Influence of the Support Nature of Copper Catalysts on Catalytic Properties in the Hydrogenation of Fatty Acid Esters
Abstract
1. Introduction
2. Results
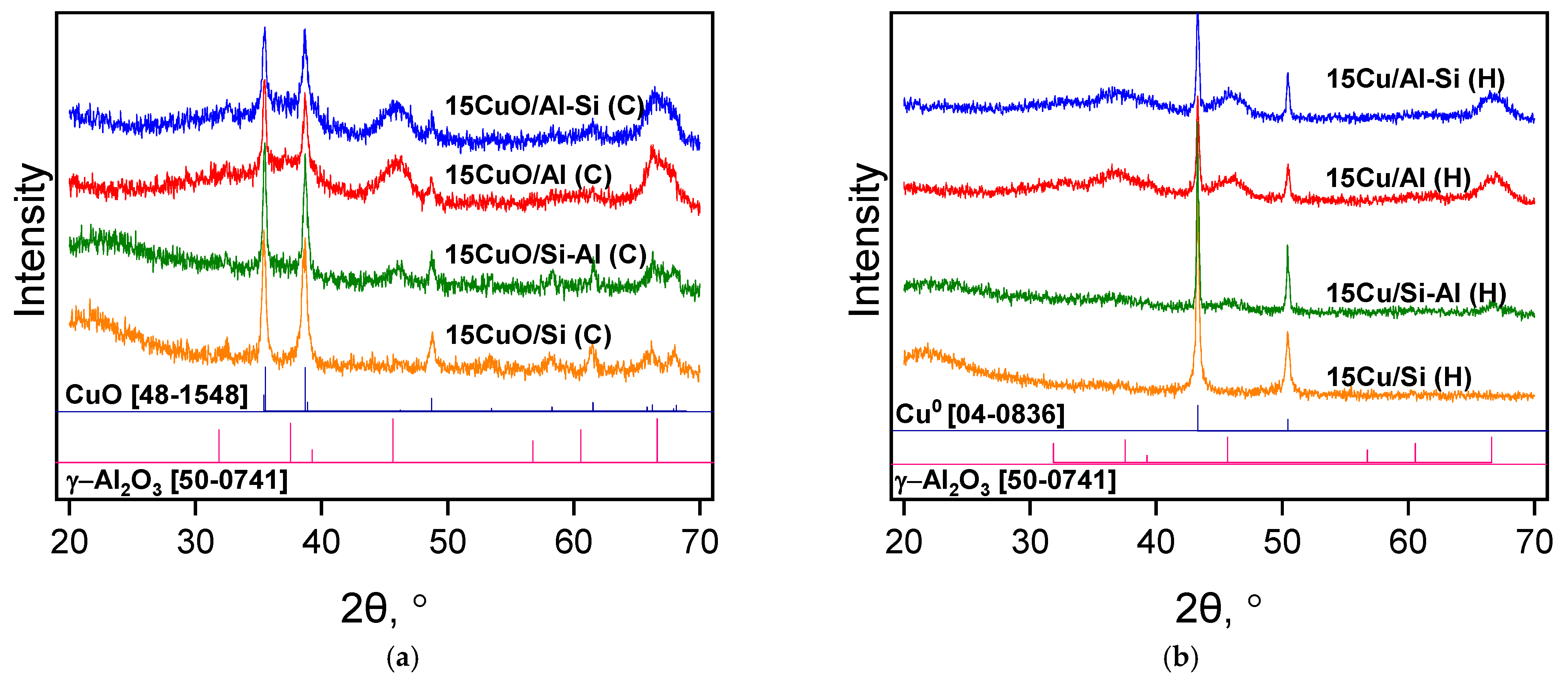
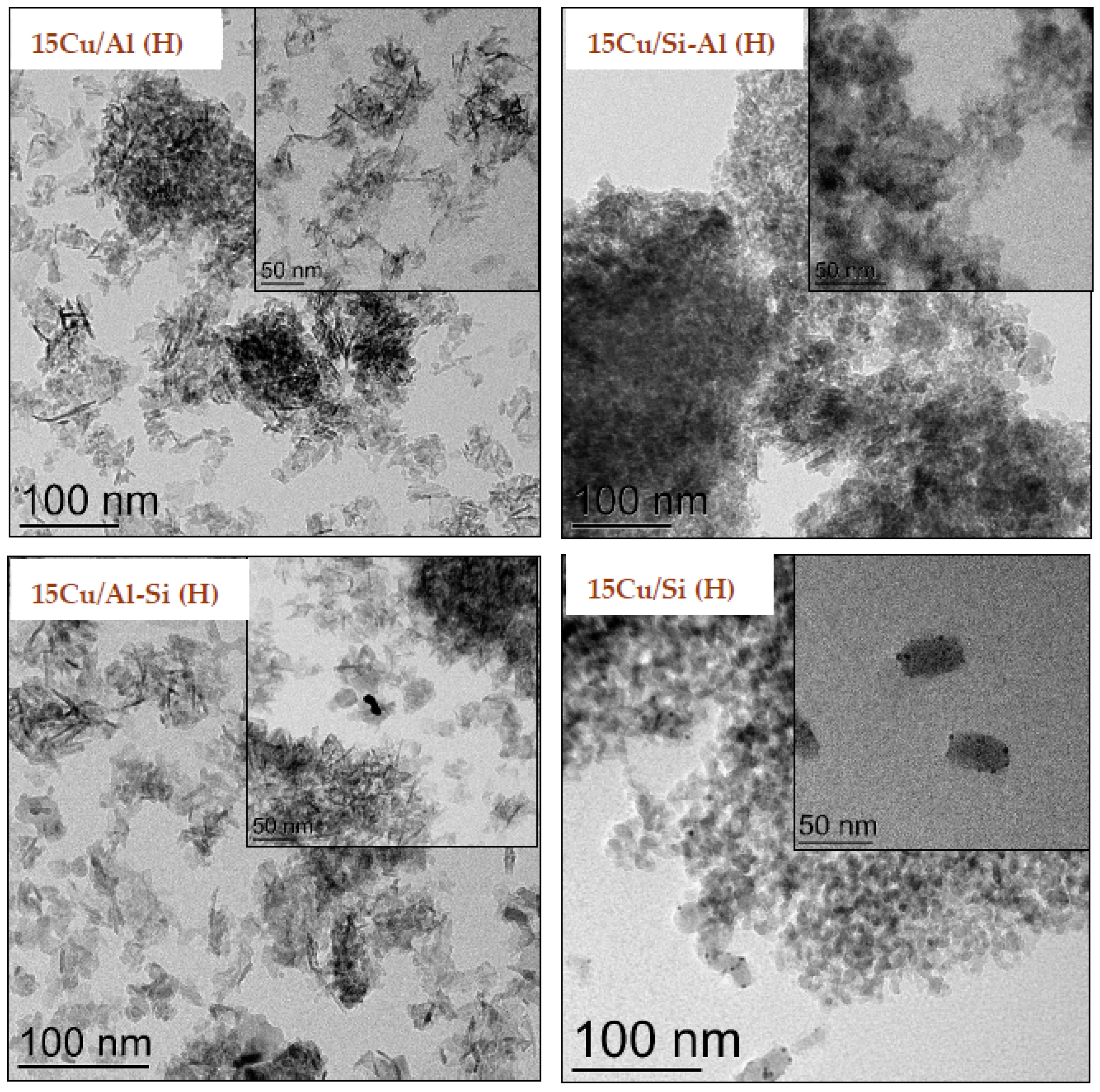
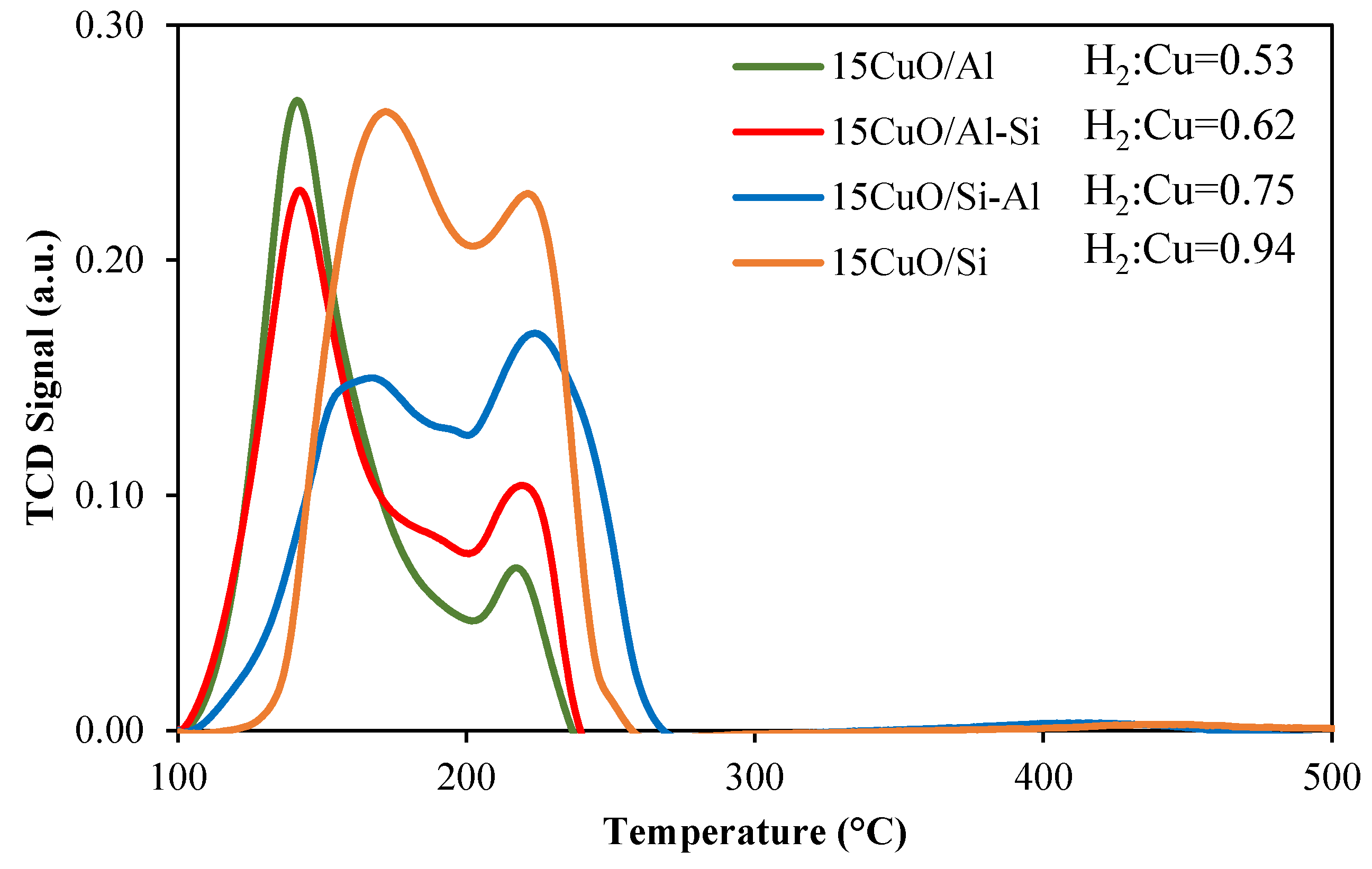
2.1. Physico-Chemical Properties of Catalysts
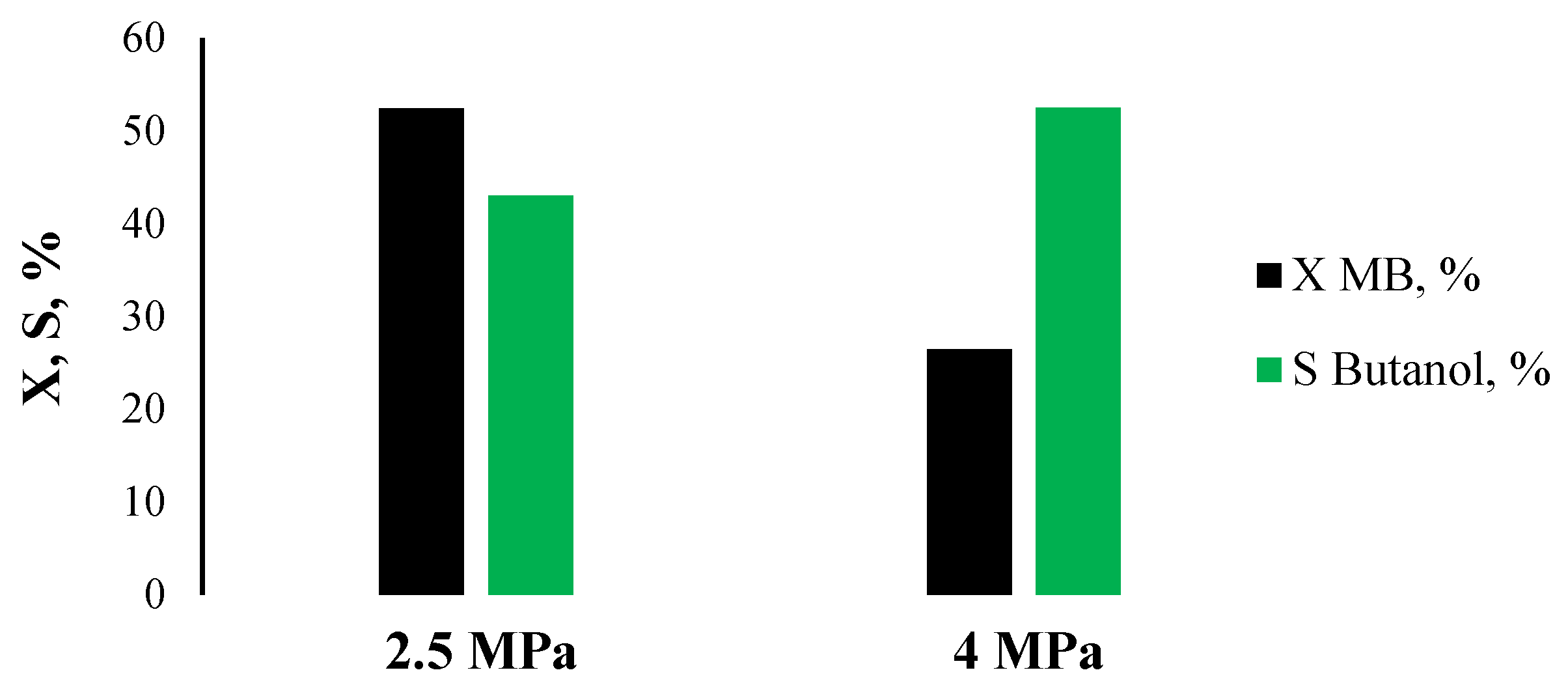
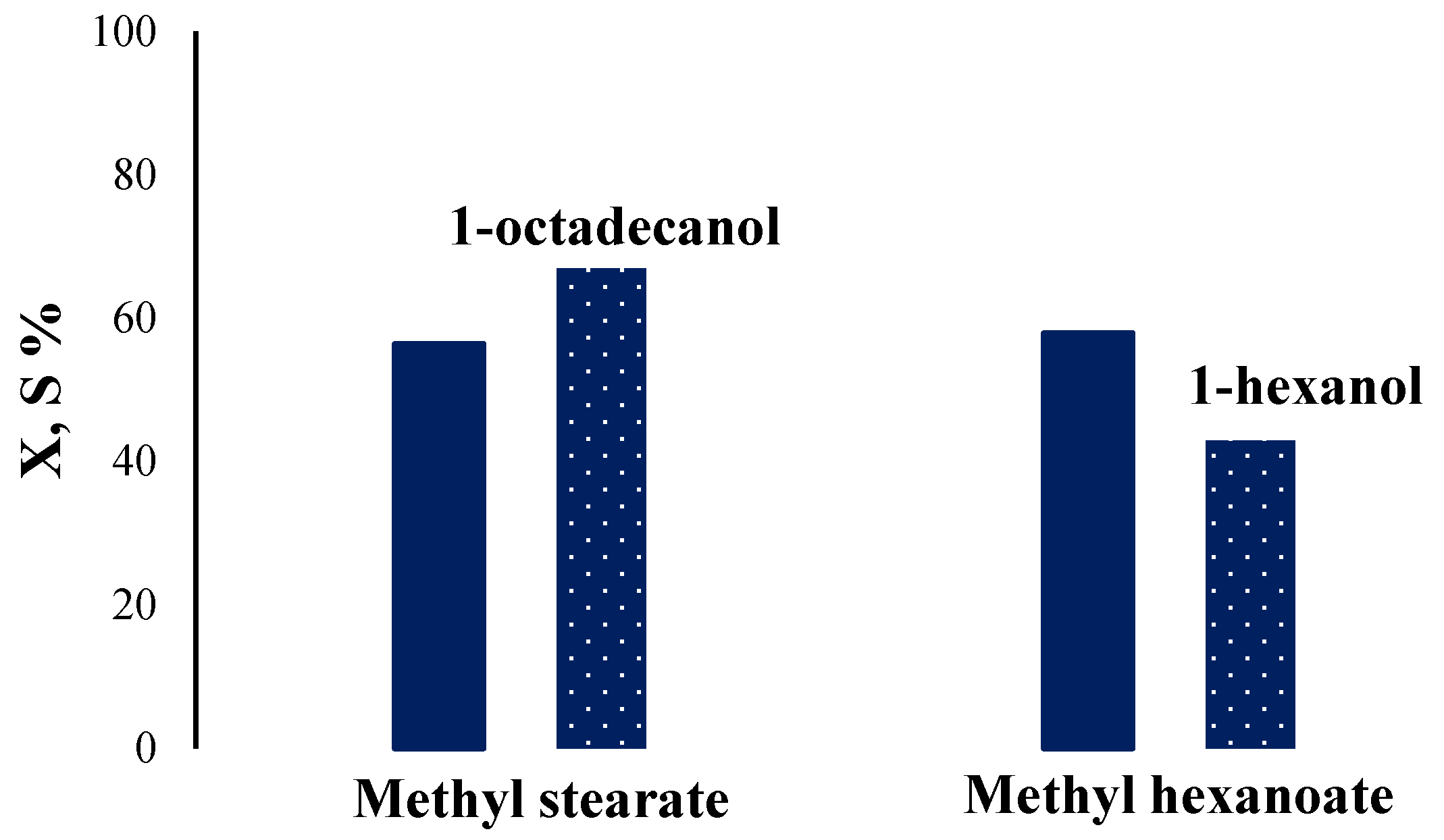
2.2. Catalytic Activity
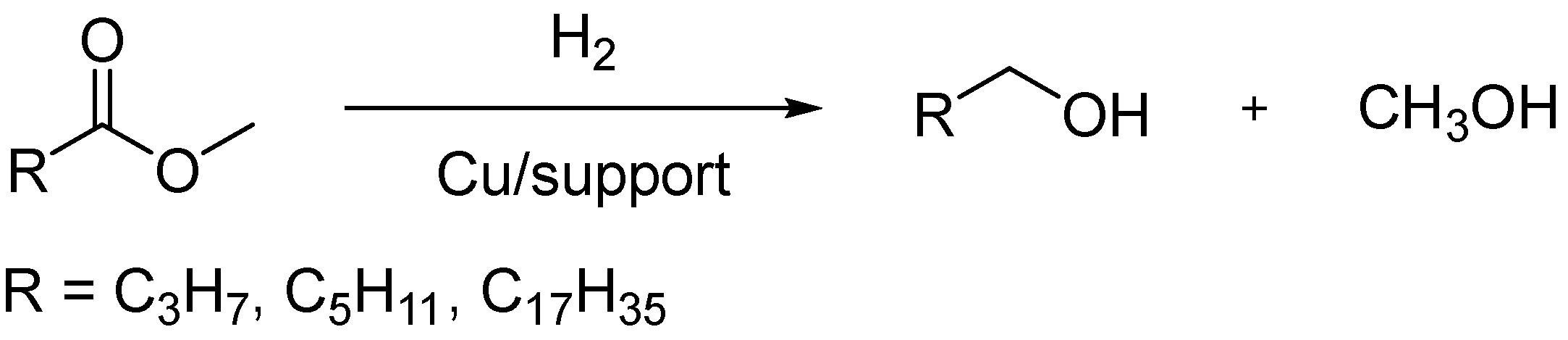
3. Materials and Methods
3.1. Materials
3.2. Catalyst Preparation
3.3. Catalyst Characterization
3.4. Catalytic Activity Test
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Zhou, Y.; Remón, J.; Jiang, Z.; Matharu, A.; Hu, C. Tuning the selectivity of natural oils and fatty acids/esters deoxygenation to biofuels and fatty alcohols: A review. Green Energy Environ. 2023, 8, 722–743. [Google Scholar]
- Strekalova, A.A.; Shesterkina, A.A.; Kustov, L.M. Recent progress in hydrogenation of esters on heterogeneous bimetallic catalysts. Catal. Sci. Technol. 2021, 11, 7229. [Google Scholar]
- Remon, J.; Zhu, G.; Budarin, V.L.; Clark, J.H. Analysis and optimisation of a microwave-assisted hydrothermal process for the production of value-added chemicals from glycerol. Green Chem. 2018, 20, 2624–2636. [Google Scholar]
- Wang, L.; Niu, X.; Chen, J. SiO2 supported Ni-In intermetallic compounds: Efficient for selective hydrogenation of fatty acid methyl esters to fatty alcohols. Appl. Catal. B. 2020, 278, 119293. [Google Scholar]
- Krishnan, A.; McNeil, B.A.; Stuart, D.T. Biosynthesis of Fatty Alcohols in Engineered Microbial Cell Factories: Advances and Limitations. Front. Bioeng. Biotechnol. 2020, 8, 610936. [Google Scholar]
- HBI Group. Global Detergent Alcohol Producers: Synthetics Stumble. 2019. Available online: https://www.oleoline.com/products/Fatty-Alcohol-Report-8.html (accessed on 21 December 2019).
- Shah, J.; Arslan, E.; Cirucci, J.; O’Brien, J.; Moss, D. Comparison of Oleo- vs. Petro-Sourcing of Fatty Alcohols via Cradle-to-Gate Life Cycle Assessment. J. Surfact. Deterg. 2016, 19, 1333–1351. [Google Scholar]
- Munkajohnpong, P.; Kesornpun, C.; Buttranon, S.; Jaroensuk, J.; Weeranoppanant, N.; Chaiyen, P. Fatty alcohol production: An opportunity of bioprocess. Biofuel Bioprod. Biorefin. 2020, 14, 899–1134. [Google Scholar]
- Gargalo, C.L.; Cheali, P.; Posada, J.A.; Carvalho, A.; Gernaey, K.V.; Sin, G. Assessing the environmental sustainability of early stage design for bioprocesses under uncertainties: An analysis of glycerol bioconversion. J. Clean. Prod. 2016, 139, 1245–1260. [Google Scholar]
- Turek, T.; Trimm, D.L.; Cant, N.W. The Catalytic Hydrogenolysis of Esters to Alcohols. Catal. Rev. 1994, 36, 645–683. [Google Scholar] [CrossRef]
- Rodina, V.O.; Ermakov, D.Y.; Saraev, A.A.; Reshetnikov, S.I.; Yakovlev, V.A. Influence of reaction conditions and kinetic analysis of the selective hydrogenation of oleic acid toward fatty alcohols on Ru-Sn-B/Al2O3 in the flow reactor. Appl. Catal. B Environ. 2017, 209, 611–620. [Google Scholar]
- Adkins, H.; Folkers, K. The catalytic hydrogenation of esters to alcohols. J. Am. Chem. Soc. 1931, 53, 1095. [Google Scholar]
- Fonseca Benítez, C.A.; Mazzieri, V.A.; Vera, C.R.; Benitez, V.M.; Pieck, C.L. Selective hydrogenation of oleic acid to fatty alcohols over a Rh–Sn–B/Al2O3 catalyst: Kinetics and optimal reaction conditions. React. Chem. Eng. 2021, 6, 726–746. [Google Scholar]
- Pritchard, J.; Filonenko, G.A.; van Putten, R.; Hensen, E.J.M.; Pidko, E.A. Heterogeneous and homogeneous catalysis for the hydrogenation of carboxylic acid derivatives: History, advances and future directions. Chem. Soc. Rev. 2015, 44, 3808–3833. [Google Scholar]
- Udomsap, P.; Eiad-Ua, A.; Chen, S.-Y.; Mochizuki, T.; Chollacoop, N.; Yoshimura, Y.; Nishi, M.; Tateno, H.; Takagi, H. Effect of Pd Precursor Salts on the Chemical State, Particle Size, and Performance of Activated Carbon-Supported Pd Catalysts for the Selective Hydrogenation of Palm Biodiesel. Int. J. Mol. Sci. 2021, 22, 1256. [Google Scholar] [CrossRef] [PubMed]
- Numwong, N.; Luengnaruemitchai, A.; Chollacoop, N.; Yoshimura, Y. Effect of metal type on partial hydrogenation of rapeseed oil-derived FAME. J. Am. Oil Chem. Soc. 2013, 90, 1431–1438. [Google Scholar] [CrossRef]
- Rungsi, A.N.; Luengnaruemitchai, A.; Chollacoop, N.; Chen, S.-Y.; Mochizuki, T.; Takagi, H.; Yoshimura, Y. Performance and sulfur poisoning of SiO2, γ-Al2O3, and SiO2-Al2O3-supported bimetallic Pd-Pt catalysts in selective hydrogenation of soybean oil-derived fatty acid methyl esters. Fuel 2023, 331, 125919. [Google Scholar]
- He, L.; Cheng, H.; Liang, G.; Yu, Y.; Zhao, F. Effect of structure of CuO/ZnO/Al2O3 composites on catalytic performance for hydrogenation of fatty acid ester. Appl. Catal. A Gen. 2013, 452, 88–93. [Google Scholar]
- Yao, Y.; Wu, X.; Gutiérrez, O.Y.; Ji, J.; Jin, P.; Wang, S.; Xu, Y.; Zhao, Y.; Wang, S.; Ma, X.; et al. Roles of Cu+ and Cu0 sites in liquid-phase hydrogenation of esters on core-shell CuZnx@ C catalysts. Appl. Catal. B Environ. 2020, 267, 118698. [Google Scholar]
- Onyestyák, G.; Harnos, S.; Kalló, D. Improving the catalytic behavior of Ni/Al2O3 by indium in reduction of carboxylic acid to alcohol. Catal. Commun. 2011, 16, 184–188. [Google Scholar]
- Cao, X.; Zhao, J.; Long, F.; Liu, P.; Jiang, X.; Zhang, X.; Xu, J.; Jiang, J. Efficient low-temperature hydrogenation of fatty acids to fatty alcohols and alkanes on a Ni-Re bimetallic catalyst: The crucial role of NiRe alloys. Appl. Catal. B Environ. 2022, 312, 121437. [Google Scholar]
- Kandel, K.; Chaudhary, U.; Nelson, N.C.; Slowing, I.I. Synergistic Interaction between oxides of copper and iron for production of fatty alcohols from fatty acids. ACS Catal. 2015, 5, 6719–6723. [Google Scholar] [CrossRef]
- Dong, X.; Lei, J.; Chen, Y.; Jiang, H.; Zhang, M. Selective hydrogenation of acetic acid to ethanol on Cu-In catalyst supported by SBA-15. Appl. Catal. B Environ. 2019, 244, 448–458. [Google Scholar] [CrossRef]
- Di, W.; Cheng, J.; Tian, S.; Li, J.; Chen, J.; Sun, Q. Synthesis and characterization of supported copper phyllosilicate catalysts for acetic ester hydrogenation to ethanol. Appl. Catal. A Gen. 2016, 510, 244–259. [Google Scholar] [CrossRef]
- Onyestyák, G.; Harnos, S.; Kalló, D. Indium, as an efficient co-catalyst of Cu/Al2O3 in the selective hydrogenation of biomass derived fatty acids to alcohols. Catal. Commun. 2012, 26, 19–24. [Google Scholar] [CrossRef]
- Pasqual Laverdura, U.; Rossi, L.; Courson, C.; Zarli, A.; Gallucci, K. Selective Catalytic Hydrogenation of Vegetable Oils over Copper-Based Catalysts Supported on Amorphous Silica. Energies 2023, 16, 7201. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, Z.; Zhang, H.; Peng, B.; Xu, Y.; Wang, Y.; Zhang, J.; Xu, Y.; Wang, S.; Ma, X. Hydrogenation of diesters on copper catalyst anchored on ordered hierarchical porous silica: Pore size effect. J. Catal. 2018, 357, 223–237. [Google Scholar] [CrossRef]
- Wang, Y.; Shen, Y.L.; Zhao, Y.J.; Lv, J.; Wang, S.P.; Ma, X.B. Insight into the balancing effect of active Cu species for hydrogenation of carbon–oxygen bonds. ACS Catal. 2015, 5, 6200. [Google Scholar] [CrossRef]
- Ma, X.G.; Yang, Z.Q.; Liu, X.B.; Tan, X.Z.; Ge, Q.J. Dynamic redox cycle of Cu0 and Cu+ over Cu/SiO2 catalyst in ester hydrogenation. RSC Adv. 2015, 5, 37581. [Google Scholar] [CrossRef]
- Zhang, C.; Wang, L.; Etim, U.J.; Song, Y.; Gazit, O.M.; Zhong, Z. Oxygen vacancies in Cu/TiO2 boost strong metal-support interaction and CO2 hydrogenation to methanol. J. Catal. 2022, 413, 284–296. [Google Scholar] [CrossRef]
- Sripada, P.; Kimpton, J.; Barlow, A.; Williams, T.; Kandasamy, S.; Bhattacharya, S. Investigating the dynamic structural changes on Cu/CeO2 catalysts observed during CO2 hydrogenation. J. Catal. 2020, 381, 415–426. [Google Scholar] [CrossRef]
- Nooto, C.; Chuaykaew, P.; Singthuen, P.; Solos, T.; Preedawichitkun, Y.; Khosukwiwat, K.; Wengwirat, K.; Promchana, P.; Kumar, R.; Chung, P.-W.; et al. Reversibly interconverted Cu+/Cu+-H species as active sites for selective hydrogenation of fatty acid methyl esters to fatty alcohol over layered double hydroxide derived CuMgAlOx catalysts. Mol. Catal. 2025, 575, 114898. [Google Scholar]
- Prasanseang, W.; Choojun, K.; Poo-arporn, Y.; Huang, A.-L.; Lin, Y.-C.; Chen, E.; Lee, H.-H.; Chung, P.-W.; Sooknoi, T. Tuning Cu+ species/brønsted acids of copper phyllosilicate by K+ doping for selective hydrogenation of methyl palmitate to hexadecanol. J. Catal. 2023, 428, 115115. [Google Scholar] [CrossRef]
- Kumar, R.; Lee, H.-H.; Chen, E.; Du, Y.-P.; Lin, C.-Y.; Prasanseang, W.; Solos, T.; Choojun, K.; Sooknoi, T.; Xie, R.-K.; et al. Facile synthesis of the atomically dispersed hydrotalcite oxide supported copper catalysts for the selective hydrogenation of 5-hydroxymethylfurfural into 2,5-bis(hydroxymethyl)furan. Appl. Catal. B. 2023, 329, 122547. [Google Scholar]
- Shesterkina, A.A.; Strekalova, A.A.; Shuvalova, E.V.; Kapustin, G.I.; Tkachenko, O.P.; Kustov, L.M. CuO-Fe2O3 Nanoparticles Supported on SiO2 and Al2O3 for Selective Hydrogenation of 2-Methyl-3-Butyn-2-ol. Catalysts 2021, 11, 625. [Google Scholar] [CrossRef]
- Yi, H.; Xue, Q.; Lu, S.; Wu, J.; Wang, Y.; Luo, G. Effect of pore structure on Ni/Al2O3 microsphere catalysts for enhanced CO2 methanation. Fuel 2022, 315, 123262. [Google Scholar]
- Mambetova, M.; Yergaziyeva, G.; Dossumov, K.; Askaruly, K.; Azat, S.; Bexeitova, K.; Anissova, M.; Baizhomartov, B. Comparative Study of Physicochemical Characteristics and Catalytic Activity of Copper Oxide over Synthetic Silicon Oxide and Silicon Oxide from Rice Husk in Non-Oxidative Dehydrogenation of Ethanol. ChemEngineering 2022, 6, 74. [Google Scholar] [CrossRef]
- Mazarío, J.; Cecilia, J.A.; Rodríguez-Castellón, E.; Domine, M.E. Highly dispersed copper oxide on silica: Towards an efficient catalyst for continuous glycerol dehydration to acetol. Appl. Catal. A 2023, 652, 119029. [Google Scholar]
- Marelli, M.; Zaccheria, F.; Ravasio, N.; Pitzalis, E.; Didi, Y.; Galarneau, A.; Scotti, N.; Evangelisti, C. Copper Oxide Nanoparticles over Hierarchical Silica Monoliths for Continuous-Flow Selective Alcoholysis of Styrene Oxide. Catalysts 2023, 13, 341. [Google Scholar] [CrossRef]


| Catalyst | Average Size of CuO Crystallites, nm | Average Size of Cu0 Crystallites, nm |
|---|---|---|
| 15Cu/Al | 22.5 | 35.6 |
| 15Cu/Al-Si | 22.5 | 30 |
| 15Cu/Si-Al | 22.7 | 42.4 |
| 15Cu/Si | 19.8 | 27.7 |
| Sample | Elements Content, %wt |
|---|---|
| Cu | |
| 15Cu/Al (H) | 11.2 |
| 15Cu/Al-Si (H) | 11.6 |
| 15Cu/Si-Al (H) | 11.4 |
| 15Cu/Si (H) | 10.7 |
| Catalyst | Theoretical Content Cu, wt.% | Received Content Cu, wt.% |
|---|---|---|
| 15Cu/Al (H) | 15 | 4.1 |
| 15Cu/Al-Si (H) | 15 | 4.3 |
| 15Cu/Si-Al (H) | 15 | 6.1 |
| 15Cu/Si (H) | 15 | 4.5 |
| Sample | BET Surface, m2/g | Vtotal, cm3/g | Vmicro, cm3/g | Average Pore Size, nm |
|---|---|---|---|---|
| Al2O3 | 254 | 0.80 | 0.005 | 12.5 |
| 15CuO/Al (C) | 321 | 1.02 | 0 | 12.6 |
| SiO2 | 236 | 0.92 | 0 | 15.5 |
| 15CuO/Si (C) | 196 | 0.79 | 0.006 | 16.0 |
| SiO2-Al2O3 | 410 | 0.60 | 0.006 | 5.8 |
| 15CuO/Si-Al (C) | 291 | 0.47 | 0 | 6.5 |
| Al2O3-SiO2 | 232 | 0.72 | 0.002 | 12.4 |
| 15CuO/Al-Si (C) | 189 | 0.59 | 0.004 | 12.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shesterkina, A.; Strekalova, A.; Mashkin, M.; Mishin, I.; Vikanova, K.; Tursunov, O.; Dunaev, S.; Kustov, A. Influence of the Support Nature of Copper Catalysts on Catalytic Properties in the Hydrogenation of Fatty Acid Esters. Int. J. Mol. Sci. 2025, 26, 3289. https://doi.org/10.3390/ijms26073289
Shesterkina A, Strekalova A, Mashkin M, Mishin I, Vikanova K, Tursunov O, Dunaev S, Kustov A. Influence of the Support Nature of Copper Catalysts on Catalytic Properties in the Hydrogenation of Fatty Acid Esters. International Journal of Molecular Sciences. 2025; 26(7):3289. https://doi.org/10.3390/ijms26073289
Chicago/Turabian StyleShesterkina, Anastasiya, Anna Strekalova, Mikhail Mashkin, Igor Mishin, Kseniia Vikanova, Obid Tursunov, Sergey Dunaev, and Alexander Kustov. 2025. "Influence of the Support Nature of Copper Catalysts on Catalytic Properties in the Hydrogenation of Fatty Acid Esters" International Journal of Molecular Sciences 26, no. 7: 3289. https://doi.org/10.3390/ijms26073289
APA StyleShesterkina, A., Strekalova, A., Mashkin, M., Mishin, I., Vikanova, K., Tursunov, O., Dunaev, S., & Kustov, A. (2025). Influence of the Support Nature of Copper Catalysts on Catalytic Properties in the Hydrogenation of Fatty Acid Esters. International Journal of Molecular Sciences, 26(7), 3289. https://doi.org/10.3390/ijms26073289








