Ex Vivo Overactivation of Lymphocyte Subsets in Fibrotic Hypersensitivity Pneumonitis Is Blunted by a Sphingosine-1-Phosphate Receptor Ligand
Abstract
1. Introduction
2. Results
2.1. Subject’s Characteristics
2.2. Increased Circulating Factors in fHP Patients
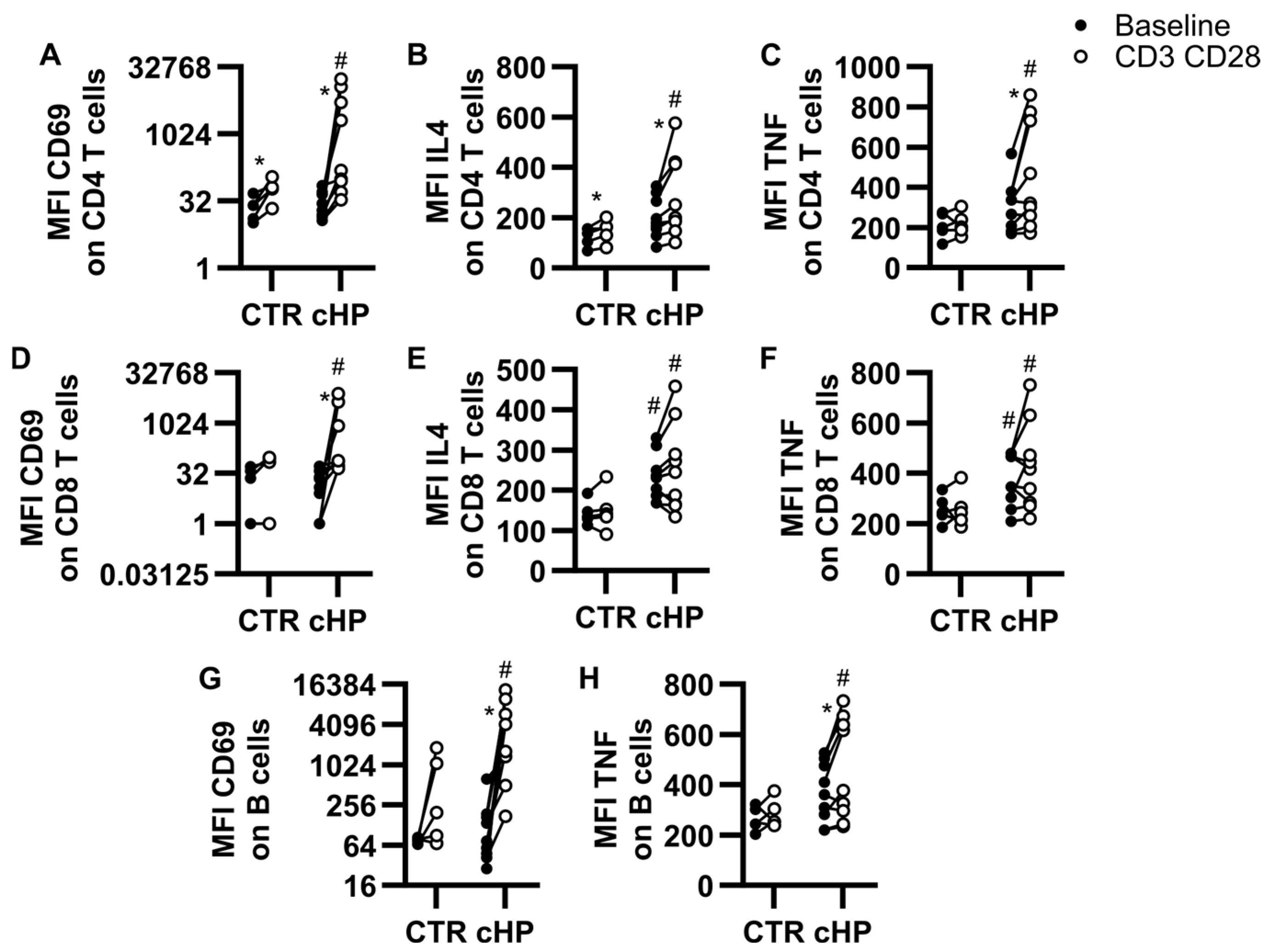
2.3. Indications That Specific Lymphocyte Subsets Are Primed and/or Activated in fHP Patients
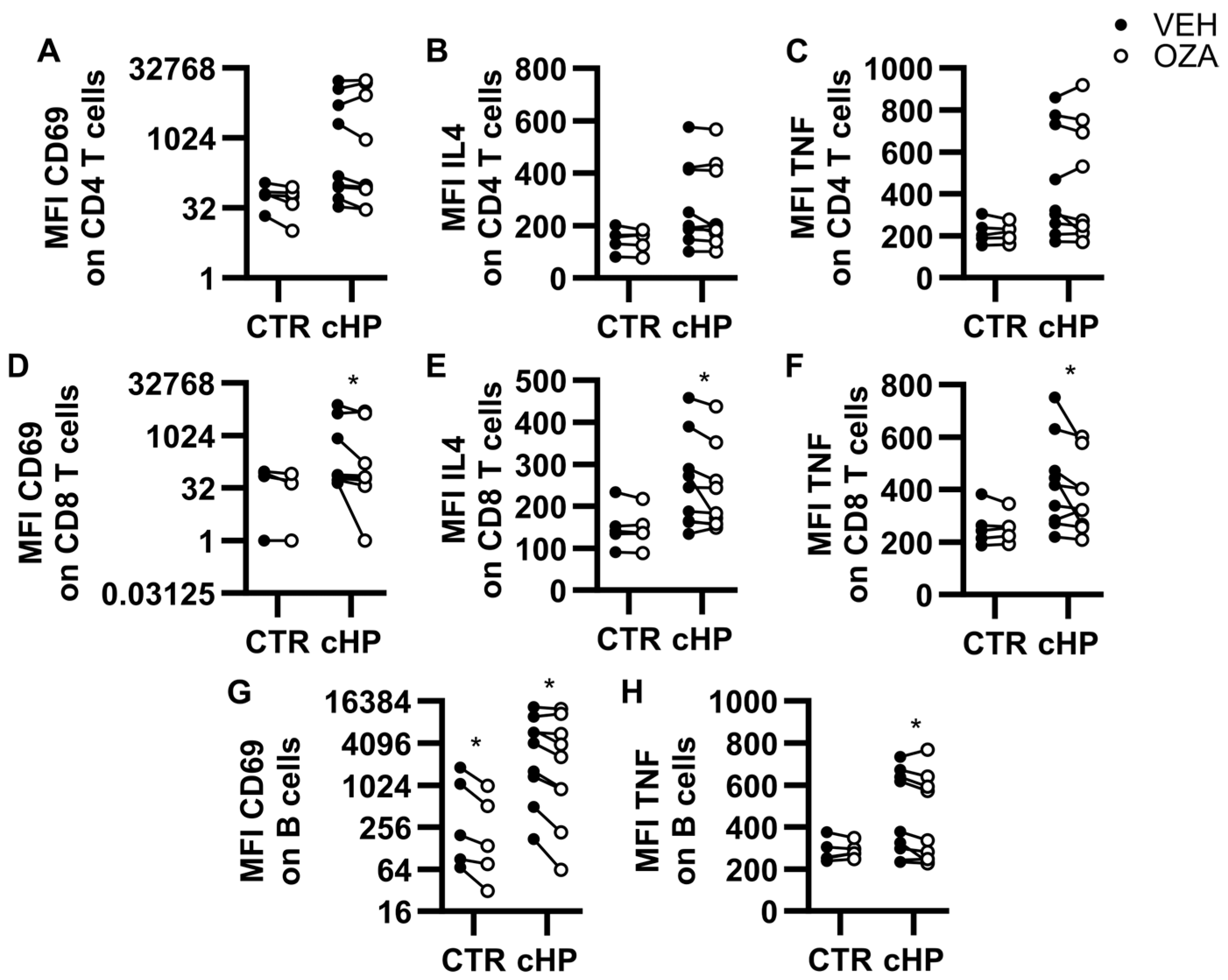
2.4. Ozanimod Inhibits CD8 T Cell Activation Ex Vivo
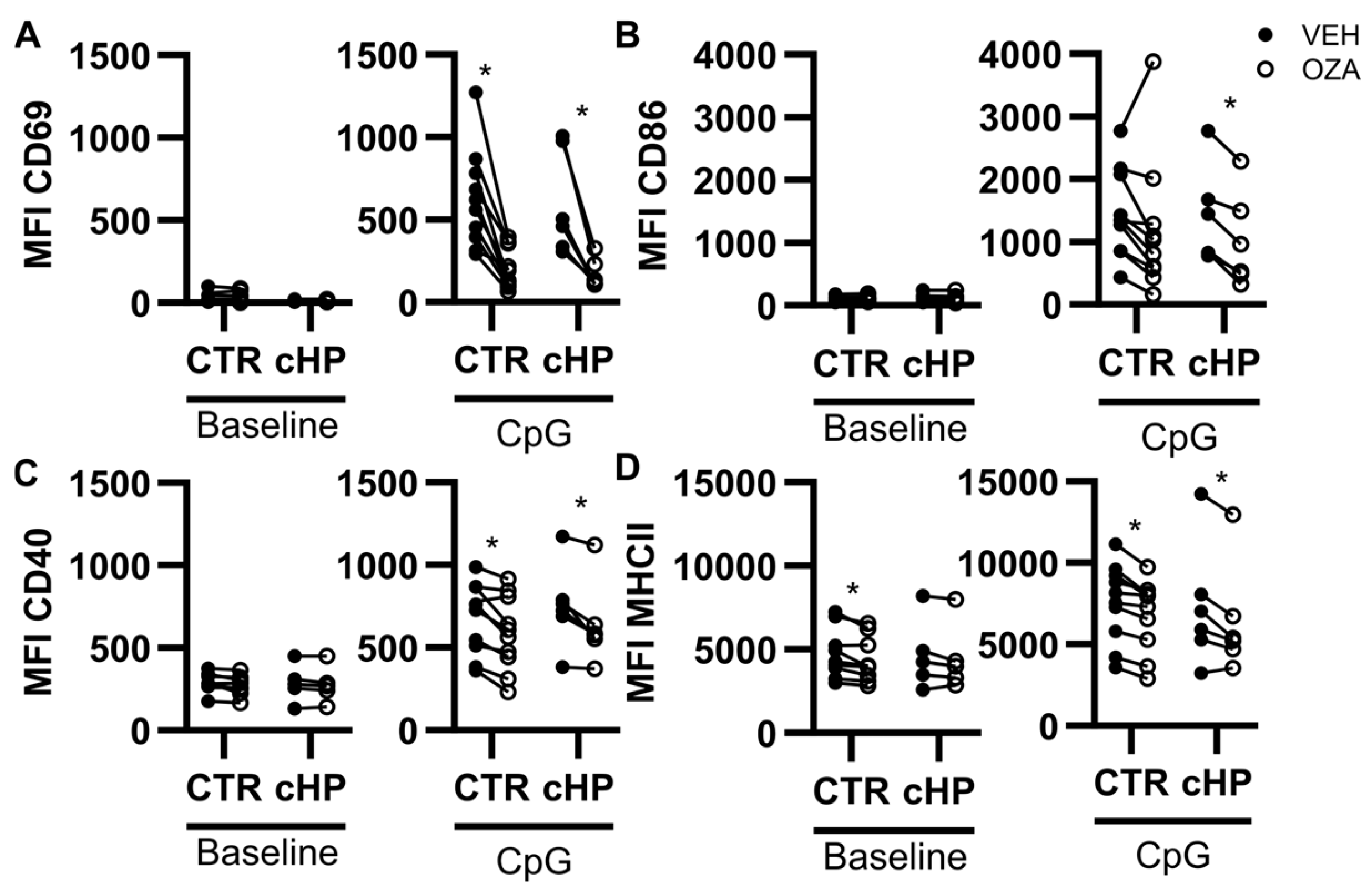
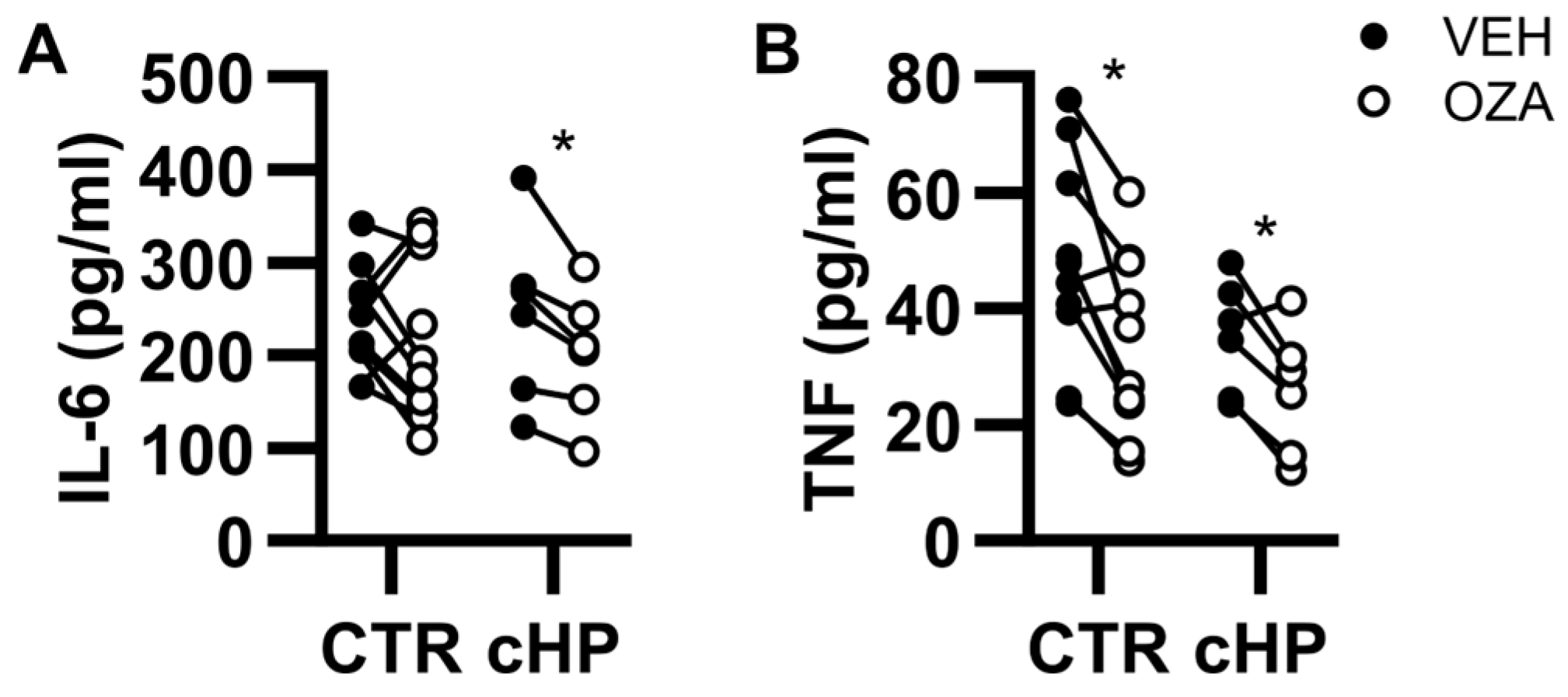
2.5. Ozanimod Perturbs B Cell Activation in Response to a TLR9 Ligand
3. Discussion
4. Materials and Methods
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vasakova, M.; Morell, F.; Walsh, S.; Leslie, K.; Raghu, G. Hypersensitivity Pneumonitis: Perspectives in Diagnosis and Management. Am. J. Respir. Crit. Care Med. 2017, 196, 680–689. [Google Scholar] [CrossRef] [PubMed]
- Thorne, P.S.; Adamcakova-Dodd, A.; Kelly, K.M.; O’Neill, M.E.; Duchaine, C. Metalworking fluid with mycobacteria and endotoxin induces hypersensitivity pneumonitis in mice. Am. J. Respir. Crit. Care Med. 2006, 173, 759–768. [Google Scholar] [CrossRef]
- Huppe, C.A.; Blais-Lecours, P.; Bernatchez, E.; Lauzon-Joset, J.F.; Duchaine, C.; Rosen, H.; Dion, G.; McNagny, K.M.; Blanchet, M.R.; Morissette, M.C.; et al. S1P1 Contributes to Endotoxin-enhanced B Cell Functions Involved in Hypersensitivity Pneumonitis. Am. J. Respir. Cell Mol. Biol. 2020, 63, 209–218. [Google Scholar] [CrossRef]
- Bueno, M.; Zank, D.; Buendia-Roldan, I.; Fiedler, K.; Mays, B.G.; Alvarez, D.; Sembrat, J.; Kimball, B.; Bullock, J.K.; Martin, J.L.; et al. PINK1 attenuates mtDNA release in alveolar epithelial cells and TLR9 mediated profibrotic responses. PLoS ONE 2019, 14, e0218003. [Google Scholar] [CrossRef] [PubMed]
- Fernandez Perez, E.R.; Kong, A.M.; Raimundo, K.; Koelsch, T.L.; Kulkarni, R.; Cole, A.L. Epidemiology of Hypersensitivity Pneumonitis among an Insured Population in the United States: A Claims-based Cohort Analysis. Ann. Am. Thorac. Soc. 2018, 15, 460–469. [Google Scholar] [CrossRef] [PubMed]
- Morell, F.; Villar, A.; Montero, M.A.; Munoz, X.; Colby, T.V.; Pipvath, S.; Cruz, M.J.; Raghu, G. Chronic hypersensitivity pneumonitis in patients diagnosed with idiopathic pulmonary fibrosis: A prospective case-cohort study. Lancet Respir. Med. 2013, 1, 685–694. [Google Scholar] [CrossRef]
- Lacasse, Y.; Girard, M.; Cormier, Y. Recent advances in hypersensitivity pneumonitis. Chest 2012, 142, 208–217. [Google Scholar] [CrossRef]
- Fernandez Perez, E.R.; Harmacek, L.D.; O’Connor, B.P.; Danhorn, T.; Vestal, B.; Maier, L.A.; Koelsch, T.L.; Leach, S.M. Prognostic accuracy of a peripheral blood transcriptome signature in chronic hypersensitivity pneumonitis. Thorax 2022, 77, 86–90. [Google Scholar] [CrossRef]
- Machahua, C.; Buendia-Roldan, I.; Ocana-Guzman, R.; Molina-Molina, M.; Pardo, A.; Chavez-Galan, L.; Selman, M. CD4+T cells in ageing-associated interstitial lung abnormalities show evidence of pro-inflammatory phenotypic and functional profile. Thorax 2021, 76, 152–160. [Google Scholar] [CrossRef]
- De Sadeleer, L.J.; Hermans, F.; De Dycker, E.; Yserbyt, J.; Verschakelen, J.A.; Verbeken, E.K.; Verleden, G.M.; Wuyts, W.A. Effects of Corticosteroid Treatment and Antigen Avoidance in a Large Hypersensitivity Pneumonitis Cohort: A Single-Centre Cohort Study. J. Clin. Med. 2018, 8, 14. [Google Scholar] [CrossRef]
- Courtemanche, O.; Huppe, C.A.; Blais Lecours, P.; Lerdu, O.; Roy, J.; Lauzon-Joset, J.F.; Blanchet, M.R.; Morissette, M.C.; Marsolais, D. Co-modulation of T cells and B cells enhances the inhibition of inflammation in experimental hypersensitivity pneumonitis. Respir. Res. 2022, 23, 275. [Google Scholar] [CrossRef]
- Lota, H.K.; Keir, G.J.; Hansell, D.M.; Nicholson, A.G.; Maher, T.M.; Wells, A.U.; Renzoni, E.A. Novel use of rituximab in hypersensitivity pneumonitis refractory to conventional treatment. Thorax 2013, 68, 780–781. [Google Scholar] [CrossRef] [PubMed]
- Tamm, A.M.; Kremens, K. Rituximab for Salvage Therapy of Refractory Hypersensitivity Pneumonitis. WMJ 2019, 118, 95–97. [Google Scholar]
- Obinata, H.; Hla, T. Sphingosine 1-phosphate and inflammation. Int. Immunol. 2019, 31, 617–625. [Google Scholar] [CrossRef] [PubMed]
- Sanna, M.G.; Liao, J.; Jo, E.; Alfonso, C.; Ahn, M.Y.; Peterson, M.S.; Webb, B.; Lefebvre, S.; Chun, J.; Gray, N.; et al. Sphingosine 1-phosphate (S1P) receptor subtypes S1P1 and S1P3, respectively, regulate lymphocyte recirculation and heart rate. J. Biol. Chem. 2004, 279, 13839–13848. [Google Scholar] [CrossRef]
- Garris, C.S.; Wu, L.; Acharya, S.; Arac, A.; Blaho, V.A.; Huang, Y.; Moon, B.S.; Axtell, R.C.; Ho, P.P.; Steinberg, G.K.; et al. Defective sphingosine 1-phosphate receptor 1 (S1P1) phosphorylation exacerbates TH17-mediated autoimmune neuroinflammation. Nat. Immunol. 2013, 14, 1166–1172. [Google Scholar] [CrossRef] [PubMed]
- Huang, M.C.; Watson, S.R.; Liao, J.J.; Goetzl, E.J. Th17 augmentation in OTII TCR plus T cell-selective type 1 sphingosine 1-phosphate receptor double transgenic mice. J. Immunol. 2007, 178, 6806–6813. [Google Scholar] [CrossRef]
- Dorsam, G.; Graeler, M.H.; Seroogy, C.; Kong, Y.; Voice, J.K.; Goetzl, E.J. Transduction of multiple effects of sphingosine 1-phosphate (S1P) on T cell functions by the S1P1 G protein-coupled receptor. J. Immunol. 2003, 171, 3500–3507. [Google Scholar]
- Schröder, M.; Richter, C.; Juan, M.H.; Maltusch, K.; Giegold, O.; Quintini, G.; Pfeilschifter, J.M.; Huwiler, A.; Radeke, H.H. The sphingosine kinase 1 and S1P1 axis specifically counteracts LPS-induced IL-12p70 production in immune cells of the spleen. Mol. Immunol. 2011, 48, 1139–1148. [Google Scholar] [CrossRef]
- Huppe, C.A.; Blais Lecours, P.; Lechasseur, A.; Gendron, D.R.; Lemay, A.M.; Bissonnette, E.Y.; Blanchet, M.R.; Duchaine, C.; Morissette, M.C.; Rosen, H.; et al. A sphingosine-1-phosphate receptor 1 agonist inhibits tertiary lymphoid tissue reactivation and hypersensitivity in the lung. Mucosal Immunol. 2018, 11, 112–119. [Google Scholar] [CrossRef]
- Raghu, G.; Remy-Jardin, M.; Ryerson, C.J.; Myers, J.L.; Kreuter, M.; Vasakova, M.; Bargagli, E.; Chung, J.H.; Collins, B.F.; Bendstrup, E.; et al. Diagnosis of Hypersensitivity Pneumonitis in Adults An Official ATS/JRS/ALAT Clinical Practice Guideline. Am. J. Respir. Crit. Care Med. 2020, 202, E36–E69. [Google Scholar] [CrossRef] [PubMed]
- Joshi, A.D.; Fong, D.J.; Oak, S.R.; Trujillo, G.; Flaherty, K.R.; Martinez, F.J.; Hogaboam, C.M. Interleukin-17-mediated immunopathogenesis in experimental hypersensitivity pneumonitis. Am. J. Respir. Crit. Care Med. 2009, 179, 705–716. [Google Scholar] [CrossRef] [PubMed]
- Bellanger, A.P.; Gbaguidi-Haore, H.; Gondoin, A.; Pallandre, J.R.; Vacheyrou, M.; Valot, B.; Soumagne, T.; Reboux, G.; Dalphin, J.C.; Millon, L. Positive fungal quantitative PCR and Th17 cytokine detection in bronchoalveolar lavage fluids: Complementary biomarkers of hypersensitivity pneumonitis? J. Immunol. Methods 2016, 434, 61–65. [Google Scholar] [CrossRef]
- Andrews, K.; Ghosh, M.C.; Schwingshackl, A.; Rapalo, G.; Luellen, C.; Waters, C.M.; Fitzpatrick, E.A. Chronic hypersensitivity pneumonitis caused by Saccharopolyspora rectivirgula is not associated with a switch to a Th2 response. Am. J. Physiol. Lung Cell Mol. Physiol. 2016, 310, L393–L402. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xie, X.; Zheng, S.; Du, G.; Chen, S.; Zhang, W.; Zhuang, J.; Lin, J.; Hu, S.; Zheng, K.; et al. Serum B-cell activating factor and lung ultrasound B-lines in connective tissue disease related interstitial lung disease. Front. Med. 2022, 9, 1066111. [Google Scholar] [CrossRef]
- Barrera, L.; Mendoza, F.; Zuniga, J.; Estrada, A.; Zamora, A.C.; Melendro, E.I.; Ramirez, R.; Pardo, A.; Selman, M. Functional diversity of T-cell subpopulations in subacute and chronic hypersensitivity pneumonitis. Am. J. Respir. Crit. Care Med. 2008, 177, 44–55. [Google Scholar] [CrossRef]
- Zhao, A.Y.; Unterman, A.; Abu Hussein, N.S.; Sharma, P.; Nikola, F.; Flint, J.; Yan, X.; Adams, T.S.; Justet, A.; Sumida, T.S.; et al. Single-Cell Analysis Reveals Novel Immune Perturbations in Fibrotic Hypersensitivity Pneumonitis. Am. J. Respir. Crit. Care Med. 2024, 210, 1252–1266. [Google Scholar] [CrossRef]
- Heron, M.; Claessen, A.M.; Grutters, J.C.; van den Bosch, J.M. T-cell activation profiles in different granulomatous interstitial lung diseases--a role for CD8+CD28(null) cells? Clin. Exp. Immunol. 2010, 160, 256–265. [Google Scholar] [CrossRef]
- Drent, M.; van Velzen-Blad, H.; Diamant, M.; Wagenaar, S.S.; Donckerwolck-Bogaert, M.; van den Bosch, J.M. Differential diagnostic value of plasma cells in bronchoalveolar lavage fluid. Chest 1993, 103, 1720–1724. [Google Scholar] [CrossRef][Green Version]
- Drent, M.; Wagenaar, S.; van Velzen-Blad, H.; Mulder, P.G.; Hoogsteden, H.C.; van den Bosch, J.M. Relationship between plasma cell levels and profile of bronchoalveolar lavage fluid in patients with subacute extrinsic allergic alveolitis. Thorax 1993, 48, 835–839. [Google Scholar] [CrossRef][Green Version]
- Evrard, M.; Wynne-Jones, E.; Peng, C.; Kato, Y.; Christo, S.N.; Fonseca, R.; Park, S.L.; Burn, T.N.; Osman, M.; Devi, S.; et al. Sphingosine 1-phosphate receptor 5 (S1PR5) regulates the peripheral retention of tissue-resident lymphocytes. J. Exp. Med. 2022, 219, e20210116. [Google Scholar] [CrossRef] [PubMed]
- Ubieta, K.; Thomas, M.J.; Wollin, L. The Effect of Nintedanib on T-Cell Activation, Subsets and Functions. Drug Des. Dev. Ther. 2021, 15, 997–1011. [Google Scholar] [CrossRef]
- Visner, G.A.; Liu, F.Z.; Bizargity, P.; Liu, H.Z.; Liu, K.F.; Yang, J.; Wang, L.Q.; Hancock, W.W. Pirfenidone Inhibits T-Cell Activation, Proliferation, Cytokine and Chemokine Production, and Host Alloresponses. Transplantation 2009, 88, 330–338. [Google Scholar] [CrossRef]
- McMurray, R.; Harisdangkul, V. Mycophenolate mofetil: Selective T cell inhibition. Am. J. Med. Sci. 2002, 323, 194–196. [Google Scholar] [CrossRef] [PubMed]
- Mandala, S.; Hajdu, R.; Bergstrom, J.; Quackenbush, E.; Xie, J.; Milligan, J.; Thornton, R.; Shei, G.J.; Card, D.; Keohane, C.; et al. Alteration of lymphocyte trafficking by sphingosine-1-phosphate receptor agonists. Science 2002, 296, 346–349. [Google Scholar] [CrossRef] [PubMed]
- Prior, T.; Wälscher, J.; Gross, B.; Bendstrup, E.; Kreuter, M. Clusters of comorbidities in fibrotic hypersensitivity pneumonitis. Respir. Res. 2022, 23, 368. [Google Scholar] [CrossRef]
- Kono, M.; Tucker, A.; Tran, J.; Bergner, J.; Turner, E.; Proia, R. Sphingosine-1-phosphate receptor 1 reporter mice reveal receptor activation sites in vivo. J. Clin. Investig. 2014, 124, 2076–2086. [Google Scholar] [CrossRef]
- Cahalan, S.M.; Gonzalez-Cabrera, P.J.; Sarkisyan, G.; Nguyen, N.; Schaeffer, M.T.; Huang, L.M.; Yeager, A.; Clemons, B.; Scott, F.; Rosen, H. Actions of a picomolar short-acting S1P(1) agonist in S1P(1)-eGFP knock-in mice. Nat. Chem. Biol. 2011, 7, 254–256. [Google Scholar] [CrossRef]
- Oo, M.; Chang, S.; Thangada, S.; Wu, M.; Rezaul, K.; Blaho, V.; Hwang, S.; Han, D.; Hla, T. Engagement of S1P1-degradative mechanisms leads to vascular leak in mice. J. Clin. Investig. 2011, 121, 2290–2300. [Google Scholar] [CrossRef]
- Scott, F.L.; Clemons, B.; Brooks, J.; Brahmachary, E.; Powell, R.; Dedman, H.; Desale, H.G.; Timony, G.A.; Martinborough, E.; Rosen, H.; et al. Ozanimod (RPC1063) is a potent sphingosine-1-phosphate receptor-1 (S1P(1)) and receptor-5 (S1P(5)) agonist with autoimmune disease-modifying activity. Br. J. Pharmacol. 2016, 173, 1778–1792. [Google Scholar] [CrossRef]
| Control Subjects (10) | Patients with fHP (12) | |
|---|---|---|
| General characteristics | ||
| Age (Year ± SEM) | 65.3 ± 2.8 | 67.1 ± 7.1 |
| Sex (Male, n *) | 6 | 7 |
| Smoker | 0 | 0 |
| Non-smoker | 8 | 6 |
| Former smoker | 2 | 6 |
| fHP treatment at time of blood sampling | N/D | |
| Mycophenolate and corticosteroids | 5 | |
| Corticosteroids | 1 | |
| Without fHP treatment | 6 | |
| Clinical data for diagnosis | ||
| Environmental exposure confirmed | N/D | 6 |
| HRCT ** | N/D | |
| Typical fHP | 9 | |
| Compatible for fHP | 1 | |
| Indeterminate for fHP | 2 | |
| Lymphocytosis | N/D | |
| Mild (10–20%) | 1 | |
| Moderate (20–40%) | 5 | |
| Important (>40%) | 3 | |
| Biopsy ** | N/D | 10 |
| Definite fHP | 6 | |
| Probable fHP | 4 | |
| Diagnosis confidence ** (Multidisciplinary team meeting) | N/D | |
| Definite fHP | 8 | |
| High Confidence fHP | 3 | |
| Low Confidence fHP | 1 |
| Cytokine | Control (pg/mL) (n = 10) | fHP (pg/mL) (n = 9) | p-Value |
|---|---|---|---|
| TNF | 0.0 (0.0–1.2) | 10.7 (0.4–53.3) | 0.020 * |
| CXCL13 | 0.9 (0.0–19.5) | 59.7 (15.1–125.5 | 0.028 * |
| BAFF | 469.3 (419.5–564.5) | 593.7 (492.7–937.0) | 0.035 * |
| IL-4 | 0.0 (0.0–0.6) | 1.7 (0.1–11.9) | 0.039 * |
| IL-21 | 0.0 (0.0–4.8) | 29.3 (0.0–101.3) | 0.050 * |
| IL-17A | 0.0 (0.0–1.3) | 1.7 (0.0–74.5) | 0.089 |
| IL-6 | 0.0 (0.0–7.6) | 2.7 (0.0–33.4) | 0.154 |
| IL-22 | 110.0 (84.7–180.2) | 212.2 (95.9–331.4) | 0.278 |
| CXCL12 | 99.9 (85.8–111.2) | 105.7 (63.43–122.4) | 0.720 |
| IFNγ | 1.0 (0.0–2.1) | 1.2 (0.0–4.7) | 0.923 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Courtemanche, O.; Huppé, C.-A.; Blais-Lecours, P.; Maranda, C.; Morissette, M.C.; Blanchet, M.-R.; Dion, G.; Marsolais, D. Ex Vivo Overactivation of Lymphocyte Subsets in Fibrotic Hypersensitivity Pneumonitis Is Blunted by a Sphingosine-1-Phosphate Receptor Ligand. Int. J. Mol. Sci. 2025, 26, 3197. https://doi.org/10.3390/ijms26073197
Courtemanche O, Huppé C-A, Blais-Lecours P, Maranda C, Morissette MC, Blanchet M-R, Dion G, Marsolais D. Ex Vivo Overactivation of Lymphocyte Subsets in Fibrotic Hypersensitivity Pneumonitis Is Blunted by a Sphingosine-1-Phosphate Receptor Ligand. International Journal of Molecular Sciences. 2025; 26(7):3197. https://doi.org/10.3390/ijms26073197
Chicago/Turabian StyleCourtemanche, Olivier, Carole-Ann Huppé, Pascale Blais-Lecours, Cloé Maranda, Mathieu C. Morissette, Marie-Renée Blanchet, Geneviève Dion, and David Marsolais. 2025. "Ex Vivo Overactivation of Lymphocyte Subsets in Fibrotic Hypersensitivity Pneumonitis Is Blunted by a Sphingosine-1-Phosphate Receptor Ligand" International Journal of Molecular Sciences 26, no. 7: 3197. https://doi.org/10.3390/ijms26073197
APA StyleCourtemanche, O., Huppé, C.-A., Blais-Lecours, P., Maranda, C., Morissette, M. C., Blanchet, M.-R., Dion, G., & Marsolais, D. (2025). Ex Vivo Overactivation of Lymphocyte Subsets in Fibrotic Hypersensitivity Pneumonitis Is Blunted by a Sphingosine-1-Phosphate Receptor Ligand. International Journal of Molecular Sciences, 26(7), 3197. https://doi.org/10.3390/ijms26073197






