Ex Vivo Plasma Application on Human Brain Microvascular Endothelial-like Cells for Blood–Brain Barrier Modeling
Abstract
1. Introduction
2. Results
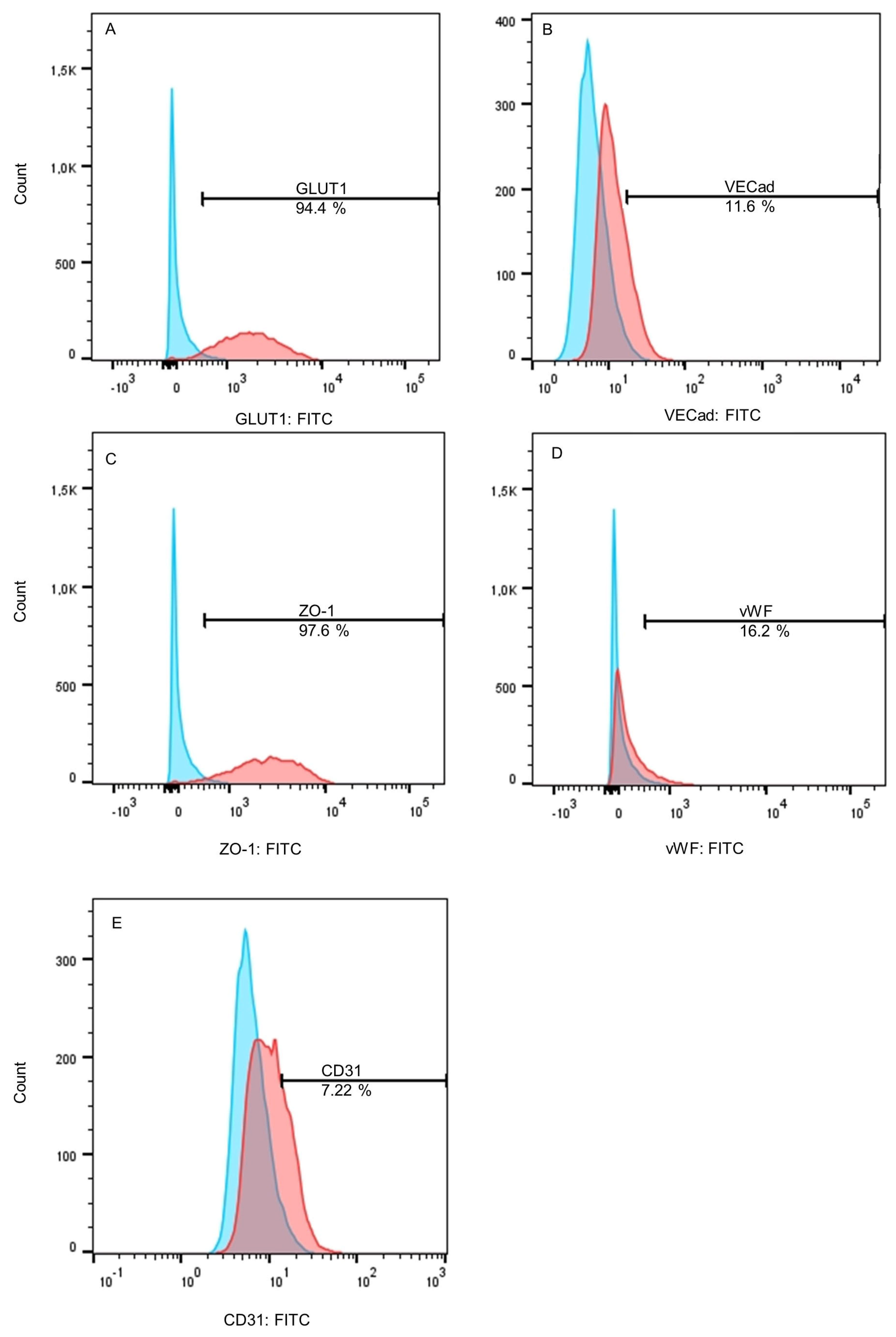
2.1. Differentiated BMEC-like Cells Lack Essential Endothelial Cell Markers
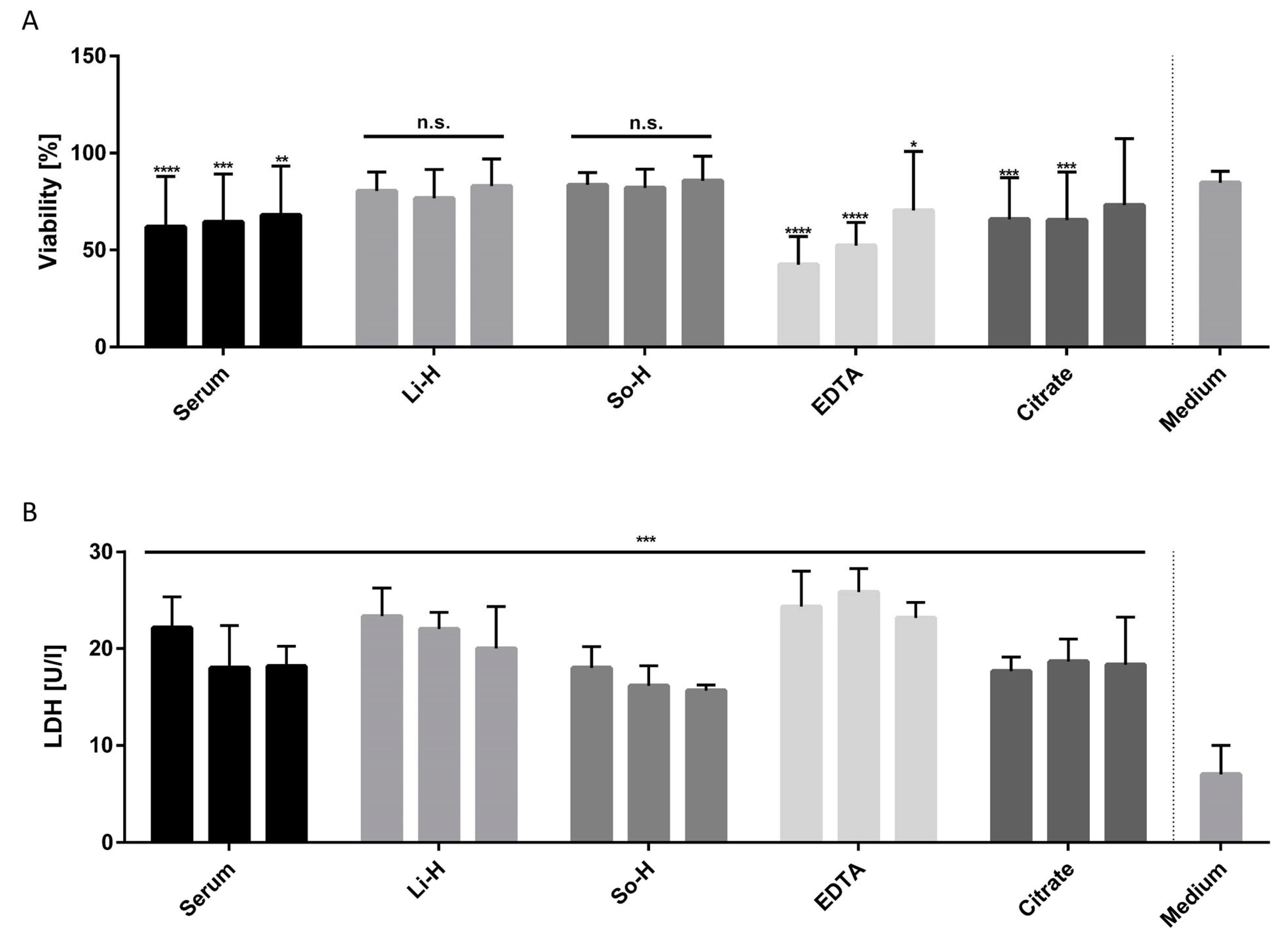
2.2. Undiluted Li-H and So-H Plasmas Can Be Applied on BMEC-like Cells Without Altering Viability
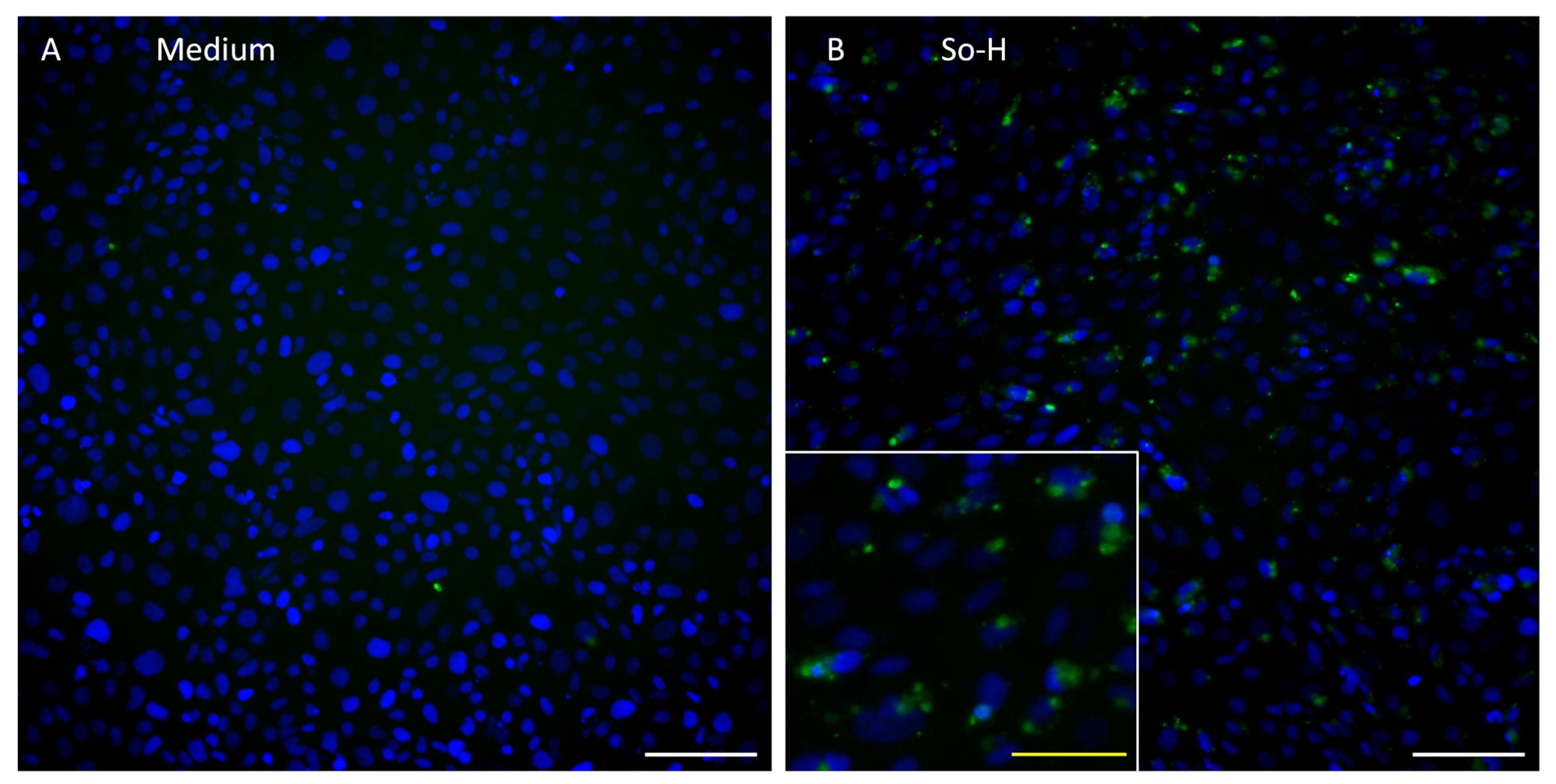
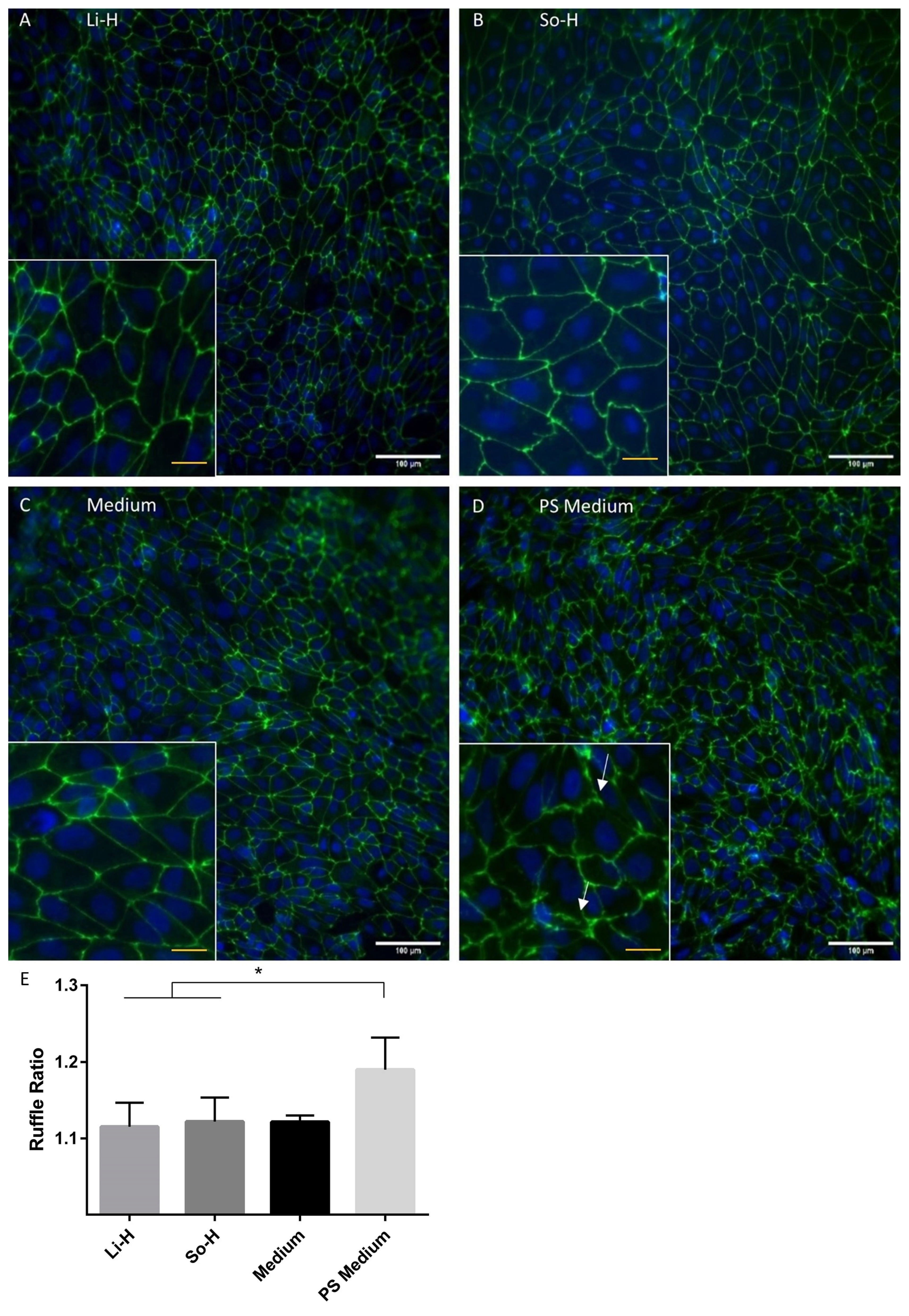
2.3. Barrier Properties Improve After Application of Undiluted So-H and Li-H Plasmas Without Significantly Altering Cell Density
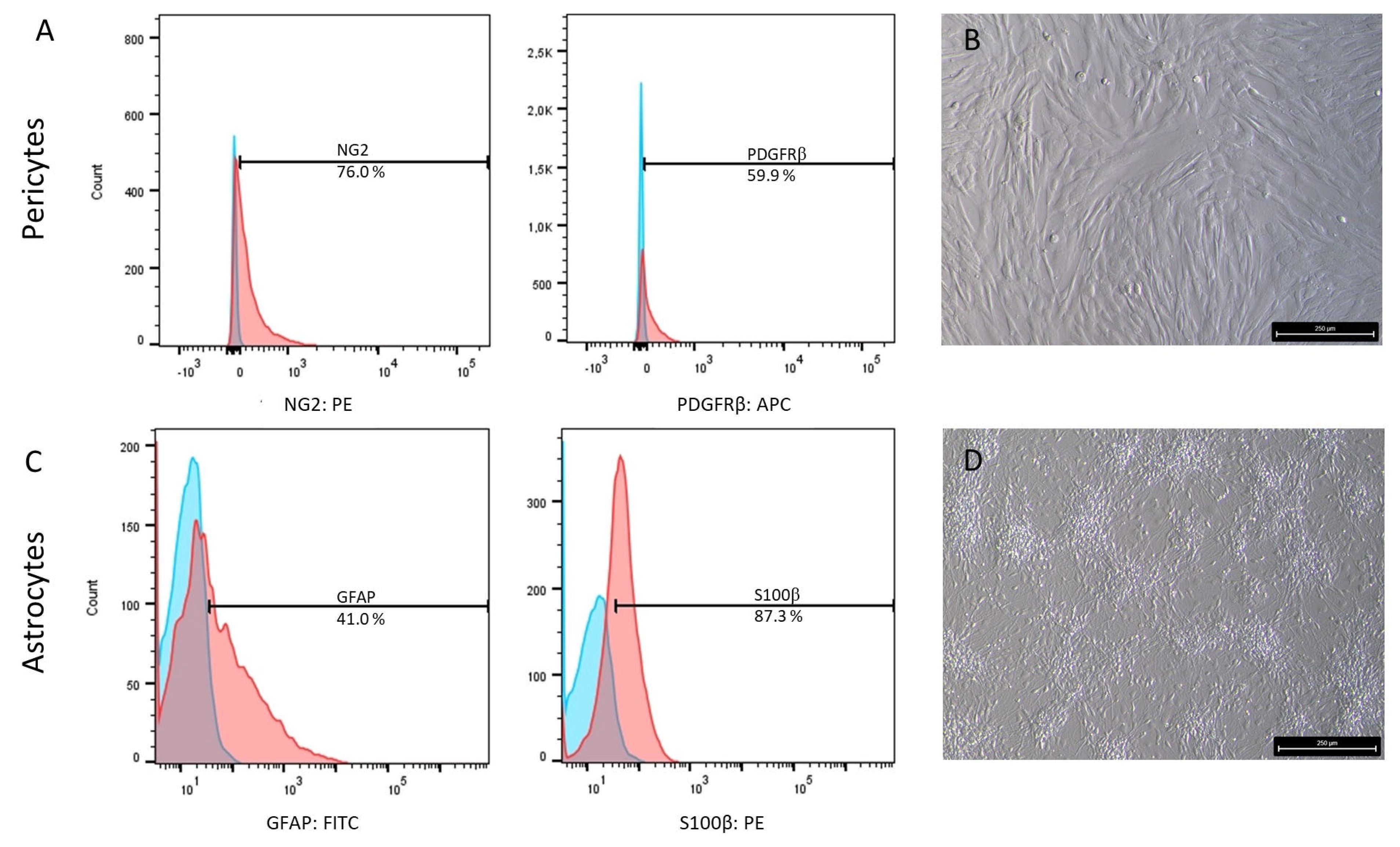
2.4. Characterization of Astrocyte and Pericyte Differentiation
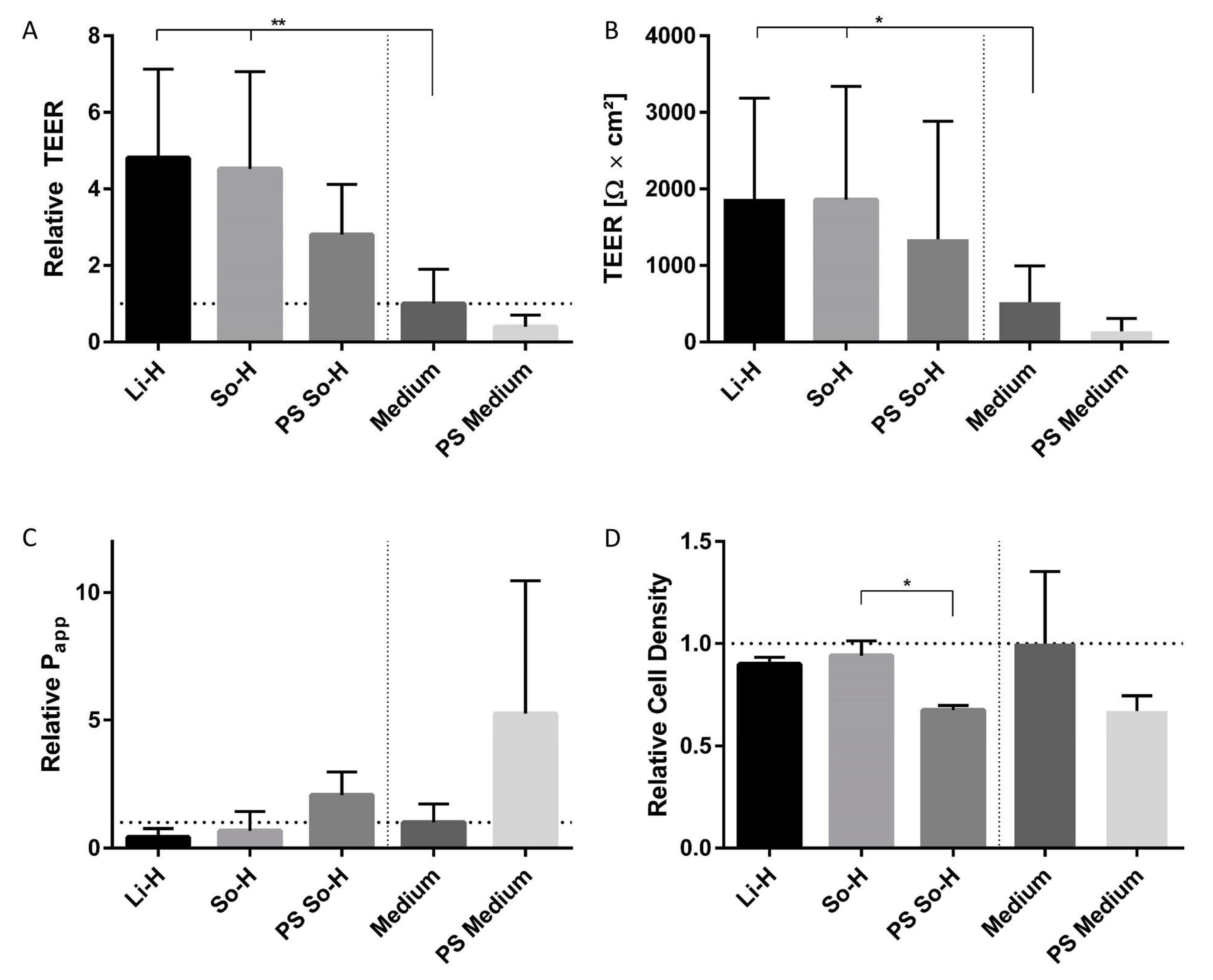
2.5. Gradual Assembly of the Coculture Model Confirms Positive Impact of Plasma Incubation and Coculture with Pericytes and Astrocytes on the Barrier Integrity of BMEC-like Cells
2.6. Continuous TEER Analysis Reveal Regenerative Effect of Coculture Under Plasma Treatment
3. Discussion
4. Materials and Methods
4.1. Human Plasma Pool
4.2. Cell Culture
4.2.1. Immortalized Human Cerebral Microvascular Endothelial Cell Line D3 (hCMEC/D3)
4.2.2. Brain Microvascular Endothelial Cell-like Cells (BMEC-like Cells)
4.2.3. Astrocytes and Pericytes
4.2.4. Establishment of a Triple-Culture BBB Model
4.3. Immunofluorescence Staining and Fluorescence-Activated Cell Sorting (FACS) Analysis
4.4. Endothelial Tube Formation Assay
4.5. Plasma and Inflammatory Treatment for Viability, LDH Secretion, and Barrier Analysis
4.6. Viability Analysis and Lactate Dehydrogenase Secretion
4.7. Barrier Functionality Analysis
4.7.1. Transendothelial Electrical Resistance
4.7.2. FITC-Dextran Permeability
4.8. Image Analysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| BBB | Blood–brain barrier |
| BMEC | Brain microvascular endothelial cells |
| CNS | Central nervous system |
| DAPI | 4′,6-diamidino-2-phenylindole |
| ECM +/+ | Endothelial culture medium with FGF and retinoic acid |
| ECM −/− | Endothelial culture medium without FGF and retinoic acid |
| ECGM | Endothelial cell growth medium |
| EDTA | Ethylenediaminetetraacetic acid |
| FACS | Fluorescence-activated cell sorting |
| FBS | Fetal bovine serum |
| FGF | Fibroblastic growth factor |
| GFAP | Glial fibrillary acidic protein |
| GLUT1 | Glucose transporter 1 |
| hiPSC | Human induced pluripotent stem cells |
| IL-1β | Interleukin-1β |
| IL-6 | Interleukin-6 |
| LDH | Lactate dehydrogenase |
| Li-H | Lithium heparin |
| LPS | Lipopolysaccharide |
| NG2 | Neural/glial antigen 2 |
| PDGFRβ | Platelet-derived growth factor receptor β |
| PS | Pathological stimulation |
| S100β | S100 calcium binding protein β |
| So-H | Sodium heparin |
| TC | Tissue culture |
| TNF-α | Tumor necrosis factor α |
| TEER | Transendothelial electrical resistance |
| VECad | Vascular endothelial cadherin |
| VEGF | Vascular endothelial growth factor |
| vWF | Von Willebrand factor |
| ZO-1 | Zonula Occludens-1 |
References
- Wu, D.; Chen, Q.; Chen, X.; Han, F.; Chen, Z.; Wang, Y. The blood-brain barrier: Structure, regulation, and drug delivery. Signal Transduct. Target. Ther. 2023, 8, 217. [Google Scholar] [CrossRef]
- Kadry, H.; Noorani, B.; Cucullo, L. A blood-brain barrier overview on structure, function, impairment, and biomarkers of integrity. Fluids Barriers CNS 2020, 17, 69. [Google Scholar] [CrossRef] [PubMed]
- Zhao, N.; Chung, T.D.; Guo, Z.; Jamieson, J.J.; Liang, L.; Linville, R.M.; Pessell, A.F.; Wang, L.; Searson, P.C. The influence of physiological and pathological perturbations on blood-brain barrier function. Front. Neurosci. 2023, 17, 1289894. [Google Scholar] [CrossRef]
- Kim, S.Y.; Buckwalter, M.; Soreq, H.; Vezzani, A.; Kaufer, D. Blood-brain barrier dysfunction-induced inflammatory signaling in brain pathology and epileptogenesis. Epilepsia 2012, 53 (Suppl. 6), 37–44. [Google Scholar] [CrossRef]
- Wei, W.; Cardes, F.; Hierlemann, A.; Modena, M.M. 3D In Vitro Blood-Brain-Barrier Model for Investigating Barrier Insults. Adv. Sci. 2023, 10, e2205752. [Google Scholar] [CrossRef]
- Sharma, B.; Luhach, K.; Kulkarni, G.T. 4—In vitro and in vivo models of BBB to evaluate brain targeting drug delivery. In Brain Targeted Drug Delivery Systems: A Focus on Nanotechnology and Nanoparticulates; Gao, H., Gao, X., Eds.; Elsevier Science & Technology: San Diego, CA, USA, 2018; pp. 53–101. ISBN 978-0-12-814001-7. [Google Scholar]
- Urich, E.; Lazic, S.E.; Molnos, J.; Wells, I.; Freskgård, P.-O. Transcriptional profiling of human brain endothelial cells reveals key properties crucial for predictive in vitro blood-brain barrier models. PLoS ONE 2012, 7, e38149. [Google Scholar] [CrossRef]
- Hoshi, Y.; Uchida, Y.; Tachikawa, M.; Inoue, T.; Ohtsuki, S.; Terasaki, T. Quantitative atlas of blood-brain barrier transporters, receptors, and tight junction proteins in rats and common marmoset. J. Pharm. Sci. 2013, 102, 3343–3355. [Google Scholar] [CrossRef]
- Wasielewska, J.M.; Da Silva Chaves, J.C.; White, A.R.; Oikari, L.E. Modeling the Blood–Brain Barrier to Understand Drug Delivery in Alzheimer’s Disease. In Alzheimer’s Disease: Drug Discovery; Huang, X., Ed.; Exon Publications: Brisbane City, QLD, Australia, 2020; ISBN 978-0-6450017-0-9. [Google Scholar]
- Qi, D.; Lin, H.; Hu, B.; Wei, Y. A review on in vitro model of the blood-brain barrier (BBB) based on hCMEC/D3 cells. J. Control. Release 2023, 358, 78–97. [Google Scholar] [CrossRef]
- Chaulagain, B.; Gothwal, A.; Lamptey, R.N.L.; Trivedi, R.; Mahanta, A.K.; Layek, B.; Singh, J. Experimental Models of In Vitro Blood-Brain Barrier for CNS Drug Delivery: An Evolutionary Perspective. Int. J. Mol. Sci. 2023, 24, 2710. [Google Scholar] [CrossRef]
- Delsing, L.; Herland, A.; Falk, A.; Hicks, R.; Synnergren, J.; Zetterberg, H. Models of the blood-brain barrier using iPSC-derived cells. Mol. Cell. Neurosci. 2020, 107, 103533. [Google Scholar] [CrossRef]
- Canfield, S.G.; Stebbins, M.J.; Faubion, M.G.; Gastfriend, B.D.; Palecek, S.P.; Shusta, E.V. An isogenic neurovascular unit model comprised of human induced pluripotent stem cell-derived brain microvascular endothelial cells, pericytes, astrocytes, and neurons. Fluids Barriers CNS 2019, 16, 25. [Google Scholar] [CrossRef] [PubMed]
- Delsing, L.; Dönnes, P.; Sánchez, J.; Clausen, M.; Voulgaris, D.; Falk, A.; Herland, A.; Brolén, G.; Zetterberg, H.; Hicks, R.; et al. Barrier Properties and Transcriptome Expression in Human iPSC-Derived Models of the Blood-Brain Barrier. Stem Cells 2018, 36, 1816–1827. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.M.; Houghton, S.; Magdeldin, T.; Durán, J.G.B.; Minotti, A.P.; Snead, A.; Sproul, A.; Nguyen, D.-H.T.; Xiang, J.; Fine, H.A.; et al. Pluripotent stem cell-derived epithelium misidentified as brain microvascular endothelium requires ETS factors to acquire vascular fate. Proc. Natl. Acad. Sci. USA 2021, 118, e2016950118. [Google Scholar] [CrossRef] [PubMed]
- Lippmann, E.S.; Azarin, S.M.; Palecek, S.P.; Shusta, E.V. Commentary on human pluripotent stem cell-based blood-brain barrier models. Fluids Barriers CNS 2020, 17, 64. [Google Scholar] [CrossRef]
- Armstrong, B. Haematology. ISBT Sci. Ser. 2020, 15, 46–53. [Google Scholar] [CrossRef]
- Andjelkovic, A.V.; Stamatovic, S.M.; Phillips, C.M.; Martinez-Revollar, G.; Keep, R.F. Modeling blood-brain barrier pathology in cerebrovascular disease in vitro: Current and future paradigms. Fluids Barriers CNS 2020, 17, 44. [Google Scholar] [CrossRef]
- Körtge, A.; Breitrück, A.; Doß, S.; Hofrichter, J.; Nelz, S.-C.; Krüsemann, H.; Wasserkort, R.; Fitzner, B.; Hecker, M.; Mitzner, S.; et al. The Utility of Miniaturized Adsorbers in Exploring the Cellular and Molecular Effects of Blood Purification: A Pilot Study with a Focus on Immunoadsorption in Multiple Sclerosis. Int. J. Mol. Sci. 2024, 25, 2590. [Google Scholar] [CrossRef]
- Lippmann, E.S.; Al-Ahmad, A.; Azarin, S.M.; Palecek, S.P.; Shusta, E.V. A retinoic acid-enhanced, multicellular human blood-brain barrier model derived from stem cell sources. Sci. Rep. 2014, 4, 4160. [Google Scholar] [CrossRef]
- Appelt-Menzel, A. Etablierung und Qualifizierung Eines Humanen Blut-Hirn-Schranken-Modells Unter Verwendung von Induziert Pluripotenten und Multipotenten Stammzellen. Ph.D. Thesis, Universität Würzburg, Würzburg, Germany, 2016. Available online: https://opus.bibliothek.uni-wuerzburg.de/frontdoor/index/index/docId/13464 (accessed on 3 March 2025).
- Zhang, P.; Policha, A.; Tulenko, T.; DiMuzio, P. Autologous human plasma in stem cell culture and cryopreservation in the creation of a tissue-engineered vascular graft. J. Vasc. Surg. 2016, 63, 805–814. [Google Scholar] [CrossRef]
- World Health Organization. Diagnostic Imaging and Laboratory Technology. In Use of Anticoagulants in Diagnostic Laboratory Investigations; WHO/DIL/LAB/99.1 Rev.2; WHO: Geneve, Switzerland, 2012; Available online: https://iris.who.int/handle/10665/65957 (accessed on 3 March 2025).
- Singh, S.; Dodt, J.; Volkers, P.; Hethershaw, E.; Philippou, H.; Ivaskevicius, V.; Imhof, D.; Oldenburg, J.; Biswas, A. Structure functional insights into calcium binding during the activation of coagulation factor XIII A. Sci. Rep. 2019, 9, 11324. [Google Scholar] [CrossRef]
- Banfi, G.; Salvagno, G.L.; Lippi, G. The role of ethylenediamine tetraacetic acid (EDTA) as in vitro anticoagulant for diagnostic purposes. Clin. Chem. Lab. Med. 2007, 45, 565–576. [Google Scholar] [CrossRef] [PubMed]
- Demuynck, T.; Grooteman, M.; ter Wee, P.; Cozzolino, M.; Meijers, B. Regional Citrate Anticoagulation: A Tale of More Than Two Stories. Semin. Nephrol. 2023, 43, 151481. [Google Scholar] [CrossRef] [PubMed]
- Heestermans, M.; Poenou, G.; Hamzeh-Cognasse, H.; Cognasse, F.; Bertoletti, L. Anticoagulants: A Short History, Their Mechanism of Action, Pharmacology, and Indications. Cells 2022, 11, 3214. [Google Scholar] [CrossRef]
- Damus, P.S.; Hicks, M.; Rosenberg, R.D. Anticoagulant action of heparin. Nature 1973, 246, 355–357. [Google Scholar] [CrossRef]
- Lai, T.-Y.; Cao, J.; Ou-Yang, P.; Tsai, C.-Y.; Lin, C.-W.; Chen, C.-C.; Tsai, M.-K.; Lee, C.-Y. Different methods of detaching adherent cells and their effects on the cell surface expression of Fas receptor and Fas ligand. Sci. Rep. 2022, 12, 5713. [Google Scholar] [CrossRef]
- Feng, H.; Guo, L.; Gao, H.; Li, X.-A. Deficiency of calcium and magnesium induces apoptosis via scavenger receptor BI. Life Sci. 2011, 88, 606–612. [Google Scholar] [CrossRef]
- Aaseth, J. Chelation Therapy in the Treatment of Metal Intoxication; Elsevier Science & Technology: San Diego, CA, USA, 2016; ISBN 9780128030738. [Google Scholar]
- Wilhelm, I.; Farkas, A.E.; Nagyoszi, P.; Váró, G.; Bálint, Z.; Végh, G.A.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Krizbai, I.A. Regulation of cerebral endothelial cell morphology by extracellular calcium. Phys. Med. Biol. 2007, 52, 6261–6274. [Google Scholar] [CrossRef]
- Talmor-Barkan, Y.; Rashid, G.; Weintal, I.; Green, J.; Bernheim, J.; Benchetrit, S. Low extracellular Ca2+: A mediator of endothelial inflammation. Nephrol. Dial. Transplant. 2009, 24, 3306–3312. [Google Scholar] [CrossRef]
- Pepe, J.; Colangelo, L.; Biamonte, F.; Sonato, C.; Danese, V.C.; Cecchetti, V.; Occhiuto, M.; Piazzolla, V.; de Martino, V.; Ferrone, F.; et al. Diagnosis and management of hypocalcemia. Endocrine 2020, 69, 485–495. [Google Scholar] [CrossRef]
- Keowmaneechai, E.; McClements, D.J. Influence of EDTA and citrate on physicochemical properties of whey protein-stabilized oil-in-water emulsions containing CaCl2. J. Agric. Food Chem. 2002, 50, 7145–7153. [Google Scholar] [CrossRef] [PubMed]
- Locatelli, L.; Fedele, G.; Castiglioni, S.; Maier, J.A. Magnesium Deficiency Induces Lipid Accumulation in Vascular Endothelial Cells via Oxidative Stress—The Potential Contribution of EDF-1 and PPARγ. Int. J. Mol. Sci. 2021, 22, 1050. [Google Scholar] [CrossRef] [PubMed]
- Kostov, K.; Halacheva, L. Role of Magnesium Deficiency in Promoting Atherosclerosis, Endothelial Dysfunction, and Arterial Stiffening as Risk Factors for Hypertension. Int. J. Mol. Sci. 2018, 19, 1724. [Google Scholar] [CrossRef] [PubMed]
- Granholm, K.; Harju, L.; Ivaska, A. Desorption of metal ions from kraft pulps. Part 2. Chelation of kraft pulps with different complexing agents and with EDTA in a reducing environment. BioResource 2010, 5, 227–243. [Google Scholar] [CrossRef]
- Narayanan, S. The preanalytic phase. An important component of laboratory medicine. Am. J. Clin. Pathol. 2000, 113, 429–452. [Google Scholar] [CrossRef]
- Carey, R.N.; Jani, C.; Johnson, C.; Pearce, J.; Hui-Ng, P.; Lacson, E. Chemistry Testing on Plasma Versus Serum Samples in Dialysis Patients: Clinical and Quality Improvement Implications. Clin. J. Am. Soc. Nephrol. 2016, 11, 1675–1679. [Google Scholar] [CrossRef]
- Guequén, A.; Zamorano, P.; Córdova, F.; Koning, T.; Torres, A.; Ehrenfeld, P.; Boric, M.P.; Salazar-Onfray, F.; Gavard, J.; Durán, W.N.; et al. Interleukin-8 Secreted by Glioblastoma Cells Induces Microvascular Hyperpermeability Through NO Signaling Involving S-Nitrosylation of VE-Cadherin and p120 in Endothelial Cells. Front. Physiol. 2019, 10, 988. [Google Scholar] [CrossRef]
- Gallagher, L.T.; LaCroix, I.; Fields, A.T.; Mitra, S.; Argabright, A.; D’Alessandro, A.; Erickson, C.; Nunez-Garcia, B.; Herrera-Rodriguez, K.; Chou, Y.C.; et al. Platelet releasates mitigate the endotheliopathy of trauma. J. Trauma Acute Care Surg. 2024, 97, 738–746. [Google Scholar] [CrossRef]
- Barichello, T.; Generoso, J.S.; Collodel, A.; Petronilho, F.; Dal-Pizzol, F. The blood-brain barrier dysfunction in sepsis. Tissue Barriers 2021, 9, 1840912. [Google Scholar] [CrossRef]
- Opal, S.M.; Scannon, P.J.; Vincent, J.L.; White, M.; Carroll, S.F.; Palardy, J.E.; Parejo, N.A.; Pribble, J.P.; Lemke, J.H. Relationship between plasma levels of lipopolysaccharide (LPS) and LPS-binding protein in patients with severe sepsis and septic shock. J. Infect. Dis. 1999, 180, 1584–1589. [Google Scholar] [CrossRef]
- Jiang, S.; Shi, D.; Bai, L.; Niu, T.; Kang, R.; Liu, Y. Inhibition of interleukin-6 trans-signaling improves survival and prevents cognitive impairment in a mouse model of sepsis. Int. Immunopharmacol. 2023, 119, 110169. [Google Scholar] [CrossRef] [PubMed]
- Skoutelis, A.T.M.; Kaleridis, V.M.; Athanassiou, G.M.P.; Kokkinis, K.I.M.; Missirlis, Y.F.P.; Bassaris, H.P.M. Neutrophil deformability in patients with sepsis, septic shock, and adult respiratory distress syndrome. Crit. Care Med. 2000, 28, 2355–2359. [Google Scholar] [CrossRef] [PubMed]
- Rosenberg-Hasson, Y.; Hansmann, L.; Liedtke, M.; Herschmann, I.; Maecker, H.T. Effects of serum and plasma matrices on multiplex immunoassays. Immunol. Res. 2014, 58, 224–233. [Google Scholar] [CrossRef] [PubMed]
- Selby, C. Interference in immunoassay. Ann. Clin. Biochem. 1999, 36 Pt 6, 704–721. [Google Scholar] [CrossRef]
- Duran-Güell, M.; Flores-Costa, R.; Casulleras, M.; López-Vicario, C.; Titos, E.; Díaz, A.; Alcaraz-Quiles, J.; Horrillo, R.; Costa, M.; Fernández, J.; et al. Albumin protects the liver from tumor necrosis factor α-induced immunopathology. FASEB J. 2021, 35, e21365. [Google Scholar] [CrossRef]
- Haferkamp, U.; Hartmann, C.; Abid, C.L.; Brachner, A.; Höchner, A.; Gerhartl, A.; Harwardt, B.; Leckzik, S.; Leu, J.; Metzger, M.; et al. Human isogenic cells of the neurovascular unit exert transcriptomic cell type-specific effects on a blood-brain barrier in vitro model of late-onset Alzheimer disease. Fluids Barriers CNS 2023, 20, 78. [Google Scholar] [CrossRef]
- Vis, M.A.M.; Ito, K.; Hofmann, S. Impact of Culture Medium on Cellular Interactions in in vitro Co-culture Systems. Front. Bioeng. Biotechnol. 2020, 8, 911. [Google Scholar] [CrossRef]
- Darland, D.C.; Massingham, L.J.; Smith, S.R.; Piek, E.; Saint-Geniez, M.; D’Amore, P.A. Pericyte production of cell-associated VEGF is differentiation-dependent and is associated with endothelial survival. Dev. Biol. 2003, 264, 275–288. [Google Scholar] [CrossRef]
- Stratman, A.N.; Malotte, K.M.; Mahan, R.D.; Davis, M.J.; Davis, G.E. Pericyte recruitment during vasculogenic tube assembly stimulates endothelial basement membrane matrix formation. Blood 2009, 114, 5091–5101. [Google Scholar] [CrossRef]
- Winkler, E.A.; Bell, R.D.; Zlokovic, B.V. Central nervous system pericytes in health and disease. Nat. Neurosci. 2011, 14, 1398–1405. [Google Scholar] [CrossRef]
- Bell, R.D.; Winkler, E.A.; Sagare, A.P.; Singh, I.; LaRue, B.; Deane, R.; Zlokovic, B.V. Pericytes control key neurovascular functions and neuronal phenotype in the adult brain and during brain aging. Neuron 2010, 68, 409–427. [Google Scholar] [CrossRef] [PubMed]
- Abbott, N.J.; Rönnbäck, L.; Hansson, E. Astrocyte-endothelial interactions at the blood-brain barrier. Nat. Rev. Neurosci. 2006, 7, 41–53. [Google Scholar] [CrossRef] [PubMed]
- Dehouck, M.P.; Méresse, S.; Delorme, P.; Fruchart, J.C.; Cecchelli, R. An easier, reproducible, and mass-production method to study the blood-brain barrier in vitro. J. Neurochem. 1990, 54, 1798–1801. [Google Scholar] [CrossRef] [PubMed]
- Nelz, S. Created in BioRender. 2025. Available online: https://BioRender.com/y15n325 (accessed on 3 March 2025).
- Sert, A. STEMdiff™ Astrocyte Differentiation Kit. Available online: https://cdn.stemcell.com/media/files/pis/10000006879-PIS_06.pdf (accessed on 8 January 2025).
- Chomiak, M. STEMdiff™ Neural Crest Differentiation Kit. Available online: https://cdn.stemcell.com/media/files/pis/10000005429-PIS_01.pdf (accessed on 8 January 2025).
- Gastfriend, B.D.; Stebbins, M.J.; Du, F.; Shusta, E.V.; Palecek, S.P. Differentiation of Brain Pericyte-Like Cells from Human Pluripotent Stem Cell-Derived Neural Crest. Curr. Protoc. 2021, 1, e21. [Google Scholar] [CrossRef]
- Protocol for Neuron-Astrocyte-Microglia 2D Co-Culture. Available online: https://www.stemcell.com/how-to-tri-culture-hpsc-derived-forebrain-neurons-astrocytes-and-microglia.html#more (accessed on 20 November 2024).
- Siflinger-Birnboim, A.; Del Vecchio, P.J.; Cooper, J.A.; Blumenstock, F.A.; Shepard, J.M.; Malik, A.B. Molecular sieving characteristics of the cultured endothelial monolayer. J. Cell. Physiol. 1987, 132, 111–117. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An open-source platform for biological-image analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef]
- Tokuda, S.; Higashi, T.; Furuse, M. ZO-1 knockout by TALEN-mediated gene targeting in MDCK cells: Involvement of ZO-1 in the regulation of cytoskeleton and cell shape. PLoS ONE 2014, 9, e104994. [Google Scholar] [CrossRef]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open source software for digital pathology image analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef]
- Weksler, B.; Romero, I.A.; Couraud, P.-O. The hCMEC/D3 cell line as a model of the human blood brain barrier. Fluids Barriers CNS 2013, 10, 16. [Google Scholar] [CrossRef]




Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nelz, S.-C.; Lück, E.; Schölzel, A.; Sauer, M.; Heskamp, J.; Doss, S. Ex Vivo Plasma Application on Human Brain Microvascular Endothelial-like Cells for Blood–Brain Barrier Modeling. Int. J. Mol. Sci. 2025, 26, 3334. https://doi.org/10.3390/ijms26073334
Nelz S-C, Lück E, Schölzel A, Sauer M, Heskamp J, Doss S. Ex Vivo Plasma Application on Human Brain Microvascular Endothelial-like Cells for Blood–Brain Barrier Modeling. International Journal of Molecular Sciences. 2025; 26(7):3334. https://doi.org/10.3390/ijms26073334
Chicago/Turabian StyleNelz, Sophie-Charlotte, Elisabeth Lück, Anne Schölzel, Martin Sauer, Jacqueline Heskamp, and Sandra Doss. 2025. "Ex Vivo Plasma Application on Human Brain Microvascular Endothelial-like Cells for Blood–Brain Barrier Modeling" International Journal of Molecular Sciences 26, no. 7: 3334. https://doi.org/10.3390/ijms26073334
APA StyleNelz, S.-C., Lück, E., Schölzel, A., Sauer, M., Heskamp, J., & Doss, S. (2025). Ex Vivo Plasma Application on Human Brain Microvascular Endothelial-like Cells for Blood–Brain Barrier Modeling. International Journal of Molecular Sciences, 26(7), 3334. https://doi.org/10.3390/ijms26073334






