D-M159 Synergistically Induces Apoptosis in HeLa Cells Through Endoplasmic Reticulum Stress and Mitochondrial Dysfunction
Abstract
1. Introduction
2. Results
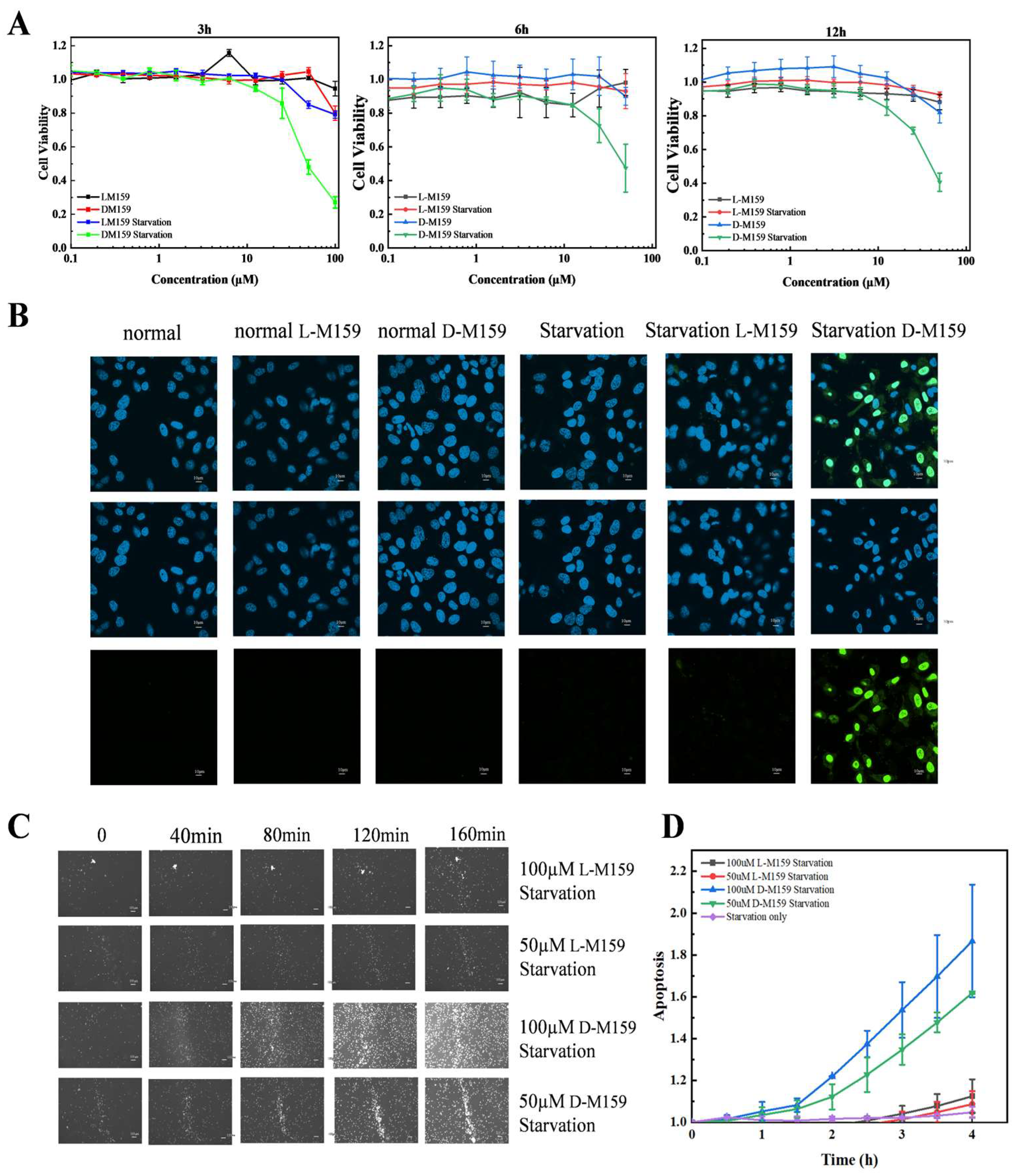
2.1. D-M159 Combined Starvation Treatment Induces Cell Death
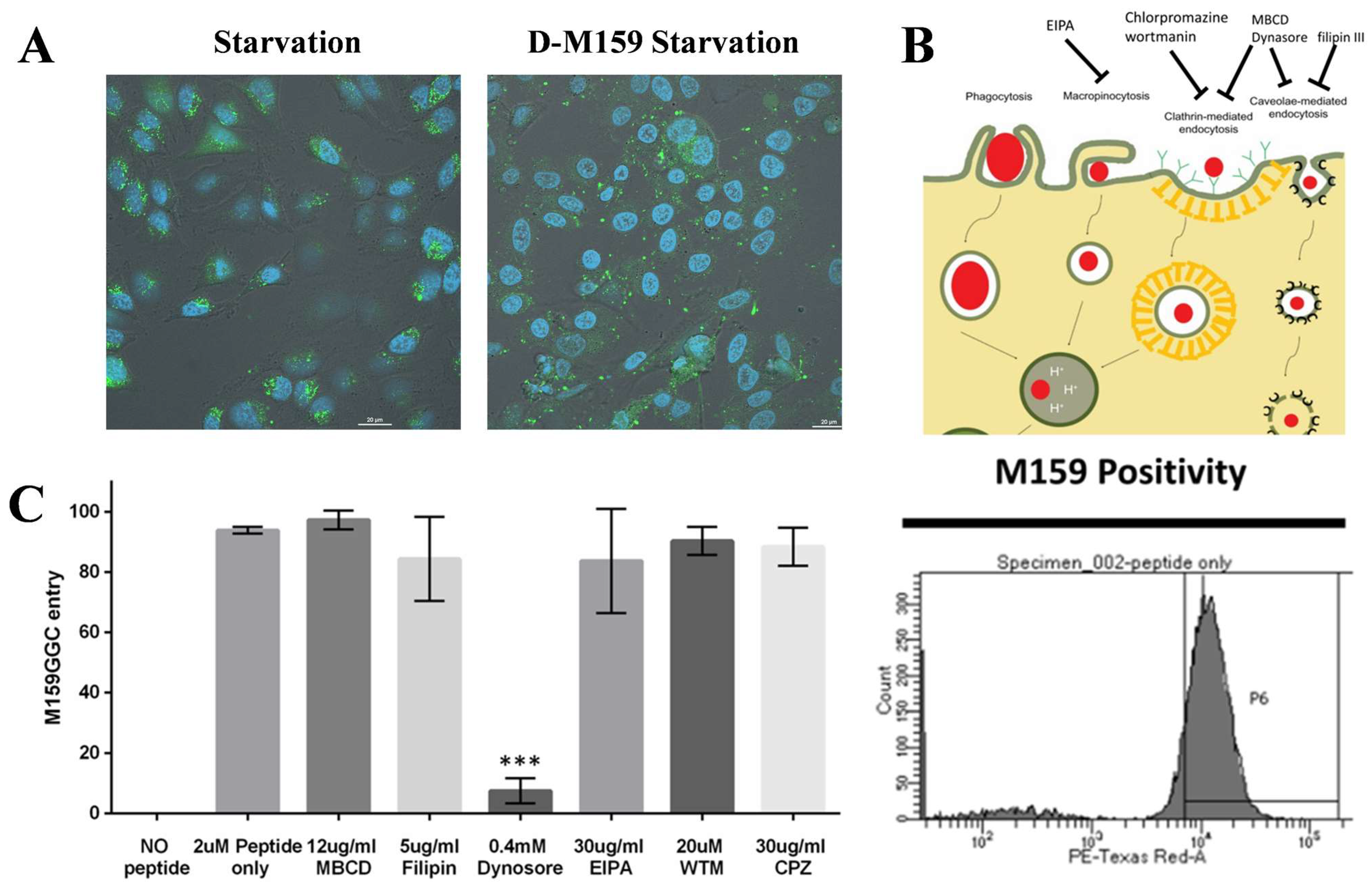
2.2. D-M159 Enters HeLa Cells by Specific Endocytosis
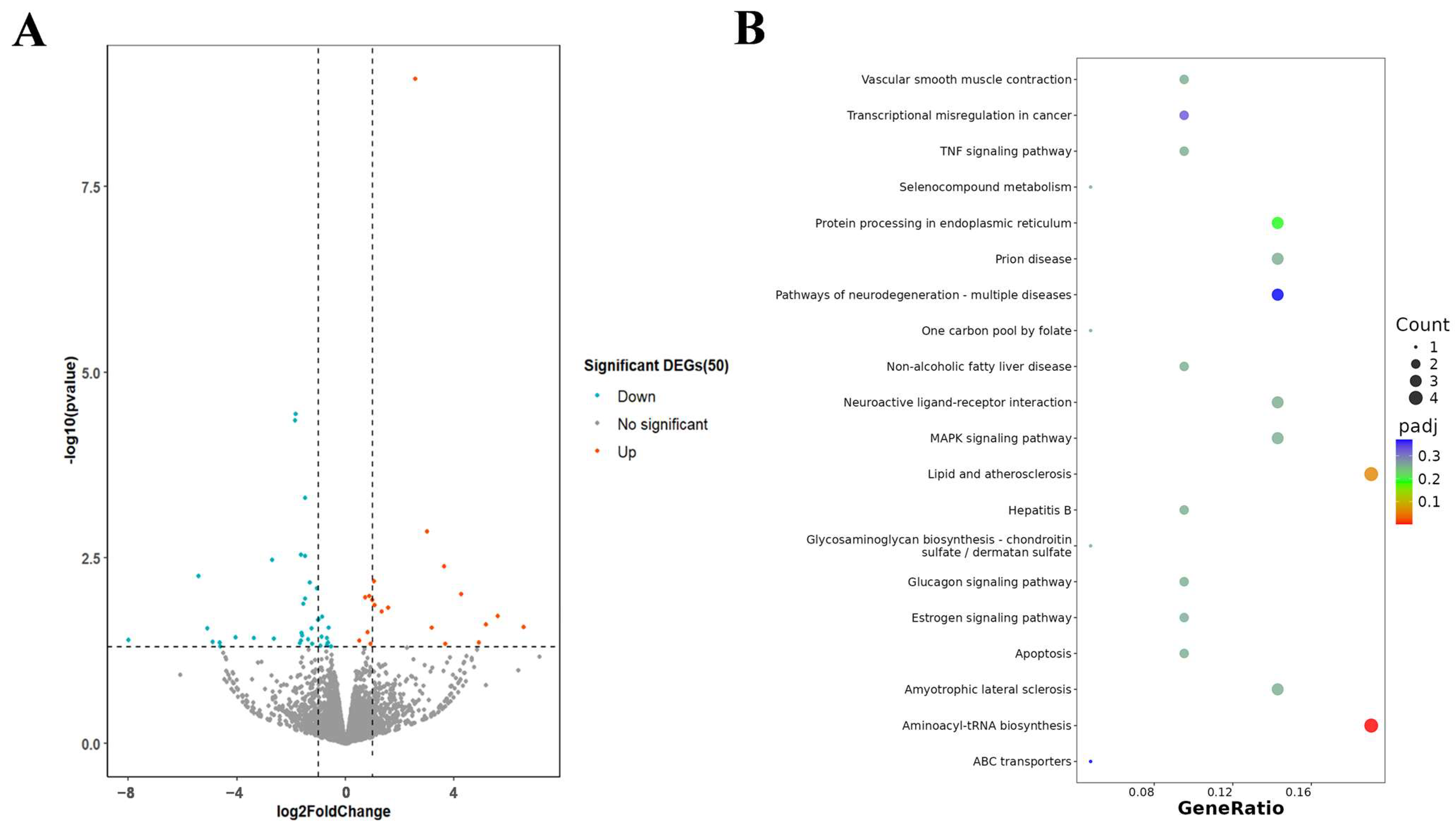
2.3. Transcriptome Analysis of HeLa After Exposure to D-MI59 and Starvation
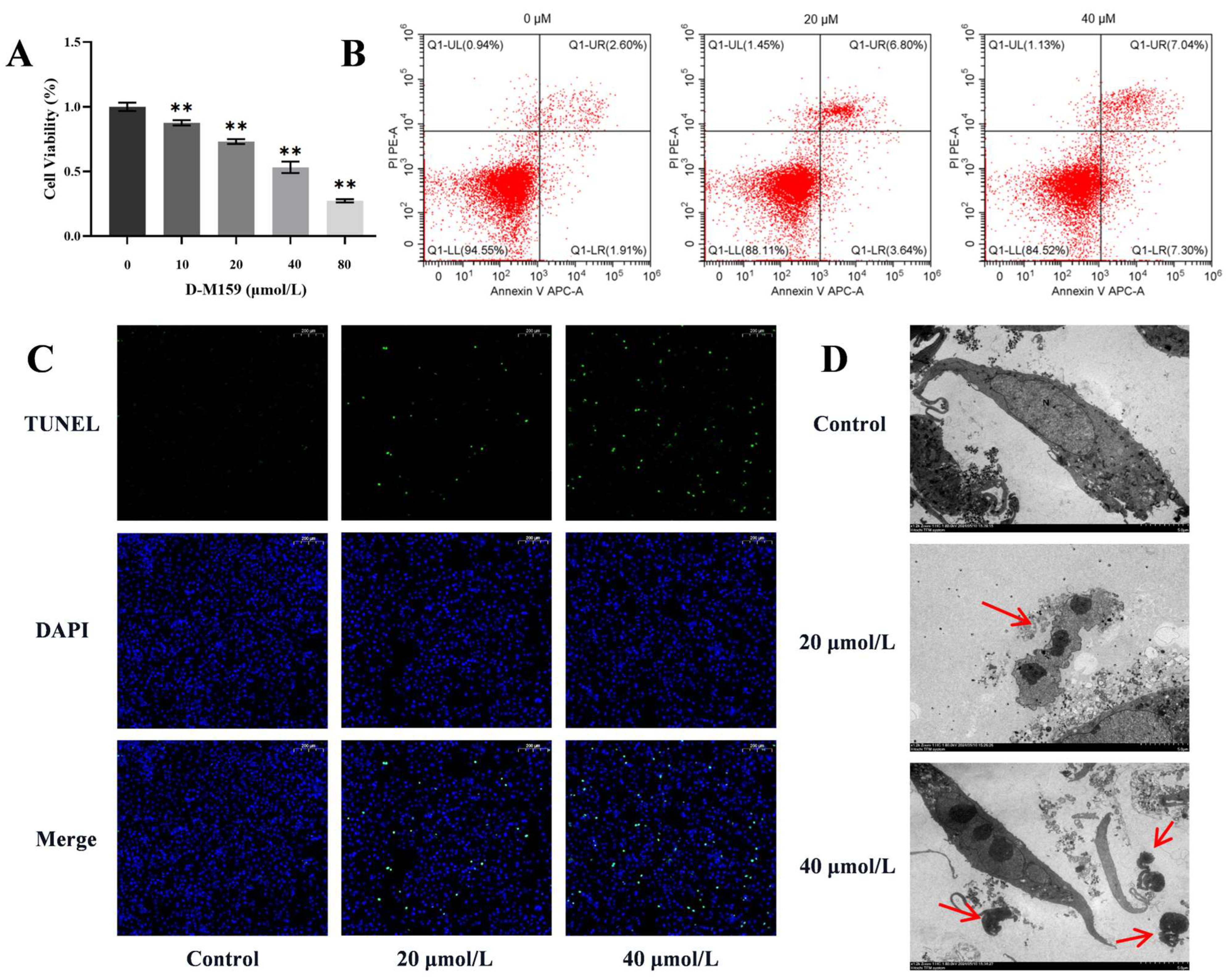
2.4. D-M159 Combined Starvation Treatment Induces Cell Apoptosis
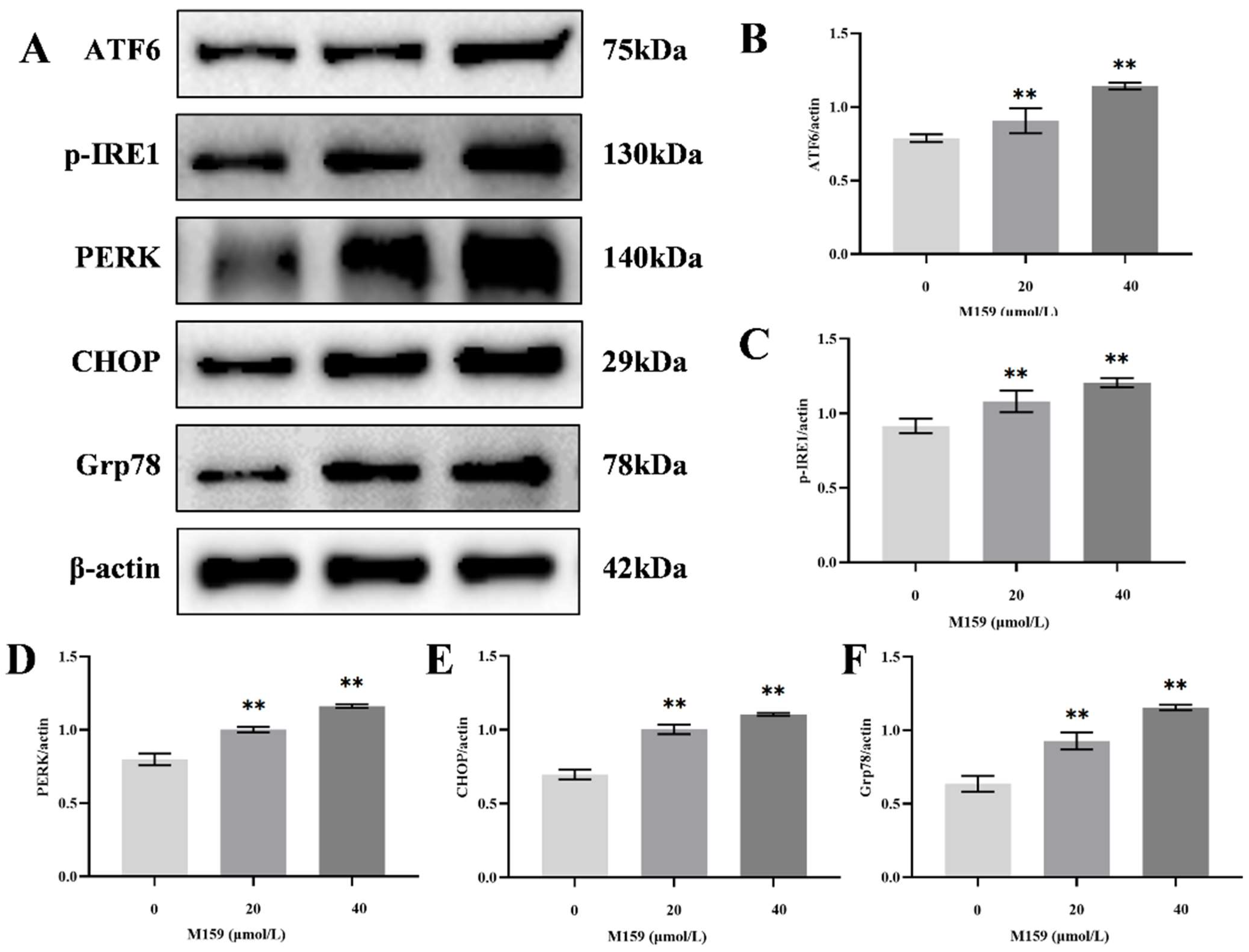
2.5. D-M159 Combined with Starvation Treatment Induces Endoplasmic Reticulum Stress in HeLa Cells
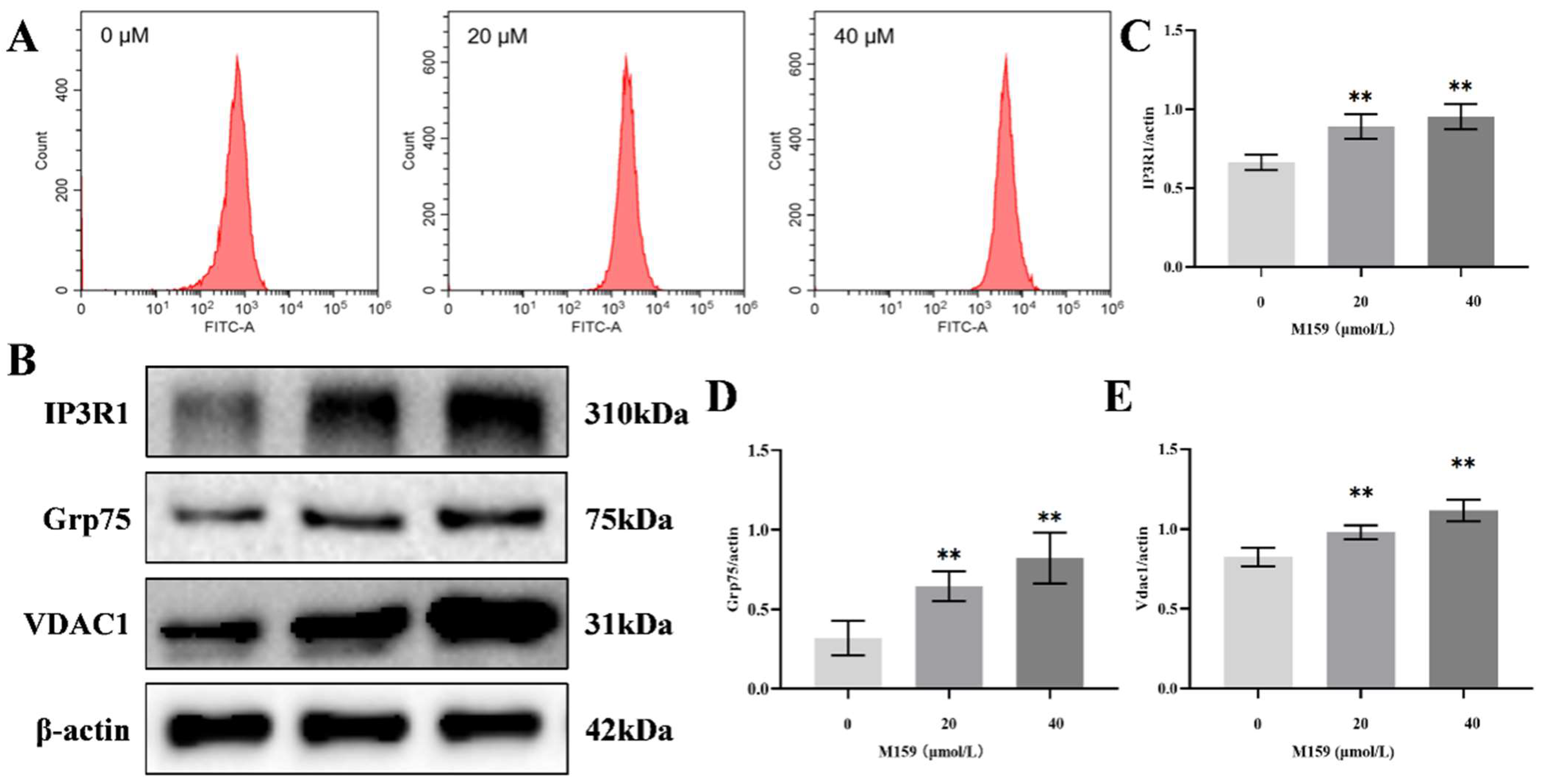
2.6. D-M159 Combined with Starvation Treatment Disrupts Intracellular Calcium Homeostasis in HeLa Cells
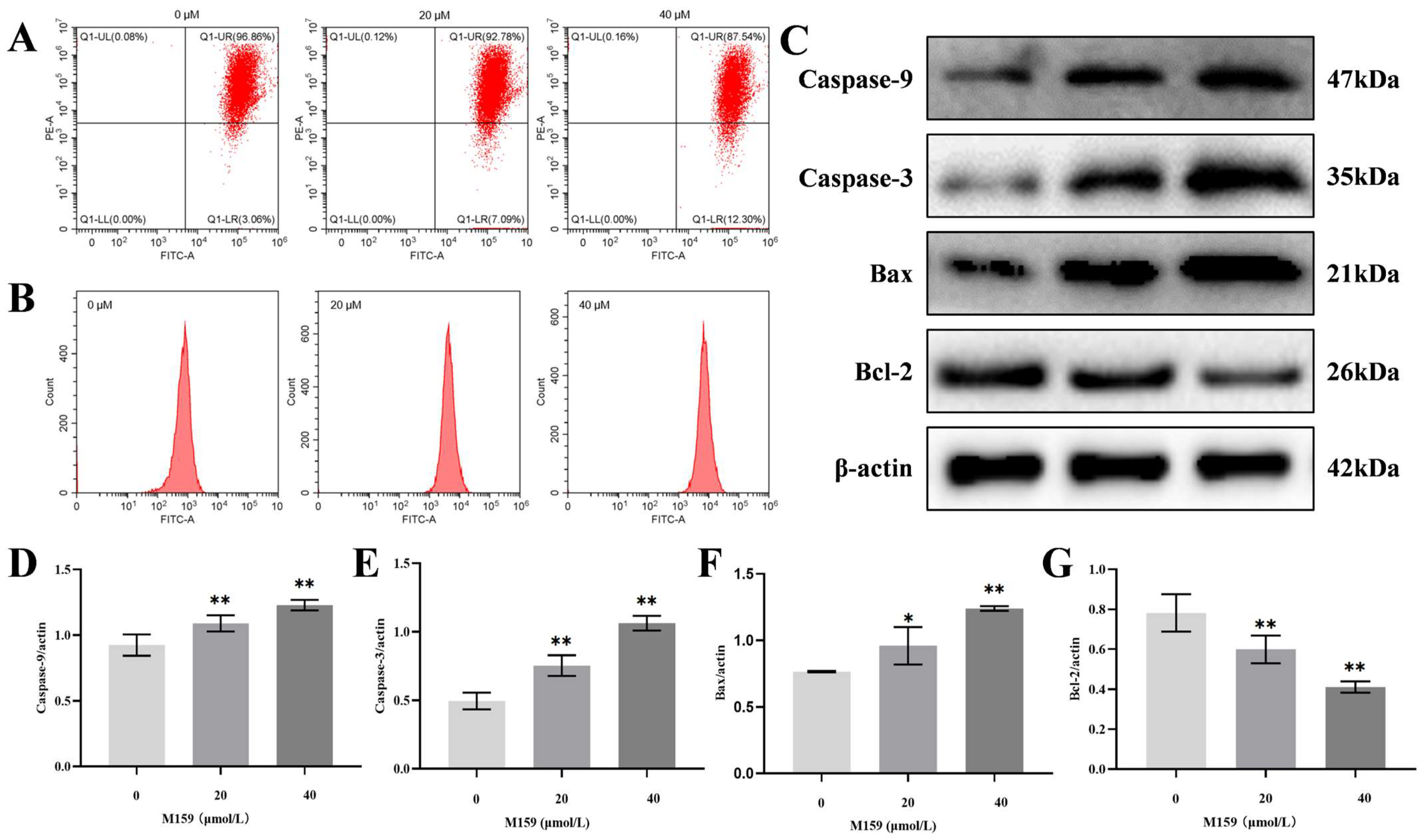
2.7. D-M159 Co-Starvation Treatment Induces Mitochondrial Pathway Apoptosis in HeLa Cells
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Cell Culture
4.3. Alamar Blue Cytotoxicity Assay
4.4. CCK-8 Cell Viability Assay
4.5. Caspase-3 Activity Assay
4.6. Detection of Apoptosis by Flow Cytometry
4.7. TUNEL Staining for Apoptosis Detection
4.8. Transmission Electron Microscopy (TEM)
4.9. Dextran Delivery
4.10. D-M159-TAMRA Conjugation
4.11. Endosomal Pathway Determination Using Inhibitors
4.12. RNA Sequencing
4.13. Western Blot Analysis
4.14. ROS Analysis
4.15. Mitochondrial Membrane Potential (ΔΨm) Measurement
4.16. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Deb, R.; Torres, M.D.T.; Boudný, M.; Koběrská, M.; Cappiello, F.; Popper, M.; Dvořáková Bendová, K.; Drabinová, M.; Hanáčková, A.; Jeannot, K.; et al. Computational Design of Pore-Forming Peptides with Potent Antimicrobial and Anticancer Activities. J. Med. Chem. 2024, 67, 14040–14061. [Google Scholar] [CrossRef]
- Devant, P.; Kagan, J.C. Molecular Mechanisms of Gasdermin D Pore-Forming Activity. Nat. Immunol. 2023, 24, 1064–1075. [Google Scholar] [CrossRef]
- Qu, B.; Yuan, J.; Liu, X.; Zhang, S.; Ma, X.; Lu, L. Anticancer Activities of Natural Antimicrobial Peptides from Animals. Front. Microbiol. 2024, 14, 1321386. [Google Scholar] [CrossRef]
- Zasloff, M. Antimicrobial Peptides of Multicellular Organisms. Nature 2002, 415, 389–395. [Google Scholar] [CrossRef] [PubMed]
- Deslouches, B.; Di, Y.P. Antimicrobial Peptides with Selective Antitumor Mechanisms: Prospect for Anticancer Applications. Oncotarget 2017, 8, 46635–46651. [Google Scholar] [CrossRef]
- Zhang, Q.-Y.; Yan, Z.-B.; Meng, Y.-M.; Hong, X.-Y.; Shao, G.; Ma, J.-J.; Cheng, X.-R.; Liu, J.; Kang, J.; Fu, C.-Y. Antimicrobial Peptides: Mechanism of Action, Activity and Clinical Potential. Mil. Med. Res. 2021, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Lyu, C.; Fang, F.; Li, B. Anti-Tumor Effects of Melittin and Its Potential Applications in Clinic. Curr. Protein Pept. Sci. 2019, 20, 240–250. [Google Scholar] [CrossRef]
- Xu, P.; Lv, D.; Wang, X.; Wang, Y.; Hou, C.; Gao, K.; Guo, X. Inhibitory Effects of Bombyx Mori Antimicrobial Peptide Cecropins on Esophageal Cancer Cells. Eur. J. Pharmacol. 2020, 887, 173434. [Google Scholar] [CrossRef]
- Bankell, E.; Liu, X.; Lundqvist, M.; Svensson, D.; Swärd, K.; Sparr, E.; Nilsson, B.-O. The Antimicrobial Peptide LL-37 Triggers Release of Apoptosis-Inducing Factor and Shows Direct Effects on Mitochondria. Biochem. Biophys. Rep. 2022, 29, 101192. [Google Scholar] [CrossRef]
- He, J.; Melnik, L.I.; Komin, A.; Wiedman, G.; Fuselier, T.; Morris, C.F.; Starr, C.G.; Searson, P.C.; Gallaher, W.R.; Hristova, K.; et al. Ebola Virus Delta Peptide Is a Viroporin. J. Virol. 2017, 91, e00438-17. [Google Scholar] [CrossRef]
- Sun, L.; Hristova, K.; Wimley, W.C. Membrane-Selective Nanoscale Pores in Liposomes by a Synthetically Evolved Peptide: Implications for Triggered Release. Nanoscale 2021, 13, 12185–12197. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.; Hristova, K.; Bondar, A.-N.; Wimley, W.C. Structural Determinants of Peptide Nanopore Formation. ACS Nano 2024, 18, 15831–15844. [Google Scholar] [CrossRef]
- Iurlaro, R.; Muñoz-Pinedo, C. Cell Death Induced by Endoplasmic Reticulum Stress. FEBS J. 2016, 283, 2640–2652. [Google Scholar] [CrossRef] [PubMed]
- Yasuhara, S.; Asai, A.; Sahani, N.D.; Martyn, J.A.J. Mitochondria, Endoplasmic Reticulum, and Alternative Pathways of Cell Death in Critical Illness. Crit. Care Med. 2007, 35, S488–S495. [Google Scholar] [CrossRef] [PubMed]
- Hetz, C. The Unfolded Protein Response: Controlling Cell Fate Decisions under ER Stress and Beyond. Nat. Rev. Mol. Cell Biol. 2012, 13, 89–102. [Google Scholar] [CrossRef]
- Nguyen, T.T.M.; Gillet, G.; Popgeorgiev, N. Caspases in the Developing Central Nervous System: Apoptosis and Beyond. Front. Cell Dev. Biol. 2021, 9, 702404. [Google Scholar] [CrossRef]
- Srinivasan, S.; Guha, M.; Kashina, A.; Avadhani, N.G. Mitochondrial Dysfunction and Mitochondrial Dynamics-The Cancer Connection. Biochim. Biophys. Acta Bioenerg. 2017, 1858, 602–614. [Google Scholar] [CrossRef]
- Mader, J.S.; Salsman, J.; Conrad, D.M.; Hoskin, D.W. Bovine Lactoferricin Selectively Induces Apoptosis in Human Leukemia and Carcinoma Cell Lines. Mol. Cancer Ther. 2005, 4, 612–624. [Google Scholar] [CrossRef]
- Hou, D.; Hu, F.; Mao, Y.; Yan, L.; Zhang, Y.; Zheng, Z.; Wu, A.; Forouzanfar, T.; Pathak, J.L.; Wu, G. Cationic Antimicrobial Peptide NRC-03 Induces Oral Squamous Cell Carcinoma Cell Apoptosis via CypD-mPTP Axis-Mediated Mitochondrial Oxidative Stress. Redox Biol. 2022, 54, 102355. [Google Scholar] [CrossRef]
- Yuan, M.; Gong, M.; He, J.; Xie, B.; Zhang, Z.; Meng, L.; Tse, G.; Zhao, Y.; Bao, Q.; Zhang, Y.; et al. IP3R1/GRP75/VDAC1 Complex Mediates Endoplasmic Reticulum Stress-Mitochondrial Oxidative Stress in Diabetic Atrial Remodeling. Redox Biol. 2022, 52, 102289. [Google Scholar] [CrossRef]
- Muttenthaler, M.; King, G.F.; Adams, D.J.; Alewood, P.F. Trends in Peptide Drug Discovery. Nat. Rev. Drug Discov. 2021, 20, 309–325. [Google Scholar] [CrossRef] [PubMed]
- San-Miguel, J.F.; Richardson, P.G.; Sonneveld, P.; Schuster, M.W.; Irwin, D.; Stadtmauer, E.A.; Facon, T.; Harousseau, J.-L.; Ben-Yehuda, D.; Lonial, S.; et al. Efficacy and Safety of Bortezomib in Patients with Renal Impairment: Results from the APEX Phase 3 Study. Leukemia 2008, 22, 842–849. [Google Scholar] [CrossRef]
- Zhang, L.; Jing, D.; Jiang, N.; Rojalin, T.; Baehr, C.M.; Zhang, D.; Xiao, W.; Wu, Y.; Cong, Z.; Li, J.J.; et al. Transformable Peptide Nanoparticles Arrest HER2 Signalling and Cause Cancer Cell Death in Vivo. Nat. Nanotechnol. 2020, 15, 145–153. [Google Scholar] [CrossRef] [PubMed]
- Costa Verdera, H.; Gitz-Francois, J.J.; Schiffelers, R.M.; Vader, P. Cellular Uptake of Extracellular Vesicles Is Mediated by Clathrin-Independent Endocytosis and Macropinocytosis. J. Control. Release 2017, 266, 100–108. [Google Scholar] [CrossRef]
- Newton, K.; Strasser, A.; Kayagaki, N.; Dixit, V.M. Cell Death. Cell 2024, 187, 235–256. [Google Scholar] [CrossRef] [PubMed]
- He, W.; Wang, S.; Yan, J.; Qu, Y.; Jin, L.; Sui, F.; Li, Y.; You, W.; Yang, G.; Yang, Q.; et al. Self-Assembly of Therapeutic Peptide into Stimuli-Responsive Clustered Nanohybrids for Cancer-Targeted Therapy. Adv. Funct. Mater. 2019, 29, 1807736. [Google Scholar] [CrossRef]
- Pan, R.; Ryan, J.; Pan, D.; Wucherpfennig, K.W.; Letai, A. Augmenting NK Cell-Based Immunotherapy by Targeting Mitochondrial Apoptosis. Cell 2022, 185, 1521–1538.e18. [Google Scholar] [CrossRef]
- Zhang, L.-L.; Xu, Y.-L.; Tang, Z.-H.; Xu, X.-H.; Chen, X.; Li, T.; Ding, C.-Y.; Huang, M.-Q.; Chen, X.-P.; Wang, Y.-T.; et al. Effects of Alisol B 23-Acetate on Ovarian Cancer Cells: G1 Phase Cell Cycle Arrest, Apoptosis, Migration and Invasion Inhibition. Phytomedicine 2016, 23, 800–809. [Google Scholar] [CrossRef]
- Meng, M.; Jiang, Y.; Wang, Y.; Huo, R.; Ma, N.; Shen, X.; Chang, G. β-Carotene Targets IP3R/GRP75/VDAC1-MCU Axis to Renovate LPS-Induced Mitochondrial Oxidative Damage by Regulating STIM1. Free Radic. Biol. Med. 2023, 205, 25–46. [Google Scholar] [CrossRef]
- Valiyari, S.; Salami, M.; Mahdian, R.; Shokrgozar, M.A.; Oloomi, M.; Mohammadi Farsani, A.; Bouzari, S. sIL-24 Peptide, a Human Interleukin-24 Isoform, Induces Mitochondrial-Mediated Apoptosis in Human Cancer Cells. Cancer Chemother. Pharmacol. 2017, 80, 451–459. [Google Scholar] [CrossRef]
- Shao, F.; Han, J.; Tian, Z.; Wang, Z.; Liu, S.; Wu, Y. Synergistic ROS Generation and Directional Overloading of Endogenous Calcium Induce Mitochondrial Dysfunction in Living Cells. Biomaterials 2023, 301, 122284. [Google Scholar] [CrossRef]
- Chen, X.; Cubillos-Ruiz, J.R. Endoplasmic Reticulum Stress Signals in the Tumour and Its Microenvironment. Nat. Rev. Cancer 2021, 21, 71–88. [Google Scholar] [CrossRef]
- Banerjee, A.; Banerjee, V.; Czinn, S.; Blanchard, T. Increased Reactive Oxygen Species Levels Cause ER Stress and Cytotoxicity in Andrographolide Treated Colon Cancer Cells. Oncotarget 2017, 8, 26142–26153. [Google Scholar] [CrossRef]
- Hu, H.; Tian, M.; Ding, C.; Yu, S. The C/EBP Homologous Protein (CHOP) Transcription Factor Functions in Endoplasmic Reticulum Stress-Induced Apoptosis and Microbial Infection. Front. Immunol. 2019, 9, 3083. [Google Scholar] [CrossRef]
- Kirsch, K.; Zeke, A.; Tőke, O.; Sok, P.; Sethi, A.; Sebő, A.; Kumar, G.S.; Egri, P.; Póti, Á.L.; Gooley, P.; et al. Co-Regulation of the Transcription Controlling ATF2 Phosphoswitch by JNK and P38. Nat. Commun. 2020, 11, 5769. [Google Scholar] [CrossRef] [PubMed]
- Badrichani, A.Z.; Stroka, D.M.; Bilbao, G.; Curiel, D.T.; Bach, F.H.; Ferran, C. Bcl-2 and Bcl-XL Serve an Anti-Inflammatory Function in Endothelial Cells through Inhibition of NF-kappaB. J. Clin. Investig. 1999, 103, 543–553. [Google Scholar] [CrossRef] [PubMed]
- Deragon, M.A.; McCaig, W.D.; Patel, P.S.; Haluska, R.J.; Hodges, A.L.; Sosunov, S.A.; Murphy, M.P.; Ten, V.S.; LaRocca, T.J. Mitochondrial ROS Prime the Hyperglycemic Shift from Apoptosis to Necroptosis. Cell Death Discov. 2020, 6, 132. [Google Scholar] [CrossRef]
- Liu, J.; Yan, J.; Jiang, S.; Wen, J.; Chen, L.; Zhao, Y.; Lin, A. Site-Specific Ubiquitination Is Required for Relieving the Transcription Factor Miz1-Mediated Suppression on TNF-α-Induced JNK Activation and Inflammation. Proc. Natl. Acad. Sci. USA 2012, 109, 191–196. [Google Scholar] [CrossRef]
- Zhao, K.; Zhang, H.; Yang, D. SIRT1 Exerts Protective Effects by Inhibiting Endoplasmic Reticulum Stress and NF-κB Signaling Pathways. Front. Cell Dev. Biol. 2024, 12, 1405546. [Google Scholar] [CrossRef]
| Pore-Forming Peptide | Sequence | IC50 (μM) | Permeability to PC Bilayers |
|---|---|---|---|
| Melittin | GIGAVLKVLTTGLPALISWIKRKRQQ | 1–5 | low |
| Melp5 | GIGAVLKVLATGLPALISWIKAAQQL | 1–5 | medium |
| M159 | GIGEVLHELATLLPELISWIKAAQQL | >200 | high |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Li, Y.; Li, D.; Jiang, Z.; Yuan, Z.; Sun, Z.; Sun, L. D-M159 Synergistically Induces Apoptosis in HeLa Cells Through Endoplasmic Reticulum Stress and Mitochondrial Dysfunction. Int. J. Mol. Sci. 2025, 26, 3172. https://doi.org/10.3390/ijms26073172
Li Y, Li D, Jiang Z, Yuan Z, Sun Z, Sun L. D-M159 Synergistically Induces Apoptosis in HeLa Cells Through Endoplasmic Reticulum Stress and Mitochondrial Dysfunction. International Journal of Molecular Sciences. 2025; 26(7):3172. https://doi.org/10.3390/ijms26073172
Chicago/Turabian StyleLi, Yuanyuan, Dingding Li, Zonghan Jiang, Zhihang Yuan, Zhiliang Sun, and Leisheng Sun. 2025. "D-M159 Synergistically Induces Apoptosis in HeLa Cells Through Endoplasmic Reticulum Stress and Mitochondrial Dysfunction" International Journal of Molecular Sciences 26, no. 7: 3172. https://doi.org/10.3390/ijms26073172
APA StyleLi, Y., Li, D., Jiang, Z., Yuan, Z., Sun, Z., & Sun, L. (2025). D-M159 Synergistically Induces Apoptosis in HeLa Cells Through Endoplasmic Reticulum Stress and Mitochondrial Dysfunction. International Journal of Molecular Sciences, 26(7), 3172. https://doi.org/10.3390/ijms26073172






