Deconvolution Analysis of G and F-Actin Unfolding: Insights into the Thermal Stability and Structural Modifications Induced by PACAP
Abstract
1. Introduction
2. Results
3. Discussion
4. Materials and Methods
4.1. Sample Preparation
4.2. Differential Scanning Calorimetry (DSC)
4.3. Deconvolution Analysis
4.4. Integration of Previous Results
4.5. Data Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Miyata, A.; Jiang, L.; Dahl, R.D.; Kitada, C.; Kubo, K.; Fujino, M.; Minamino, N.; Arimura, A. Isolation of a Neuropeptide Corresponding to the N-Terminal 27 Residues of the Pituitary Adenylate Cyclase Activating Polypeptide with 38 Residues (PACAP38). Biochem. Biophys. Res. Commun. 1990, 170, 643–648. [Google Scholar] [CrossRef]
- Ohkubo, S.; Chiharukimura; Ogi, K.; Okazaki, K.; Masakihosoya; Onda, H.; Miyata, A.; Arimura, A.; Fujino, M. Primary Structure and Characterization of the Precursor to Human Pituitary Adenylate Cyclase Activating Polypeptide. DNA Cell Biol. 1992, 11, 21–30. [Google Scholar] [CrossRef] [PubMed]
- Miyata, A.; Arimura, A.; Dahl, R.R.; Minamino, N.; Uehara, A.; Jiang, L.; Culler, M.D.; Coy, D.H. Isolation of a Novel 38 Residue-Hypothalamic Polypeptide Which Stimulates Adenylate Cyclase in Pituitary Cells. Biochem. Biophys. Res. Commun. 1989, 164, 567–574. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Song, X.; Zhang, D.; Chen, X.; Li, X.; Sun, Y.; Li, C.; Song, Y.; Ding, Y.; Ren, R.; et al. Cryo-EM Structures of PAC1 Receptor Reveal Ligand Binding Mechanism. Cell Res. 2020, 30, 436–445. [Google Scholar] [CrossRef]
- Hamelink, C.; Lee, H.-W.; Chen, Y.; Grimaldi, M.; Eiden, L.E. Coincident Elevation of cAMP and Calcium Influx by PACAP-27 Synergistically Regulates Vasoactive Intestinal Polypeptide Gene Transcription through a Novel PKA-Independent Signaling Pathway. J. Neurosci. 2002, 22, 5310–5320. [Google Scholar] [CrossRef] [PubMed]
- Handbook of Biologically Active Peptides. Available online: https://www.nhbs.com/handbook-of-biologically-active-peptides-book-2 (accessed on 28 December 2022).
- Arimura, A. Pituitary Adenylate Cyclase Activating Polypeptide (PACAP): Discovery and Current Status of Research. Regul. Pept. 1992, 37, 285–303. [Google Scholar] [CrossRef]
- Vaudry, D.; Falluel-Morel, A.; Bourgault, S.; Basille, M.; Burel, D.; Wurtz, O.; Fournier, A.; Chow, B.K.C.; Hashimoto, H.; Galas, L.; et al. Pituitary Adenylate Cyclase-Activating Polypeptide and Its Receptors: 20 Years after the Discovery. Pharmacol. Rev. 2009, 61, 283–357. [Google Scholar] [CrossRef]
- Sherwood, N.M.; Krueckl, S.L.; McRory, J.E. The Origin and Function of the Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)/Glucagon Superfamily. Endocr. Rev. 2000, 21, 619–670. [Google Scholar] [CrossRef]
- Lelièvre, V.; Pineau, N.; Du, J.; Wen, C.-H.; Nguyen, T.; Janet, T.; Muller, J.-M.; Waschek, J.A. Differential Effects of Peptide Histidine Isoleucine (PHI) and Related Peptides on Stimulation and Suppression of Neuroblastoma Cell Proliferation: A Novel Vip-Independent Action of Phi via Map Kinase. J. Biol. Chem. 1998, 273, 19685–19690. [Google Scholar] [CrossRef]
- Sun, C.; Song, D.; Davis-Taber, R.A.; Barrett, L.W.; Scott, V.E.; Richardson, P.L.; Pereda-Lopez, A.; Uchic, M.E.; Solomon, L.R.; Lake, M.R.; et al. Solution Structure and Mutational Analysis of Pituitary Adenylate Cyclase-Activating Polypeptide Binding to the Extracellular Domain of PAC1-RS. Proc. Natl. Acad. Sci. USA 2007, 104, 7875–7880. [Google Scholar] [CrossRef]
- Culler, M.D.; Paschall, C.S. Pituitary Adenylate Cyclase-Activating Polypeptide (Pacap) Potentiates the Gonadotropin-Releasing Activity of Luteinizing Hormone-Releasing Hormone. Endocrinology 1991, 129, 2260–2262. [Google Scholar] [CrossRef] [PubMed]
- Suzuki, Y.; Kasai, K.; Takekoshi, K.; Oka, M.; Banba, N.; Numao, T.; Sugimura, H.; Iizuka, M.; Shimoda, S.-I. Effects of Pituitary Adenylate Cyclase Activating Polypeptide (PACAP) on the Cardiovascular System. Regul. Pept. 1993, 47, 213–220. [Google Scholar] [CrossRef] [PubMed]
- Starr, E.R.; Margiotta, J.F. PACAP Modulates Distinct Neuronal Components to Induce Cell-Specific Plasticity at Central and Autonomic Synapses. In Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Reglodi, D., Tamas, A., Eds.; Current Topics in Neurotoxicity; Springer International Publishing: Cham, Switzerland, 2016; pp. 83–107. ISBN 978-3-319-35135-3. [Google Scholar]
- Braas, K.M.; May, V.; Harakall, S.A.; Hardwick, J.C.; Parsons, R.L. Pituitary Adenylate Cyclase-Activating Polypeptide Expression and Modulation of Neuronal Excitability in Guinea Pig Cardiac Ganglia. J. Neurosci. 1998, 18, 9766–9779. [Google Scholar] [CrossRef]
- Reglodi, D.; Tamas, A. (Eds.) Pituitary Adenylate Cyclase Activating Polypeptide—PACAP; Current Topics in Neurotoxicity; Springer International Publishing: Cham, Switzerland, 2016; Volume 11, ISBN 978-3-319-35133-9. [Google Scholar]
- Chamoux, E.; Breault, L.; LeHoux, J.G.; Gallo-Payet, N. Comparative Effects of ACTH, PACAP, and VIP on Fetal Human Adrenal Cells. Endocr. Res. 1998, 24, 943–946. [Google Scholar] [CrossRef] [PubMed]
- Kellogg, D.L.; Zhao, J.L.; Wu, Y.; Johnson, J.M. VIP/PACAP Receptor Mediation of Cutaneous Active Vasodilation during Heat Stress in Humans. J. Appl. Physiol. 2010, 109, 95–100. [Google Scholar] [CrossRef]
- Kellogg, D.L.; Zhao, J.L.; Wu, Y.; Johnson, J.M. Nitric Oxide and Receptors for VIP and PACAP in Cutaneous Active Vasodilation during Heat Stress in Humans. J. Appl. Physiol. 2012, 113, 1512–1518. [Google Scholar] [CrossRef]
- Masmoudi-Kouki, O.; Gandolfo, P.; Castel, H.; Leprince, J.; Fournier, A.; Dejda, A.; Vaudry, H.; Tonon, M.-C. Role of PACAP and VIP in Astroglial Functions. Peptides 2007, 28, 1753–1760. [Google Scholar] [CrossRef] [PubMed]
- Reglodi, D.; Vaczy, A.; Rubio-Beltran, E.; MaassenVanDenBrink, A. Protective Effects of PACAP in Ischemia. J. Headache Pain 2018, 19, 19. [Google Scholar] [CrossRef]
- Tamas, A.; Reglodi, D.; Farkas, O.; Kovesdi, E.; Pal, J.; Povlishock, J.T.; Schwarcz, A.; Czeiter, E.; Szanto, Z.; Doczi, T.; et al. Effect of PACAP in Central and Peripheral Nerve Injuries. Int. J. Mol. Sci. 2012, 13, 8430–8448. [Google Scholar] [CrossRef]
- Kóvesdi, E.; Tamás, A.; Reglodi, D.; Farkas, O.; PáL, J.; Tóth, G.; Bukovics, P.; Dóczi, T.; Büki, A. Posttraumatic Administration of Pituitary Adenylate Cyclase Activating Polypeptide in Central Fluid Percussion Injury in Rats. Neurotox. Res. 2008, 13, 71–78. [Google Scholar] [CrossRef]
- Falluel-Morel, A.; Vaudry, D.; Aubert, N.; Galas, L.; Benard, M.; Basille, M.; Fontaine, M.; Fournier, A.; Vaudry, H.; Gonzalez, B.J. Pituitary Adenylate Cyclase-Activating Polypeptide Prevents the Effects of Ceramides on Migration, Neurite Outgrowth, and Cytoskeleton Remodeling. Proc. Natl. Acad. Sci. USA 2005, 102, 2637–2642. [Google Scholar] [CrossRef] [PubMed]
- Falluel-Morel, A.; Vaudry, D.; Aubert, N.; Galas, L.; Benard, M.; Basille, M.; Fontaine, M.; Fournier, A.; Vaudry, H.; Gonzalez, B.J. PACAP and Ceramides Exert Opposite Effects on Migration, Neurite Outgrowth, and Cytoskeleton Remodeling. Ann. N. Y. Acad. Sci. 2006, 1070, 265–270. [Google Scholar] [CrossRef] [PubMed]
- Toth, D.; Tamas, A.; Reglodi, D. The Neuroprotective and Biomarker Potential of PACAP in Human Traumatic Brain Injury. Int. J. Mol. Sci. 2020, 21, 827. [Google Scholar] [CrossRef] [PubMed]
- Bukovics, P.; Czeiter, E.; Amrein, K.; Kovacs, N.; Pal, J.; Tamas, A.; Bagoly, T.; Helyes, Z.; Buki, A.; Reglodi, D. Changes of PACAP Level in Cerebrospinal Fluid and Plasma of Patients with Severe Traumatic Brain Injury. Peptides 2014, 60, 18–22. [Google Scholar] [CrossRef]
- Reglodi, D.; Kiss, P.; Lubics, A.; Tamas, A. Review on the Protective Effects of PACAP in Models of Neurodegenerative Diseases In Vitro and In Vivo. Curr. Pharm. Des. 2011, 17, 962–972. [Google Scholar] [CrossRef]
- Rat, D.; Schmitt, U.; Tippmann, F.; Dewachter, I.; Theunis, C.; Wieczerzak, E.; Postina, R.; van Leuven, F.; Fahrenholz, F.; Kojro, E. Neuropeptide Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP) Slows down Alzheimer’s Disease-like Pathology in Amyloid Precursor Protein-Transgenic Mice. FASEB J. 2011, 25, 3208–3218. [Google Scholar] [CrossRef]
- Pinhasov, A.; Nesher, E.; Gross, M.; Turgeman, G.; Kreinin, A.; Yadid, G. The Role of the PACAP Signaling System in Depression. Curr. Pharm. Des. 2011, 17, 990–1001. [Google Scholar] [CrossRef]
- Hammack, S.E.; Cheung, J.; Rhodes, K.M.; Schutz, K.C.; Falls, W.A.; Braas, K.M.; May, V. Chronic Stress Increases Pituitary Adenylate Cyclase-Activating Peptide (PACAP) and Brain-Derived Neurotrophic Factor (BDNF) mRNA Expression in the Bed Nucleus of the Stria Terminalis (BNST): Roles for PACAP in Anxiety-like Behavior. Psychoneuroendocrinology 2009, 34, 833–843. [Google Scholar] [CrossRef]
- Stricker, J.; Falzone, T.; Gardel, M.L. Mechanics of the F-Actin Cytoskeleton. J. Biomech. 2010, 43, 9–14. [Google Scholar] [CrossRef]
- The Structure of F-Actin-Proquest. Available online: https://www.proquest.com/openview/2cf5572f1584cf28668c15ca8f84af11/1?cbl=18750&diss=y&pq-origsite=gscholar&parentSessionId=FGbQVYuoDnSooddQFM2OGWoDU62Ufp3Fv537ksmtSgw%3D (accessed on 10 November 2023).
- Hanson, J.; Lowy, J. The Structure of F-Actin and of Actin Filaments Isolated from Muscle. J. Mol. Biol. 1963, 6, 46-IN5. [Google Scholar] [CrossRef]
- Riedl, J.; Crevenna, A.H.; Kessenbrock, K.; Yu, J.H.; Neukirchen, D.; Bista, M.; Bradke, F.; Jenne, D.; Holak, T.A.; Werb, Z.; et al. Lifeact: A Versatile Marker to Visualize F-Actin. Nat. Methods 2008, 5, 605–607. [Google Scholar] [CrossRef]
- Galkin, V.E.; Orlova, A.; Schröder, G.F.; Egelman, E.H. Structural Polymorphism in F-Actin. Nat. Struct. Mol. Biol. 2010, 17, 1318–1323. [Google Scholar] [CrossRef]
- Hayden, S.M.; Miller, P.S.; Brauweiler, A.; Bamburg, J.R. Analysis of the Interactions of Actin Depolymerizing Factor with G- and F-Actin. Biochemistry 1993, 32, 9994–10004. [Google Scholar] [CrossRef] [PubMed]
- Robaszkiewicz, K.; Ostrowska, Z.; Marchlewicz, K.; Moraczewska, J. Tropomyosin Isoforms Differentially Modulate the Regulation of Actin Filament Polymerization and Depolymerization by Cofilins. FEBS J. 2016, 283, 723–737. [Google Scholar] [CrossRef] [PubMed]
- Bezanilla, M.; Gladfelter, A.S.; Kovar, D.R.; Lee, W.-L. Cytoskeletal Dynamics: A View from the Membrane. J. Cell Biol. 2015, 209, 329–337. [Google Scholar] [CrossRef]
- Civelekoglu, G.; Edelstein-Keshet, L. Modelling the Dynamics of F-Actin in the Cell. Bull. Math. Biol. 1994, 56, 587–616. [Google Scholar] [CrossRef] [PubMed]
- Maruthamuthu, V.; Aratyn-Schaus, Y.; Gardel, M.L. Conserved F-Actin Dynamics and Force Transmission at Cell Adhesions. Curr. Opin. Cell Biol. 2010, 22, 583–588. [Google Scholar] [CrossRef]
- Lemieux, M.G.; Janzen, D.; Hwang, R.; Roldan, J.; Jarchum, I.; Knecht, D.A. Visualization of the Actin Cytoskeleton: Different F-Actin-Binding Probes Tell Different Stories. Cytoskeleton 2014, 71, 157–169. [Google Scholar] [CrossRef]
- Tatunashvili, L.V.; Privalov, P.L. Calorimetric study of G-actin denaturation. Biofizika 1984, 29, 583–585. [Google Scholar]
- Le Bihan, T.; Gicquaud, C. Kinetic Study of the Thermal Denaturation of G Actin Using Differential Scanning Calorimetry and Intrinsic Fluorescence Spectroscopy. Biochem. Biophys. Res. Commun. 1993, 194, 1065–1073. [Google Scholar] [CrossRef]
- Bertazzon, A.; Tian, G.H.; Lamblin, A.; Tsong, T.Y. Enthalpic and Entropic Contributions to Actin Stability: Calorimetry, Circular Dichroism, and Fluorescence Study and Effects of Calcium. Biochemistry 1990, 29, 291–298. [Google Scholar] [CrossRef]
- Lőrinczy, D.; Belágyi, J. Scanning Calorimetric and EPR Studies on the Thermal Stability of Actin. Thermochim. Acta 1995, 259, 153–164. [Google Scholar] [CrossRef]
- Lőrinczy, D.; Könczöl, F.; Gaszner, B.; Belagyi, J. Structural Stability of Actin Filaments as Studied by DSC and EPR. Thermochim. Acta 1998, 322, 95–100. [Google Scholar] [CrossRef]
- Lőrinczy, D.; Vértes, Z.; Könczöl, F.; Belágyi, J. Thermal Transitions of Actin. J. Therm. Anal. Calorim. 2009, 95, 713–719. [Google Scholar] [CrossRef]
- Lőrinczy, D.; Vértes, Z.; Belagyi, J. The Effect of Nucleotides (ADP and ADP + Vi) on the Thermal Stability of Rat Uterus. Thermochim. Acta 2001, 376, 109–115. [Google Scholar] [CrossRef]
- Könczöl, F.; Lőrinczy, D.; Vértes, Z.; Hegyi, G.; Belagyi, J. Inter-Monomer Cross-Linking Affects the Thermal Transitions in F-Actin. J. Therm. Anal. Calorim. 2010, 101, 549–553. [Google Scholar] [CrossRef]
- Szatmári, D.; Lőrinczy, D. Alterations of Inter-Domain Flexibility in Actin Monomers during Cyclophosphamide Treatment. J. Therm. Anal. Calorim. 2022, 147, 7799–7810. [Google Scholar] [CrossRef]
- Lőrinczy, D. Cyclophosphamide Treatment Evoked Side Effects on Skeletal Muscle Monitored by DSC. J. Therm. Anal. Calorim. 2020, 142, 1897–1901. [Google Scholar] [CrossRef]
- Lőrinczy, D.; Szatmári, D. Dose-Dependent Effect of Cyclophosphamide Treatment on Actin. J. Therm. Anal. Calorim. 2022, 147, 10403–10409. [Google Scholar] [CrossRef]
- Takács-Kollár, V.; Nyitrai, M.; Lőrinczy, D.; Hild, G. Calorimetric Characterisation of the Toxofilin–G-Actin Complex. J. Therm. Anal. Calorim. 2018, 131, 1307–1311. [Google Scholar] [CrossRef]
- Takács-Kollár, V.; Nyitrai, M.; Lőrinczy, D.; Hild, G. Resolving the Similarities and Differences between the Effect of Structurally Different Actin-Binding Proteins on the Thermodynamic Properties of G-Actin. J. Therm. Anal. Calorim. 2017, 127, 1261–1266. [Google Scholar] [CrossRef]
- Takács-Kollár, V.; Lőrinczy, D.; Nyitrai, M.; Hild, G. Spectroscopic Characterization of the Effect of Mouse Twinfilin-1 on Actin Filaments at Different pH Values. J. Photochem. Photobiol. B Biol. 2016, 164, 276–282. [Google Scholar] [CrossRef] [PubMed]
- Bukovics, P.; Tamás, A.; Tóth, G.; Lőrinczy, D. Investigating the Impact of PACAP on Thermal Stability of G-Actin by Differential Scanning Calorimetry. J. Therm. Anal. Calorim. 2024. [Google Scholar] [CrossRef]
- Bukovics, P.; Lőrinczy, D. Exploring the Response of PACAP on Thermal Endurance of F-Actin by Differential Scanning Calorimetry. J. Therm. Anal. Calorim. 2024, 149, 8059–8065. [Google Scholar] [CrossRef]
- Vékony, R.G.; Tamás, A.; Lukács, A.; Ujfalusi, Z.; Lőrinczy, D.; Takács-Kollár, V.; Bukovics, P. Exploring the Role of Neuropeptide PACAP in Cytoskeletal Function Using Spectroscopic Methods. Int. J. Mol. Sci. 2024, 25, 8063. [Google Scholar] [CrossRef]
- Perez, V.; Bouschet, T.; Fernandez, C.; Bockaert, J.; Journot, L. Dynamic Reorganization of the Astrocyte Actin Cytoskeleton Elicited by cAMP and PACAP: A Role for phosphatidylInositol 3-Kinase Inhibition. Eur. J. Neurosci. 2005, 21, 26–32. [Google Scholar] [CrossRef]
- Sakai, Y.; Hashimoto, H.; Shintani, N.; Katoh, H.; Negishi, M.; Kawaguchi, C.; Kasai, A.; Baba, A. PACAP Activates Rac1 and Synergizes with NGF to Activate ERK1/2, Thereby Inducing Neurite Outgrowth in PC12 Cells. Brain Res. Mol. Brain Res. 2004, 123, 18–26. [Google Scholar] [CrossRef] [PubMed]
- Li, H.-X.; Feng, J.; Liu, Q.; Ou, B.-Q.; Lu, S.-Y.; Ma, Y. PACAP-Derived Mutant Peptide MPAPO Protects Trigeminal Ganglion Cell and the Retina from Hypoxic Injury through Anti-Oxidative Stress, Anti-Apoptosis, and Promoting Axon Regeneration. Biochim. Biophys. Acta Gen. Subj. 2021, 1865, 130018. [Google Scholar] [CrossRef]
- Fabian, E.; Reglodi, D.; Horvath, G.; Opper, B.; Toth, G.; Fazakas, C.; Vegh, A.G.; Wilhelm, I.; Krizbai, I.A. Pituitary Adenylate Cyclase Activating Polypeptide Acts against Neovascularization in Retinal Pigment Epithelial Cells. Ann. N. Y. Acad. Sci. 2019, 1455, 160–172. [Google Scholar] [CrossRef]
- Héraud, C.; Hilairet, S.; Muller, J.-M.; Leterrier, J.-F.; Chadéneau, C. Neuritogenesis Induced by Vasoactive Intestinal Peptide, Pituitary Adenylate Cyclase-Activating Polypeptide, and Peptide Histidine Methionine in SH-SY5y Cells Is Associated with Regulated Expression of Cytoskeleton mRNAs and Proteins. J. Neurosci. Res. 2004, 75, 320–329. [Google Scholar] [CrossRef]
- Toriyama, M.; Mizuno, N.; Fukami, T.; Iguchi, T.; Toriyama, M.; Tago, K.; Itoh, H. Phosphorylation of Doublecortin by Protein Kinase A Orchestrates Microtubule and Actin Dynamics to Promote Neuronal Progenitor Cell Migration. J. Biol. Chem. 2012, 287, 12691–12702. [Google Scholar] [CrossRef] [PubMed]
- Waschek, J.A.; Casillas, R.A.; Nguyen, T.B.; DiCicco-Bloom, E.M.; Carpenter, E.M.; Rodriguez, W.I. Neural Tube Expression of Pituitary Adenylate Cyclase-Activating Peptide (PACAP) and Receptor: Potential Role in Patterning and Neurogenesis. Proc. Natl. Acad. Sci. USA 1998, 95, 9602–9607. [Google Scholar] [CrossRef]
- May, V.; Lutz, E.; MacKenzie, C.; Schutz, K.C.; Dozark, K.; Braas, K.M. Pituitary Adenylate Cyclase-Activating Polypeptide (PACAP)/PAC1HOP1 Receptor Activation Coordinates Multiple Neurotrophic Signaling Pathways: Akt Activation through Phosphatidylinositol 3-Kinase Gamma and Vesicle Endocytosis for Neuronal Survival. J. Biol. Chem. 2010, 285, 9749–9761. [Google Scholar] [CrossRef] [PubMed]
- Bayón, R.; García-Rojas, R.; Rojas, E.; Rodríguez-García, M.M. Assessment of Isoconversional Methods and Peak Functions for the Kinetic Analysis of Thermogravimetric Data and Its Application to Degradation Processes of Organic Phase Change Materials. J. Therm. Anal. Calorim. 2024, 149, 13879–13899. [Google Scholar] [CrossRef]
- Ferencz, A.; Vértes, Z.; Lőrinczy, D. Deconvoluted DSC Curves of Intestinal Muscle Layer Following Warm and Cold Ischaemic Injury. J. Therm. Anal. Calorim. 2023, 148, 831–836. [Google Scholar] [CrossRef]
- Lőrinczy, D.; Moezzi, M.; Ferencz, A. Deconvoluted Plasma DSC Curves on Patients with Psoriasis. J. Therm. Anal. Calorim. 2020, 142, 789–796. [Google Scholar] [CrossRef]
- Lőrinczy, D.; Belagyi, J. Nucleotide Binding Induces Global and Local Structural Changes of Myosin Head in Muscle Fibres. Eur. J. Biochem. 2001, 268, 5970–5976. [Google Scholar] [CrossRef] [PubMed]
- Telek, E.; Ujfalusi, Z.; Nyitrai, M.; Bogner, P.; Lukács, A.; Németh, T.; Hild, G.; Hild, G. Deconvolution Analysis of the Non-Ionic Iomeprol, Iobitridol and Iodixanol Contrast Media-Treated Human Whole Blood Thermograms: A Comparative Study. Diagnostics 2023, 13, 2523. [Google Scholar] [CrossRef]
- Vega, S.; Garcia-Gonzalez, M.A.; Lanas, A.; Velazquez-Campoy, A.; Abian, O. Deconvolution Analysis for Classifying Gastric Adenocarcinoma Patients Based on Differential Scanning Calorimetry Serum Thermograms. Sci. Rep. 2015, 5, 7988. [Google Scholar] [CrossRef]
- Ferencz, A.; Szatmári, D.; Lőrinczy, D. Thermodynamic Sensitivity of Blood Plasma Components in Patients Afflicted with Skin, Breast and Pancreatic Forms of Cancer. Cancers 2022, 14, 6147. [Google Scholar] [CrossRef]
- Lőrinczy, D.; Ferencz, A. Comparison of Deconvoluted Plasma DSC Curves on Patients with Solid Tumors. J. Therm. Anal. Calorim. 2020, 142, 1243–1248. [Google Scholar] [CrossRef]
- Spudich, J.A.; Watt, S. The Regulation of Rabbit Skeletal Muscle Contraction. I. Biochemical Studies of the Interaction of the Tropomyosin-Troponin Complex with Actin and the Proteolytic Fragments of Myosin. J. Biol. Chem. 1971, 246, 4866–4871. [Google Scholar] [PubMed]
- Vig, A.T.; Földi, I.; Szikora, S.; Migh, E.; Gombos, R.; Tóth, M.Á.; Huber, T.; Pintér, R.; Talián, G.C.; Mihály, J.; et al. The Activities of the C-Terminal Regions of the Formin Protein Disheveled-Associated Activator of Morphogenesis (DAAM) in Actin Dynamics. J. Biol. Chem. 2017, 292, 13566–13583. [Google Scholar] [CrossRef] [PubMed]
- Tóth, M.Á.; Majoros, A.K.; Vig, A.T.; Migh, E.; Nyitrai, M.; Mihály, J.; Bugyi, B. Biochemical Activities of the Wiskott-Aldrich Syndrome Homology Region 2 Domains of Sarcomere Length Short (SALS) Protein. J. Biol. Chem. 2016, 291, 667–680. [Google Scholar] [CrossRef]
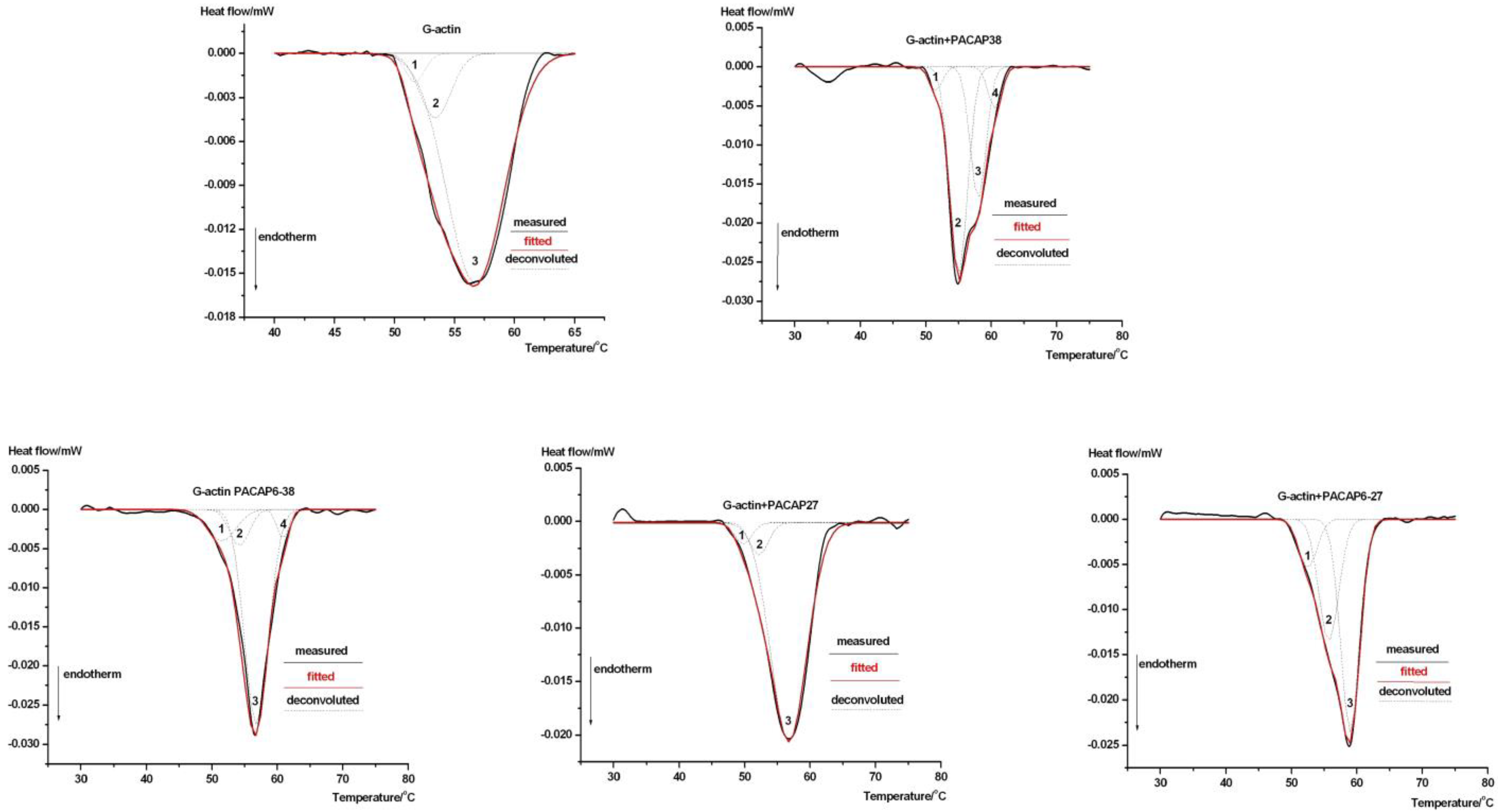
| Decomposition | T1 (°C) | T2 (°C) | T3 (°C) | T4 (°C) | ΔHTcal (J g−1) |
|---|---|---|---|---|---|
| G-actin | 51.7 | 53.4 | 56.7 | - | 0.023 ± 0.002 |
| % Area | 8.9 | 19.95 | 71.2 | - | |
| G-PACAP38 | 51.5 | 55.0 | 58.0 | 60.55 | 0.037 ± 0.004 |
| % Area | 5.90 | 51.30 | 32.63 | 10.25 | |
| G-PACAP6-38 | 51.5 | 54.2 | 56.8 | 60.85 | 0.038 ± 0.004 |
| % Area | 10.05 | 11.55 | 69.03 | 9.39 | |
| G-PACAP27 | 49.8 | 52.2 | 56.75 | - | 0.035 ± 0.004 |
| % Area | 7.83 | 11.94 | 80.23 | - | |
| G-PACAP6-27 | 52.5 | 55.7 | 59.0 | - | 0.031 ± 0.003 |
| % Area | 12.03 | 34.68 | 53.3 | - |
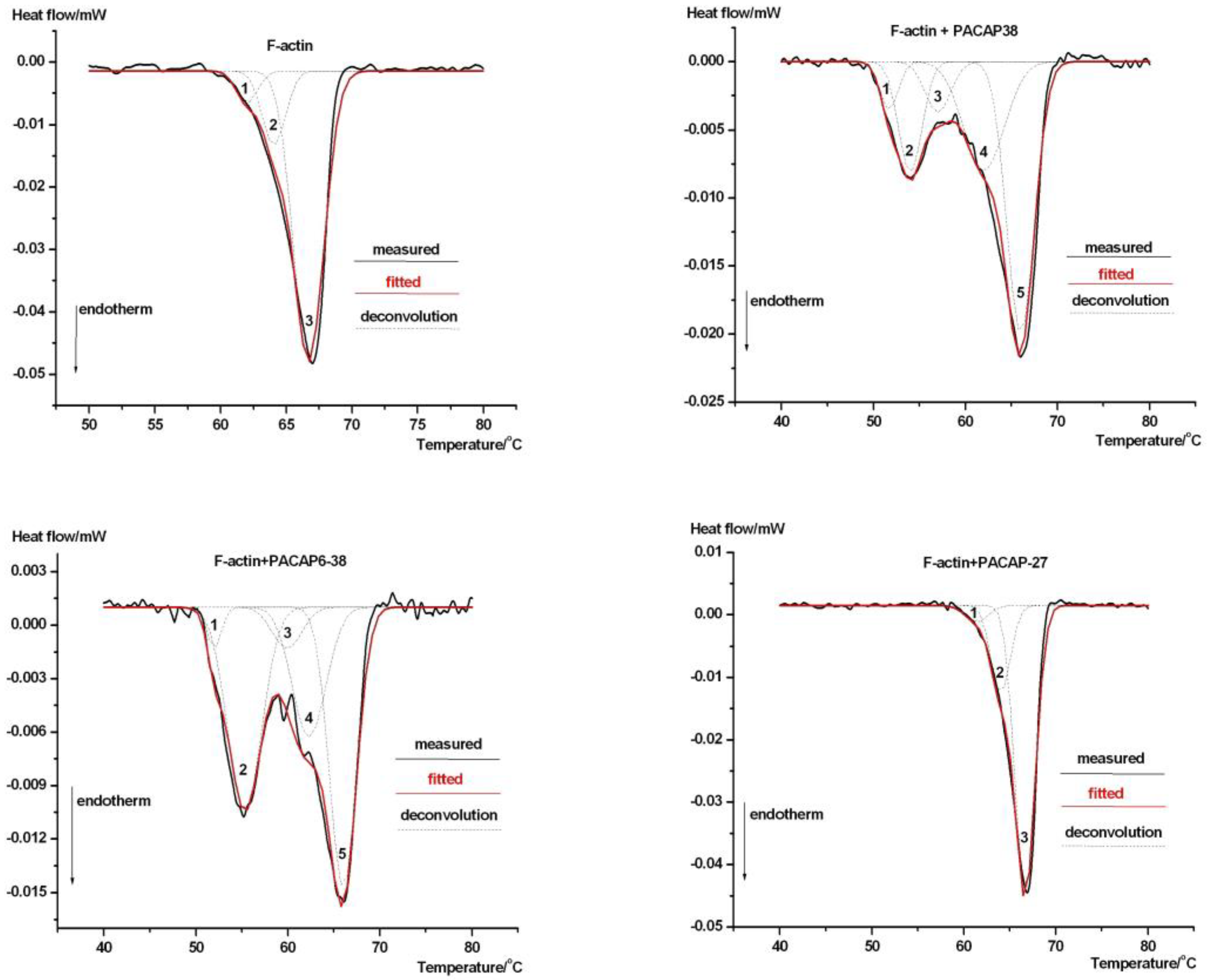
| Decomposition | T1 (°C) | T2 (°C) | T3 (°C) | T4 (°C) | T5 (°C) | ΔHTcal (J g−1) |
|---|---|---|---|---|---|---|
| F-actin | 62.0 | 64.1 | 66.7 | - | - | 0.039 ± 0.002 |
| % Area | 7.88 | 18.77 | 77.34 | - | - | |
| F-PACAP38 | 51.7 | 54.0 | 57.0 | 62.0 | 66.0 | 0.038 ± 0.003 |
| % Area | 8.09 | 18.84 | 8.55 | 18.50 | 46.24 | |
| F-PACAP6-38 | 52.0 | 55.3 | 59.9 | 62.3 | 66.0 | 0.032 ± 0.002 |
| % Area | 5.89 | 29.17 | 5.89 | 18.68 | 40.38 | |
| F-PACAP27 | 61.4 | 64.0 | 66.7 | - | - | 0.037 ± 0.003 |
| % Area | 4.20 | 22.10 | 73.20 | - | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Bukovics, P.; Lőrinczy, D. Deconvolution Analysis of G and F-Actin Unfolding: Insights into the Thermal Stability and Structural Modifications Induced by PACAP. Int. J. Mol. Sci. 2025, 26, 3336. https://doi.org/10.3390/ijms26073336
Bukovics P, Lőrinczy D. Deconvolution Analysis of G and F-Actin Unfolding: Insights into the Thermal Stability and Structural Modifications Induced by PACAP. International Journal of Molecular Sciences. 2025; 26(7):3336. https://doi.org/10.3390/ijms26073336
Chicago/Turabian StyleBukovics, Péter, and Dénes Lőrinczy. 2025. "Deconvolution Analysis of G and F-Actin Unfolding: Insights into the Thermal Stability and Structural Modifications Induced by PACAP" International Journal of Molecular Sciences 26, no. 7: 3336. https://doi.org/10.3390/ijms26073336
APA StyleBukovics, P., & Lőrinczy, D. (2025). Deconvolution Analysis of G and F-Actin Unfolding: Insights into the Thermal Stability and Structural Modifications Induced by PACAP. International Journal of Molecular Sciences, 26(7), 3336. https://doi.org/10.3390/ijms26073336








