Recent Progress in N-Acylethanolamine Research: Biological Functions and Metabolism Regulated by Two Distinct N-Acyltransferases: cPLA2ε and PLAAT Enzymes
Abstract
1. Introduction
2. cPLA2ε
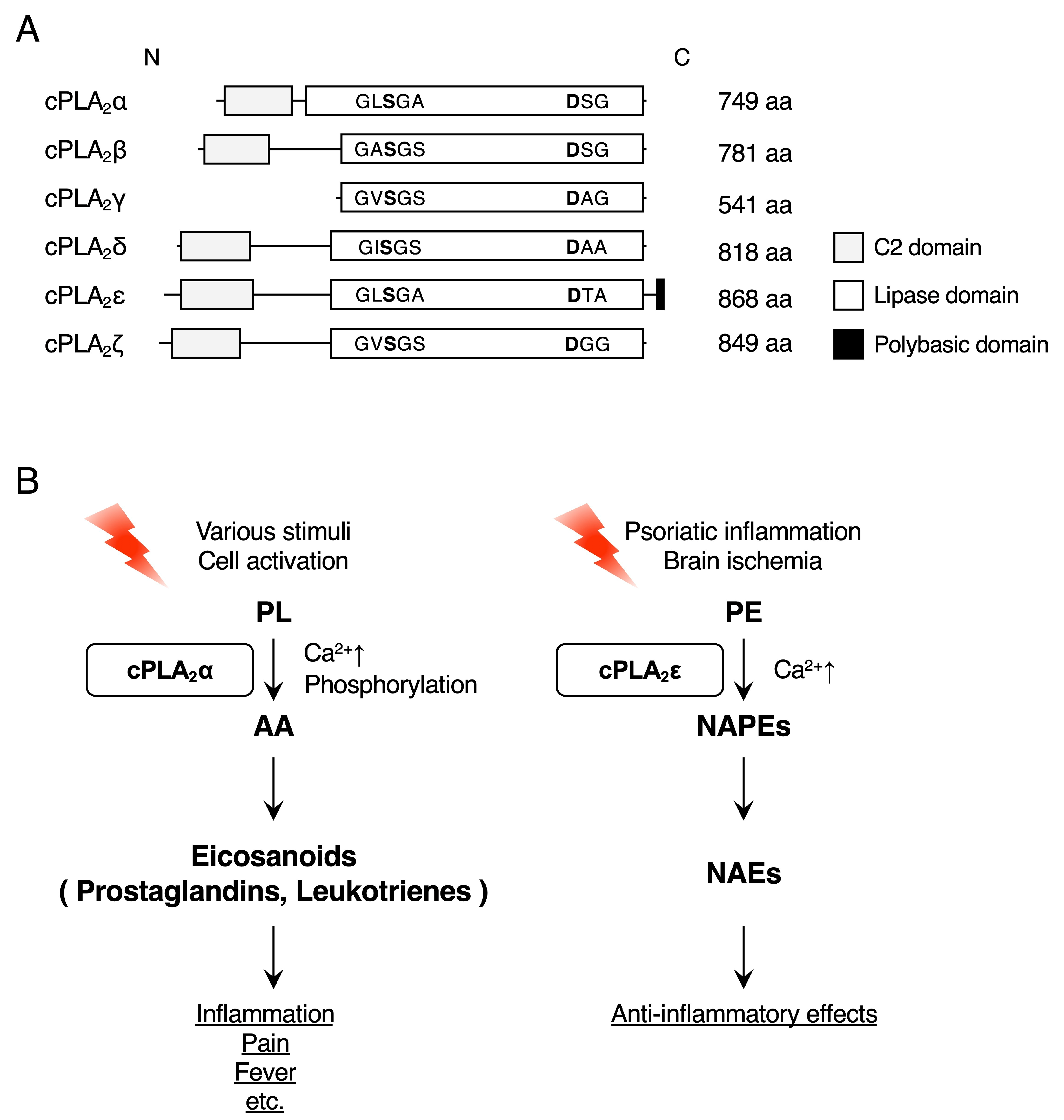
2.1. cPLA2ε Functions as a Ca2+-Dependent N-Acyltransferase
2.2. cPLA2ε in the Brain
2.2.1. Neonatal Development
2.2.2. Ischemia
2.3. cPLA2ε in the Skin
2.4. cPLA2ε in Other Tissues
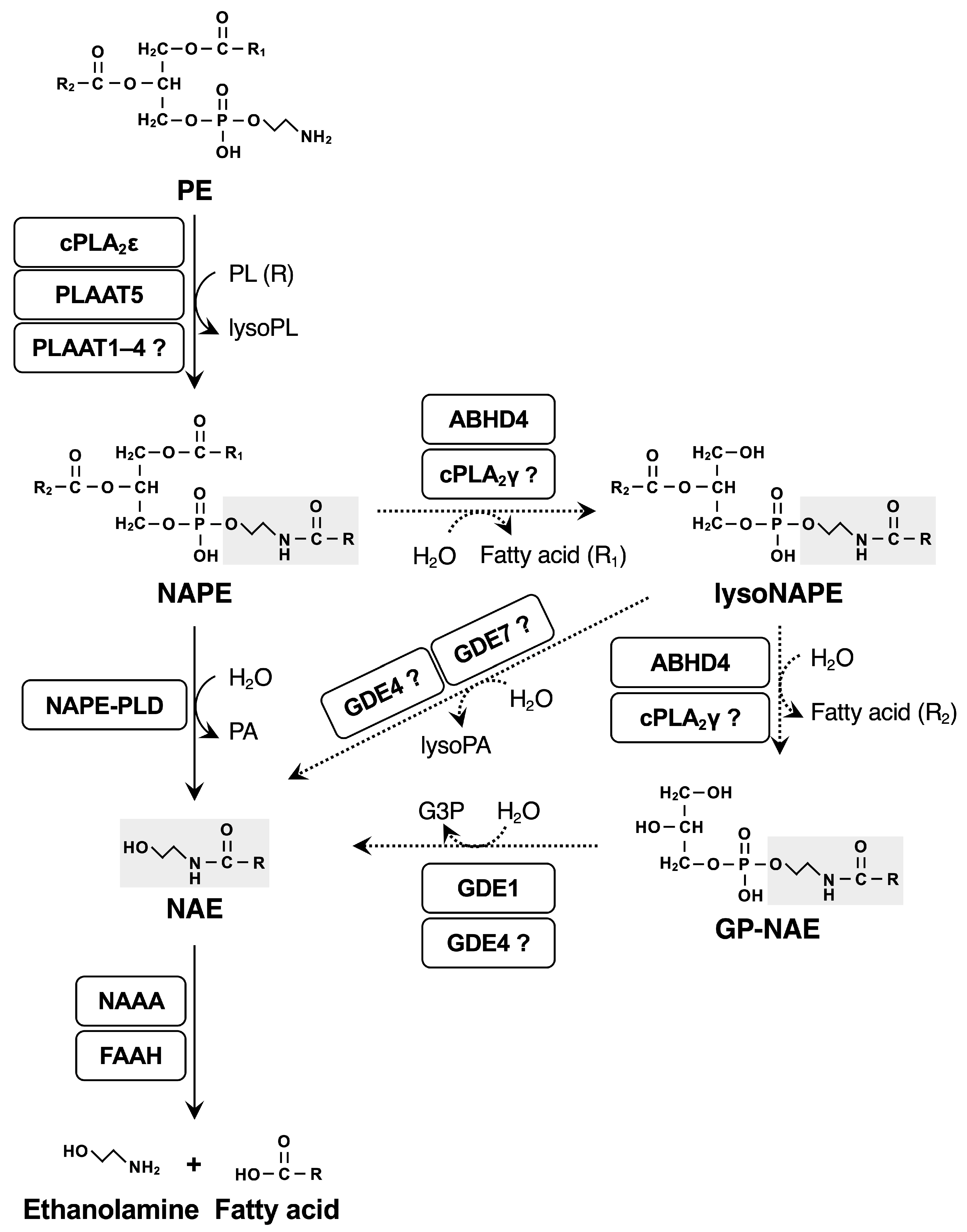
2.5. cPLA2γ and ABHD4 Potentially Contribute to the Conversion of NAPEs to NAEs
3. The PLAAT Family
3.1. The PLAAT Family Functions as Ca2+-Independent N-/O-Acyltransferases and PLA1/A2s
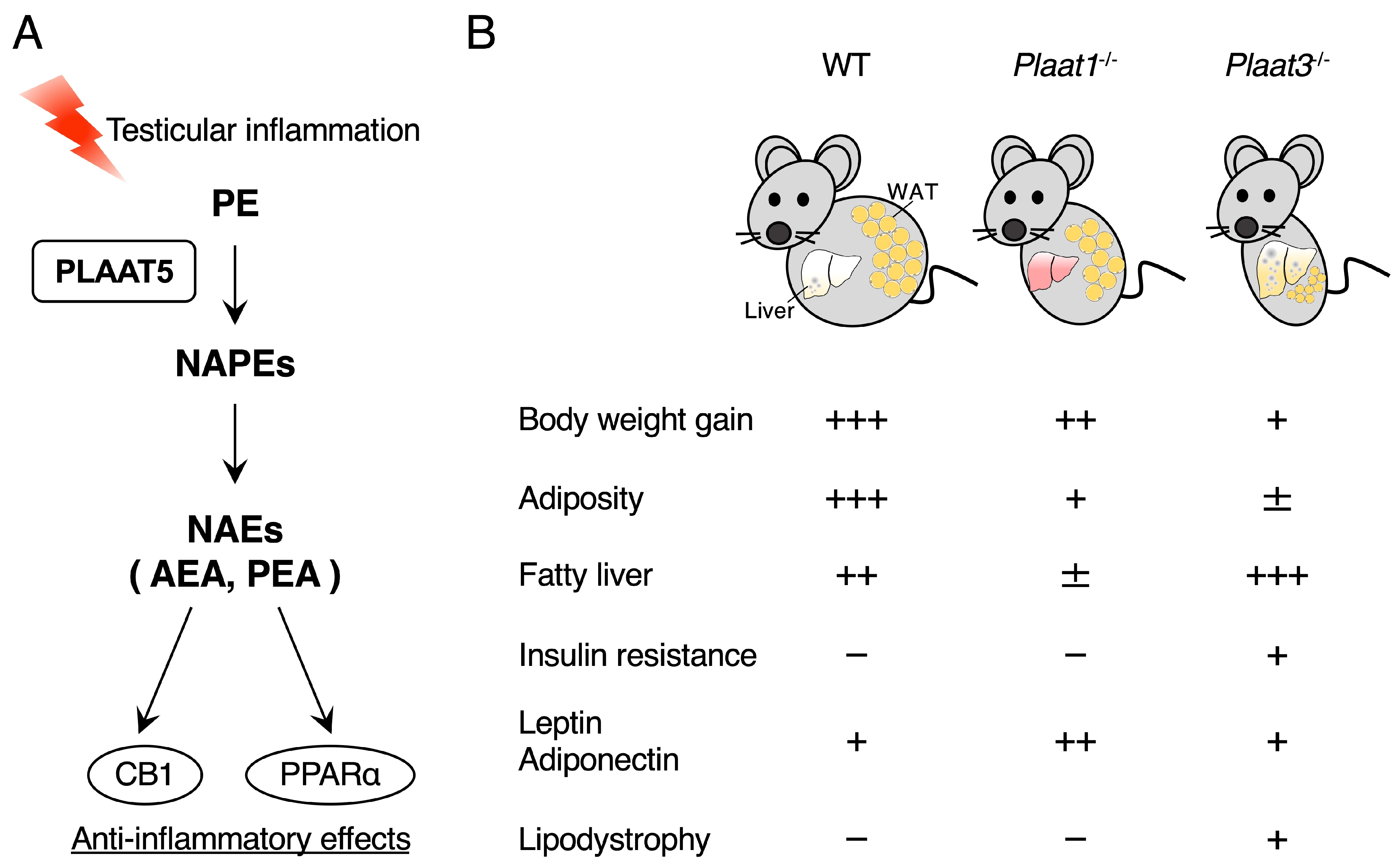
3.2. PLAAT5 Functions as a Ca2+-Independent N-Acyltransferase Producing Anti-Inflammatory NAEs in the Testis
3.3. Deficiency of PLAAT1 or PLAAT3 Ameliorates High-Fat Diet-Induced Obesity
3.4. Roles of PLAAT Proteins in Organellar Membrane Degradation
3.5. Other PLAATs
4. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ABHD4 | α/β-hydrolase domain-containing 4 |
| AD | Alzheimer’s disease |
| AdPLA2 | adipose-specific PLA2 |
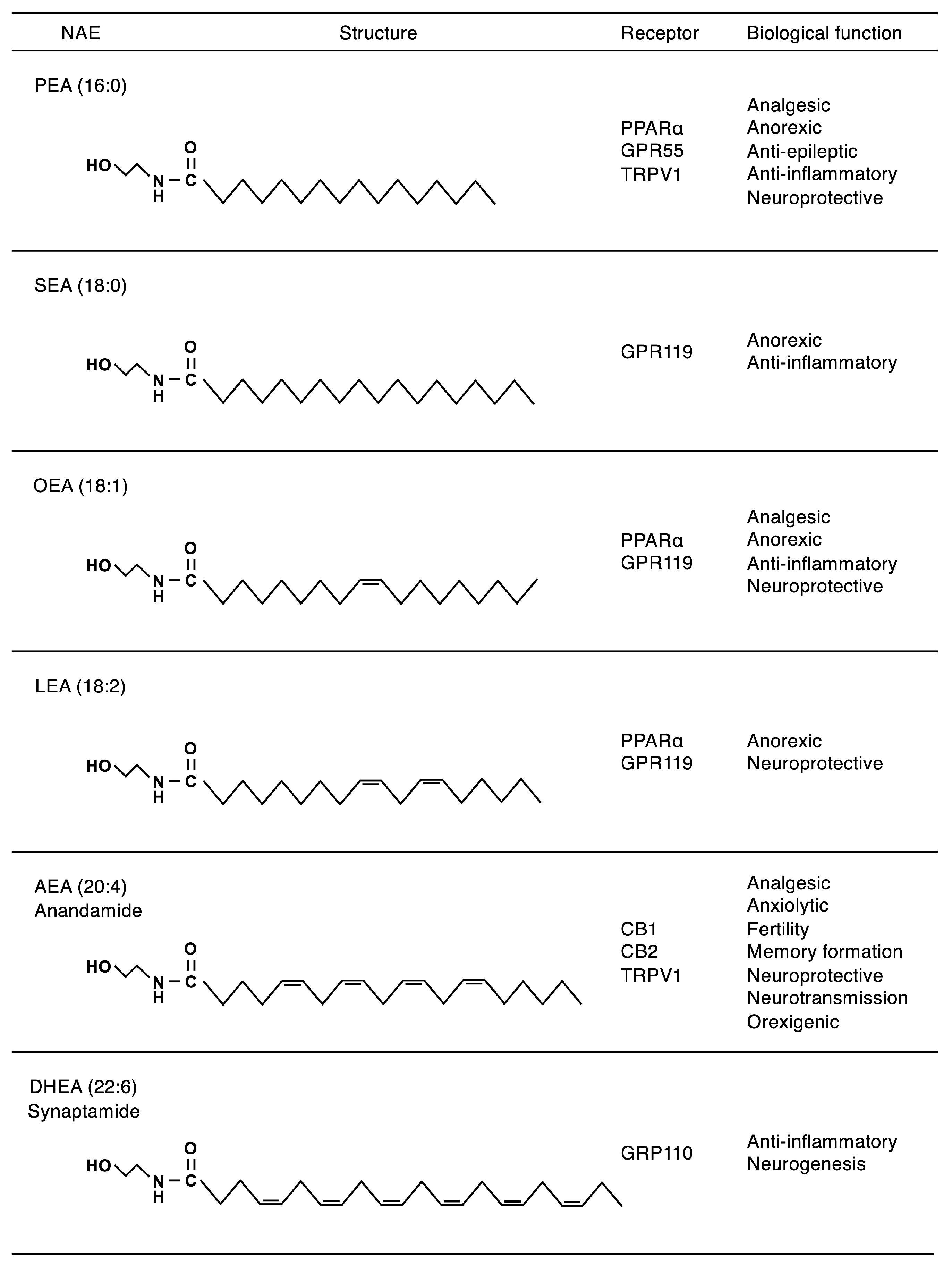
| AEA | N-arachidonoylethanolamine |
| CdCl2 | cadmium chloride |
| cPLA2 | cytosolic phospholipase A2 |
| DHA | docosahexaenoic acid |
| DHEA | N-docosahexaenoylethanolamine |
| DPEA | N-docosapentaenoylethanolamine |
| EPA | eicosapentaenoic acid |
| GDE | glycerophosphodiesterase |
| GP-NAE | glycerophospho-NAE |
| HFD | high-fat diet |
| IMQ | imiquimod |
| iPLA2 | Ca2+-independent phospholipase A2 |
| LRAT | lecithin-retinol acyltransferase |
| lysoPA | lysophosphatidic acid |
| NAE | N-acylethanolamine |
| NAPE | N-acyl-phosphatidylethanolamine |
| NAPE-PLD | NAPE-hydrolyzing phospholipase D |
| NAPS | N-acyl-phosphatidylserine |
| OEA | N-oleoylethanolamine |
| PA | phosphatidic acid |
| PC | phosphatidylcholine |
| PE | phosphatidylethanolamine |
| PEA | N-palmitoylethanolamine |
| PI | phosphatidylinositol |
| PIP2 | phosphatidylinositol 4,5-bisphosphate |
| PLA1 | phospholipase A1 |
| PLA2 | phospholipase A2 |
| PLAAT | phospholipase A and acyltransferase |
| PPARα | peroxisome proliferator-activated receptor α |
| PS | phosphatidylserine |
| SEA | N-stearoylethanolamine |
| WAT | white adipose tissue |
| WT | wild type |
References
- Mock, E.D.; Gagestein, B.; van der Stelt, M. Anandamide and other N-acylethanolamines: A class of signaling lipids with therapeutic opportunities. Prog. Lipid Res. 2023, 89, 101194. [Google Scholar] [CrossRef] [PubMed]
- Piomelli, D.; Mabou Tagne, A. Endocannabinoid-Based Therapies. Annu. Rev. Pharmacol. Toxicol. 2022, 62, 483–507. [Google Scholar] [CrossRef]
- Rahman, S.M.K.; Uyama, T.; Hussain, Z.; Ueda, N. Roles of Endocannabinoids and Endocannabinoid-Like Molecules in Energy Homeostasis and Metabolic Regulation: A Nutritional Perspective. Annu. Rev. Nutr. 2021, 41, 177–202. [Google Scholar] [CrossRef] [PubMed]
- Devane, W.A.; Hanus, L.; Breuer, A.; Pertwee, R.G.; Stevenson, L.A.; Griffin, G.; Gibson, D.; Mandelbaum, A.; Etinger, A.; Mechoulam, R. Isolation and structure of a brain constituent that binds to the cannabinoid receptor. Science 1992, 258, 1946–1949. [Google Scholar] [CrossRef]
- Devane, W.A.; Axelrod, J. Enzymatic synthesis of anandamide, an endogenous ligand for the cannabinoid receptor, by brain membranes. Proc. Natl. Acad. Sci. USA 1994, 91, 6698–6701. [Google Scholar] [CrossRef]
- Maccarrone, M.; Di Marzo, V.; Gertsch, J.; Grether, U.; Howlett, A.C.; Hua, T.; Makriyannis, A.; Piomelli, D.; Ueda, N.; van der Stelt, M. Goods and Bads of the Endocannabinoid System as a Therapeutic Target: Lessons Learned after 30 Years. Pharmacol. Rev. 2023, 75, 885–958. [Google Scholar] [CrossRef]
- Calignano, A.; La Rana, G.; Giuffrida, A.; Piomelli, D. Control of pain initiation by endogenous cannabinoids. Nature 1998, 394, 277–281. [Google Scholar] [CrossRef]
- Rodriguez de Fonseca, F.; Navarro, M.; Gomez, R.; Escuredo, L.; Nava, F.; Fu, J.; Murillo-Rodriguez, E.; Giuffrida, A.; LoVerme, J.; Gaetani, S.; et al. An anorexic lipid mediator regulated by feeding. Nature 2001, 414, 209–212. [Google Scholar] [CrossRef]
- Lee, J.W.; Huang, B.X.; Kwon, H.; Rashid, M.A.; Kharebava, G.; Desai, A.; Patnaik, S.; Marugan, J.; Kim, H.Y. Orphan GPR110 (ADGRF1) targeted by N-docosahexaenoylethanolamine in development of neurons and cognitive function. Nat. Commun. 2016, 7, 13123. [Google Scholar] [CrossRef]
- Kim, H.Y.; Spector, A.A. N-Docosahexaenoylethanolamine: A neurotrophic and neuroprotective metabolite of docosahexaenoic acid. Mol. Aspects. Med. 2018, 64, 34–44. [Google Scholar] [CrossRef]
- Swamy, M.J.; Tarafdar, P.K.; Kamlekar, R.K. Structure, phase behaviour and membrane interactions of N-acylethanolamines and N-acylphosphatidylethanolamines. Chem. Phys. Lipids 2010, 163, 266–279. [Google Scholar] [CrossRef] [PubMed]
- Ogura, Y.; Parsons, W.H.; Kamat, S.S.; Cravatt, B.F. A calcium-dependent acyltransferase that produces N-acyl phosphatidylethanolamines. Nat. Chem. Biol. 2016, 12, 669–671. [Google Scholar] [CrossRef] [PubMed]
- Uyama, T.; Ikematsu, N.; Inoue, M.; Shinohara, N.; Jin, X.H.; Tsuboi, K.; Tonai, T.; Tokumura, A.; Ueda, N. Generation of N-acylphosphatidylethanolamine by members of the phospholipase A/acyltransferase (PLA/AT) family. J. Biol. Chem. 2012, 287, 31905–31919. [Google Scholar] [CrossRef]
- Natarajan, V.; Reddy, P.V.; Schmid, P.C.; Schmid, H.H. On the biosynthesis and metabolism of N-acylethanolamine phospholipids in infarcted dog heart. Biochim. Biophys. Acta 1981, 664, 445–448. [Google Scholar] [CrossRef] [PubMed]
- Natarajan, V.; Schmid, P.C.; Reddy, P.V.; Zuzarte-Augustin, M.L.; Schmid, H.H. Biosynthesis of N-acylethanolamine phospholipids by dog brain preparations. J. Neurochem. 1983, 41, 1303–1312. [Google Scholar] [CrossRef]
- Jin, X.H.; Okamoto, Y.; Morishita, J.; Tsuboi, K.; Tonai, T.; Ueda, N. Discovery and characterization of a Ca2+-independent phosphatidylethanolamine N-acyltransferase generating the anandamide precursor and its congeners. J. Biol. Chem. 2007, 282, 3614–3623. [Google Scholar] [CrossRef]
- Cadas, H.; di Tomaso, E.; Piomelli, D. Occurrence and biosynthesis of endogenous cannabinoid precursor, N-arachidonoyl phosphatidylethanolamine, in rat brain. J. Neurosci. 1997, 17, 1226–1242. [Google Scholar] [CrossRef]
- Cadas, H.; Gaillet, S.; Beltramo, M.; Venance, L.; Piomelli, D. Biosynthesis of an endogenous cannabinoid precursor in neurons and its control by calcium and cAMP. J. Neurosci. 1996, 16, 3934–3942. [Google Scholar] [CrossRef]
- Binte Mustafiz, S.S.; Uyama, T.; Morito, K.; Takahashi, N.; Kawai, K.; Hussain, Z.; Tsuboi, K.; Araki, N.; Yamamoto, K.; Tanaka, T.; et al. Intracellular Ca2+-dependent formation of N-acyl-phosphatidylethanolamines by human cytosolic phospholipase A2ε. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 158515. [Google Scholar] [CrossRef]
- Hussain, Z.; Uyama, T.; Kawai, K.; Binte Mustafiz, S.S.; Tsuboi, K.; Araki, N.; Ueda, N. Phosphatidylserine-stimulated production of N-acyl-phosphatidylethanolamines by Ca2+-dependent N-acyltransferase. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2018, 1863, 493–502. [Google Scholar] [CrossRef]
- Murakami, M. The phospholipase A2 superfamily as a central hub of bioactive lipids and beyond. Pharmacol. Ther. 2023, 244, 108382. [Google Scholar] [CrossRef] [PubMed]
- Kita, Y.; Shindou, H.; Shimizu, T. Cytosolic phospholipase A2 and lysophospholipid acyltransferases. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 838–845. [Google Scholar] [CrossRef] [PubMed]
- Shimizu, T. Lipid mediators in health and disease: Enzymes and receptors as therapeutic targets for the regulation of immunity and inflammation. Annu. Rev. Pharmacol. Toxicol. 2009, 49, 123–150. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Uyama, T.; Rahman, S.M.K.; Sikder, M.M.; Hussain, Z.; Tsuboi, K.; Miyake, M.; Ueda, N. Involvement of the gamma Isoform of cPLA2 in the Biosynthesis of Bioactive N-Acylethanolamines. Molecules 2021, 26, 5213. [Google Scholar] [CrossRef]
- Liang, L.; Takamiya, R.; Miki, Y.; Heike, K.; Taketomi, Y.; Sugimoto, N.; Yamaguchi, M.; Shitara, H.; Nishito, Y.; Kobayashi, T.; et al. Group IVE cytosolic phospholipase A2 limits psoriatic inflammation by mobilizing the anti-inflammatory lipid N-acylethanolamine. FASEB J. 2022, 36, e22301. [Google Scholar] [CrossRef]
- Binte Mustafiz, S.S.; Uyama, T.; Hussain, Z.; Kawai, K.; Tsuboi, K.; Araki, N.; Ueda, N. The role of intracellular anionic phospholipids in the production of N-acyl-phosphatidylethanolamines by cytosolic phospholipase A2ε. J. Biochem. 2019, 165, 343–352. [Google Scholar] [CrossRef]
- Capestrano, M.; Mariggio, S.; Perinetti, G.; Egorova, A.V.; Iacobacci, S.; Santoro, M.; Di Pentima, A.; Iurisci, C.; Egorov, M.V.; Di Tullio, G.; et al. Cytosolic phospholipase A2ε drives recycling through the clathrin-independent endocytic route. J. Cell Sci. 2014, 127, 977–993. [Google Scholar] [CrossRef]
- Murakami, M.; Takamiya, R.; Miki, Y.; Sugimoto, N.; Nagasaki, Y.; Suzuki-Yamamoto, T.; Taketomi, Y. Segregated functions of two cytosolic phospholipase A2 isoforms (cPLA2α and cPLA2ε) in lipid mediator generation. Biochem. Pharmacol. 2022, 203, 115176. [Google Scholar] [CrossRef]
- Rahman, S.M.K.; Hussain, Z.; Morito, K.; Takahashi, N.; Sikder, M.M.; Tanaka, T.; Ohta, K.I.; Ueno, M.; Takahashi, H.; Yamamoto, T.; et al. Formation of N-acyl-phosphatidylethanolamines by cytosolic phospholipase A2ε in an ex vivo murine model of brain ischemia. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2022, 1867, 159222. [Google Scholar] [CrossRef]
- Natarajan, V.; Schmid, P.C.; Schmid, H.H. N-acylethanolamine phospholipid metabolism in normal and ischemic rat brain. Biochim. Biophys. Acta 1986, 878, 32–41. [Google Scholar] [CrossRef]
- Moesgaard, B.; Petersen, G.; Jaroszewski, J.W.; Hansen, H.S. Age dependent accumulation of N-acyl-ethanolamine phospholipids in ischemic rat brain. A 31P NMR and enzyme activity study. J. Lipid Res. 2000, 41, 985–990. [Google Scholar] [CrossRef] [PubMed]
- Luptakova, D.; Baciak, L.; Pluhacek, T.; Skriba, A.; Sediva, B.; Havlicek, V.; Juranek, I. Membrane depolarization and aberrant lipid distributions in the neonatal rat brain following hypoxic-ischaemic insult. Sci. Rep. 2018, 8, 6952. [Google Scholar] [CrossRef]
- Janfelt, C.; Wellner, N.; Leger, P.L.; Kokesch-Himmelreich, J.; Hansen, S.H.; Charriaut-Marlangue, C.; Hansen, H.S. Visualization by mass spectrometry of 2-dimensional changes in rat brain lipids, including N-acylphosphatidylethanolamines, during neonatal brain ischemia. FASEB J. 2012, 26, 2667–2673. [Google Scholar] [CrossRef]
- Patel, S.; Carrier, E.J.; Ho, W.S.; Rademacher, D.J.; Cunningham, S.; Reddy, D.S.; Falck, J.R.; Cravatt, B.F.; Hillard, C.J. The postmortal accumulation of brain N-arachidonylethanolamine (anandamide) is dependent upon fatty acid amide hydrolase activity. J. Lipid Res. 2005, 46, 342–349. [Google Scholar] [CrossRef]
- Kempe, K.; Hsu, F.F.; Bohrer, A.; Turk, J. Isotope dilution mass spectrometric measurements indicate that arachidonylethanolamide, the proposed endogenous ligand of the cannabinoid receptor, accumulates in rat brain tissue post mortem but is contained at low levels in or is absent from fresh tissue. J. Biol. Chem. 1996, 271, 17287–17295. [Google Scholar] [CrossRef]
- Kilaru, A.; Tamura, P.; Garg, P.; Isaac, G.; Baxter, D.; Duncan, R.S.; Welti, R.; Koulen, P.; Chapman, K.D.; Venables, B.J. Changes in N-acylethanolamine Pathway Related Metabolites in a Rat Model of Cerebral Ischemia/Reperfusion. J. Glycom. Lipidom. 2011, 1, 101. [Google Scholar] [CrossRef]
- Sun, Y.; Alexander, S.P.; Garle, M.J.; Gibson, C.L.; Hewitt, K.; Murphy, S.P.; Kendall, D.A.; Bennett, A.J. Cannabinoid activation of PPARα; a novel neuroprotective mechanism. Br. J. Pharmacol. 2007, 152, 734–743. [Google Scholar]
- Perez-Gonzalez, M.; Mendioroz, M.; Badesso, S.; Sucunza, D.; Roldan, M.; Espelosin, M.; Ursua, S.; Lujan, R.; Cuadrado-Tejedor, M.; Garcia-Osta, A. PLA2G4E, a candidate gene for resilience in Alzheimer s disease and a new target for dementia treatment. Prog. Neurobiol. 2020, 191, 101818. [Google Scholar] [CrossRef]
- Morimoto, Y.; Shimada-Sugimoto, M.; Otowa, T.; Yoshida, S.; Kinoshita, A.; Mishima, H.; Yamaguchi, N.; Mori, T.; Imamura, A.; Ozawa, H.; et al. Whole-exome sequencing and gene-based rare variant association tests suggest that PLA2G4E might be a risk gene for panic disorder. Transl. Psychiatry 2018, 8, 41. [Google Scholar] [CrossRef]
- Shao, S.; Chen, J.; Swindell, W.R.; Tsoi, L.C.; Xing, X.; Ma, F.; Uppala, R.; Sarkar, M.K.; Plazyo, O.; Billi, A.C.; et al. Phospholipase A2 enzymes represent a shared pathogenic pathway in psoriasis and pityriasis rubra pilaris. JCI Insight 2021, 6, e151911. [Google Scholar] [CrossRef]
- Sasso, O.; Summa, M.; Armirotti, A.; Pontis, S.; De Mei, C.; Piomelli, D. The N-Acylethanolamine Acid Amidase Inhibitor ARN077 Suppresses Inflammation and Pruritus in a Mouse Model of Allergic Dermatitis. J. Investig. Dermatol. 2018, 138, 562–569. [Google Scholar] [CrossRef] [PubMed]
- Ueharaguchi, Y.; Honda, T.; Kusuba, N.; Hanakawa, S.; Adachi, A.; Sawada, Y.; Otsuka, A.; Kitoh, A.; Dainichi, T.; Egawa, G.; et al. Thromboxane A2 facilitates IL-17A production from Vγ4+ γδ T cells and promotes psoriatic dermatitis in mice. J. Allergy Clin. Immunol. 2018, 142, 680–683. [Google Scholar] [CrossRef] [PubMed]
- Sumida, H.; Yanagida, K.; Kita, Y.; Abe, J.; Matsushima, K.; Nakamura, M.; Ishii, S.; Sato, S.; Shimizu, T. Interplay between CXCR2 and BLT1 facilitates neutrophil infiltration and resultant keratinocyte activation in a murine model of imiquimod-induced psoriasis. J. Immunol. 2014, 192, 4361–4369. [Google Scholar] [CrossRef]
- Ghafouri, N.; Ghafouri, B.; Larsson, B.; Stensson, N.; Fowler, C.J.; Gerdle, B. Palmitoylethanolamide and stearoylethanolamide levels in the interstitium of the trapezius muscle of women with chronic widespread pain and chronic neck-shoulder pain correlate with pain intensity and sensitivity. Pain 2013, 154, 1649–1658. [Google Scholar] [CrossRef]
- Laleh, P.; Yaser, K.; Alireza, O. Oleoylethanolamide: A novel pharmaceutical agent in the management of obesity-an updated review. J. Cell Physiol. 2019, 234, 7893–7902. [Google Scholar] [CrossRef]
- Rastelli, M.; Van Hul, M.; Terrasi, R.; Lefort, C.; Regnier, M.; Beiroa, D.; Delzenne, N.M.; Everard, A.; Nogueiras, R.; Luquet, S.; et al. Intestinal NAPE-PLD contributes to short-term regulation of food intake via gut-to-brain axis. Am. J. Physiol. Endocrinol. Metab. 2020, 319, E647–E657. [Google Scholar] [CrossRef]
- Everard, A.; Plovier, H.; Rastelli, M.; Van Hul, M.; de Wouters d’Oplinter, A.; Geurts, L.; Druart, C.; Robine, S.; Delzenne, N.M.; Muccioli, G.G.; et al. Intestinal epithelial N-acylphosphatidylethanolamine phospholipase D links dietary fat to metabolic adaptations in obesity and steatosis. Nat. Commun. 2019, 10, 457. [Google Scholar] [CrossRef]
- Misto, A.; Provensi, G.; Vozella, V.; Passani, M.B.; Piomelli, D. Mast Cell-Derived Histamine Regulates Liver Ketogenesis via Oleoylethanolamide Signaling. Cell Metab. 2019, 29, 91–102. [Google Scholar] [CrossRef]
- Asai, K.; Hirabayashi, T.; Houjou, T.; Uozumi, N.; Taguchi, R.; Shimizu, T. Human group IVC phospholipase A2 (cPLA2γ). Roles in the membrane remodeling and activation induced by oxidative stress. J. Biol. Chem. 2003, 278, 8809–8814. [Google Scholar] [CrossRef]
- Lord, C.C.; Thomas, G.; Brown, J.M. Mammalian alpha beta hydrolase domain (ABHD) proteins: Lipid metabolizing enzymes at the interface of cell signaling and energy metabolism. Biochim. Biophys. Acta. 2013, 1831, 792–802. [Google Scholar] [CrossRef]
- Bononi, G.; Tuccinardi, T.; Rizzolio, F.; Granchi, C. alpha/beta-Hydrolase Domain (ABHD) Inhibitors as New Potential Therapeutic Options against Lipid-Related Diseases. J. Med. Chem. 2021, 64, 9759–9785. [Google Scholar] [CrossRef] [PubMed]
- Simon, G.M.; Cravatt, B.F. Endocannabinoid biosynthesis proceeding through glycerophospho-N-acyl ethanolamine and a role for alpha/beta-hydrolase 4 in this pathway. J. Biol. Chem. 2006, 281, 26465–26472. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.C.; Simon, G.M.; Cravatt, B.F. ABHD4 regulates multiple classes of N-acyl phospholipids in the mammalian central nervous system. Biochemistry 2015, 54, 2539–2549. [Google Scholar] [CrossRef] [PubMed]
- Milman, G.; Maor, Y.; Abu-Lafi, S.; Horowitz, M.; Gallily, R.; Batkai, S.; Mo, F.M.; Offertaler, L.; Pacher, P.; Kunos, G.; et al. N-arachidonoyl L-serine, an endocannabinoid-like brain constituent with vasodilatory properties. Proc. Natl. Acad. Sci. USA 2006, 103, 2428–2433. [Google Scholar] [CrossRef]
- Arul Prakash, S.; Kamlekar, R.K. Function and therapeutic potential of N-acyl amino acids. Chem. Phys. Lipids 2021, 239, 105114. [Google Scholar] [CrossRef]
- Duncan, R.E.; Sarkadi-Nagy, E.; Jaworski, K.; Ahmadian, M.; Sul, H.S. Identification and functional characterization of adipose-specific phospholipase A2 (AdPLA). J. Biol. Chem. 2008, 283, 25428–25436. [Google Scholar] [CrossRef]
- Mashhadi, Z.; Yin, L.; Dosoky, N.S.; Chen, W.; Davies, S.S. Plaat1l1 controls feeding induced NAPE biosynthesis and contributes to energy balance regulation in zebrafish. Prostaglandins Other Lipid Mediat. 2024, 174, 106869. [Google Scholar] [CrossRef]
- Shinohara, N.; Uyama, T.; Jin, X.H.; Tsuboi, K.; Tonai, T.; Houchi, H.; Ueda, N. Enzymological analysis of the tumor suppressor A-C1 reveals a novel group of phospholipid-metabolizing enzymes. J. Lipid Res. 2011, 52, 1927–1935. [Google Scholar] [CrossRef]
- Jin, X.H.; Uyama, T.; Wang, J.; Okamoto, Y.; Tonai, T.; Ueda, N. cDNA cloning and characterization of human and mouse Ca2+-independent phosphatidylethanolamine N-acyltransferases. Biochim. Biophys. Acta 2009, 1791, 32–38. [Google Scholar] [CrossRef]
- Uyama, T.; Morishita, J.; Jin, X.H.; Okamoto, Y.; Tsuboi, K.; Ueda, N. The tumor suppressor gene H-Rev107 functions as a novel Ca2+-independent cytosolic phospholipase A1/2 of the thiol hydrolase type. J. Lipid Res. 2009, 50, 685–693. [Google Scholar] [CrossRef]
- Golczak, M.; Kiser, P.D.; Sears, A.E.; Lodowski, D.T.; Blaner, W.S.; Palczewski, K. Structural basis for the acyltransferase activity of lecithin:retinol acyltransferase-like proteins. J. Biol. Chem. 2012, 287, 23790–23807. [Google Scholar] [CrossRef] [PubMed]
- Pang, X.Y.; Cao, J.; Addington, L.; Lovell, S.; Battaile, K.P.; Zhang, N.; Rao, J.; Dennis, E.A.; Moise, A.R. Structure/function relationships of adipose phospholipase A2 containing a cys-his-his catalytic triad. J. Biol. Chem. 2012, 287, 35260–35274. [Google Scholar] [CrossRef] [PubMed]
- Uyama, T.; Inoue, M.; Okamoto, Y.; Shinohara, N.; Tai, T.; Tsuboi, K.; Inoue, T.; Tokumura, A.; Ueda, N. Involvement of phospholipase A/acyltransferase-1 in N-acylphosphatidylethanolamine generation. Biochim. Biophys. Acta 2013, 1831, 1690–1701. [Google Scholar] [CrossRef] [PubMed]
- Uyama, T.; Jin, X.H.; Tsuboi, K.; Tonai, T.; Ueda, N. Characterization of the human tumor suppressors TIG3 and HRASLS2 as phospholipid-metabolizing enzymes. Biochim. Biophys. Acta 2009, 1791, 1114–1124. [Google Scholar] [CrossRef]
- Kiser, P.D.; Palczewski, K. Retinoids and Retinal Diseases. Annu. Rev. Vis. Sci. 2016, 2, 197–234. [Google Scholar] [CrossRef]
- Martin Ask, N.; Leung, M.; Radhakrishnan, R.; Lobo, G.P. Vitamin A Transporters in Visual Function: A Mini Review on Membrane Receptors for Dietary Vitamin A Uptake, Storage, and Transport to the Eye. Nutrients 2021, 13, 3987. [Google Scholar] [CrossRef]
- Ruiz, A.; Winston, A.; Lim, Y.H.; Gilbert, B.A.; Rando, R.R.; Bok, D. Molecular and biochemical characterization of lecithin retinol acyltransferase. J. Biol. Chem. 1999, 274, 3834–3841. [Google Scholar] [CrossRef]
- Zolfaghari, R.; Ross, A.C. Lecithin:retinol acyltransferase from mouse and rat liver. cDNA cloning and liver-specific regulation by dietary vitamin A and retinoic acid. J. Lipid Res. 2000, 41, 2024–2034. [Google Scholar] [CrossRef]
- Golczak, M.; Sears, A.E.; Kiser, P.D.; Palczewski, K. LRAT-specific domain facilitates vitamin A metabolism by domain swapping in HRASLS3. Nat. Chem. Biol. 2015, 11, 26–32. [Google Scholar] [CrossRef]
- Hansen, H.S.; Moesgaard, B.; Hansen, H.H.; Petersen, G. N-Acylethanolamines and precursor phospholipids—Relation to cell injury. Chem. Phys. Lipids 2000, 108, 135–150. [Google Scholar] [CrossRef]
- Sikder, M.M.; Sasaki, S.; Miki, Y.; Nagasaki, Y.; Ohta, K.I.; Hussain, Z.; Saiga, H.; Ohmura-Hoshino, M.; Hoshino, K.; Ueno, M.; et al. PLAAT5 as an N-acyltransferase responsible for the generation of anti-inflammatory N-acylethanolamines in testis. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2025, 1870, 159583. [Google Scholar] [CrossRef] [PubMed]
- Schuel, H.; Burkman, L.J.; Lippes, J.; Crickard, K.; Forester, E.; Piomelli, D.; Giuffrida, A. N-Acylethanolamines in human reproductive fluids. Chem. Phys. Lipids 2002, 121, 211–227. [Google Scholar] [CrossRef] [PubMed]
- Balvers, M.G.; Verhoeckx, K.C.; Meijerink, J.; Wortelboer, H.M.; Witkamp, R.F. Measurement of palmitoylethanolamide and other N-acylethanolamines during physiological and pathological conditions. CNS. Neurol. Disord. Drug Targets 2013, 12, 23–33. [Google Scholar] [CrossRef] [PubMed]
- Tyrtyshnaia, A.; Konovalova, S.; Ponomarenko, A.; Egoraeva, A.; Manzhulo, I. Fatty Acid-Derived N-acylethanolamines Dietary Supplementation Attenuates Neuroinflammation and Cognitive Impairment in LPS Murine Model. Nutrients 2022, 14, 3879. [Google Scholar] [CrossRef]
- Piomelli, D.; Scalvini, L.; Fotio, Y.; Lodola, A.; Spadoni, G.; Tarzia, G.; Mor, M. N-Acylethanolamine Acid Amidase (NAAA): Structure, Function, and Inhibition. J. Med. Chem. 2020, 63, 7475–7490. [Google Scholar] [CrossRef]
- Siu, E.R.; Mruk, D.D.; Porto, C.S.; Cheng, C.Y. Cadmium-induced testicular injury. Toxicol. Appl. Pharmacol. 2009, 238, 240–249. [Google Scholar] [CrossRef]
- Ogawa, Y.; Itoh, M.; Hirai, S.; Suna, S.; Naito, M.; Qu, N.; Terayama, H.; Ikeda, A.; Miyaso, H.; Matsuno, Y.; et al. Cadmium exposure increases susceptibility to testicular autoimmunity in mice. J. Appl. Toxicol. 2013, 33, 652–660. [Google Scholar] [CrossRef]
- Hishikawa, D.; Valentine, W.J.; Iizuka-Hishikawa, Y.; Shindou, H.; Shimizu, T. Metabolism and functions of docosahexaenoic acid-containing membrane glycerophospholipids. FEBS Lett. 2017, 591, 2730–2744. [Google Scholar] [CrossRef]
- Sato, H.; Taketomi, Y.; Isogai, Y.; Miki, Y.; Yamamoto, K.; Masuda, S.; Hosono, T.; Arata, S.; Ishikawa, Y.; Ishii, T.; et al. Group III secreted phospholipase A2 regulates epididymal sperm maturation and fertility in mice. J. Clin. Investig. 2010, 120, 1400–1414. [Google Scholar] [CrossRef]
- Iizuka-Hishikawa, Y.; Hishikawa, D.; Sasaki, J.; Takubo, K.; Goto, M.; Nagata, K.; Nakanishi, H.; Shindou, H.; Okamura, T.; Ito, C.; et al. Lysophosphatidic acid acyltransferase 3 tunes the membrane status of germ cells by incorporating docosahexaenoic acid during spermatogenesis. J. Biol. Chem. 2017, 292, 12065–12076. [Google Scholar] [CrossRef]
- Shishikura, K.; Kuroha, S.; Matsueda, S.; Iseki, H.; Matsui, T.; Inoue, A.; Arita, M. Acyl-CoA synthetase 6 regulates long-chain polyunsaturated fatty acid composition of membrane phospholipids in spermatids and supports normal spermatogenic processes in mice. FASEB J. 2019, 33, 14194–14203. [Google Scholar] [CrossRef] [PubMed]
- Hussain, Z.; Uyama, T.; Kawai, K.; Rahman, I.A.; Tsuboi, K.; Araki, N.; Ueda, N. Comparative analyses of isoforms of the calcium-independent phosphatidylethanolamine N-acyltransferase PLAAT-1 in humans and mice. J. Lipid Res. 2016, 57, 2051–2060. [Google Scholar] [CrossRef] [PubMed]
- Jaworski, K.; Ahmadian, M.; Duncan, R.E.; Sarkadi-Nagy, E.; Varady, K.A.; Hellerstein, M.K.; Lee, H.Y.; Samuel, V.T.; Shulman, G.I.; Kim, K.H.; et al. AdPLA ablation increases lipolysis and prevents obesity induced by high-fat feeding or leptin deficiency. Nat. Med. 2009, 15, 159–168. [Google Scholar] [CrossRef]
- Rahman, S.M.K.; Sasaki, S.; Uyama, T.; Hussain, Z.; Sikder, M.M.; Saiga, H.; Ohmura-Hoshino, M.; Ohta, K.I.; Miki, Y.; Hoshino, K.; et al. PLAAT1 deficiency alleviates high-fat diet-induced hepatic lipid accumulation in mice. FASEB J. 2023, 37, e23032. [Google Scholar] [CrossRef]
- Mann, J.P.; Savage, D.B. What lipodystrophies teach us about the metabolic syndrome. J. Clin. Investig. 2019, 129, 4009–4021. [Google Scholar] [CrossRef]
- Schuermans, N.; El Chehadeh, S.; Hemelsoet, D.; Gautheron, J.; Vantyghem, M.C.; Nouioua, S.; Tazir, M.; Vigouroux, C.; Auclair, M.; Bogaert, E.; et al. Loss of phospholipase PLAAT3 causes a mixed lipodystrophic and neurological syndrome due to impaired PPARγ signaling. Nat. Genet. 2023, 55, 1929–1940. [Google Scholar] [CrossRef]
- Otto, A.C.; Gan-Schreier, H.; Zhu, X.; Tuma-Kellner, S.; Staffer, S.; Ganzha, A.; Liebisch, G.; Chamulitrat, W. Group VIA phospholipase A2 deficiency in mice chronically fed with high-fat-diet attenuates hepatic steatosis by correcting a defect of phospholipid remodeling. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 662–676. [Google Scholar] [CrossRef]
- Song, H.; Wohltmann, M.; Bao, S.; Ladenson, J.H.; Semenkovich, C.F.; Turk, J. Mice deficient in group VIB phospholipase A2 (iPLA2γ) exhibit relative resistance to obesity and metabolic abnormalities induced by a Western diet. Am. J. Physiol. Endocrinol. Metab. 2010, 298, E1097–E1114. [Google Scholar] [CrossRef]
- Mancuso, D.J.; Sims, H.F.; Yang, K.; Kiebish, M.A.; Su, X.; Jenkins, C.M.; Guan, S.; Moon, S.H.; Pietka, T.; Nassir, F.; et al. Genetic ablation of calcium-independent phospholipase A2γ prevents obesity and insulin resistance during high fat feeding by mitochondrial uncoupling and increased adipocyte fatty acid oxidation. J. Biol. Chem. 2010, 285, 36495–36510. [Google Scholar] [CrossRef]
- Morishita, H.; Eguchi, T.; Mizushima, N. A new insight into the lens: Cytosolic PLAAT phospholipases degrade organelles to make the lens transparent. Autophagy 2021, 17, 2645–2647. [Google Scholar] [CrossRef]
- Uyama, T.; Tsuboi, K.; Ueda, N. An involvement of phospholipase A/acyltransferase family proteins in peroxisome regulation and plasmalogen metabolism. FEBS Lett. 2017, 591, 2745–2760. [Google Scholar] [CrossRef] [PubMed]
- Uyama, T.; Ichi, I.; Kono, N.; Inoue, A.; Tsuboi, K.; Jin, X.H.; Araki, N.; Aoki, J.; Arai, H.; Ueda, N. Regulation of peroxisomal lipid metabolism by catalytic activity of tumor suppressor H-rev107. J. Biol. Chem. 2012, 287, 2706–2718. [Google Scholar] [CrossRef] [PubMed]
- Uyama, T.; Kawai, K.; Kono, N.; Watanabe, M.; Tsuboi, K.; Inoue, T.; Araki, N.; Arai, H.; Ueda, N. Interaction of Phospholipase A/Acyltransferase-3 with Pex19p: A Possible Involvement in The Down-Regulation of Peroxisomes. J. Biol. Chem. 2015, 290, 17520–17534. [Google Scholar] [CrossRef]
- Watanabe, S.; Nihongaki, Y.; Itoh, K.; Uyama, T.; Toda, S.; Watanabe, S.; Inoue, T. Defunctionalizing intracellular organelles such as mitochondria and peroxisomes with engineered phospholipase A/acyltransferases. Nat. Commun. 2022, 13, 4413. [Google Scholar] [CrossRef]
- Sikder, M.M.; Uyama, T.; Sasaki, S.; Kawai, K.; Araki, N.; Ueda, N. PLAAT1 expression triggers fragmentation of mitochondria in an enzyme activity-dependent manner. J. Biochem. 2023, 175, 101–113. [Google Scholar] [CrossRef]
- Morishita, H.; Eguchi, T.; Tsukamoto, S.; Sakamaki, Y.; Takahashi, S.; Saito, C.; Koyama-Honda, I.; Mizushima, N. Organelle degradation in the lens by PLAAT phospholipases. Nature 2021, 592, 634–638. [Google Scholar] [CrossRef]
- Yamamuro, T.; Kawabata, T.; Fukuhara, A.; Saita, S.; Nakamura, S.; Takeshita, H.; Fujiwara, M.; Enokidani, Y.; Yoshida, G.; Tabata, K.; et al. Age-dependent loss of adipose Rubicon promotes metabolic disorders via excess autophagy. Nat. Commun. 2020, 11, 4150. [Google Scholar] [CrossRef]
- Minami, S.; Nakamura, S.; Yoshimori, T. Rubicon in Metabolic Diseases and Ageing. Front. Cell Dev. Biol. 2021, 9, 816829. [Google Scholar] [CrossRef]
- Yamamuro, T.; Nakamura, S.; Yanagawa, K.; Tokumura, A.; Kawabata, T.; Fukuhara, A.; Teranishi, H.; Hamasaki, M.; Shimomura, I.; Yoshimori, T. Loss of RUBCN/rubicon in adipocytes mediates the upregulation of autophagy to promote the fasting response. Autophagy 2022, 18, 2686–2696. [Google Scholar] [CrossRef]
- Staring, J.; von Castelmur, E.; Blomen, V.A.; van den Hengel, L.G.; Brockmann, M.; Baggen, J.; Thibaut, H.J.; Nieuwenhuis, J.; Janssen, H.; van Kuppeveld, F.J.; et al. PLA2G16 represents a switch between entry and clearance of Picornaviridae. Nature 2017, 541, 412–416. [Google Scholar] [CrossRef]
- Elling, U.; Wimmer, R.A.; Leibbrandt, A.; Burkard, T.; Michlits, G.; Leopoldi, A.; Micheler, T.; Abdeen, D.; Zhuk, S.; Aspalter, I.M.; et al. A reversible haploid mouse embryonic stem cell biobank resource for functional genomics. Nature 2017, 550, 114–118. [Google Scholar] [CrossRef]
- DiSepio, D.; Ghosn, C.; Eckert, R.L.; Deucher, A.; Robinson, N.; Duvic, M.; Chandraratna, R.A.; Nagpal, S. Identification and characterization of a retinoid-induced class II tumor suppressor/growth regulatory gene. Proc. Natl. Acad. Sci. USA 1998, 95, 14811–14815. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.L.; Shyu, R.Y.; Yeh, M.Y.; Jiang, S.Y. Cloning and characterization of a novel retinoid-inducible gene 1(RIG1) deriving from human gastric cancer cells. Mol. Cell Endocrinol. 2000, 159, 15–24. [Google Scholar] [CrossRef] [PubMed]
- Casanova, B.; de la Fuente, M.T.; Garcia-Gila, M.; Sanz, L.; Silva, A.; Garcia-Marco, J.A.; Garcia-Pardo, A. The class II tumor-suppressor gene RARRES3 is expressed in B cell lymphocytic leukemias and down-regulated with disease progression. Leukemia 2001, 15, 1521–1526. [Google Scholar] [CrossRef] [PubMed]
- Yamane, D.; Feng, H.; Rivera-Serrano, E.E.; Selitsky, S.R.; Hirai-Yuki, A.; Das, A.; McKnight, K.L.; Misumi, I.; Hensley, L.; Lovell, W.; et al. Basal expression of interferon regulatory factor 1 drives intrinsic hepatocyte resistance to multiple RNA viruses. Nat. Microbiol. 2019, 4, 1096–1104. [Google Scholar] [CrossRef]
- Zhao, J.Y.; Yuan, X.K.; Luo, R.Z.; Wang, L.X.; Gu, W.; Yamane, D.; Feng, H. Phospholipase A and acyltransferase 4/retinoic acid receptor responder 3 at the intersection of tumor suppression and pathogen restriction. Front. Immunol. 2023, 14, 1107239. [Google Scholar] [CrossRef]
- Rinkenberger, N.; Abrams, M.E.; Matta, S.K.; Schoggins, J.W.; Alto, N.M.; Sibley, L.D. Overexpression screen of interferon-stimulated genes identifies RARRES3 as a restrictor of Toxoplasma gondii infection. eLife 2021, 10, e73137. [Google Scholar] [CrossRef]
- Sturniolo, M.T.; Dashti, S.R.; Deucher, A.; Rorke, E.A.; Broome, A.M.; Chandraratna, R.A.; Keepers, T.; Eckert, R.L. A novel tumor suppressor protein promotes keratinocyte terminal differentiation via activation of type I transglutaminase. J. Biol. Chem. 2003, 278, 48066–48073. [Google Scholar] [CrossRef]
- Scharadin, T.M.; Eckert, R.L. TIG3: An important regulator of keratinocyte proliferation and survival. J. Investig. Dermatol. 2014, 134, 1811–1816. [Google Scholar] [CrossRef]


| Name Enzyme (Gene) | Enzyme Activity | Ca2+ Dependency | ||
|---|---|---|---|---|
| PLA1/A2 | Lysophospholipase | Transacylase | ||
| cPLA2α (PLA2G4A) | ++ (PLA2) | ± | ± | Yes |
| cPLA2β (PLA2G4B) | + (PLA1 = PLA2) | ++ | ND | Yes |
| cPLA2γ (PLA2G4C) | ++ (PLA2) | ++ | ++ (O-acyltransferase) | No |
| cPLA2δ (PLA2G4D) | + (PLA1 > PLA2) | + | + (O-acyltransferase) | Yes |
| cPLA2ε (PLA2G4E) | ± | − | ++ (N-acyltransferase) | Yes |
| cPLA2ζ (PLA2G4F) | ++ (PLA2) | + | ND | Yes |
| Name | Synonym | Enzyme Activity | Organelle- Degrading Activity | ||
|---|---|---|---|---|---|
| PLA1/A2 | N-Acyltransferase | O-Acyltransferase | |||
| PLAAT1 | A-C1, HRASLS1 | + | ++ | ++ | Yes |
| PLAAT2 | HRASLS2 | ++ | +++ | ++ | Yes |
| PLAAT3 | AdPLA, HRASLS3, H-rev107, PLA2G16 | +++ | ± | + | Yes |
| PLAAT4 | HRASLS4, RARRE3, RIG1, TIG3 | ++ | ± | + | Yes |
| PLAAT5 | HRASLS5, iNAT | + | + | + | ND |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Uyama, T.; Sasaki, S.; Okada-Iwabu, M.; Murakami, M. Recent Progress in N-Acylethanolamine Research: Biological Functions and Metabolism Regulated by Two Distinct N-Acyltransferases: cPLA2ε and PLAAT Enzymes. Int. J. Mol. Sci. 2025, 26, 3359. https://doi.org/10.3390/ijms26073359
Uyama T, Sasaki S, Okada-Iwabu M, Murakami M. Recent Progress in N-Acylethanolamine Research: Biological Functions and Metabolism Regulated by Two Distinct N-Acyltransferases: cPLA2ε and PLAAT Enzymes. International Journal of Molecular Sciences. 2025; 26(7):3359. https://doi.org/10.3390/ijms26073359
Chicago/Turabian StyleUyama, Toru, Sumire Sasaki, Miki Okada-Iwabu, and Makoto Murakami. 2025. "Recent Progress in N-Acylethanolamine Research: Biological Functions and Metabolism Regulated by Two Distinct N-Acyltransferases: cPLA2ε and PLAAT Enzymes" International Journal of Molecular Sciences 26, no. 7: 3359. https://doi.org/10.3390/ijms26073359
APA StyleUyama, T., Sasaki, S., Okada-Iwabu, M., & Murakami, M. (2025). Recent Progress in N-Acylethanolamine Research: Biological Functions and Metabolism Regulated by Two Distinct N-Acyltransferases: cPLA2ε and PLAAT Enzymes. International Journal of Molecular Sciences, 26(7), 3359. https://doi.org/10.3390/ijms26073359






