An Acylhydrazone Fluorescent Sensor: Bifunctional Detection of Thorium (IV) and Vanadyl Ions over Uranyl and Lanthanide Ions
Abstract
1. Introduction
2. Results and Discussion
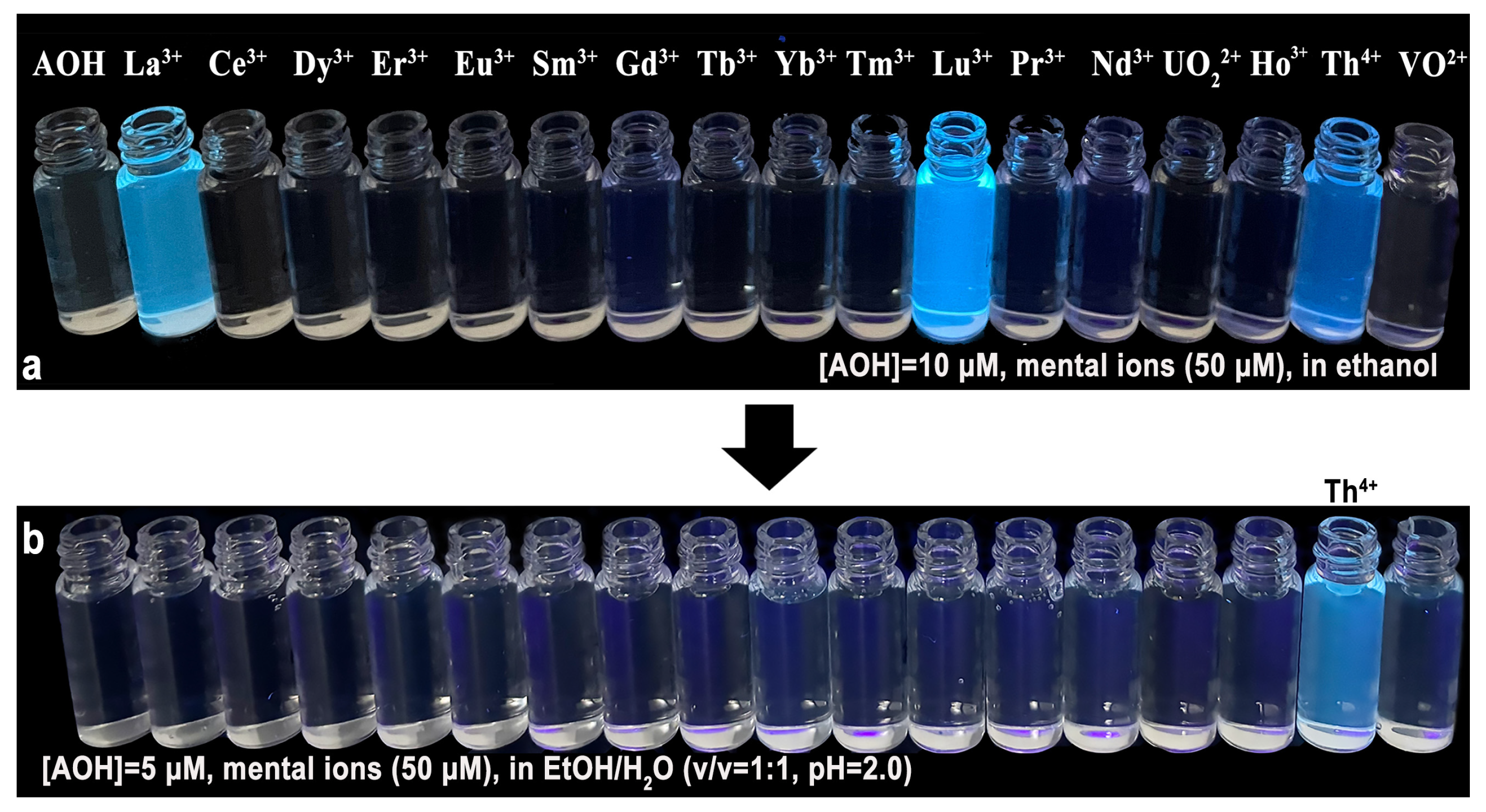
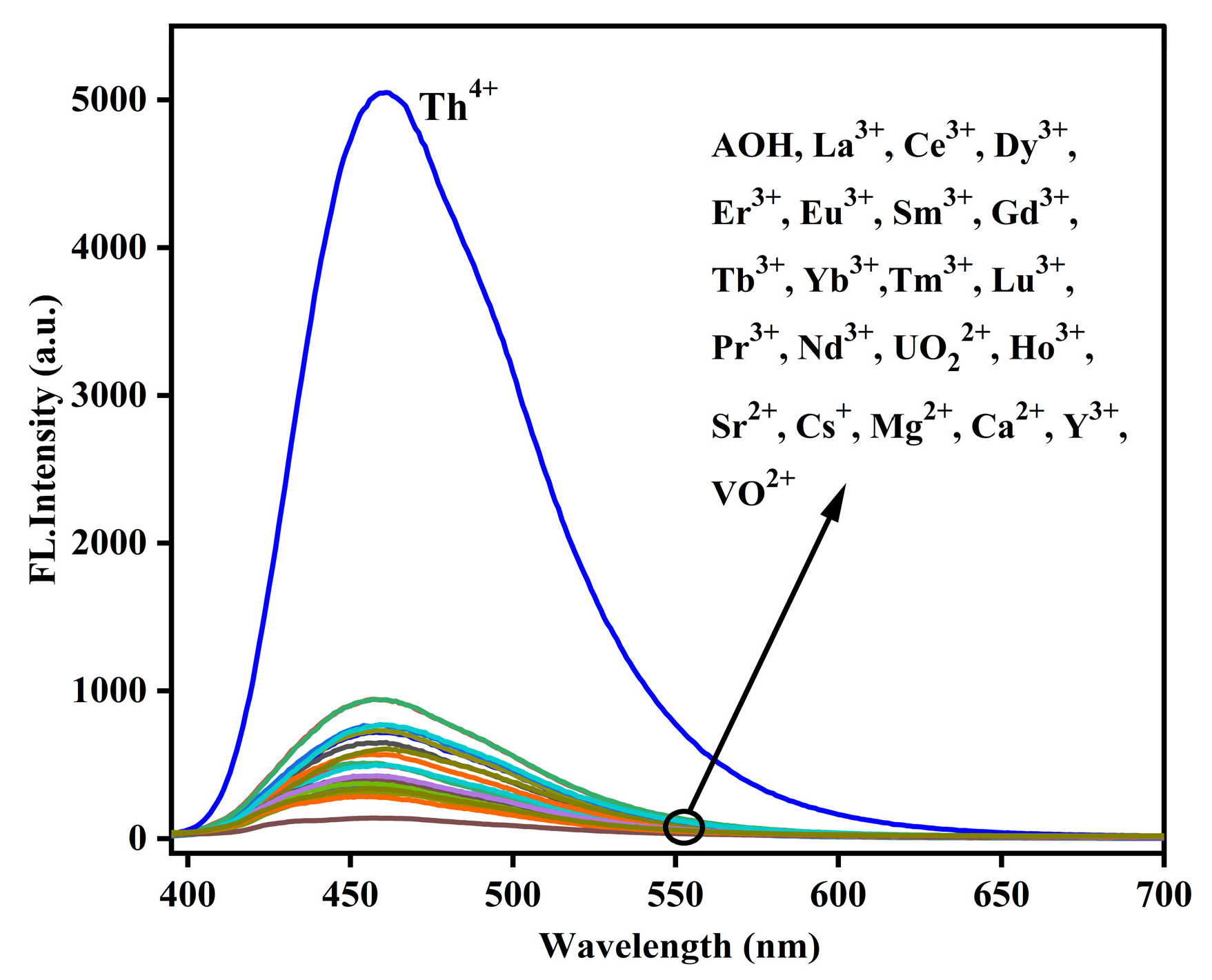
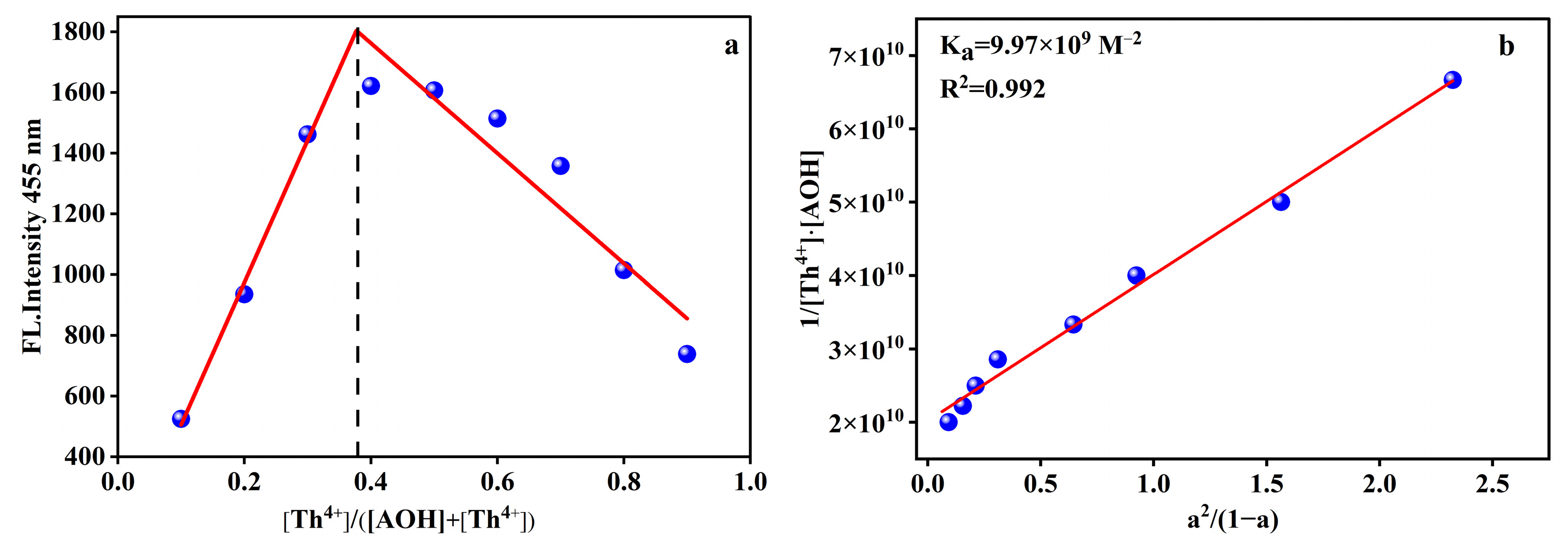
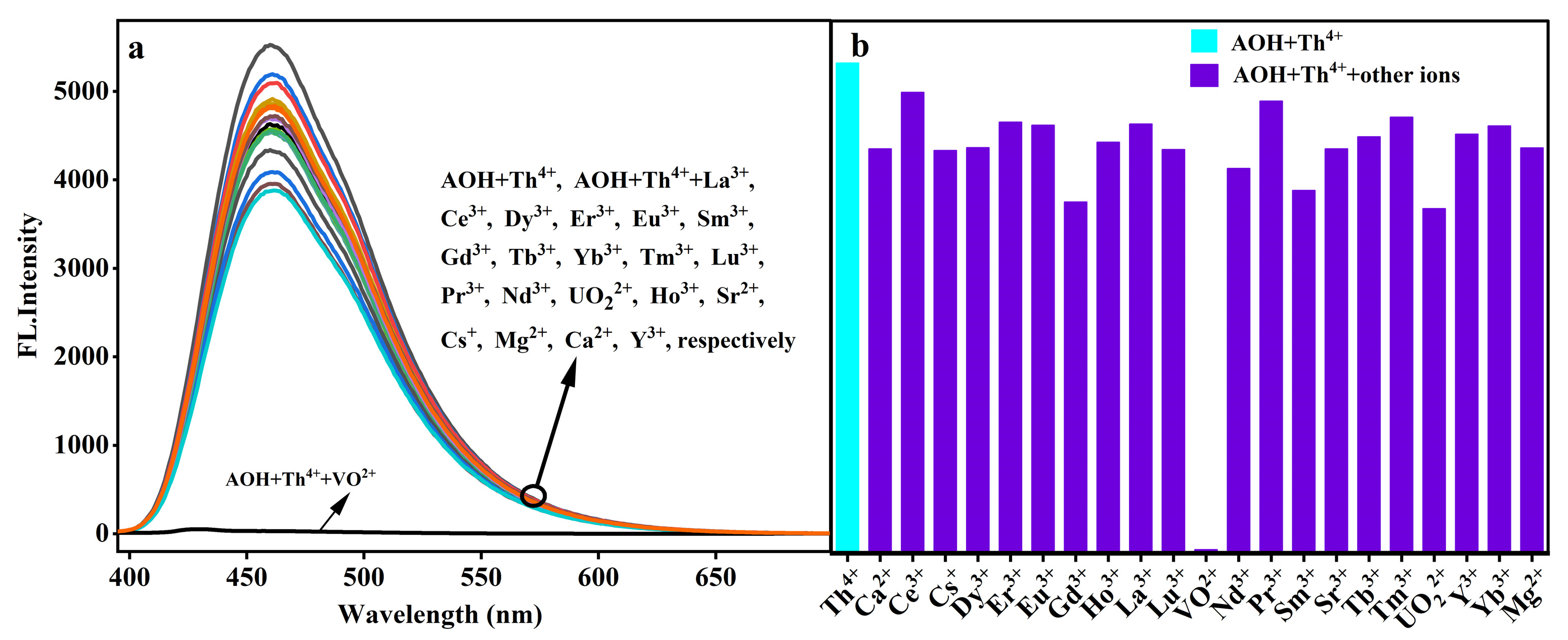
2.1. Selectivity of AOH Towards Th4+
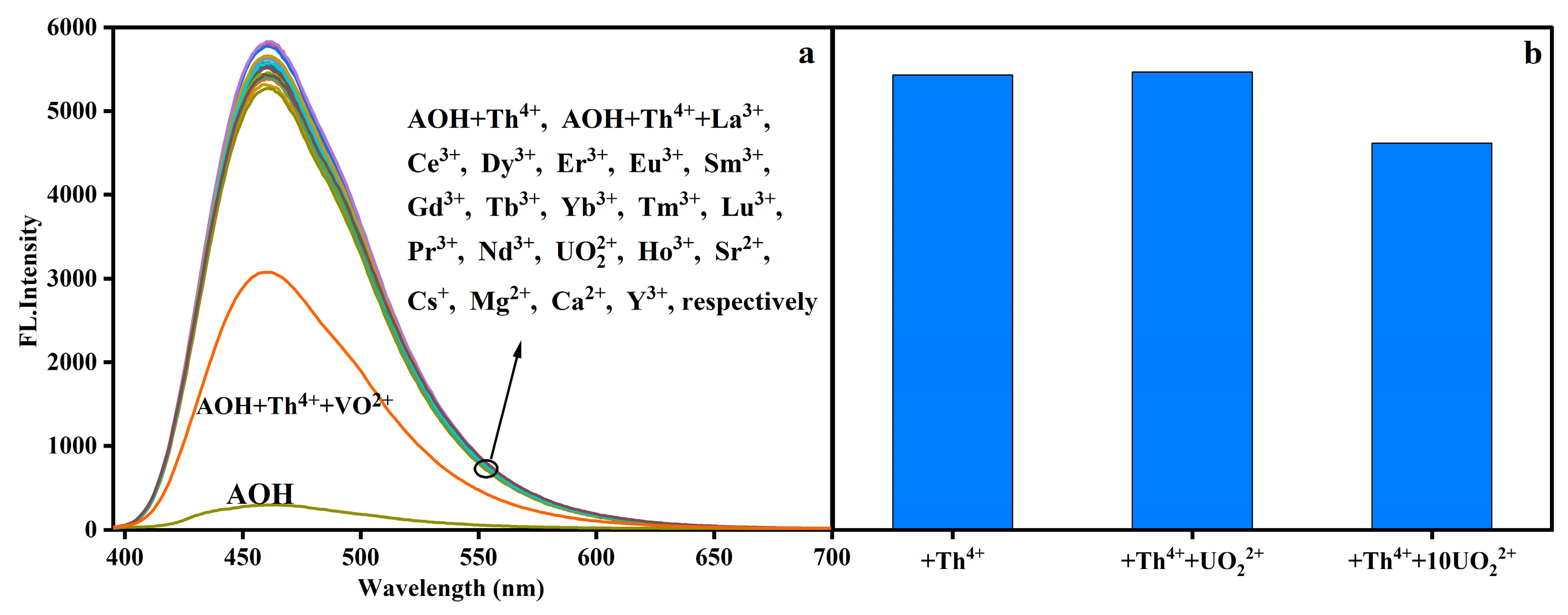
2.2. Interfering Ions Effect on Th4+ Response
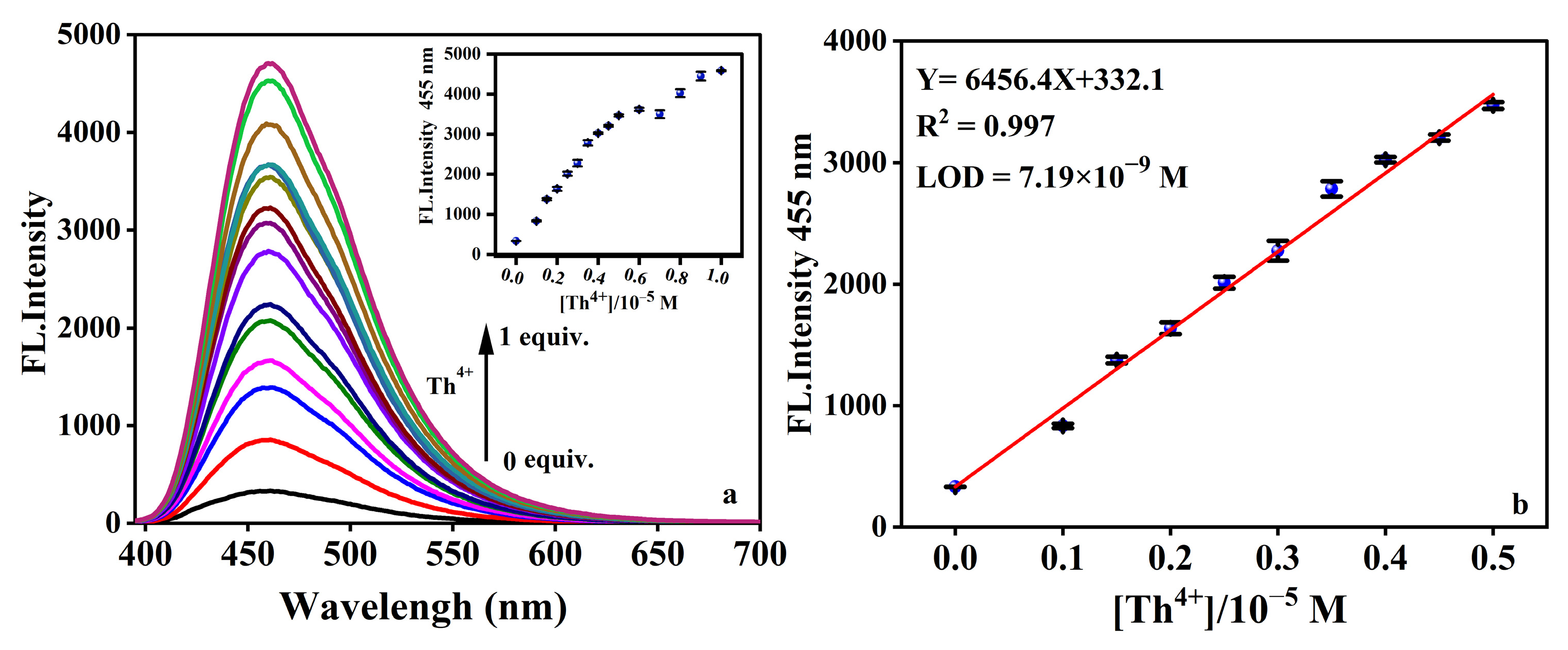
2.3. Limit of Detection (LOD) on Th4+ Response
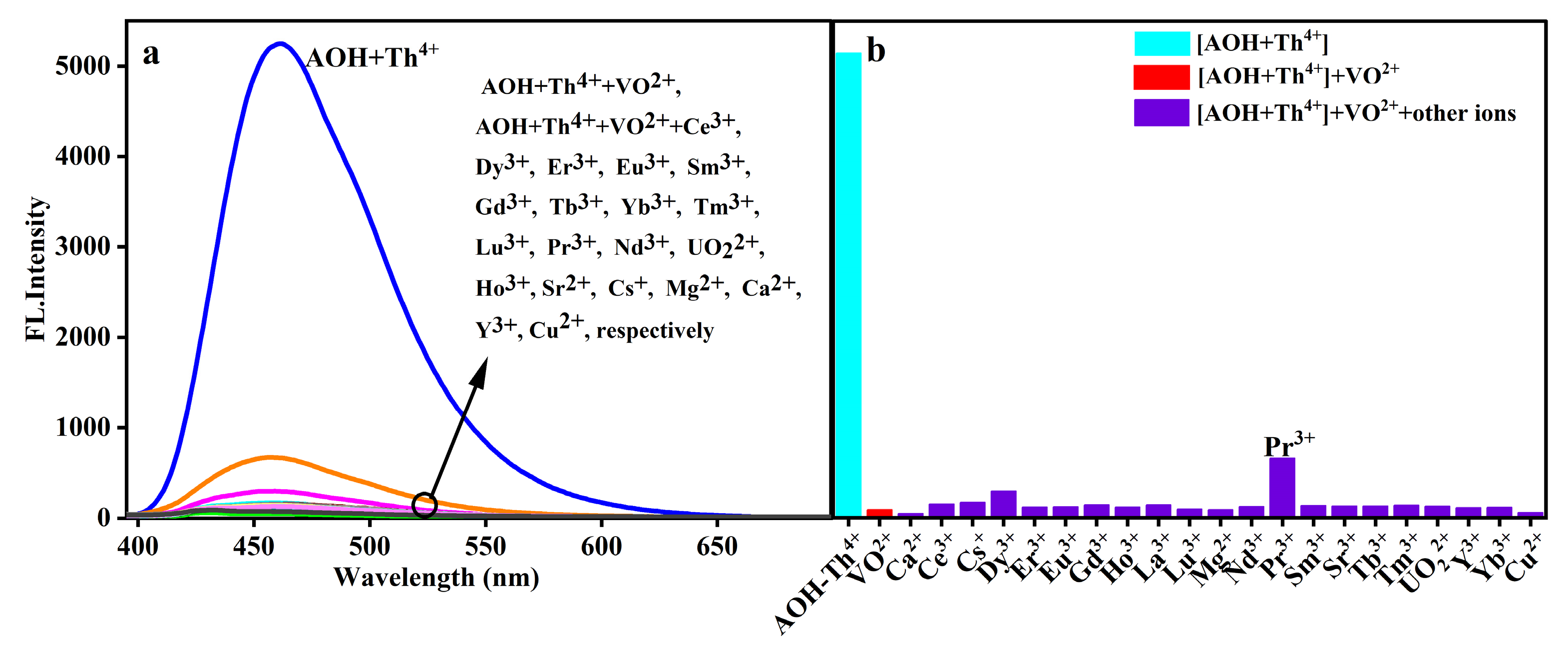
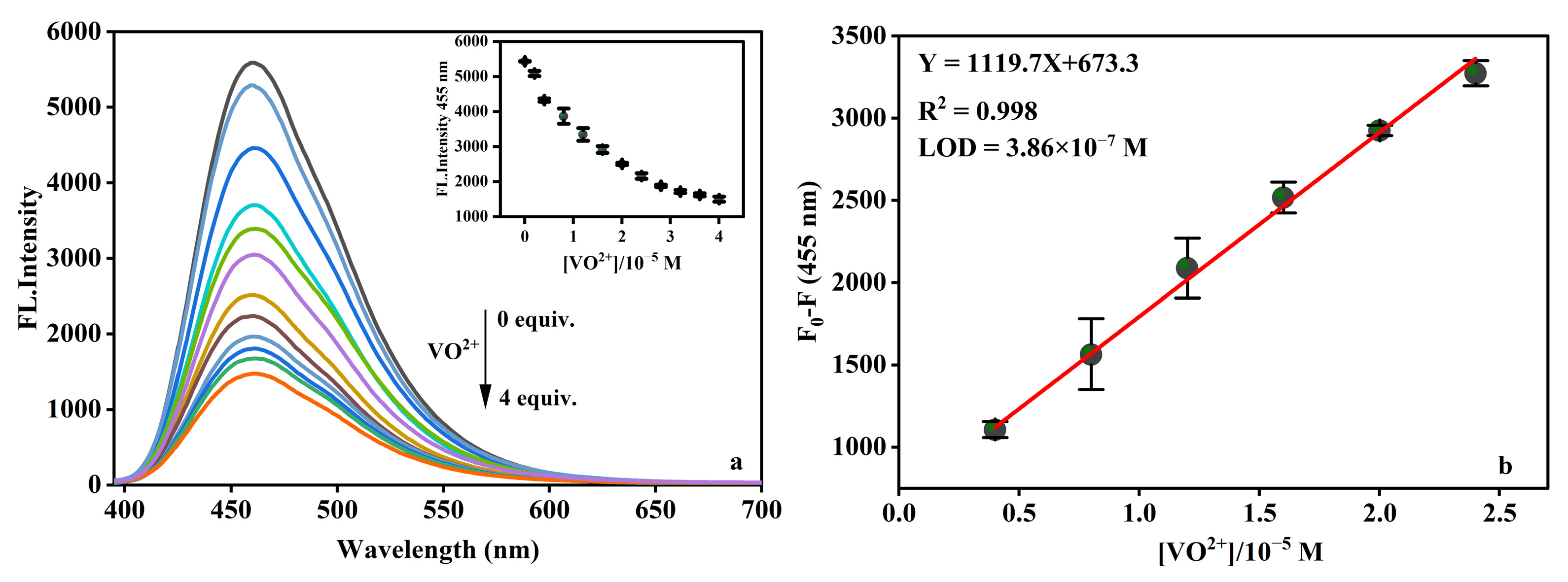
2.4. Selectivity on VO2+ Response
2.5. Interfering Ions Effect on VO2+ Response
2.6. LOD of VO2+
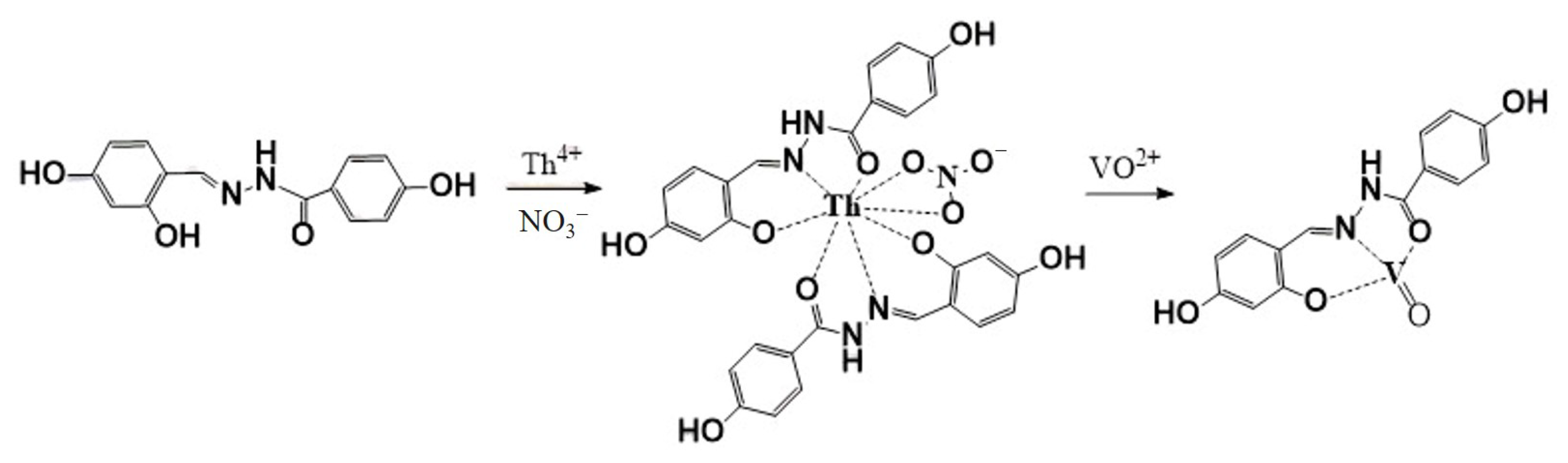
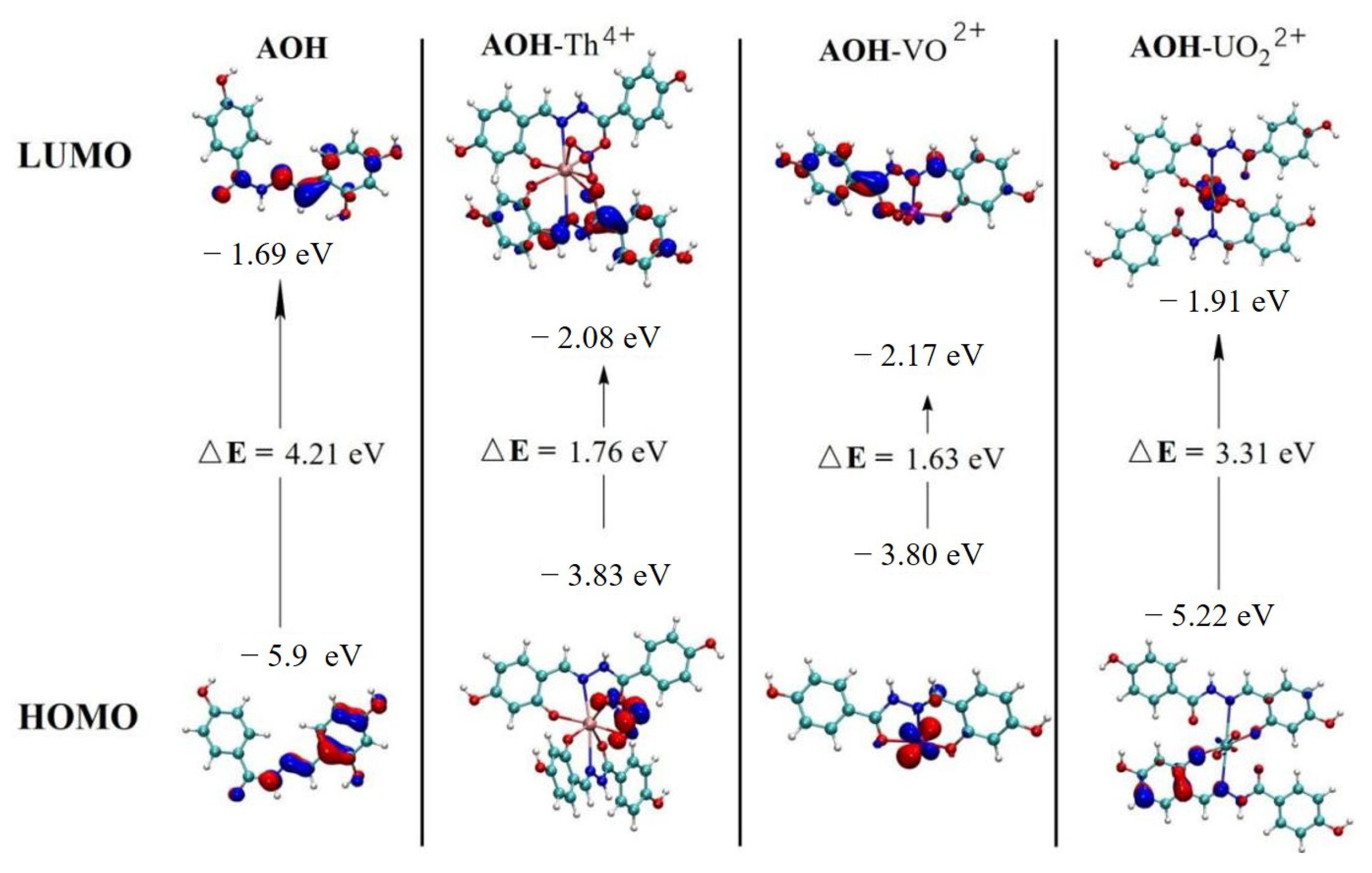
2.7. Recognition Mechanism of AOH on Th4+ and VO2+ Response
2.8. Detection Comparison to Reported Sensors on Th4+ or VO2+
2.9. Th4+ and VO2+ Determination in Real Water
3. Materials and Methods
3.1. Materials and Reagents
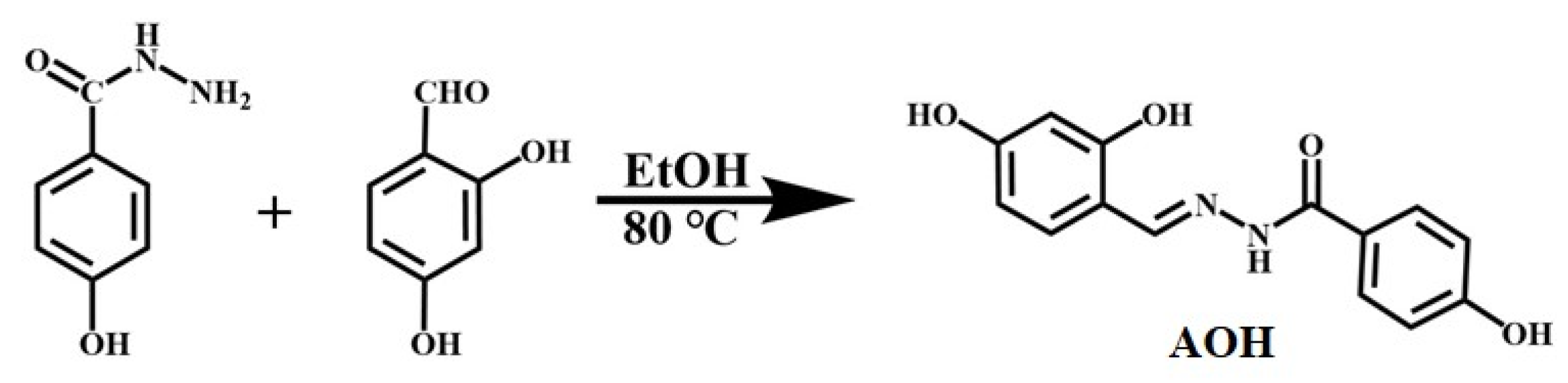
3.2. Synthesis of AOH
3.3. Apparatus
3.4. Solution Preparation and Testing Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| AOH | Acylhydrazone, N’-(2,4-dihydroxybenzylidene)-4-hydroxylphenylhydrazide |
| ICT | Intramolecular charge transfer |
| LOD | Limit of detection |
References
- Mohd Salehuddin, A.H.J.; Ismail, A.F.; Che Zainul Bahri, C.N.A.; Aziman, E.S. Economic analysis of thorium extraction from monazite. Nucl. Eng. Technol. 2019, 51, 631–640. [Google Scholar]
- Humphrey, U.E.; Khandaker, M.U. Viability of thorium-based nuclear fuel cycle for the next generation nuclear reactor: Issues and prospects. Renew. Sustain. Energy Rev. 2018, 97, 259–275. [Google Scholar]
- Javoy, M.; Kaminski, E. Earth’s Uranium and Thorium content and geoneutrinos fluxes based on enstatite chondrites. Earth Planet. Sc. Lett. 2014, 407, 1–8. [Google Scholar]
- Sun, J.; Fu, H.; Jing, H.; Hu, X.; Chen, D.; Li, F.; Liu, Y.; Qin, X.; Huang, W. Synergistic Integration of Halide Perovskite and Rare-Earth Ions toward Photonics. Adv. Mater. 2025, 37, 2417397. [Google Scholar]
- Chen, X.; Cen, C.; Zhou, L.; Cao, R.; Yi, Z.; Tang, Y. Magnetic properties and reverse magnetization process of anisotropic nanocomposite permanent magnet. J. Magn. Magn. Mater. 2019, 483, 152–157. [Google Scholar] [CrossRef]
- Moeller, T.; Schweitzer, G.K.; Starr, D.D. The analytical aspects of thorium chemistry. Chem. Rev. 1948, 42, 63–105. [Google Scholar] [PubMed]
- Fang, Y.; Dehaen, W. Small-molecule-based fluorescent probes for f-block metal ions: A new frontier in chemosensors. Coordin. Chem. Rev. 2021, 427, 213524. [Google Scholar]
- Galson, D.A.; Atkin, B.P.; Harvey, P.K. The determination of low concentrations of U, Th and K by XRF spectrometry. Chem. Geol. 1983, 38, 225–237. [Google Scholar]
- Grinberg, P.; Willie, S.; Sturgeon, R.E. Determination of thorium and uranium in ultrapure lead by inductively coupled plasma mass spectrometry. Anal. Chem. 2005, 77, 2432–2436. [Google Scholar]
- Alian, A.; Shabana, R. Neutron activation analysis by standard addition and solvent extraction Determination of impurities in thorium and iron. Talanta 1968, 15, 1109–1119. [Google Scholar]
- Shinotsuka, K.; Ebihara, M. Precise determination of rare earth elements, thorium and uranium in chondritic meteorites by inductively coupled plasma mass spectrometry—A comparative study with radiochemical neutron activation analysis. Anal. Chim. Acta 1997, 338, 237–246. [Google Scholar]
- Benedik, L.; Pilar, A.M.; Prosen, H.; Jaćimović, R.; Povinec, P.P. Determination of ultra-trace levels of uranium and thorium in electrolytic copper using radiochemical neutron activation analysis. Appl. Radiat. Isot. 2021, 175, 109801. [Google Scholar]
- Roy, P. Fluorescent chemosensors based on 4-methyl-2,6-diformylphenol. Coordin. Chem. Rev. 2021, 427, 213562. [Google Scholar]
- Alam, P.; Leung, N.L.C.; Zhang, J.; Kwok, R.T.K.; Lam, J.W.Y.; Tang, B.Z. AIE-based luminescence probes for metal ion detection. Coordin. Chem. Rev. 2021, 429, 213693. [Google Scholar] [CrossRef]
- Sawminathan, S.; Kulathu Iyer, S. Phenanthridine based rapid “turn-on” fluorescent sensor for selective detection of Th4+ ion and its real-time application. Spectrochim. Acta. A 2022, 265, 120403. [Google Scholar] [CrossRef]
- Kumar, R.S.; Kumar, S.K.A.; Vijayakrishna, K.; Sivaramakrishna, A.; Brahmmananda Rao, C.V.S.; Sivaraman, N.; Sahoo, S.K. Development of the Smartphone-Assisted Colorimetric Detection of Thorium by Using New Schiff’s Base and Its Applications to Real Time Samples. Inorg. Chem. 2018, 57, 15270–15279. [Google Scholar]
- Gomaa, H.; Emran, M.Y.; Elsenety, M.M.; Abdel-Rahim, R.D.; Deng, Q.; Gadallah, M.I.; Saad, M.; Almohiy, H.; Ezzeldien, M.; Seaf El-Nasr, T.A.; et al. Detection and Selective Removal Strategy of Thorium Ions Using a Novel Fluorescent Ligand and Hybrid Mesoporous γ-Al2O3-like Nanoneedles. ACS Sustain. Chem. Eng. 2023, 11, 2127–2138. [Google Scholar]
- Kumar, R.S.; Bhaskar, R.; Sharma, H.K.; Ashok Kumar, S.K.; Sahoo, S.K. Historical overview and recent progress on supramolecular sensors for thorium recognition. Trends Analyt. Chem. 2024, 172, 117551. [Google Scholar]
- Liu, B.; Tan, Y.; Hu, Q.; Wang, Y.; Mao, Y.; Tao, P.; Wang, H. A new ratiometric and colorimetric fluorescent Th4+ probe under extreme acidity and cell imaging. Sensor Actuat. B Chem. 2019, 296, 126675. [Google Scholar] [CrossRef]
- Kumar, V.; Kumar, P.; Kumar, S.; Singhal, D.; Gupta, R. Turn-On Fluorescent Sensors for the Selective Detection of Al3+ (and Ga3+) and PPi Ions. Inorg. Chem. 2019, 58, 10364–10376. [Google Scholar]
- Elabd, A.A.; Elhefnawy, O.A. A potential sensor for assessing thorium (IV) based on Albuterol sulfate fluorescence enhancement: A density functional theory (DFT) study. Inorg. Chem. Commun. 2022, 145, 110001. [Google Scholar]
- Kim, J.; Tsouris, C.; Mayes, R.T.; Oyola, Y.; Saito, T.; Janke, C.J.; Dai, S.; Schneider, E.; Sachde, D. Recovery of Uranium from Seawater: A Review of Current Status and Future Research Needs. Sep. Sci. Technol. 2013, 48, 367–387. [Google Scholar]
- Ahmed, B.; Ahmad, Z.; Ihsan, A.; Khan, M.A.; Fazal, T. Biomaterials as promising biosorbents for efficient uranium extraction from seawater: A comprehensive review. Sep. Purif. Technol. 2024, 338, 126507. [Google Scholar]
- Gao, J.; Yuan, Y.; Yu, Q.; Yan, B.; Qian, Y.; Wen, J.; Ma, C.; Jiang, S.; Wang, X.; Wang, N. Bio-inspired antibacterial cellulose paper–poly(amidoxime) composite hydrogel for highly efficient uranium(vi) capture from seawater. Chem. Commun. 2020, 56, 3935–3938. [Google Scholar]
- Cao, M.; Luo, G.; Peng, Q.; Wang, L.; Wang, Y.; Zhao, S.; Wang, H.; Zhang, J.; Yuan, Y.; Wang, N. Poly(amidoxime)/polyzwitterionic semi-interpenetrating network hydrogel with robust salt-shrinkage resistance for enhanced uranium extraction from seawater. Chem. Eng. J. 2024, 481, 148536. [Google Scholar]
- Li, J.B.; Li, D.; Liu, Y.Y.; Cao, A.; Wang, H. Cytotoxicity of vanadium dioxide nanoparticles to human embryonic kidney cell line: Compared with vanadium(IV/V) ions. Environ. Toxicol. Phar. 2024, 106, 104378. [Google Scholar]
- Hashmi, K.; Satya; Gupta, S.; Siddique, A.; Khan, T.; Joshi, S. Medicinal applications of vanadium complexes with Schiff bases. J. Trace Elem. Med. Biol. 2023, 79, 127245. [Google Scholar]
- Berdiyeva, P.; Oreiro, S.N.; Fenini, F.; Petrov, M.; Rahimi, M.; Papaharalabos, G.; Bentien, A. Facile and robust assessment of membrane transport properties in course of standard electrochemical tests of vanadium redox flow batteries. J. Power Sources 2024, 614, 234974. [Google Scholar]
- Varadaraju, C.; Tamilselvan, G.; Enoch, I.V.M.V.; Srinivasadesikan, V.; Lee, S.-L.; Selvakumar, P.M. The first highly selective turn “ON” fluorescent sensor for vanadyl (VO2+) ions: DFT studies and molecular logic gate behavior. New J. Chem. 2018, 42, 3833–3839. [Google Scholar]
- Huo, F.J.; Su, J.; Sun, Y.Q.; Yin, C.X.; Tong, H.B.; Nie, Z.X. A rhodamine-based dual chemosensor for the visual detection of copper and the ratiometric fluorescent detection of vanadium. Dyes Pigment. 2010, 86, 50–55. [Google Scholar]
- He, W.; Hua, D. Spectrographic sensors for uranyl detection in the environment. Talanta 2019, 201, 317–329. [Google Scholar] [PubMed]
- Zhong, W.; Wang, M.; Hu, H.; Qian, J.; Wang, S.; Su, X.; Xiao, S.; Xu, H.; Gao, Y. Ultrahigh-Efficient N,O-Functionalized covalent organic framework towards thorium adsorption from uranium and rare earth elements. Sep. Purif. Technol. 2024, 347, 127603. [Google Scholar]
- Maniak, H.; Matyja, K.; Pląskowska, E.; Jarosz, J.; Majewska, P.; Wietrzyk, J.; Gołębiowska, H.; Trusek, A.; Giurg, M. 4-Hydroxybenzoic Acid-Based Hydrazide–Hydrazones as Potent Growth Inhibition Agents of Laccase-Producing Phytopathogenic Fungi That Are Useful in the Protection of Oilseed Crops. Molecules 2024, 29, 2212. [Google Scholar] [CrossRef]
- Branković, J.; Milivojević, N.; Milovanović, V.; Simijonović, D.; Petrović, Z.D.; Marković, Z.; Šeklić, D.S.; Živanović, M.N.; Vukić, M.D.; Petrović, V.P. Evaluation of antioxidant and cytotoxic properties of phenolic N-acylhydrazones: Structure–activity relationship. Roy. Soc. Open Sci. 2022, 9, 211853. [Google Scholar]
- Humelnicu, D.; Pui, A.; Malutan, C.; Malutan, T.; Humelnicu, I. Synthesis, characterization and theoretical investigations of new uranium (VI) and thorium (IV) complexes with 1-furfurylaldehyde-derived Schiff bases as ligands. J. Saudi Chem. Soc. 2020, 24, 451–460. [Google Scholar]
- Shilpa, A.S.; Thangadurai, T.D.; Bhalerao, G.M.; Maji, S. Tailor-designed carbon-based novel fluorescent architecture for nanomolar detection of radioactive elements U(VI) and Th(IV) in pH ± 5.0. Talanta 2024, 272, 125783. [Google Scholar]
- Gao, R.; Hu, J.; Zhang, K.; He, Y.; Liu, P.; Luo, S.; Yang, Y.; Yang, L.; Feng, W.; Yuan, L. Highly Selective Fluorescent Recognition towards Th4+ Based on Coumarin-derivatized Crescent Aromatic Oligoamide. Chin. J. Chem. 2013, 31, 689–694. [Google Scholar]
- Huang, Z.W.; Li, Z.J.; Zheng, L.R.; Wu, W.S.; Chai, Z.F.; Shi, W.Q. Adsorption of Eu(III) and Th(IV) on three-dimensional graphene-based macrostructure studied by spectroscopic investigation. Environ. Pollut. 2019, 248, 82–89. [Google Scholar]
- Mincher, B.J.; Modolo, G.; Mezyk, S.P. Review: The Effects of Radiation Chemistry on Solvent Extraction 4: Separation of the Trivalent Actinides and Considerations for Radiation-Resistant Solvent Systems. Solvent Extr. Ion Exc. 2010, 28, 415–436. [Google Scholar]
- Khayatzadeh Mahani, M.; Divsar, F.; Chaloosi, M.; Maragheh, M.G.; Khanchi, A.R.; Rofouei, M.K. Simultaneous determination of thorium and uranyl ions by optode spectra and chemometric techniques. Sensor Actuat. B-Chem. 2008, 133, 632–637. [Google Scholar]
- Zheng, S.; Wang, H.; Hu, Q.; Wang, Y.; Hu, J.; Zhou, F.; Liu, P. “Turn-On” fluorescent chemosensor based on β-diketone for detecting Th4+ ions in Aqueous Solution and application in living cell imaging. Sensor Actuat. B-Chem. 2017, 253, 766–772. [Google Scholar]
- Orabi, A.H.; Falila, N.I.; Ismaiel, D.A.; Abdulmoteleb, S.S. An innovative spectrophotometric method for determination of uranium and thorium using 3-aminomethylalizarn-N-N diacetic acid in some geological samples. J. Radioanal. Nucl. Chem. 2020, 327, 239–250. [Google Scholar]
- Yu, L.; Lin, Z.; Cheng, X.; Chu, J.; Li, X.; Chen, C.; Zhu, T.; Li, W.; Lin, W.; Tang, W. Thorium inhibits human respiratory chain complex IV (cytochrome c oxidase). J. Hazard. Mater. 2022, 424, 127546. [Google Scholar]
- Orabi, A.H.; Abdou, A.A.; Ahmed, S.H.; Mahmoud, W.H.; Weheish, H.L. A New Spectrophotometric Method for Thorium Determination Using 1,4-Dihydroxyanthraquinone. J. Anal. Chem. 2021, 76, 322–329. [Google Scholar]
- Elabd, A.A.; Elhefnawy, O.A. A new benzeneacetic acid derivative-based sensor for assessing thorium (IV) in aqueous solution based on aggregation caused quenching (ACQ) and aggregation induced emission (AIE). J. Photoch. Photobio. A 2022, 428, 113866. [Google Scholar]
- Xiong, H.; Liang, H.; Dai, K.; Tian, Q.; Dai, X.; Su, H.; Royal, G. Acylhydrazones as sensitive fluorescent sensors for discriminative detection of thorium(IV) from uranyl and lanthanide ions. Spectrochim. Acta. A 2023, 293, 122501. [Google Scholar]
- Sun, J.; Ye, B.; Xia, G.; Wang, H. A multi-responsive squaraine-based “turn on” fluorescent chemosensor for highly sensitive detection of Al3+, Zn2+ and Cd2+ in aqueous media and its biological application. Sensor Actuat. B Chem. 2017, 249, 386–394. [Google Scholar]
- Jiang, D.; Xue, X.; Zhang, G.; Wang, Y.; Zhang, H.; Feng, C.; Wang, Z.; Zhao, H. Simple and efficient rhodamine-derived VO2+ and Cu2+-responsive colorimetric and reversible fluorescent chemosensors toward the design of multifunctional materials. J. Mater. Chem. C 2019, 7, 3576–3589. [Google Scholar]
- Chakraborty, D.; Singh, R. Novel fluorescent vanadylmoxifloxacinato complexes as sensors for Cu2+. J. Photoch. Photobio. A 2017, 335, 211–216. [Google Scholar]
- Kajinehbaf, T.; Alizadeh, N. A selective fluorescent probe based on citrate doped polypyrrole for dual determination of VO2+/Fe3+ in biological samples. New J. Chem. 2022, 46, 1763–1769. [Google Scholar]
- Tulcan, R.X.S.; Ouyang, W.; Lin, C.; He, M.; Wang, B. Vanadium pollution and health risks in marine ecosystems: Anthropogenic sources over natural contributions. Water Res. 2021, 207, 117838. [Google Scholar] [CrossRef] [PubMed]
- Cao, X.; Dolg, M. Valence basis sets for relativistic energy-consistent small-core lanthanide pseudopotentials. J. Chem. Phys. 2001, 115, 7348–7355. [Google Scholar] [CrossRef]
- Lu, T.; Chen, F. Multiwfn: A multifunctional wavefunction analyzer. J. Comput. Chem. 2011, 33, 580–592. [Google Scholar] [CrossRef]
- Lu, T. A comprehensive electron wavefunction analysis toolbox for chemists, Multiwfn. J. Chem. Phys. 2024, 161, 082503. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Jiang, S.; Guo, H.; Yang, F. “Turn-on” fluorescent sensor for Th4+ in aqueous media based on a combination of PET-AIE effect. Spectrochim. Acta. A 2021, 248, 119191. [Google Scholar] [CrossRef]
- Sawminathan, S.; Munusamy, S.; Manickam, S.; Jothi, D.; KulathuIyer, S. Azine based fluorescent rapid “off-on” chemosensor for detecting Th4+ and Fe3+ ions and its real-time application. Dyes Pigment. 2021, 196, 109755. [Google Scholar] [CrossRef]
- Wen, J.; Dong, L.; Tian, J.; Jiang, T.; Yang, Y.Q.; Huang, Z.; Yu, X.Q.; Hu, C.W.; Hu, S.; Yang, T.Z.; et al. Fluorescent BINOL-based sensor for thorium recognition and a density functional theory investigation. J. Hazard. Mater. 2013, 263, 638–642. [Google Scholar] [CrossRef]
- Yang, Z.; Wu, D.; Dai, K.; Cao, S.; Li, Z.; Huang, H.; Shang, Z.; Liang, H.; Yan, M.; Xie, S. A facile accessible acylhydrazone as Al3+ sensor with excellent sensitivity and selectivity. J. Mol. Struct. 2020, 1201, 127155. [Google Scholar] [CrossRef]
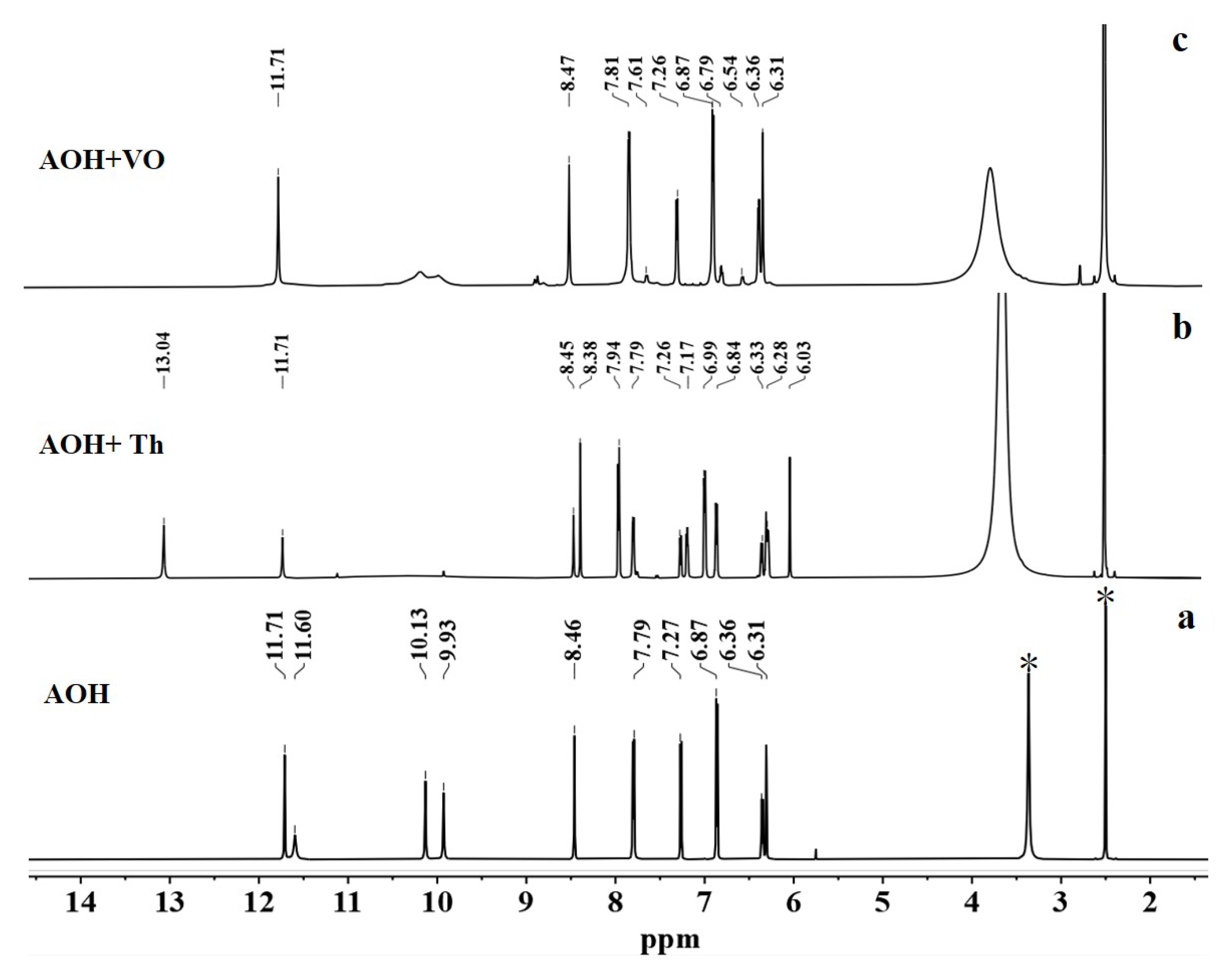
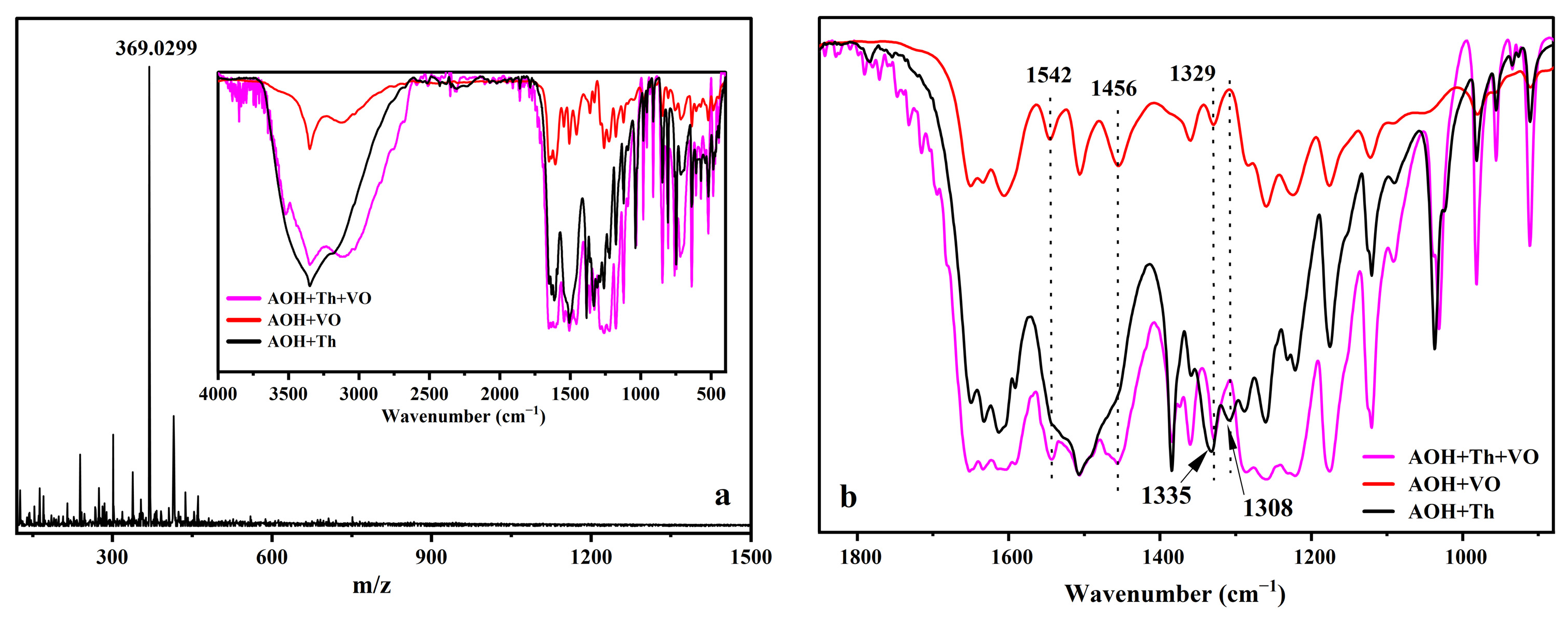
| Sensor (Ref.) | Detecting Object | Media (v/v) | Ka | LOD | Interfering Ions |
|---|---|---|---|---|---|
| [19] | Th4+ | MeOH/H2O (7:3) | 2.03 × 104 M−1 | 20.0 nM | UO22+, Cu2+ |
| [41] | Th4+ | MeOH/H2O (7:3) | 2.46 × 104 M−1 | 34.4 nM | UO22+ |
| [56] | Th4+ | CH3CN/H2O (9:1) | 5.67 × 103 M−1 | 2.10 nM | Fe3+ |
| [57] | Th4+ | MeOH/H2O (1:1) | NR | 0.60 μM | Cu2+, Fe3+, UO22+, Lu3+ |
| [46] | Th4+ | EtOH/H2O (7:3, pH 2.0) | 6.64 × 109 M−2 | 29.2 nM | NO |
| [48] | VO2+ | DMSO-Tris-HCl (7:3, pH 7.4) | NR | 3.65 nM | Cu2+ |
| [50] | VO2+ | H2O | 4.40 × 103 M−1 | 0.34 μM | Fe3+ |
| This work | Th4+, VO2+ | EtOH/H2O (1:1, pH 2.0) | 9.97 × 109 M−2 4.54 × 104 M−1 | 7.19 nM 0.386 μM | NO NO |
| Sample | Th4+ Added (μM) | Th4+ Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap water | 25.0 | 25.0 | 100.0 | 0.9 |
| 25.0 | 25.3 | 101.2 | ||
| 25.0 | 24.8 | 99.3 | ||
| Pool water | 25.0 | 24.9 | 99.5 | 0.9 |
| 25.0 | 25.3 | 101.3 | ||
| 25.0 | 25.1 | 100.3 |
| Sample | Th4+ Added (μM) | Th4+ Found (μM) | Recovery (%) | RSD (%) |
|---|---|---|---|---|
| Tap water | 25.0 | 24.9 | 99.8 | 0.6 |
| 25.0 | 25.1 | 100.3 | ||
| 25.0 | 25.3 | 101.0 | ||
| Pool water | 25.0 | 25.1 | 100.4 | 1.2 |
| 25.0 | 25.7 | 102.7 | ||
| 25.0 | 25.3 | 101.0 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lin, X.; Liang, H.; Dai, K.; Zhou, J.; Tian, Q.; Xiang, Y.; Guo, Z.; Almásy, L. An Acylhydrazone Fluorescent Sensor: Bifunctional Detection of Thorium (IV) and Vanadyl Ions over Uranyl and Lanthanide Ions. Int. J. Mol. Sci. 2025, 26, 3231. https://doi.org/10.3390/ijms26073231
Lin X, Liang H, Dai K, Zhou J, Tian Q, Xiang Y, Guo Z, Almásy L. An Acylhydrazone Fluorescent Sensor: Bifunctional Detection of Thorium (IV) and Vanadyl Ions over Uranyl and Lanthanide Ions. International Journal of Molecular Sciences. 2025; 26(7):3231. https://doi.org/10.3390/ijms26073231
Chicago/Turabian StyleLin, Xin, Hua Liang, Ke Dai, Jing Zhou, Qiang Tian, Yuge Xiang, Zhicheng Guo, and László Almásy. 2025. "An Acylhydrazone Fluorescent Sensor: Bifunctional Detection of Thorium (IV) and Vanadyl Ions over Uranyl and Lanthanide Ions" International Journal of Molecular Sciences 26, no. 7: 3231. https://doi.org/10.3390/ijms26073231
APA StyleLin, X., Liang, H., Dai, K., Zhou, J., Tian, Q., Xiang, Y., Guo, Z., & Almásy, L. (2025). An Acylhydrazone Fluorescent Sensor: Bifunctional Detection of Thorium (IV) and Vanadyl Ions over Uranyl and Lanthanide Ions. International Journal of Molecular Sciences, 26(7), 3231. https://doi.org/10.3390/ijms26073231







