. 2003, 64, 82–91. [Google Scholar] [CrossRef]
- Trimarchi, H.; Mongitore, M.; Baglioni, P.; Forrester, M.; Freixas, E.A.R.; Schropp, M.; Pereyra, H.; Alonso, M. N-acetylcysteine reduces malondialdehyde levels in chronic hemodialysis patients—A pilot study. Clin. Nephrol. 2003, 59, 441–446. [Google Scholar] [CrossRef] [PubMed]
- Thaha, M.; Widodo Pranawa, W.; Yogiantoro, M.; Tomino, Y. Intravenous N-acetylcysteine during hemodialysis reduces asym-metric dimethylarginine level in end-stage renal disease patients. Clin. Nephrol. 2008, 69, 24–32. [Google Scholar] [CrossRef]
- Coombes, J.S.; Fassett, R.G. Antioxidant therapy in hemodialysis patients: A systematic review. Kidney Int. 2012, 81, 233–246. [Google Scholar] [CrossRef]
- Hsu, S.P.; Chiang, C.K.; Yang, S.Y.; Chien, C.T. N-acetylcysteine for the management of anemia and oxidative stress in hemodialysis patients. Nephron Clin. Pract. 2010, 116, c207–c216. [Google Scholar] [CrossRef]
- Tepel, M.; van der Giet, M.; Statz, M.; Jankowski, J.; Zidek, W. The antioxidant acetylcysteine reduces cardiovascular events in patients with end-stage renal failure. A randomized, controlled trial. Circulation 2003, 107, 992–995. [Google Scholar] [CrossRef]
- Lee, E.; Seo, E.Y.; Kwon, Y.; Ha, H. Rapid and Reliable Measurement for Evaluating Directly the Reactivity of N-Acetylcysteine with Glucose Degradation Products in Peritoneal Dialysis Fluids. Anal. Chem. 2011, 83, 1518–1522. [Google Scholar] [CrossRef]
- Hung, K.Y.; Liu, S.Y.; Yang, T.C.; Liao, T.L.; Kao, S.H. High-Dialysate-Glucose-Induced Oxidative Stress and Mitochondrial-Mediated Apoptosis in Human Peritoneal Mesothelial Cells. Oxidative Med. Cell. Longev. 2014, 2014, 642793. [Google Scholar]
- Kuo, H.T.; Lee, J.J.; Hsiao, H.H.; Chen, H.W.; Chen, H.C. N-Acetylcysteine Prevents Mitochondria from Oxidative Injury Induced by Conventional Peritoneal Dialysate in Human Peritoneal Mesothelial Cells. Am. J. Nephrol. 2009, 30, 179–185. [Google Scholar] [PubMed]
- Purwanto, B.; Prasetyo, D.H. Effect of oral N-acetylcysteine treatment on immune system in continuous ambulatory peritoneal dialysis patients. Acta Medica Indones. 2012, 44, 140–144. [Google Scholar]
- Najafi, F.; Mousavi-Roknabadi, R.S.; Pirdehghan, A.; Rahimian, M.; Nourimajalan, N. Effect of N-Acetylcysteine on hsCRP in Patients on Continues Ambulatory Peritoneal Dialysis: A Quasi-Experimental Study. Nephro-Urol. Mon. 2021, 13, e113990. [Google Scholar]
- Feldman, L.; Shani, M.; Efrati, S.; Beberashvili, I.; Yakov–Hai, I.; Abramov, E.; Sinuani, I.; Rosenberg, R.; Weissgarten, J. N-Acetylcysteine Improves Residual Renal Function in Peritoneal Dialysis Patients: A Pilot Study. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2011, 31, 545–550. [Google Scholar] [CrossRef]
- Nascimento, M.M.; Suliman, M.E.; Silva, M.; Chinaglia, T.; Marchioro, J.; Hayashi, S.Y.; Riella, M.C.; Lindholm, B.; Anderstam, B. Effect of Oral N-Acetylcysteine Treatment on Plasma Inflammatory and Oxidative Stress Markers in Peritoneal Dialysis Patients: A Placebo-Controlled Study. Perit. Dial. Int. J. Int. Soc. Perit. Dial. 2010, 30, 336–342. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).