Modeling Spinal Cord Injury in a Dish with Hyperosmotic Stress: Population-Specific Effects and the Modulatory Role of Mesenchymal Stromal Cell Secretome
Abstract
1. Introduction
2. Results
2.1. Development of a Mixed-Cell Spinal Cord Culture System
2.2. Characterization of Cell Adhesion and Growth
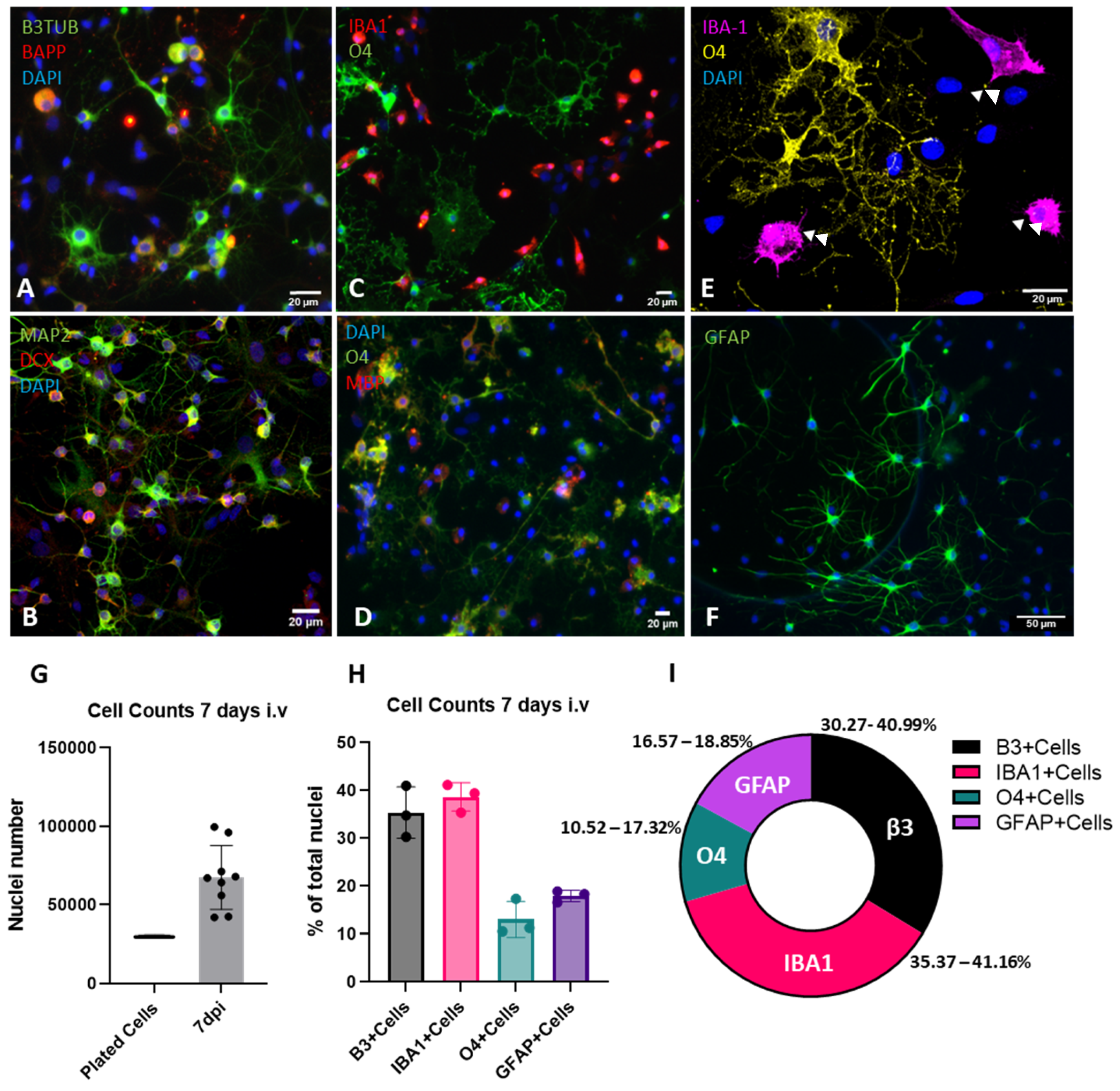
2.3. Characterization of Cellular Populations
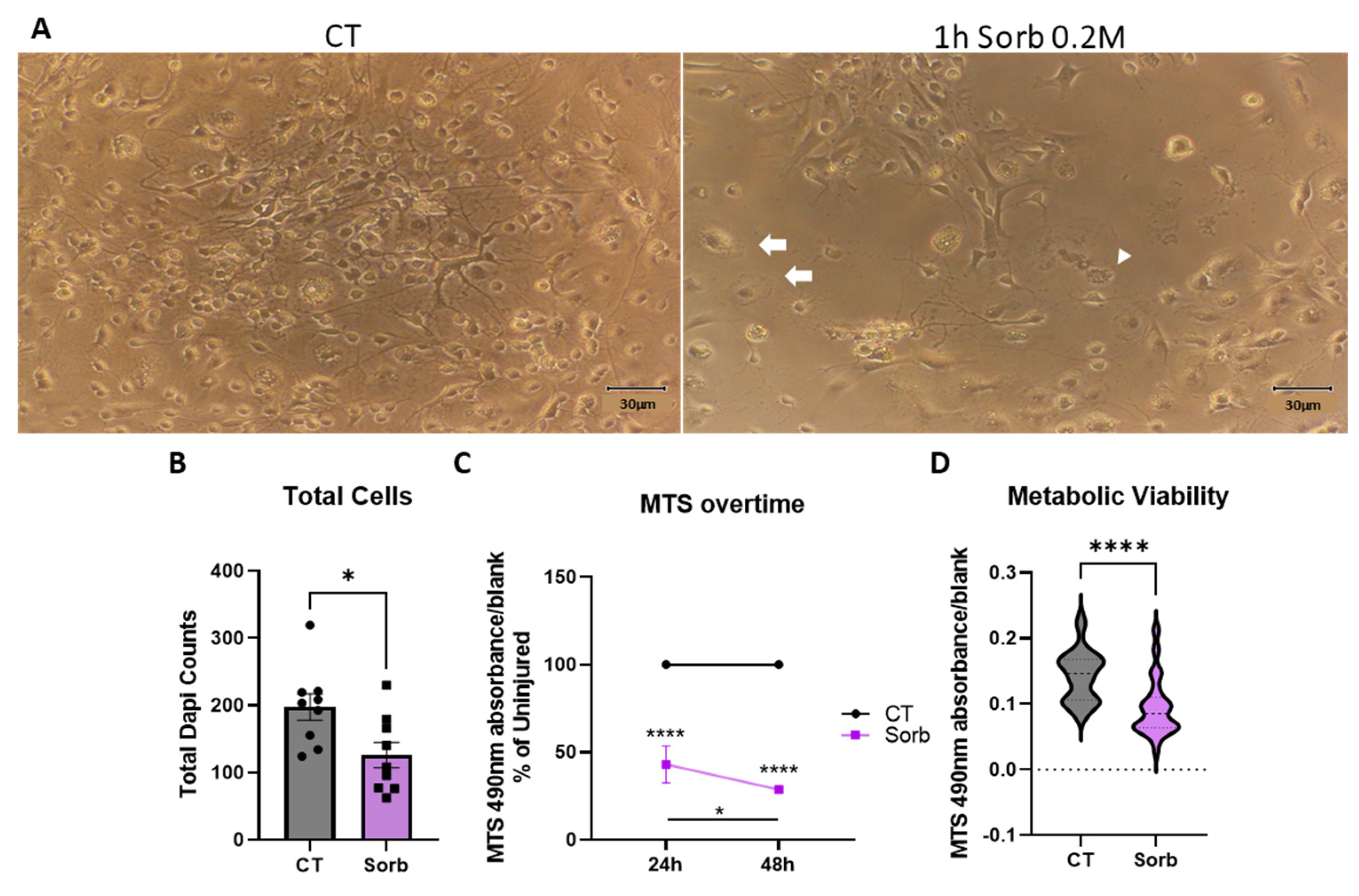
2.4. Development of a Hypertonic Osmotic Shock SCI Model in a Dish
2.5. The Secretome from Adipose-Derived Mesenchymal Stromal Cells Is Cytoprotective Against Hyperosmolar Stress in Mixed Spinal Cord Cells
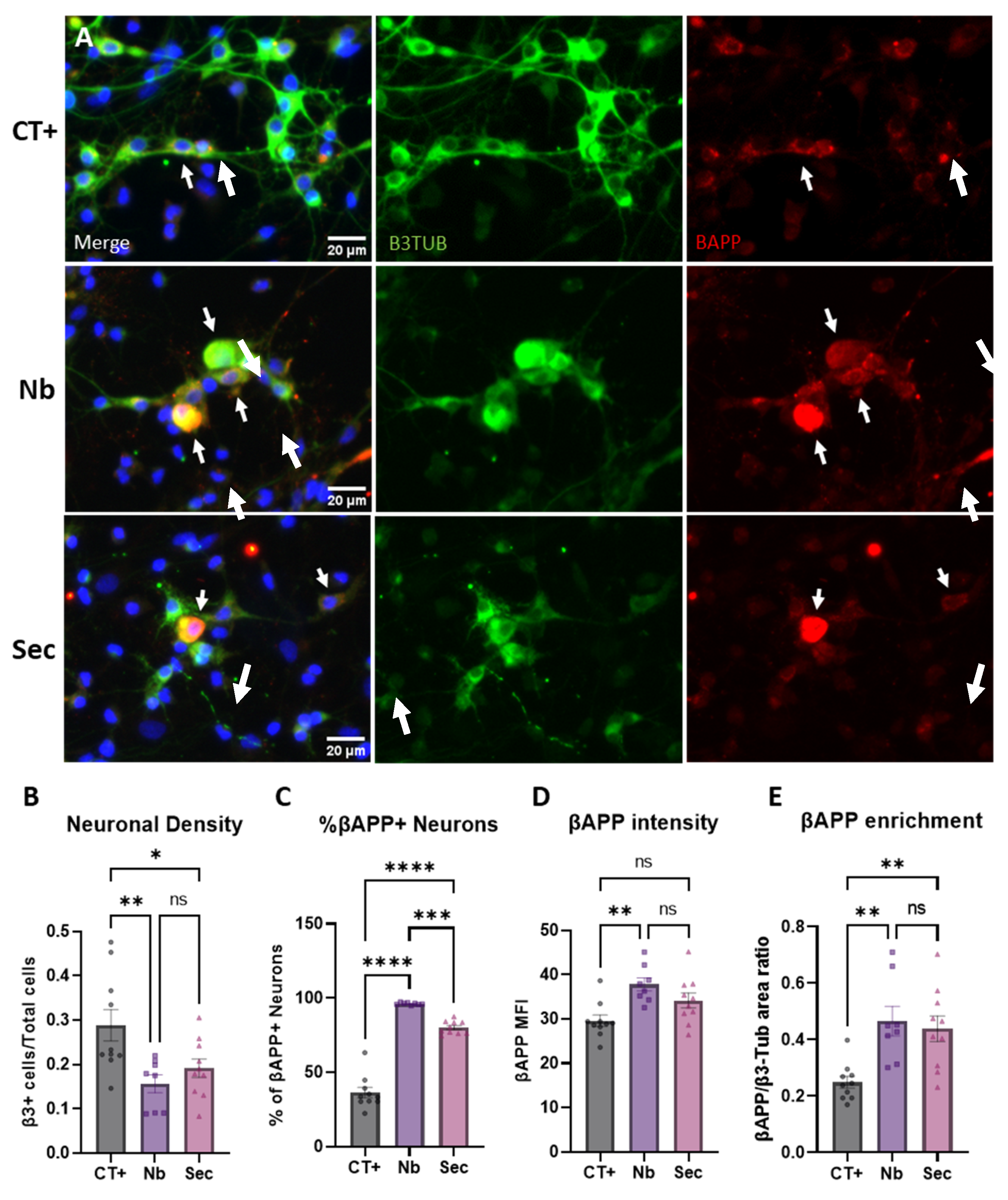
2.6. Neuronal Phenotypic Response to Sorbitol Injury and Effects of the ASC Secretome
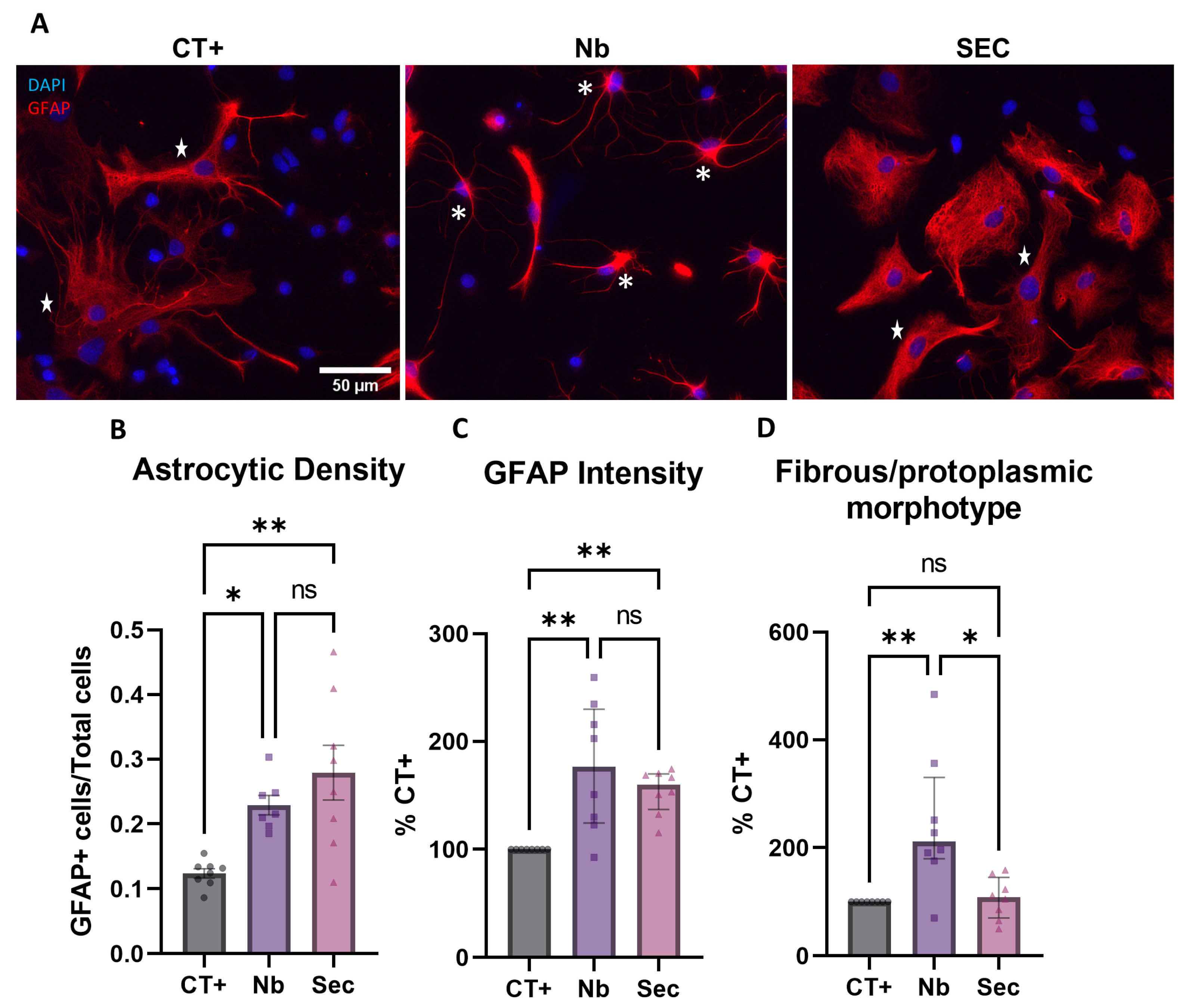
2.7. Astroglial Phenotypic Response to Sorbitol Injury and Effects of the hASC Secretome
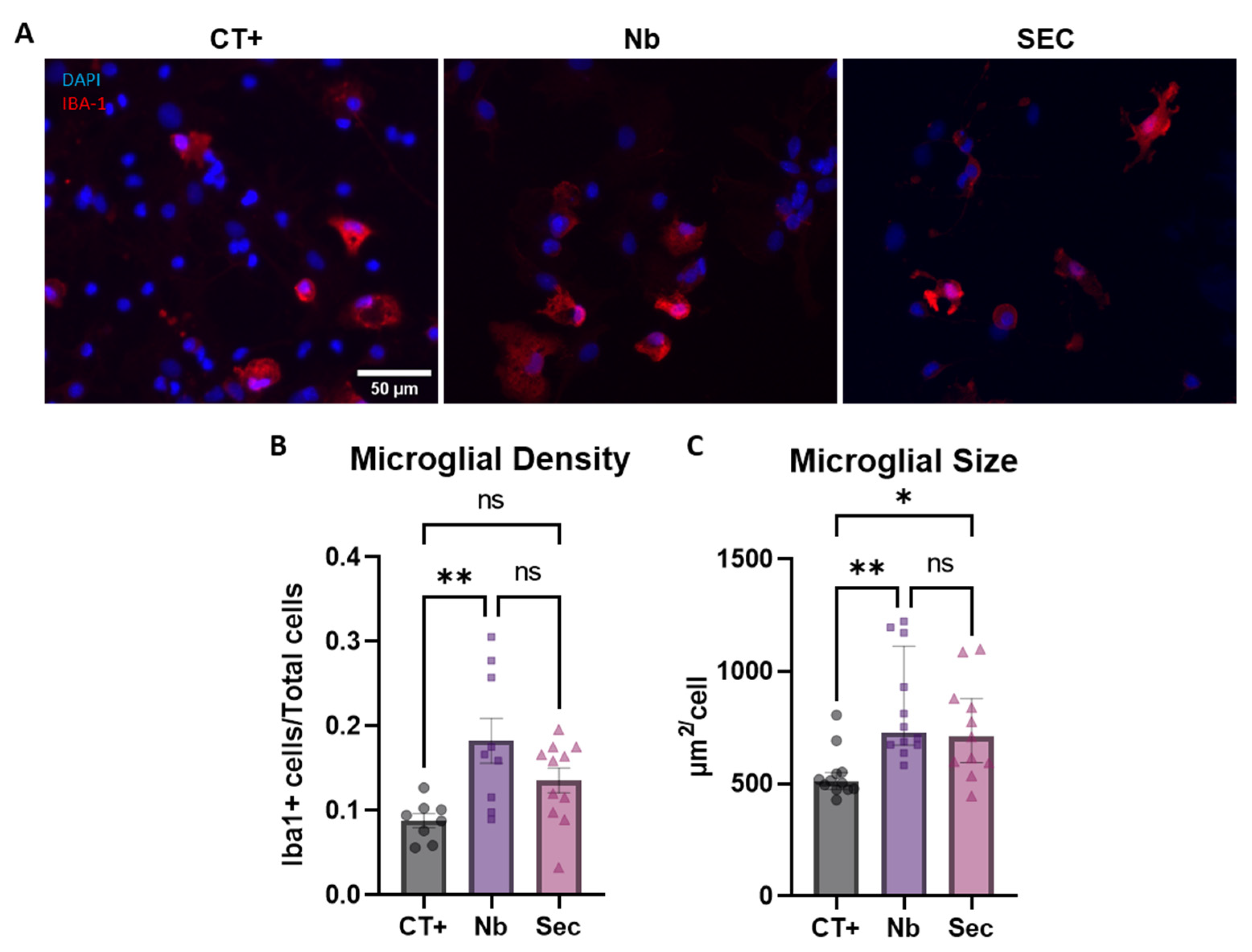
2.8. Microglial Phenotypic Response to Sorbitol Injury and Effects of the hASC Secretome
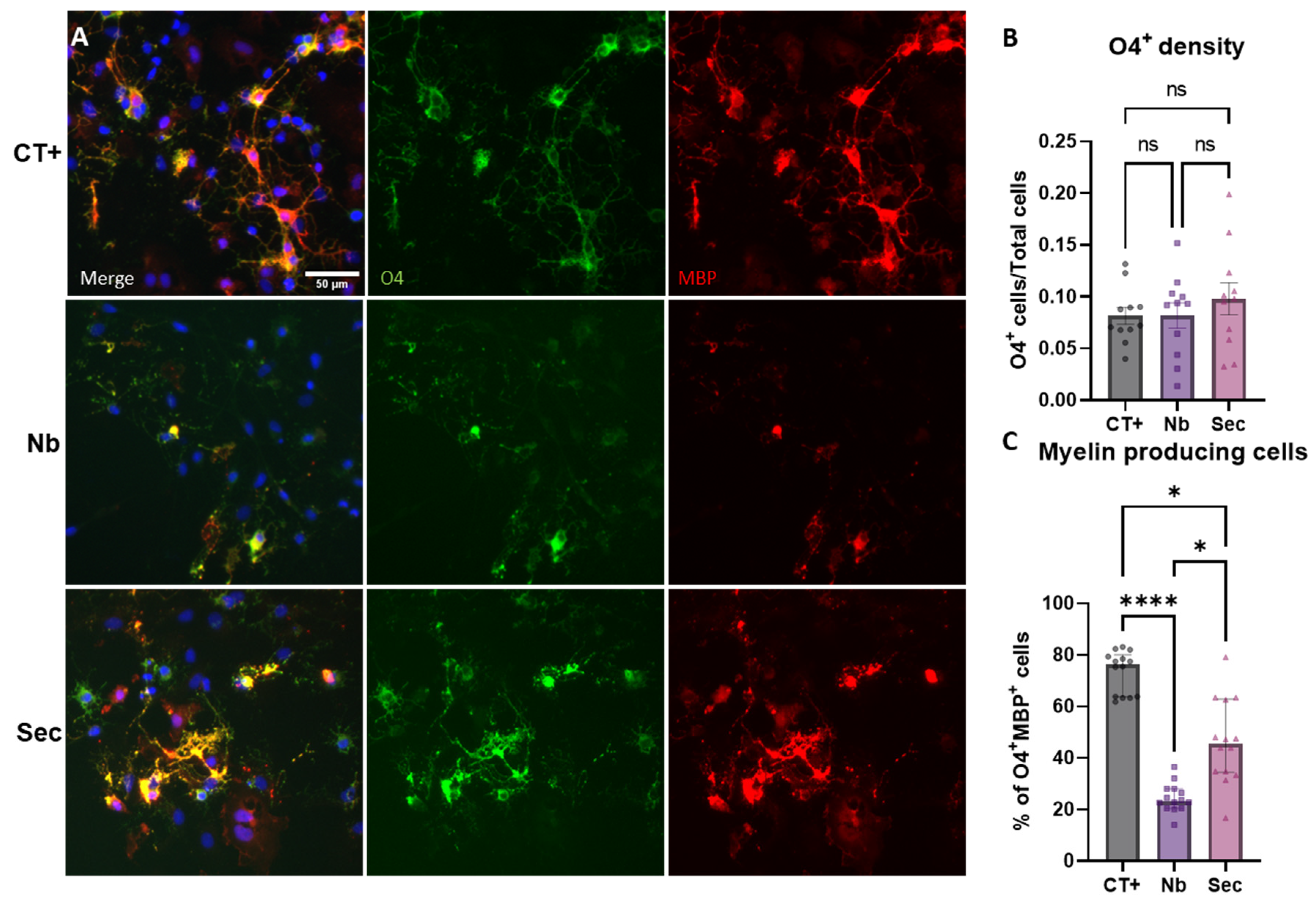
2.9. Oligodendrocytes’ Phenotypic Responses to Hypertonic Stress Injury and the Effects of the hASC Secretome
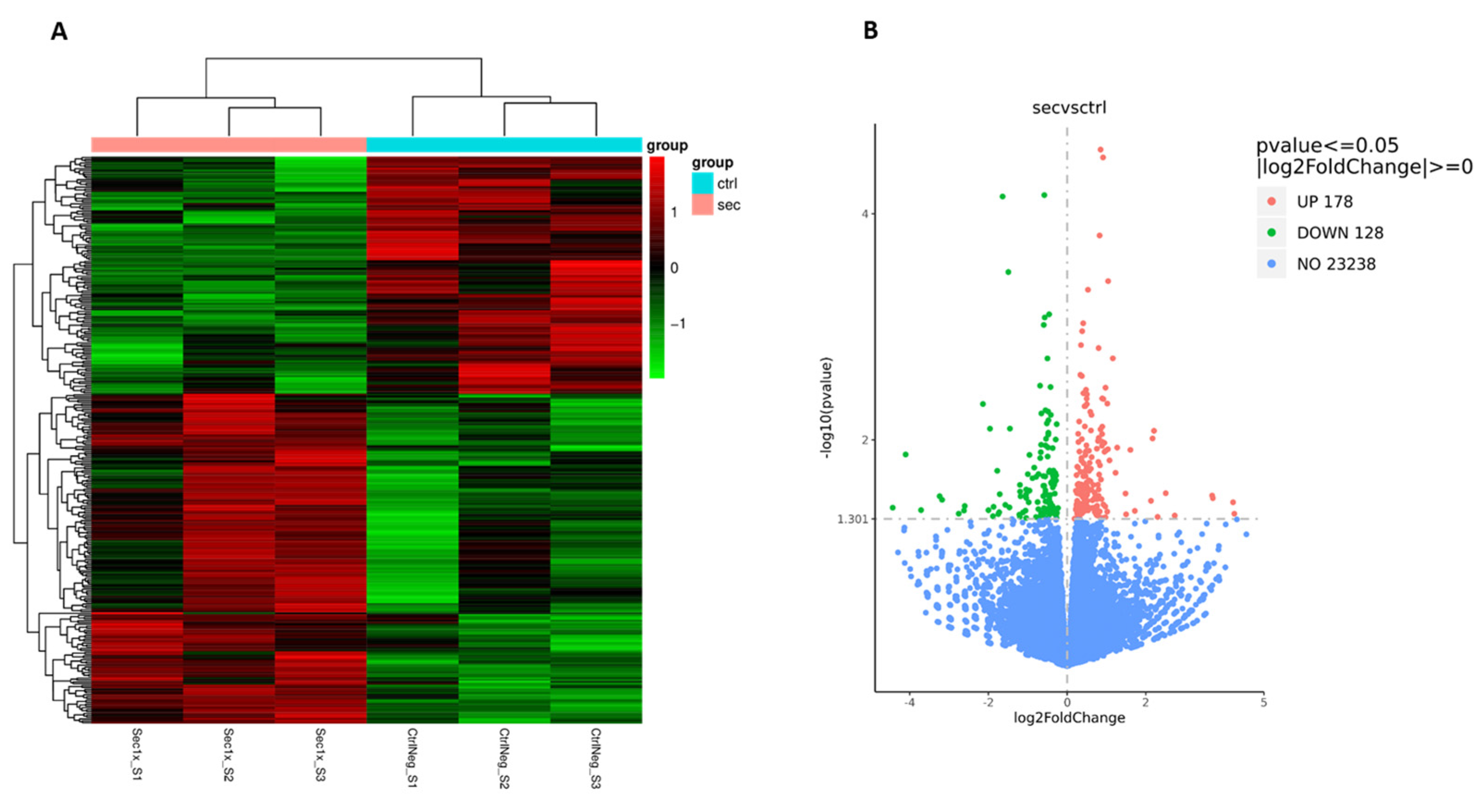
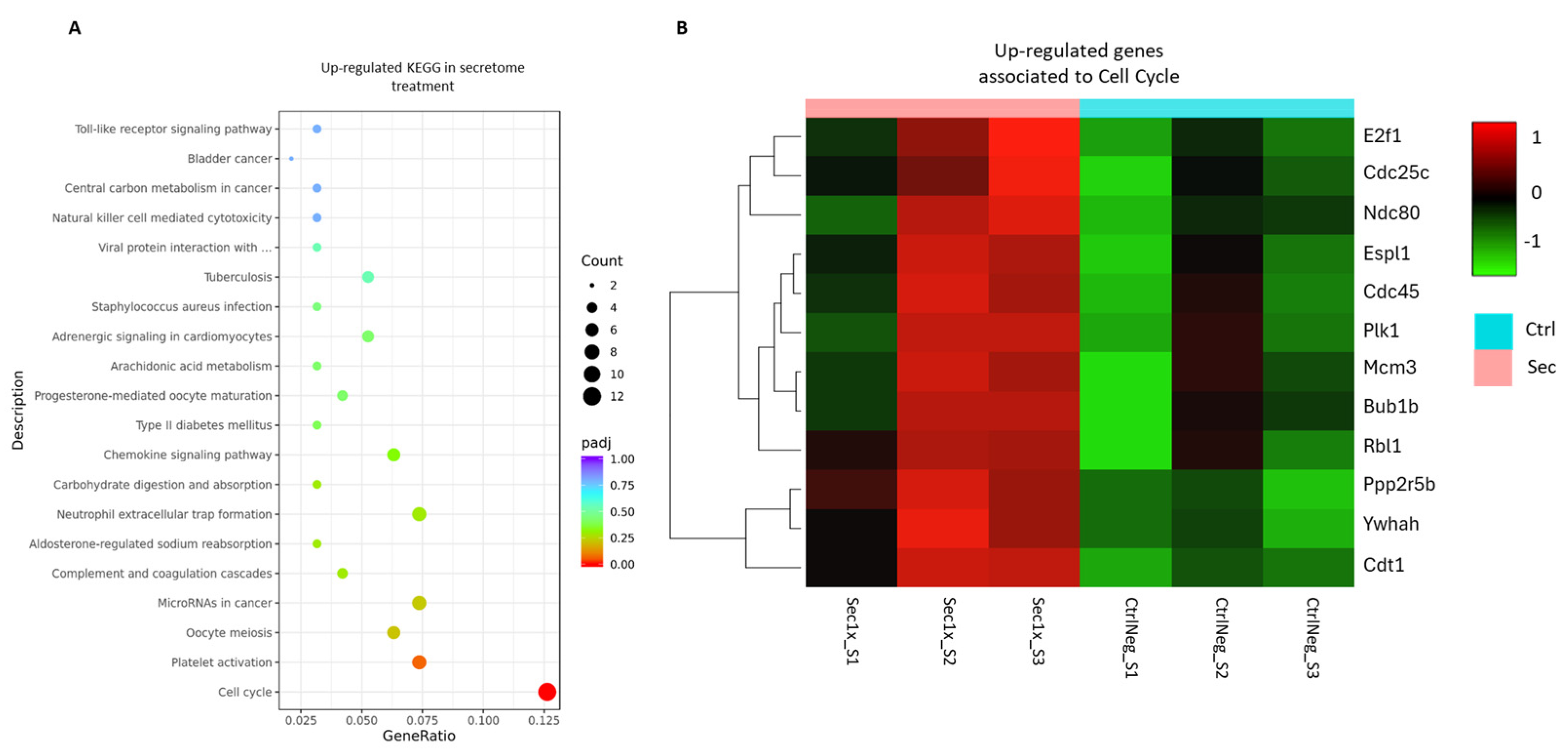
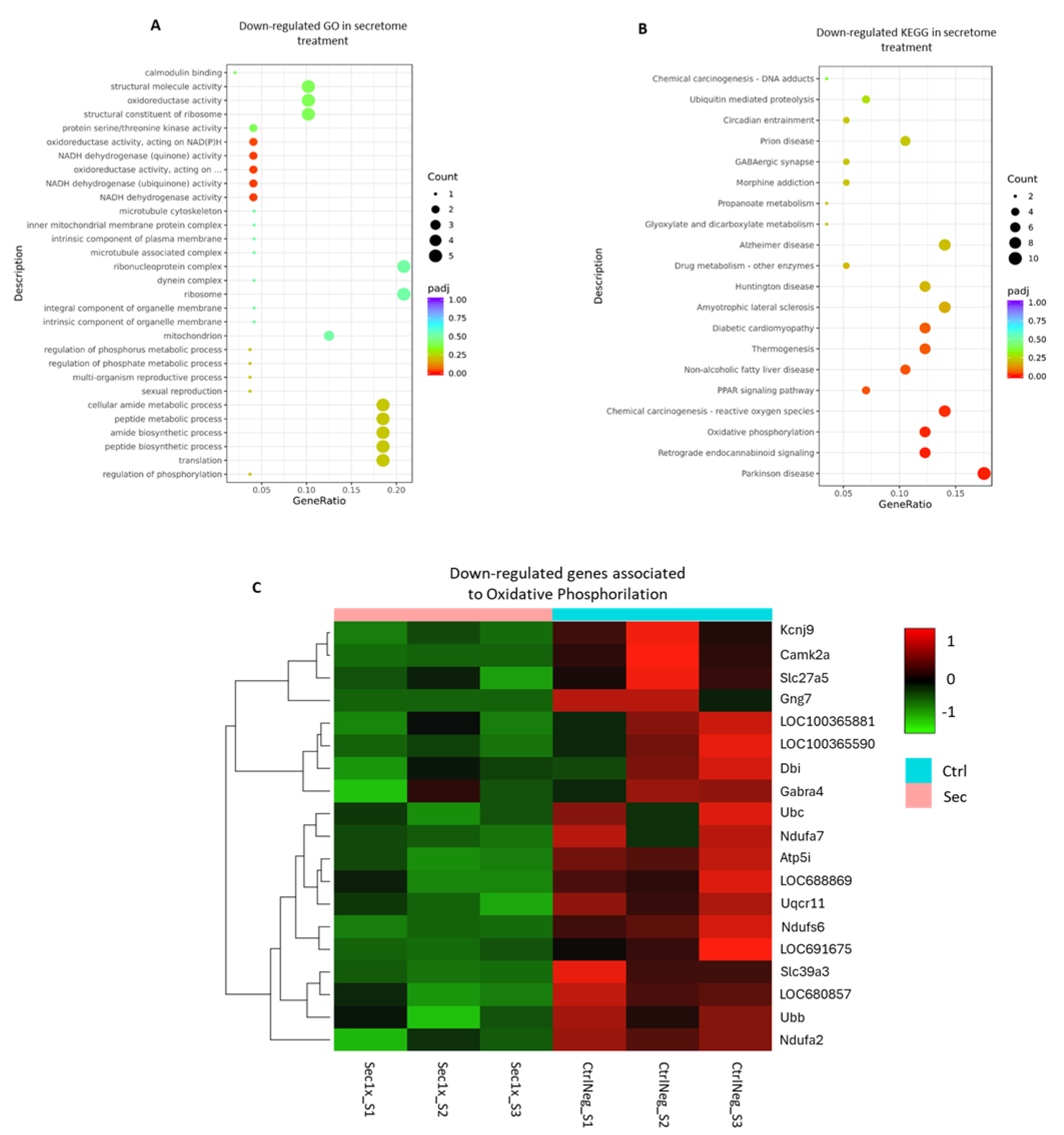
2.10. The Secretome from hASC Modulates the Transcriptional Response of the Mixed Spinal Cord Cells to Hyperosmolar Stress
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Spinal Cord Cells’ Isolation and Maintenance
4.3. hASC Cell Culture
4.4. Cell Density Quantification
4.5. Assessment of Metabolic Viability
4.6. Immunofluorescence and Lineage-Dependent Characterization
4.7. Sorbitol Hyperosmotic Injury
4.8. Secretome Collection and Treatments After Injury
4.9. Image Analysis of Injury-Induced Phenotypic Changes
4.10. RNA-Sequencing Analysis After Sorbitol Injury
4.11. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Data Availability Statement
Conflicts of Interest
References
- James, S.L.; Theadom, A.; Ellenbogen, R.G.; Bannick, M.S.; Montjoy-Venning, W.; Lucchesi, L.R.; Abbasi, N.; Abdulkader, R.; Abraha, H.N.; Adsuar, J.C.; et al. Global, Regional, and National Burden of Traumatic Brain Injury and Spinal Cord Injury, 1990–2016: A Systematic Analysis for the Global Burden of Disease Study 2016. Lancet Neurol. 2019, 18, 56–87. [Google Scholar] [CrossRef]
- Khorasanizadeh, M.; Yousefifard, M.; Eskian, M.; Lu, Y.; Chalangari, M.; Harrop, J.S.; Jazayeri, S.B.; Seyedpour, S.; Khodaei, B.; Hosseini, M.; et al. Neurological Recovery Following Traumatic Spinal Cord Injury: A Systematic Review and Meta-Analysis. J. Neurosurg. Spine 2019, 30, 683–699. [Google Scholar] [CrossRef] [PubMed]
- Lu, Y.; Shang, Z.; Zhang, W.; Pang, M.; Hu, X.; Dai, Y.; Shen, R.; Wu, Y.; Liu, C.; Luo, T.; et al. Global Incidence and Characteristics of Spinal Cord Injury since 2000–2021: A Systematic Review and Meta-Analysis. BMC Med. 2024, 22, 285. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H.; Fehlings, M.G. Review of the Secondary Injury Theory of Acute Spinal Cord Trauma with Emphasis on Vascular Mechanisms. J. Neurosurg. 1991, 75, 15–26. [Google Scholar] [CrossRef] [PubMed]
- Tator, C.H.; Koyanagi, I. Vascular Mechanisms in the Pathophysiology of Human Spinal Cord Injury. J. Neurosurg. 1997, 86, 483–492. [Google Scholar] [CrossRef]
- Christie, S.D.; Comeau, B.; Myers, T.; Sadi, D.; Purdy, M.; Mendez, I. Duration of Lipid Peroxidation after Acute Spinal Cord Injury in Rats and the Effect of Methylprednisolone. Neurosurg. Focus. 2008, 25, E5. [Google Scholar] [CrossRef]
- Faden, A.I.; Simon, R.P. A Potential Role for Excitotoxins in the Pathophysiology of Spinal Cord Injury. Ann. Neurol. 1988, 23, 623–626. [Google Scholar] [CrossRef]
- Ahuja, C.S.; Wilson, J.R.; Nori, S.; Kotter, M.R.N.; Druschel, C.; Curt, A.; Fehlings, M.G. Traumatic Spinal Cord Injury. Nat. Rev. Dis. Primers 2017, 3, 1–21. [Google Scholar] [CrossRef]
- Campos, J.; Silva, N.A.; Salgado, A.J. Nutritional Interventions for Spinal Cord Injury: Preclinical Efficacy and Molecular Mechanisms. Nutr. Rev. 2022, 80, 1206–1221. [Google Scholar] [CrossRef]
- Lima, R.; Monteiro, A.; Salgado, A.J.; Monteiro, S.; Silva, N.A. Pathophysiology and Therapeutic Approaches for Spinal Cord Injury. Int. J. Mol. Sci. 2022, 23, 13833. [Google Scholar] [CrossRef]
- Silva, N.A.; Sousa, N.; Reis, R.L.; Salgado, A.J. From Basics to Clinical: A Comprehensive Review on Spinal Cord Injury. Prog. Neurobiol. 2014, 114, 25–57. [Google Scholar] [CrossRef]
- Tran, A.P.; Warren, P.M.; Silver, J. The Biology of Regeneration Failure and Success After Spinal Cord Injury. Physiol. Rev. 2018, 98, 881–917. [Google Scholar] [CrossRef]
- Alizadeh, A.; Dyck, S.M.; Karimi-Abdolrezaee, S. Traumatic Spinal Cord Injury: An Overview of Pathophysiology, Models and Acute Injury Mechanisms. Front. Neurol. 2019, 10, 282. [Google Scholar] [CrossRef]
- McCanney, G.A.; Whitehead, M.J.; McGrath, M.A.; Lindsay, S.L.; Barnett, S.C. Neural Cell Cultures to Study Spinal Cord Injury. Drug Discov. Today Dis. Models 2017, 25–26, 11–20. [Google Scholar] [CrossRef]
- Andersen, J.; Revah, O.; Miura, Y.; Thom, N.; Amin, N.D.; Kelley, K.W.; Singh, M.; Chen, X.; Thete, M.V.; Walczak, E.M.; et al. Generation of Functional Human 3D Cortico-Motor Assembloids. Cell 2020, 183, 1913–1929.e26. [Google Scholar] [CrossRef] [PubMed]
- Slovinska, L.; Blasko, J.; Nagyova, M.; Szekiova, E.; Cizkova, D. In Vitro Models of Spinal Cord Injury. In Recovery of Motor Following Spinal Cord Injury; IntechOpen: Lodon, UK, 2016; ISBN 978-953-51-2497-9. [Google Scholar]
- Omelchenko, A.; Singh, N.K.; Firestein, B.L. Current Advances in in Vitro Models of Central Nervous System Trauma. Curr. Opin. Biomed. Eng. 2020, 14, 34–41. [Google Scholar] [CrossRef] [PubMed]
- Oliveira, J.M. Complex in Vitro Models: Do Not Complicate It. In Vitro Models 2023, 2, 67–68. [Google Scholar] [CrossRef] [PubMed]
- Hale, C.; Yonan, J.; Batarseh, R.; Chaar, R.; Jonak, C.R.; Ge, S.; Binder, D.; Rodgers, V.G.J. Implantable Osmotic Transport Device Can Reduce Edema After Severe Contusion Spinal Cord Injury. Front. Bioeng. Biotechnol. 2020, 8, 806. [Google Scholar] [CrossRef]
- Seblani, M.; Decherchi, P.; Brezun, J.-M. Edema after CNS Trauma: A Focus on Spinal Cord Injury. Int. J. Mol. Sci. 2023, 24, 7159. [Google Scholar] [CrossRef]
- Hock, E.-M.; Maniecka, Z.; Hruska-Plochan, M.; Reber, S.; Laferrière, F.; Sahadevan, M.K.S.; Ederle, H.; Gittings, L.; Pelkmans, L.; Dupuis, L.; et al. Hypertonic Stress Causes Cytoplasmic Translocation of Neuronal, but Not Astrocytic, FUS Due to Impaired Transportin Function. Cell Rep. 2018, 24, 987–1000.e7. [Google Scholar] [CrossRef]
- Chiong, M.; Parra, V.; Eisner, V.; Ibarra, C.; Maldonado, C.; Criollo, A.; Bravo, R.; Quiroga, C.; Contreras, A.; Vicencio, J.M.; et al. Parallel Activation of Ca(2+)-Induced Survival and Death Pathways in Cardiomyocytes by Sorbitol-Induced Hyperosmotic Stress. Apoptosis 2010, 15, 887–903. [Google Scholar] [CrossRef] [PubMed]
- Canat, A.; Atilla, D.; Torres-Padilla, M.-E. Hyperosmotic Stress Induces 2-Cell-like Cells through ROS and ATR Signaling. EMBO Rep. 2023, 24, e56194. [Google Scholar] [CrossRef]
- Gao, C.; Gu, J.; Zhang, H.; Jiang, K.; Tang, L.; Liu, R.; Zhang, L.; Zhang, P.; Liu, C.; Dai, B.; et al. Hyperosmotic-Stress-Induced Liquid-Liquid Phase Separation of ALS-Related Proteins in the Nucleus. Cell Rep. 2022, 40, 111086. [Google Scholar] [CrossRef]
- Harley, J.; Patani, R. Stress-Specific Spatiotemporal Responses of RNA-Binding Proteins in Human Stem Cell-Derived Motor Neurons. Int. J. Mol. Sci. 2020, 21, 8346. [Google Scholar] [CrossRef]
- Dewey, C.M.; Cenik, B.; Sephton, C.F.; Dries, D.R.; Mayer, P.; Good, S.K.; Johnson, B.A.; Herz, J.; Yu, G. TDP-43 Is Directed to Stress Granules by Sorbitol, a Novel Physiological Osmotic and Oxidative Stressor. Mol. Cell Biol. 2011, 31, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Anderson, P.; Kedersha, N. Stress Granules: The Tao of RNA Triage. Trends Biochem. Sci. 2008, 33, 141–150. [Google Scholar] [CrossRef] [PubMed]
- Assunção Silva, R.C.; Pinto, L.; Salgado, A.J. Cell Transplantation and Secretome Based Approaches in Spinal Cord Injury Regenerative Medicine. Med. Res. Rev. 2022, 42, 850–896. [Google Scholar] [CrossRef]
- Silva, D.; Schirmer, L.; Pinho, T.S.; Atallah, P.; Cibrão, J.R.; Lima, R.; Afonso, J.; B-Antunes, S.; Marques, C.R.; Dourado, J.; et al. Sustained Release of Human Adipose Tissue Stem Cell Secretome from Star-Shaped Poly(Ethylene Glycol) Glycosaminoglycan Hydrogels Promotes Motor Improvements after Complete Transection in Spinal Cord Injury Rat Model. Adv. Heal. Mater. 2023, 12, e2202803. [Google Scholar] [CrossRef]
- Pinho, A.G.; Cibrão, J.R.; Lima, R.; Gomes, E.D.; Serra, S.C.; Lentilhas-Graça, J.; Ribeiro, C.; Lanceros-Mendez, S.; Teixeira, F.G.; Monteiro, S.; et al. Immunomodulatory and Regenerative Effects of the Full and Fractioned Adipose Tissue Derived Stem Cells Secretome in Spinal Cord Injury. Exp. Neurol. 2022, 351, 113989. [Google Scholar] [CrossRef]
- Lilley, E.; Andrews, M.R.; Bradbury, E.J.; Elliott, H.; Hawkins, P.; Ichiyama, R.M.; Keeley, J.; Michael-Titus, A.T.; Moon, L.D.F.; Pluchino, S.; et al. Refining Rodent Models of Spinal Cord Injury. Exp. Neurol. 2020, 328, 113273. [Google Scholar] [CrossRef]
- Richner, M.; Jager, S.B.; Siupka, P.; Vaegter, C.B. Hydraulic Extrusion of the Spinal Cord and Isolation of Dorsal Root Ganglia in Rodents. J. Vis. Exp. 2017, 119, 55226. [Google Scholar] [CrossRef]
- Dawkins, E.; Small, D.H. Insights into the Physiological Function of the β-Amyloid Precursor Protein: Beyond Alzheimer’s Disease. J. Neurochem. 2014, 129, 756–769. [Google Scholar] [CrossRef]
- Pinho, A.G.; Monteiro, A.; Fernandes, S.; de Sousa, N.; Salgado, A.J.; Silva, N.A.; Monteiro, S. The Central Nervous System Source Modulates Microglia Function and Morphology In Vitro. Int. J. Mol. Sci. 2023, 24, 7685. [Google Scholar] [CrossRef] [PubMed]
- Lundgaard, I.; Osório, M.J.; Kress, B.; Sanggaard, S.; Nedergaard, M. White Matter Astrocytes in Health and Disease. Neuroscience 2014, 276, 161–173. [Google Scholar] [CrossRef] [PubMed]
- Köhler, S.; Winkler, U.; Junge, T.; Lippmann, K.; Eilers, J.; Hirrlinger, J. Gray and White Matter Astrocytes Differ in Basal Metabolism but Respond Similarly to Neuronal Activity. Glia 2023, 71, 229–244. [Google Scholar] [CrossRef]
- Verkhratsky, A.; Butt, A.; Li, B.; Illes, P.; Zorec, R.; Semyanov, A.; Tang, Y.; Sofroniew, M.V. Astrocytes in Human Central Nervous System Diseases: A Frontier for New Therapies. Sig Transduct. Target. Ther. 2023, 8, 1–37. [Google Scholar] [CrossRef]
- Dabrowski, W.; Siwicka-Gieroba, D.; Robba, C.; Bielacz, M.; Sołek-Pastuszka, J.; Kotfis, K.; Bohatyrewicz, R.; Jaroszyński, A.; Malbrain, M.L.N.G.; Badenes, R. Potentially Detrimental Effects of Hyperosmolality in Patients Treated for Traumatic Brain Injury. J. Clin. Med. 2021, 10, 4141. [Google Scholar] [CrossRef]
- Norenberg, M.D. A Hypothesis of Osmotic Endothelial Injury: A Pathogenetic Mechanism in Central Pontine Myelinolysis. Arch. Neurol. 1983, 40, 66–69. [Google Scholar] [CrossRef]
- Park, W.; Wei, S.; Kim, B.-S.; Kim, B.; Bae, S.-J.; Chae, Y.C.; Ryu, D.; Ha, K.-T. Diversity and Complexity of Cell Death: A Historical Review. Exp. Mol. Med. 2023, 55, 1573–1594. [Google Scholar] [CrossRef]
- Marina, N.; Christie, I.N.; Korsak, A.; Doronin, M.; Brazhe, A.; Hosford, P.S.; Wells, J.A.; Sheikhbahaei, S.; Humoud, I.; Paton, J.F.R.; et al. Astrocytes Monitor Cerebral Perfusion and Control Systemic Circulation to Maintain Brain Blood Flow. Nat. Commun. 2020, 11, 131. [Google Scholar] [CrossRef]
- Yan, X.; Liu, J.; Wang, X.; Li, W.; Chen, J.; Sun, H. Pretreatment with AQP4 and NKCC1 Inhibitors Concurrently Attenuated Spinal Cord Edema and Tissue Damage after Spinal Cord Injury in Rats. Front. Physiol. 2018, 9, 6. [Google Scholar] [CrossRef] [PubMed]
- Berliner, J.A.; Lam, M.A.; Najafi, E.; Hemley, S.J.; Bilston, L.E.; Stoodley, M.A. Aquaporin-4 Expression and Modulation in a Rat Model of Post-Traumatic Syringomyelia. Sci. Rep. 2023, 13, 9662. [Google Scholar] [CrossRef] [PubMed]
- Cibelli, A.; Ballesteros-Gomez, D.; McCutcheon, S.; Yang, G.L.; Bispo, A.; Krawchuk, M.; Piedra, G.; Spray, D.C. Astrocytes Sense Glymphatic-Level Shear Stress through the Interaction of Sphingosine-1-Phosphate with Piezo1. iScience 2024, 27, 110069. [Google Scholar] [CrossRef]
- Pischiutta, F.; Cavaleiro, H.; Caruso, E.; Tribuzio, F.; Di Marzo, N.; Moro, F.; Kobeissy, F.; Wang, K.K.; Salgado, A.J.; Zanier, E.R. A Novel Organotypic Cortical Slice Culture Model for Traumatic Brain Injury: Molecular Changes Induced by Injury and Mesenchymal Stromal Cell Secretome Treatment. Front. Cell Neurosci. 2023, 17, 1217987. [Google Scholar] [CrossRef]
- Lee, J.-H.; Shin, H.; Shaker, M.R.; Kim, H.J.; Park, S.-H.; Kim, J.H.; Lee, N.; Kang, M.; Cho, S.; Kwak, T.H.; et al. Production of Human Spinal-Cord Organoids Recapitulating Neural-Tube Morphogenesis. Nat. Biomed. Eng. 2022, 6, 435–448. [Google Scholar] [CrossRef]
- Azari, H.; Reynolds, B.A. In Vitro Models for Neurogenesis. Cold Spring Harb. Perspect. Biol. 2016, 8, a021279. [Google Scholar] [CrossRef] [PubMed]
- Hopkins, A.M.; DeSimone, E.; Chwalek, K.; Kaplan, D.L. 3D in Vitro Modeling of the Central Nervous System. Prog. Neurobiol. 2015, 125, 1–25. [Google Scholar] [CrossRef]
- Huang, W.L.; King, V.R.; Curran, O.E.; Dyall, S.C.; Ward, R.E.; Lal, N.; Priestley, J.V.; Michael-Titus, A.T. A Combination of Intravenous and Dietary Docosahexaenoic Acid Significantly Improves Outcome after Spinal Cord Injury. Brain 2007, 130, 3004–3019. [Google Scholar] [CrossRef]
- Sama, R.R.K.; Ward, C.L.; Kaushansky, L.J.; Lemay, N.; Ishigaki, S.; Urano, F.; Bosco, D.A. FUS/TLS Assembles into Stress Granules and Is a Prosurvival Factor during Hyperosmolar Stress. J. Cell. Physiol. 2013, 228, 2222–2231. [Google Scholar] [CrossRef]
- Liddelow, S.A.; Guttenplan, K.A.; Clarke, L.E.; Bennett, F.C.; Bohlen, C.J.; Schirmer, L.; Bennett, M.L.; Münch, A.E.; Chung, W.-S.; Peterson, T.C.; et al. Neurotoxic Reactive Astrocytes Are Induced by Activated Microglia. Nature 2017, 541, 481–487. [Google Scholar] [CrossRef]
- Rock, K.L.; Kono, H. The Inflammatory Response to Cell Death. Annu. Rev. Pathol. 2008, 3, 99–126. [Google Scholar] [CrossRef]
- Chen, G.Y.; Nuñez, G. Sterile Inflammation: Sensing and Reacting to Damage. Nat. Rev. Immunol. 2010, 10, 826–837. [Google Scholar] [CrossRef]
- Huntemer-Silveira, A.; Patil, N.; Brickner, M.A.; Parr, A.M. Strategies for Oligodendrocyte and Myelin Repair in Traumatic CNS Injury. Front. Cell. Neurosci. 2021, 14, 619707. [Google Scholar] [CrossRef]
- Patar, A.; Dockery, P.; Howard, L.; McMahon, S.S. Cell Viability in Three Ex Vivo Rat Models of Spinal Cord Injury. J. Anat. 2019, 234, 244–251. [Google Scholar] [CrossRef] [PubMed]
- Han, Z.; Mou, Z.; Jing, Y.; Jiang, R.; Sun, T. CircSmox Knockdown Alleviates PC12 Cell Apoptosis and Inflammation in Spinal Cord Injury by miR-340-5p/Smurf1 Axis. Immun. Inflamm. Dis. 2023, 11, e824. [Google Scholar] [CrossRef]
- Wei, Z.; Liu, J.; Xie, H.; Wang, B.; Wu, J.; Zhu, Z. MiR-122-5p Mitigates Inflammation, Reactive Oxygen Species and SH-SY5Y Apoptosis by Targeting CPEB1 After Spinal Cord Injury Via the PI3K/AKT Signaling Pathway. Neurochem. Res. 2021, 46, 992–1005. [Google Scholar] [CrossRef] [PubMed]
- Li, R.; Thode, S.; Zhou, J.; Richard, N.; Pardinas, J.; Rao, M.S.; Sah, D.W. Motoneuron Differentiation of Immortalized Human Spinal Cord Cell Lines. J. Neurosci. Res. 2000, 59, 342–352. [Google Scholar] [CrossRef]
- Pandamooz, S.; Salehi, M.S.; Zibaii, M.I.; Safari, A.; Nabiuni, M.; Ahmadiani, A.; Dargahi, L. Modeling Traumatic Injury in Organotypic Spinal Cord Slice Culture Obtained from Adult Rat. Tissue Cell 2019, 56, 90–97. [Google Scholar] [CrossRef]
- Taylor, A.M.; Blurton-Jones, M.; Rhee, S.W.; Cribbs, D.H.; Cotman, C.W.; Jeon, N.L. A Microfluidic Culture Platform for CNS Axonal Injury, Regeneration and Transport. Nat. Methods 2005, 2, 599–605. [Google Scholar] [CrossRef]
- Xu, J.; Fang, S.; Deng, S.; Li, H.; Lin, X.; Huang, Y.; Chung, S.; Shu, Y.; Shao, Z. Generation of Neural Organoids for Spinal-Cord Regeneration via the Direct Reprogramming of Human Astrocytes. Nat. Biomed. Eng. 2023, 7, 253–269. [Google Scholar] [CrossRef]
- Lopez-Suarez, L.; Awabdh, S.A.; Coumoul, X.; Chauvet, C. The SH-SY5Y Human Neuroblastoma Cell Line, a Relevant in Vitro Cell Model for Investigating Neurotoxicology in Human: Focus on Organic Pollutants. NeuroToxicology 2022, 92, 131–155. [Google Scholar] [CrossRef] [PubMed]
- Liu, J.; Mai, P.; Yang, Z.; Wang, Z.; Yang, W.; Wang, Z. Piceatannol Protects PC-12 Cells against Oxidative Damage and Mitochondrial Dysfunction by Inhibiting Autophagy via SIRT3 Pathway. Nutrients 2023, 15, 2973. [Google Scholar] [CrossRef]
- Goshi, N.; Morgan, R.K.; Lein, P.J.; Seker, E. A Primary Neural Cell Culture Model to Study Neuron, Astrocyte, and Microglia Interactions in Neuroinflammation. J. Neuroinflamm. 2020, 17, 155. [Google Scholar] [CrossRef] [PubMed]
- Estévez, A.G.; Spear, N.; Manuel, S.M.; Radi, R.; Henderson, C.E.; Barbeito, L.; Beckman, J.S. Nitric Oxide and Superoxide Contribute to Motor Neuron Apoptosis Induced by Trophic Factor Deprivation. J. Neurosci. 1998, 18, 923–931. [Google Scholar] [CrossRef]
- Domeniconi, M.; Hempstead, B.L.; Chao, M.V. Pro-NGF Secreted by Astrocytes Promotes Motor Neuron Cell Death. Mol. Cell Neurosci. 2007, 34, 271–279. [Google Scholar] [CrossRef] [PubMed]
- Caroni, P.; Schwab, M.E. Antibody against Myelin-Associated Inhibitor of Neurite Growth Neutralizes Nonpermissive Substrate Properties of CNS White Matter. Neuron 1988, 1, 85–96. [Google Scholar] [CrossRef]
- Chen, M.S.; Huber, A.B.; van der Haar, M.E.; Frank, M.; Schnell, L.; Spillmann, A.A.; Christ, F.; Schwab, M.E. Nogo-A Is a Myelin-Associated Neurite Outgrowth Inhibitor and an Antigen for Monoclonal Antibody IN-1. Nature 2000, 403, 434–439. [Google Scholar] [CrossRef]
- Assunção-Silva, R.C.; Mendes-Pinheiro, B.; Patrício, P.; Behie, L.A.; Teixeira, F.G.; Pinto, L.; Salgado, A.J. Exploiting the Impact of the Secretome of MSCs Isolated from Different Tissue Sources on Neuronal Differentiation and Axonal Growth. Biochimie 2018, 155, 83–91. [Google Scholar] [CrossRef]
- Lentilhas-Graça, J.; Santos, D.J.; Afonso, J.; Monteiro, A.; Pinho, A.G.; Mendes, V.M.; Dias, M.S.; Gomes, E.D.; Lima, R.; Fernandes, L.S.; et al. The Secretome of Macrophages Has a Differential Impact on Spinal Cord Injury Recovery According to the Polarization Protocol. Front. Immunol. 2024, 15, 1354479. [Google Scholar] [CrossRef]
- Krassioukov, A.V.; Ackery, A.; Schwartz, G.; Adamchik, Y.; Liu, Y.; Fehlings, M.G. An in Vitro Model of Neurotrauma in Organotypic Spinal Cord Cultures from Adult Mice. Brain Res. Protoc. 2002, 10, 60–68. [Google Scholar] [CrossRef]
- Mazzone, G.L.; Nistri, A. S100β as an Early Biomarker of Excitotoxic Damage in Spinal Cord Organotypic Cultures. J. Neurochem. 2014, 130, 598–604. [Google Scholar] [CrossRef]
- Martins, L.F.; Costa, R.O.; Pedro, J.R.; Aguiar, P.; Serra, S.C.; Teixeira, F.G.; Sousa, N.; Salgado, A.J.; Almeida, R.D. Mesenchymal Stem Cells Secretome-Induced Axonal Outgrowth Is Mediated by BDNF. Sci. Rep. 2017, 7, 4153. [Google Scholar] [CrossRef]
- Costa, R.O.; Martins, H.; Martins, L.F.; Cwetsch, A.W.; Mele, M.; Pedro, J.R.; Tomé, D.; Jeon, N.L.; Cancedda, L.; Jaffrey, S.R.; et al. Synaptogenesis Stimulates a Proteasome-Mediated Ribosome Reduction in Axons. Cell Rep. 2019, 28, 864–876.e6. [Google Scholar] [CrossRef] [PubMed]
- Mesquida-Veny, F.; Martínez-Torres, S.; Del Río, J.A.; Hervera, A. Genetic Control of Neuronal Activity Enhances Axonal Growth Only on Permissive Substrates. Mol. Med. 2022, 28, 97. [Google Scholar] [CrossRef]
- Hopkins, H.K.; Traverse, E.M.; Barr, K.L. Methodologies for Generating Brain Organoids to Model Viral Pathogenesis in the CNS. Pathogens 2021, 10, 1510. [Google Scholar] [CrossRef] [PubMed]
- Marx, V. Reality Check for Organoids in Neuroscience. Nat. Methods 2020, 17, 961–964. [Google Scholar] [CrossRef]
- Koyanagi, I.; Tator, C.H.; Lea, P.J. Three-Dimensional Analysis of the Vascular System in the Rat Spinal Cord with Scanning Electron Microscopy of Vascular Corrosion Casts. Part 1: Normal Spinal Cord. Neurosurgery 1993, 33, 277–283; discussion 283–284. [Google Scholar]
- Burg, M.B.; Ferraris, J.D. Intracellular Organic Osmolytes: Function and Regulation. J. Biol. Chem. 2008, 283, 7309–7313. [Google Scholar] [CrossRef]
- Noble, L.J.; Wrathall, J.R. Distribution and Time Course of Protein Extravasation in the Rat Spinal Cord after Contusive Injury. Brain Res. 1989, 482, 57–66. [Google Scholar] [CrossRef]
- Hsu, C.Y.; Hogan, E.L.; Gadsden, R.H.; Spicer, K.M.; Shi, M.P.; Cox, R.D. Vascular Permeability in Experimental Spinal Cord Injury. J. Neurol. Sci. 1985, 70, 275–282. [Google Scholar] [CrossRef]
- Farooque, M.; Zhang, Y.; Holtz, A.; Olsson, Y. Exudation of Fibronectin and Albumin after Spinal Cord Injury in Rats. Acta Neuropathol. 1992, 84, 613–620. [Google Scholar] [CrossRef] [PubMed]
- Yu, W.R.; Westergren, H.; Farooque, M.; Holtz, A.; Olsson, Y. Systemic Hypothermia Following Compression Injury of Rat Spinal Cord: Reduction of Plasma Protein Extravasation Demonstrated by Immunohistochemistry. Acta Neuropathol. 1999, 98, 15–21. [Google Scholar] [CrossRef] [PubMed]
- Brocker, C.; Thompson, D.C.; Vasiliou, V. The Role of Hyperosmotic Stress in Inflammation and Disease. Biomol. Concepts 2012, 3, 345–364. [Google Scholar] [CrossRef] [PubMed]
- Venkatesh, K.; Ghosh, S.K.; Mullick, M.; Manivasagam, G.; Sen, D. Spinal Cord Injury: Pathophysiology, Treatment Strategies, Associated Challenges, and Future Implications. Cell Tissue Res. 2019, 377, 125–151. [Google Scholar] [CrossRef]
- Blaschke, S.J.; Demir, S.; König, A.; Abraham, J.-A.; Vay, S.U.; Rabenstein, M.; Olschewski, D.N.; Hoffmann, C.; Hoffmann, M.; Hersch, N.; et al. Substrate Elasticity Exerts Functional Effects on Primary Microglia. Front. Cell. Neurosci. 2020, 14, 590500. [Google Scholar] [CrossRef]
- Liu, Y.; Zhang, J.; Li, Y.; Zhao, Y.; Kuermanbayi, S.; Zhuang, J.; Zhang, H.; Xu, F.; Li, F. Matrix Stiffness-Dependent Microglia Activation in Response to Inflammatory Cues: In Situ Investigation by Scanning Electrochemical Microscopy. Chem. Sci. 2024, 15, 171–184. [Google Scholar] [CrossRef]
- C. Benincasa, J.; Madias, M.I.; Kandell, R.M.; Delgado-Garcia, L.M.; Engler, A.J.; Kwon, E.J.; Porcionatto, M.A. Mechanobiological Modulation of In Vitro Astrocyte Reactivity Using Variable Gel Stiffness. ACS Biomater. Sci. Eng. 2024, 10, 4279–4296. [Google Scholar] [CrossRef]
- Wilson, C.L.; Hayward, S.L.; Kidambi, S. Astrogliosis in a Dish: Substrate Stiffness Induces Astrogliosis in Primary Rat Astrocytes. RSC Adv. 2016, 6, 34447–34457. [Google Scholar] [CrossRef]
- Palomares, T.; Cordero, M.; Bruzos-Cidon, C.; Torrecilla, M.; Ugedo, L.; Alonso-Varona, A. The Neuroprotective Effect of Conditioned Medium from Human Adipose-Derived Mesenchymal Stem Cells Is Impaired by N-Acetyl Cysteine Supplementation. Mol. Neurobiol. 2018, 55, 13–25. [Google Scholar] [CrossRef]
- Pires, A.O.; Mendes-Pinheiro, B.; Teixeira, F.G.; Anjo, S.I.; Ribeiro-Samy, S.; Gomes, E.D.; Serra, S.C.; Silva, N.A.; Manadas, B.; Sousa, N.; et al. Unveiling the Differences of Secretome of Human Bone Marrow Mesenchymal Stem Cells, Adipose Tissue-Derived Stem Cells, and Human Umbilical Cord Perivascular Cells: A Proteomic Analysis. Stem Cells Dev. 2016, 25, 1073–1083. [Google Scholar] [CrossRef]
- Mountian, I.; Waelkens, E.; Missiaena, L.; van Driessche, W. Changes in Actin Cytoskeleton during Volume Regulation in C6 Glial Cells. Eur. J. Cell Biol. 1998, 77, 196–204. [Google Scholar] [CrossRef]
- Chernova, I.; Song, W.; Steach, H.; Hafez, O.; Al Souz, J.; Chen, P.-M.; Chandra, N.; Cantley, L.; Veselits, M.; Clark, M.R.; et al. The Ion Transporter Na+-K+-ATPase Enables Pathological B Cell Survival in the Kidney Microenvironment of Lupus Nephritis. Sci. Adv. 2023, 9, eadf8156. [Google Scholar] [CrossRef]
- Wetzel, R.K.; Pascoa, J.L.; Arystarkhova, E. Stress-Induced Expression of the Gamma Subunit (FXYD2) Modulates Na,K-ATPase Activity and Cell Growth. J. Biol. Chem. 2004, 279, 41750–41757. [Google Scholar] [CrossRef] [PubMed]
- Izumi, Y.; Yang, W.; Zhu, J.; Burg, M.B.; Ferraris, J.D. RNA-Seq Analysis of High NaCl-Induced Gene Expression. Physiol. Genom. 2015, 47, 500–513. [Google Scholar] [CrossRef]
- Wang, R.; Zhou, R.; Chen, Z.; Gao, S.; Zhou, F. The Glial Cells Respond to Spinal Cord Injury. Front. Neurol. 2022, 13, 844497. [Google Scholar] [CrossRef]
- Gavasso, S.; Kråkenes, T.; Olsen, H.; Evjenth, E.C.; Ytterdal, M.; Haugsøen, J.B.; Kvistad, C.E. The Therapeutic Mechanisms of Mesenchymal Stem Cells in MS—A Review Focusing on Neuroprotective Properties. Int. J. Mol. Sci. 2024, 25, 1365. [Google Scholar] [CrossRef]
- Fletcher, J.L.; Murray, S.S.; Xiao, J. Brain-Derived Neurotrophic Factor in Central Nervous System Myelination: A New Mechanism to Promote Myelin Plasticity and Repair. Int. J. Mol. Sci. 2018, 19, 4131. [Google Scholar] [CrossRef] [PubMed]
- Ye, P.; Carson, J.; D’Ercole, A.J. In Vivo Actions of Insulin-like Growth Factor-I (IGF-I) on Brain Myelination: Studies of IGF-I and IGF Binding Protein-1 (IGFBP-1) Transgenic Mice. J. Neurosci. 1995, 15, 7344–7356. [Google Scholar] [CrossRef]
- Palazuelos, J.; Klingener, M.; Aguirre, A. TGFβ Signaling Regulates the Timing of CNS Myelination by Modulating Oligodendrocyte Progenitor Cell Cycle Exit through SMAD3/4/FoxO1/Sp1. J. Neurosci. 2014, 34, 7917–7930. [Google Scholar] [CrossRef]
- Madine, M.A.; Khoo, C.-Y.; Mills, A.D.; Laskey, R.A. MCM3 Complex Required for Cell Cycle Regulation of DNA Replication in Vertebrate Cells. Nature 1995, 375, 421–424. [Google Scholar] [CrossRef]
- Simon, A.C.; Sannino, V.; Costanzo, V.; Pellegrini, L. Structure of Human Cdc45 and Implications for CMG Helicase Function. Nat. Commun. 2016, 7, 11638. [Google Scholar] [CrossRef] [PubMed]
- Zhang, N.; Pati, D. Biology and Insights into the Role of Cohesin Protease Separase in Human Malignancies. Biol. Rev. 2017, 92, 2070–2083. [Google Scholar] [CrossRef]
- Pearson, R.J.; Blake, D.G.; Mezna, M.; Fischer, P.M.; Westwood, N.J.; McInnes, C. The Meisenheimer Complex as a Paradigm in Drug Discovery: Reversible Covalent Inhibition through C67 of the ATP Binding Site of PLK1. Cell Chem. Biol. 2018, 25, 1107–1116.e4. [Google Scholar] [CrossRef] [PubMed]
- Denechaud, P.-D.; Fajas, L.; Giralt, A. E2F1, a Novel Regulator of Metabolism. Front. Endocrinol. 2017, 8, 311. [Google Scholar] [CrossRef]
- Burg, M.B.; Ferraris, J.D.; Dmitrieva, N.I. Cellular Response to Hyperosmotic Stresses. Physiol. Rev. 2007, 87, 1441–1474. [Google Scholar] [CrossRef]
- Kültz, D. Chapter 12—Osmotic Regulation of DNA Activity and the Cell Cycle. In Cell and Molecular Response to Stress; Storey, K.B., Storey, J.M., Eds.; Environmental Stressors and Gene Responses; Elsevier: Amsterdam, The Netherlands, 2000; Volume 1, pp. 157–179. [Google Scholar]
- Ahuja, C.S.; Nori, S.; Tetreault, L.; Wilson, J.; Kwon, B.; Harrop, J.; Choi, D.; Fehlings, M.G. Traumatic Spinal Cord Injury-Repair and Regeneration. Neurosurgery 2017, 80, S9–S22. [Google Scholar] [CrossRef]
- Silver, J.; Schwab, M.E.; Popovich, P.G. Central Nervous System Regenerative Failure: Role of Oligodendrocytes, Astrocytes, and Microglia. Cold Spring Harb. Perspect. Biol. 2015, 7, a020602. [Google Scholar] [CrossRef]
- Cooper, G.M. The Mechanism of Oxidative Phosphorylation. In The Cell: A Molecular Approach, 2nd ed.; Sinauer Associates: Sunderland, MA, USA, 2000. [Google Scholar]
- Ma, W.; Tian, Y.; Shi, L.; Liang, J.; Ouyang, Q.; Li, J.; Chen, H.; Sun, H.; Ji, H.; Liu, X.; et al. N-Acetyltransferase 10 Represses Uqcr11 and Uqcrb Independently of ac4C Modification to Promote Heart Regeneration. Nat. Commun. 2024, 15, 2137. [Google Scholar] [CrossRef]
- Wang, W.; Shen, W.; Zhang, S.; Luo, G.; Wang, K.; Xu, Y.; Zhang, H. The Role of CaMKII Overexpression and Oxidation in Atrial Fibrillation-A Simulation Study. Front. Physiol. 2020, 11, 607809. [Google Scholar] [CrossRef]
- PPARs: Diverse Regulators in Energy Metabolism and Metabolic Diseases | Cell Research. Available online: https://www.nature.com/articles/cr201013 (accessed on 28 October 2024).
- Muzio, G.; Barrera, G.; Pizzimenti, S. Peroxisome Proliferator-Activated Receptors (PPARs) and Oxidative Stress in Physiological Conditions and in Cancer. Antioxidants 2021, 10, 1734. [Google Scholar] [CrossRef]
- Ribeiro, C.A.; Salgado, A.J.; Fraga, J.S.; Silva, N.A.; Reis, R.L.; Sousa, N. The Secretome of Bone Marrow Mesenchymal Stem Cells-Conditioned Media Varies with Time and Drives a Distinct Effect on Mature Neurons and Glial Cells (Primary Cultures). J. Tissue Eng. Regen. Med. 2011, 5, 668–672. [Google Scholar] [CrossRef]
- Campos, J.; Sampaio-Marques, B.; Santos, D.; Barata-Antunes, S.; Ribeiro, M.; Serra, S.C.; Pinho, T.S.; Canto-Gomes, J.; Marote, A.; Cortez, M.; et al. Lipid Priming of Adipose Mesenchymal Stromal Cells with Docosahexaenoic Acid: Impact on Cell Differentiation, Senescence and the Secretome Neuroregulatory Profile. Tissue Eng. Regen. Med. 2024, 22, 113–128. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed]
- Otsu, N. A Threshold Selection Method from Gray-Level Histograms. IEEE Trans. Syst. Man. Cybern. 1979, 9, 62–66. [Google Scholar] [CrossRef]
- Sternberg, S.R. Cellular Computers and Biomedical Image Processing. In Proceedings of the Biomedical Images and Computers; Sklansky, J., Bisconte, J.-C., Eds.; Springer: Berlin/Heidelberg, Germany, 1982; pp. 294–319. [Google Scholar]
- Huang, L.-K.; Wang, M.-J.J. Image Thresholding by Minimizing the Measures of Fuzziness. Pattern Recognit. 1995, 28, 41–51. [Google Scholar] [CrossRef]
- Schwartz, L.; Guais, A.; Pooya, M.; Abolhassani, M. Is Inflammation a Consequence of Extracellular Hyperosmolarity? J. Inflamm. 2009, 6, 21. [Google Scholar] [CrossRef]
- Mandyartha, E.P.; Anggraeny, F.T.; Muttaqin, F.; Akbar, F.A. Global and Adaptive Thresholding Technique for White Blood Cell Image Segmentation. J. Phys. Conf. Ser. 2020, 1569, 022054. [Google Scholar] [CrossRef]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Campos, J.; Palha, A.T.; Fernandes, L.S.; Cibrão, J.R.; Pinho, T.S.; Serra, S.C.; Silva, N.A.; Michael-Titus, A.T.; Salgado, A.J. Modeling Spinal Cord Injury in a Dish with Hyperosmotic Stress: Population-Specific Effects and the Modulatory Role of Mesenchymal Stromal Cell Secretome. Int. J. Mol. Sci. 2025, 26, 3298. https://doi.org/10.3390/ijms26073298
Campos J, Palha AT, Fernandes LS, Cibrão JR, Pinho TS, Serra SC, Silva NA, Michael-Titus AT, Salgado AJ. Modeling Spinal Cord Injury in a Dish with Hyperosmotic Stress: Population-Specific Effects and the Modulatory Role of Mesenchymal Stromal Cell Secretome. International Journal of Molecular Sciences. 2025; 26(7):3298. https://doi.org/10.3390/ijms26073298
Chicago/Turabian StyleCampos, Jonas, Ana T. Palha, Luís S. Fernandes, Jorge R. Cibrão, Tiffany S. Pinho, Sofia C. Serra, Nuno A. Silva, Adina T. Michael-Titus, and António J. Salgado. 2025. "Modeling Spinal Cord Injury in a Dish with Hyperosmotic Stress: Population-Specific Effects and the Modulatory Role of Mesenchymal Stromal Cell Secretome" International Journal of Molecular Sciences 26, no. 7: 3298. https://doi.org/10.3390/ijms26073298
APA StyleCampos, J., Palha, A. T., Fernandes, L. S., Cibrão, J. R., Pinho, T. S., Serra, S. C., Silva, N. A., Michael-Titus, A. T., & Salgado, A. J. (2025). Modeling Spinal Cord Injury in a Dish with Hyperosmotic Stress: Population-Specific Effects and the Modulatory Role of Mesenchymal Stromal Cell Secretome. International Journal of Molecular Sciences, 26(7), 3298. https://doi.org/10.3390/ijms26073298







