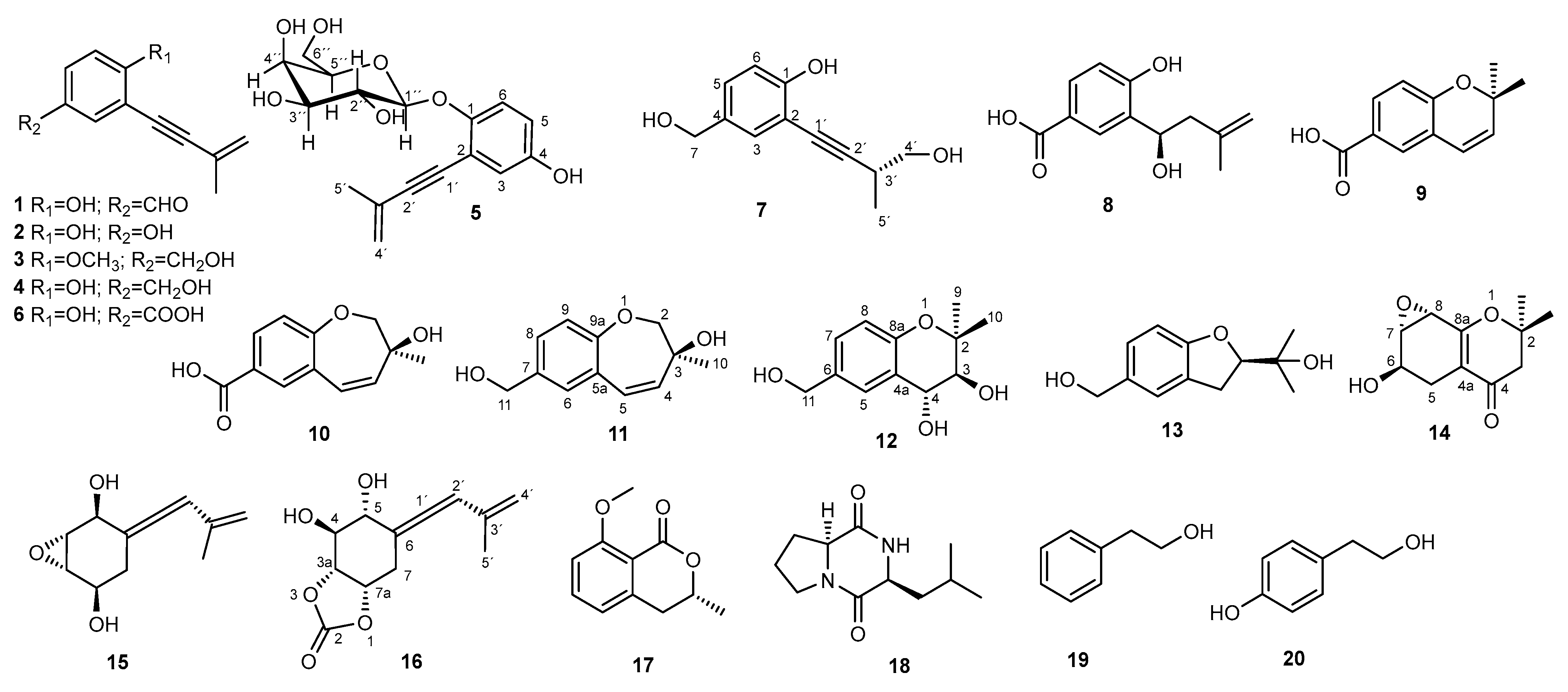
Discovery of Novel Phenolic Compounds from Eutypa lata Through OSMAC Approach: Structural Elucidation and Antibiotic Potential
Abstract
1. Introduction
2. Results
In Vitro Phytotoxic and Antimicrobial Assays
3. Discussion
4. Materials and Methods
4.1. General Experimental Procedures
4.2. Fungal Material
4.3. General Culture Conditions
4.4. Liquid Culture Fermentation
4.4.1. PDB Medium Fermentation
4.4.2. Czapek–Dox Medium Fermentation
4.5. Solid Culture Fermentation
4.5.1. PDA Medium Fermentation
4.5.2. AM Medium Fermentation
4.5.3. Rice Medium
4.6. Computational Details of EDC Calculations
4.7. In Vitro Phytotoxic Assays
4.8. In Vitro Antimicrobial Assays
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fontaine, F.; Gramaje, D.; Armengol, J.; Smart, R.; Nagy, Z.A.; Borgo, M.; Rego, C.; Corio-Costet, M.-F. Grapevine Trunk Diseases. A Review; International Organisation of Vine and Wine (OIV): Paris, France, 2016; p. 25. [Google Scholar]
- Martín, L.; García-García, B.; Alguacil, M.d.M. Interactions of the Fungal Community in the Complex Patho-System of Esca, a Grapevine Trunk Disease. Int. J. Mol. Sci. 2022, 23, 14726. [Google Scholar] [CrossRef] [PubMed]
- Kenfaoui, J.; Radouane, N.; Mennani, M.; Tahiri, A.; El Ghadraoui, L.; Belabess, Z.; Fontaine, F.; El Hamss, H.; Amiri, S.; Lahlali, R.; et al. A Panoramic View on Grapevine Trunk Diseases Threats: Case of Eutypa Dieback, Botryosphaeria Dieback, and Esca Disease. J. Fungi 2022, 8, 595. [Google Scholar] [CrossRef] [PubMed]
- Gramaje, D.; Urbez-Torres, J.R.; Sosnowski, M.R. Managing Grapevine Trunk Diseases with Respect to Etiology and Epidemiology: Current Strategies and Future Prospects. Plant Dis. 2018, 102, 12–39. [Google Scholar] [CrossRef] [PubMed]
- Lorrain, B.; Ky, I.; Pasquier, G.; Jourdes, M.; Dubrana, L.G.; Gény, L.; Rey, P.; Donèche, B.; Teissedre, P.L. Effect of Esca Disease on the Phenolic and Sensory Attributes of Cabernet Sauvignon Grapes, Musts and Wines. Aust. J. Grape Wine Res. 2012, 18, 64–72. [Google Scholar] [CrossRef]
- Hillis, V.; Lubell, M.; Kaplan, J.; Baumgartner, K. Preventative Disease Management and Grower Decision Making: A Case Study of California Wine-Grape Growers. Phytopathology 2017, 107, 704–710. [Google Scholar] [CrossRef]
- Munkvold, G.P.; Duthie, J.A.; Marois, J.J. Reductions in Yield and Vegetative Growth of Grapevines Due to Eutypa Dieback. Phytopathology 1994, 84, 186–192. [Google Scholar] [CrossRef]
- Mondello, V.; Songy, A.; Battiston, E.; Pinto, C.; Coppin, C.; Trotel-Aziz, P.; Clément, C.; Mugnai, L.; Fontaine, F. Grapevine Trunk Diseases: A Review of Fifteen Years of Trials for Their Control with Chemicals and Biocontrol Agents. Plant Dis. 2018, 102, 1189–1217. [Google Scholar] [CrossRef]
- Blundell, R.; Eskalen, A. Evaluation of Biological and Chemical Pruning Wound Protectants to Control Grapevine Trunk Disease Pathogens Eutypa lata and Neofusicoccum parvum. Plant Health Prog. 2022, 23, 197–205. [Google Scholar] [CrossRef]
- Mondello, V.; Fernandez, O.; Guise, J.F.; Trotel-Aziz, P.; Fontaine, F. In Planta Activity of the Novel Copper Product HA + Cu(II) Based on a Biocompatible Drug Delivery System on Vine Physiology and Trials for the Control of Botryosphaeria Dieback. Front. Plant Sci. 2021, 12, 693995. [Google Scholar] [CrossRef]
- Andolfi, A.; Mugnai, L.; Luque, J.; Surico, G.; Cimmino, A.; Evidente, A. Phytotoxins Produced by Fungi Associated with Grapevine Trunk Diseases. Toxins 2011, 3, 1569–1605. [Google Scholar] [CrossRef]
- Singh, A.; Singh, D.K.; Kharwar, R.N.; White, J.F.; Gond, S.K. Fungal Endophytes as Efficient Sources of Plant-Derived Bioactive Compounds and Their Prospective Applications in Natural Product Drug Discovery: Insights, Avenues, and Challenges. Microorganisms 2021, 9, 197. [Google Scholar] [CrossRef] [PubMed]
- Keshri, P.K.; Rai, N.; Verma, A.; Kamble, S.C.; Barik, S.; Mishra, P.; Singh, S.K.; Salvi, P.; Gautam, V. Biological Potential of Bioactive Metabolites Derived from Fungal Endophytes Associated with Medicinal Plants. Mycol. Prog. 2021, 20, 577–594. [Google Scholar] [CrossRef]
- Hardoim, P.R.; van Overbeek, L.S.; Berg, G.; Pirttilä, A.M.; Compant, S.; Campisano, A.; Döring, M.; Sessitsch, A. The Hidden World within Plants: Ecological and Evolutionary Considerations for Defining Functioning of Microbial Endophytes. Microbiol. Mol. Biol. Rev. 2015, 79, 293–320. [Google Scholar] [CrossRef] [PubMed]
- Hashem, A.H.; Attia, M.S.; Kandil, E.K.; Fawzi, M.M.; Abdelrahman, A.S.; Khader, M.S.; Khodaira, M.A.; Emam, A.E.; Goma, M.A.; Abdelaziz, A.M. Bioactive Compounds and Biomedical Applications of Endophytic Fungi: A Recent Review. Microb. Cell Factories 2023, 22, 107. [Google Scholar] [CrossRef]
- Mahoney, N.; Molyneux, R.J.; Smith, L.R.; Schoch, T.K.; Rolshausen, P.E.; Gubler, W.D. Dying-Arm Disease in Grapevines: Diagnosis of Infection with Eutypa lata by Metabolite Analysis. J. Agric. Food Chem. 2005, 53, 8148–8155. [Google Scholar] [CrossRef]
- Masi, M.; Cimmino, A.; Reveglia, P.; Mugnai, L.; Surico, G.; Evidente, A. Advances on Fungal Phytotoxins and Their Role in Grapevine Trunk Diseases. J. Agric. Food Chem. 2018, 66, 5948–5958. [Google Scholar] [CrossRef]
- Muntean, M.D.; Drăgulinescu, A.M.; Tomoiagă, L.L.; Comșa, M.; Răcoare, H.S.; Sîrbu, A.D.; Chedea, V.S. Fungal Grapevine Trunk Diseases in Romanian Vineyards in the Context of the International Situation. Pathogens 2022, 11, 1006. [Google Scholar] [CrossRef]
- Kovács, C.; Sándor, E. The Increasing Importance of Grapevine Trunk Diseases. Int. J. Hortic. Sci. 2016, 22, 21–30. [Google Scholar] [CrossRef]
- Agustí-Brisach, C.; López-Moral, A.; Raya-Ortega, M.C.; Franco, R.; Roca-Castillo, L.F.; Trapero, A. Occurrence of Grapevine Trunk Diseases Affecting the Native Cultivar Pedro Ximénez in Southern Spain. Eur. J. Plant Pathol. 2019, 153, 599–625. [Google Scholar] [CrossRef]
- Azzollini, A.; Boggia, L.; Boccard, J.; Sgorbini, B.; Lecoultre, N.; Allard, P.-M.; Rubiolo, P.; Rudaz, S.; Gindro, K.; Bicchi, C.; et al. Evaluation of Biocontrol Agents for Grapevine Pruning Wound Protection against Trunk Pathogen Infection. Phytopathol. Mediterr. 2011, 50, S176–S190. [Google Scholar] [CrossRef]
- Moreno-Sanz, P.; Lucchetta, G.; Zanzotto, A.; Loureiro, M.D.; Suarez, B.; Angelini, E. Fungi Associated to Grapevine Trunk Diseases in Young Plants in Asturias (Northern Spain). Hortic. Sci. 2013, 40, 138–144. [Google Scholar] [CrossRef]
- Rolshausen, P.E.; Greve, L.C.; Labavitch, J.M.; Mahoney, N.E.; Molyneux, R.J.; Gubler, W.D. Pathogenesis of Eutypa lata in Grapevine: Identification of Virulence Factors and Biochemical Characterization of Cordon Dieback. Phytopathology 2008, 98, 222–229. [Google Scholar] [CrossRef] [PubMed]
- Smith, L.R.; Mahoney, N.; Molyneux, R.J. Synthesis and Structure—Phytotoxicity Relationships of Acetylenic Phenols and Chromene Metabolites, and Their Analogues, from the Grapevine Pathogen Eutypa lata. J. Nat. Prod. 2003, 66, 169–176. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.H.; Mahoney, N.; Chan, K.L.; Molyneux, R.J.; Campbell, B.C. Secondary Metabolites of the Grapevine Pathogen Eutypa lata Inhibit Mitochondrial respiration, Based on a Model Bioassay Using the Yeast Saccharomyces cerevisiae. Curr. Microbiol. 2004, 49, 282–287. [Google Scholar] [CrossRef]
- Lardner, R.; Mahoney, N.; Zanker, T.P.; Molyneux, R.J.; Scott, E.S. Secondary Metabolite Production by the Fungal Pathogen Eutypa lata: Analysis of Extracts from Grapevine Cultures and Detection of Those Metabolites in Planta. Aust. J. Grape Wine Res. 2006, 12, 107–114. [Google Scholar] [CrossRef]
- Mahoney, N.; Lardner, R.; Molyneux, R.J.; Scott, E.S.; Smith, L.R.; Schoch, T.K. Phenolic and Heterocyclic Metabolite Profiles of the Grapevine Pathogen Eutypa lata. Phytochemistry 2003, 64, 475–484. [Google Scholar] [CrossRef]
- Renaud, J.-M.; Tsoupras, G.; Stoeckli-Evans, H.; Tabacchi, R. A Novel Allenic Epoxycyclohexane and Related Compounds from Eutypa lata (Pers: F.) Tul. Helv. Chim. Acta 1989, 72, 1262–1267. [Google Scholar] [CrossRef]
- Renaud, J.-M.; Tsoupras, G.; Tabacchi, R. Biologically Active Natural Acetylenic Compounds from Eutypa lata (Pers: F.) TUL. Helv. Chim. Acta 1989, 72, 929–932. [Google Scholar] [CrossRef]
- Walter, H.; Tobler, H.; Gribkov, D.; Corsi, C. Sedaxane, isopyrazam and SolatenolTM: Novel Broad-Spectrum Fungicides Inhibiting Succinate Dehydrogenase (SDH)—Synthesis Challenges and Biological Aspects. Chimia 2015, 69, 425–433. [Google Scholar] [CrossRef]
- Hewage, R.T.; Aree, T.; Mahidol, C.; Ruchirawat, S.; Kittakoop, P. One Strain-Many Compounds (OSMAC) Method for Production of Polyketides, Azaphilones, and an Isochromanone Using the Endophytic Fungus Dothideomycete sp. Phytochemistry 2014, 108, 87–94. Phytochemistry 2014, 108, 87–94. [Google Scholar] [CrossRef]
- Bode, H.B.; Bethe, B.; Höfs, R.; Zeeck, A. Big Effects from Small Changes: Possible Ways to Explore Nature’s Chemical Diversity. ChemBioChem 2002, 3, 619–627. [Google Scholar] [CrossRef] [PubMed]
- Kang, S.J.; Zhao, L.; Wang, H.; Gao, J.; Qi, J. Chemical Structures, Biological Activities, and Biosynthetic Analysis of Secondary Metabolites of the Diatrypaceae Family: A Comprehensive Review. Mycology 2024, 15, 322–344. [Google Scholar] [CrossRef] [PubMed]
- Molyneux, R.J.; Mahoney, N.; Bayman, P.; Wong, R.Y.; Meyer, K.; Irelan, N. Eutypa Dieback in Grapevines: Differential Production of Acetylenic Phenol Metabolites by Strains of Eutypa lata. J. Agric. Food Chem. 2002, 50, 1393–1399. [Google Scholar] [CrossRef] [PubMed]
- Travadon, R.; Baumgartner, K.; Rolshausen, P.E.; Gubler, W.D.; Sosnowski, M.R.; Lecomte, P.; Halleen, F.; Péros, J.-P. Genetic Structure of the Fungal Grapevine Pathogen Eutypa lata from Four Continents. Plant Pathol. 2012, 61, 85–95. [Google Scholar] [CrossRef]
- Onetto, C.A.; Sosnowski, M.R.; Van Den Heuvel, S.; Borneman, A.R. Population Genomics of the Grapevine Pathogen Eutypa lata Reveals Evidence for Population Expansion and Intraspecific Differences in Secondary Metabolite Gene Clusters. PLoS Genet. 2022, 18, e1010153. [Google Scholar] [CrossRef]
- Kupka, J.; Anke, T.; Steglich, W.; Zechlin, L. Antibiotics from Basidiomycetes. XI the Biological Activity of Siccayne, Isolated from the Marine Fungus Halocyphina villosa J. & E. Kohlmeyer. J. Antibiot. 1981, 34, 298–304. [Google Scholar] [CrossRef]
- Adamczeski, M.; Reed, A.R.; Crews, P. New and Known Diketopiperazines from the Caribbean Sponge, Calyx Cf. Podatypa. J. Nat. Prod. 1995, 58, 201–208. [Google Scholar] [CrossRef]
- Marković, A.K.; Torić, J.; Barbarić, M.; Brala, C.J. Hydroxytyrosol, Tyrosol and Derivatives and Their Potential Effects on Human Health. Molecules 2019, 24, 2001. [Google Scholar] [CrossRef]
- Islam, M.S.; Ishigami, K.; Watanabe, H. Synthesis of (-)-Mellein, (+)-Ramulosin, and Related Natural Products. Tetrahedron 2007, 63, 1074–1079. [Google Scholar] [CrossRef]
- Ishibashi, K.; Nose, K.; Shindo, T.; Arai, M.; Mishima, H. Siccayne: A Novel Acetylenic Metabolite of Helminthosporium siccans. Ann. Sankyo Res. Lab. 1968, 20, 76–79. [Google Scholar]
- Sun, L.; Li, D.; Tao, M.; Chen, Y.; Zhang, Q.; Dan, F.; Zhang, W. Two new polyketides from a marine sediment-derived fungus Eutypella scoparia FS26. Nat. Prod. Res. 2013, 27, 1298–1304. [Google Scholar] [CrossRef] [PubMed]
- Mann, E.; Chiara, J.L.; Verkhnyatskaya, S. Synthesis of 1,3,4,6-Tetra-O-Acetyl-2-Azido-2-Deoxy-α,β-D-Galactopyranose. In Carbohydrate Chemistry: Proven Synthetic Methods; CRC Press: Boca Raton, FL, USA, 2020; pp. 121–128. [Google Scholar] [CrossRef]
- Li, X.-C.; Ferreira, D.; Ding, Y. Determination of Absolute Configuration of Natural Products: Theoretical Calculation of Electronic Circular Dichroism as a Tool. Curr. Org. Chem. 2010, 14, 1678–1697. [Google Scholar] [CrossRef] [PubMed]
- Siless, G.E.; Gallardo, G.L.; Rodriguez, M.A.; Rincón, Y.A.; Godeas, A.M.; Cabrera, G.M. Metabolites from the Dark Septate Endophyte Drechslera sp. Evaluation by LC/MS and Principal Component Analysis of Culture Extracts with Histone Deacetylase Inhibitors. Chem. Biodivers. 2018, 15, e1800133. [Google Scholar] [CrossRef] [PubMed]
- Tanaka, Y.M.; Takechi, C.U. Constituents of Anodendron affine. III. Structure of a New Benzopyran Compound. Chem. Pharm. Bull. 1994, 17, 1460–1462. [Google Scholar] [CrossRef][Green Version]
- Prompanya, C.; Dethoup, T.; Gales, L.; Lee, M.; Pereira, J.A.C.; Silva, A.M.S.; Pinto, M.M.M.; Kijjoa, A. New Polyketides and New Benzoic Acid Derivatives from the Marine Sponge-Associated Fungus Neosartorya quadricincta KUFA 0081. Mar. Drugs 2016, 14, 134. [Google Scholar] [CrossRef]
- Cho, T.Y.; Wang, G.J.; Ju, Y.M.; Chen, M.C.; Lee, T.H. Chemical Constituents from Termite-Associated Xylaria acuminatilongissima YMJ623. J. Chin. Chem. Soc. 2016, 63, 404–409. [Google Scholar] [CrossRef]
- Abraham, W.; Arfmann, H.; Word, K. Hydroxy-(Methylbutenylyl)-Benzoic acid and Derivatives from Curvularia fallax. Phytochemistry 1990, 29, 2641–2644. [Google Scholar] [CrossRef]
- Ayer, W.A.; Trifonov, L.S. Aromatic Compounds from Liquid Cultures of Lactarius deliciosus. J. Nat. Prod. 1994, 57, 839–841. [Google Scholar] [CrossRef]
- Rosselli, S.; Maggio, A.; Bellone, G.; Bruno, M. The First Example of Natural Cyclic Carbonate in Terpenoids. Tetrahedron Lett. 2006, 47, 7047–7050. [Google Scholar] [CrossRef]
- El-Demerdash, A.; Genta-Jouve, G.; Bärenstrauch, M.; Kunz, C.; Baudouin, E.; Prado, S. Highly Oxygenated Isoprenylated Cyclohexanoids from the Fungus Parastagonospora nodorum SN15. Phytochemistry 2019, 166, 112056. [Google Scholar] [CrossRef]
- Gordon, J.; Tabacchi, R. Stereospecific Synthesis of a Novel Allenic Cyclohexanoid Epoxide from the Fungus Eutypa lata. J. Org. Chem. 1992, 57, 4728–4731. [Google Scholar] [CrossRef]
- De Amorim, M.R.; Somensi, A.; Araujo, A.R.; Bonifácio, B.V.; Bauab, T.M.; Santos, L.C.D. Compounds of Anthostomella brabeji, an Endophytic Fungus Isolated from Paepalanthus planifolius (Eriocaulaceae). J. Braz. Chem. Soc. 2016, 27, 1048–1054. [Google Scholar] [CrossRef]
- Eagle, B.Y.H.; Musselman, A.D. The Rate of Bactericidal Action of Penicillin in vitro as a Function of its Concentration, and at High Cobcentrations Against Certain Organisms. J. Exp. Med. 1948, 88, 99–131. [Google Scholar] [CrossRef] [PubMed]
- Prasetyoputri, A.; Jarrad, A.M.; Cooper, M.A.; Blaskovich, M.A.T. The Eagle Effect and Antibiotic-Induced Persistence: Two Sides of the Same Coin? Trends Microbiol. 2019, 27, 339–354. [Google Scholar] [CrossRef]
- Valero, C.; Colabardini, A.C.; de Castro, P.A.; Amich, J.; Bromley, M.J.; Goldman, G.H. The Caspofungin Paradoxical Effect is a Tolerant “Eagle Effect” in the Filamentous Fungal Pathogen Aspergillus fumigatus. MBio 2022, 13, e0044722. [Google Scholar] [CrossRef]
- Pan, R.; Bai, X.; Chen, J.; Zhang, H.; Wang, H.; Aon, J.C. Exploring Structural Diversity of Microbe Secondary Metabolites Using OSMAC Strategy: A Literature Review. Front. Microbiol. 2019, 10, 294. [Google Scholar] [CrossRef]
- Reveglia, P.; Savocchia, S.; Billones-Baaijens, R.; Masi, M.; Cimmino, A.; Evidente, A. Phytotoxic Metabolites by Nine Species of Botryosphaeriaceae Involved in Grapevine Dieback in Australia and Identification of Those Produced by Diplodia mutila, Diplodia seriata, Neofusicoccum australe and Neofusicoccum luteum. Nat. Prod. Res. 2019, 33, 2223–2229. [Google Scholar] [CrossRef]
- Reveglia, P.; Pacetti, A.; Masi, M.; Cimmino, A.; Carella, G.; Marchi, G.; Mugnai, L.; Evidente, A. Phytotoxic Metabolites Produced by Diaporthe eres Involved in Cane Blight of Grapevine in Italy. Nat. Prod. Res. 2021, 35, 2872–2880. [Google Scholar] [CrossRef]
- Evidente, A.; Punzo, B.; Andolfi, A.; Cimmino, A.; Melck, D.; Luque, J. Lipophilic Phytotoxins Produced by Neofusicoccum parvum, a Grapevine Canker Agent. Phytopathol. Mediterr. 2010, 49, 74–79. Available online: https://www.jstor.org/stable/26458570 (accessed on 23 September 2024).
- Abou-Mansour, E.; Débieux, J.L.; Ramírez-Suero, M.; Bénard-Gellon, M.; Magnin-Robert, M.; Spagnolo, A.; Chong, J.; Farine, S.; Bertsch, C.; L’Haridon, F.; et al. Phytotoxic Metabolites from Neofusicoccum parvum, a Pathogen of Botryosphaeria Dieback of Grapevine. Phytochemistry 2015, 115, 207–215. [Google Scholar] [CrossRef]
- Liu, S.; Guo, L.; Che, Y.; Liu, L. Pestaloficiols Q–S from the Plant Endophytic Fungus Pestalotiopsis fici. Fitoterapia 2013, 85, 114–118. [Google Scholar] [CrossRef] [PubMed]
- Sadorn, K.; Saepua, S.; Boonyuen, N.; Komwijit, S.; Rachtawee, P.; Pittayakhajonwut, P. Phenolic Glucosides and Chromane Analogs from the Insect Fungus Conoideocrella Krungchingensis BCC53666. Tetrahedron 2019, 75, 3463–3471. [Google Scholar] [CrossRef]
- Fan, W.; Li, E.; Ren, J.; Wang, W.; Liu, X.; Zhang, Y. Cordycepamides A−E and Cordyglycoside A, New Alkaloidal and Glycoside Metabolites from the Entomopathogenic Fungus Cordyceps sp. Fitoterapia 2020, 142, 104525. [Google Scholar] [CrossRef] [PubMed]
- Suyanto, E.; Gorantla, J.N.; Santi, M.; Fatchiyah, F.; Ketudat-Cairns, M.; Talabnin, C.; Ketudat Cairns, J.R. Enzymatic Synthesis of Phenolic Acid Glucosyl Esters to Test Activities on Cholangiocarcinoma Cells. Appl. Microbiol. Biotechnol. 2024, 108, 1–13. [Google Scholar] [CrossRef]
- Baldoqui, D.C.; Kato, M.J.; Cavalheiro, A.J.; Bolzani, V.D.S.; Young, M.C.M.; Furlan, M. A Chromene and Prenylated Benzoic Acid from Piper aduncum. Phytochemistry 1999, 51, 899–902. [Google Scholar] [CrossRef]
- Abdelwahab, M.F.; Kurtán, T.; Mándi, A.; Müller, W.E.G.; Fouad, M.A.; Kamel, M.S.; Liu, Z.; Ebrahim, W.; Daletos, G.; Proksch, P. Induced Secondary Metabolites from the Endophytic Fungus Aspergillus versicolor through Bacterial Co-Culture and OSMAC Approaches. Tetrahedron Lett. 2018, 59, 2647–2652. [Google Scholar] [CrossRef]
- Gao, S.S.; Shang, Z.; Li, X.M.; Li, C.S.; Cui, C.M.; Wang, B.G. Secondary Metabolites Produced by Solid Fermentation of the Marine-Derived Fungus Penicillium commune QSD-17. Biosci. Biotechnol. Biochem. 2012, 76, 358–360. [Google Scholar] [CrossRef]
- Song, J.; Zhang, Z.; Han, B.; Hu, S.; Li, W.; Xie, Y. Synthesis of Cyclic Carbonates from Epoxides and CO2 Catalyzed by Potassium Halide in the Presence of β-Cyclodextrin. Green Chem. 2008, 10, 1337–1341. [Google Scholar] [CrossRef]
- Zalomaeva, O.V.; Chibiryaev, A.M.; Kovalenko, K.A.; Kholdeeva, O.A.; Balzhinimaev, B.S.; Fedin, V.P. Cyclic Carbonates Synthesis from Epoxides and CO2 over Metal-Organic Framework Cr-MIL-101. J. Catal. 2013, 298, 179–185. [Google Scholar] [CrossRef]
- Yue, Y.; Yu, H.; Li, R.; Xing, R.; Liu, S.; Li, P. Exploring the Antibacterial and Antifungal Potential of Jellyfish-Associated Marine Fungi by Cultivation-Dependent Approaches. PLoS ONE 2015, 10, e0144394. [Google Scholar] [CrossRef]
- Liu, Y.; Li, X.M.; Meng, L.H.; Jiang, W.L.; Xu, G.M.; Huang, C.G.; Wang, B.G. Bisthiodiketopiperazines and Acorane Sesquiterpenes Produced by the Marine-Derived Fungus Penicillium adametzioides AS-53 on Different Culture Media. J. Nat. Prod. 2015, 78, 1294–1299. [Google Scholar] [CrossRef] [PubMed]
- Tey-Rulh, P.; Philippe, I.; Renaud, J.-M.; Tsoupras, G.; de Angelis, P.; Fallot, J.; Tabacchi, R. Eutypine, a Phytotoxin Produced by Eutypa lata the Causal Agent of Dying-Arm Disease of Grapevine. Phytochemistry 1991, 30, 471–473. [Google Scholar] [CrossRef]
- Martin, M.T.; Cobos, R. Identification of Fungi Associated with Grapevine Decline in Castillo y León (Spain). Phytopathol. Mediterr. 2007, 46, 18–25. Available online: http://digital.casalini.it/10.1400/68063 (accessed on 23 September 2024).
- White, T.J.; Bruns, T.; Lee, S.; Taylor, J. Amplification and Direct Sequencing of Fungal Ribosomal RNA Genes for Phylogenetics. In PCR Protocols: A Guide to Methods and Applications; Academic Press: San Diego, CA, USA, 1990; pp. 315–322. [Google Scholar]
- Liu, W.; Wang, L.; Wang, B.; Xu, Y.; Zhu, G.; Lan, M.; Zhu, W.; Sun, K. Diketopiperazine and Diphenylether Derivatives from Marine Algae-Derived Aspergillus versicolor OUCMDZ-2738 by Epigenetic Activation. Mar. Drugs 2019, 17, 6. [Google Scholar] [CrossRef]
- Stewart, J.J.P. Optimization of Parameters for Semiempirical Methods V: Modification of NDDO Approximations and Application to 70 Elements. J. Mol. Model. 2007, 13, 1173–1213. [Google Scholar] [CrossRef]
- Frisch, M.; Trucks, G.W.; Schlegel, H.B.; Scuseria, G.E.; Robb, M.A.; Cheeseman, J.R.; Scalmani, G.; Barone, V.; Mennucci, B.; Petersson, G.A. Gaussian 09; Revision D. 01; Gaussian, Inc.: Wallingford, CT, USA, 2009. [Google Scholar]
- Lee, C.; Yang, W.; Parr, R.G. Development of the Colle-Salvetti Correlation-Energy Formula into a Functional of the Electron Density. Phys. Rev. B 1988, 37, 785–789. [Google Scholar] [CrossRef]
- Becke, A.D. Density-functional Thermochemistry. III. The Role of Exact Exchange. J. Chem. Phys. 1993, 98, 5648–5652. [Google Scholar] [CrossRef]
- Casida, M.E.; Jamorski, C.; Casida, K.C.; Salahub, D.R. Molecular Excitation Energies to High-Lying Bound States from Time-Dependent Density-Functional Response Theory: Characterization and Correction of the Time-Dependent Local Density Approximation Ionization Threshold. J. Chem. Phys. 1998, 108, 4439–4449. [Google Scholar] [CrossRef]
- Bauernschmitt, R.; Ahlrichs, R. Treatment of Electronic Excitations within the Adiabatic Approximation of Time Dependent Density Functional Theory. Chem. Phys. Lett. 1996, 256, 454–464. [Google Scholar] [CrossRef]
- Mennucci, B.; Cancès, E.; Tomasi, J. Evaluation of Solvent Effects in Isotropic and Anisotropic Dielectrics and in Ionic Solutions with a Unified Integral Equation Method: Theoretical Bases, Computational Implementation, and Numerical Applications. J. Phys. Chem. B 1997, 101, 10506–10517. [Google Scholar] [CrossRef]
- Cancès, E.; Mennucci, B.; Tomasi, J. A New Integral Equation Formalism for the Polarizable Continuum Model: Theoretical Background and Applications to Isotropic and Anisotropic Dielectrics. J. Chem. Phys. 1997, 107, 3032–3041. [Google Scholar] [CrossRef]
- Tomasi, J.; Mennucci, B.; Cammi, R. Quantum Mechanical Continuum Solvation Models. Chem. Rev. 2005, 105, 2999–3093. [Google Scholar] [CrossRef] [PubMed]
- Colmenares, A.J.; Aleu, J.; Durán-Patrón, R.; Collado, I.G.; Hernández-Galán, R. The Putative Role of Botrydial and Related Metabolites in the Infection Mechanism of Botrytis cinerea. J. Chem. Ecol. 2002, 28, 997–1005. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Ravipati, A.S.; Koyyalamudi, S.R.; Jeong, S.C.; Reddy, N.; Bartlett, J.; Smith, P.T.; de la Cruz, M.; Monteiro, M.C.; Melguizo, Á.; et al. Anti-Fungal and Anti-Bacterial Activities of Ethanol Extracts of Selected Traditional Chinese Medicinal Herbs. Asian Pac. J. Trop. Med. 2013, 6, 673–681. [Google Scholar] [CrossRef]
- Blanco-Ulate, B.; Rolshausen, P.E.; Cantu, D. Draft Genome Sequence of the Grapevine Dieback Fungus Eutypa lata UCR-EL1. Genome Announc. 2013, 1, e00228-13. [Google Scholar] [CrossRef]
 HMBC spectra correlations for 5.
HMBC spectra correlations for 5.
 NOESY spectra correlations for 5.
NOESY spectra correlations for 5.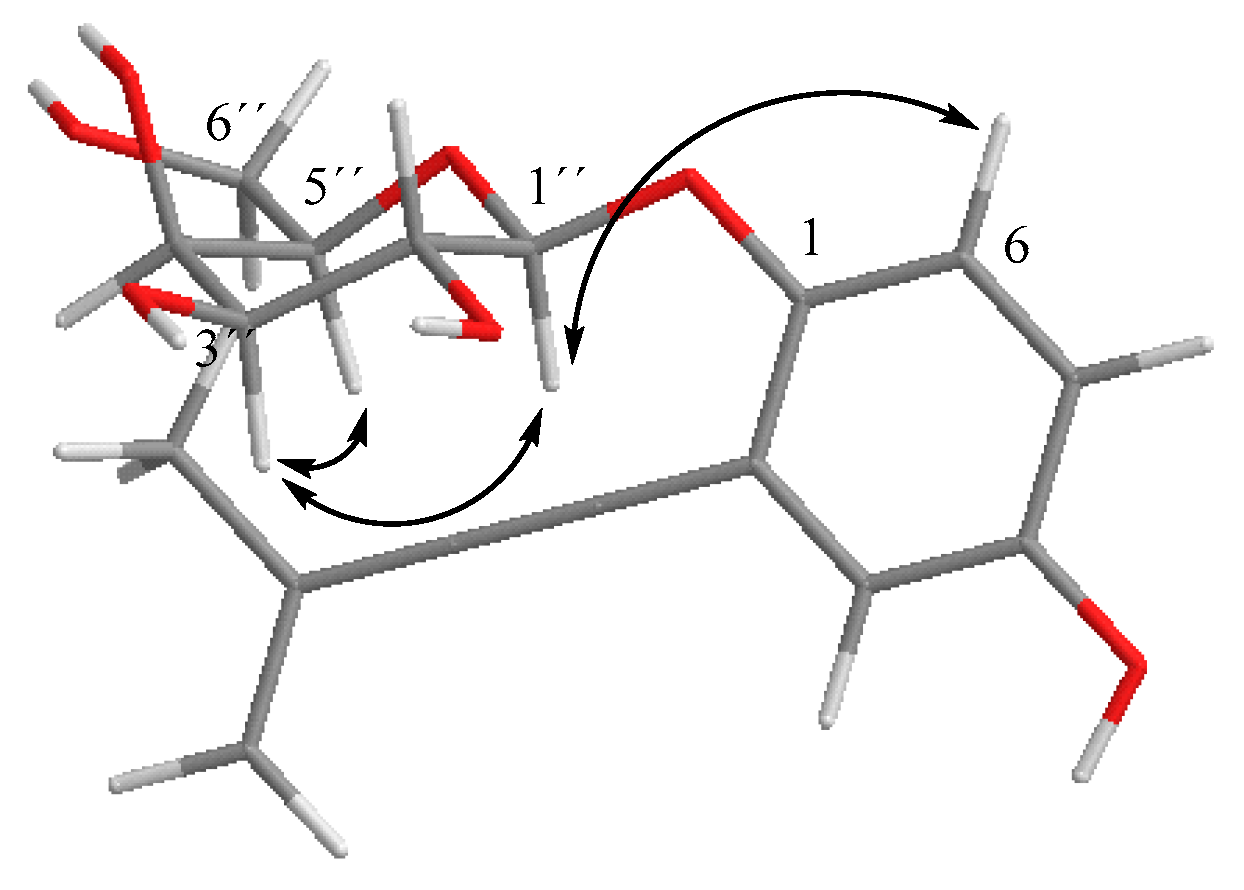
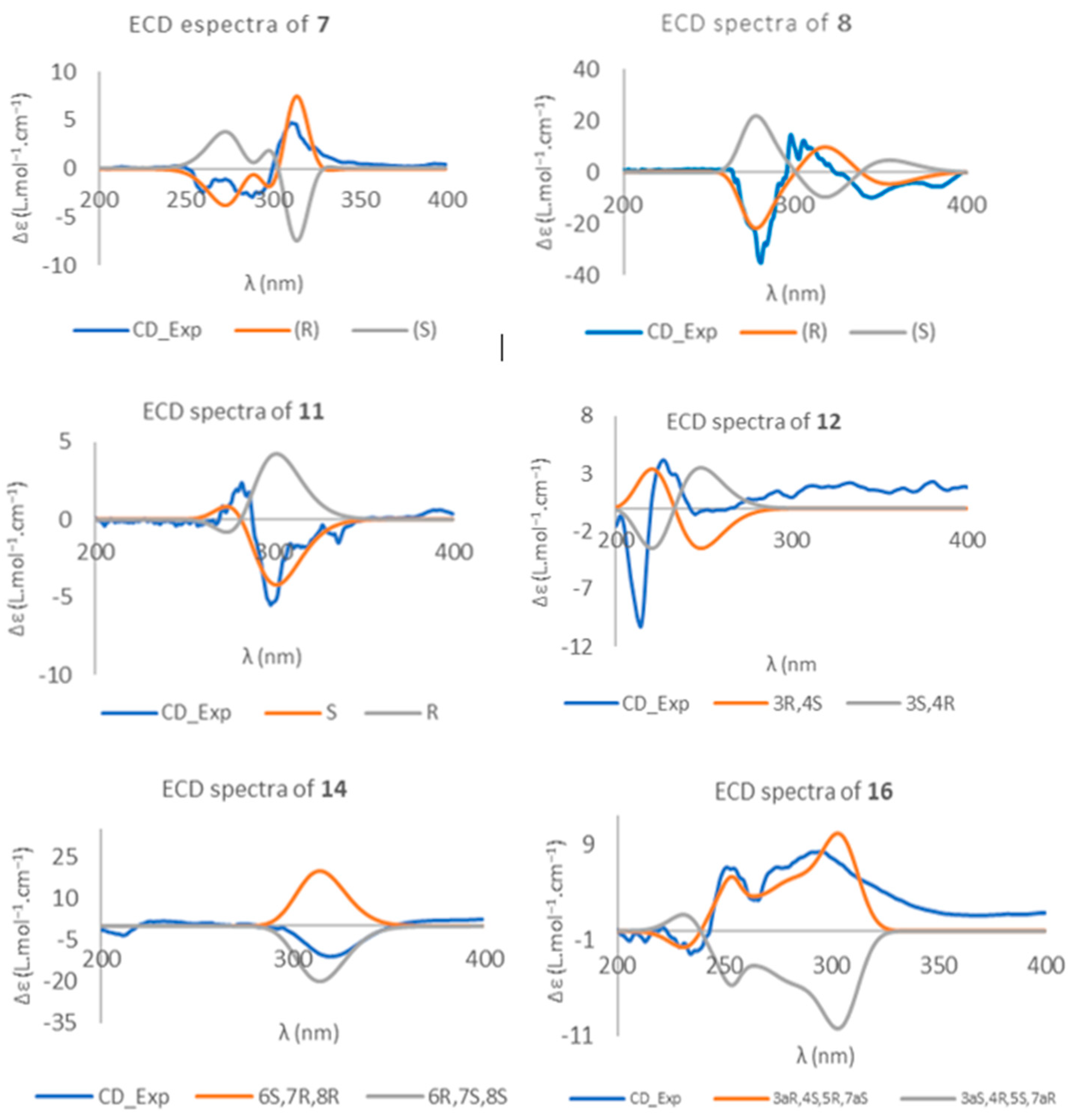
 HMBC and
HMBC and  COSY spectra correlations for 7.
COSY spectra correlations for 7.
 HMBC and
HMBC and  COSY spectra correlations for 11.
COSY spectra correlations for 11.
 HMBC and
HMBC and  COSY spectra correlations for 16.
COSY spectra correlations for 16.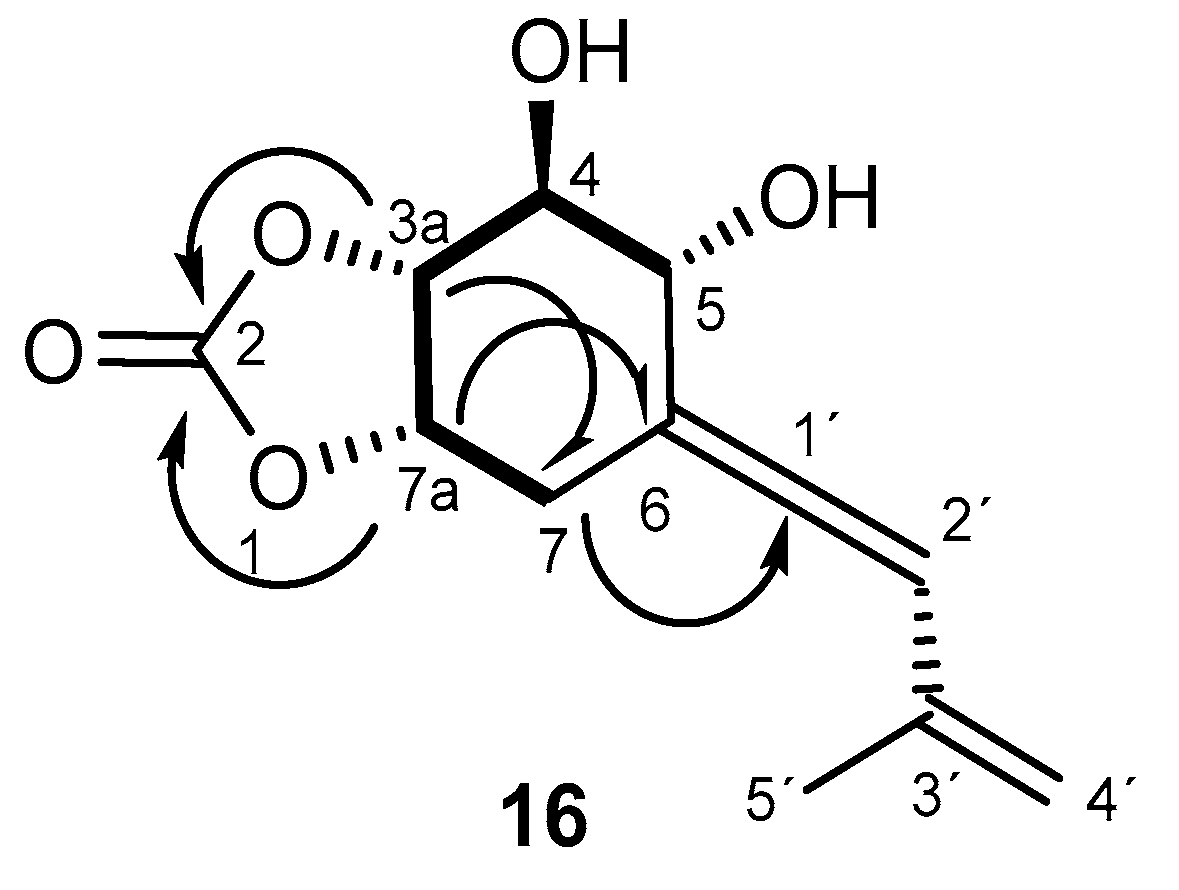
 Selected NOE interactions and correlations exhibited by 16.
Selected NOE interactions and correlations exhibited by 16.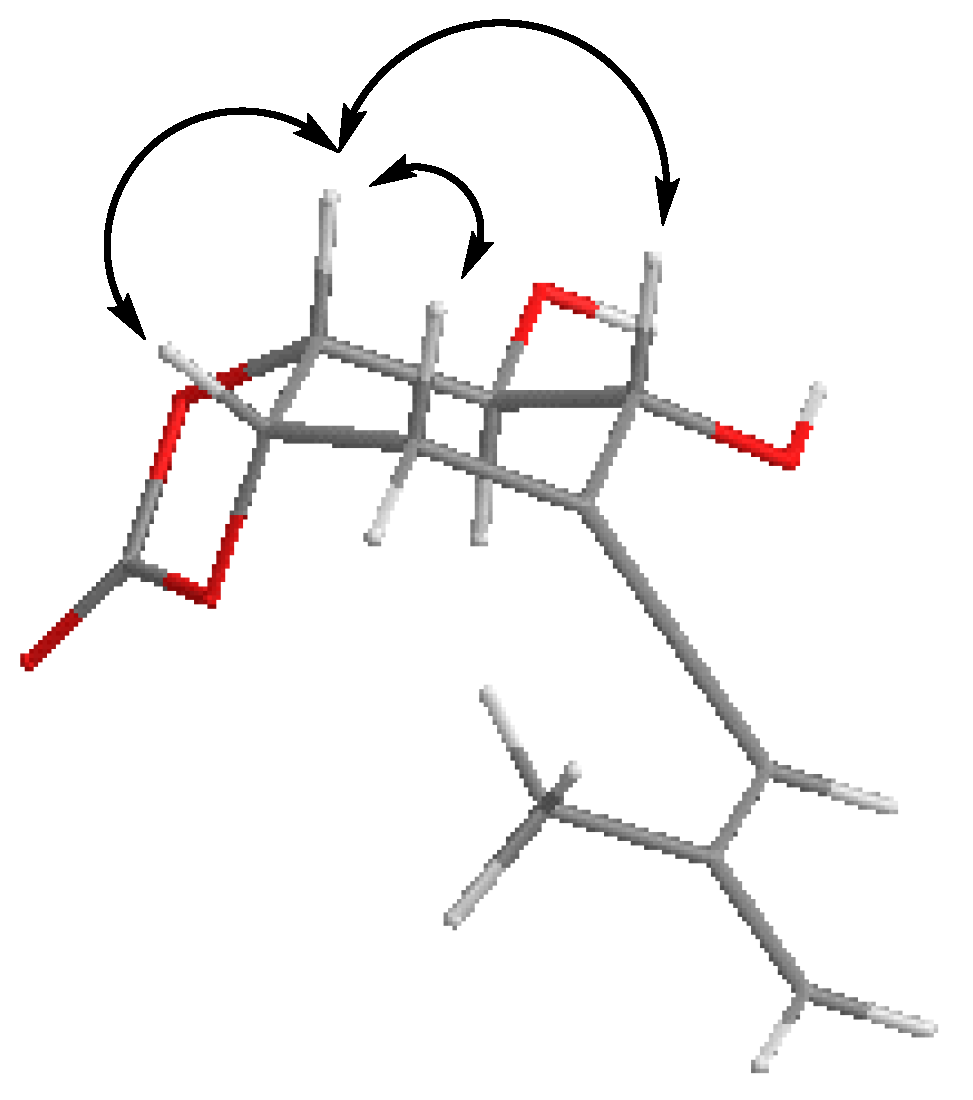


| Position | δH, Mult (J in Hz) a | δC, Type b |
|---|---|---|
| 1 | - | 152.2, C |
| 2 | - | 115.9, C |
| 3 | 6.75, d (3), 1H | C 119.7, CH |
| 4 | - | 153.4, C |
| 5 | 6.71, dd (8.9, 3.0), 1H | 117.6, CH |
| 6 | 7.06, d (8.9), 1H | 119.5, CH |
| 1′ | - | 85.8, C |
| 2′ | - | 95.2, C |
| 3′ | - | 128.5, C |
| 4′a | 5.37, dq (2.1, 1.0), 1H | 122.2, CH2 |
| 4′b | 5.28, m, 1H | |
| 5′ | 1.97, dd (1.6, 1.0), 3H | 23.6, CH3 |
| 1″ | 4.81, d (7.7), 1H | 103.7, C |
| 2″ | 3.81, dd (9.7, 7.7), 1H | 72.4, CH |
| 3″ | 3.56, dd (9.7, 3.4), 1H | 74.9, CH |
| 4″ | 3.87, dd (3.4, 1.1), 1H | 70.1, CH |
| 5″ | 3.60, ddd (6.7, 5.5, 1.1), 1H | 76.9, CH |
| 6″ | 3.74, m, 2H | 62.3, CH2 |
| Position | δH, Mult (J in Hz) a | δC, Type a |
|---|---|---|
| 1 | 156.5, C | |
| 2 | 109.6, C | |
| 3 | 7.31, d (br), 1H | 130.1, CH |
| 4 | 132.6, C | |
| 5 | 7.21, dd (8.3, 2.1), 1H | 129.1, CH |
| 6 | 6.91, d (8.3), 1H | 114.6, CH |
| 7 | 4.57, s, 2H | 64.7, CH2 |
| 1′ | 76.5, C | |
| 2′ | 99.1, C | |
| 3′ | 2.96, m, 1H | 30.1, CH |
| 4′a 4′b | 3.73, dd (10.3, 5.5), 1H 3.64, dd (10.3, 7.2), 1H | 66.7, CH2 |
| 5′ | 1.29, d (7.0), 3H | 16.9, CH3 |
| Position | δH, Mult (J in Hz) a | δC, Type a |
|---|---|---|
| 2α 2β | 4.12, dd (11.9, 1.8), 1H 3.87, d (11.9), 1H | 78.5, CH2 |
| 3 | 72.6, C | |
| 4 | 5.93, dd (11.9, 1.7), 1H | 136.5, CH |
| 5 | 6.25, dd (11.9, 0.6), 1H | 126.5, CH |
| 5a | 135.6, C | |
| 6 | 7.19, d (br) (2.3), 1H | 131.5, CH |
| 7 | 126.3, C | |
| 8 | 7.20, dd (8.0, 2.3), 1H | |
| 9 | 7.02, d (8.0), 1H | 120.2, C |
| 9a | 158.4, C | |
| 10 | 1.33, s, 3H | 24.1, CH3 |
| 11 | 4.63, s, 2H | 64.8, CH2 |
| Position | δH, Mult (J in Hz) a | δC, Type a |
|---|---|---|
| 2 | 154.0, C | |
| 3a | 4.62, td (7.0, 0.6), 1H | 79.6, CH |
| 4 | 3.82, dd, (9.0, 7.0), 1H | 75.9, CH |
| 5 | 4.04, dd (9.0, 3.4), 1H | 69.7, CH |
| 6 | 100.0, C | |
| 7α 7β | 2.94, dd, (16, 3.3), 1H 2.66 m, 1H. | 29.2, CH2 |
| 7a | 4.90, ddd (7.0, 4.5, 3.3), 1H | 75.4, CH |
| 1′ | 200.8, C | |
| 2′ | 6.30, t (3.5), 1H | 103.9, CH |
| 3′ | 137.6, C | |
| 4′a 4′b | 5.04, dq (1.6, 0.8), 1H 4.96, dq (1.6, 1.5), 1H | 116.5, CH2 |
| 5′ | 1.75, dd (1.5, 0.8), 3H | 19.6, CH3 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cotán, A.; Izquierdo-Bueno, I.; Ezzanad, A.; Martín, L.; Delgado, M.; Collado, I.G.; Pinedo-Rivilla, C. Discovery of Novel Phenolic Compounds from Eutypa lata Through OSMAC Approach: Structural Elucidation and Antibiotic Potential. Int. J. Mol. Sci. 2025, 26, 5774. https://doi.org/10.3390/ijms26125774
Cotán A, Izquierdo-Bueno I, Ezzanad A, Martín L, Delgado M, Collado IG, Pinedo-Rivilla C. Discovery of Novel Phenolic Compounds from Eutypa lata Through OSMAC Approach: Structural Elucidation and Antibiotic Potential. International Journal of Molecular Sciences. 2025; 26(12):5774. https://doi.org/10.3390/ijms26125774
Chicago/Turabian StyleCotán, Ana, Inmaculada Izquierdo-Bueno, Abdellah Ezzanad, Laura Martín, Manuel Delgado, Isidro G. Collado, and Cristina Pinedo-Rivilla. 2025. "Discovery of Novel Phenolic Compounds from Eutypa lata Through OSMAC Approach: Structural Elucidation and Antibiotic Potential" International Journal of Molecular Sciences 26, no. 12: 5774. https://doi.org/10.3390/ijms26125774
APA StyleCotán, A., Izquierdo-Bueno, I., Ezzanad, A., Martín, L., Delgado, M., Collado, I. G., & Pinedo-Rivilla, C. (2025). Discovery of Novel Phenolic Compounds from Eutypa lata Through OSMAC Approach: Structural Elucidation and Antibiotic Potential. International Journal of Molecular Sciences, 26(12), 5774. https://doi.org/10.3390/ijms26125774







