Cancer-Associated Fibroblasts as the “Architect” of the Lung Cancer Immune Microenvironment: Multidimensional Roles and Synergistic Regulation with Radiotherapy
Abstract
1. Introduction
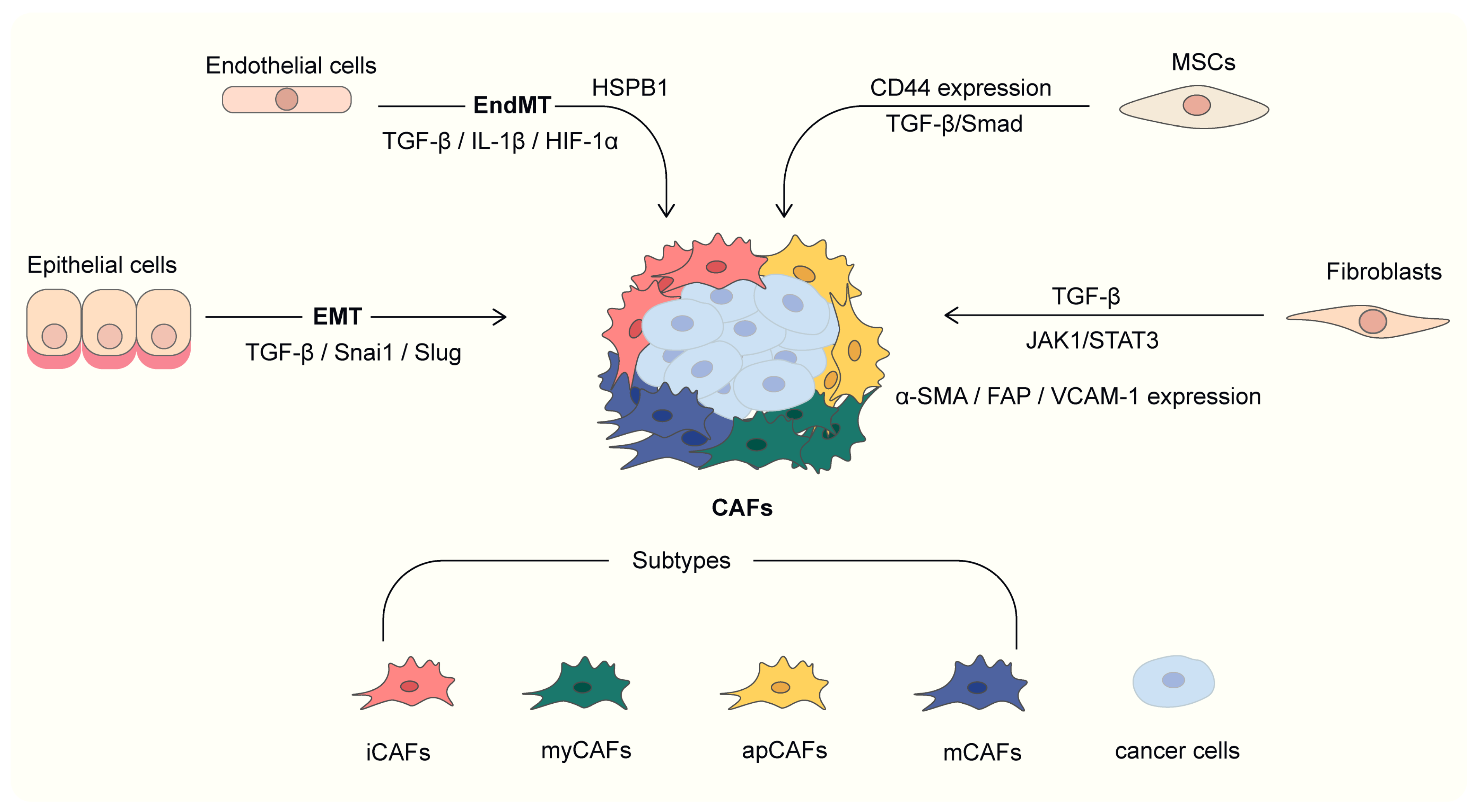
2. Heterogeneity of CAF in Lung Cancer
2.1. Heterogeneity of CAF Origin
2.1.1. Fibroblast
2.1.2. Epithelial-Mesenchymal Transition (EMT)
2.1.3. Endothelial-Mesenchymal Transition (EndMT)
2.1.4. Bone Marrow-Derived Mesenchymal Stem Cells (BM-MSC)
2.1.5. Methods for Separating CAFs from TME
2.2. Heterogeneity of Molecular Phenotype
2.2.1. Classical Molecular Markers of CAFs
- 1.
- FAP
- 2.
- α-SMA
- 3.
- PDGFR-α/β
2.2.2. Subtypes of CAF Based on Molecular Phenotypes
- 1.
- MyCAF
- 2.
- iCAF
- 3.
- Other subtypes
2.2.3. Subtypes of CAFs in Different Lung Cancer Types
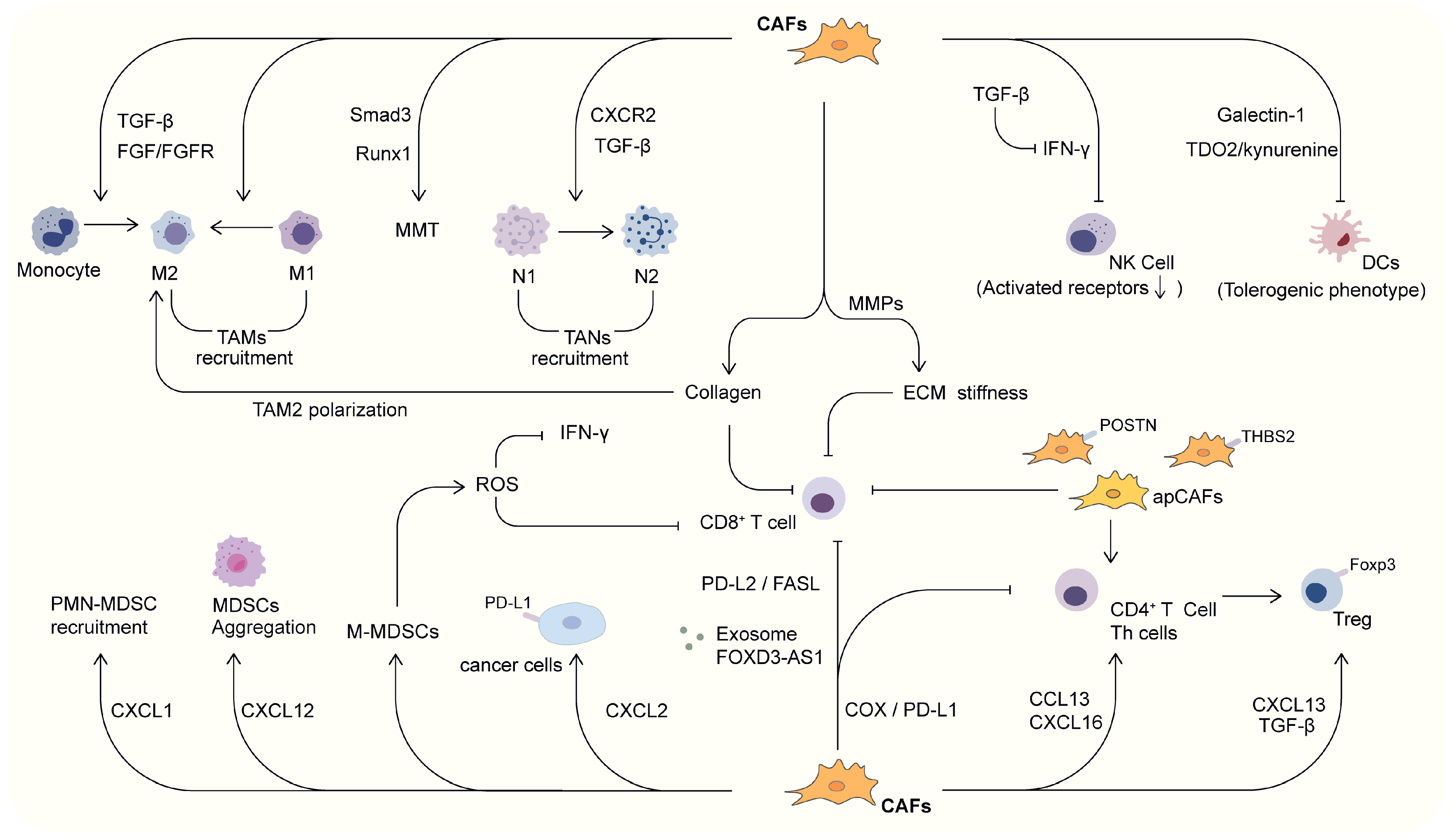
3. CAF and Tumor Immune Microenvironment in Lung Cancer
3.1. Connection Between CAF and Innate Immune Cells
3.1.1. CAF and TAMs
3.1.2. CAF and TANs
3.1.3. CAF and NK Cells
3.1.4. CAF and DCs
3.1.5. CAF and MDSCs
3.2. Connection Between CAF and Adaptive Immune Cells
3.2.1. CAF and Treg Cells
3.2.2. CAF and Th Cells
3.2.3. CAF and CTLs
3.3. Interactions Between CAF and Other Factors in TIME
4. Relationship Between CAF and Radiotherapy
5. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| CAF | Cancer-associated fibroblasts |
| ECM | Extracellular matrix |
| NSCLC | Non-small cell lung cancer |
| SCLC | Small cell lung cancer |
| BM-MSC | Bone marrow-derived mesenchymal stem cells |
| EGFR | Epidermal growth factor receptor |
| ALK | Activin receptor-like kinase |
| ROS | Reactive oxygen species |
| TME | Tumor microenvironment |
| TIME | Tumor immune microenvironment |
| TGF-β | Transforming growth factor-β |
| α-SMA | α-smooth muscle actin |
| FAP | Fibroblast activation protein |
| VCAM-1 | Vascular cell adhesion molecule-1 |
| CAF-CM | CAF-conditioned medium |
| NF-CM | Normal fibroblast-conditioned medium |
| EMT | Epithelial-mesenchymal transition |
| SDF-1 | stromal cell-derived factor-1 |
| EndMT | Endothelial-mesenchymal transition |
| FSP1 | Fibroblast-specific protein-1 |
| HMEC-1 | Human microvascular endothelial cells |
| ILK | Integrin-linked kinase |
| MRTF | Myocardin-related transcription factor |
| FAPIs | FAP inhibitors |
| CXCL12 | C-X-C Motif Chemokine Ligand 12 |
| IL-6 | Interleukin-6 |
| COL1A1 | Collagen type I alpha 1 chain |
| PDGFR | Platelet-derived growth factor receptor |
| SCC | Lung squamous cell carcinoma |
| myCAFs | Myofibroblast-like cancer-associated fibroblasts |
| PDAC | Pancreatic ductal adenocarcinoma |
| HNSCC | Head and neck squamous cell carcinoma |
| LUAD | Lung adenocarcinoma |
| MMPs | Matrix metalloproteinases |
| iCAFs | Inflammatory CAFs |
| HGF | Hepatocyte growth factor |
| IMC | Imaging mass cytometry |
| non-NE | Non-neuroendocrine |
| apCAF | Antigen-presenting CAFs |
| mCAF | Matrix CAF |
| TAMs | Tumor-associated macrophages |
| TNF | Tumor necrosis factor |
| MMT | Macrophage-myofibroblast transformation |
| TANs | Tumor-associated neutrophils |
| CXCR2 | C-X-C chemokine receptor 2 |
| G-CSF | Granulocyte colony-stimulating factor |
| GM-CSF | Granulocyte-macrophage colony-stimulating factor |
| NK | Natural killer |
| IFN-γ | Interferon-γ |
| DCs | Dendritic cells |
| TDO2 | Tryptophan 2,3-dioxygenase |
| MDSCs | Myeloid-derived suppressor cells |
| Fgl2 | Fibrinogen-like protein 2 |
| PMN-MDSC | Polymorphonuclear myeloid-derived suppressor cells |
| M-MDSCs | Mononuclear myeloid-derived suppressor cells |
| Th | Helper T |
| CTL | Cytotoxic T lymphocytes |
| Foxp3 | Forkhead box P3 |
| CFR | CD8+ T cell/CAF ratio |
| MMPs | Matrix metalloproteinases |
| RT | Radiotherapy |
| RIPF | Radiation-induced pulmonary fibrosis |
| HD-RT | High-dose radiotherapy |
| MCs | Mast cells |
| SCF | Stem cell factor |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Inamura, K. Lung Cancer: Understanding Its Molecular Pathology and the 2015 WHO Classification. Front. Oncol. 2017, 7, 193. [Google Scholar]
- Vinod, S.K.; Hau, E. Radiotherapy treatment for lung cancer: Current status and future directions. Respirology 2020, 25 (Suppl. S2), 61–71. [Google Scholar] [CrossRef]
- Duma, N.; Santana-Davila, R.; Molina, J.R. Non-Small Cell Lung Cancer: Epidemiology, Screening, Diagnosis, and Treatment. Mayo Clin. Proc. 2019, 94, 1623–1640. [Google Scholar] [CrossRef] [PubMed]
- Roszkowska, M. Multilevel Mechanisms of Cancer Drug Resistance. Int. J. Mol. Sci. 2024, 25, 12402. [Google Scholar] [CrossRef]
- Bejarano, L.; Jordāo, M.J.C.; Joyce, J.A. Therapeutic Targeting of the Tumor Microenvironment. Cancer Discov. 2021, 11, 933–959. [Google Scholar] [CrossRef]
- Tan, Z.; Xue, H.; Sun, Y.; Zhang, C.; Song, Y.; Qi, Y. The Role of Tumor Inflammatory Microenvironment in Lung Cancer. Front. Pharmacol. 2021, 12, 688625. [Google Scholar] [CrossRef]
- Murciano-Goroff, Y.R.; Warner, A.B.; Wolchok, J.D. The future of cancer immunotherapy: Microenvironment-targeting combinations. Cell Res. 2020, 30, 507–519. [Google Scholar] [CrossRef]
- O’Donnell, J.S.; Teng, M.W.L.; Smyth, M.J. Cancer immunoediting and resistance to T cell-based immunotherapy. Nat. Rev. Clin. Oncol. 2019, 16, 151–167. [Google Scholar] [CrossRef]
- Genova, C.; Dellepiane, C.; Carrega, P.; Sommariva, S.; Ferlazzo, G.; Pronzato, P.; Gangemi, R.; Filaci, G.; Coco, S.; Croce, M. Therapeutic Implications of Tumor Microenvironment in Lung Cancer: Focus on Immune Checkpoint Blockade. Front. Immunol. 2021, 12, 799455. [Google Scholar] [CrossRef]
- Larroquette, M.; Guegan, J.P.; Besse, B.; Cousin, S.; Brunet, M.; Le Moulec, S.; Le Loarer, F.; Rey, C.; Soria, J.C.; Barlesi, F.; et al. Spatial transcriptomics of macrophage infiltration in non-small cell lung cancer reveals determinants of sensitivity and resistance to anti-PD1/PD-L1 antibodies. J. Immunother. Cancer 2022, 10, e003890. [Google Scholar] [CrossRef]
- Du, X.; Yang, S.; Bian, J.; Zhang, Y.; Wang, Y.; Lv, Z. Role of vascular endothelial growth factor D in lung adenocarcinoma immunotherapy response. Am. J. Transl. Res. 2024, 16, 2263–2277. [Google Scholar] [CrossRef] [PubMed]
- Sun, C.; Yin, M.; Cheng, Y.; Kuang, Z.; Liu, X.; Wang, G.; Wang, X.; Yuan, K.; Min, W.; Dong, J.; et al. Novel Small-Molecule PD-L1 Inhibitor Induces PD-L1 Internalization and Optimizes the Immune Microenvironment. J. Med. Chem. 2023, 66, 2064–2083. [Google Scholar] [CrossRef] [PubMed]
- Lu, C.; Gao, Z.; Wu, D.; Zheng, J.; Hu, C.; Huang, D.; He, C.; Liu, Y.; Lin, C.; Peng, T.; et al. Understanding the dynamics of TKI-induced changes in the tumor immune microenvironment for improved therapeutic effect. J. Immunother. Cancer 2024, 12, e009165. [Google Scholar] [CrossRef]
- Gao, Y.H.; Lovreković, V.; Kussayeva, A.; Chen, D.Y.; Margetić, D.; Chen, Z.L. The photodynamic activities of dimethyl 13(1)-[2-(guanidinyl)ethylamino] chlorin e(6) photosensitizers in A549 tumor. Eur. J. Med. Chem. 2019, 177, 144–152. [Google Scholar] [CrossRef]
- Guo, D.; Xu, S.; Huang, Y.; Jiang, H.; Yasen, W.; Wang, N.; Su, Y.; Qian, J.; Li, J.; Zhang, C.; et al. Platinum(IV) complex-based two-in-one polyprodrug for a combinatorial chemo-photodynamic therapy. Biomaterials 2018, 177, 67–77. [Google Scholar] [CrossRef] [PubMed]
- Ai, X.; Mu, J.; Xing, B. Recent Advances of Light-Mediated Theranostics. Theranostics 2016, 6, 2439–2457. [Google Scholar] [CrossRef]
- Dai, J.; Li, Y.; Long, Z.; Jiang, R.; Zhuang, Z.; Wang, Z.; Zhao, Z.; Lou, X.; Xia, F.; Tang, B.Z. Efficient Near-Infrared Photosensitizer with Aggregation-Induced Emission for Imaging-Guided Photodynamic Therapy in Multiple Xenograft Tumor Models. ACS Nano 2020, 14, 854–866. [Google Scholar] [CrossRef]
- Zhang, Q.; Wu, L.; Liu, S.; Chen, Q.; Zeng, L.; Chen, X.; Zhang, Q. Targeted nanobody complex enhanced photodynamic therapy for lung cancer by overcoming tumor microenvironment. Cancer Cell Int. 2020, 20, 570. [Google Scholar] [CrossRef]
- Zhao, Y.; Guo, S.; Deng, J.; Shen, J.; Du, F.; Wu, X.; Chen, Y.; Li, M.; Chen, M.; Li, X.; et al. VEGF/VEGFR-Targeted Therapy and Immunotherapy in Non-small Cell Lung Cancer: Targeting the Tumor Microenvironment. Int. J. Biol. Sci. 2022, 18, 3845–3858. [Google Scholar] [CrossRef]
- Chen, F.; Zhuang, X.; Lin, L.; Yu, P.; Wang, Y.; Shi, Y.; Hu, G.; Sun, Y. New horizons in tumor microenvironment biology: Challenges and opportunities. BMC Med. 2015, 13, 45. [Google Scholar] [CrossRef]
- Paluskievicz, C.M.; Cao, X.; Abdi, R.; Zheng, P.; Liu, Y.; Bromberg, J.S. T Regulatory Cells and Priming the Suppressive Tumor Microenvironment. Front. Immunol. 2019, 10, 2453. [Google Scholar] [CrossRef]
- Quail, D.F.; Joyce, J.A. Microenvironmental regulation of tumor progression and metastasis. Nat. Med. 2013, 19, 1423–1437. [Google Scholar] [CrossRef]
- Schulz, M.; Salamero-Boix, A.; Niesel, K.; Alekseeva, T.; Sevenich, L. Microenvironmental Regulation of Tumor Progression and Therapeutic Response in Brain Metastasis. Front. Immunol. 2019, 10, 1713. [Google Scholar] [CrossRef]
- Giraldo, N.A.; Sanchez-Salas, R.; Peske, J.D.; Vano, Y.; Becht, E.; Petitprez, F.; Validire, P.; Ingels, A.; Cathelineau, X.; Fridman, W.H.; et al. The clinical role of the TME in solid cancer. Br. J. Cancer 2019, 120, 45–53. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Fan, X.; Houghton, J. Tumor microenvironment: The role of the tumor stroma in cancer. J. Cell Biochem. 2007, 101, 805–815. [Google Scholar] [CrossRef]
- Chen, Z.; Zhou, L.; Liu, L.; Hou, Y.; Xiong, M.; Yang, Y.; Hu, J.; Chen, K. Single-cell RNA sequencing highlights the role of inflammatory cancer-associated fibroblasts in bladder urothelial carcinoma. Nat. Commun. 2020, 11, 5077. [Google Scholar] [CrossRef] [PubMed]
- Mao, X.; Xu, J.; Wang, W.; Liang, C.; Hua, J.; Liu, J.; Zhang, B.; Meng, Q.; Yu, X.; Shi, S. Crosstalk between cancer-associated fibroblasts and immune cells in the tumor microenvironment: New findings and future perspectives. Mol. Cancer 2021, 20, 131. [Google Scholar] [CrossRef]
- Lv, B.; Wang, Y.; Ma, D.; Cheng, W.; Liu, J.; Yong, T.; Chen, H.; Wang, C. Immunotherapy: Reshape the Tumor Immune Microenvironment. Front. Immunol. 2022, 13, 844142. [Google Scholar] [CrossRef]
- Chen, Y.; McAndrews, K.M.; Kalluri, R. Clinical and therapeutic relevance of cancer-associated fibroblasts. Nat. Rev. Clin. Oncol. 2021, 18, 792–804. [Google Scholar]
- Desbois, M.; Wang, Y. Cancer-associated fibroblasts: Key players in shaping the tumor immune microenvironment. Immunol. Rev. 2021, 302, 241–258. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Li, W.; Lin, S.; Liu, B.; Wu, P.; Li, L. Fibroblast diversity and plasticity in the tumor microenvironment: Roles in immunity and relevant therapies. Cell Commun. Signal 2023, 21, 234. [Google Scholar]
- Pei, L.; Liu, Y.; Liu, L.; Gao, S.; Gao, X.; Feng, Y.; Sun, Z.; Zhang, Y.; Wang, C. Roles of cancer-associated fibroblasts (CAFs) in anti- PD-1/PD-L1 immunotherapy for solid cancers. Mol. Cancer 2023, 22, 29. [Google Scholar] [PubMed]
- Raaijmakers, K.; Adema, G.J.; Bussink, J.; Ansems, M. Cancer-associated fibroblasts, tumor and radiotherapy: Interactions in the tumor micro-environment. J. Exp. Clin. Cancer Res. 2024, 43, 323. [Google Scholar]
- Bremnes, R.M.; Dønnem, T.; Al-Saad, S.; Al-Shibli, K.; Andersen, S.; Sirera, R.; Camps, C.; Marinez, I.; Busund, L.T. The role of tumor stroma in cancer progression and prognosis: Emphasis on carcinoma-associated fibroblasts and non-small cell lung cancer. J. Thorac. Oncol. 2011, 6, 209–217. [Google Scholar] [CrossRef] [PubMed]
- Öhlund, D.; Elyada, E.; Tuveson, D. Fibroblast heterogeneity in the cancer wound. J. Exp. Med. 2014, 211, 1503–1523. [Google Scholar]
- Navab, R.; Strumpf, D.; Bandarchi, B.; Zhu, C.Q.; Pintilie, M.; Ramnarine, V.R.; Ibrahimov, E.; Radulovich, N.; Leung, L.; Barczyk, M.; et al. Prognostic gene-expression signature of carcinoma-associated fibroblasts in non-small cell lung cancer. Proc. Natl. Acad. Sci. USA 2011, 108, 7160–7165. [Google Scholar]
- Albrengues, J.; Bertero, T.; Grasset, E.; Bonan, S.; Maiel, M.; Bourget, I.; Philippe, C.; Herraiz Serrano, C.; Benamar, S.; Croce, O.; et al. Epigenetic switch drives the conversion of fibroblasts into proinvasive cancer-associated fibroblasts. Nat. Commun. 2015, 6, 10204. [Google Scholar] [CrossRef]
- Zhou, Z.; Zhou, Q.; Wu, X.; Xu, S.; Hu, X.; Tao, X.; Li, B.; Peng, J.; Li, D.; Shen, L.; et al. VCAM-1 secreted from cancer-associated fibroblasts enhances the growth and invasion of lung cancer cells through AKT and MAPK signaling. Cancer Lett. 2020, 473, 62–73. [Google Scholar] [CrossRef]
- Procopio, M.G.; Laszlo, C.; Al Labban, D.; Kim, D.E.; Bordignon, P.; Jo, S.H.; Goruppi, S.; Menietti, E.; Ostano, P.; Ala, U.; et al. Combined CSL and p53 downregulation promotes cancer-associated fibroblast activation. Nat. Cell Biol. 2015, 17, 1193–1204. [Google Scholar]
- Iwano, M.; Plieth, D.; Danoff, T.M.; Xue, C.; Okada, H.; Neilson, E.G. Evidence that fibroblasts derive from epithelium during tissue fibrosis. J. Clin. Investig. 2002, 110, 341–350. [Google Scholar] [PubMed]
- Saito, A.; Horie, M.; Nagase, T. TGF-β Signaling in Lung Health and Disease. Int. J. Mol. Sci. 2018, 19, 2460. [Google Scholar] [CrossRef] [PubMed]
- Yang, F.; Yan, Y.; Yang, Y.; Hong, X.; Wang, M.; Yang, Z.; Liu, B.; Ye, L. MiR-210 in exosomes derived from CAFs promotes non-small cell lung cancer migration and invasion through PTEN/PI3K/AKT pathway. Cell. Signal. 2020, 73, 109675. [Google Scholar] [CrossRef]
- You, J.; Li, M.; Cao, L.M.; Gu, Q.H.; Deng, P.B.; Tan, Y.; Hu, C.P. Snail1-dependent cancer-associated fibroblasts induce epithelial-mesenchymal transition in lung cancer cells via exosomes. QJM 2019, 112, 581–590. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Cao, L.; Wang, H.; Liu, B.; Zhang, Q.; Meng, Z.; Wu, X.; Zhou, Q.; Xu, K. Cancer-associated fibroblasts enhance metastatic potential of lung cancer cells through IL-6/STAT3 signaling pathway. Oncotarget 2017, 8, 76116–76128. [Google Scholar] [CrossRef]
- Wang, Y.; Lan, W.; Xu, M.; Song, J.; Mao, J.; Li, C.; Du, X.; Jiang, Y.; Li, E.; Zhang, R.; et al. Cancer-associated fibroblast-derived SDF-1 induces epithelial-mesenchymal transition of lung adenocarcinoma via CXCR4/β-catenin/PPARδ signalling. Cell Death Dis. 2021, 12, 214. [Google Scholar]
- Shintani, Y.; Abulaiti, A.; Kimura, T.; Funaki, S.; Nakagiri, T.; Inoue, M.; Sawabata, N.; Minami, M.; Morii, E.; Okumura, M. Pulmonary fibroblasts induce epithelial mesenchymal transition and some characteristics of stem cells in non-small cell lung cancer. Ann. Thorac. Surg. 2013, 96, 425–433. [Google Scholar] [CrossRef]
- Zeisberg, E.M.; Potenta, S.; Xie, L.; Zeisberg, M.; Kalluri, R. Discovery of endothelial to mesenchymal transition as a source for carcinoma-associated fibroblasts. Cancer Res. 2007, 67, 10123–10128. [Google Scholar] [CrossRef]
- Ciszewski, W.M.; Sobierajska, K.; Wawro, M.E.; Klopocka, W.; Chefczyńska, N.; Muzyczuk, A.; Siekacz, K.; Wujkowska, A.; Niewiarowska, J. The ILK-MMP9-MRTF axis is crucial for EndMT differentiation of endothelial cells in a tumor microenvironment. Biochim. Biophys. Acta Mol. Cell Res. 2017, 1864, 2283–2296. [Google Scholar] [CrossRef]
- Choi, S.H.; Nam, J.K.; Kim, B.Y.; Jang, J.; Jin, Y.B.; Lee, H.J.; Park, S.; Ji, Y.H.; Cho, J.; Lee, Y.J. HSPB1 Inhibits the Endothelial-to-Mesenchymal Transition to Suppress Pulmonary Fibrosis and Lung Tumorigenesis. Cancer Res. 2016, 76, 1019–1030. [Google Scholar] [CrossRef]
- Wen, S.; Niu, Y.; Yeh, S.; Chang, C. BM-MSCs promote prostate cancer progression via the conversion of normal fibroblasts to cancer-associated fibroblasts. Int. J. Oncol. 2015, 47, 719–727. [Google Scholar] [PubMed]
- Iwamoto, C.; Ohuchida, K.; Shinkawa, T.; Okuda, S.; Otsubo, Y.; Okumura, T.; Sagara, A.; Koikawa, K.; Ando, Y.; Shindo, K.; et al. Bone marrow-derived macrophages converted into cancer-associated fibroblast-like cells promote pancreatic cancer progression. Cancer Lett. 2021, 512, 15–27. [Google Scholar] [CrossRef]
- Moratin, H.; Böhm, S.; Hagen, R.; Scherzad, A.; Hackenberg, S. Influence of Wound Fluid on the Transdifferentiation of Human Mesenchymal Bone Marrow Stem Cells into Cancer-Associated Fibroblasts. Cells Tissues Organs 2023, 212, 304–316. [Google Scholar] [CrossRef] [PubMed]
- Quante, M.; Tu, S.P.; Tomita, H.; Gonda, T.; Wang, S.S.; Takashi, S.; Baik, G.H.; Shibata, W.; Diprete, B.; Betz, K.S.; et al. Bone marrow-derived myofibroblasts contribute to the mesenchymal stem cell niche and promote tumor growth. Cancer Cell 2011, 19, 257–272. [Google Scholar] [PubMed]
- Spaeth, E.L.; Labaff, A.M.; Toole, B.P.; Klopp, A.; Andreeff, M.; Marini, F.C. Mesenchymal CD44 expression contributes to the acquisition of an activated fibroblast phenotype via TWIST activation in the tumor microenvironment. Cancer Res. 2013, 73, 5347–5359. [Google Scholar] [PubMed]
- Peng, Y.; Li, Z.; Li, Z. GRP78 secreted by tumor cells stimulates differentiation of bone marrow mesenchymal stem cells to cancer-associated fibroblasts. Biochem. Biophys. Res. Commun. 2013, 440, 558–563. [Google Scholar] [CrossRef] [PubMed]
- Sha, M.; Jeong, S.; Qiu, B.J.; Tong, Y.; Xia, L.; Xu, N.; Zhang, J.J.; Xia, Q. Isolation of cancer-associated fibroblasts and its promotion to the progression of intrahepatic cholangiocarcinoma. Cancer Med. 2018, 7, 4665–4677. [Google Scholar] [CrossRef]
- Zawieracz, K.; Eckert, M.A. Isolation of Normal and Cancer-Associated Fibroblasts. Methods Mol. Biol. 2022, 2424, 155–165. [Google Scholar] [CrossRef]
- Sharon, Y.; Alon, L.; Glanz, S.; Servais, C.; Erez, N. Isolation of normal and cancer-associated fibroblasts from fresh tissues by Fluorescence Activated Cell Sorting (FACS). J. Vis. Exp. 2013, 71, e4425. [Google Scholar] [CrossRef]
- Jiang, R.; Agrawal, S.; Aghaamoo, M.; Parajuli, R.; Agrawal, A.; Lee, A.P. Rapid isolation of circulating cancer associated fibroblasts by acoustic microstreaming for assessing metastatic propensity of breast cancer patients. Lab Chip 2021, 21, 875–887. [Google Scholar] [CrossRef]
- Nurmik, M.; Ullmann, P.; Rodriguez, F.; Haan, S.; Letellier, E. In search of definitions: Cancer-associated fibroblasts and their markers. Int. J. Cancer 2020, 146, 895–905. [Google Scholar] [CrossRef] [PubMed]
- Hu, D.; Li, Z.; Zheng, B.; Lin, X.; Pan, Y.; Gong, P.; Zhuo, W.; Hu, Y.; Chen, C.; Chen, L.; et al. Cancer-associated fibroblasts in breast cancer: Challenges and opportunities. Cancer Commun. 2022, 42, 401–434. [Google Scholar] [CrossRef]
- Peltier, A.; Seban, R.D.; Buvat, I.; Bidard, F.C.; Mechta-Grigoriou, F. Fibroblast heterogeneity in solid tumors: From single cell analysis to whole-body imaging. Semin. Cancer Biol. 2022, 86 Pt 3, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Mathieson, L.; Koppensteiner, L.; Dorward, D.A.; O’Connor, R.A.; Akram, A.R. Cancer-associated fibroblasts expressing fibroblast activation protein and podoplanin in non-small cell lung cancer predict poor clinical outcome. Br. J. Cancer 2024, 130, 1758–1769. [Google Scholar] [CrossRef]
- Sugimoto, H.; Mundel, T.M.; Kieran, M.W.; Kalluri, R. Identification of fibroblast heterogeneity in the tumor microenvironment. Cancer Biol. Ther. 2006, 5, 1640–1646. [Google Scholar] [CrossRef]
- Samson, S.C.; Rojas, A.; Zitnay, R.G.; Carney, K.R.; Hettinga, W.; Schaelling, M.C.; Sicard, D.; Zhang, W.; Gilbert-Ross, M.; Dy, G.K.; et al. Tenascin-C in the early lung cancer tumor microenvironment promotes progression through integrin αvβ1 and FAK. bioRxiv 2024. [Google Scholar] [CrossRef]
- Hu, W.W.; Chen, P.C.; Chen, J.M.; Wu, Y.M.; Liu, P.Y.; Lu, C.H.; Lin, Y.F.; Tang, C.H.; Chao, C.C. Periostin promotes epithelial-mesenchymal transition via the MAPK/miR-381 axis in lung cancer. Oncotarget 2017, 8, 62248–62260. [Google Scholar] [CrossRef]
- Li, K.; Wang, R.; Liu, G.W.; Peng, Z.Y.; Wang, J.C.; Xiao, G.D.; Tang, S.C.; Du, N.; Zhang, J.; Zhang, J.; et al. Refining the optimal CAF cluster marker for predicting TME-dependent survival expectancy and treatment benefits in NSCLC patients. Sci. Rep. 2024, 14, 16766. [Google Scholar] [CrossRef]
- Zhu, G.; Wang, Y.; Wang, Y.; Huang, H.; Li, B.; Chen, P.; Chen, C.; Zhang, H.; Li, Y.; Liu, H.; et al. Myofibroblasts derived type V collagen promoting tissue mechanical stress and facilitating metastasis and therapy resistance of lung adenocarcinoma cells. Cell Death Dis. 2024, 15, 493. [Google Scholar] [CrossRef]
- Zhang, Q.; Yang, J.; Bai, J.; Ren, J. Reverse of non-small cell lung cancer drug resistance induced by cancer-associated fibroblasts via a paracrine pathway. Cancer Sci. 2018, 109, 944–955. [Google Scholar] [CrossRef]
- Sugai, M.; Yanagawa, N.; Shikanai, S.; Hashimoto, M.; Saikawa, H.; Osakabe, M.; Saito, H.; Maemondo, M.; Sugai, T. Correlation of tumor microenvironment-related markers with clinical outcomes in patients with squamous cell carcinoma of the lung. Transl. Lung Cancer Res. 2022, 11, 975–990. [Google Scholar] [CrossRef] [PubMed]
- Puré, E.; Blomberg, R. Pro-tumorigenic roles of fibroblast activation protein in cancer: Back to the basics. Oncogene 2018, 37, 4343–4357. [Google Scholar]
- Kilvaer, T.K.; Rakaee, M.; Hellevik, T.; Østman, A.; Strell, C.; Bremnes, R.M.; Busund, L.T.; Dønnem, T.; Martinez-Zubiaurre, I. Tissue analyses reveal a potential immune-adjuvant function of FAP-1 positive fibroblasts in non-small cell lung cancer. PLoS ONE 2018, 13, e0192157. [Google Scholar]
- Chen, L.; Chen, M.; Han, Z.; Jiang, F.; Xu, C.; Qin, Y.; Ding, N.; Liu, Y.; Zhang, T.; An, Z.; et al. Clinical significance of FAP-α on microvessel and lymphatic vessel density in lung squamous cell carcinoma. J. Clin. Pathol. 2018, 71, 721–728. [Google Scholar] [CrossRef]
- Fang, Y.; Wang, Y.J.; Zhao, H.L.; Huang, X.; Fang, Y.N.; Chen, W.Y.; Han, R.Z.; Zhao, A.; Gao, J.M. Development of FAP-Targeted Chimeric Antigen Receptor NK-92 Cells for Non-Small Cell Lung Cancer. Discov. Med. 2023, 35, 405–417. [Google Scholar] [CrossRef] [PubMed]
- Li, H.; Qiu, L.; Liu, Q.; Ma, Z.; Xie, X.; Luo, Y.; Wu, X. Senescent Fibroblasts Generate a CAF Phenotype through the Stat3 Pathway. Genes 2022, 13, 1579. [Google Scholar] [CrossRef]
- Lu, Y.; Li, H.; Zhao, P.; Tian, L.; Liu, Y.; Sun, X.; Cheng, Y. Dynamic phenotypic reprogramming and chemoresistance induced by lung fibroblasts in small cell lung cancer. Sci. Rep. 2024, 14, 2884. [Google Scholar] [CrossRef]
- Paulsson, J.; Ehnman, M.; Östman, A. PDGF receptors in tumor biology: Prognostic and predictive potential. Future Oncol. 2014, 10, 1695–1708. [Google Scholar] [CrossRef]
- Akiyama, T.; Yasuda, T.; Uchihara, T.; Yasuda-Yoshihara, N.; Tan, B.J.Y.; Yonemura, A.; Semba, T.; Yamasaki, J.; Komohara, Y.; Ohnishi, K.; et al. Stromal Reprogramming through Dual PDGFRα/β Blockade Boosts the Efficacy of Anti-PD-1 Immunotherapy in Fibrotic Tumors. Cancer Res. 2023, 83, 753–770. [Google Scholar] [CrossRef]
- Sun, C.; Zhang, K.; Ni, C.; Wan, J.; Duan, X.; Lou, X.; Yao, X.; Li, X.; Wang, M.; Gu, Z.; et al. Transgelin promotes lung cancer progression via activation of cancer-associated fibroblasts with enhanced IL-6 release. Oncogenesis 2023, 12, 18. [Google Scholar]
- Zeltz, C.; Alam, J.; Liu, H.; Erusappan, P.M.; Hoschuetzky, H.; Molven, A.; Parajuli, H.; Cukierman, E.; Costea, D.E.; Lu, N.; et al. α11β1 Integrin is Induced in a Subset of Cancer-Associated Fibroblasts in Desmoplastic Tumor Stroma and Mediates In Vitro Cell Migration. Cancers 2019, 11, 765. [Google Scholar] [CrossRef]
- Vasiukov, G.; Zou, Y.; Senosain, M.F.; Rahman, J.S.M.; Antic, S.; Young, K.M.; Grogan, E.L.; Kammer, M.N.; Maldonado, F.; Reinhart-King, C.A.; et al. Cancer-associated fibroblasts in early-stage lung adenocarcinoma correlate with tumor aggressiveness. Sci. Rep. 2023, 13, 17604. [Google Scholar]
- Nabeshima, K.; Inoue, T.; Shimao, Y.; Sameshima, T. Matrix metalloproteinases in tumor invasion: Role for cell migration. Pathol. Int. 2002, 52, 255–264. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Chan, W.N.; Xie, F.; Mui, C.W.; Liu, X.; Cheung, A.H.K.; Lung, R.W.M.; Chow, C.; Zhang, Z.; Fang, C.; et al. The molecular classification of cancer-associated fibroblasts on a pan-cancer single-cell transcriptional atlas. Clin. Transl. Med. 2023, 13, e1516. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Zhou, J.; Xiao, Q.; Fujiwara, K.; Zhang, M.; Mo, G.; Gong, W.; Zheng, L. Cancer-associated fibroblast heterogeneity is associated with organ-specific metastasis in pancreatic ductal adenocarcinoma. J. Hematol. Oncol. 2021, 14, 184. [Google Scholar] [CrossRef] [PubMed]
- Xu, K.; Wang, H.; Zou, Y.X.; Zhang, H.H.; Wang, Y.N.; Ren, X.R.; Wang, H.Q.; Xu, Y.H.; Li, J.J.; Tang, H.; et al. Distinct fibroblast subpopulations associated with bone, brain or intrapulmonary metastasis in advanced non-small-cell lung cancer. Clin. Transl. Med. 2024, 14, e1605. [Google Scholar] [CrossRef]
- Cords, L.; Engler, S.; Haberecker, M.; Rüschoff, J.H.; Moch, H.; de Souza, N.; Bodenmiller, B. Cancer-associated fibroblast phenotypes are associated with patient outcome in non-small cell lung cancer. Cancer Cell 2024, 42, 396–412.e5. [Google Scholar] [CrossRef]
- Zhao, Y.; Jia, Y.; Wang, J.; Chen, X.; Han, J.; Zhen, S.; Yin, S.; Lv, W.; Yu, F.; Wang, J.; et al. circNOX4 activates an inflammatory fibroblast niche to promote tumor growth and metastasis in NSCLC via FAP/IL-6 axis. Mol. Cancer 2024, 23, 47. [Google Scholar]
- Lu, Y.; Li, H.; Zhao, P.; Wang, X.; Shao, W.; Liu, Y.; Tian, L.; Zhong, R.; Liu, H.; Cheng, Y. Crosstalk between cancer-associated fibroblasts and non-neuroendocrine tumor cells in small cell lung cancer involves in glycolysis and antigen-presenting features. Mol. Med. 2024, 30, 274. [Google Scholar] [CrossRef]
- Ma, C.; Yang, C.; Peng, A.; Sun, T.; Ji, X.; Mi, J.; Wei, L.; Shen, S.; Feng, Q. Pan-cancer spatially resolved single-cell analysis reveals the crosstalk between cancer-associated fibroblasts and tumor microenvironment. Mol. Cancer 2023, 22, 170. [Google Scholar] [CrossRef]
- Wu, F.; Fan, J.; He, Y.; Xiong, A.; Yu, J.; Li, Y.; Zhang, Y.; Zhao, W.; Zhou, F.; Li, W.; et al. Single-cell profiling of tumor heterogeneity and the microenvironment in advanced non-small cell lung cancer. Nat. Commun. 2021, 12, 2540. [Google Scholar] [CrossRef]
- Hu, H.; Piotrowska, Z.; Hare, P.J.; Chen, H.; Mulvey, H.E.; Mayfield, A.; Noeen, S.; Kattermann, K.; Greenberg, M.; Williams, A.; et al. Three subtypes of lung cancer fibroblasts define distinct therapeutic paradigms. Cancer Cell 2021, 39, 1531–1547.e1510. [Google Scholar] [CrossRef] [PubMed]
- Hanley, C.J.; Waise, S.; Ellis, M.J.; Lopez, M.A.; Pun, W.Y.; Taylor, J.; Parker, R.; Kimbley, L.M.; Chee, S.J.; Shaw, E.C.; et al. Single-cell analysis reveals prognostic fibroblast subpopulations linked to molecular and immunological subtypes of lung cancer. Nat. Commun. 2023, 14, 387. [Google Scholar] [CrossRef]
- Si, Y.; Zhao, Z.; Meng, X.; Zhao, K. RNA-seq and bulk RNA-seq data analysis of cancer-related fibroblasts (CAF) in LUAD to construct a CAF-based risk signature. Sci. Rep. 2024, 14, 23243. [Google Scholar] [CrossRef] [PubMed]
- Bota-Rabassedas, N.; Banerjee, P.; Niu, Y.; Cao, W.; Luo, J.; Xi, Y.; Tan, X.; Sheng, K.; Ahn, Y.H.; Lee, S.; et al. Contextual cues from cancer cells govern cancer-associated fibroblast heterogeneity. Cell Rep. 2021, 35, 109009. [Google Scholar] [CrossRef]
- Zhang, X.; Xiao, Q.; Zhang, C.; Zhou, Q.; Xu, T. Construction of a prognostic model with CAFs for predicting the prognosis and immunotherapeutic response of lung squamous cell carcinoma. J. Cell. Mol. Med. 2024, 28, e18262. [Google Scholar] [CrossRef]
- Fang, X.; Li, D.; Wan, S.; Hu, J.; Zhang, P.; Jie, D.; Chen, L.; Jiang, G.; Song, N. Insights into the heterogeneity of the tumor microenvironment in lung adenocarcinoma and squamous carcinoma through single-cell transcriptomic analysis: Implications for distinct immunotherapy outcomes. J. Gene Med. 2024, 26, e3694. [Google Scholar] [CrossRef]
- Yang, Y.; Shao, X.; Li, Z.; Zhang, L.; Yang, B.; Jin, B.; Hu, X.; Qu, X.; Che, X.; Liu, Y. Prognostic heterogeneity of Ki67 in non-small cell lung cancer: A comprehensive reappraisal on immunohistochemistry and transcriptional data. J. Cell. Mol. Med. 2024, 28, e18521. [Google Scholar] [CrossRef]
- Liu, T.; Han, C.; Wang, S.; Fang, P.; Ma, Z.; Xu, L.; Yin, R. Cancer-associated fibroblasts: An emerging target of anti-cancer immunotherapy. J. Hematol. Oncol. 2019, 12, 86. [Google Scholar] [CrossRef]
- Harper, J.; Sainson, R.C. Regulation of the anti-tumour immune response by cancer-associated fibroblasts. Semin. Cancer Biol. 2014, 25, 69–77. [Google Scholar] [CrossRef]
- Ziani, L.; Chouaib, S.; Thiery, J. Alteration of the Antitumor Immune Response by Cancer-Associated Fibroblasts. Front. Immunol. 2018, 9, 414. [Google Scholar] [CrossRef] [PubMed]
- Kim, R.; Emi, M.; Tanabe, K. Cancer immunosuppression and autoimmune disease: Beyond immunosuppressive networks for tumour immunity. Immunology 2006, 119, 254–264. [Google Scholar] [CrossRef]
- Wang, S.; Fan, G.; Li, L.; He, Y.; Lou, N.; Xie, T.; Dai, L.; Gao, R.; Yang, M.; Shi, Y.; et al. Integrative analyses of bulk and single-cell RNA-seq identified cancer-associated fibroblasts-related signature as a prognostic factor for immunotherapy in NSCLC. Cancer Immunol. Immunother. 2023, 72, 2423–2442. [Google Scholar] [CrossRef]
- Mantovani, A.; Sica, A.; Sozzani, S.; Allavena, P.; Vecchi, A.; Locati, M. The chemokine system in diverse forms of macrophage activation and polarization. Trends Immunol. 2004, 25, 677–686. [Google Scholar] [CrossRef]
- Shapouri-Moghaddam, A.; Mohammadian, S.; Vazini, H.; Taghadosi, M.; Esmaeili, S.A.; Mardani, F.; Seifi, B.; Mohammadi, A.; Afshari, J.T.; Sahebkar, A. Macrophage plasticity, polarization, and function in health and disease. J. Cell. Physiol. 2018, 233, 6425–6440. [Google Scholar] [CrossRef]
- Allavena, P.; Sica, A.; Garlanda, C.; Mantovani, A. The Yin-Yang of tumor-associated macrophages in neoplastic progression and immune surveillance. Immunol. Rev. 2008, 222, 155–161. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Allavena, P.; Sica, A.; Balkwill, F. Cancer-related inflammation. Nature 2008, 454, 436–444. [Google Scholar] [CrossRef] [PubMed]
- Herrera, M.; Herrera, A.; Domínguez, G.; Silva, J.; García, V.; García, J.M.; Gómez, I.; Soldevilla, B.; Muñoz, C.; Provencio, M.; et al. Cancer-associated fibroblast and M2 macrophage markers together predict outcome in colorectal cancer patients. Cancer Sci. 2013, 104, 437–444. [Google Scholar] [CrossRef]
- Tan, B.; Shi, X.; Zhang, J.; Qin, J.; Zhang, N.; Ren, H.; Qian, M.; Siwko, S.; Carmon, K.; Liu, Q.; et al. Inhibition of Rspo-Lgr4 Facilitates Checkpoint Blockade Therapy by Switching Macrophage Polarization. Cancer Res. 2018, 78, 4929–4942. [Google Scholar] [CrossRef]
- Comito, G.; Giannoni, E.; Segura, C.P.; Barcellos-de-Souza, P.; Raspollini, M.R.; Baroni, G.; Lanciotti, M.; Serni, S.; Chiarugi, P. Cancer-associated fibroblasts and M2-polarized macrophages synergize during prostate carcinoma progression. Oncogene 2014, 33, 2423–2431. [Google Scholar] [CrossRef]
- Cohen, N.; Shani, O.; Raz, Y.; Sharon, Y.; Hoffman, D.; Abramovitz, L.; Erez, N. Fibroblasts drive an immunosuppressive and growth-promoting microenvironment in breast cancer via secretion of Chitinase 3-like 1. Oncogene 2017, 36, 4457–4468. [Google Scholar] [CrossRef] [PubMed]
- Hegab, A.E.; Ozaki, M.; Kameyama, N.; Gao, J.; Kagawa, S.; Yasuda, H.; Soejima, K.; Yin, Y.; Guzy, R.D.; Nakamura, Y.; et al. Effect of FGF/FGFR pathway blocking on lung adenocarcinoma and its cancer-associated fibroblasts. J. Pathol. 2019, 249, 193–205. [Google Scholar] [CrossRef]
- Suzuki, J.; Aokage, K.; Neri, S.; Sakai, T.; Hashimoto, H.; Su, Y.; Yamazaki, S.; Nakamura, H.; Tane, K.; Miyoshi, T.; et al. Relationship between podoplanin-expressing cancer-associated fibroblasts and the immune microenvironment of early lung squamous cell carcinoma. Lung Cancer 2021, 153, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Guo, Q.; Liu, Y.; Hou, Q.; Liao, M.; Guo, Y.; Zang, Y.; Wang, F.; Liu, H.; Luan, X.; et al. Single-cell and spatial transcriptomics reveal POSTN(+) cancer-associated fibroblasts correlated with immune suppression and tumour progression in non-small cell lung cancer. Clin. Transl. Med. 2023, 13, e1515. [Google Scholar] [CrossRef]
- Tang, P.C.; Chung, J.Y.; Xue, V.W.; Xiao, J.; Meng, X.M.; Huang, X.R.; Zhou, S.; Chan, A.S.; Tsang, A.C.; Cheng, A.S.; et al. Smad3 Promotes Cancer-Associated Fibroblasts Generation via Macrophage-Myofibroblast Transition. Adv. Sci. 2022, 9, e2101235. [Google Scholar] [CrossRef] [PubMed]
- Tang, P.C.; Chan, M.K.; Chung, J.Y.; Chan, A.S.; Zhang, D.; Li, C.; Leung, K.T.; Ng, C.S.; Wu, Y.; To, K.F.; et al. Hematopoietic Transcription Factor RUNX1 is Essential for Promoting Macrophage-Myofibroblast Transition in Non-Small-Cell Lung Carcinoma. Adv. Sci. 2024, 11, e2302203. [Google Scholar] [CrossRef]
- Zhou, J.; Liu, H.; Jiang, S.; Wang, W. Role of tumor-associated neutrophils in lung cancer (Review). Oncol. Lett. 2023, 25, 2. [Google Scholar] [CrossRef]
- Piccard, H.; Muschel, R.J.; Opdenakker, G. On the dual roles and polarized phenotypes of neutrophils in tumor development and progression. Crit. Rev. Oncol. Hematol. 2012, 82, 296–309. [Google Scholar] [CrossRef]
- Sionov, R.V.; Fridlender, Z.G.; Granot, Z. The Multifaceted Roles Neutrophils Play in the Tumor Microenvironment. Cancer Microenviron. 2015, 8, 125–158. [Google Scholar] [CrossRef]
- Coffelt, S.B.; Wellenstein, M.D.; de Visser, K.E. Neutrophils in cancer: Neutral no more. Nat. Rev. Cancer 2016, 16, 431–446. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Albelda, S.M. Tumor-associated neutrophils: Friend or foe? Carcinogenesis 2012, 33, 949–955. [Google Scholar] [CrossRef] [PubMed]
- Tsai, Y.M.; Wu, K.L.; Liu, Y.W.; Chang, W.A.; Huang, Y.C.; Chang, C.Y.; Tsai, P.H.; Liao, S.H.; Hung, J.Y.; Hsu, Y.L. Cooperation Between Cancer and Fibroblasts in Vascular Mimicry and N2-Type Neutrophil Recruitment via Notch2-Jagged1 Interaction in Lung Cancer. Front. Oncol. 2021, 11, 696931. [Google Scholar] [CrossRef]
- Raman, D.; Baugher, P.J.; Thu, Y.M.; Richmond, A. Role of chemokines in tumor growth. Cancer Lett. 2007, 256, 137–165. [Google Scholar] [CrossRef]
- Fridlender, Z.G.; Sun, J.; Kim, S.; Kapoor, V.; Cheng, G.; Ling, L.; Worthen, G.S.; Albelda, S.M. Polarization of tumor-associated neutrophil phenotype by TGF-beta: “N1” versus “N2” TAN. Cancer Cell 2009, 16, 183–194. [Google Scholar] [CrossRef]
- Mucciolo, G.; Araos Henríquez, J.; Jihad, M.; Pinto Teles, S.; Manansala, J.S.; Li, W.; Ashworth, S.; Lloyd, E.G.; Cheng, P.S.W.; Luo, W.; et al. EGFR-activated myofibroblasts promote metastasis of pancreatic cancer. Cancer Cell 2024, 42, 101–118.e111. [Google Scholar] [CrossRef]
- Park, Y.; Chung, C. Immune Evasion of G-CSF and GM-CSF in Lung Cancer. Tuberc. Respir. Dis. 2024, 87, 22–30. [Google Scholar] [CrossRef]
- Guillerey, C.; Huntington, N.D.; Smyth, M.J. Targeting natural killer cells in cancer immunotherapy. Nat. Immunol. 2016, 17, 1025–1036. [Google Scholar] [CrossRef]
- Souza-Fonseca-Guimaraes, F.; Cursons, J.; Huntington, N.D. The Emergence of Natural Killer Cells as a Major Target in Cancer Immunotherapy. Trends Immunol. 2019, 40, 142–158. [Google Scholar] [CrossRef]
- Myers, J.A.; Miller, J.S. Exploring the NK cell platform for cancer immunotherapy. Nat. Rev. Clin. Oncol. 2021, 18, 85–100. [Google Scholar] [CrossRef]
- Chiossone, L.; Dumas, P.Y.; Vienne, M.; Vivier, E. Natural killer cells and other innate lymphoid cells in cancer. Nat. Rev. Immunol. 2018, 18, 671–688. [Google Scholar] [CrossRef]
- Sivori, S.; Vacca, P.; Del Zotto, G.; Munari, E.; Mingari, M.C.; Moretta, L. Human NK cells: Surface receptors, inhibitory checkpoints, and translational applications. Cell Mol. Immunol. 2019, 16, 430–441. [Google Scholar] [CrossRef] [PubMed]
- Chen, X.; Song, E. Turning foes to friends: Targeting cancer-associated fibroblasts. Nat. Rev. Drug Discov. 2019, 18, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Turley, S.J.; Cremasco, V.; Astarita, J.L. Immunological hallmarks of stromal cells in the tumour microenvironment. Nat. Rev. Immunol. 2015, 15, 669–682. [Google Scholar] [CrossRef] [PubMed]
- Yang, N.; Lode, K.; Berzaghi, R.; Islam, A.; Martinez-Zubiaurre, I.; Hellevik, T. Irradiated Tumor Fibroblasts Avoid Immune Recognition and Retain Immunosuppressive Functions Over Natural Killer Cells. Front. Immunol. 2020, 11, 602530. [Google Scholar] [CrossRef]
- Flavell, R.A.; Sanjabi, S.; Wrzesinski, S.H.; Licona-Limón, P. The polarization of immune cells in the tumour environment by TGFbeta. Nat. Rev. Immunol. 2010, 10, 554–567. [Google Scholar] [CrossRef]
- Batlle, E.; Massagué, J. Transforming Growth Factor-β Signaling in Immunity and Cancer. Immunity 2019, 50, 924–940. [Google Scholar] [CrossRef]
- Trotta, R.; Dal Col, J.; Yu, J.; Ciarlariello, D.; Thomas, B.; Zhang, X.; Allard, J., 2nd; Wei, M.; Mao, H.; Byrd, J.C.; et al. TGF-beta utilizes SMAD3 to inhibit CD16-mediated IFN-gamma production and antibody-dependent cellular cytotoxicity in human NK cells. J. Immunol. 2008, 181, 3784–3792. [Google Scholar] [CrossRef]
- Lee, Y.S.; Radford, K.J. The role of dendritic cells in cancer. Int. Rev. Cell Mol. Biol. 2019, 348, 123–178. [Google Scholar] [CrossRef]
- Gardner, A.; Ruffell, B. Dendritic Cells and Cancer Immunity. Trends Immunol. 2016, 37, 855–865. [Google Scholar] [CrossRef]
- Berzaghi, R.; Tornaas, S.; Lode, K.; Hellevik, T.; Martinez-Zubiaurre, I. Ionizing Radiation Curtails Immunosuppressive Effects From Cancer-Associated Fibroblasts on Dendritic Cells. Front. Immunol. 2021, 12, 662594. [Google Scholar] [CrossRef]
- Kuo, P.L.; Hung, J.Y.; Huang, S.K.; Chou, S.H.; Cheng, D.E.; Jong, Y.J.; Hung, C.H.; Yang, C.J.; Tsai, Y.M.; Hsu, Y.L.; et al. Lung cancer-derived galectin-1 mediates dendritic cell anergy through inhibitor of DNA binding 3/IL-10 signaling pathway. J. Immunol. 2011, 186, 1521–1530. [Google Scholar] [CrossRef] [PubMed]
- Hsu, Y.L.; Hung, J.Y.; Chiang, S.Y.; Jian, S.F.; Wu, C.Y.; Lin, Y.S.; Tsai, Y.M.; Chou, S.H.; Tsai, M.J.; Kuo, P.L. Lung cancer-derived galectin-1 contributes to cancer associated fibroblast-mediated cancer progression and immune suppression through TDO2/kynurenine axis. Oncotarget 2016, 7, 27584–27598. [Google Scholar] [CrossRef] [PubMed]
- Ohshio, Y.; Teramoto, K.; Hanaoka, J.; Tezuka, N.; Itoh, Y.; Asai, T.; Daigo, Y.; Ogasawara, K. Cancer-associated fibroblast-targeted strategy enhances antitumor immune responses in dendritic cell-based vaccine. Cancer Sci. 2015, 106, 134–142. [Google Scholar] [CrossRef] [PubMed]
- Gabrilovich, D.I. Myeloid-Derived Suppressor Cells. Cancer Immunol. Res. 2017, 5, 3–8. [Google Scholar] [CrossRef]
- Adah, D.; Hussain, M.; Qin, L.; Qin, L.; Zhang, J.; Chen, X. Implications of MDSCs-targeting in lung cancer chemo-immunotherapeutics. Pharmacol. Res. 2016, 110, 25–34. [Google Scholar] [CrossRef]
- Xiang, H.; Ramil, C.P.; Hai, J.; Zhang, C.; Wang, H.; Watkins, A.A.; Afshar, R.; Georgiev, P.; Sze, M.A.; Song, X.S.; et al. Cancer-Associated Fibroblasts Promote Immunosuppression by Inducing ROS-Generating Monocytic MDSCs in Lung Squamous Cell Carcinoma. Cancer Immunol. Res. 2020, 8, 436–450. [Google Scholar] [CrossRef]
- Zhu, Y.; Zhang, L.; Zha, H.; Yang, F.; Hu, C.; Chen, L.; Guo, B.; Zhu, B. Stroma-derived Fibrinogen-like Protein 2 Activates Cancer-associated Fibroblasts to Promote Tumor Growth in Lung Cancer. Int. J. Biol. Sci. 2017, 13, 804–814. [Google Scholar] [CrossRef]
- Kumar, V.; Donthireddy, L.; Marvel, D.; Condamine, T.; Wang, F.; Lavilla-Alonso, S.; Hashimoto, A.; Vonteddu, P.; Behera, R.; Goins, M.A.; et al. Cancer-Associated Fibroblasts Neutralize the Anti-tumor Effect of CSF1 Receptor Blockade by Inducing PMN-MDSC Infiltration of Tumors. Cancer Cell 2017, 32, 654–668.e655. [Google Scholar] [CrossRef]
- Kumar, B.V.; Connors, T.J.; Farber, D.L. Human T Cell Development, Localization, and Function throughout Life. Immunity 2018, 48, 202–213. [Google Scholar] [CrossRef]
- O’Connor, R.A.; Chauhan, V.; Mathieson, L.; Titmarsh, H.; Koppensteiner, L.; Young, I.; Tagliavini, G.; Dorward, D.A.; Prost, S.; Dhaliwal, K.; et al. T cells drive negative feedback mechanisms in cancer associated fibroblasts, promoting expression of co-inhibitory ligands, CD73 and IL-27 in non-small cell lung cancer. Oncoimmunology 2021, 10, 1940675. [Google Scholar] [CrossRef]
- Tanaka, A.; Sakaguchi, S. Regulatory T cells in cancer immunotherapy. Cell Res. 2017, 27, 109–118. [Google Scholar] [CrossRef] [PubMed]
- Kinoshita, T.; Ishii, G.; Hiraoka, N.; Hirayama, S.; Yamauchi, C.; Aokage, K.; Hishida, T.; Yoshida, J.; Nagai, K.; Ochiai, A. Forkhead box P3 regulatory T cells coexisting with cancer associated fibroblasts are correlated with a poor outcome in lung adenocarcinoma. Cancer Sci. 2013, 104, 409–415. [Google Scholar] [CrossRef]
- O’Connor, R.A.; Martinez, B.R.; Koppensteiner, L.; Mathieson, L.; Akram, A.R. Cancer-associated fibroblasts drive CXCL13 production in activated T cells via TGF-beta. Front. Immunol. 2023, 14, 1221532. [Google Scholar] [CrossRef]
- Zhu, J. T Helper Cell Differentiation, Heterogeneity, and Plasticity. Cold Spring Harb. Perspect. Biol. 2018, 10, a030338. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, Y.; Gu, W.; He, L.; Sun, B. Th1/Th2 cell’s function in immune system. Adv. Exp. Med. Biol. 2014, 841, 45–65. [Google Scholar] [CrossRef] [PubMed]
- Koeck, S.; Amann, A.; Kern, J.; Zwierzina, M.; Lorenz, E.; Sopper, S.; Zwierzina, H.; Mildner, F.; Sykora, M.; Sprung, S.; et al. Whole stromal fibroblast signature is linked to specific chemokine and immune infiltration patterns and to improved survival in NSCLC. Oncoimmunology 2023, 12, 2274130. [Google Scholar] [CrossRef] [PubMed]
- Miyai, Y.; Sugiyama, D.; Hase, T.; Asai, N.; Taki, T.; Nishida, K.; Fukui, T.; Chen-Yoshikawa, T.F.; Kobayashi, H.; Mii, S.; et al. Meflin-positive cancer-associated fibroblasts enhance tumor response to immune checkpoint blockade. Life Sci. Alliance 2022, 5, e202101230. [Google Scholar] [CrossRef]
- Freeman, P.; Mielgo, A. Cancer-Associated Fibroblast Mediated Inhibition of CD8+ Cytotoxic T Cell Accumulation in Tumours: Mechanisms and Therapeutic Opportunities. Cancers 2020, 12, 2687. [Google Scholar] [CrossRef]
- Yang, H.; Sun, B.; Fan, L.; Ma, W.; Xu, K.; Hall, S.R.R.; Wang, Z.; Schmid, R.A.; Peng, R.W.; Marti, T.M.; et al. Multi-scale integrative analyses identify THBS2(+) cancer-associated fibroblasts as a key orchestrator promoting aggressiveness in early-stage lung adenocarcinoma. Theranostics 2022, 12, 3104–3130. [Google Scholar] [CrossRef]
- Sakai, T.; Aokage, K.; Neri, S.; Nakamura, H.; Nomura, S.; Tane, K.; Miyoshi, T.; Sugano, M.; Kojima, M.; Fujii, S.; et al. Link between tumor-promoting fibrous microenvironment and an immunosuppressive microenvironment in stage I lung adenocarcinoma. Lung Cancer 2018, 126, 64–71. [Google Scholar] [CrossRef]
- Grout, J.A.; Sirven, P.; Leader, A.M.; Maskey, S.; Hector, E.; Puisieux, I.; Steffan, F.; Cheng, E.; Tung, N.; Maurin, M.; et al. Spatial Positioning and Matrix Programs of Cancer-Associated Fibroblasts Promote T-cell Exclusion in Human Lung Tumors. Cancer Discov. 2022, 12, 2606–2625. [Google Scholar] [CrossRef] [PubMed]
- Lee, H.; Hwang, M.; Jang, S.; Um, S.W. Immune Regulatory Function of Cancer- Associated Fibroblasts in Non-small Cell Lung Cancer. Tuberc. Respir. Dis. 2023, 86, 304–318. [Google Scholar] [CrossRef]
- Mao, G.; Liu, J. Research on the mechanism of exosomes from different sources influencing the progression of lung cancer. Environ. Toxicol. 2024, 39, 4231–4248. [Google Scholar] [CrossRef] [PubMed]
- Zheng, X.; Jiang, K.; Xiao, W.; Zeng, D.; Peng, W.; Bai, J.; Chen, X.; Li, P.; Zhang, L.; Zheng, X.; et al. CD8(+) T cell/cancer-associated fibroblast ratio stratifies prognostic and predictive responses to immunotherapy across multiple cancer types. Front. Immunol. 2022, 13, 974265. [Google Scholar] [CrossRef]
- Koppensteiner, L.; Mathieson, L.; Neilson, L.; O’Connor, R.A.; Akram, A.R. IFNγ and TNFα drive an inflammatory secretion profile in cancer-associated fibroblasts from human non-small cell lung cancer. FEBS Lett. 2025, 599, 713–723. [Google Scholar] [CrossRef]
- Thommen, D.S.; Schumacher, T.N. T Cell Dysfunction in Cancer. Cancer Cell 2018, 33, 547–562. [Google Scholar] [CrossRef]
- Thommen, D.S.; Schreiner, J.; Müller, P.; Herzig, P.; Roller, A.; Belousov, A.; Umana, P.; Pisa, P.; Klein, C.; Bacac, M.; et al. Progression of Lung Cancer Is Associated with Increased Dysfunction of T Cells Defined by Coexpression of Multiple Inhibitory Receptors. Cancer Immunol. Res. 2015, 3, 1344–1355. [Google Scholar] [CrossRef]
- Sun, C.; Mezzadra, R.; Schumacher, T.N. Regulation and Function of the PD-L1 Checkpoint. Immunity 2018, 48, 434–452. [Google Scholar] [CrossRef]
- Lakins, M.A.; Ghorani, E.; Munir, H.; Martins, C.P.; Shields, J.D. Cancer-associated fibroblasts induce antigen-specific deletion of CD8 (+) T Cells to protect tumour cells. Nat. Commun. 2018, 9, 948. [Google Scholar] [CrossRef]
- Nazareth, M.R.; Broderick, L.; Simpson-Abelson, M.R.; Kelleher, R.J., Jr.; Yokota, S.J.; Bankert, R.B. Characterization of human lung tumor-associated fibroblasts and their ability to modulate the activation of tumor-associated T cells. J. Immunol. 2007, 178, 5552–5562. [Google Scholar] [CrossRef]
- Zhao, Y.; Liu, Y.; Jia, Y.; Wang, X.; He, J.; Zhen, S.; Wang, J.; Liu, L. Fibroblast activation protein in the tumor microenvironment predicts outcomes of PD-1 blockade therapy in advanced non-small cell lung cancer. J. Cancer Res. Clin. Oncol. 2023, 149, 3469–3483. [Google Scholar] [CrossRef] [PubMed]
- Bu, Y.; Zhang, L.; Ma, X.; Wang, R.; Zhang, X.; Li, J. Systematic Analysis of the Oncogenic Role of WDR62 in Human Tumors. Dis. Markers 2021, 2021, 9940274. [Google Scholar] [CrossRef] [PubMed]
- Inoue, C.; Miki, Y.; Saito, R.; Hata, S.; Abe, J.; Sato, I.; Okada, Y.; Sasano, H. PD-L1 Induction by Cancer-Associated Fibroblast-Derived Factors in Lung Adenocarcinoma Cells. Cancers 2019, 11, 1257. [Google Scholar] [CrossRef]
- Eble, J.A.; Niland, S. The extracellular matrix in tumor progression and metastasis. Clin. Exp. Metastasis 2019, 36, 171–198. [Google Scholar] [CrossRef]
- Ozbek, S.; Balasubramanian, P.G.; Chiquet-Ehrismann, R.; Tucker, R.P.; Adams, J.C. The evolution of extracellular matrix. Mol. Biol. Cell 2010, 21, 4300–4305. [Google Scholar] [CrossRef]
- Miles, F.L.; Sikes, R.A. Insidious changes in stromal matrix fuel cancer progression. Mol. Cancer Res. 2014, 12, 297–312. [Google Scholar] [CrossRef] [PubMed]
- Chakravarthy, A.; Khan, L.; Bensler, N.P.; Bose, P.; De Carvalho, D.D. TGF-β-associated extracellular matrix genes link cancer-associated fibroblasts to immune evasion and immunotherapy failure. Nat. Commun. 2018, 9, 4692. [Google Scholar] [CrossRef]
- Sorokin, L. The impact of the extracellular matrix on inflammation. Nat. Rev. Immunol. 2010, 10, 712–723. [Google Scholar] [CrossRef]
- Joyce, J.A.; Fearon, D.T. T cell exclusion, immune privilege, and the tumor microenvironment. Science 2015, 348, 74–80. [Google Scholar] [CrossRef]
- Salmon, H.; Franciszkiewicz, K.; Damotte, D.; Dieu-Nosjean, M.C.; Validire, P.; Trautmann, A.; Mami-Chouaib, F.; Donnadieu, E. Matrix architecture defines the preferential localization and migration of T cells into the stroma of human lung tumors. J. Clin. Investig. 2012, 122, 899–910. [Google Scholar] [CrossRef]
- Stahl, M.; Schupp, J.; Jäger, B.; Schmid, M.; Zissel, G.; Müller-Quernheim, J.; Prasse, A. Lung collagens perpetuate pulmonary fibrosis via CD204 and M2 macrophage activation. PLoS ONE 2013, 8, e81382. [Google Scholar] [CrossRef]
- Zhang, J.; Lu, S.; Lu, T.; Han, D.; Zhang, K.; Gan, L.; Wu, X.; Li, Y.; Zhao, X.; Li, Z.; et al. Single-cell analysis reveals the COL11A1(+) fibroblasts are cancer-specific fibroblasts that promote tumor progression. Front. Pharmacol. 2023, 14, 1121586. [Google Scholar] [CrossRef]
- Berzaghi, R.; Gundersen, K.; Dille Pedersen, B.; Utne, A.; Yang, N.; Hellevik, T.; Martinez-Zubiaurre, I. Immunological signatures from irradiated cancer-associated fibroblasts. Front. Immunol. 2024, 15, 1433237. [Google Scholar] [CrossRef]
- Weichselbaum, R.R.; Liang, H.; Deng, L.; Fu, Y.X. Radiotherapy and immunotherapy: A beneficial liaison? Nat. Rev. Clin. Oncol. 2017, 14, 365–379. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, S.R.; Ardiani, A.; Kwilas, A.; Hodge, J.W. Radiation-induced survival responses promote immunogenic modulation to enhance immunotherapy in combinatorial regimens. Oncoimmunology 2014, 3, e28643. [Google Scholar] [CrossRef]
- Meng, J.; Li, Y.; Wan, C.; Sun, Y.; Dai, X.; Huang, J.; Hu, Y.; Gao, Y.; Wu, B.; Zhang, Z.; et al. Targeting senescence-like fibroblasts radiosensitizes non-small cell lung cancer and reduces radiation-induced pulmonary fibrosis. JCI Insight 2021, 6, e146334. [Google Scholar] [CrossRef] [PubMed]
- Gorchs, L.; Hellevik, T.; Bruun, J.A.; Camilio, K.A.; Al-Saad, S.; Stuge, T.B.; Martinez-Zubiaurre, I. Cancer-associated fibroblasts from lung tumors maintain their immunosuppressive abilities after high-dose irradiation. Front. Oncol. 2015, 5, 87. [Google Scholar] [CrossRef]
- Akanda, M.R.; Ahn, E.J.; Kim, Y.J.; Salam, S.M.A.; Noh, M.G.; Kim, S.S.; Jung, T.Y.; Kim, I.Y.; Kim, C.H.; Lee, K.H.; et al. Different Expression and Clinical Implications of Cancer-Associated Fibroblast (CAF) Markers in Brain Metastases. J. Cancer 2023, 14, 464–479. [Google Scholar] [CrossRef]
- Hellevik, T.; Pettersen, I.; Berg, V.; Winberg, J.O.; Moe, B.T.; Bartnes, K.; Paulssen, R.H.; Busund, L.T.; Bremnes, R.; Chalmers, A.; et al. Cancer-associated fibroblasts from human NSCLC survive ablative doses of radiation but their invasive capacity is reduced. Radiat. Oncol. 2012, 7, 59. [Google Scholar] [CrossRef]
- Hodkinson, P.S.; Elliott, T.; Wong, W.S.; Rintoul, R.C.; Mackinnon, A.C.; Haslett, C.; Sethi, T. ECM overrides DNA damage-induced cell cycle arrest and apoptosis in small-cell lung cancer cells through beta1 integrin-dependent activation of PI3-kinase. Cell Death Differ. 2006, 13, 1776–1788. [Google Scholar] [CrossRef]
- Carracedo, S.; Lu, N.; Popova, S.N.; Jonsson, R.; Eckes, B.; Gullberg, D. The fibroblast integrin alpha11beta1 is induced in a mechanosensitive manner involving activin A and regulates myofibroblast differentiation. J. Biol. Chem. 2010, 285, 10434–10445. [Google Scholar] [CrossRef]
- Puthawala, K.; Hadjiangelis, N.; Jacoby, S.C.; Bayongan, E.; Zhao, Z.; Yang, Z.; Devitt, M.L.; Horan, G.S.; Weinreb, P.H.; Lukashev, M.E.; et al. Inhibition of integrin alpha(v)beta6, an activator of latent transforming growth factor-beta, prevents radiation-induced lung fibrosis. Am. J. Respir. Crit. Care Med. 2008, 177, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Barcellos-Hoff, M.H.; Derynck, R.; Tsang, M.L.; Weatherbee, J.A. Transforming growth factor-beta activation in irradiated murine mammary gland. J. Clin. Investig. 1994, 93, 892–899. [Google Scholar] [CrossRef] [PubMed]
- Neuzillet, C.; Tijeras-Raballand, A.; Cohen, R.; Cros, J.; Faivre, S.; Raymond, E.; de Gramont, A. Targeting the TGFβ pathway for cancer therapy. Pharmacol. Ther. 2015, 147, 22–31. [Google Scholar] [CrossRef]
- Yao, F.; Shi, W.; Fang, F.; Lv, M.Y.; Xu, M.; Wu, S.Y.; Huang, C.L. Exosomal miR-196a-5p enhances radioresistance in lung cancer cells by downregulating NFKBIA. Kaohsiung J. Med. Sci. 2023, 39, 554–564. [Google Scholar] [CrossRef]
- Wang, Y.; Gan, G.; Wang, B.; Wu, J.; Cao, Y.; Zhu, D.; Xu, Y.; Wang, X.; Han, H.; Li, X.; et al. Cancer-associated Fibroblasts Promote Irradiated Cancer Cell Recovery Through Autophagy. EBioMedicine 2017, 17, 45–56. [Google Scholar] [CrossRef]
- Berzaghi, R.; Ahktar, M.A.; Islam, A.; Pedersen, B.D.; Hellevik, T.; Martinez-Zubiaurre, I. Fibroblast-Mediated Immunoregulation of Macrophage Function Is Maintained after Irradiation. Cancers 2019, 11, 689. [Google Scholar] [CrossRef] [PubMed]
- Domogauer, J.D.; de Toledo, S.M.; Howell, R.W.; Azzam, E.I. Acquired radioresistance in cancer associated fibroblasts is concomitant with enhanced antioxidant potential and DNA repair capacity. Cell Commun. Signal. 2021, 19, 30. [Google Scholar] [CrossRef]
- Li, H.; Zhang, Q.; Wu, Q.; Cui, Y.; Zhu, H.; Fang, M.; Zhou, X.; Sun, Z.; Yu, J. Interleukin-22 secreted by cancer-associated fibroblasts regulates the proliferation and metastasis of lung cancer cells via the PI3K-Akt-mTOR signaling pathway. Am. J. Transl. Res. 2019, 11, 4077–4088. [Google Scholar]
- Ceuppens, H.; Pombo Antunes, A.R.; Navarro, L.; Ertveldt, T.; Berdal, M.; Nagachinta, S.; De Ridder, K.; Lahoutte, T.; Keyaerts, M.; Devoogdt, N.; et al. Efficient α and β(-) radionuclide therapy targeting fibroblast activation protein-α in an aggressive preclinical mouse tumour model. Eur. J. Nucl. Med. Mol. Imaging 2025, 52, 444–457. [Google Scholar] [CrossRef]
- Xie, Y.; Ma, J.; Tang, W.; Zhang, Y.; Zhang, C.; Chen, Y. Efficacy and Safety Evaluation of 177Lu-FAP-2286 in the Treatment of Advanced Lung Cancer. Clin. Nucl. Med. 2024, 49, 830–837. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Jakobsson, V.; Wang, J.; Zhao, T.; Peng, X.; Li, B.; Xue, J.; Liang, N.; Zhu, Z.; Chen, X.; et al. Dual targeting PET tracer [(68)Ga]Ga-FAPI-RGD in patients with lung neoplasms: A pilot exploratory study. Theranostics 2023, 13, 2979–2992. [Google Scholar] [CrossRef] [PubMed]
- Lee, I.K.; Noguera-Ortega, E.; Xiao, Z.; Todd, L.; Scholler, J.; Song, D.; Liousia, M.; Lohith, K.; Xu, K.; Edwards, K.J.; et al. Monitoring Therapeutic Response to Anti-FAP CAR T Cells Using [18F]AlF-FAPI-74. Clin. Cancer Res. 2022, 28, 5330–5342. [Google Scholar] [CrossRef]
- Huang, J.; Zhang, X.; Liu, Q.; Gong, F.; Huang, Y.; Huang, S.; Fu, L.; Tang, G. (68)Ga/(177)Lu-Labeled Theranostic Pair for Targeting Fibroblast Activation Protein with Improved Tumor Uptake and Retention. J. Med. Chem. 2024, 67, 17785–17795. [Google Scholar] [CrossRef] [PubMed]
- Giesel, F.L.; Kratochwil, C.; Schlittenhardt, J.; Dendl, K.; Eiber, M.; Staudinger, F.; Kessler, L.; Fendler, W.P.; Lindner, T.; Koerber, S.A.; et al. Head-to-head intra-individual comparison of biodistribution and tumor uptake of (68)Ga-FAPI and (18)F-FDG PET/CT in cancer patients. Eur. J. Nucl. Med. Mol. Imaging 2021, 48, 4377–4385. [Google Scholar] [CrossRef] [PubMed]
- Gong, X.; Li, J.; Xu, X.; Wu, Y.; Lei, Y.; Liu, H.; Qian, X.; Li, Y.; Zhang, Z. Microvesicle-inspired oxygen-delivering nanosystem potentiates radiotherapy-mediated modulation of tumor stroma and antitumor immunity. Biomaterials 2022, 290, 121855. [Google Scholar] [CrossRef]
- Kolset, S.O.; Pejler, G. Serglycin: A structural and functional chameleon with wide impact on immune cells. J. Immunol. 2011, 187, 4927–4933. [Google Scholar] [CrossRef]
- Beer, T.W.; Ng, L.B.; Murray, K. Mast cells have prognostic value in Merkel cell carcinoma. Am. J. Dermatopathol. 2008, 30, 27–30. [Google Scholar] [CrossRef]
- Medina, V.; Cricco, G.; Nuñez, M.; Martín, G.; Mohamad, N.; Correa-Fiz, F.; Sanchez-Jimenez, F.; Bergoc, R.; Rivera, E.S. Histamine-mediated signaling processes in human malignant mammary cells. Cancer Biol. Ther. 2006, 5, 1462–1471. [Google Scholar] [CrossRef]
- Carmeliet, P.; Jain, R.K. Molecular mechanisms and clinical applications of angiogenesis. Nature 2011, 473, 298–307. [Google Scholar] [CrossRef]
- Ribatti, D.; Crivellato, E. Mast cells, angiogenesis and cancer. Adv. Exp. Med. Biol. 2011, 716, 270–288. [Google Scholar] [CrossRef] [PubMed]
- Maltby, S.; Khazaie, K.; McNagny, K.M. Mast cells in tumor growth: Angiogenesis, tissue remodelling and immune-modulation. Biochim. Biophys. Acta 2009, 1796, 19–26. [Google Scholar] [CrossRef] [PubMed]
- Alashkar Alhamwe, B.; Ponath, V.; Alhamdan, F.; Dörsam, B.; Landwehr, C.; Linder, M.; Pauck, K.; Miethe, S.; Garn, H.; Finkernagel, F.; et al. BAG6 restricts pancreatic cancer progression by suppressing the release of IL33-presenting extracellular vesicles and the activation of mast cells. Cell Mol. Immunol. 2024, 21, 918–931. [Google Scholar] [CrossRef] [PubMed]
| Marker | Function | Specific Role in Lung Cancer | Clinical Significance | Refs |
|---|---|---|---|---|
| α-SMA | Cell shrinkage, matrix remodeling | A core marker of CAF activation that promotes tumor invasion and fibrosis. | High expression is associated with poor prognosis | [63] |
| FAP | Extracellular matrix degradation, immunomodulation | Promote tumor growth and metastasis and inhibit anti-tumor immune response. | Associated with chemotherapy resistance and shortened survival | [45] |
| PDGFRβ | Cell proliferation, migration | High expression of CAF in lung cancer drives mesenchymal remodeling and angiogenesis. | Potential markers for targeted therapies | [64] |
| FSP1 (S100A4) | Cell migration, metastasis | Specifically labeling lung cancer CAF subpopulations, metabolic plasticity can drive tumor invasion and metastasis in non-small cell lung cancer cells. | Targeted metabolic reprogramming provides therapeutic window for enhanced glycolysis inhibition | [65] |
| Tenascin-C | Cell migration, metastasis | Significantly expressed in the microenvironment of lung cancer, promoting cancer cell invasion and metastasis. | High expression predicts advanced staging | [66] |
| Periostin | EMT | Periostin expression was positively correlated with the EMT markers Snail and Twist and with lung cancer stage, in which mature epithelial cells undergo phenotypic morphological changes and become invasive, motile cells. | Higher periostin levels correlate with poorer overall survival | [67] |
| Vimentin | Cell migration and EMT | High expression in CAFs enhances lung cancer cell metastasis by inducing EMT signaling; correlates with immunosuppression in the tumor microenvironment. | Associated with late staging and increased risk of metastasis | [45] |
| COL1A1 | Malignant progression and metastasis | Higher proportion of COL1A1-positive CAFs associated with shorter patient survival. | Predicting TME-dependent survival expectancy and treatment benefits | [68] |
| COL5A1 | Regulates collagen fiber assembly and matrix structure | COL5A1 promotes metastasis by regulating protease activity and migration-associated proteins, and inhibition of its expression reduces invasiveness and enhances chemosensitivity. | Potential therapeutic targets | [69] |
| P-glycoprotein | Reduces drug retention and increases drug outflow | Induced by the AKT/Sox2 signaling pathway, P-GP expression induced chemoresistance in NSCLC cells. | Associated with chemotherapy resistance | [70] |
| Adipocyte enhancer-binding protein 1 (AEBP1) | Promotes proliferation, migration, invasion and transfer | AEBP1 is not only oncogenic in epithelial tumor cells, but may also be oncogenic in stromal cells. | Overexpression is associated with the prediction of poor prognosis in patients with pulmonary SCC | [71] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Z.; Hu, C.; Li, Q.; Sun, C. Cancer-Associated Fibroblasts as the “Architect” of the Lung Cancer Immune Microenvironment: Multidimensional Roles and Synergistic Regulation with Radiotherapy. Int. J. Mol. Sci. 2025, 26, 3234. https://doi.org/10.3390/ijms26073234
Shi Z, Hu C, Li Q, Sun C. Cancer-Associated Fibroblasts as the “Architect” of the Lung Cancer Immune Microenvironment: Multidimensional Roles and Synergistic Regulation with Radiotherapy. International Journal of Molecular Sciences. 2025; 26(7):3234. https://doi.org/10.3390/ijms26073234
Chicago/Turabian StyleShi, Zheng, Cuilan Hu, Qiang Li, and Chao Sun. 2025. "Cancer-Associated Fibroblasts as the “Architect” of the Lung Cancer Immune Microenvironment: Multidimensional Roles and Synergistic Regulation with Radiotherapy" International Journal of Molecular Sciences 26, no. 7: 3234. https://doi.org/10.3390/ijms26073234
APA StyleShi, Z., Hu, C., Li, Q., & Sun, C. (2025). Cancer-Associated Fibroblasts as the “Architect” of the Lung Cancer Immune Microenvironment: Multidimensional Roles and Synergistic Regulation with Radiotherapy. International Journal of Molecular Sciences, 26(7), 3234. https://doi.org/10.3390/ijms26073234






