The Use of Potent Populations of Expanded Fetal Human Placental Stromal Cells for the Treatment of Dextran Sodium Sulfate-Induced Colitis in a Mouse Model
Abstract
1. Introduction
2. Results
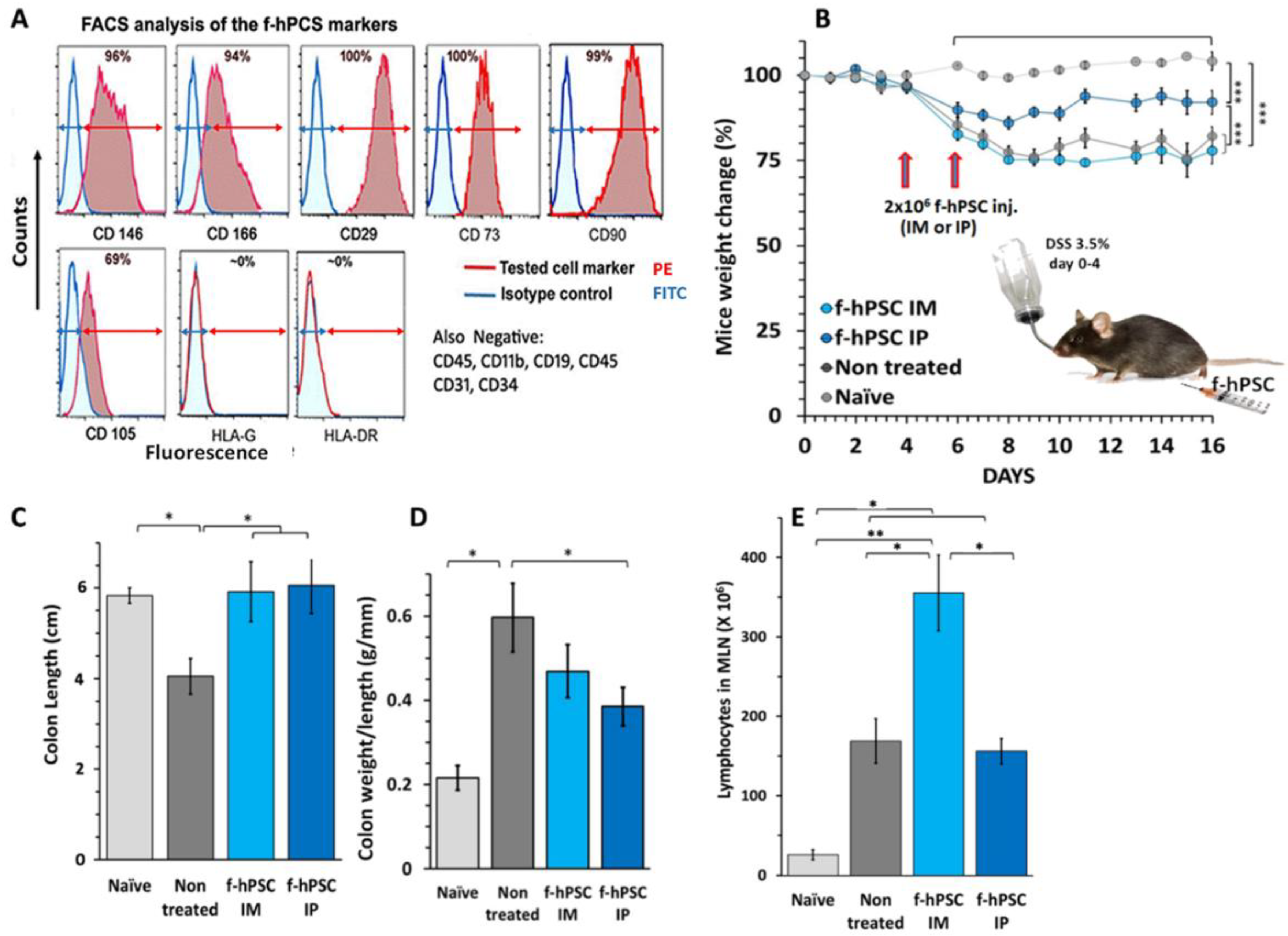
2.1. Characterization of the f-hPSCs
2.2. DSS Induced IBD
2.3. The Influence of f-hPSCs Injection on the Mice Colon
2.4. The Influence of f-hPSCs Treatments on the Total Mesenteric Lymph Nodes (MLN) Cell Counts
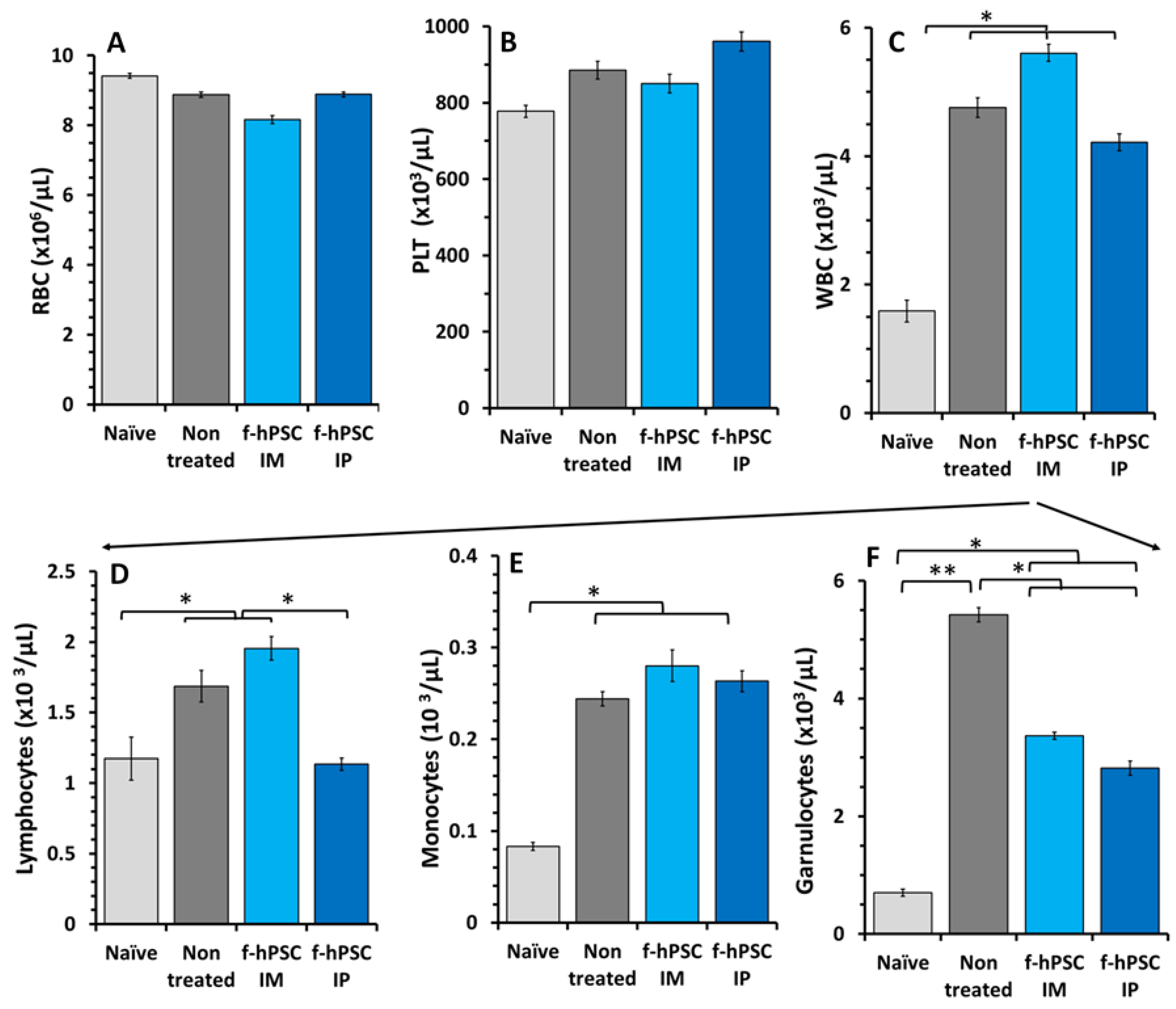
2.5. Effect of the DSS and f-hPSCs Treatments on the Peripheral Blood Cell Counts
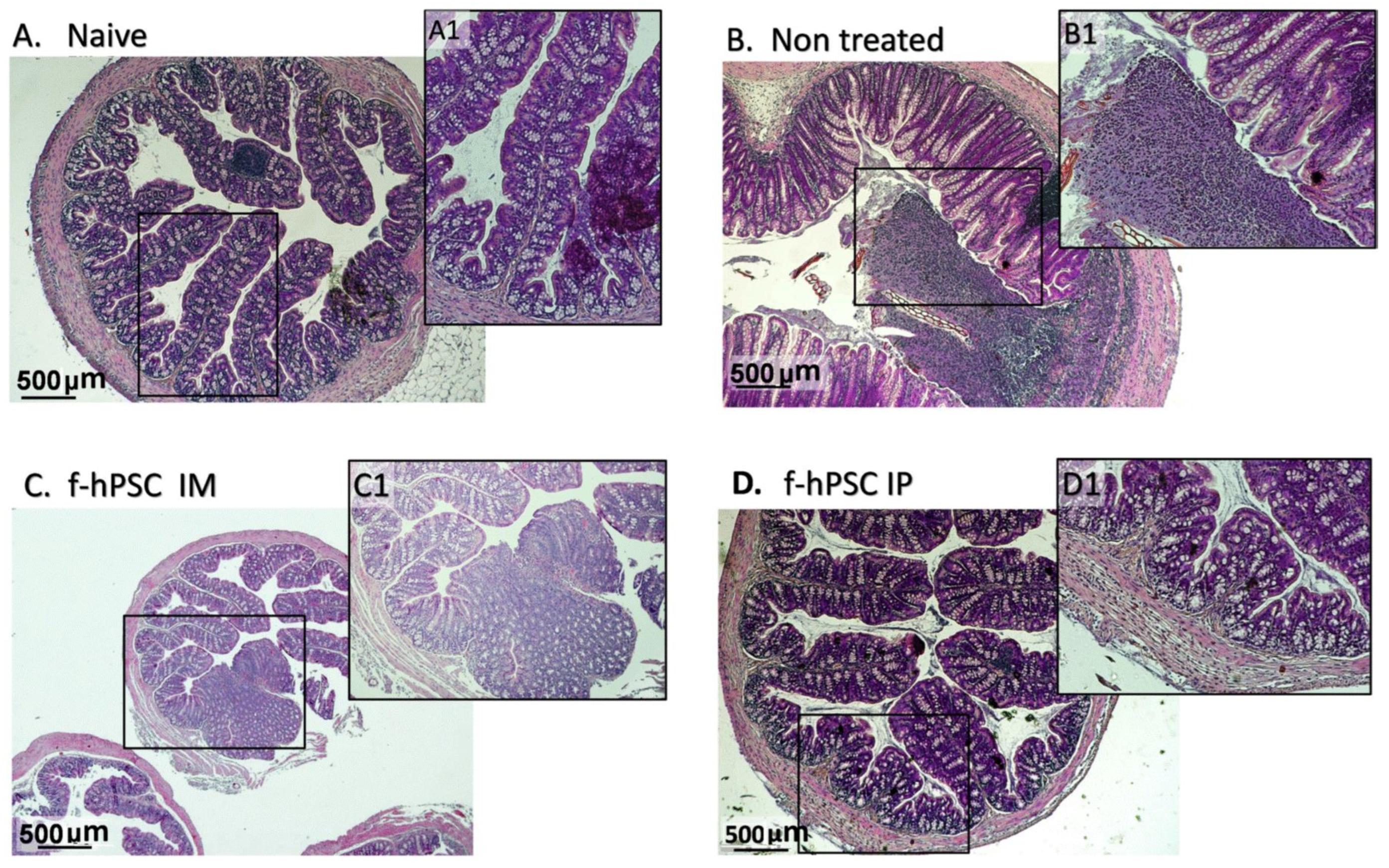
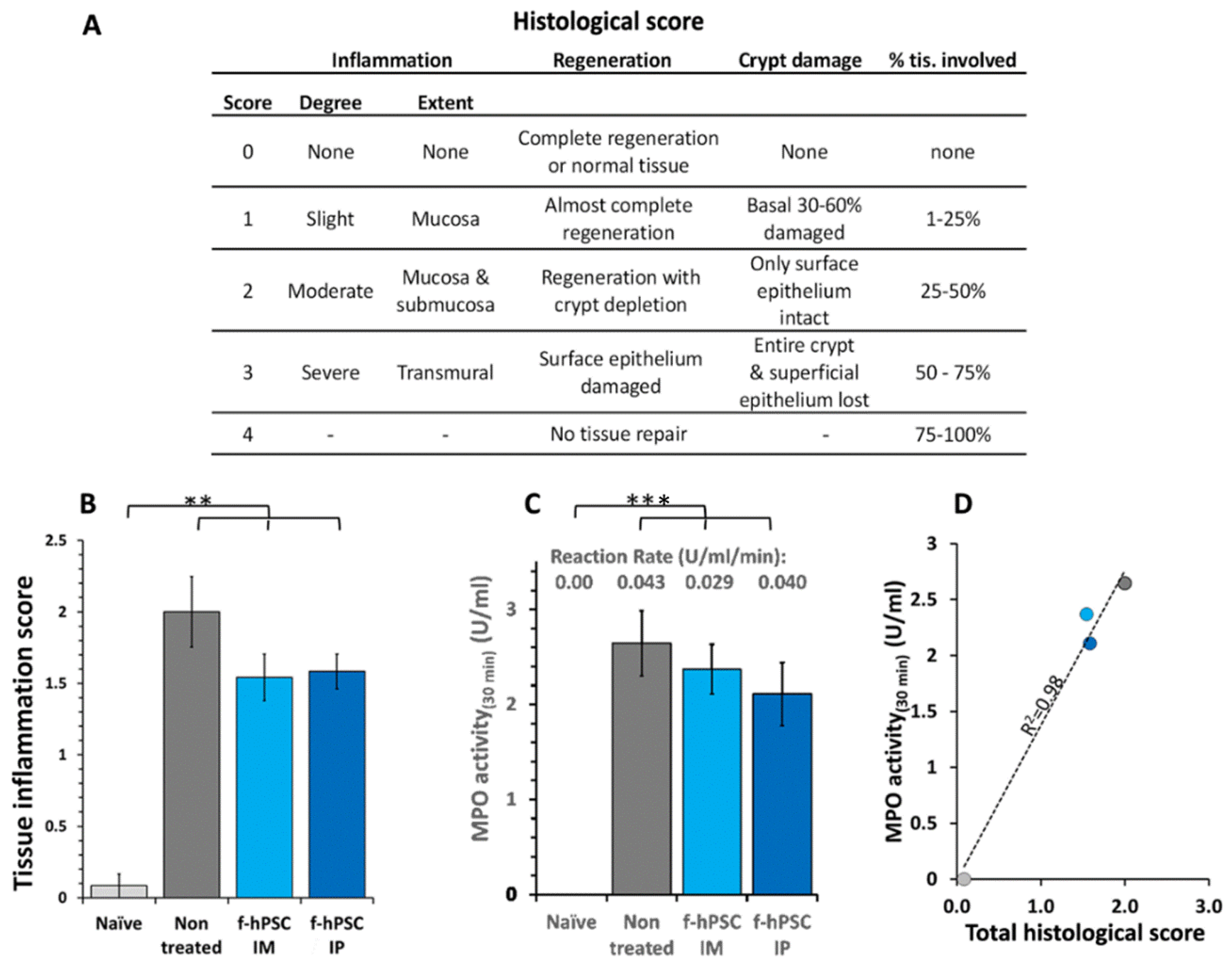
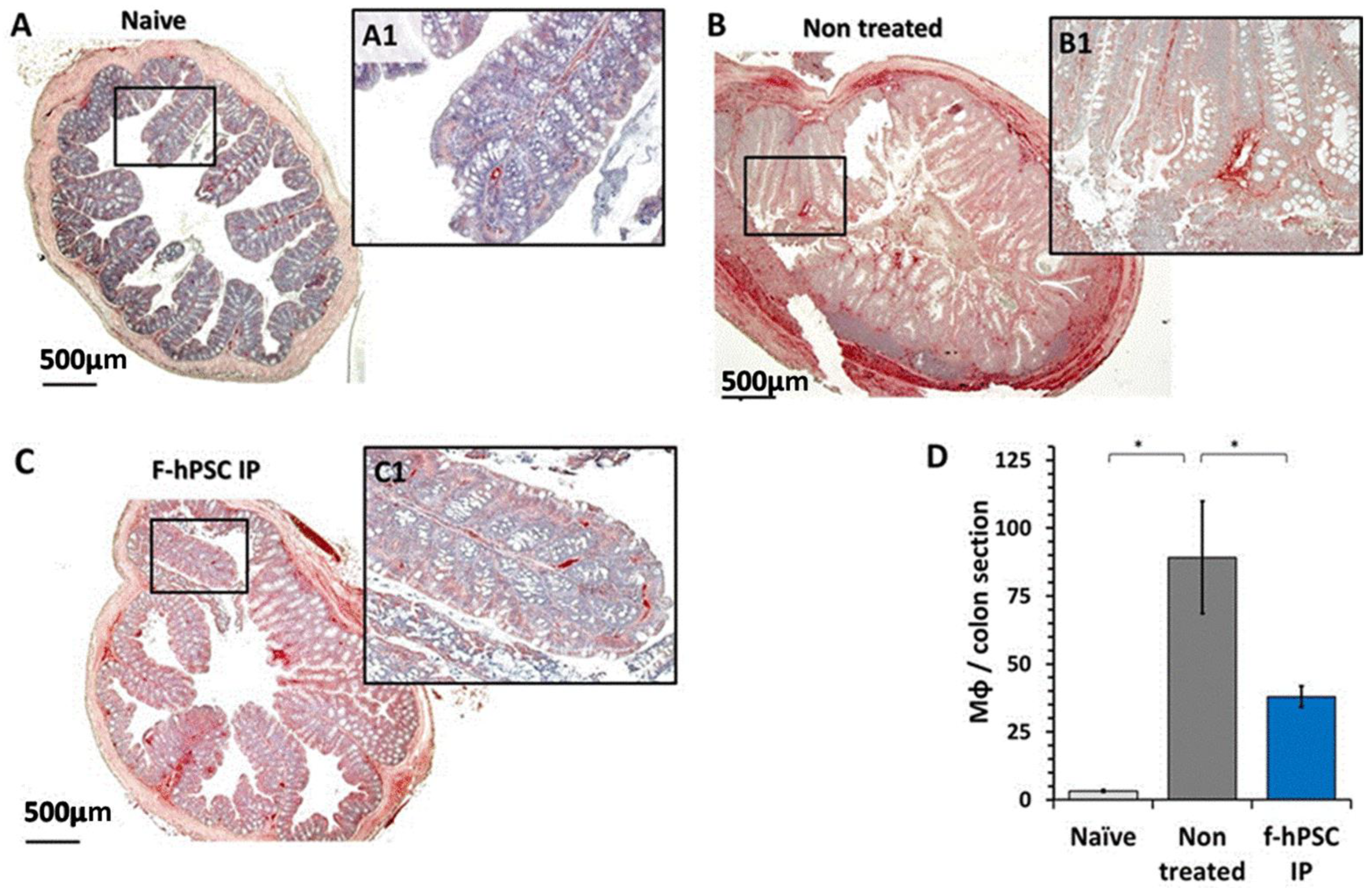
2.6. Colon Histology
2.7. MPO Assay for Neutrophils Activity
3. Discussion
4. Methods and Materials
4.1. The Mouse Model
4.2. f-hPSCs Preparation and Their Cell Surface Phenotype
4.3. The Induction of IBD by DSS in Drinking Water
4.4. f-hPSCs Injections
4.5. Termination of the Experiments and Samples Collection
4.6. Assessment of Inflammatory Process by MLN Cell Count and WBC Cellularity
4.7. Assessment of the Severity of Inflammatory Processes by Neutrophil Activity by MPO Activity Assay in the Colon Samples
4.8. Colon Histology for Evaluation of Inflammatory Process
4.9. Statistics
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Hold, G.L.; Smith, M.; Grange, C.; Watt, E.R.; El-Omar, E.M.; Mukhopadhya, I. Role of the gut microbiota in inflammatory bowel disease pathogenesis: What have we learnt in the past 10 years? World J. Gastroenterol. 2014, 20, 1192–1210. [Google Scholar] [PubMed]
- Strober, W. Impact of the gut microbiome on mucosal inflammation. Trends Immunol. 2013, 34, 423–430. [Google Scholar] [PubMed]
- Bandzar, S.; Gupta, S.; Platt, M.O. Crohn’s disease: A review of treatment options and current research. Cell. Immunol. 2013, 286, 45–52. [Google Scholar]
- Singh, S.; Andersen, N.N.; Andersson, M.; Loftus, E.V.; Jess, T., Jr. Comparison of infliximab with adalimumab in 827 biologic-naive patients with Crohn’s disease: A population-based Danish cohort study. Aliment. Pharmacol. Ther. 2018, 47, 596–604. [Google Scholar]
- Feagan, B.G.; Rutgeerts, P.; Sands, B.E.; Hanauer, S.; Colombel, J.F.; Sandborn, W.J.; Van Assche, G.; Axler, J.; Kim, H.J.; Danese, S.; et al. Vedolizumab as induction and maintenance therapy for ulcerative colitis. N. Engl. J. Med. 2013, 369, 699–710. [Google Scholar]
- Sands, B.E.; Feagan, B.G.; Peyrin-Biroulet, L.; Danese, S.; Rubin, D.T.; Luo, A.; Nguyen, D.D.; Lu, J.; Yen, M.; Leszczyszyn, J.; et al. Phase 2 trial of anti-TL1A monoclonal antibody Tulisokibart for ulcerative colitis. N. Engl. J. Med. 2024, 391, 1119–1129. [Google Scholar]
- Mishra, R.; Dhawan, P.; Srivastava, A.S.; Singh, A.B. Inflammatory bowel disease: Therapeutic limitations and prospective of the stem cell therapy. World J. Stem Cells 2020, 12, 1050–1066. [Google Scholar] [PubMed]
- Mayer, L.; Pandak, W.M.; Melmed, G.Y.; Hanauer, S.B.; Johnson, K.; Payne, D.; Faleck, H.; Hariri, R.J.; Fischkoff, S.A. Safety and tolerability of human placenta-derived cells (PDA001) in treatment-resistant crohn’s disease: A phase 1 study. Inflamm. Bowel Dis. 2013, 19, 754–760. [Google Scholar]
- Pak, S.; Hwang, S.W.; Shim, I.K.; Bae, S.M.; Ryu, Y.M.; Kim, H.B.; Do, E.J.; Son, H.N.; Choi, E.J.; Park, S.H.; et al. Endoscopic transplantation of mesenchymal stem cell sheets in experimental colitis in rats. Sci. Rep. 2018, 8, 11314. [Google Scholar]
- Liao, Y.; Lei, J.; Liu, M.; Lin, W.; Hong, D.; Tuo, Y.; Jiang, M.H.; Xia, H.; Wang, M.; Huang, W.; et al. Mesenchymal stromal cells mitigate experimental colitis via insulin-like growth factor binding protein 7-mediated Immunosuppression. Mol. Ther. J. Am. Soc. Gene Ther. 2016, 24, 1860–1872. [Google Scholar]
- Trebol, J.; Georgiev-Hristov, T.; Pascual-Miguelanez, I.; Guadalajara, H.; Garcia-Arranz, M.; Garcia-Olmo, D. Stem cell therapy applied for digestive anastomosis: Current state and future perspectives. World J. Stem Cells 2022, 14, 117–141. [Google Scholar] [CrossRef]
- El-Nakeep, S. Stem cell therapy for the treatment of Crohn’s disease; Current obstacles and future hopes. Curr. Stem Cell Res. Ther. 2022, 17, 727–733. [Google Scholar] [CrossRef]
- Melmed, G.Y.; Pandak, W.M.; Casey, K.; Abraham, B.; Valentine, J.; Schwartz, D.; Awais, D.; Bassan, I.; Lichtiger, S.; Sands, B.; et al. Human placenta-derived cells (PDA-001) for the treatment of moderate-to-severe Crohn’s Disease: A Phase 1b/2a Study, Inflamm. Bowel Dis. 2015, 21, 1809–1816. [Google Scholar] [CrossRef] [PubMed]
- Caplan, A.I. Mesenchymal Stem Cells: Time to change the name! Stem Cells Transl. Med. 2017, 6, 1445–1451. [Google Scholar] [CrossRef] [PubMed]
- Lee, R.H.; Pulin, A.A.; Seo, M.J.; Kota, D.J.; Ylostalo, J.; Larson, B.L.; Semprun-Prieto, L.; Delafontaine, P.; Prockop, D.J. Intravenous hMSCs improve myocardial infarction in mice because cells embolized in lung are activated to secrete the anti-inflammatory protein TSG-6. Cell Stem Cell 2009, 5, 54–63. [Google Scholar] [CrossRef]
- Gaberman, E.; Pinzur, L.; Levdansky, L.; Tsirlin, M.; Netzer, N.; Aberman, Z.; Gorodetsky, R. Mitigation of Lethal Radiation Syndrome in Mice by Intramuscular Injection of 3D Cultured Adherent Human Placental Stromal Cells. PLoS ONE 2013, 8, e66549. [Google Scholar] [CrossRef] [PubMed]
- Volinsky, E.; Lazmi-Hailu, A.; Cohen, N.; Adani, B.; Faroja, M.; Grunewald, M.; Gorodetsky, R. Alleviation of acute radiation-induced bone marrow failure in mice with human fetal placental stromal cell therapy. Stem Cell Res Ther 2020, 11, 337. [Google Scholar] [CrossRef]
- Adani, B.; Sapir, E.; Volinsky, E.; Lazmi-Hailu, A.; Gorodetsky, R. Alleviation of severe skin insults following high-dose irradiation with isolated human fetal placental stromal cells. Cryobiology 2022, 89, 100–103. [Google Scholar] [CrossRef]
- Pinzur, L.; Akyuez, L.; Levdansky, L.; Blumenfeld, M.; Volinsky, E.; Aberman, Z.; Reinke, P.; Ofir, R.; Volk, H.D.; Gorodetsky, R. Rescue from lethal acute radiation syndrome (ARS) with severe weight loss by secretome of intramuscularly injected human placental stromal cells. J. Cachexia Sarcopenia Muscle 2018, 9, 1079–1092. [Google Scholar]
- Amend, B.; Buttgereit, L.; Abruzzese, T.; Harland, N.; Abele, H.; Jakubowski, P.; Stenzl, A.; Gorodetsky, R.; Aicher, W.K. Regulation of immune checkpoint antigen CD276 (B7-H3) on human placenta-derived mesenchymal stromal cells in GMP-compliant cell culture media. Int. J. Mol. Sci. 2023, 24, 16422. [Google Scholar] [CrossRef]
- Shapira, I.; Fainstein, N.; Tsirlin, M.; Stav, I.; Volinsky, E.; Moresi, C.; Ben-Hur, T.; Gorodetsky, R. Placental stromal cell therapy for experimental autoimmune encephalomyelitis: The role of route of cell delivery. Stem Cells Transl. Med. 2016, 6, 1286–1294. [Google Scholar] [PubMed]
- Pampalone, M.; Corrao, S.; Amico, G.; Vitale, G.; Alduino, R.; Conaldi, P.G.; Pietrosi, G. Human amnion-derived mesenchymal stromal cells in cirrhotic patients with refractory ascites: A possible anti-inflammatory therapy for preventing spontaneous bacterial Peritonitis. Stem Cell Rev. Rep. 2021, 17, 981–998. [Google Scholar] [CrossRef] [PubMed]
- Abumaree, M.H.; Al Jumah, M.A.; Kalionis, B.; Jawdat, D.; Al Khaldi, A.; AlTalabani, A.A.; Knawy, B.A. Phenotypic and functional characterization of mesenchymal stem cells from chorionic villi of human term placenta. Stem Cell Rev. 2013, 9, 16–31. [Google Scholar]
- Duan, L.; Huang, H.; Zhao, X.; Zhou, M.; Chen, S.; Wang, C.; Han, Z.; Han, Z.C.; Guo, Z.; Li, Z.; et al. Extracellular vesicles derived from human placental mesenchymal stem cells alleviate experimental colitis in mice by inhibiting inflammation and oxidative stress. Int. J. Mol. Med. 2020, 46, 1551–1561. [Google Scholar] [PubMed]
- Eiro, N.; Fraile, M.; Gonzalez-Jubete, A.; Gonzalez, L.O.; Vizoso, F.J. Mesenchymal (Stem) stromal Cells based as new therapeutic alternative in inflammatory bowel disease: Basic mechanisms. experimental and clinical evidence and challenges. Int. J. Mol. Sci. 2022, 23, 8905. [Google Scholar] [CrossRef]
- Fu, Y.; Zhang, C.; Xie, H.; Wu, Z.; Tao, Y.; Wang, Z.; Gu, M.; Wei, P.; Lin, S.; Li, R.; et al. Human umbilical cord mesenchymal stem cells alleviated TNBS-induced colitis in mice by restoring the balance of intestinal microbes and immunoregulation. Life Sci. 2023, 334, 122189. [Google Scholar]
- Ke, C.; Biao, H.; Qianqian, L.; Yunwei, S.; Xiaohua, J. Mesenchymal stem cell therapy for inflammatory bowel diseases: Promise and challenge. Curr. Stem Cell Res. Ther. 2015, 10, 499–508. [Google Scholar] [CrossRef]
- Lightner, A.L.; Irving, P.M.; Lord, G.M.; Betancourt, A. Stem cells and stem cell-derived factors for the treatment of inflammatory bowel disease with a particular focus on perianal fistulizing disease: A Minireview on future perspectives. BioDrugs 2024, 38, 527–539. [Google Scholar]
- Cao, X.; Duan, L.; Hou, H.; Liu, Y.; Chen, S.; Zhang, S.; Liu, Y.; Wang, C.; Qi, X.; Liu, N.; et al. IGF-1C hydrogel improves the therapeutic effects of MSCs on colitis in mice through PGE(2)-mediated M2 macrophage polarization. Theranostics 2020, 10, 7697–7709. [Google Scholar] [CrossRef]
- Bai, K.; Li, X.; Zhong, J.; Ng, E.H.Y.; Yeung, W.S.B.; Lee, C.L.; Chiu, P.C.N. Placenta-derived exosomes as a modulator in maternal immune tolerance during pregnancy. Front. Immunol. 2021, 12, 671093. [Google Scholar]
- Sendon-Lago, J.; Rio, L.G.; Eiro, N.; Diaz-Rodriguez, P.; Avila, L.; Gonzalez, L.O.; Vizoso, F.J.; Perez-Fernandez, R.; Landin, M. Tailored hydrogels as delivery platforms for conditioned medium from mesenchymal stem cells in a model of acute colitis in mice. Pharmaceutics 2021, 13, 1127. [Google Scholar] [CrossRef]
- Perse, M.; Cerar, A. Dextran sodium sulphate colitis mouse model: Traps and tricks. J. Biomed. Biotechnol. 2012, 2012, 718617. [Google Scholar] [CrossRef]
- Chassaing, B.; Aitken, J.D.; Malleshappa, M.; Vijay-Kumar, M. Dextran sulfate sodium (DSS)-induced colitis in mice. Curr. Protoc. Immunol. 2014, 104, 15–25. [Google Scholar] [CrossRef] [PubMed]
- Galvez, J. Role of Th17 cells in the pathogenesis of human IBD. ISRN Inflamm. 2014, 2014, 928461. [Google Scholar] [CrossRef] [PubMed]
- Abomaray, F.M.; Al Jumah, M.A.; Alsaad, K.O.; Jawdat, D.; Al Khaldi, A.; AlAskar, A.S.; Al Harthy, S.; Al Subayyil, A.M.; Khatlani, T.; Alawad, A.O.; et al. Phenotypic and functional characterization of mesenchymal stem/multipotent stromal cells from Decidua Basalis of human term placenta. Stem Cells Int. 2016, 2016, 5184601. [Google Scholar] [CrossRef]
- Yi, Q.; Wang, J.; Song, Y.; Guo, Z.; Lei, S.; Yang, X.; Li, L.; Gao, C.; Zhou, Z. Ascl2 facilitates IL-10 production in Th17 cells to restrain their pathogenicity in inflammatory bowel disease. Biochem. Biophys. Res. Commun. 2019, 510, 435–441. [Google Scholar] [CrossRef] [PubMed]
- Riddell, M.R.; Winkler-Lowen, B.; Chakrabarti, S.; Dunk, C.; Davidge, S.T.; Guilbert, L.J. The characterization of fibrocyte-like cells: A novel fibroblastic cell of the placenta. Placenta 2012, 33, 143–150. [Google Scholar] [CrossRef]
- Zhao, Y.; Gillen, J.R.; Harris, D.A.; Kron, I.L.; Murphy, M.P.; Lau, C.L. Treatment with placenta-derived mesenchymal stem cells mitigates development of bronchiolitis obliterans in a murine model. J. Thorac. Cardiovasc. Surg. 2014, 147, 1668–1677.e5. [Google Scholar] [CrossRef]
- Lahiani, A.; Zahavi, E.; Netzer, N.; Ofir, R.; Pinzur, L.; Raveh, S.; Arien-Zakay, H.; Yavin, E.; Lazarovici, P. Human placental eXpanded (PLX) mesenchymal-like adherent stromal cells confer neuroprotection to nerve growth factor (NGF)-differentiated PC12 cells exposed to ischemia by secretion of IL-6 and VEGF. Biochim. Biophys. Acta 2015, 1853, 422–430. [Google Scholar] [CrossRef]
- Petrou, P.; Gothelf, Y.; Argov, Z.; Gotkine, M.; Levy, Y.S.; Kassis, I.; Vaknin-Dembinsky, A.; Ben-Hur, T.; Offen, D.; Abramsky, O.; et al. Safety and clinical effects of mesenchymal stem cells secreting neurotrophic factor transplantation in patients with amyotrophic lateral Sclerosis: Results of Phase 1/2 and 2a Clinical Trials. JAMA Neurol. 2016, 73, 337–344. [Google Scholar] [CrossRef]
- Adams, K.M.; Yan, Z.; Stevens, A.M.; Nelson, J.L. The changing maternal “self” hypothesis: A mechanism for maternal tolerance of the fetus. Placenta 2007, 28, 378–382. [Google Scholar]
- Chang, C.J.; Yen, M.L.; Chen, Y.C.; Chien, C.C.; Huang, H.I.; Bai, C.H.; Yen, B.L. Placenta-derived multipotent cells exhibit immunosuppressive properties that are enhanced in the presence of interferon-gamma. Stem Cells 2006, 24, 2466–2477. [Google Scholar]
- Gorodetsky, R.; Aicher, W.K. Allogenic use of human placenta-derived stromal cells as a highly active subtype of mesenchymal stromal cells for cell-based therapies. Int. J. Mol. Sci. 2021, 22, 5302. [Google Scholar] [CrossRef]
- Maier, C.L.; Pober, J.S. Human placental pericytes poorly stimulate and actively regulate allogeneic CD4 T cell responses. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 183–189. [Google Scholar]
- Macholdova, K.; Machackova, E.; Proskova, V.; Hromadnikova, I.; Klubal, R. Latest findings on the placenta from the point of view of immunology. tolerance and mesenchymal stem cells, Ceska gynekologie/Ceska lekarska spolecnost. J. Ev. Purkyne 2019, 84, 154–160. [Google Scholar]
- Forbes, G.M. Mesenchymal Stromal Cell Therapy in Crohn’s Disease. Dig Dis 2017, 35, 115–122. [Google Scholar]
- Magatti, M.; De Munari, S.; Vertua, E.; Gibelli, L.; Wengler, G.S.; Parolini, O. Human amnion mesenchyme harbors cells with allogeneic T-cell suppression and stimulation capabilities. Stem Cells 2008, 26, 182–192. [Google Scholar] [CrossRef]
- Wang, Y.; Zhao, X.; Li, Z.; Wang, W.; Jiang, Y.; Zhang, H.; Liu, X.; Ren, Y.; Xu, X.; Hu, X. Decidual natural killer cells dysfunction is caused by IDO downregulation in dMDSCs with Toxoplasma gondii infection. Commun Biol 2024, 7, 669. [Google Scholar] [CrossRef]
- Kshirsagar, S.K.; Alam, S.M.; Jasti, S.; Hodes, H.; Nauser, T.; Gilliam, M.; Billstrand, C.; Hunt, J.S.; Petroff, M.G. Immunomodulatory molecules are released from the first trimester and term placenta via exosomes. Placenta 2012, 33, 982–990. [Google Scholar] [CrossRef]
- Chen, X.; Zhao, X.; Wang, H.; Yang, Z.; Li, J.; Suo, H. Prevent Effects of Lactobacillus Fermentum HY01 on Dextran Sulfate Sodium-Induced Colitis in Mice. Nutrients 2017, 9, 545. [Google Scholar] [CrossRef]
- Nordgren, S.; McPheeters, G.; Svaninger, G.; Öresland, T.; HulténInt, L. Small bowel length in inflammatory bowel disease. J. Colorect. Dis. 1997, 12, 230–234. [Google Scholar] [CrossRef]
- Sydora, B.C.; Albert, E.J.; Foshaug, R.R.; Doyle, J.S.; Churchill, T.A.; Fedorak, R.N. Intravenous injection of endogenous microbial components abrogates DSS-induced colitis. Dig. Dis. Sci. 2012, 57, 345–354. [Google Scholar] [PubMed]
- Li, C.; Zhang, W.; Jiang, X.; Mao, N. Human-placenta-derived mesenchymal stem cells inhibit proliferation and function of allogeneic immune cells. Cell Tissue Res. 2007, 330, 437–446. [Google Scholar] [PubMed]
- Liu, W.; Morschauser, A.; Zhang, X.; Lu, X.; Gleason, J.; He, S.; Chen, H.J.; Jankovic, V.; Ye, Q.; Labazzo, K.; et al. Human placenta-derived adherent cells induce tolerogenic immune responses. Clin. Transl. Immunol. 2014, 3, e14. [Google Scholar]
- Ouyang, Y.; Mouillet, J.F.; Coyne, C.B.; Sadovsky, Y. Review: Placenta-specific microRNAs in exosomes—Good things come in nano-packages. Placenta 2014, 35, S69–S73. [Google Scholar]
- McGuirk, J.P.; Metheny, L.; Pineiro, L.; Litzow, M.; Rowley, S.D.; Avni, B.; Tamari, R.; Lazarus, H.M.; Rowe, J.M.; Sheleg, M.; et al. Placental expanded mesenchymal-like cells (PLX-R18) for poor graft function after hematopoietic cell transplantation: A phase I study. Bone Marrow Transpl. 2023, 58, 1189–1196. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorodetsky, R.; Lazmi Hailu, A.; Volinsky, E.; Adani, B.; Pappo, O.; Israeli, E. The Use of Potent Populations of Expanded Fetal Human Placental Stromal Cells for the Treatment of Dextran Sodium Sulfate-Induced Colitis in a Mouse Model. Int. J. Mol. Sci. 2025, 26, 3222. https://doi.org/10.3390/ijms26073222
Gorodetsky R, Lazmi Hailu A, Volinsky E, Adani B, Pappo O, Israeli E. The Use of Potent Populations of Expanded Fetal Human Placental Stromal Cells for the Treatment of Dextran Sodium Sulfate-Induced Colitis in a Mouse Model. International Journal of Molecular Sciences. 2025; 26(7):3222. https://doi.org/10.3390/ijms26073222
Chicago/Turabian StyleGorodetsky, Raphael, Astar Lazmi Hailu, Evgenia Volinsky, Boaz Adani, Orit Pappo, and Eran Israeli. 2025. "The Use of Potent Populations of Expanded Fetal Human Placental Stromal Cells for the Treatment of Dextran Sodium Sulfate-Induced Colitis in a Mouse Model" International Journal of Molecular Sciences 26, no. 7: 3222. https://doi.org/10.3390/ijms26073222
APA StyleGorodetsky, R., Lazmi Hailu, A., Volinsky, E., Adani, B., Pappo, O., & Israeli, E. (2025). The Use of Potent Populations of Expanded Fetal Human Placental Stromal Cells for the Treatment of Dextran Sodium Sulfate-Induced Colitis in a Mouse Model. International Journal of Molecular Sciences, 26(7), 3222. https://doi.org/10.3390/ijms26073222








