Investigation of Anti-Cancer Properties of Novel Curcuminoids in Leukemic Cells and Dalton Lymphoma Ascites Model
Abstract
1. Introduction
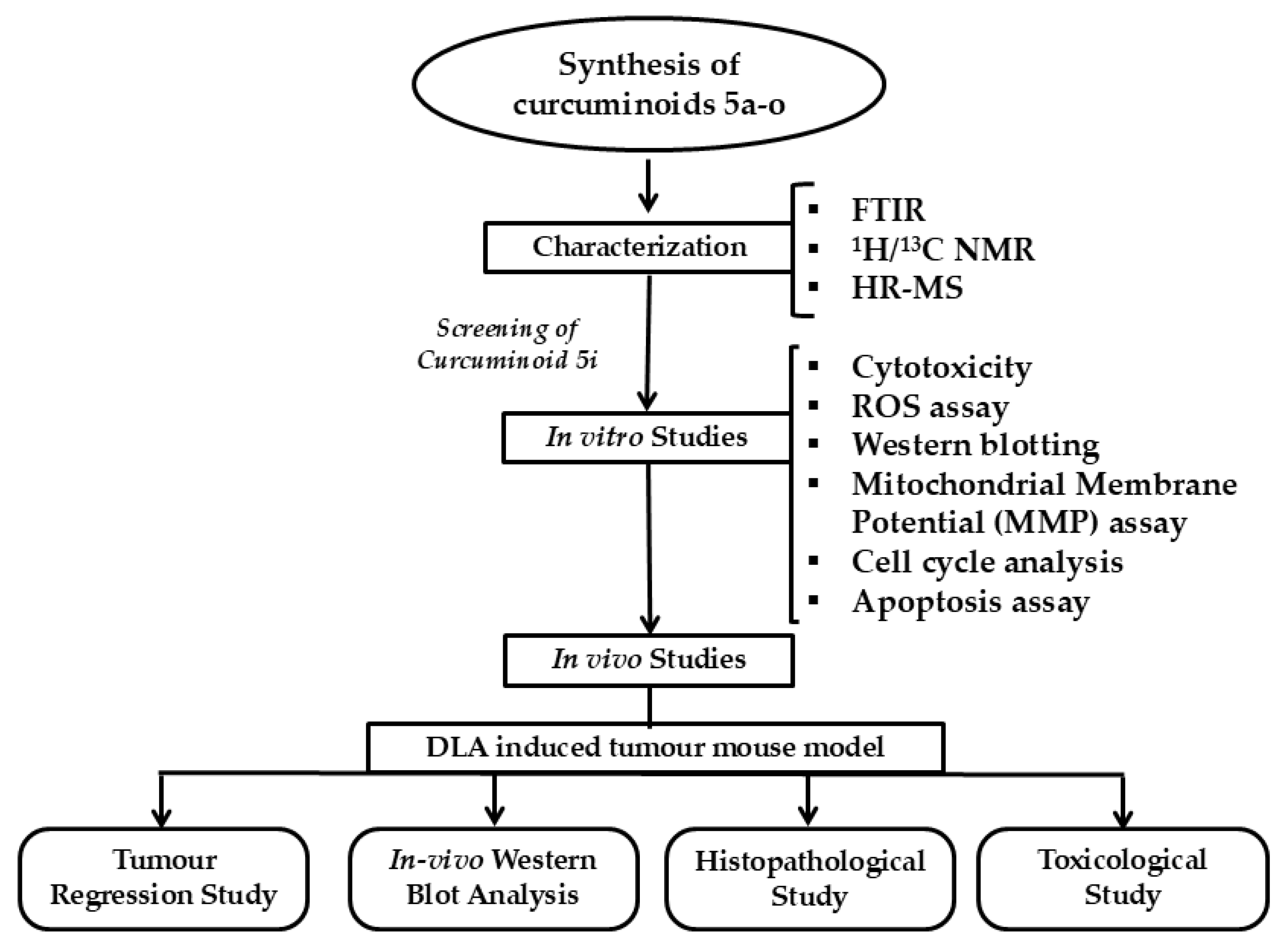
2. Results
2.1. Chemistry
Synthesis and Characterisation of Curcuminoids
2.2. In Vitro Studies
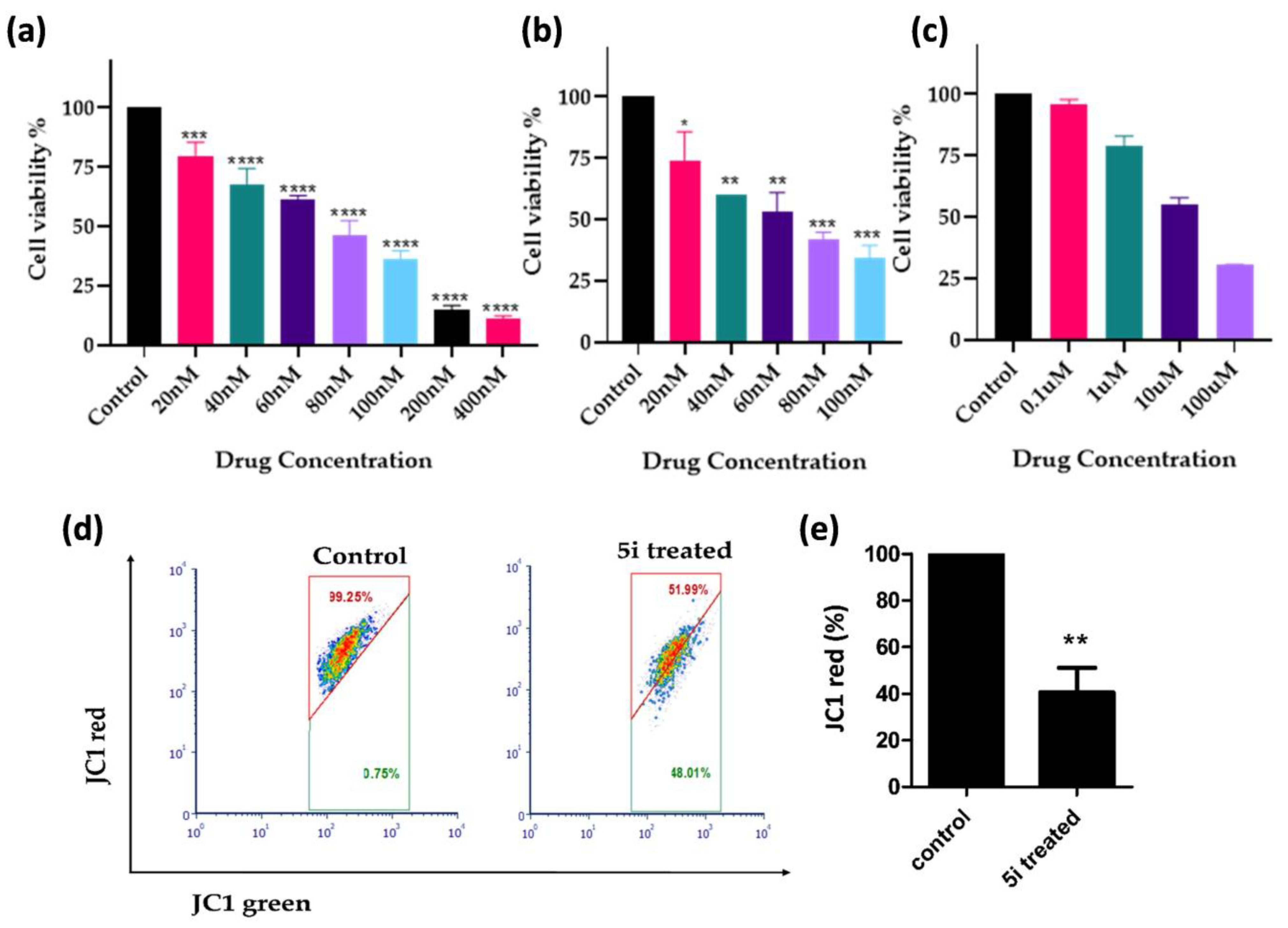
2.2.1. In Vitro Cytotoxicity Evaluation
2.2.2. In Vitro Cytotoxicity Evaluation in Cell Lines
2.2.3. 5i Alters Mitochondrial Membrane Potential (MMP; ∆ψm)
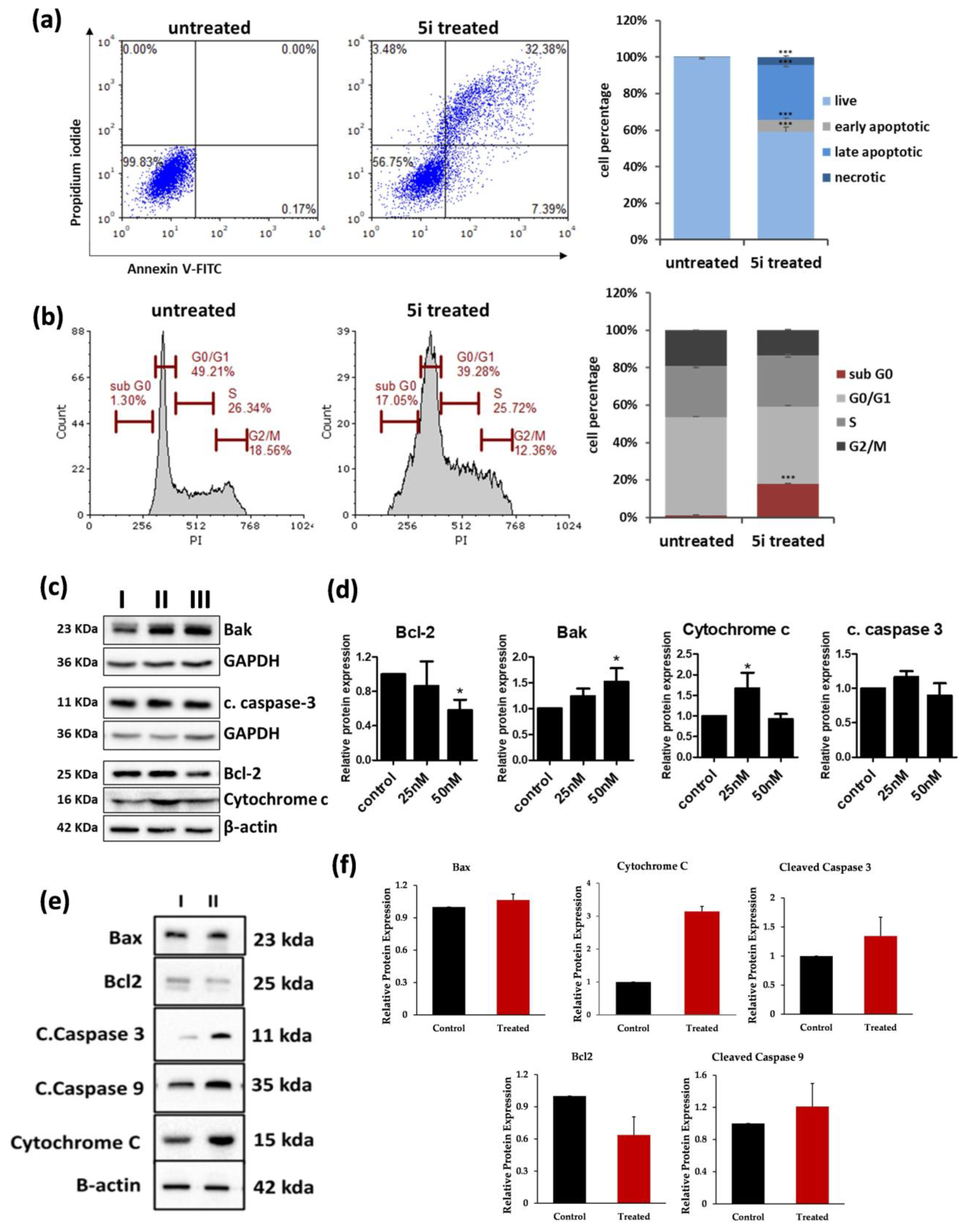
2.2.4. 5i Treatment-Induced Apoptotic Activation in MOLT-4 Cells
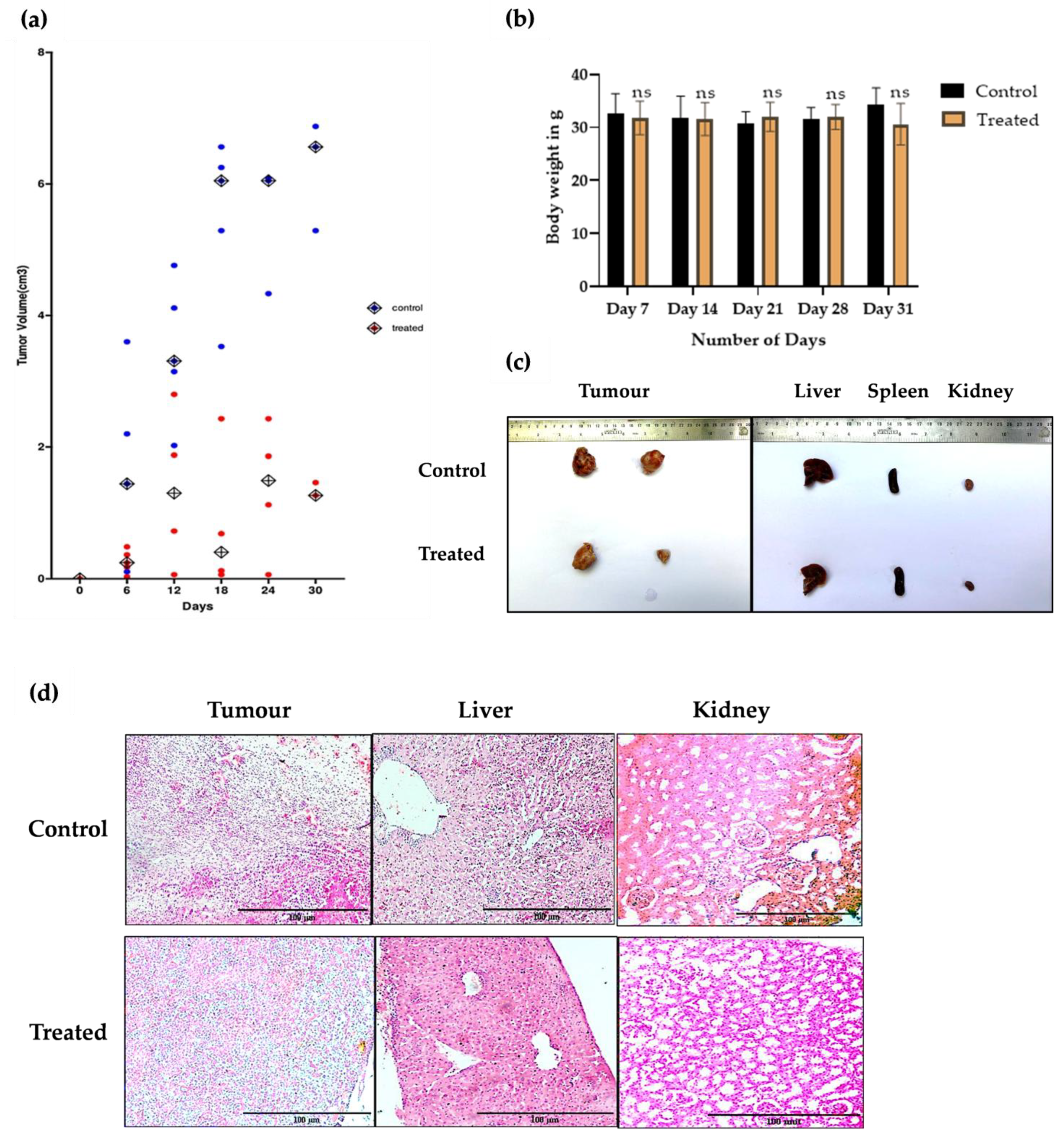
2.3. In Vivo Studies
5i Induced Tumour Regression in DLAMouse Model Without Observable Toxicity
3. Discussion
4. Materials and Methods
4.1. Chemicals and Instruments
4.2. General Procedure for Synthesis of 1-Phenyl-2-(1-(4-substituted phenyl)ethylidene)hydrazine(3a–e)
4.2.1. (E)-1-Phenyl-2-(1-phenylethylidene)hydrazine 3a
4.2.2. (E)-1-(1-(4-Methoxyphenyl)ethylidene)-2-phenylhydrazine 3b
4.2.3. (E)-1-Phenyl-2-(1-(p-tolyl)ethylidene)hydrazine 3c
4.2.4. (E)-1-(1-(4-Nitrophenyl)ethylidene)-2-phenylhydrazine 3d
4.2.5. (E)-1-(1-(4-Chlorophenyl)ethylidene)-2-phenylhydrazine 3e
4.3. General Procedure for the Synthesis of 3-(4-Substituted phenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde (4a–e)
4.3.1. 1,3-Diphenyl-1H-pyrazole-4-carbaldehyde 4a
4.3.2. 3-(4-Methoxyphenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde 4b
4.3.3. 1-Phenyl-3-(p-tolyl)-1H-pyrazole-4-carbaldehyde 4c
4.3.4. 3-(4-Nitrophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde 4d
4.3.5. 3-(4-Chlorophenyl)-1-phenyl-1H-pyrazole-4-carbaldehyde 4e
4.4. General Procedure for Synthesis of 2,6-Bis((3-(4-substituted phenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)ketone (5a–o)
4.4.1. (2E,6E)-2,6-Bis((1,3-diphenyl-1H-pyrazol-4-yl)methylene)cyclohexan-1-one 5a
4.4.2. (2E,6E)-2,6-Bis((3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)methylene) cyclohexan-1-one 5b
4.4.3. (2E,6E)-2,6-Bis((1-phenyl-3-(p-tolyl)-1H-pyrazol-4-yl)methylene)cyclohexan-1-one 5c
4.4.4. (2E,6E)-2,6-Bis((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)cyclohexan-1-one 5d
4.4.5. (2E,6E)-2,6-Bis((3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene) cyclohexan-1-one 5e
4.4.6. (2E,5E)-2,5-Bis((1,3-diphenyl-1H-pyrazol-4-yl)methylene)cyclopentan-1-one 5f
4.4.7. (2E,5E)-2,5-Bis((3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4yl)methylene)cyclopentan-1-one 5g
4.4.8. (2E,5E)-2,5-Bis((1-phenyl-3-(p-tolyl)-1H-pyrazol-4-yl)methylene)cyclopentan-1-one 5h
4.4.9. (2E,5E)-2,5-Bis((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)cyclopentan-1-one 5i
4.4.10. (2E,5E)-2,5-Bis((3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene) cyclopentan-1-one 5j
4.4.11. (2E,7E)-2,7-Bis((1,3-diphenyl-1H-pyrazol-4-yl)methylene)cycloheptan-1-one 5k
4.4.12. (2E,7E)-2,7-Bis((3-(4-methoxyphenyl)-1-phenyl-1H-pyrazol-4-yl)methylene) cycloheptan-1-one 5l
4.4.13. (2E,7E)-2,7-Bis((1-phenyl-3-(p-tolyl)-1H-pyrazol-4-yl)methylene)cycloheptan-1-one 5m
4.4.14. (2E,7E)-2,7-Bis((3-(4-nitrophenyl)-1-phenyl-1H-pyrazol-4yl)methylene)cycloheptan-1-one 5n
4.4.15. (2E,7E)-2,7-Bis((3-(4-chlorophenyl)-1-phenyl-1H-pyrazol-4-yl)methylene)-cycloheptan-1-one 5o
4.5. In Vitro Studies
4.5.1. Cell Lines and Cultures
4.5.2. MTT Assay
4.5.3. Resazurin Assay
4.5.4. Cell Cycle Analysis
4.5.5. Apoptosis Assay
4.5.6. JC-1 Mitochondrial Membrane Potential (ΔΨm) Assay
4.5.7. Western Blotting
4.6. In Vivo Studies
4.6.1. Animals
4.6.2. Investigating the Anticancer Potential of 5i in Mice Models
4.6.3. Histological Evaluation of Tumour and Organs (Haematoxylin and Eosin Staining)
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| SAR | Structure–activity relationship |
| FTIR | Fourier Transform Infrared Spectroscopy |
| NMR | Nuclear Magnetic Resonance |
| HR-MS | High-ResolutionMass Spectrometry |
| MTT | 3-(4,5-Dimethylthiazol-2-yl)-2,5-diphenyltetrazolium bromide |
| IC50 | Half-maximal inhibitory concentration |
| DMSO | Dimethyl sulfoxide |
| CDCl3 | Deuterated chloroform |
| JC1 | 5,5′,6,6′-Tetrachloro-1,1′,3,3′-tetraethylbenzimidazolylcarbocyanine chloride |
| MMP | Mitochondrial membrane potential |
| DLA | Dalton lymphoma ascites |
| GF254 | SILICA GEL GF 254 |
| TLC | Thin Layer Chromatography |
| NCCS | National Centre for Cell Science |
| RPMI | Roswell Park Memorial Institute Medium |
| FBS | Foetal Bovine Serum |
| SDS | Sodium dodecyl sulphate |
| SDS-PAGE | Sodium dodecylsulphate–polyacrylamide gel electrophoresis |
| PVDF | Polyvinylidene fluoride |
| ROS | Reactive oxygen species |
| H&E | Haematoxylin and eosin stain |
References
- Du, M.; Chen, W.; Liu, K.; Wang, L.; Hu, Y.; Mao, Y.; Sun, X.; Luo, Y.; Shi, J.; Shao, K.; et al. The Global Burden of Leukemia and Its Attributable Factors in 204 Countries and Territories: Findings from the Global Burden of Disease 2019 Study and Projections to 2030. J. Oncol. 2022, 2022, 1612702. [Google Scholar] [CrossRef] [PubMed]
- Major Cancer Types by Deaths Worldwide 2020. Available online: https://www.statista.com/statistics/288580/number-of-cancer-deaths-worldwide-by-type/ (accessed on 18 May 2024).
- Gunz, F.W.; Hough, R.F. Acute Leukemia Over the Age of Fifty: A Study of Its Incidence and Natural History. Blood 1956, 11, 882–901. [Google Scholar] [PubMed]
- Tebbi, C.K. Etiology of Acute Leukemia: A Review. Cancers 2021, 13, 2256. [Google Scholar] [CrossRef]
- Othman, E.M.; Fayed, E.A.; Husseiny, E.M.; Abulkhair, H.S. Apoptosis Induction, PARP-1 Inhibition, and Cell Cycle Analysis of Leukemia Cancer Cells Treated with Novel Synthetic 1,2,3-Triazole-Chalcone Conjugates. Bioorg. Chem. 2022, 123, 105762. [Google Scholar]
- Mazumder, K.; Aktar, A.; Roy, P.; Biswas, B.; Hossain, M.d.E.; Sarkar, K.K.; Bachar, S.C.; Ahmed, F.; Monjur-Al-Hossain, A.S.M.; Fukase, K. A Review on Mechanistic Insight of Plant Derived Anticancer Bioactive Phytocompounds and Their Structure Activity Relationship. Molecules 2022, 27, 3036. [Google Scholar] [CrossRef]
- Kunnumakkara, A.B.; Hegde, M.; Parama, D.; Girisa, S.; Kumar, A.; Daimary, U.D.; Garodia, P.; Yenisetti, S.C.; Oommen, O.V.; Aggarwal, B.B. Role of Turmeric and Curcumin in Prevention and Treatment of Chronic Diseases: Lessons Learned from Clinical Trials. ACS Pharmacol. Transl. Sci. 2023, 6, 447–518. [Google Scholar]
- Jacob, S.; Kather, F.; Morsy, M.; Boddu, S.; Attimarad, M.; Shah, J.; Shinu, P.; Nair, A. Advances in Nanocarrier Systems for Overcoming Formulation Challenges of Curcumin: Current Insights. Nanomaterials 2024, 14, 672. [Google Scholar] [CrossRef]
- Wilken, R.; Veena, M.S.; Wang, M.B.; Srivatsan, E.S. Curcumin: A Review of Anti-Cancer Properties and Therapeutic Activity in Head and Neck Squamous Cell Carcinoma. Mol. Cancer 2011, 10, 12. [Google Scholar] [CrossRef]
- Nishimura, F.G.; Sampaio, B.B.; do Couto, G.O.; da Silva, A.D.; da Silva, W.J.; Peronni, K.C.; Evangelista, A.F.; Hossain, M.; Dimmock, J.R.; Bandy, B.; et al. The Transcriptome of BT-20 Breast Cancer Cells Exposed to Curcumin Analog NC2603 Reveals a Relationship between EGR3 Gene Modulation and Cell Migration Inhibition. Molecules 2024, 29, 1366. [Google Scholar] [CrossRef]
- MaruYama, T.; Yamakoshi, H.; Iwabuchi, Y.; Shibata, H. Mono-Carbonyl Curcumin Analogs for Cancer Therapy. Biol. Pharm. Bull. 2023, 46, 756–763. [Google Scholar]
- Srivastava, S.; Mishra, S.; Surolia, A.; Panda, D. C1, a Highly Potent Novel Curcumin Derivative, Binds to Tubulin, Disrupts Microtubule Network and Induces Apoptosis. Biosci. Rep. 2016, 36, e00323. [Google Scholar]
- Kaur, K.; Al-Khazaleh, A.K.; Bhuyan, D.J.; Li, F.; Li, C.G. A Review of Recent Curcumin Analogues and Their Antioxidant, Anti-Inflammatory and Anticancer Activities. Antioxidants 2024, 13, 1092. [Google Scholar] [CrossRef] [PubMed]
- Jha, N.S.; Mishra, S.; Jha, S.K.; Surolia, A. Antioxidant Activity and Electrochemical Elucidation of the Enigmatic Redox Behavior of Curcumin and Its Structurally Modified Analogues. Electrochim. Acta 2015, 151, 574–583. [Google Scholar]
- Chakraborti, S.; Dhar, G.; Dwivedi, V.; Das, A.; Poddar, A.; Chakraborti, G.; Basu, G.; Chakrabarti, P.; Surolia, A.; Bhattacharyya, B. Stable and Potent Analogues Derived from the Modification of the Dicarbonyl Moiety of Curcumin. Biochemistry 2013, 52, 7449–7460. [Google Scholar]
- Marchiani, A.; Rozzo, C.; Fadda, A.; Delogu, G.; Ruzza, P. Curcumin and Curcumin-like Molecules: From Spice to Drugs. Curr. Med. Chem. 2013, 21, 204–222. [Google Scholar] [CrossRef]
- Xu, F.; Chen, M.; Chen, H.; Wu, N.; Qi, Q.; Jiang, X.; Fang, D.; Feng, Q.; Jin, R.; Jiang, L. The Curcumin Analog Da0324 Inhibits the Proliferation of Gastric Cancer Cells Via HOTAIRM1/MiR-29b-1-5p/PHLPP1 Axis. J. Cancer 2022, 13, 2644–2655. [Google Scholar] [CrossRef]
- Liang, G.; Shao, L.; Wang, Y.; Zhao, C.; Chu, Y.; Xiao, J.; Zhao, Y.; Li, X.; Yang, S. Exploration and Synthesis of Curcumin Analogues with Improved Structural Stability Both in vitro and in vivo as Cytotoxic Agents. Bioorg. Med. Chem. 2009, 17, 2623–2631. [Google Scholar]
- Ramana Reddy, P.V.; Shivakumar, E.; Ramachandran, D. Design, Synthesis and Anticancer Evaluation of Substituted Aryl-1,3-Oxazole Incorporated Pyrazole-Thiazole Derivatives as Anticancer Agents. Chem. Data Coll. 2024, 51, 101127. [Google Scholar]
- Edukondalu, P.; Sireesha, R.; Kavuluri, P.; Suresh, P.; Rao, C.; Chandrasekhar, C.; Raju, R.R. Design, Synthesis and Biological Evaluation of Sulfonamide Derivatives of Benzothiazol-Quinoline-Pyrazoles as Anticancer Agents. Chem. Data Coll. 2024, 51, 101136. [Google Scholar]
- Lusardi, M.; Signorello, M.G.; Russo, E.; Caviglia, D.; Ponassi, M.; Iervasi, E.; Rosano, C.; Brullo, C.; Spallarossa, A. Structure–Activity Relationship Studies on Highly Functionalized Pyrazole Hydrazones and Amides as Antiproliferative and Antioxidant Agents. Int. J. Mol. Sci. 2024, 25, 4607. [Google Scholar] [CrossRef]
- Boshta, N.M.; Temirak, A.; El-Shahid, Z.A.; Shafiq, Z.; Ahmed Soliman, A.F. Design, Synthesis, Molecular Docking and Biological Evaluation of 1,3,5-Trisubstituted-1H-Pyrazole Derivatives as Anticancer Agents with Cell Cycle Arrest, ERK and RIPK3- Kinase Activities. Bioorg. Chem. 2024, 143, 107058. [Google Scholar]
- Kachhot, K.D.; Vaghela, F.H.; Dhamal, C.H.; Vegal, N.K.; Bhatt, T.D.; Joshi, H.S. Water-Promoted Synthesis of Pyrazole-Thiazole-Derivatives as Potent Antioxidants and Their Anti-Cancer Activity: ADMET and SAR Studies. ChemistrySelect 2024, 9, e202303521. [Google Scholar]
- Zhang, L.; Li, C. Eco-Friendly Green Synthesis of N-Pyrazole Amino Chitosan Using PEG-400 as an Anticancer Agent against Gastric Cancer Cells via Inhibiting EGFR. In Vitro Cell Dev. Biol. Anim. 2024, 60, 365–373. [Google Scholar] [PubMed]
- Husseiny, E.M.; Hamada, S.A.; El-Dydamony, N.M.; Anwer, K.E. Exploring the Cytotoxic Effect and CDK-9 Inhibition Potential of Novel Sulfaguanidine-Based Azopyrazolidine-3,5-Diones and 3,5-Diaminoazopyrazoles. Bioorg. Chem. 2023, 133, 106397. [Google Scholar]
- Malebari, A.M.; Ahmed, H.E.A.; Ihmaid, S.K.; Omar, A.M.; Muhammad, Y.A.; Althagfan, S.S.; Aljuhani, N.; El-Sayed, A.-A.A.A.; Halawa, A.H.; El-Tahir, H.M.; et al. Exploring the Dual Effect of Novel 1,4-Diarylpyranopyrazoles as Antiviral and Anti-Inflammatory for the Management of SARS-CoV-2 and Associated Inflammatory Symptoms. Bioorg. Chem. 2023, 130, 106255. [Google Scholar]
- Noureddin, S.A.; El-Shishtawy, R.M.; Al-Footy, K.O. Curcumin Analogues and Their Hybrid Molecules as Multifunctional Drugs. Eur. J. Med. Chem. 2019, 182, 111631. [Google Scholar]
- Othman, E.M.; Fayed, E.A.; Husseiny, E.M.; Abulkhair, H.S. The Effect of Novel Synthetic Semicarbazone- and Thiosemicarbazone-Linked 1,2,3-Triazoles on the Apoptotic Markers, VEGFR-2, and Cell Cycle of Myeloid Leukemia. Bioorg. Chem. 2022, 127, 105968. [Google Scholar]
- Pandey, M.K.; Kumar, S.; Thimmulappa, R.K.; Parmar, V.S.; Biswal, S.; Watterson, A.C. Design, Synthesis and Evaluation of Novel PEGylated Curcumin Analogs as Potent Nrf2 Activators in Human Bronchial Epithelial Cells. Eur. J. Pharm. Sci. 2011, 43, 16–24. [Google Scholar]
- Murwanti, R.; Rahmadani, A.; Ritmaleni, R.; Hermawan, A.; Sudarmanto, A.B.S. Curcumin Analogs Induce Apoptosis and G2/M Arrest in 4T1 Murine Triple-Negative Breast Cancer Cells. Indones. J. Pharm. 2020, 31, 11–18. [Google Scholar]
- Yeap, S.K.; Mohd Ali, N.; Akhtar, M.N.; Razak, N.A.; Chong, Z.X.; Ho, W.Y.; Boo, L.; Zareen, S.; Kurniawan, T.A.; Avtar, R.; et al. Induction of Apoptosis and Regulation of MicroRNA Expression by (2E,6E)-2,6-Bis-(4-Hydroxy-3-Methoxybenzylidene)-Cyclohexanone (BHMC) Treatment on MCF-7 Breast Cancer Cells. Molecules 2021, 26, 1277. [Google Scholar] [CrossRef]
- Gan, X.; Wu, Y.; Zhu, M.; Liu, B.; Kong, M.; Xi, Z.; Li, K.; Wang, H.; Su, T.; Yao, J.; et al. Design, Synthesis, and Evaluation of Cyclic C7-Bridged Monocarbonyl Curcumin Analogs Containing an O-Methoxy Phenyl Group as Potential Agents against Gastric Cancer. J. Enzyme Inhib. Med. Chem. 2024, 39, 2314233. [Google Scholar] [PubMed]
- Wang, L.; Wang, C.; Tao, Z.; Zhao, L.; Zhu, Z.H.; Wu, W.; He, Y.; Chen, H.; Zheng, B.; Huang, X.; et al. Curcumin Derivative WZ35 Inhibits Tumor Cell Growth via ROS-YAP-JNK Signaling Pathway in Breast Cancer. J. Exp. Clin. Cancer Res. 2019, 38, 460. [Google Scholar] [PubMed]
- Zhang, X.; Zhang, H.Q.; Zhu, G.H.; Wang, Y.H.; Yu, X.C.; Zhu, X.B.; Liang, G.; Xiao, J.; Li, X.K. A Novel Mono-Carbonyl Analogue of Curcumin Induces Apoptosis in Ovarian Carcinoma Cells via Endoplasmic Reticulum Stress and Reactive Oxygen Species Production. Mol. Med. Rep. 2012, 5, 739–744. [Google Scholar] [PubMed]
- Manohar, S.; Khan, S.I.; Kandi, S.K.; Raj, K.; Sun, G.; Yang, X.; Calderon Molina, A.D.; Ni, N.; Wang, B.; Rawat, D.S. Synthesis, Antimalarial Activity and Cytotoxic Potential of New Monocarbonyl Analogues of Curcumin. Bioorg. Med. Chem. Lett. 2013, 23, 112–116. [Google Scholar]
- Adams, B.K.; Ferstl, E.M.; Davis, M.C.; Herold, M.; Kurtkaya, S.; Camalier, R.F.; Hollingshead, M.G.; Kaur, G.; Sausville, E.A.; Rickles, F.R.; et al. Synthesis and Biological Evaluation of Novel Curcumin Analogs as Anti-Cancer and Anti-Angiogenesis Agents. Bioorg. Med. Chem. 2004, 12, 3871–3883. [Google Scholar]
- Wang, J.; Qi, L.; Zheng, S.; Wu, T. Curcumin Induces Apoptosis through the Mitochondria-Mediated Apoptotic Pathway in HT-29 Cells. J. Zhejiang Univ. Sci. B 2009, 10, 93–102. [Google Scholar]
- Ahmed, M.; Aatif, M.; Muteeb, G.; Alam, M.W.; El Oirdi, M.; Farhan, M. Curcumin and Its Derivatives Induce Apoptosis in Human Cancer Cells by Mobilizing and Redox Cycling Genomic Copper Ions. Molecules 2022, 27, 7410. [Google Scholar] [CrossRef]
- Wang, H.; Xu, Y.; Sun, J.; Sui, Z. The Novel Curcumin Derivative 1g Induces Mitochondrial and ER-Stress-Dependent Apoptosis in Colon Cancer Cells by Induction of ROS Production. Front. Oncol. 2021, 11, 644197. [Google Scholar]
- Bleicken, S.; Classen, M.; Pulagam, V.L.P.; Ishikawa, T.; Zeth, K.; Steinhoff, H.-J.; Bordignon, E. Molecular Details of Bax Activation, Oligomerization, and Membrane Insertion. J. Biol. Chem. 2010, 285, 6636–6647. [Google Scholar]
- Ali, I.; Haque, A.; Saleem, K.; Hsieh, M.F. Curcumin-I Knoevenagel’s Condensates and Their Schiff’s Bases as Anticancer Agents: Synthesis, Pharmacological and Simulation Studies. Bioorg. Med. Chem. 2013, 21, 3808–3820. [Google Scholar]
- Zhang, M.; Shang, Z.-R.; Li, X.-T.; Zhang, J.-N.; Wang, Y.; Li, K.; Li, Y.-Y.; Zhang, Z.-H. Simple and Efficient Approach for Synthesis of Hydrazones from Carbonyl Compounds and Hydrazides Catalyzed by Meglumine. Synth. Commun. 2016, 47, 178–187. [Google Scholar] [CrossRef]
- Hajipour, A.R.; Mohammadpoor-Baltork, I.; Bigdeli, M. A Convenient and Mild Procedure for the Synthesis of Hydrazones and Semicarbazones from Aldehydes or Ketones under Solvent-Free Conditions. J. Chem. Res. Synop. 1999, 9, 570–571. [Google Scholar]
- Rector, D.L.; Folz, S.D.; Conklin, R.D.; Nowakowski, L.H.; Kaugars, G. Structure-Activity Relationships in a Broad-Spectrum Anthelmintic Series. Acid Chloride Phenylhydrazones. I. Aryl Substitutions and Chloride Variations. J. Med. Chem. 1981, 24, 532–538. [Google Scholar] [CrossRef] [PubMed]
- Vora, J.J.; Vasava, S.B.; Parmar, K.C.; Chauhan, S.; Sharma, S. Synthesis, Spectral and Microbial Studies of Some Novel Schiff Base Derivatives of 4-Methylpyridin-2-Amine. E-J. Chem. 2009, 6, 1205–1210. [Google Scholar] [CrossRef]
- Prakash, O.; Pannu, K.; Kumar, A. Synthesis of Some New 2-(3-Aryl-1-Phenyl-4-Pyrazolyl)-Benzoxazoles Using Hypervalent Iodine Mediated Oxidative Cyclization of Schiff’s Bases. Molecules 2006, 11, 43–48. [Google Scholar] [CrossRef]
- Koroth, J.; Nirgude, S.; Tiwari, S.; Gopalakrishnan, V.; Mahadeva, R.; Kumar, S.; Karki, S.S.; Choudhary, B. Investigation of Anti-Cancer and Migrastatic Properties of Novel Curcumin Derivatives on Breast and Ovarian Cancer Cell Lines. BMC Complement. Altern. Med. 2019, 19, 273. [Google Scholar] [CrossRef]
- Murugesan, K.; Koroth, J.; Srinivasan, P.P.; Singh, A.; Mukundan, S.; Karki, S.S.; Choudhary, B.; Gupta, C.M. Effects of Green Synthesized Silver Nanoparticles (ST06-AgNPs) Using Curcumin Derivative (ST06) on Human Cervical Cancer Cells (HeLa) in Vitro and EAC Tumor Bearing Mice Models. Int. J. Nanomed. 2019, 14, 5257–5270. [Google Scholar] [CrossRef]
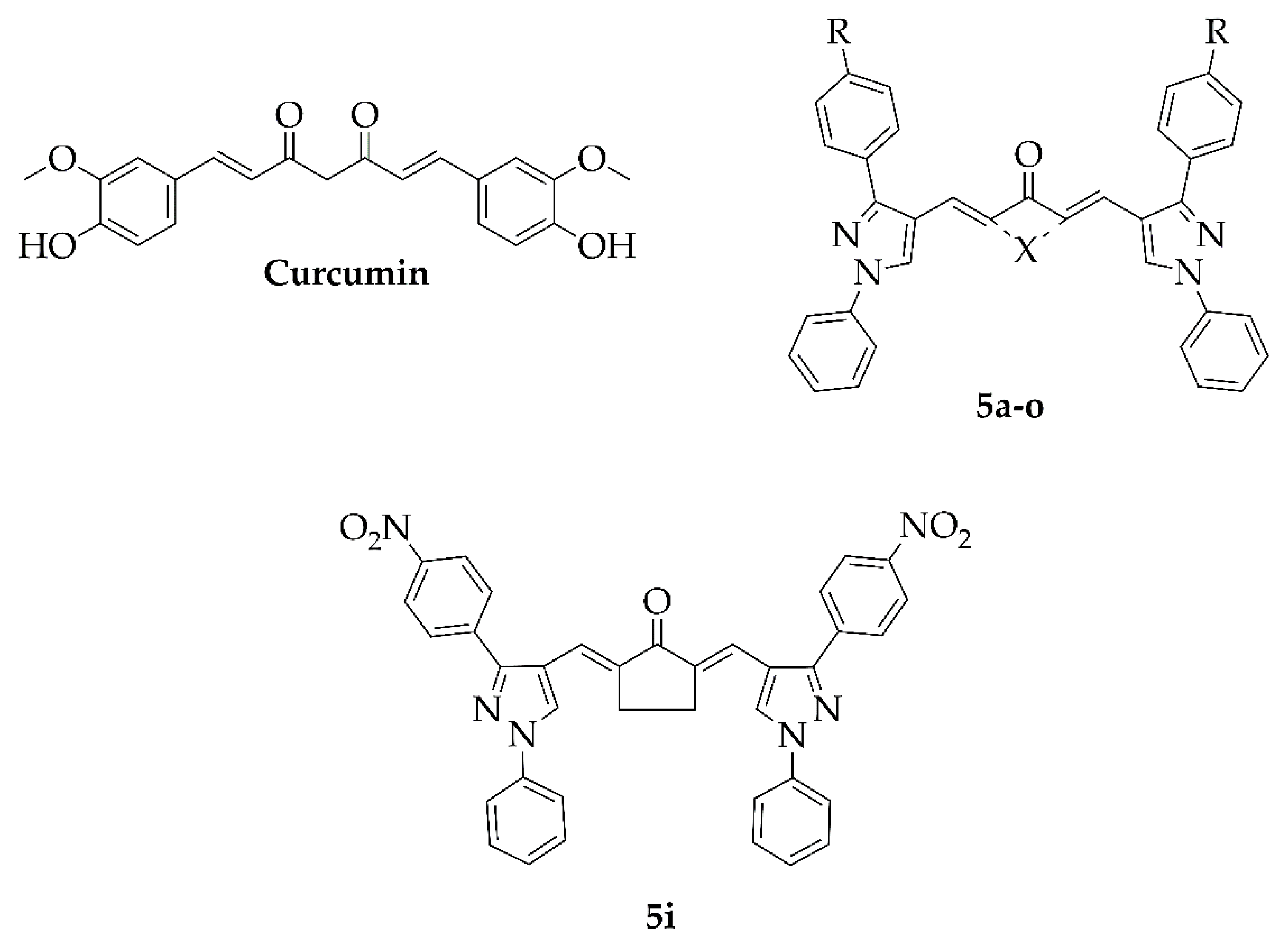
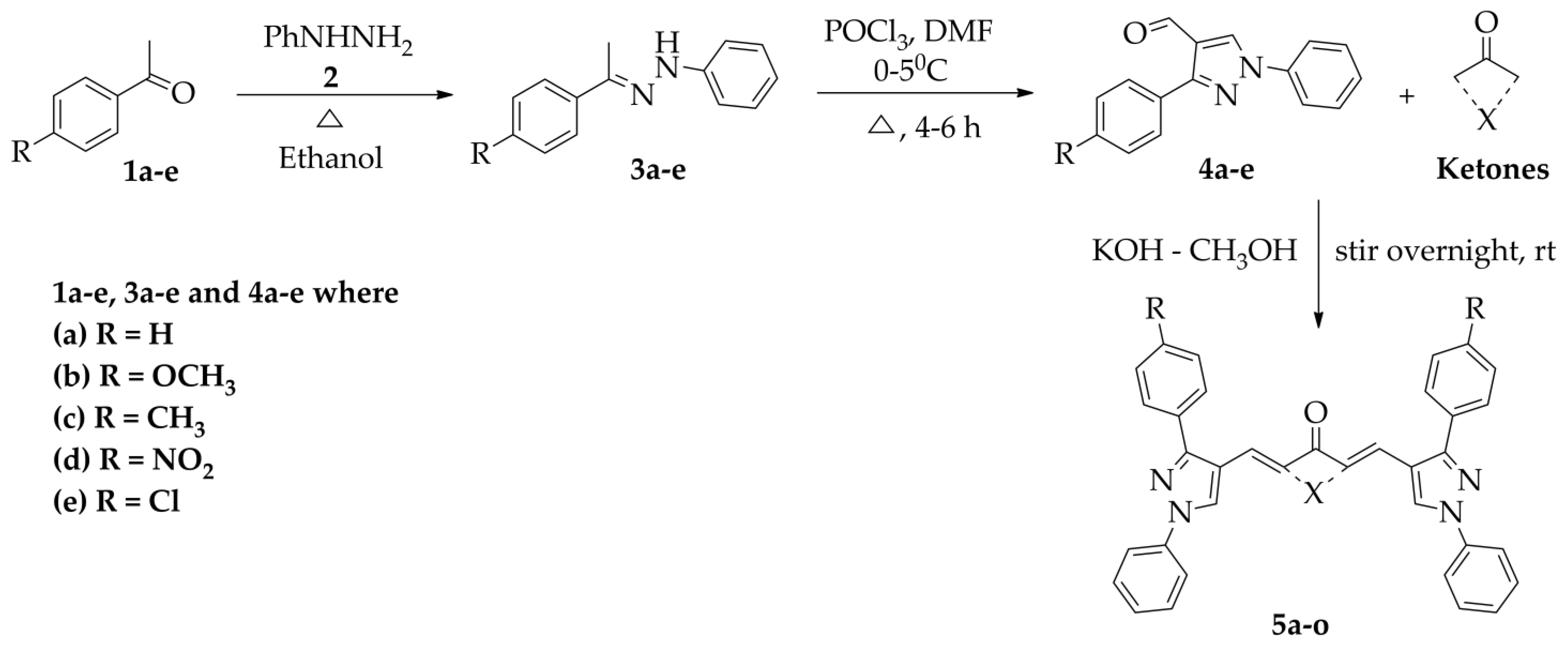
| Code | R | X |
|---|---|---|
| 5a | H | -(CH2)3- |
| 5b | OCH3 | -(CH2)3- |
| 5c | CH3 | -(CH2)3- |
| 5d | NO2 | -(CH2)3- |
| 5e | Cl | -(CH2)3- |
| 5f | H | -(CH2)2- |
| 5g | OCH3 | -(CH2)2- |
| 5h | CH3 | -(CH2)2- |
| 5i | NO2 | -(CH2)2- |
| 5j | Cl | -(CH2)2- |
| 5k | H | -(CH2)4- |
| 5l | OCH3 | -(CH2)4- |
| 5m | CH3 | -(CH2)4- |
| 5n | NO2 | -(CH2)4- |
| 5o | Cl | -(CH2)4- |
| Compound | IC50 (µM) |
|---|---|
| 5a | 34.36 ± 3.24 |
| 5b | 134.59 ± 1.55 |
| 5c | >100 |
| 5d | 36.39 ± 1.78 |
| 5e | >100 |
| 5f | 0.92 ± 0.00 |
| 5g | 12.44 ± 0.44 |
| 5h | 49.98 ± 10.77 |
| 5i | 0.10 ± 0.01 |
| 5j | 11.91 ± 1.48 |
| 5k | 0.58 ± 0.00 |
| 5l | 16.15 ± 0.31 |
| 5m | 46.24 ± 4.18 |
| 5n | 8.97 ± 2.62 |
| 5o | 9.23 ± 0.04 |
| Curcumin | 61.66 ± 2.26 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sudarshan, V.; Shyamjith, P.; Kumar, S.; Ravindran, F.; Choudhary, B.; Karki, S.S. Investigation of Anti-Cancer Properties of Novel Curcuminoids in Leukemic Cells and Dalton Lymphoma Ascites Model. Int. J. Mol. Sci. 2025, 26, 3186. https://doi.org/10.3390/ijms26073186
Sudarshan V, Shyamjith P, Kumar S, Ravindran F, Choudhary B, Karki SS. Investigation of Anti-Cancer Properties of Novel Curcuminoids in Leukemic Cells and Dalton Lymphoma Ascites Model. International Journal of Molecular Sciences. 2025; 26(7):3186. https://doi.org/10.3390/ijms26073186
Chicago/Turabian StyleSudarshan, Vijayalakshmi, P. Shyamjith, Sujeet Kumar, Febina Ravindran, Bibha Choudhary, and Subhas S. Karki. 2025. "Investigation of Anti-Cancer Properties of Novel Curcuminoids in Leukemic Cells and Dalton Lymphoma Ascites Model" International Journal of Molecular Sciences 26, no. 7: 3186. https://doi.org/10.3390/ijms26073186
APA StyleSudarshan, V., Shyamjith, P., Kumar, S., Ravindran, F., Choudhary, B., & Karki, S. S. (2025). Investigation of Anti-Cancer Properties of Novel Curcuminoids in Leukemic Cells and Dalton Lymphoma Ascites Model. International Journal of Molecular Sciences, 26(7), 3186. https://doi.org/10.3390/ijms26073186








