Rescue of a Rotenone Model of Parkinson’s Disease in C. elegans by the Mitochondrial Na+/Ca2+ Exchanger Inhibitor CGP37157
Abstract
1. Introduction
2. Results
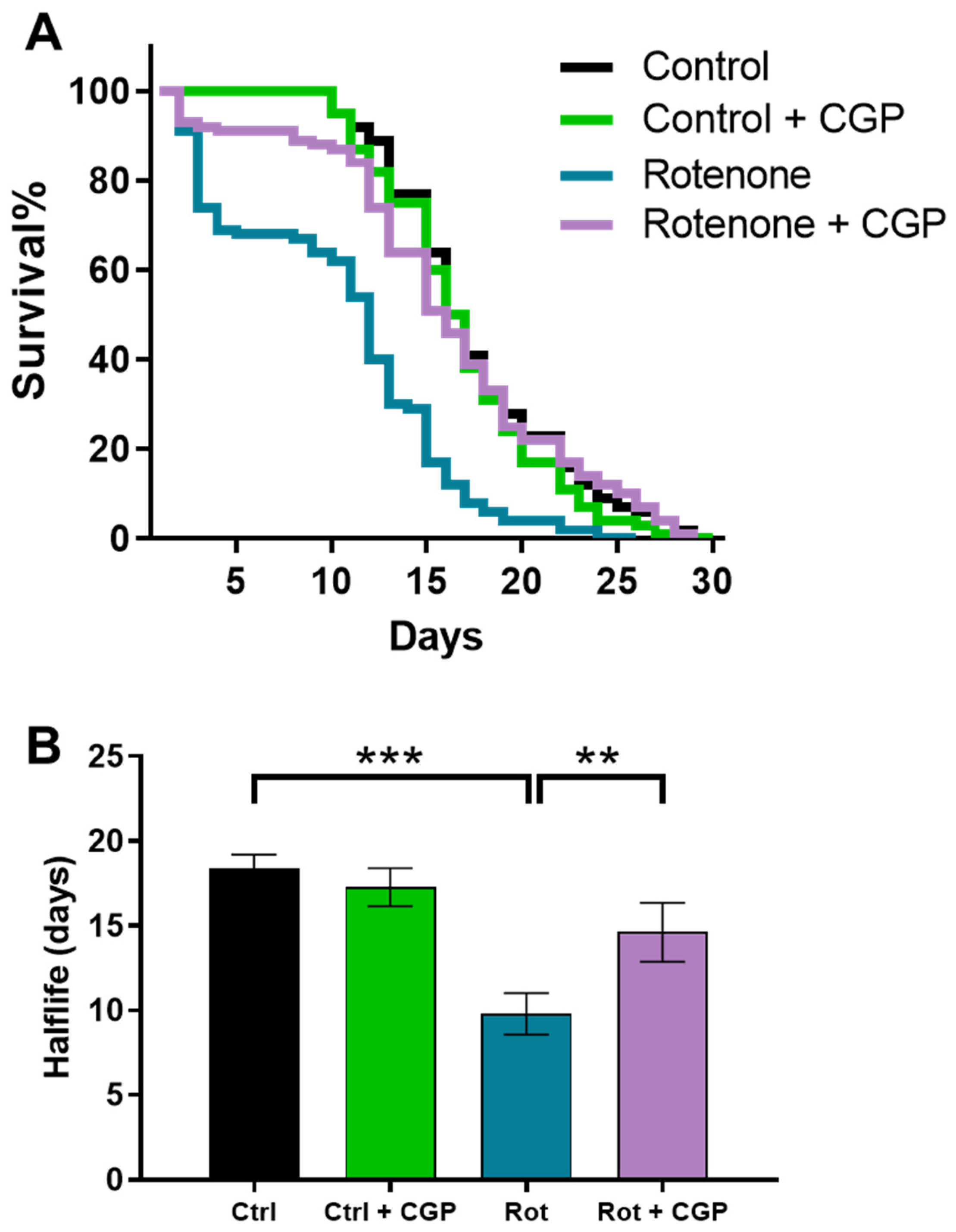
2.1. Effects on Lifespan
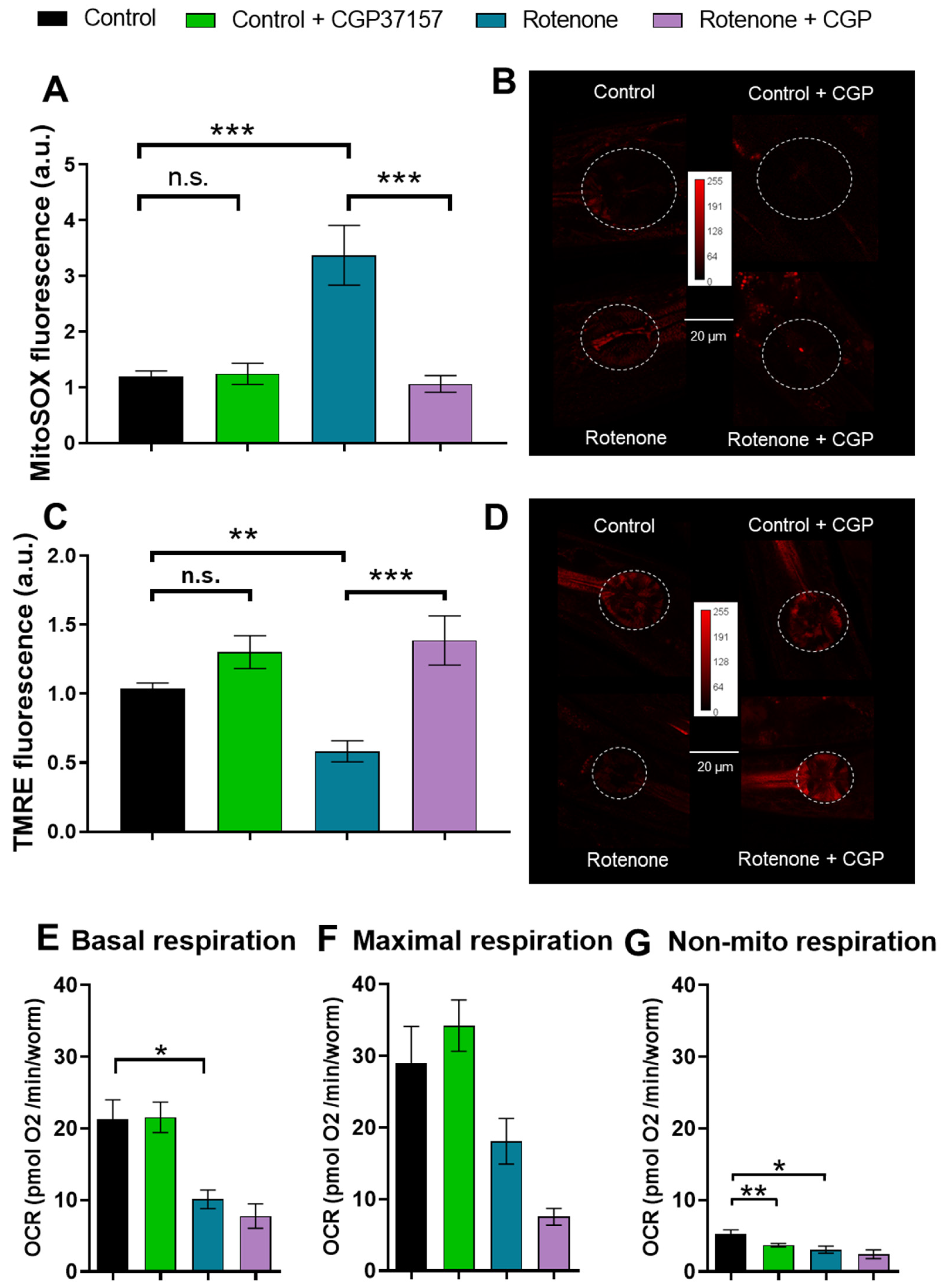
2.2. Effects on ROS Production
2.3. Effects on Mitochondrial Membrane Potential
2.4. Effects on Oxygen Consumption Rate
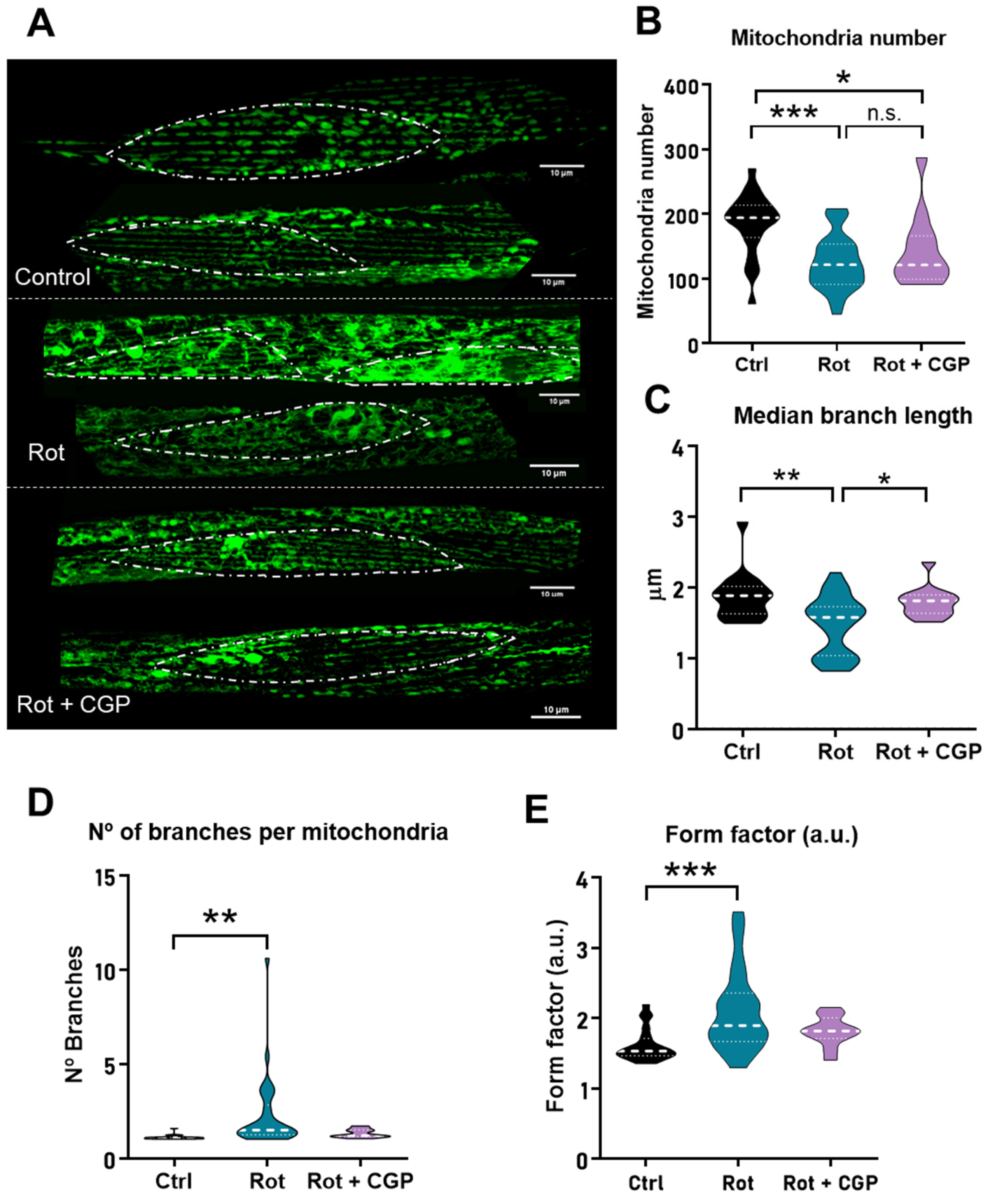
2.5. Effects on Mitochondrial Structure
2.6. Effects on Dopaminergic Neurons
2.7. Effects on Associative Learning
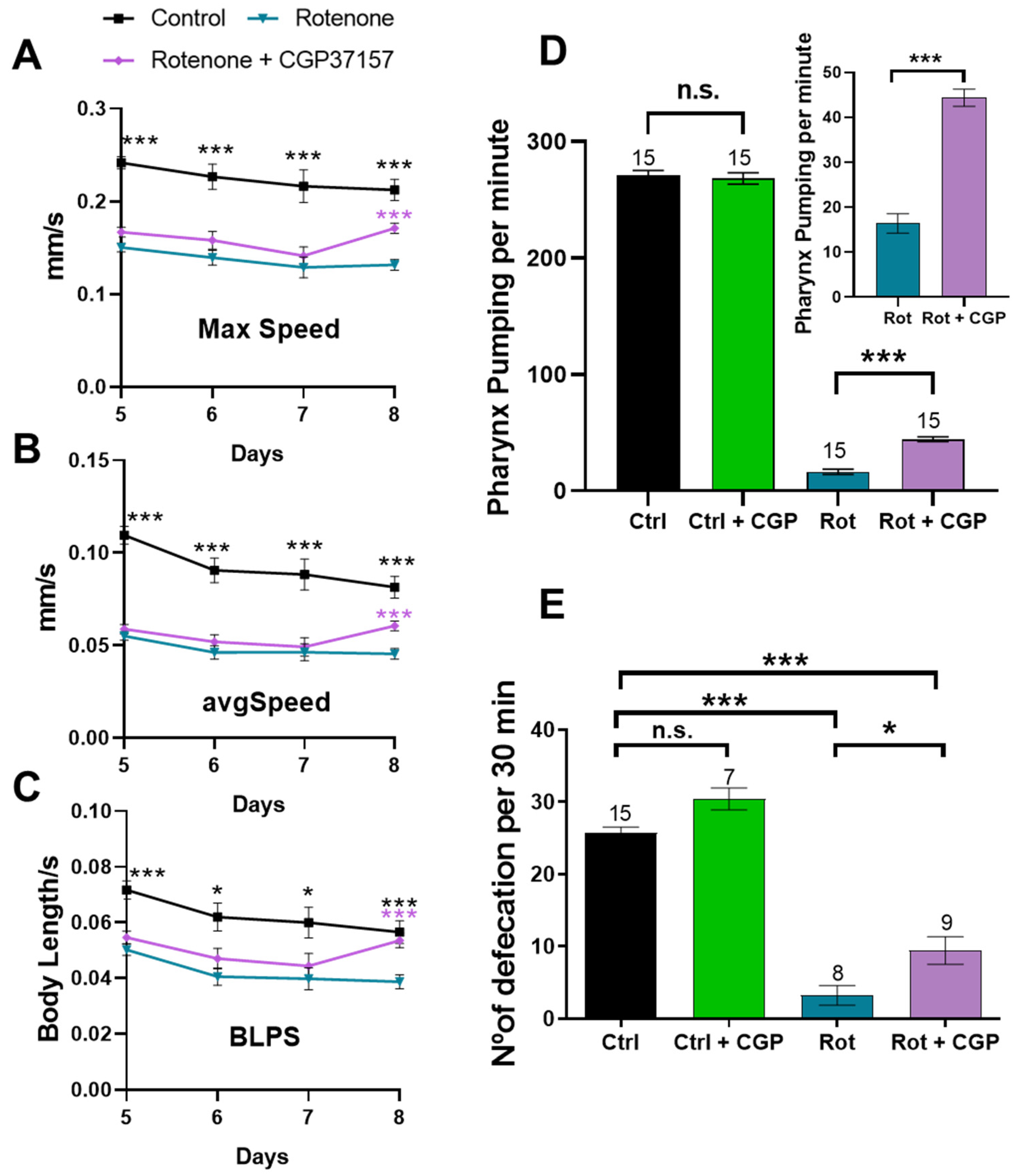
2.8. Effects on Muscle Activity: Mobility, Pharynx Pumping, and Defecation
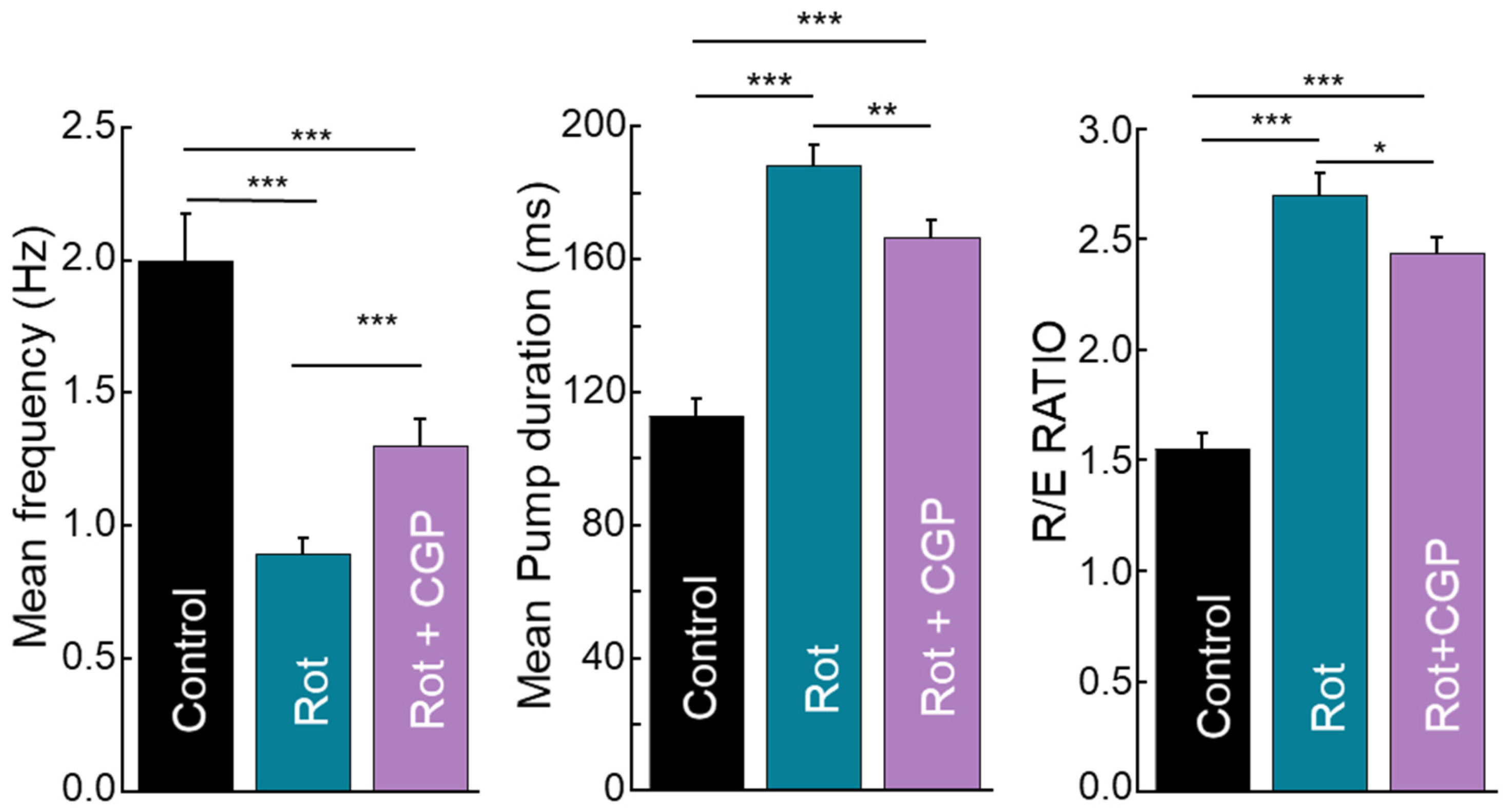
2.9. Effects on Electropharyngeogram
2.10. Effects on Size and Fertility
3. Discussion
4. Materials and Methods
4.1. C. elegans Strains and Maintenance
4.2. Administration of Rotenone and CGP37157
4.3. C. elegans Lifespan Assays
4.4. Mitochondrial ROS
4.5. Mitochondrial Membrane Potential
4.6. Measurement of Oxygen Consumption Rate
4.7. Mitochondrial Structure
4.8. Fluorescence from Dopaminergic Neurons
4.9. Associative Learning
4.10. Tracking and Mobility
4.11. Pharynx Pumping
4.12. Defecation
4.13. Electropharyngeogram
4.14. Fertility
4.15. Materials
4.16. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Poewe, W.; Seppi, K.; Tanner, C.M.; Halliday, G.M.; Brundin, P.; Volkmann, J.; Schrag, A.E.; Lang, A.E. Parkinson disease. Nat. Rev. Dis. Prim. 2017, 3, 17013. [Google Scholar] [PubMed]
- Thirugnanam, T.; Santhakumar, K. Chemically induced models of Parkinson’s disease. Comp. Biochem. Physiol. C Toxicol. Pharmacol. 2022, 252, 109213. [Google Scholar]
- Langston, J.W. The MPTP Story. J. Park. Dis. 2017, 7, S11–S19. [Google Scholar] [CrossRef]
- Zeng, X.S.; Geng, W.S.; Jia, J.J. Neurotoxin-Induced Animal Models of Parkinson Disease: Pathogenic Mechanism and Assessment. ASN Neuro 2018, 10, 1759091418777438. [Google Scholar] [PubMed]
- Chia, S.J.; Tan, E.K.; Chao, Y.X. Historical Perspective: Models of Parkinson’s Disease. Int. J. Mol. Sci. 2020, 21, 2464. [Google Scholar] [CrossRef] [PubMed]
- Ibarra-Gutiérrez, M.T.; Serrano-García, N.; Orozco-Ibarra, M. Rotenone-Induced Model of Parkinson’s Disease: Beyond Mitochondrial Complex I Inhibition. Mol. Neurobiol. 2023, 60, 1929–1948. [Google Scholar]
- Sherer, T.B.; Betarbet, R.; Testa, C.M.; Seo, B.B.; Richardson, J.R.; Kim, J.H.; Miller, G.W.; Yagi, T.; Matsuno-Yagi, A.; Greenamyre, J.T. Mechanism of toxicity in rotenone models of Parkinson’s disease. J. Neurosci. 2003, 23, 10756–10764. [Google Scholar]
- Sanders, L.H.; Greenamyren, J.T. Oxidative damage to macromolecules in human Parkinson disease and the rotenone model. Free Radic. Biol. Med. 2013, 62, 111–120. [Google Scholar]
- Greenamyre, J.T.; Cannon, J.R.; Drolet, R.; Mastroberardino, P.G. Lessons from the rotenone model of Parkinson’s disease. Trends Pharmacol. Sci. 2010, 31, 141–142. [Google Scholar]
- Gonzalez-Hunt, C.P.; Luz, A.L.; Ryde, I.T.; Turner, E.A.; Ilkayeva, O.R.; Bhatt, D.P.; Hirschey, M.D.; Meyer, J.N. Multiple metabolic changes mediate the response of Caenorhabditis elegans to the complex I inhibitor rotenone. Toxicology 2021, 447, 152630. [Google Scholar]
- Martinez, B.A.; Caldwell, K.A.; Caldwell, G.A. C. elegans as a model system to accelerate discovery for Parkinson disease. Curr. Opin. Genet. Dev. 2017, 44, 102–109. [Google Scholar] [CrossRef] [PubMed]
- da Silva, L.P.D.; da Cruz Guedes, E.; Fernandes, I.C.O.; Pedroza, L.A.L.; da Silva Pereira, G.J.; Gubert, P. Exploring Caenorhabditis elegans as Parkinson’s Disease Model: Neurotoxins and Genetic Implications. Neurotox. Res. 2024, 42, 11. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Minobe, E.; Kameyama, M. Ca2+ Dyshomeostasis Links Risk Factors to Neurodegeneration in Parkinson’s Disease. Front. Cell. Neurosci. 2022, 16, 867385. [Google Scholar]
- Imberechts, D.; Kinnart, I.; Wauters, F.; Terbeek, J.; Manders, L.; Wierda, K.; Eggermont, K.; Madeiro, R.F.; Sue, C.; Verfaillie, C.; et al. DJ-1 is an essential downstream mediator in PINK1/parkin-dependent mitophagy. Brain 2022, 145, 4368–4384. [Google Scholar]
- Lim, D.; Dematteis, G.; Tapella, L.; Genazzani, A.A.; Calì, T.; Brini, M.; Verkhratsky, A. Ca2+ handling at the mitochondria-ER contact sites in neurodegeneration. Cell Calcium 2021, 98, 102453. [Google Scholar]
- Barazzuol, L.; Giamogante, F.; Calì, T. Mitochondria Associated Membranes (MAMs): Architecture and physiopathological role. Cell Calcium 2021, 94, 102343. [Google Scholar]
- Betzer, C.; Lassen, L.B.; Olsen, A.; Kofoed, R.H.; Reimer, L.; Gregersen, E.; Zheng, J.; Calì, T.; Gai, W.; Chen, T.; et al. Alpha-synuclein aggregates activate calcium pump SERCA leading to calcium dysregulation. EMBO Rep. 2018, 19, e44617. [Google Scholar]
- García-Casas, P.; Arias-del-Val, J.; Alvarez-Illera, P.; Fonteriz, R.I.; Montero, M.; Alvarez, J. Inhibition of Sarco-Endoplasmic Reticulum Ca2+ ATPase Extends the Lifespan in C. elegans Worms. Front. Pharmacol. 2018, 9, 669. [Google Scholar] [CrossRef]
- García-Casas, P.; Arias-Del-Val, J.; Alvarez-Illera, P.; Wojnicz, A.; De Los Ríos, C.; Fonteriz, R.I.; Montero, M.; Alvarez, J. The Neuroprotector Benzothiazepine CGP37157 Extends Lifespan in C. elegans Worms. Front. Aging Neurosci. 2019, 10, 440. [Google Scholar]
- García-Casas, P.; Alvarez-Illera, P.; Fonteriz, R.I.; Montero, M.; Alvarez, J. Mechanism of the lifespan extension induced by submaximal SERCA inhibition in C. elegans. Mech. Ageing Dev. 2021, 196, 111474. [Google Scholar] [CrossRef]
- García-Casas, P.; Alvarez-Illera, P.; Gómez-Orte, E.; Cabello, J.; Fonteriz, R.I.; Montero, M.; Alvarez, J. The Mitochondrial Na+/Ca2+ Exchanger Inhibitor CGP37157 Preserves Muscle Structure and Function to Increase Lifespan and Healthspan in Caenorhabditis elegans. Front. Pharmacol. 2021, 12, 695687. [Google Scholar]
- Romero-Sanz, S.; Caldero-Escudero, E.; Álvarez-Illera, P.; Santo-Domingo, J.; Fonteriz, R.I.; Montero, M.; Álvarez, J. SERCA inhibition improves lifespan and healthspan in a chemical model of Parkinson disease in Caenorhabditis elegans. Front. Pharmacol. 2023, 14, 1182428. [Google Scholar] [CrossRef] [PubMed]
- Vrijsen, S.; Besora-Casals, L.; Van Veen, S.; Zielich, J.; Van Den Haute, C.; Hamouda, N.N.; Fischer, C.; Ghesquière, B.; Tournev, I.; Agostinis, P.; et al. ATP13A2-mediated endo-lysosomal polyamine export counters mitochondrial oxidative stress. Proc. Nat. Acad. Sci. USA 2020, 117, 31198–31207. [Google Scholar] [PubMed]
- Nass, R.; Hall, D.H.; Miller, D.M.; Blakely, R.D. Neurotoxin-induced degeneration of dopamine neurons in Caenorhabditis elegans. Proc. Nat. Acad. Sci. USA 2002, 99, 3264–3269. [Google Scholar] [PubMed]
- Maulik, M.; Mitra, S.; Bult-Ito, A.; Taylor, B.E.; Vayndorf, E.M. Behavioral Phenotyping and Pathological Indicators of Parkinson’s Disease in C. elegans Models. Front. Genet. 2017, 8, 77. [Google Scholar]
- Smith, L.L.; Ryde, I.T.; Hartman, J.H.; Romersi, R.F.; Markovich, Z.; Meyer, J.N. Strengths and limitations of morphological and behavioral analyses in detecting dopaminergic deficiency in Caenorhabditis elegans. Neurotoxicology 2019, 74, 209–220. [Google Scholar] [CrossRef]
- Lee, J.; Jee, C.; McIntire, S.L. Ethanol Preference in C. elegans. Genes Brain Behav. 2009, 8, 578. [Google Scholar]
- Thomas, J.H. Genetic analysis of defecation in Caenorhabditis elegans. Genetics 1990, 124, 855–872. [Google Scholar]
- Baron, K.T.; Thayer, S.A. CGP37157 modulates mitochondrial Ca2+ homeostasis in cultured rat dorsal root ganglion neurons. Eur. J. Pharmacol. 1997, 340, 295–300. [Google Scholar]
- Moreno-Ortega, A.J.; Martínez-Sanz, F.J.; Lajarín-Cuesta, R.; De Los Rios, C.; Cano-Abad, M.F. Benzothiazepine CGP37157 and its 2′-isopropyl analogue modulate Ca2+ entry through CALHM1. Neuropharmacology 2015, 95, 503–510. [Google Scholar] [CrossRef]
- Czyz, A.; Kiedrowski, L. Inhibition of plasmalemmal Na+/Ca2+ exchange by mitochondrial Na+/Ca2+ exchange inhibitor 7-chloro-5-(2-chlorophenyl)-1,5-dihydro-4,1-benzothiazepin-2(3H)-one (CGP-37157) in cerebellar granule cells. Biochem. Pharmacol. 2003, 66, 2409–2411. [Google Scholar] [PubMed]
- Nicolau, S.M.; De Diego, A.M.G.; Cortés, L.; Egea, J.; González, J.C.; Mosquera, M.; López, M.G.; Hernández-Guijo, J.M.; García, A.G. Mitochondrial Na+/Ca2+-exchanger blocker CGP37157 protects against chromaffin cell death elicited by veratridine. J. Pharmacol. Exp. Ther. 2009, 330, 844–854. [Google Scholar] [PubMed]
- Nicolau, S.M.; Egea, J.; López, M.G.; García, A.G. Mitochondrial Na+/Ca2+ exchanger, a new target for neuroprotection in rat hippocampal slices. Biochem. Biophys. Res. Commun. 2010, 400, 140–144. [Google Scholar] [PubMed]
- González-Lafuente, L.; Egea, J.; León, R.; Martínez-Sanz, F.J.; Monjas, L.; Perez, C.; Merino, C.; García-De Diego, A.M.; Rodríguez-Franco, M.I.; García, A.G.; et al. Benzothiazepine CGP37157 and its isosteric 2′-ethyl analogue provide neuroprotection and block cell calcium entry. ACS Chem. Neurosci. 2012, 3, 519–529. [Google Scholar] [CrossRef] [PubMed]
- Martínez-Sanz, F.J.; Lajarín-Cuesta, R.; Moreno-Ortega, A.J.; González-Lafuente, L.; Fernández-Morales, J.C.; López-Arribas, R.; Cano-Abad, M.F.; De Los Ríos, C. Benzothiazepine CGP37157 Analogues Exert Cytoprotection in Various in Vitro Models of Neurodegeneration. ACS Chem. Neurosci. 2015, 6, 1626–1636. [Google Scholar] [CrossRef]
- Ruiz, A.; Alberdi, E.; Matute, C. CGP37157, an inhibitor of the mitochondrial Na+/Ca2+ exchanger, protects neurons from excitotoxicity by blocking voltage-gated Ca2+ channels. Cell Death Dis. 2014, 5, e1156. [Google Scholar] [PubMed]
- Bastioli, G.; Piccirillo, S.; Castaldo, P.; Magi, S.; Tozzi, A.; Amoroso, S.; Calabresi, P. Selective inhibition of mitochondrial sodium-calcium exchanger protects striatal neurons from α-synuclein plus rotenone induced toxicity. Cell Death Dis. 2019, 10, 80. [Google Scholar]
- Zhang, Z.; Shen, Y.; Luo, H.; Zhang, F.; Peng, D.; Jing, L.; Wu, Y.; Xia, X.; Song, Y.; Li, W.; et al. MANF protects dopamine neurons and locomotion defects from a human α-synuclein induced Parkinson’s disease model in C. elegans by regulating ER stress and autophagy pathways. Exp. Neurol. 2018, 308, 59–71. [Google Scholar]
- Alvarez, J.; Alvarez-Illera, P.; Santo-Domingo, J.; Fonteriz, R.I.; Montero, M. Modeling Alzheimer’s Disease in Caenorhabditis elegans. Biomedicines 2022, 10, 288. [Google Scholar] [CrossRef]
- Stiernagle, T. Maintenance of C. elegans. In WormBook; WormBook Research Community: Pasadena, CA, USA, 2006; pp. 1–11. [Google Scholar]
- Luz, A.L.; Smith, L.L.; Rooney, J.P.; Meyer, J.N. Seahorse Xfe 24 Extracellular Flux Analyzer-Based Analysis of Cellular Respiration in Caenorhabditis elegans. Curr. Protoc. Toxicol. 2015, 66, 25.7.1–25.7.15. [Google Scholar]
- Chaudhry, A.; Shi, R.; Luciani, D.S. A pipeline for multidimensional confocal analysis of mitochondrial morphology, function, and dynamics in pancreatic β-cells. Am. J. Physiol.-Endocrinol. Metab. 2020, 318, E87. [Google Scholar] [CrossRef] [PubMed]
- Nussbaum-Krammer, C.I.; Neto, M.F.; Brielmann, R.M.; Pedersen, J.S.; Morimoto, R.I. Investigating the spreading and toxicity of prion-like proteins using the metazoan model organism C. Elegans. J. Vis. Exp. 2015, 95, e52321. [Google Scholar]
- Álvarez-Illera, P.; García-Casas, P.; Fonteriz, R.I.; Montero, M.; Alvarez, J. Mitochondrial Ca2+ dynamics in MCU knockout C. Elegans worms. Int. J. Mol. Sci. 2020, 21, 8622. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Romero-Sanz, S.; Caldero-Escudero, E.; Álvarez-Illera, P.; Santo-Domingo, J.; de la Fuente, S.; García-Casas, P.; Fonteriz, R.I.; Montero, M.; Álvarez, J. Rescue of a Rotenone Model of Parkinson’s Disease in C. elegans by the Mitochondrial Na+/Ca2+ Exchanger Inhibitor CGP37157. Int. J. Mol. Sci. 2025, 26, 3371. https://doi.org/10.3390/ijms26073371
Romero-Sanz S, Caldero-Escudero E, Álvarez-Illera P, Santo-Domingo J, de la Fuente S, García-Casas P, Fonteriz RI, Montero M, Álvarez J. Rescue of a Rotenone Model of Parkinson’s Disease in C. elegans by the Mitochondrial Na+/Ca2+ Exchanger Inhibitor CGP37157. International Journal of Molecular Sciences. 2025; 26(7):3371. https://doi.org/10.3390/ijms26073371
Chicago/Turabian StyleRomero-Sanz, Silvia, Elena Caldero-Escudero, Pilar Álvarez-Illera, Jaime Santo-Domingo, Sergio de la Fuente, Paloma García-Casas, Rosalba I. Fonteriz, Mayte Montero, and Javier Álvarez. 2025. "Rescue of a Rotenone Model of Parkinson’s Disease in C. elegans by the Mitochondrial Na+/Ca2+ Exchanger Inhibitor CGP37157" International Journal of Molecular Sciences 26, no. 7: 3371. https://doi.org/10.3390/ijms26073371
APA StyleRomero-Sanz, S., Caldero-Escudero, E., Álvarez-Illera, P., Santo-Domingo, J., de la Fuente, S., García-Casas, P., Fonteriz, R. I., Montero, M., & Álvarez, J. (2025). Rescue of a Rotenone Model of Parkinson’s Disease in C. elegans by the Mitochondrial Na+/Ca2+ Exchanger Inhibitor CGP37157. International Journal of Molecular Sciences, 26(7), 3371. https://doi.org/10.3390/ijms26073371










