Abstract
Metabolic syndrome (MetS) associated with Osteoarthritis (OA) is an increasingly recognised entity. Whilst the degenerative pattern in cuff-tear arthropathy (CTA) has been well documented, the biological processes behind primary shoulder OA and CTA remain less understood. This study investigates transcriptomic differences in these conditions, alongside the impact of MetS in patients undergoing total shoulder replacement. In a multi-centre study, 20 OA patients undergoing total shoulder replacement were included based on specific treatment indications for OA and cuff-tear arthropathy as well as 25 patients undergoing rotator cuff repair (RCR) as a comparator group. Tissues from subchondral bone, capsule (OA and RCR), and synovium were biopsied, and RNA sequencing was performed using Illumina platforms. Differential gene expression was conducted using DESeq2, adjusting for demographic factors, followed by pathway enrichment using the mitch package. Gene expressions in CTA and primary OA was differentially affected. CTA showed mitochondrial dysfunction, GATD3A downregulation, and increased cartilage degradation, while primary OA was marked by upregulated inflammatory and catabolic pathways. The effect of MetS on these pathologies was further shown. MetS further disrupted WNT/β-catenin signalling in CTA, and in OA. Genes such as ACAN, PANX3, CLU, and VAT1L were upregulated, highlighting potential biomarkers for early OA detection. This transcriptomic analysis reveals key differences between end-stage CTA and primary glenohumeral OA. CTA shows heightened metabolic/protein synthesis activity with less immune-driven inflammation. Under MetS, mitochondrial dysfunction (including GATD3A downregulation) and altered Wnt/β-catenin signalling intensify cartilage and bone damage. In contrast, primary OA features strong complement activation, inflammatory gene expression, and collagen remodelling. MetS worsens both conditions via oxidative stress, advanced glycation end products, and ECM disruption—particularly, increased CS/DS degradation. These distinctions support targeted treatments, from antioxidants and Wnt modulators to aggrecanase inhibitors or clusterin augmentation. Addressing specific molecular disruptions, especially those amplified by MetS, may preserve shoulder function, delay surgical intervention, and improve long-term patient outcomes.
1. Introduction
Osteoarthritis is a leading cause of disability worldwide, affecting over 527 million people. Despite the socioeconomic burden of OA [1], current conservative treatment is largely limited to pain management and intra-articular glucocorticoid injections [2], underscoring the need for deeper mechanistic insights to guide therapeutic development. Once considered a primarily age-related degenerative condition, OA is increasingly prevalent in younger populations due to factors such as obesity, occupational strain, sports participation, genetic predisposition, and anatomical variations [3], with metabolic disorders contributing to its onset and progression [4,5]. Obesity has traditionally been considered a risk factor due to increased joint loading [6]; however, given the association with hand OA, despite its non-weight-bearing nature, systemic metabolic influences on joint pathology are suspected [7].
MetS is characterized by visceral adiposity, hypertension, dyslipidaemia, and insulin resistance, all of which contribute to a state of chronic low-grade inflammation that disrupts joint homeostasis and accelerates cartilage degradation [4,8,9,10,11,12]. Further, OA patients have a 5.26-fold increased risk of coexisting metabolic syndrome (MetS), independent of obesity [13]. The role of inflammatory gene modulation in OA pathogenesis is well documented [14,15,16], with transcriptomic studies revealing disease-specific genetic signatures in knee and hip OA [17]. However, the molecular landscape of shoulder OA remains largely unexplored.
Emerging transcriptomic analyses suggest that increased cartilage catabolism rather than reduced anabolism, drives MetS-associated OA [18]. For instance, Casagrande et al. identified increased expression of connexin 43 (Cx43), ADAMTS5, and pro-inflammatory cytokines in osteoarthritic cartilage [19], while Aleem et al. reported upregulation of inflammatory mediators such as CCL3, CHST11, GPR22, PRKAR2B, and PTGS2 in shoulder OA compared to instability cases [20].
To further investigate the role of MetS and systemic inflammation in shoulder OA, this study compares subchondral bone and capsular biopsies from patients with primary shoulder OA and cuff-tear arthropathy (CTA), with and without MetS. We hypothesize that CTA will exhibit less pronounced inflammatory gene expression than primary OA, particularly in patients without concomitant MetS.
2. Main Points
2.1. Inflammation and Mechanical Stress in Joint Degeneration
CTA demonstrates heightened metabolic and protein synthesis activity, reflecting ongoing remodelling, and relatively lower immune-driven inflammation, likely due to altered biomechanics from chronic rotator cuff deficiency. In contrast, primary OA features persistent complement activation, inflammatory gene upregulation, and collagen biosynthetic changes. Both conditions demonstrate distinct transcriptomic patterns under MetS.
2.2. MetS’s Impact on OA and CTA
MetS accelerates degeneration via oxidative stress, glycation end products, and dysregulated gene expression. In OA, MetS intensifies collagen crosslinking and chondroitin sulphate degradation, whereas CTA is marked by mitochondrial dysfunction (including GATD3A downregulation) and altered Wnt/β-catenin signalling. These differences highlight distinct pathways driving tissue deterioration.
2.3. Targeted Therapeutic Opportunities
Addressing inflammation, mitochondrial dysfunction, and ECM breakdown may mitigate disease progression. Antioxidant strategies (e.g., mitochondria-targeted agents) could reduce oxidative damage in CTA. In OA, inhibiting Wnt/β-catenin or aggrecanase, plus using anabolic factors (e.g., sprifermin), may help restore cartilage. Tailoring interventions to each condition’s transcriptomic profile under MetS could preserve shoulder function and delay surgical intervention.
3. Results
The transcriptomic drivers of CTA compared to primary shoulder OA demonstrate a predominance of catabolic and inflammatory genes in subchondral bone biopsies (Table 1 and Figure 1). GATD3A, a top differentially expressed gene (DEG), was downregulated in both bone and capsular tissue of study patients with CTA as compared to patients with primary OA (Table 1 and Table 2).

Table 1.
Top DEGs in subchondral bone for patients with CTA compared to primary OA, corrected for age, sex, and CRP. Likely gene effect is described with pathophysiological description and references shown.
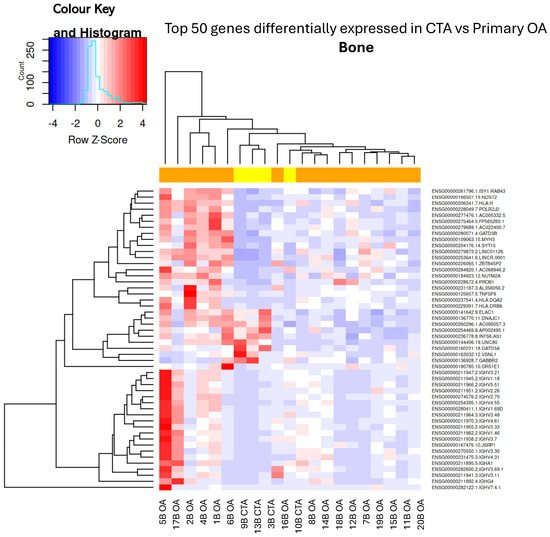
Figure 1.
Hierarchical unsupervised clustering (not ordered prior to analysis; genes and samples are positioned based on their observed similarity) gene expression heatmap and colour histogram. Top 50 DEGs in CTA compared with shoulder OA. Bone biopsies for patients 1 through to 20. B = bone, OA = OA, CTA = cuff-tear arthropathy.

Table 2.
Top DEGs in capsular tissue for patients with CTA compared to primary OA, corrected for age, sex, and CRP.
When comparing capsular tissue biopsies between CTA and primary OA, the downregulation of anabolic genes such as MAS1, ACSM3, and LINC01229 was noted, as well as the downregulation of several anti-inflammatory genes (Table 2 and Figure 2).
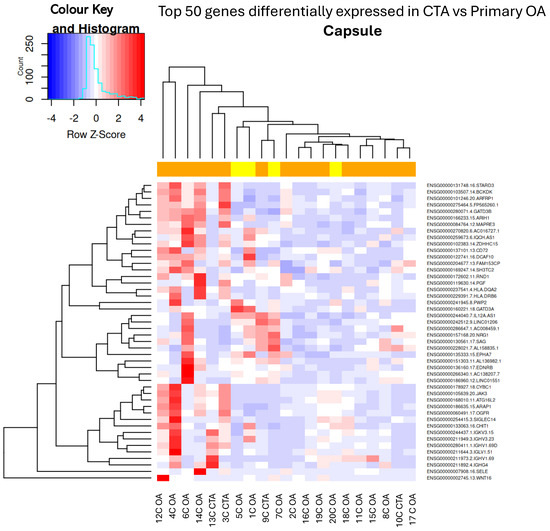
Figure 2.
Hierarchical unsupervised clustering (not ordered prior to analysis; genes and samples are positioned based on their observed similarity) gene expression heatmap and colour histogram. Top 50 DEGs in CTA compared with shoulder OA. Capsular tissue biopsies for patients 1 through to 20. C = capsule, OA = OA, CTA = cuff-tear arthropathy.
Comparison of capsular tissue biopsies between CTA and primary OA demonstrated a singular differentially expressed gene, specifically the downregulation of GATD3A (Figure 3).
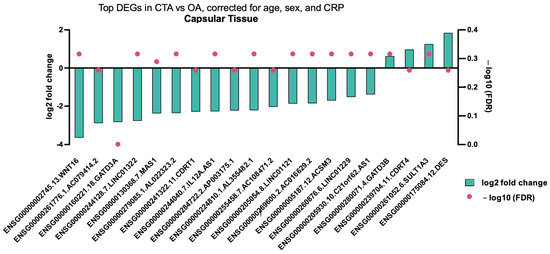
Figure 3.
Top DEGs in CTA compared with shoulder OA, adjusted for age, sex, and CRP in capsular tissue biopsies. A single differentially expressed gene (adjusted p value < 0.05) is shown.
When analysing specific gene variation in subchondral bone tissue biopsies between CTA and primary OA, several significantly DEGs were demonstrated, including inflammatory genes and those involved in mitochondrial homeostasis, including GATD3A (Figure 4). When analysing all tissue types, subchondral bone, capsule, and synovium, a predominance of downregulated anabolic-associated genes was demonstrated, with up- and downregulated inflammatory and catabolic genes also shown (Table 3).
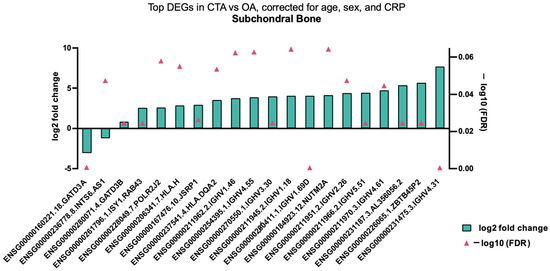
Figure 4.
Top DEGs in CTA compared with shoulder OA, adjusted for age, sex, and CRP in bone tissue biopsies.

Table 3.
Top DEGs that are altered in all tissues (subchondral bone, capsule, synovium) of MetS-associated CTA.
Top DEGs involved in all tissue types biopsied, including subchondral bone, capsule, and synovium in CTA in patients with MetS, are shown in Figure 5. Significantly, DEGs impacted by MetS involve cellular and chondrocyte proliferation, bone mineralization, modulation of WNT/β-catenin signalling, low-grade inflammation, weight gain, and increased cholesterol uptake; see Table 3 and Figure 5.
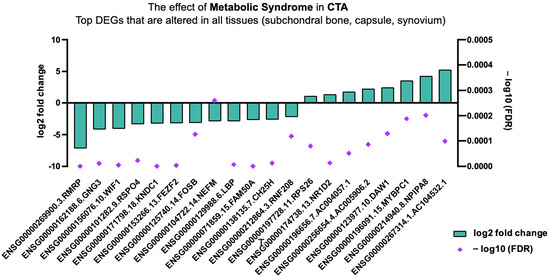
Figure 5.
Top DEGs in CTA altered in all tissue types given MetS.
When analysing the effect of MetS on primary shoulder OA, subchondral bone biopsies analysed with mitch demonstrated upregulation of collagen-associated anabolic pathways (Figure 5).
Subchondral bone biopsies in MetS-associated CTA show differential expression profiles dominated by downregulation of anabolic genes as well as several inflammatory genes, including ZC3H13, DNER, and MX1 (Table 4).

Table 4.
Top DEGs in subchondral bone of MetS-associated CTA, corrected for age, sex, and CRP.
Subchondral bone biopsies in MetS-associated primary shoulder OA show a more inflammatory profile; upregulated anabolic and catabolic processes also demonstrated this (Table 5).

Table 5.
Top DEGs in subchondral bone of MetS-associated primary OA, corrected for age, sex, and CRP.
The effect of MetS on CTA and primary shoulder OA with respect to Reactome gene pathway activity as analysed with mitch demonstrated the upregulation of apoptotic pathways (Table 6), those pertaining to energy production, and the maintenance of genomic stability (see Figure 6 and Figure 7).

Table 6.
Top N = 50 Reactome pathways implicated in MetS-associated primary OA.
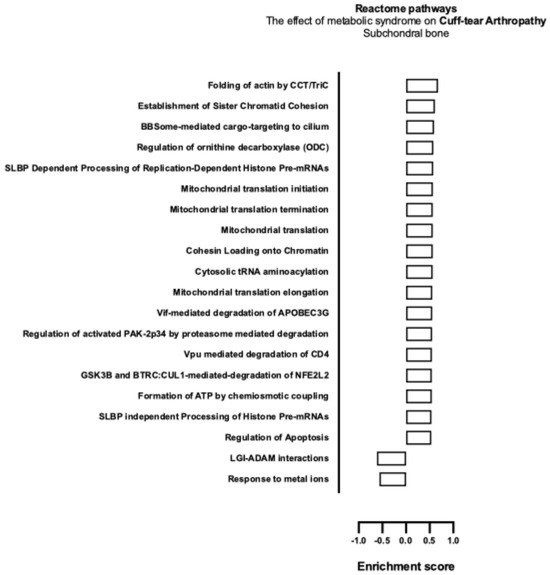
Figure 6.
Biological enrichment processes in CTA demonstrating the effect of MetS in subchondral bone biopsies.
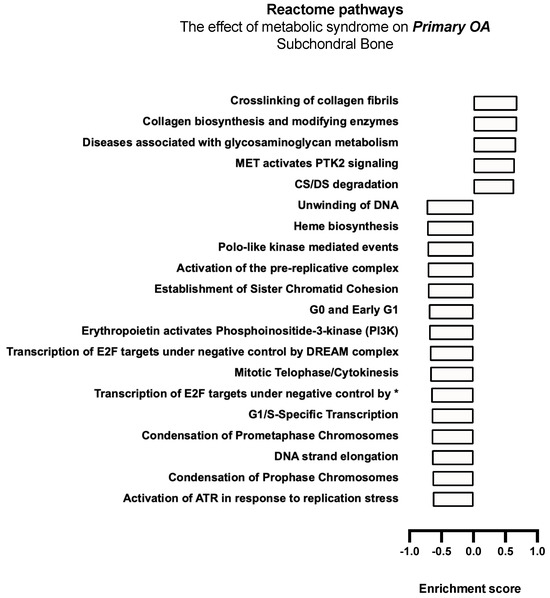
Figure 7.
Biological enrichment processes in primary OA demonstrating the effect of MetS in subchondral bone biopsies. * Transcription of E2F targets under negative control by p107 (RBL1) and p130 (RBL2) in complex with HDAC1.
MetS-associated primary OA demonstrated a significant downregulation of anabolic and cell cycle progression, DNA replication, and DNA repair pathways, including decreased G0 and early G1 Reactome, G1/S transcription, prometaphase chromosomal condensation, DNA unwinding, as well as the transcription of E2F targets under negative control by the DREAM complex pathway, indicating a significant reduction in anabolic processes in subchondral bone biopsies. Upregulation of the chondroitin sulphate (CS) and dermatan sulphate (DS) degradation pathway was also noted (Figure 7).
When comparing primary OA to RCT (see Figure 8), OA exhibited upregulation of pathways related to translation and protein synthesis (e.g., ribosomal scanning, translation initiation, and elongation) and RNA processing (e.g., nonsense-mediated decay), while immune activation, complement signalling, glycolysis, and chaperone activity were downregulated.
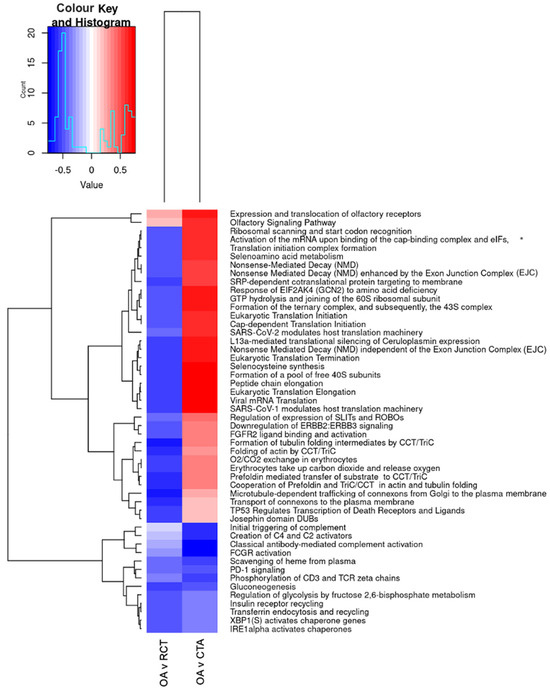
Figure 8.
Differential pathway regulation in primary shoulder OA, RCT, and cuff-tear arthropathy (CTA). * Activation of the mRNA upon binding of the cap-binding complex and eIFs, and subsequent binding to 43S. The heatmap displays significantly enriched pathways across tissue comparisons: OA vs. RC (first column, OA v RCT) and CTA vs. OA (second column, OA v CTA). The colour scale represents the extent of pathway upregulation (red) or downregulation (blue), with the histogram in the top left corner showing the distribution of values.
When comparing CTA to primary OA, similar increases in translation and metabolic activity were observed, with additional upregulation of Wnt/β-catenin signalling and extracellular matrix remodelling pathways (SLIT/ROBO regulation). In contrast, immune response pathways, complement activation, and glucose metabolism were significantly downregulated. These results suggest that CTA exhibits increased metabolic and translational activity, but a reduced immune response compared to primary OA, whereas OA shows greater immune activation and metabolic dysregulation compared to RCTs.
4. Discussion
Primary glenohumeral OA involves progressive cartilage wear with typical features like osteophyte formation and subchondral sclerosis, whereas CTA arises specifically following a chronic massive RCT that alters joint biomechanics. Additionally, the loss of the normal sealed (“water-tight”) joint compartment in CTA can compromise synovial nutrition to the cartilage, further accelerating degeneration. In CTA, the deficient rotator cuff causes abnormal glenohumeral loading (e.g., superior migration of the humeral head) and instability, resulting in characteristic wear patterns, as explained by Hamada [95]. Gene regulation in shoulder OA and CTA associated with MetS remains unexplored. This study focuses on the transcriptomic characteristics of these conditions and the role of MetS, revealing that MetS impacts CTA and primary OA differently. In CTA, cartilage anabolism is coupled with upregulated degradation pathways, impaired DNA repair, and disrupted cell cycle progression. In contrast, primary OA involves the upregulation of inflammatory and catabolic genes linked to collagen biosynthesis. Further, MetS in the primary OA group is associated with the upregulation of the chondroitin sulphate (CS) and dermatan sulphate (DS) degradation pathway, responsible for the breakdown of glycosaminoglycans (GAGs), a crucial element of cartilage.
4.1. Common Molecular Pathways Involved in Primary OA and CTA
ERK1/2 Pathway Activation
The ERK1/2 pathway is activated in both CTA and primary OA, particularly in the presence of MetS. The PI3K/Akt signalling pathway plays a pivotal role in regulating chondrocyte proliferation and apoptosis in OA. The downregulation of the PI3K pathway in MetS-associated OA is both a notable and anticipated finding. Previous studies have proposed that inflammation may inhibit chondrocyte proliferation and the autophagy rate [96]. Ghrelin, a neuropeptide known for its anti-inflammatory properties, has been found to exist in lower levels in individuals with a higher prevalence of MetS [97]. Additionally, ghrelin has been shown to protect against OA by downregulating inflammatory responses [98]. Consequently, it is plausible that mitigating inflammatory mediators associated with MetS could activate the PI3K/Akt pathway, thereby reducing chondrocyte apoptosis.
Further, Simonaro et al. have posited that inflammation significantly contributes to chondrocyte apoptosis through the accumulation of glycosaminoglycans (GAGs) in various bone and joint diseases [99]. Although the precise mechanisms underlying chondrocyte apoptosis in OA remain elusive, in vitro studies indicate that normal chondrocytes exposed to accumulated GAGs exhibit increased levels of the pro-apoptotic lipid ceramide, suggesting a direct impact of GAG fragments on chondrocyte viability [100].
GAGs, including hyaluronan, have recently been implicated in several pathological processes, including the inflammatory response, diet-induced insulin resistance, adipogenesis, and autoimmunity in type 1 diabetes [101]. The observed association between dysregulated GAG metabolism and MetS in patients with primary shoulder OA suggests that the inflammatory effects of MetS contribute to the progression towards end-stage disease, although elucidating the potential causative mechanisms warrants further research.
4.2. Distinct Gene Expression and Molecular Pathways Differentiating Primary OA and CTA
CTA is marked by mitochondrial dysfunction and GATD3A downregulation. Mitochondrial dysfunction in CTA contributes to oxidative stress, ATP depletion, and cartilage degeneration, making it a promising target for therapeutic intervention. In this condition, inflammatory and catabolic genes are activated alongside mitochondrial gene dysfunction, similarly seen in knee OA [21]. GATD3A plays a role in mitochondrial homeostasis and regulates advanced glycation end products (AGEs), which accumulate when GATD3A is knocked down, leading to non-enzymatic glycation of collagen [24,102]. This impairs osteoblast differentiation and bone remodelling [103]. Interestingly, GATD3A was not differentially expressed in MetS samples where glycaemic control was poorer, suggesting alternative pathogenic mechanisms in CTA other than non-enzymatic glycation. Human OA chondrocytes with impaired mitophagy exhibit increased ATP production and mitochondrial dysfunction, activating inflammasomes and releasing pro-inflammatory cytokines [22,23], leading to extracellular matrix breakdown [104]. GATD3A downregulation intensifies mitochondrial dysfunction, possibly accelerating OA progression by inducing chondrocyte death and activating apoptotic pathways [24,63]. These findings indicate that GATD3A downregulation and mitochondrial dysfunction contribute to end-stage CTA.
Enhancing antioxidant defences (e.g., SOD2 upregulation) and activating SIRT3/AMPK signalling can improve mitochondrial quality control and energy production, protecting chondrocytes from apoptosis [105]. Mitochondria-targeted antioxidants, including Mito-TEMPO, melatonin, quercetin, and dihydromyricetin (DHM), show chondroprotective effects by scavenging ROS and preserving mitochondrial function. DHM specifically enhances antioxidant capacity and matrix synthesis (aggrecan, collagen II) via SIRT3 activation, while resveratrol stabilizes membrane potential and ATP levels, preventing chondrocyte apoptosis [105].
Although not yet standard therapy for OA or CTA, these approaches could mitigate oxidative stress in cuff-tear arthropathy, improve cartilage resilience, and slow disease progression, complementing current surgical and symptom-based treatments.
One of the most notable findings was the increased translation and protein synthesis activity in CTA compared to OA. The upregulation of pathways related to translation initiation, elongation, and ribosomal function suggests heightened chondrocyte or fibroblast activity, likely contributing to greater extracellular matrix (ECM) remodelling in CTA. This may reflect a more dynamic tissue repair or remodelling process, distinguishing it from achondrocytic ECM synthesis and cartilage matrix degradation driven by inflammation in primary OA [106]. Another key difference was the significant downregulation of immune and complement activation pathways (Creation of C4 and C2 activators, classical antibody-mediated complement activation) in CTA. In patients undergoing hip, knee, and shoulder arthroplasty for primary and post-traumatic OA and rheumatoid arthritis, complement factors were detected in osteochondral tissue and were further upregulated in response to IL-1β stimulation, implicating the alternative complement pathway in OA progression [107]. The downregulation of complement system components (C4/C2 activation, classical antibody-mediated complement activation) and Fc receptor (FCGR) signalling in our study suggests that CTA exhibits a lower inflammatory immune response compared to OA. This contrasts with the chronic low-grade inflammation characteristic of OA. Findings from our previous study demonstrated that complement activation is both a localized process and a distinct feature of primary shoulder OA [108]. These findings underscore the distinct role of complement-driven inflammation in OA pathogenesis, setting it apart from CTA, which appears to be driven more by mechanical and metabolic factors rather than immune-mediated inflammation
4.3. The Effect of MetS on CTA
WIF1 and RSPO4 Downregulation
The Wnt/β-catenin pathway, a crucial regulator of skeletal development and tissue homeostasis, becomes dysregulated in OA. In healthy adult joints, Wnt signalling is relatively quiescent, maintaining a balance between cartilage anabolic and catabolic activities. In OA (including shoulder OA and, as we demonstrate, CTA), excessive Wnt/β-catenin activity has been observed in articular cartilage, subchondral bone, and synovium [109]. Aberrant activation of canonical Wnt signalling drives chondrocytes towards a hypertrophic and catabolic phenotype. Specifically, Wnt target genes such as WISP-1 (Wnt1-inducible signalling protein-1) are upregulated in human and experimental OA, and they stimulate the production of matrix-degrading enzymes, breaking down collagen and aggrecan [109]. Specifically, Wnt5a (a non-canonical Wnt) has been shown to reduce aggrecan (ACAN) expression while increasing MMP-1 and MMP-13, underscoring how Wnt pathways tilt the balance towards matrix catabolism [110].
Alongside enzymatic degradation, excessive Wnt/β-catenin signalling promotes chondrocyte term hypertrophy, which promotes shedding and calcification [111].
Wnt agonists initiate excessive bone remodelling, whilst Wnt antagonists have a protective effect on cartilage [112]. Interestingly, in cuff-tear arthropathy, radiographic analyses note a relative lack of osteophytes and, instead, the presence of subchondral osteopenia and even humeral head collapse in late stages [113]. This suggests that the bone response in CTA may differ from typical OA, potentially due to disuse (from reduced joint loading) or altered mechanotransduction. However, the principle remains that Wnt signalling drives aberrant remodelling: when overactive, it encourages bone formation at joint margins (osteophytes) and when deficient, bone loss can occur. Animal models support that a need for balanced Wnt signalling both excessive activation and complete inhibition of β-catenin in chondrocytes can worsen cartilage outcomes [109]. Thus, too much Wnt causes breakdown, but too little can impair repair and lead to degeneration, indicating a U-shaped relationship. This nuanced understanding is important for therapeutics, as simply blocking Wnt might have unintended consequences if not carefully controlled.
MetS-associated CTA disrupts key pathways involved in cartilage and bone health. WIF1 and RSPO4 were downregulated across all tissues, impairing WNT/β-catenin signalling, which is essential for regulating chondrocyte proliferation, bone mineralization, and inflammation. This pathway, crucial in OA pathogenesis [114], remains underexplored in CTA. Additionally, the downregulation of INTS6.AS1, C21orf62.AS1, and WNT16, a β-catenin inhibitor, further influences WNT signalling in OA [38]. In contrast, the upregulation of NOTUM, a WNT inhibitor, in MetS-associated primary OA suggests it may play a protective role in OA [115].
In the early stages of OA, articular chondrocytes exhibit reduced metabolic activity, accompanied by hypertrophy and apoptosis. This is marked by a gradual decline in cartilage-specific gene expression, including Col2a1 and aggrecan, alongside an upregulation of hypertrophic markers like Runx2 and Col10a1. Additionally, there is an increase in the expression of catabolic enzymes such as Mmp13, Adamts4, and Adamts5, which contribute to cartilage breakdown [109]. We found that in CTA, COL2A1 expression is upregulated within the assembly of collagen fibrils and other multimeric structures pathway, suggesting a potential compensatory response to cartilage degradation. Additionally, genes associated with OA progression, including the hypertrophic marker COL10A1 and catabolic enzymes MMP13, MMP9, and MMP3, are also upregulated, indicating an active extracellular matrix remodelling process consistent with previous findings in OA pathology [116,117].
4.4. Fine-Tuning Wnt/β-Catenin Signaling: Balancing Cartilage Remodeling in OA and CTA
Given Wnt/β-catenin’s central role in joint tissue remodelling, it represents an attractive but challenging therapeutic target. The goal is to dial down the pathological Wnt signalling seen in OA/CTA without abolishing its normal protective effects. Early experimental therapies include small-molecule Wnt pathway inhibitors and biologics that sequester Wnt ligands. One notable example is SM04690 (lorecivivint), an intra-articular Wnt pathway inhibitor previously investigated in knee OA. In preclinical models, SM04690 promoted chondrocyte differentiation and was chondroprotective, suggesting a disease-modifying potential [118]. Phase 2 studies in humans indicated some benefit in pain scores and radiographic stabilization of the joint [119]. Further, using intra-articular Wnt antagonists such as sclerostin (Wnt signalling inhibitor) was shown to have anti-catabolic effects on cartilage in animal models [109]. Whilst no Wnt-targeted therapy has been investigated in shoulder arthritis, these efforts suggest that by fine-tuning Wnt/β-catenin activity, we may be able to curtail cartilage breakdown as well as abnormal bony remodelling in degenerative shoulder conditions. Such therapy would need to be carefully calibrated, given the pathway’s dual-edged nature.
Proteasome degradation of β-catenin through activation of the canonical WNT pathway saturates the destruction complex, allowing β-catenin accumulation and factor-dependent transcription [120]. Future studies should seek to delineate the biomolecular mechanism of transcription-related outcomes on cartilage homeostasis. In contrast to GATD3A-related mitochondrial dysfunction in end-stage CTA, WNT/β-catenin signalling acts in primary OA progression due to the disruption of the balance of its regulatory factors. A precise balance of WNT signalling is crucial for cartilage homeostasis, as both suppression and excessive β-catenin activation contribute to cartilage degradation in OA [110]. WISP1, a key regulator of Wnt signalling, is elevated in OA and promotes cartilage breakdown by inducing MMP-3, MMP-13, ADAMTS-4, and ADAMTS-5 [110].
4.5. Impaired Metabolic and Cellular Stress Responses
The intricate interplay of differentially expressed pathways in CTA in patients with MetS reveals chronic inflammation, metabolic dysregulation, and oxidative stress: key features of both conditions. In MetS, oxidative stress and metabolic overload impair mitochondrial function and protein folding [121], reducing ATP production and contributing to cellular dysfunction and disease progression. This oxidative stress also compromises mitochondrial function in OA, reducing energy levels and increasing apoptosis in chondrocytes, which accelerates cartilage degradation [122].
In CTA, we demonstrated the upregulation of ornithine decarboxylase (ODC) and the downregulation of cellular response to metal ions, highlighting active cellular stress responses in this condition. Hypoxia-associated pathways, positively enriched in CTA, suggest an adaptive response to low oxygen levels, as has been demonstrated in early rotator cuff disease [123]. Key pathways differentially expressed in CTA, including mitochondrial translation, ATP formation, the degradation of NFE2L2 (Nrf2) by GSK3B and BTRC, and apoptosis regulation, are all influenced by hypoxia and oxidative phosphorylation [124,125,126,127]. The stress response protein Nrf2, known for its antioxidative and anti-apoptotic roles in osteoarthritic chondrocytes, is considered a potential therapeutic target for managing OA [126,128]. Further, disrupted DNA replication and repair processes lead to genomic instability and impaired cell proliferation [129], which we have demonstrated to be features of CTA, given MetS with the differential expression of the establishment of sister chromatid cohesion, cohesin loading onto chromatin, and SLBP-dependent processing of replication-dependent histone pre-mRNAs.
4.6. Hypoxia-Responsive Pathways and Emerging Pharmacological Options for Shoulder OA
The activation of those molecular pathways responsive to hypoxic conditions may signal cellular stress and initiate physiological adaptations aimed at coping with reduced oxygen availability. However, the dysregulation or overactivation of these pathways may create a detrimental feedback loop, perpetuating or exacerbating hypoxia. In the near term, several pharmacologic agents being trialled for OA in general could be applied to shoulder joints. Antioxidant and mitochondria-targeted drugs could reduce oxidative cartilage damage, as evidenced by compounds like melatonin and taurine improving chondrocyte survival and matrix synthesis in preclinical models [105].
4.7. The Effect of MetS on Primary OA
MetS Upregulates ACAN, PANX3, CLU, and VAT1L
Aggrecan is the major proteoglycan of articular cartilage, responsible for the tissue’s compressive stiffness and hydration. In shoulder OA, as in other forms of OA, the aggrecan content is progressively lost from the cartilage matrix due to enzymatic degradation [130]. Aggrecanases (primarily ADAMTS-5 and ADAMTS-4) cleave aggrecan, leading to depletion of this key molecule and loss of cartilage resilience [130]. Wnt/β-catenin overactivation exacerbates this problem by upregulating those aggrecan-degrading enzymes [109] and by downregulating expression in chondrocytes [110]. The end result is a vicious cycle: cartilage with reduced aggrecan is more vulnerable to mechanical stress, which, in turn, can further injure chondrocytes and perpetuate inflammation.
In MetS-associated OA, several genes are upregulated, reflecting complex interactions between cartilage regulation, inflammation, and metabolism. ACAN, known for its anabolic role in cartilage, is promoted by MIA, which inhibits ERK signalling [88]. Although heightened ACAN expression is observed in shoulder OA linked to MetS, contrasting with reduced SOX9 activation in knee OA [131], increased ACAN expression does not always signify proper cartilage maintenance. It also indicates increased catabolic processes affecting aggrecan [132].
4.8. Potential Therapeutic Strategies Targetting Aggrecan and PANX3 in Osteoarthritis
To preserve and restore aggrecan in arthritic cartilage, two strategies are being explored, inhibiting aggrecan breakdown and stimulating aggrecan synthesis. On the anti-catabolic front, pharmaceutical companies have developed ADAMTS-5 inhibitors as potential disease-modifying OA drugs. For example, GLPG1972/S201086 is an orally available ADAMTS-5 inhibitor that was tested in a phase 2 trial (the ROCCELLA study) for knee OA [133]. The rationale is that blocking the major aggrecanase will slow aggrecan depletion and cartilage erosion. Although most trials so far have focused on weight-bearing joints, the same principle could apply to the shoulder. Another anti-catabolic approach is using biologic agents (e.g., monoclonal antibodies or nanobodies) against aggrecanases. Specifically, M6495, a nanobody targeting ADAMTS-5, has shown safety in early trials [134]. Further, growth factor therapies endeavour to enhance chondrocyte production of aggrecan and collagen. Sprifermin (rhFGF18) is an anabolic therapy that has reached clinical trials, and stimulates chondrocyte proliferation and matrix synthesis, increasing cartilage thickness in a dose-dependent manner while also reducing catabolic enzyme activity in knee OA [135]. By promoting aggrecan and collagen II deposition, sprifermin effectively attempts to tilt the scales towards joint repair. Though these interventions are not routinely available, they demonstrate the translational potential of research aimed at finding effective disease-modifying OA drugs (DMOADs). Further exploration targeting aggrecanase inhibition represents a promising avenue for disease-modifying treatments in OA [133].
Pannexin 3 is a channel-forming glycoprotein expressed in cartilage and bone cells, involved in mechanotransduction and ATP/calcium signalling between cells. Recent studies have identified PANX3 as a pro-catabolic factor in OA pathology. Notably, PANX3 expression is low in healthy articular cartilage but is strongly upregulated in osteoarthritic cartilage lesions in both mice and humans [91]. Specifically, mice lacking PANX3 in cartilage were resistant to developing post-traumatic OA after injury (destabilization of the meniscus) [91]. This suggests that PANX3 contributes to cartilage degeneration under stress conditions, possibly by facilitating the release of inflammatory ATP signals or by altering chondrocyte mechanosensitivity. Interestingly, the role of PANX3 may be context-dependent, with some evidence indicating that while PANX3 drives injury-induced OA, its absence in natural ageing may have complex effects, as PANX3 knockout mice showed accelerated spontaneous cartilage degeneration [136]. Nonetheless, in the setting of relative joint concentricity, such as MetS-OA in our study, PANX3 upregulation, we posit, promotes the joint’s degenerative response. PANX3 is vital for chondrogenesis and osteogenesis [91], activating PI3K/AKT signalling and stimulating connexin 43 (Cx43), a key pro-inflammatory factor, particularly in shoulder OA [19,90,137,138].
4.9. Novel Targets and Therapeutic Approaches: Inhibiting PANX3/Cx43 and Harnessing Clusterin
Targeting Panx3 and Cx43 might offer therapeutic options to mitigate inflammation in primary shoulder OA. The success in preventing OA progression without altering skeletal structure in Panx3 knockdown mice highlights its potential for gene therapy [91]. PANX3 represents a novel target for chondroprotective therapy, wherein inhibiting this channel could slow cartilage breakdown. Pharmacologically, drugs that block pannexin channels are being considered. Probenecid is known to broadly inhibit pannexin 1 channels and has been used in research to inhibit pannexin-mediated signalling [139]. The future development of selective PANX3 inhibitors or blocking antibodies might provide a more targeted approach. From a genetic standpoint, experimental methods like siRNA or gene editing could knock down PANX3 expression in joint tissues. The proof-of-concept stems from the Panx3 knockout mice previously discussed, which showed cartilage protection in injury models [91]. The translational challenge will be to achieve tissue-specific inhibition in the human joint, for example, an intra-articular injection of a PANX3 antisense oligonucleotide or a viral vector carrying a dominant-negative PANX3. As these are early-stage ideas, safety and off-target effects (given PANX3’s roles in normal physiology, including bone growth) must be carefully evaluated.
Clusterin (CLU), a chaperone protein, is associated with joint inflammation and synovitis, particularly in obesity [49,140]. It plays a protective role in early OA but decreases in advanced stages [141], acting as a molecular chaperone and cellular stress protein facilitating the clearance of cellular debris, inhibiting protein aggregation, and modulating apoptosis and inflammation [142]. Under inflammatory stress (e.g., exposure to IL-1β), chondrocytes increase the production of clusterin, suggesting a stress response; however, evidence suggests that in late-stage OA cartilage, overall clusterin expression can be reduced through chondrocyte senescence or the exhaustion of this protective mechanism [141].
The functional importance of CLU in cartilage was highlighted by a recent in vitro study: adding exogenous clusterin to human OA chondrocytes significantly blunted inflammation and cell death. Clusterin treatment suppressed the production of inflammatory mediators (NO, IL-6, TNF-α) and the pro-apoptotic effector caspase-3, while upregulating key anabolic genes like SOX9 and ACAN aggrecan [143]. It also downregulated catabolic and inflammatory genes (NF-κB, MMP-13, IL-6) via activation of the pro-survival PI3K/Akt pathway [143].
These findings illustrate that clusterin is a natural dampener of OA pathogenesis, counteracting the cartilage-destructive milieu. As OA progresses, chondrocytes shift from a stable phenotype (COL2A1, ACAN) to a hypertrophic state (COL10A1, MMP13) liable to shedding [144,145].
4.10. CLU as a Potential Synovial and Systemic Biomarker: Therapeutic Pathways in MetS-Associated OA
CLU silencing in OA chondrocytes has been shown to increase MMP13 and COL10A1 expression, suggesting a protective role in maintaining chondrocyte stability [146]. Further, anti-inflammatory properties position CLU as a potential synovial and systemic biomarker of OA [147]. Augmenting clusterin levels or activity in the joint is a promising therapeutic concept to harness its chondroprotective effects. Pharmacologically, one might envision administering recombinant clusterin protein into the joint space to supplement what osteoarthritic chondrocytes may lack. Given that CLU is secreted and works extracellularly (and at the cell surface), an injected form might directly influence the cartilage surface and synovium. Another approach may be to use small molecules to upregulate the CLU gene expression in chondrocytes. From a genetic therapy perspective, gene delivery of CLU via viral vectors could ensure a sustained local increase in clusterin. For instance, an adeno-associated virus (AAV) carrying the human CLU gene could be injected into the shoulder joint, leading to chondrocytes and synoviocytes secreting higher amounts of clusterin protein. Such gene therapy approaches are speculative but increasingly feasible as techniques improve; notably, gene therapies for joint diseases (like the IL-1 receptor antagonist gene for arthritis) have been tested in clinical trials. Encouragingly, the recent in vitro data make a strong case that boosting clusterin could protect cartilage, with the authors concluding that CLU is a promising target for therapeutic intervention in OA [143]. Moving this forward will require in vivo studies to see if clusterin delivery can indeed slow or reverse cartilage degeneration in animal models of shoulder arthritis. If successful, clusterin-based therapy might provide an anti-inflammatory and anti-apoptotic treatment option distinct from traditional steroids or NSAIDs.
Adiponectin, which is typically beneficial in MetS, is paradoxically linked to increased OA risk [148,149]. VAT1L, associated with bone metabolism, is upregulated in MetS-associated OA. Its relationship with single-nucleotide polymorphisms, which correlate with higher plasma adiponectin levels, may reveal its role in cartilage regeneration and potential as a biomarker for OA progression [76]. These findings highlight the intricate balance between anabolic and catabolic processes in MetS-associated OA, suggesting that targeting specific genes involved in cartilage regulation, inflammation, and metabolism could offer new therapeutic approaches for managing disease progression and severity.
4.11. Pathways in MetS and Primary Shoulder OA
In MetS-associated OA, we observed downregulation of G0, early G1, and G1/S transcriptional activity in subchondral bone, indicating impaired chondrocyte cell cycle progression. This aligns with existing knowledge that WNT/β-catenin signalling regulates chondrocyte proliferation by halting the cell cycle at the G1/S restriction point in a glucosamine-dependent manner [114]. WNT/β-catenin also regulates Cx43 expression, a gene linked to inflammation in OA [90]. Furthermore, variations in the FRZB gene, especially in women with hip OA, reduce WNT pathway antagonism, further disrupting cartilage maintenance [150].
MetS impacts the expression of extracellular matrix (ECM) biosynthetic pathways. Among these, the collagen fibril crosslinking pathway emerges as the most positively enriched pathway affected by MetS in primary OA. This pathway is crucial for organising the ECM, serving as a cornerstone for maintaining the mechanical strength and structural integrity of various tissues throughout the body. Understanding how MetS alters these pathways can lead to novel interventions aimed at restoring ECM homeostasis and potentially mitigating the severity of shoulder OA. Research conducted by Steinberg et al. (2021) on cartilage and synovial biopsies from patients undergoing total knee replacement revealed distinct disease processes that are specific to the tissue type. The analysis indicated that the synovium exhibited unique expression profiles related to ECM receptor interactions and focal cell adhesion pathways [151], aligning with our finding of pronounced upregulation of collagen fibril crosslinking pathways in MetS-OA. This excessive crosslinking leads to the stiffening of cartilage, diminishing its flexibility at the articular surface and the cartilage–bone interface [152]. Such alterations impair the cartilage’s capacity to absorb mechanical stress, resulting in accelerated catabolism of chondrocytes while simultaneously hindering their anabolic processes in OA [153]. This dual effect exacerbates the progression of OA, highlighting the need for targeted therapeutic strategies that can mitigate these biochemical changes and restore normal cartilage function.
Moreover, the accumulation of advanced glycation end products (AGEs) is a critical factor in this context. AGEs, which can form crosslinks with collagen, contribute to the “age-related failure” of collagen in human articular cartilage, rendering it more susceptible to structural damage [154]. Notably, elevated levels of AGEs have been documented in individuals with MetS, potentially exacerbating joint space narrowing in knee OA [155]. Our observations underscore the significant differential expression of collagen fibril crosslinking pathways in primary shoulder OA associated with MetS, highlighting a potential link between AGEs and the progression of OA.
4.12. Chondroitin Sulphate Degradation Pathways in MetS-Associated OA Pathogenesis
The downregulation of critical pathways in MetS-associated OA is expected to adversely affect DNA repair mechanisms, cell cycle progression, and transcriptional regulation, while also disrupting cellular metabolism in end-stage shoulder OA. These alterations contribute to diminished chondrocyte functionality, increased apoptosis, impaired cartilage repair processes, and accelerated joint degeneration. Furthermore, the degradation pathways of chondroitin sulphate/delta-sulphated (CD/DS) were upregulated in MetS-associated OA, influencing various aspects of cartilage homeostasis and joint function. Hyperglycaemia associated with MetS can alter the function of the disaccharide-containing CS and DS and is linked to altered ECM function in a range of tissues that drive complications associated with MetS [156]. Despite the substantial differences between these diseases, our findings indicate that the upregulation of CD/DS degradation pathways due to MetS implies a role for hyperglycaemia and/or inflammatory pathways in this process.
4.13. Translational Insight, GLP-1 Agonists
Recent evidence posits the beneficial role GLP-1 Agonists, used in the treatment of diabetes, may have in alleviating OA progression through interactions between cartilage, synovium, and subchondral bone through homeostatic mechanisms via the gut–bone axis, and locally by suppressing pro-degradative and inflammatory enzymatic processes [157].
4.14. Limitations
This study is the first transcriptomic analysis comparing gene expression in MetS-associated primary OA and CTA patients as well as 25 patients undergoing RCR as a comparator group; we analysed 45 patients in total and 85 biopsies, one of the largest datasets of its kind. Due to ethical constraints, samples were limited to patients requiring shoulder arthroplasty, reducing variance in confounding factors like age and occupational demands. Future studies should validate findings with larger, more diverse cohorts.
Technical constraints capped RNA sequencing and PCR at 15 million reads per sample, limiting comprehensive sequence coverage. Full diversity requires up to 500 million reads, exceeding industry standards [158]. Bulk RNA-seq measures average gene expression across samples, unlike single-cell RNA-seq, which accounts for cellular heterogeneity. Despite this, RNA-seq is a robust method, often eliminating the need for RT-qPCR validation, as demonstrated by strong concordance in prior studies [159,160].
Our data reflect transcriptomic profiles of end-stage OA and CTA in patients undergoing joint replacement, leaving early disease mechanisms uncertain. Variable gene expression across a lifetime, as seen with clusterin, remains unexplored [141]. Nevertheless, our findings establish a foundation for future research on early OA and chronic RCTs, potentially aiding in early diagnosis and targeted intervention, particularly for MetS-associated pathologies.
5. Materials and Methods
Clinical Trial Number: This investigation has been registered with the Australian New Zealand Clinical Trials Registry (ANZCTR), registered on the 26 March 2018, registration number 12618000431224, accessible from https://anzctr.org.au/Trial/Registration/TrialReview.aspx?id=374665&isReview=true.
5.1. Patients
This was a prospective, longitudinal, multi-centre clinical and laboratory-based study. A total of 20 patients with shoulder OA undergoing either anatomic or reverse total shoulder replacement were included. Eligible patients had at least six months of symptoms with radiographic evidence of OA. Those diagnosed with cuff-tear arthropathy (CTA) based on the Hamada classification [161] underwent reverse total shoulder replacement (n = 4), whereas those without full-thickness rotator cuff tears (RCTs) diagnosed on ultrasound or MRI underwent anatomic total shoulder replacement (n = 16). Patients with inflammatory arthritis, prior shoulder fractures or dislocations, or corticosteroid injections within three months were excluded.
Given the well-established progression from chronic RCTs to CTA, a control group of 25 patients with chronic RCTs was included to provide a comparative baseline. These patients had symptomatic, full-thickness RCTs for over six months, confirmed by ultrasound or MRI. Exclusion criteria included inflammatory arthritis, prior shoulder trauma, or corticosteroid injection within three months.
Written informed consent was obtained from all patients. Surgeries were performed by three specialized shoulder surgeons, who obtained tissue biopsies from subchondral bone, capsule, and synovial tissue in the primary OA and CTA groups, aggregating 60 separate tissue samples for analysis. In the RCT group, capsular tissue (3 × 3 mm) was collected to ensure comparable tissue types were analysed across pathologies in bioinformatic analysis.
This study design enables comparative analysis of molecular differences between primary OA, CTA, and chronic RCTs, providing insights into disease progression and distinct pathological mechanisms.
5.2. Clinical Data Collection
Data on preoperative patient characteristics, including age, sex, and smoking status, were collected along with clinical data, including body mass index (BMI), American Society of Anesthesiologists (ASA) physical status score [162], presence or absence of MetS and its constituents, and preoperative renal function.
5.3. RNA Extraction and Transcriptomics Analysis
All tissues underwent RNA extraction using a commercially available kit (Qiagen, Hilden, Germany), and the quantity and quality of RNA were measured using the Agilent Bioanalyzer (Santa Clara, CA, USA). The Illumina TruSeq RNA preparation kit (San Diego, CA, USA) was used to generate a library from 1 μg of RNA. Briefly, RNA was reverse transcribed to generate complementary DNA (cDNA), with reverse transcriptase supplemented with actinomycin-D during the first strand, followed by second strand synthesis. Double-stranded cDNA libraries were then sequenced using Illumina NovaSeq at the Deakin Genomics Facility, Geelong, Australia.
The resulting fastq files underwent QC analysis using FastQC (v0.12.1) and MultiQC (v1.26) tools [163,164]. Fastq files were quality trimmed using Skewer to remove 3′ ends with quality scores lower than 10 [162]. Trimmed reads were then mapped to the human Gencode transcriptome (version 378) [165] using Kallisto (v0.48.0), a transcript isoform-aware aligner [166]. Transcript counts were read into R (v4.4.1) and aggregated to the gene level for statistical analysis. This analysis was conducted using DESeq (v1.40.2) [167] and corrected for demographic information such as sex, age, and CRP. Principal component analysis was used to identify outliers and potential confounders.
Functional enrichment analysis approaches were used to determine pathway-specific alterations in gene expression, to provide new insights into the pathogenesis of OA and CTA. The pathway database was downloaded from Reactome [168]. The DESeq2 t-statistic was used to score differential expression for each gene, and the enrichment was evaluated using the mitch bioconductor package (v1.16.0) [169].
Differentially expressed genes (DEGs) and pathways (log fold change (LFC) ± 1.5 and FDR ≤ 0.01) were examined and compared in capsular tissue, subchondral bone, and capsule within and between the OA and CTA groups. Differential analysis with DESeq2 was performed, taking into consideration the sex, age, and CRP of the patients. Multi-contrast pathway analysis with mitch was undertaken, referencing Reactome pathways to identify differentially expressed pathways enriched in OA, and CTA in patients with and without MetS [169]. Further pathway analysis with mitch was undertaken, referencing Reactome pathways to identify differentially expressed pathways enriched in capsular tissue, comparing RCR to OA, and CTA. Nonparametric Wilcox tests were undertaken to reduce the batch effect. Pathway analysis was undertaken using results from DESeq2 and Wilcox testing. The code is available from https://github.com/markziemann/shoulder-instability-osteroarthritis/blob/main/metab_syndr.Rmd, accessed on 8 November 2024.
6. Conclusions
In conclusion, this study elucidates distinct transcriptomic signatures and molecular pathways differentiating primary glenohumeral OA from CTA, with particular emphasis on the modulatory role of MetS. Our findings highlight significant divergences, notably CTA’s association with mitochondrial dysfunction, impaired DNA repair, increased extracellular matrix remodelling, and reduced immune-mediated inflammatory responses compared to primary OA, which demonstrates prominent inflammatory gene activation and heightened extracellular matrix degradation through collagen biosynthesis pathways.
MetS exerts differential effects in these conditions. In CTA, MetS contributes to the disruption of WNT/β-catenin signalling via the downregulation of WIF1 and RSPO4, impacting cartilage and bone homeostasis. Conversely, in primary OA, MetS upregulates critical factors, including ACAN, PANX3, CLU, and VAT1L, enhancing cartilage catabolism, inflammation, and extracellular matrix stiffness, mediated by the accumulation of advanced glycation end products (AGEs). Notably, the chondroitin and dermatan sulphate degradation pathways, essential for cartilage integrity, are significantly upregulated in primary OA with MetS, underscoring the pathogenic role of metabolic dysregulation.
From a translational perspective, our study highlights promising therapeutic targets: mitochondrial antioxidants to combat oxidative stress in CTA; WNT pathway modulation through agents like lorecivivint and sclerostin for balancing cartilage remodelling; aggrecanase inhibitors to prevent cartilage breakdown; and clusterin augmentation for its chondroprotective potential. Additionally, targeting PANX3-mediated inflammatory signalling presents a novel intervention pathway. The nuanced understanding of gene expression and metabolic disruptions identified here provides a foundational platform for future research into early diagnostic markers and personalized, disease-modifying therapies for both primary shoulder OA and CTA, particularly within MetS-associated populations.
Author Contributions
S.J.L. conceptualised the analysis, performed RNA extraction, analysed and interpreted the data, and was the major contributor to the writing of the manuscript and creation of the tables and figures. Z.L. performed literature searches, created tables, and contributed to early drafts of the manuscript. M.Z. undertook the bioinformatic analysis, interpreted the data, and contributed to the creation of the figures and to the editing of the manuscript. S.D.G. contributed to the writing and editing of the manuscript. S.L.M. contributed to the writing and editing of the manuscript and the interpretation of the data. R.S.P. conceptualised the study and contributed to sample collection and the writing and editing of the manuscript. All authors have read and agreed to the published version of the manuscript.
Funding
Funding support for this work was obtained from the Australian Orthopaedic Association Research Foundation (AOARF), Grant I.D.: 25-Reg.
Institutional Review Board Statement
The study protocol conformed to the ethical guidelines of the 1975 Declaration of Helsinki as reflected in a prior approval, obtained through the Research Administration Officer, Research Ethics, Governance & Integrity (REGI) Unit of Barwon Health Human Research Ethics Committee (HREC), Reference number 15.15, on the 13 June 2019.
Informed Consent Statement
Consenting patients were required to read and understand the English-language Patient Information Form and give voluntary written informed consent. Participation in the study was voluntary and without financial incentive.
Data Availability Statement
The data presented in this study are available on request from the corresponding author.
Acknowledgments
We thank Sally Beattie for her help in tissue collection and processing and data collection and management. This research was supported by use of the Nectar Research Cloud, a collaborative Australian research platform supported by the NCRIS-funded Australian Research Data Commons (ARDC). The authors gratefully acknowledge the contribution to this work of the Victorian Operational Infrastructure Support Program received by the Burnet Institute.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| AMPK | Adenosine monophosphate-activated protein kinase |
| BMP-2 | Bone morphogenetic protein-2 |
| CS | Chondroitin sulphate |
| C21orf62.AS1 | Chromosome 21 Open Reading Frame 62 antisense RNA 1 |
| cDNA | Complimentary Deoxyribonucleic acid |
| Cx43 | Connexin 43 |
| CTA | Cuff-tear arthropathy |
| DS | Dermatan sulphate |
| DKK1 | Dickkopf-1 |
| DEGs | Differentially expressed genes |
| FRZB | Frizzled motif associated with bone development |
| FOSB | FosB Proto-Oncogene |
| INTS6.AS1 | Integrator complex subunit 6 antisense RNA 1 |
| KNDC1 | Kinase Non-Catalytic C-Lobe Domain Containing 1 |
| Panx3 | Pannexin 3 |
| MIA | Melanoma inhibitory activity |
| MAPK | Mitogen-activated protein kinase |
| OA | Osteoarthritis |
| CD-RAP | Retinoic acid-sensitive protein |
| SIRT-1 | Sirtuin-1 |
| STC2 | Stanniocalcin-2 |
| TGF-β3 | Transforming growth factor β3 |
| VAT1L | Vesicle Amine Transport 1-Like |
References
- Krakowski, P.; Rejniak, A.; Sobczyk, J.; Karpiński, R. Cartilage Integrity: A Review of Mechanical and Frictional Properties and Repair Approaches in Osteoarthritis. Healthcare 2024, 12, 1648. [Google Scholar] [CrossRef]
- Sharma, L. Osteoarthritis of the Knee. N. Engl. J. Med. 2021, 384, 51–59. [Google Scholar] [CrossRef] [PubMed]
- Quicke, J.G.; Conaghan, P.G.; Corp, N.; Peat, G. Osteoarthritis year in review 2021: Epidemiology & therapy. Osteoarthr. Cartil. 2022, 30, 196–206. [Google Scholar] [CrossRef]
- Kluzek, S.; Newton, J.; Arden, N. Is osteoarthritis a metabolic disorder? Br. Med. Bull. 2015, 115, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Wallace, I.J.; Worthington, S.; Felson, D.T.; Jurmain, R.D.; Wren, K.T.; Maijanen, H.; Woods, R.J.; Lieberman, D.E. Knee osteoarthritis has doubled in prevalence since the mid-20th century. Proc. Natl. Acad. Sci. USA 2017, 114, 9332–9336. [Google Scholar]
- Eajazi, A.; Kussman, S.; LeBedis, C.; Guermazi, A.; Kompel, A.; Jawa, A.; Murakami, A.M. Rotator Cuff Tear Arthropathy: Pathophysiology, Imaging Characteristics, and Treatment Options. Am. J. Roentgenol. 2015, 205, W502–W511. [Google Scholar] [CrossRef]
- Grotle, M.; Hagen, K.B.; Natvig, B.; Dahl, F.A.; Kvien, T.K. Obesity and osteoarthritis in knee, hip and/or hand: An epidemiological study in the general population with 10 years follow-up. BMC Musculoskelet. Disord. 2008, 9, 132. [Google Scholar] [CrossRef]
- Fahed, G.; Aoun, L.; Bou Zerdan, M.; Allam, S.; Bou Zerdan, M.; Bouferraa, Y.; Assi, H.I. Metabolic syndrome: Updates on pathophysiology and management in 2021. Int. J. Mol. Sci. 2022, 23, 786. [Google Scholar] [CrossRef]
- Courties, A.; Gualillo, O.; Berenbaum, F.; Sellam, J. Metabolic stress-induced joint inflammation and osteoarthritis. Osteoarthr. Cartil. 2015, 23, 1955–1965. [Google Scholar] [CrossRef]
- Jeffries, M.A. Osteoarthritis year in review 2018: Genetics and epigenetics. Osteoarthr. Cartil. 2019, 27, 371–377. [Google Scholar] [CrossRef]
- Wang, X.; Hunter, D.; Xu, J.; Ding, C. Metabolic triggered inflammation in osteoarthritis. Osteoarthr. Cartil. 2015, 23, 22–30. [Google Scholar] [CrossRef] [PubMed]
- Zhuo, Q.; Yang, W.; Chen, J.; Wang, Y. Metabolic syndrome meets osteoarthritis. Nat. Rev. Rheumatol. 2012, 8, 729–737. [Google Scholar] [CrossRef]
- Puenpatom, R.A.; Victor, T.W. Increased prevalence of metabolic syndrome in individuals with osteoarthritis: An analysis of NHANES III data. Postgrad. Med. 2009, 121, 9–20. [Google Scholar] [CrossRef]
- Chapman, K.; Valdes, A.M. Genetic factors in OA pathogenesis. Bone 2012, 51, 258–264. [Google Scholar] [CrossRef]
- Fernández-Moreno, M.; Rego, I.; Carreira-Garcia, V.; Blanco, F.J. Genetics in osteoarthritis. Curr. Genom. 2008, 9, 542–547. [Google Scholar] [CrossRef] [PubMed]
- Loughlin, J. Genetic contribution to osteoarthritis development: Current state of evidence. Curr. Opin. Rheumatol. 2015, 27, 284. [Google Scholar] [CrossRef]
- Reynard, L.N.; Barter, M.J. Osteoarthritis year in review 2019: Genetics, genomics and epigenetics. Osteoarthr. Cartil. 2020, 28, 275–284. [Google Scholar] [CrossRef]
- Onkarappa, R.S.; Chauhan, D.K.; Saikia, B.; Karim, A.; Kanojia, R.K. Metabolic Syndrome and Its Effects on Cartilage Degeneration vs Regeneration: A Pilot Study Using Osteoarthritis Biomarkers. Indian J. Orthop. 2020, 54 (Suppl. S1), 20–24. [Google Scholar] [CrossRef] [PubMed]
- Casagrande, D.; Stains, J.P.; Murthi, A.M. Identification of shoulder osteoarthritis biomarkers: Comparison between shoulders with and without osteoarthritis. J. Shoulder Elb. Surg. 2015, 24, 382–390. [Google Scholar] [CrossRef]
- Aleem, A.W.; Rai, M.F.; Cai, L.; Brophy, R.H. Gene Expression in Glenoid Articular Cartilage Varies Across Acute Instability, Chronic Instability, and Osteoarthritis. J. Bone Jt. Surg. Am. 2023, 105, 990–1000. [Google Scholar] [CrossRef]
- Ansari, M.Y.; Ahmad, N.; Voleti, S.; Wase, S.J.; Novak, K.; Haqqi, T.M. Mitochondrial dysfunction triggers a catabolic response in chondrocytes via ROS-mediated activation of the JNK/AP1 pathway. J. Cell Sci. 2020, 133, jcs247353. [Google Scholar] [CrossRef] [PubMed]
- Blanco, F.J.; Valdes, A.M.; Rego-Pérez, I. Mitochondrial DNA variation and the pathogenesis of osteoarthritis phenotypes. Nat. Rev. Rheumatol. 2018, 14, 327–340. [Google Scholar] [CrossRef] [PubMed]
- Dalmao-Fernández, A.; Hermida-Gómez, T.; Lund, J.; Vazquez-Mosquera, M.E.; Rego-Pérez, I.; Garesse, R.; Blanco, F.J.; Fernández-Moreno, M. Mitochondrial DNA from osteoarthritic patients drives functional impairment of mitochondrial activity: A study on transmitochondrial cybrids. Cytotherapy 2021, 23, 399–410. [Google Scholar] [CrossRef] [PubMed]
- Smith, A.J.; Advani, J.; Brock, D.C.; Nellissery, J.; Gumerson, J.; Dong, L.; Aravind, L.; Kennedy, B.; Swaroop, A. GATD3A, a mitochondrial deglycase with evolutionary origins from gammaproteobacteria, restricts the formation of advanced glycation end products. BMC Biol. 2022, 20, 68. [Google Scholar] [CrossRef]
- Sun, K.; Xu, L.; Jing, Y.; Han, Z.; Chen, X.; Cai, C.; Zhao, P.; Zhao, X.; Yang, L.; Wei, L. Autophagy-deficient Kupffer cells promote tumorigenesis by enhancing mtROS-NF-κB-IL1α/β-dependent inflammation and fibrosis during the preneoplastic stage of hepatocarcinogenesis. Cancer Lett. 2017, 388, 198–207. [Google Scholar] [CrossRef]
- Nong, S.; Chen, X.; Wang, Z.; Xu, G.; Wei, W.; Peng, B.; Zhou, L.; Wei, L.; Zhao, J.; Wei, Q.; et al. Potential lncRNA Biomarkers for HBV-Related Hepatocellular Carcinoma Diagnosis Revealed by Analysis on Coexpression Network. Biomed Res. Int. 2021, 2021, 9972011. [Google Scholar] [CrossRef]
- Lui, K.Y.; Zhao, H.; Qiu, C.; Li, C.; Zhang, Z.; Peng, H.; Fu, R.; Chen, H.-a.; Lu, M.-q. Integrator complex subunit 6 (INTS6) inhibits hepatocellular carcinoma growth by Wnt pathway and serve as a prognostic marker. BMC Cancer 2017, 17, 644. [Google Scholar] [CrossRef]
- Geng, N.; Yun, D.; Liu, D.; Liu, P. AB0053 LncRNA NUTM2A-AS1 Alleviated Osteoarthritis by Regulating miR-183-5p/TGFA Pathway. Ann. Rheum. Dis. 2022, 81 (Suppl. S1), 1161. [Google Scholar] [CrossRef]
- Dayal, A.A.; Medvedeva, N.V.; Nekrasova, T.M.; Duhalin, S.D.; Surin, A.K.; Minin, A.A. Desmin Interacts Directly with Mitochondria. Int. J. Mol. Sci. 2020, 21, 8122. [Google Scholar] [CrossRef]
- Hoffmann, B.R.; Stodola, T.J.; Wagner, J.R.; Didier, D.N.; Exner, E.C.; Lombard, J.H.; Greene, A.S. Mechanisms of Mas1 Receptor-Mediated Signaling in the Vascular Endothelium. Arter. Thromb. Vasc. Biol. 2017, 37, 433–445. [Google Scholar] [CrossRef]
- Zheng, X.; Wu, J.; Song, L.; Huang, B. ACSM3 suppresses proliferation and induces apoptosis and cell cycle arrest in acute myeloid leukemia cells via the regulation of IGF2BP2. Exp. Ther. Med. 2023, 25, 177. [Google Scholar] [CrossRef]
- Hao, H.; Nakayamada, S.; Ohkubo, N.; Yamagata, K.; Zhang, M.; Shan, Y.; Iwata, S.; Zhang, T.; Tanaka, Y. Involvement of lncRNA IL21-AS1 in interleukin-2 and T follicular regulatory cell activation in systemic lupus erythematosus. Arthritis Res. Ther. 2021, 23, 302. [Google Scholar] [CrossRef]
- Chen, Q.; Hu, Z.; Zhang, X.; Wei, Z.; Fu, H.; Yang, D.; Cai, Q. A four-lncRNA signature for predicting prognosis of recurrence patients with gastric cancer. Open Med. 2021, 16, 540–552. [Google Scholar] [CrossRef]
- Chen, X.; Li, Z.; Xu, D.; Li, S. LINC01121 induced intervertebral disc degeneration via modulating miR-150-5p/MMP16 axis. J. Gene Med. 2020, 22, e3231. [Google Scholar] [CrossRef]
- Dubin, R.L.; Hall, C.M.; Pileri, C.L.; Kudlacek, P.E.; Li, X.Y.; Yee, J.A.; Johnson, M.L.; Anderson, R.J. Thermostable (SULT1A1) and thermolabile (SULT1A3) phenol sulfotransferases in human osteosarcoma and osteoblast cells. Bone 2001, 28, 617–624. [Google Scholar] [CrossRef]
- Leask, M.; Dowdle, A.; Salvesen, H.; Topless, R.; Fadason, T.; Wei, W.; Schierding, W.; Marsman, J.; Antony, J.; O’Sullivan, J.M.; et al. Functional Urate-Associated Genetic Variants Influence Expression of lincRNAs LINC01229 and MAFTRR. Front. Genet. 2019, 9, 733. [Google Scholar] [CrossRef]
- Nishikawa, K.; Nakashima, T.; Takeda, S.; Isogai, M.; Hamada, M.; Kimura, A.; Kodama, T.; Yamaguchi, A.; Owen, M.J.; Takahashi, S.; et al. Maf promotes osteoblast differentiation in mice by mediating the age-related switch in mesenchymal cell differentiation. J. Clin. Investig. 2010, 120, 3455–3465. [Google Scholar] [CrossRef]
- Nalesso, G.; Thomas, B.L.; Sherwood, J.C.; Yu, J.; Addimanda, O.; Eldridge, S.E.; Thorup, A.S.; Dale, L.; Schett, G.; Zwerina, J.; et al. WNT16 antagonises excessive canonical WNT activation and protects cartilage in osteoarthritis. Ann. Rheum. Dis. 2017, 76, 218–226. [Google Scholar] [CrossRef]
- Yu, S.; Shen, J.; Fei, J.; Zhu, X.; Yin, M.; Zhou, J. KNDC1 is a predictive marker of malignant transformation in borderline ovarian tumors. OncoTargets Ther. 2020, 13, 709–718. [Google Scholar]
- Zhang, C.; Zhen, Y.Z.; Lin, Y.J.; Liu, J.; Wei, J.; Xu, R.; Hu, G. KNDC1 knockdown protects human umbilical vein endothelial cells from senescence. Mol. Med. Rep. 2014, 10, 82–88. [Google Scholar] [CrossRef]
- Kim, K.A.; Zhao, J.; Andarmani, S.; Kakitani, M.; Oshima, T.; Binnerts, M.E.; Abo, A.; Tomizuka, K.; Funk, W.D. R-Spondin proteins: A novel link to beta-catenin activation. Cell Cycle 2006, 5, 23–26. [Google Scholar] [CrossRef]
- Lu, J.-F.; Qi, L.-G.; Zhu, X.-B.; Shen, Y.-X. LncRNA RMRP knockdown promotes proliferation and inhibits apoptosis in osteoarthritis chondrocytes by miR-206/CDK9 axis. Pharm. Int. J. Pharm. Sci. 2020, 75, 500–504. [Google Scholar]
- Zhang, S.; Li, J.; Lea, R.; Vleminckx, K.; Amaya, E. Fezf2 promotes neuronal differentiation through localised activation of Wnt/β-catenin signalling during forebrain development. Development 2014, 141, 4794–4805. [Google Scholar] [CrossRef]
- Zhu, Z.; Bai, X.; Wang, H.; Li, X.; Sun, G.; Zhang, P. A study on the mechanism of Wnt inhibitory factor 1 in osteoarthritis. Arch. Med. Sci. 2020, 16, 898–906. [Google Scholar] [CrossRef]
- Won, Y.; Yang, J.I.; Park, S.; Chun, J.S. Lipopolysaccharide Binding Protein and CD14, Cofactors of Toll-like Receptors, Are Essential for Low-Grade Inflammation-Induced Exacerbation of Cartilage Damage in Mouse Models of Posttraumatic Osteoarthritis. Arthritis Rheumatol. 2021, 73, 1451–1460. [Google Scholar] [CrossRef]
- Zhu, H.; Zhu, X.; Liu, Y.; Jiang, F.; Chen, M.; Cheng, L.; Cheng, X. Gene Expression Profiling of Type 2 Diabetes Mellitus by Bioinformatics Analysis. Comput. Math. Methods Med. 2020, 2020, 9602016. [Google Scholar] [CrossRef]
- Choi, W.-S.; Lee, G.; Song, W.-H.; Koh, J.-T.; Yang, J.; Kwak, J.-S.; Kim, H.-E.; Kim, S.K.; Son, Y.-O.; Nam, H.; et al. The CH25H–CYP7B1–RORα axis of cholesterol metabolism regulates osteoarthritis. Nature 2019, 566, 254–258. [Google Scholar] [CrossRef]
- Ko, J.-Y.; Wang, F.-S.; Lian, W.-S.; Fang, H.-C.; Kuo, S.-J. Cartilage-specific knockout of miRNA-128a expression normalizes the expression of circadian clock genes (CCGs) and mitigates the severity of osteoarthritis. Biomed. J. 2023, 47, 100629. [Google Scholar] [CrossRef]
- Abed, É.; Chan, T.F.; Delalandre, A.; Martel-Pelletier, J.; Pelletier, J.-P.; Lajeunesse, D. R-spondins are newly recognized players in osteoarthritis that regulate Wnt signaling in osteoblasts. Arthritis Rheum. 2011, 63, 3865–3875. [Google Scholar] [CrossRef]
- Cui, D.; Li, L.; Lou, H.; Sun, H.; Ngai, S.M.; Shao, G.; Tang, J. The ribosomal protein S26 regulates p53 activity in response to DNA damage. Oncogene 2014, 33, 2225–2235. [Google Scholar] [CrossRef]
- Pang, K.; Park, J.; Ahn, S.G.; Lee, J.; Park, Y.; Ooshima, A.; Mizuno, S.; Yamashita, S.; Park, K.-S.; Lee, S.-Y.; et al. RNF208, an estrogen-inducible E3 ligase, targets soluble Vimentin to suppress metastasis in triple-negative breast cancers. Nat. Commun. 2019, 10, 5805. [Google Scholar] [CrossRef]
- Haasper, C.; Jagodzinski, M.; Drescher, M.; Meller, R.; Wehmeier, M.; Krettek, C.; Hesse, E. Cyclic strain induces FosB and initiates osteogenic differentiation of mesenchymal cells. Exp. Toxicol. Pathol. 2008, 59, 355–363. [Google Scholar] [CrossRef] [PubMed]
- Inoue, D.; Kido, S.; Matsumoto, T. Transcriptional induction of FosB/DeltaFosB gene by mechanical stress in osteoblasts. J. Biol. Chem. 2004, 279, 49795–49803. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhang, S.; Si, H.; Zeng, Y.; Wu, Y.; Liu, Y.; Li, M.; Wu, L.; Shen, B. A genetic correlation scan identifies blood proteins associated with bone mineral density. BMC Musculoskelet. Disord. 2022, 23, 530. [Google Scholar] [CrossRef]
- Li, D.; Zhao, W.; Zhang, X.; Lv, H.; Li, C.; Sun, L. NEFM DNA methylation correlates with immune infiltration and survival in breast cancer. Clin. Epigenetics 2021, 13, 112. [Google Scholar] [CrossRef]
- Adamczyk, M. Transglutaminase 2 in cartilage homoeostasis: Novel links with inflammatory osteoarthritis. Amino Acids 2017, 49, 625–633. [Google Scholar] [CrossRef]
- Liu, F.; Wu, M.; Wu, X.; Chen, D.; Xie, M.; Pan, H. TGM2 accelerates migration and differentiation of BMSCs by activating Wnt/β-catenin signaling. J. Orthop. Surg. Res. 2023, 18, 168. [Google Scholar] [CrossRef]
- Xin, L.; Wen, Y.; Song, J.; Chen, T.; Zhai, Q. Bone regeneration strategies based on organelle homeostasis of mesenchymal stem cells. Front. Endocrinol. 2023, 14, 1151691. [Google Scholar] [CrossRef]
- Yu, W.M.; Liu, X.; Shen, J.; Jovanovic, O.; Pohl, E.E.; Gerson, S.L.; Finkel, T.; Broxmeyer, H.E.; Qu, C.K. Metabolic regulation by the mitochondrial phosphatase PTPMT1 is required for hematopoietic stem cell differentiation. Cell Stem Cell 2013, 12, 62–74. [Google Scholar] [CrossRef]
- Kim, P.; Park, J.; Lee, D.-J.; Mizuno, S.; Shinohara, M.; Hong, C.P.; Jeong, Y.; Yun, R.; Park, H.; Park, S.; et al. Mast4 determines the cell fate of MSCs for bone and cartilage development. Nat. Commun. 2022, 13, 3960. [Google Scholar] [CrossRef]
- Lu, Y.; Zhang, H.; Pan, H.; Zhang, Z.; Zeng, H.; Xie, H.; Yin, J.; Tang, W.; Lin, R.; Zeng, C.; et al. Expression pattern analysis of m6A regulators reveals IGF2BP3 as a key modulator in osteoarthritis synovial macrophages. J. Transl. Med. 2023, 21, 339. [Google Scholar] [CrossRef]
- Karlsen, T.; Jakobsen, R.; Mikkelsen, T.; JE, B. microRNA-140 Targets RALA and Regulates Chondrogenic Differentiation of Human Mesenchymal Stem Cells by Translational Enhancement of SOX9 and ACAN. Stem Cells Dev. 2014, 23, 290–304. [Google Scholar] [CrossRef]
- Alvarez-Garcia, O.; Matsuzaki, T.; Olmer, M.; Plate, L.; Kelly, J.W.; Lotz, M.K. Regulated in Development and DNA Damage Response 1 Deficiency Impairs Autophagy and Mitochondrial Biogenesis in Articular Cartilage and Increases the Severity of Experimental Osteoarthritis. Arthritis Rheumatol. 2017, 69, 1418–1428. [Google Scholar] [CrossRef]
- Alvarez-Garcia, O.; Olmer, M.; Akagi, R.; Akasaki, Y.; Fisch, K.M.; Shen, T.; Su, A.I.; Lotz, M.K. Suppression of REDD1 in osteoarthritis cartilage, a novel mechanism for dysregulated mTOR signaling and defective autophagy. Osteoarthr. Cartil. 2016, 24, 1639–1647. [Google Scholar] [CrossRef]
- Geyer, M.; Grässel, S.; Straub, R.H.; Schett, G.; Dinser, R.; Grifka, J.; Gay, S.; Neumann, E.; Müller-Ladner, U. Differential transcriptome analysis of intraarticular lesional vs intact cartilage reveals new candidate genes in osteoarthritis pathophysiology. Osteoarthr. Cartil. 2009, 17, 328–335. [Google Scholar] [CrossRef]
- Giordano, R.; Petersen, K.K.; Andersen, H.H.; Simonsen, O.; Arendt-Nielsen, L. Serum Inflammatory Markers in Patients With Knee Osteoarthritis: A Proteomic Approach. Clin. J. Pain 2020, 36, 229–237. [Google Scholar] [CrossRef]
- Shu, L.; Li, Y.; Liu, Y.; Zhu, Z.; Huang, H.; Chen, S.; Wu, X.; Liang, Y. CD320 Regulates Bone Marrow Angiogenesis in Multiple Myeloma Via HGF-ERK Axis. Blood 2022, 140 (Suppl. S1), 12466–12467. [Google Scholar] [CrossRef]
- Jin, Q.; Liu, Y.; Zhang, Z.; Wen, X.; Chen, Z.; Tian, H.; Kang, Z.; Wu, X.; Xu, H. MYC promotes fibroblast osteogenesis by regulating ALP and BMP2 to participate in ectopic ossification of ankylosing spondylitis. Arthritis Res. Ther. 2023, 25, 28. [Google Scholar] [CrossRef]
- Shkhyan, R.; Van Handel, B.; Bogdanov, J.; Lee, S.; Yu, Y.; Scheinberg, M.; Banks, N.W.; Limfat, S.; Chernostrik, A.; Franciozi, C.E.; et al. Drug-induced modulation of gp130 signalling prevents articular cartilage degeneration and promotes repair. Ann. Rheum. Dis. 2018, 77, 760–769. [Google Scholar] [CrossRef]
- Wang, G.; He, L.; Xiang, Y.; Jia, D.; Li, Y. Long noncoding and micro-RNA expression in a model of articular chondrocyte degeneration induced by stromal cell-derived factor-1. Asian Biomed. (Res. Rev. News) 2022, 16, 169–179. [Google Scholar] [CrossRef]
- van der Crabben, S.N.; Hennus, M.P.; McGregor, G.A.; Ritter, D.I.; Nagamani, S.C.; Wells, O.S.; Harakalova, M.; Chinn, I.K.; Alt, A.; Vondrova, L.; et al. Destabilized SMC5/6 complex leads to chromosome breakage syndrome with severe lung disease. J. Clin. Investig. 2016, 126, 2881–2892. [Google Scholar] [CrossRef]
- Tang, Z.; Feng, H.; Chen, X.; Shao, S.; Li, C. SNORC knockdown alleviates inflammation, autophagy defect and matrix degradation of chondrocytes in osteoarthritis development. Mol. Cell. Biochem. 2023, 479, 2323–2335. [Google Scholar] [CrossRef]
- Liu, G.; He, G.; Zhang, J.; Zhang, Z.; Wang, L. Identification of SCRG1 as a Potential Therapeutic Target for Human Synovial Inflammation. Front. Immunol. 2022, 13, 893301. [Google Scholar] [CrossRef]
- Schneider, C.V.; Schneider, K.M.; Conlon, D.M.; Park, J.; Vujkovic, M.; Zandvakili, I.; Ko, Y.A.; Trautwein, C.; Center, R.; Carr, R.M.; et al. A genome-first approach to mortality and metabolic phenotypes in MTARC1 p.Ala165Thr (rs2642438) heterozygotes and homozygotes. Med 2021, 2, 851–863.e853. [Google Scholar] [CrossRef]
- Mucientes, A.; Herranz, E.; Lois, P.; Blanco, F.J.; Abasolo, L.; Rodriguez, L.R.; Lamas, J.R.; Fernandez, B. AB0077 Contribution of Notum to the Development of Osteoarthritis. Ann. Rheum. Dis. 2020, 79 (Suppl. S1), 1338–1339. [Google Scholar] [CrossRef]
- Coltell, O.; Ortega-Azorín, C.; Sorlí, J.V.; Portolés, O.; Asensio, E.M.; Saiz, C.; Barragán, R.; Estruch, R.; Corella, D. Circulating Adiponectin and Its Association with Metabolic Traits and Type 2 Diabetes: Gene-Diet Interactions Focusing on Selected Gene Variants and at the Genome-Wide Level in High-Cardiovascular Risk Mediterranean Subjects. Nutrients 2021, 13, 541. [Google Scholar] [CrossRef]
- de Boer, T.N.; van Spil, W.E.; Huisman, A.M.; Polak, A.A.; Bijlsma, J.W.; Lafeber, F.P.; Mastbergen, S.C. Serum adipokines in osteoarthritis; comparison with controls and relationship with local parameters of synovial inflammation and cartilage damage. Osteoarthr. Cartil. 2012, 20, 846–853. [Google Scholar] [CrossRef]
- Laurberg, T.B.; Frystyk, J.; Ellingsen, T.; Hansen, I.T.; Jørgensen, A.; Tarp, U.; Hetland, M.L.; Hørslev-Petersen, K.; Hornung, N.; Poulsen, J.H.; et al. Plasma Adiponectin in Patients with Active, Early, and Chronic Rheumatoid Arthritis Who Are Steroid- and Disease-Modifying Antirheumatic Drug-Naive Compared with Patients with Osteoarthritis and Controls. J. Rheumatol. 2009, 36, 1885–1891. [Google Scholar] [CrossRef]
- Tang, C.H.; Chiu, Y.C.; Tan, T.W.; Yang, R.S.; Fu, W.M. Adiponectin enhances IL-6 production in human synovial fibroblast via an AdipoR1 receptor, AMPK, p38, and NF-kappa B pathway. J. Immunol. 2007, 179, 5483–5492. [Google Scholar] [CrossRef]
- Tong, K.-M.; Chen, C.-P.; Huang, K.-C.; Shieh, D.-C.; Cheng, H.-C.; Tzeng, C.-Y.; Chen, K.-H.; Chiu, Y.-C.; Tang, C.-H. Adiponectin increases MMP-3 expression in human chondrocytes through adipor1 signaling pathway. J. Cell. Biochem. 2011, 112, 1431–1440. [Google Scholar] [CrossRef]
- Whitehead, J.P.; Richards, A.A.; Hickman, I.J.; Macdonald, G.A.; Prins, J.B. Adiponectin--a key adipokine in the metabolic syndrome. Diabetes Obes. Metab. 2006, 8, 264–280. [Google Scholar] [CrossRef]
- Ungsudechachai, T.; Honsawek, S.; Jittikoon, J.; Udomsinprasert, W. Clusterin exacerbates interleukin-1β-induced inflammation via suppressing PI3K/Akt pathway in human fibroblast-like synoviocytes of knee osteoarthritis. Sci. Rep. 2022, 12, 9963. [Google Scholar] [CrossRef]
- Roughley, P.J.; Mort, J.S. The role of aggrecan in normal and osteoarthritic cartilage. J. Exp. Orthop. 2014, 1, 8. [Google Scholar] [CrossRef]
- Ma, B.; Xu, X.; He, S.; Zhang, J.; Wang, X.; Wu, P.; Liu, J.; Jiang, H.; Zheng, M.; Li, W.; et al. STC2 modulates ERK1/2 signaling to suppress adipogenic differentiation of human bone marrow mesenchymal stem cells. Biochem. Biophys. Res. Commun. 2020, 524, 163–168. [Google Scholar] [CrossRef]
- Lee, H.; Kim, Y.I.; Nirmala, F.S.; Kim, J.S.; Seo, H.D.; Ha, T.Y.; Jang, Y.J.; Jung, C.H.; Ahn, J. MiR-141-3p promotes mitochondrial dysfunction in ovariectomy-induced sarcopenia via targeting Fkbp5 and Fibin. Aging 2021, 13, 4881–4894. [Google Scholar] [CrossRef]
- Rajasekaran, S.; Soundararajan, D.C.R.; Nayagam, S.M.; Tangavel, C.; Raveendran, M.; Thippeswamy, P.B.; Djuric, N.; Anand, S.V.; Shetty, A.P.; Kanna, R.M. Modic changes are associated with activation of intense inflammatory and host defense response pathways–molecular insights from proteomic analysis of human intervertebral discs. Spine J. 2022, 22, 19–38. [Google Scholar] [CrossRef]
- Yang, B.; Xu, L.; Wang, S. Regulation of lncRNA-H19/miR-140-5p in cartilage matrix degradation and calcification in osteoarthritis. Ann. Palliat. Med. 2020, 9, 1896–1904. [Google Scholar]
- Schubert, T.; Schlegel, J.; Schmid, R.; Opolka, A.; Grassel, S.; Humphries, M.; Bosserhoff, A.K. Modulation of cartilage differentiation by melanoma inhibiting activity/cartilage-derived retinoic acid-sensitive protein (MIA/CD-RAP). Exp. Mol. Med. 2010, 42, 166–174. [Google Scholar] [CrossRef]
- Tscheudschilsuren, G.; Bosserhoff, A.K.; Schlegel, J.; Vollmer, D.; Anton, A.; Alt, V.; Schnettler, R.; Brandt, J.; Proetzel, G. Regulation of mesenchymal stem cell and chondrocyte differentiation by MIA. Exp. Cell Res. 2006, 312, 63–72. [Google Scholar] [CrossRef]
- Ishikawa, M.; Yamada, Y. The Role of Pannexin 3 in Bone Biology. J. Dent. Res. 2017, 96, 372–379. [Google Scholar] [CrossRef]
- Moon, P.M.; Penuela, S.; Barr, K.; Khan, S.; Pin, C.L.; Welch, I.; Attur, M.; Abramson, S.B.; Laird, D.W.; Beier, F. Deletion of Panx3 Prevents the Development of Surgically Induced Osteoarthritis. J. Mol. Med. 2015, 93, 845–856. [Google Scholar] [CrossRef]
- Kawatsu, M.; Takeshita, N.; Takimoto, A.; Yoshimoto, Y.; Seiryu, M.; Ito, A.; Kimura, S.; Kawamoto, T.; Hiraki, Y.; Shukunami, C.; et al. Scleraxis upregulated by transforming growth factor-β1 signaling inhibits tension-induced osteoblast differentiation of priodontal ligament cells via ephrin A2. Bone 2021, 149, 115969. [Google Scholar] [CrossRef]
- Hattori, K.; Takahashi, N.; Terabe, K.; Ohashi, Y.; Kishimoto, K.; Yokota, Y.; Suzuki, M.; Kojima, T.; Imagama, S. Activation of transient receptor potential vanilloid 4 protects articular cartilage against inflammatory responses via CaMKK/AMPK/NF-κB signaling pathway. Sci. Rep. 2021, 11, 15508. [Google Scholar] [CrossRef]
- Hu, K.; Sun, H.; Gui, B.; Sui, C. TRPV4 functions in flow shear stress induced early osteogenic differentiation of human bone marrow mesenchymal stem cells. Biomed. Pharmacother. 2017, 91, 841–848. [Google Scholar] [CrossRef]
- Hamada, K.; Fukuda, H.; Mikasa, M.; Kobayashi, Y. Roentgenographic findings in massive rotator cuff tears. A long-term observation. Clin. Orthop. Relat. Res. 1990, 254, 92–96. [Google Scholar] [CrossRef]
- Xue, J.-F.; Shi, Z.-M.; Zou, J.; Li, X.-L. Inhibition of PI3K/AKT/mTOR signaling pathway promotes autophagy of articular chondrocytes and attenuates inflammatory response in rats with osteoarthritis. Biomed. Pharmacother. 2017, 89, 1252–1261. [Google Scholar]
- Pulkkinen, L.; Ukkola, O.; Kolehmainen, M.; Uusitupa, M. Ghrelin in diabetes and metabolic syndrome. Int. J. Pept. 2010, 2010, 248948. [Google Scholar] [CrossRef] [PubMed]
- Qu, R.; Chen, X.; Wang, W.; Qiu, C.; Ban, M.; Guo, L.; Vasilev, K.; Chen, J.; Li, W.; Zhao, Y. Ghrelin protects against osteoarthritis through interplay with Akt and NF-κB signaling pathways. FASEB J. 2018, 32, 1044–1058. [Google Scholar]
- Simonaro, C.M.; D’Angelo, M.; He, X.; Eliyahu, E.; Shtraizent, N.; Haskins, M.E.; Schuchman, E.H. Mechanism of glycosaminoglycan-mediated bone and joint disease: Implications for the mucopolysaccharidoses and other connective tissue diseases. Am. J. Pathol. 2008, 172, 112–122. [Google Scholar] [CrossRef]
- Simonaro, C.M.; Haskins, M.E.; Schuchman, E.H. Articular chondrocytes from animals with a dermatan sulfate storage disease undergo a high rate of apoptosis and release nitric oxide and inflammatory cytokines: A possible mechanism underlying degenerative joint disease in the mucopolysaccharidoses. Lab. Investig. 2001, 81, 1319–1328. [Google Scholar]
- Drygalski, K.; Lecoutre, S.; Clément, K.; Dugail, I. Hyaluronan in Adipose Tissue, Metabolic Inflammation, and Diabetes: Innocent Bystander or Guilty Party? Diabetes 2023, 72, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Thornalley, P.J. Protein and nucleotide damage by glyoxal and methylglyoxal in physiological systems-role in ageing and disease. Drug Metab. Drug Interact. 2008, 23, 125–150. [Google Scholar] [CrossRef]
- Alikhani, M.; Alikhani, Z.; Boyd, C.; MacLellan, C.M.; Raptis, M.; Liu, R.; Pischon, N.; Trackman, P.C.; Gerstenfeld, L.; Graves, D.T. Advanced glycation end products stimulate osteoblast apoptosis via the MAP kinase and cytosolic apoptotic pathways. Bone 2007, 40, 345–353. [Google Scholar] [CrossRef] [PubMed]
- Goldring, M.B.; Otero, M. Inflammation in osteoarthritis. Curr. Opin. Rheumatol. 2011, 23, 471–478. [Google Scholar] [CrossRef]
- Mao, X.; Fu, P.; Wang, L.; Xiang, C. Mitochondria: Potential Targets for Osteoarthritis. Front. Med. 2020, 7, 581402. [Google Scholar] [CrossRef]
- Maldonado, M.; Nam, J. The role of changes in extracellular matrix of cartilage in the presence of inflammation on the pathology of osteoarthritis. Biomed Res. Int. 2013, 2013, 284873. [Google Scholar] [CrossRef] [PubMed]
- Assirelli, E.; Dolzani, P.; Pulsatelli, L.; Addimanda, O.; Lisignoli, G.; Mariani, E.; Meliconi, R. Complement factor expression in osteoarthritis joint compartments. Osteoarthr. Cartil. 2016, 24, S2383–S2384. [Google Scholar] [CrossRef]
- Lynskey, S.J.; Gill, S.D.; McGee, S.L.; Ziemann, M.; Page, R.S. ‘QuickDASH’ to find unique genes and biological processes associated with shoulder osteoarthritis: A prospective case–control study. BMC Res. Notes 2024, 17, 361. [Google Scholar] [CrossRef]
- Zhou, Y.; Wang, T.; Hamilton, J.L.; Chen, D. Wnt/β-catenin Signaling in Osteoarthritis and in Other Forms of Arthritis. Curr. Rheumatol. Rep. 2017, 19, 53. [Google Scholar] [CrossRef]
- Wang, Y.; Fan, X.; Xing, L.; Tian, F. Wnt signaling: A promising target for osteoarthritis therapy. Cell Commun. Signal. 2019, 17, 97. [Google Scholar] [CrossRef]
- Usami, Y.; Gunawardena, A.T.; Iwamoto, M.; Enomoto-Iwamoto, M. Wnt signaling in cartilage development and diseases: Lessons from animal studies. Lab. Investig. 2016, 96, 186–196. [Google Scholar] [CrossRef] [PubMed]
- Kovács, B.; Vajda, E.; Nagy, E.E. Regulatory Effects and Interactions of the Wnt and OPG-RANKL-RANK Signaling at the Bone-Cartilage Interface in Osteoarthritis. Int. J. Mol. Sci. 2019, 20, 4653. [Google Scholar] [CrossRef]
- Hacking, C.Y.J.; Elfeky, M.; Yap, J. Rotator Cuff Tear Arthropathy: Radiopaedia.org. 1 September 2015. Available online: https://radiopaedia.org/articles/39335 (accessed on 17 March 2025).
- Ma, Y.; Zheng, W.; Chen, H.; Shao, X.; Lin, P.; Liu, X.; Li, X.; Ye, H. Glucosamine promotes chondrocyte proliferation via the Wnt/β-catenin signaling pathway. Int. J. Mol. Med. 2018, 42, 61–70. [Google Scholar] [CrossRef]
- Mucientes, A.; Herranz, E.; Lois, P.; Candelas, G.; Abasolo, L.; Rodriguez-Rodriguez, L.; Lamas, J.R.; Fernández-Gutiérrez, B. Contribution of Notum and Glypicans to the Development of Osteoarthritis. In Arthritis & Rheumatology; Wiley: Hoboken, NJ, USA, 2020. [Google Scholar]
- Chen, J.; Chen, F.; Wu, X.; Bian, H.; Chen, C.; Zhang, X.; Hei, R.; Yuan, H.; Wang, Q.; Lu, Y.; et al. DLX5 promotes Col10a1 expression and chondrocyte hypertrophy and is involved in osteoarthritis progression. Genes Dis. 2023, 10, 2097–2108. [Google Scholar] [CrossRef]
- Wang, M.; Shen, J.; Jin, H.; Im, H.J.; Sandy, J.; Chen, D. Recent progress in understanding molecular mechanisms of cartilage degeneration during osteoarthritis. Ann. N. Y. Acad. Sci. 2011, 1240, 61–69. [Google Scholar] [CrossRef] [PubMed]
- Deshmukh, V.; Hu, H.; Barroga, C.; Bossard, C.; Kc, S.; Dellamary, L.; Stewart, J.; Chiu, K.; Ibanez, M.; Pedraza, M.; et al. A small-molecule inhibitor of the Wnt pathway (SM04690) as a potential disease modifying agent for the treatment of osteoarthritis of the knee. Osteoarthr. Cartil. 2018, 26, 18–27. [Google Scholar] [CrossRef]
- Yazici, Y.; McAlindon, T.E.; Gibofsky, A.; Lane, N.E.; Latterman, C.; Skrepnik, N.; Swearingen, C.J.; DiFrancesco, A.; Tambiah, J.R.; Hochberg, M.C. Efficacy and safety from a phase 2B trial of SM04690, a novel, intra-articular, WNT pathway inhibitor for the treatment of osteoarthritis of the knee. Osteoarthr. Cartil. 2019, 27, S503. [Google Scholar] [CrossRef]
- Clevers, H.; Nusse, R. Wnt/-Catenin Signaling and Disease. Cell 2012, 149, 1192–1205. [Google Scholar] [CrossRef]
- Masenga, S.K.; Kabwe, L.S.; Chakulya, M.; Kirabo, A. Mechanisms of Oxidative Stress in Metabolic Syndrome. Int. J. Mol. Sci. 2023, 24, 7898. [Google Scholar] [CrossRef]
- Loeser, R.F. Aging and osteoarthritis: The role of chondrocyte senescence and aging changes in the cartilage matrix. Osteoarthr. Cartil. 2009, 17, 971–979. [Google Scholar] [CrossRef]
- Tashjian, R.Z.; Lock, I.; Granger, E.K.; Wang, Y.; Lee, Y.; Chalmers, P.N.; Jones, K.B. Gene Expression in Torn Rotator Cuff Tendons Determined by RNA Sequencing. Orthop. J. Sports Med. 2020, 8, 2325967120927480. [Google Scholar] [CrossRef] [PubMed]
- Lenihan, C.R.; Taylor, C.T. The impact of hypoxia on cell death pathways. Biochem. Soc. Trans. 2013, 41, 657–663. [Google Scholar] [CrossRef]
- Svensson, K.J.; Welch, J.E.; Kucharzewska, P.; Bengtson, P.; Bjurberg, M.; Pahlman, S.; Ten Dam, G.B.; Persson, L.; Belting, M. Hypoxia-Mediated Induction of the Polyamine System Provides Opportunities for Tumor Growth Inhibition by Combined Targeting of Vascular Endothelial Growth Factor and Ornithine Decarboxylase. Cancer Res. 2008, 68, 9291–9301. [Google Scholar] [CrossRef]
- Khan, N.M.; Ahmad, I.; Haqqi, T.M. Nrf2/ARE pathway attenuates oxidative and apoptotic response in human osteoarthritis chondrocytes by activating ERK1/2/ELK1-P70S6K-P90RSK signaling axis. Free Radic. Biol. Med. 2018, 116, 159–171. [Google Scholar] [CrossRef] [PubMed]
- Bhatti, J.S.; Bhatti, G.K.; Reddy, P.H. Mitochondrial dysfunction and oxidative stress in metabolic disorders—A step towards mitochondria based therapeutic strategies. Biochim. Biophys. Acta Mol. Basis Dis. 2017, 1863, 1066–1077. [Google Scholar] [CrossRef]
- Ma, Q. Role of nrf2 in oxidative stress and toxicity. Annu. Rev. Pharmacol. Toxicol. 2013, 53, 401–426. [Google Scholar] [CrossRef] [PubMed]
- Mahjoub, S.; Masrour-Roudsari, J. Role of oxidative stress in pathogenesis of metabolic syndrome. Casp. J. Intern. Med. 2012, 3, 386–396. [Google Scholar]
- Nagase, H.; Kashiwagi, M. Aggrecanases and cartilage matrix degradation. Arthritis Res. Ther. 2003, 5, 94–103. [Google Scholar] [CrossRef]
- Bar Oz, M.; Kumar, A.; Elayyan, J.; Reich, E.; Binyamin, M.; Kandel, L.; Liebergall, M.; Steinmeyer, J.; Lefebvre, V.; Dvir-Ginzberg, M. Acetylation reduces SOX9 nuclear entry and ACAN gene transactivation in human chondrocytes. Aging Cell 2016, 15, 499–508. [Google Scholar] [CrossRef]
- Bay-Jensen, A.C.; Mobasheri, A.; Thudium, C.S.; Kraus, V.B.; Karsdal, M.A. Blood and urine biomarkers in osteoarthritis—An update on cartilage associated type II collagen and aggrecan markers. Curr. Opin. Rheumatol. 2022, 34, 54–60. [Google Scholar] [CrossRef]
- Cuffaro, D.; Ciccone, L.; Rossello, A.; Nuti, E.; Santamaria, S. Targeting Aggrecanases for Osteoarthritis Therapy: From Zinc Chelation to Exosite Inhibition. J. Med. Chem. 2022, 65, 13505–13532. [Google Scholar] [CrossRef] [PubMed]
- Bihlet, A.R.; Balchen, T.; Goteti, K.; Sonne, J.; Ladel, C.; Karsdal, M.A.; Ona, V.; Moreau, F.; Waterhouse, R.; Bay-Jensen, A.-C.; et al. Safety, Tolerability, and Pharmacodynamics of the ADAMTS-5 Nanobody M6495: Two Phase 1, Single-Center, Double-Blind, Randomized, Placebo-Controlled Studies in Healthy Subjects and Patients With Osteoarthritis. ACR Open Rheumatol. 2024, 6, 205–213. [Google Scholar] [CrossRef] [PubMed]
- Song, Z.; Li, Y.; Shang, C.; Shang, G.; Kou, H.; Li, J.; Chen, S.; Liu, H. Sprifermin: Effects on Cartilage Homeostasis and Therapeutic Prospects in Cartilage-Related Diseases. Front. Cell Dev. Biol. 2021, 9, 786546. [Google Scholar] [CrossRef]
- Moon, P.M.; Shao, Z.Y.; Wambiekele, G.; Appleton, C.T.G.; Laird, D.W.; Penuela, S.; Beier, F. Global Deletion of Pannexin 3 Resulting in Accelerated Development of Aging-Induced Osteoarthritis in Mice. Arthritis Rheumatol. 2021, 73, 1178–1188. [Google Scholar] [CrossRef]
- Han, Y.; Cho, D.H.; Chung, D.J.; Lee, K.Y. Osterix plays a critical role in BMP4-induced promoter activity of connexin43. Biochem. Biophys. Res. Commun. 2016, 478, 683–688. [Google Scholar] [CrossRef]
- Gupta, A.; Niger, C.; Buo, A.M.; Eidelman, E.R.; Chen, R.J.; Stains, J.P. Connexin43 enhances the expression of osteoarthritis-associated genes in synovial fibroblasts in culture. BMC Musculoskelet. Disord. 2014, 15, 425. [Google Scholar] [CrossRef]
- Luo, Y.; Zheng, S.; Xiao, W.; Zhang, H.; Li, Y. Pannexins in the musculoskeletal system: New targets for development and disease progression. Bone Res. 2024, 12, 26. [Google Scholar] [CrossRef] [PubMed]
- Ungsudechachai, T.; Honsawek, S.; Jittikoon, J.; Udomsinprasert, W. Clusterin Is Associated with Systemic and Synovial Inflammation in Knee Osteoarthritis. Cartilage 2021, 13 (Suppl. S1), 1557s–1565s. [Google Scholar] [CrossRef]
- Connor, J.R.; Kumar, S.; Sathe, G.; Mooney, J.; O’Brien, S.P.; Mui, P.; Murdock, P.R.; Gowen, M.; Lark, M.W. Clusterin expression in adult human normal and osteoarthritic articular cartilage. Osteoarthr. Cartil. 2001, 9, 727–737. [Google Scholar] [CrossRef]
- Kovács, P.; Pushparaj, P.N.; Takács, R.; Mobasheri, A.; Matta, C. The clusterin connectome: Emerging players in chondrocyte biology and putative exploratory biomarkers of osteoarthritis. Front. Immunol. 2023, 14, 1103097. [Google Scholar] [CrossRef]
- Ungsudechachai, T.; Jittikoon, J.; Honsawek, S.; Udomsinprasert, W. Protective effect of clusterin against interleukin-1β-induced apoptosis and inflammation in human knee osteoarthritis chondrocytes. Clin. Transl. Sci. 2024, 17, e13881. [Google Scholar] [CrossRef] [PubMed]
- Van der Kraan, P.; Van den Berg, W. Chondrocyte hypertrophy and osteoarthritis: Role in initiation and progression of cartilage degeneration? Osteoarthr. Cartil. 2012, 20, 223–232. [Google Scholar] [CrossRef] [PubMed]
- Von der Mark, K.; Kirsch, T.; Nerlich, A.; Kuss, A.; Weseloh, G.; Glückert, K.; Stöss, H. Type X collagen synthesis in human osteoarthritic cartilage. Indication of chondrocyte hypertrophy. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1992, 35, 806–811. [Google Scholar]
- Tarquini, C.; Pucci, S.; Scioli, M.G.; Doldo, E.; Agostinelli, S.; D’Amico, F.; Bielli, A.; Ferlosio, A.; Caredda, E.; Tarantino, U.; et al. Clusterin exerts a cytoprotective and antioxidant effect in human osteoarthritic cartilage. Aging 2020, 12, 10129–10146. [Google Scholar] [CrossRef]
- Kalvaityte, U.; Matta, C.; Bernotiene, E.; Pushparaj, P.N.; Kiapour, A.M.; Mobasheri, A. Exploring the translational potential of clusterin as a biomarker of early osteoarthritis. J. Orthop. Transl. 2022, 32, 77–84. [Google Scholar] [CrossRef] [PubMed]
- Kacso, I.M.; Bondor, C.I.; Kacso, G. Plasma adiponectin is related to the progression of kidney disease in type 2 diabetes patients. Scand. J. Clin. Lab. Investig. 2012, 72, 333–339. [Google Scholar]
- Tootsi, K.; Kals, J.; Zilmer, M.; Paapstel, K.; Märtson, A. Severity of Osteoarthritis Is Associated with Increased Arterial Stiffness. Int. J. Rheumatol. 2016, 2016, 6402963. [Google Scholar] [CrossRef]
- Loughlin, J.; Dowling, B.; Chapman, K.; Marcelline, L.; Mustafa, Z.; Southam, L.; Ferreira, A.; Ciesielski, C.; Carson, D.A.; Corr, M. Functional variants within the secreted frizzled-related protein 3 gene are associated with hip osteoarthritis in females. Proc. Natl. Acad. Sci. USA 2004, 101, 9757–9762. [Google Scholar] [CrossRef]
- Steinberg, J.; Southam, L.; Fontalis, A.; Clark, M.J.; Jayasuriya, R.L.; Swift, D.; Shah, K.M.; Brooks, R.A.; McCaskie, A.W.; Wilkinson, J.M.; et al. Linking chondrocyte and synovial transcriptional profile to clinical phenotype in osteoarthritis. Ann. Rheum. Dis. 2021, 80, 1070–1074. [Google Scholar] [CrossRef]
- Wen, C.-Y.; Wu, C.-B.; Tang, B.; Wang, T.; Yan, C.-H.; Lu, W.; Pan, H.; Hu, Y.; Chiu, K.-Y. Collagen fibril stiffening in osteoarthritic cartilage of human beings revealed by atomic force microscopy. Osteoarthr. Cartil. 2012, 20, 916–922. [Google Scholar]
- Kim, J.-H.; Lee, G.; Won, Y.; Lee, M.; Kwak, J.-S.; Chun, C.-H.; Chun, J.-S. Matrix cross-linking–mediated mechanotransduction promotes posttraumatic osteoarthritis. Proc. Natl. Acad. Sci. USA 2015, 112, 9424–9429. [Google Scholar] [PubMed]
- Verzijl, N.; DeGroot, J.; Zaken, C.B.; Braun-Benjamin, O.; Maroudas, A.; Bank, R.A.; Mizrahi, J.; Schalkwijk, C.G.; Thorpe, S.R.; Baynes, J.W. Crosslinking by advanced glycation end products increases the stiffness of the collagen network in human articular cartilage: A possible mechanism through which age is a risk factor for osteoarthritis. Arthritis Rheum. 2002, 46, 114–123. [Google Scholar] [PubMed]
- Eaton, C.B.; Sayeed, S.M.; Roberts, M.; Lapane, K.; Waring, M.; Yang, S.; Driban, J.; McAlindon, T. Metabolic syndrome, advanced glycation end products and knee osteoarthritis progression: A report from OAI. Osteoarthr. Cartil. 2013, 21, S165–S166. [Google Scholar] [CrossRef][Green Version]
- Kaur, G.; Song, Y.; Xia, K.; McCarthy, K.; Zhang, F.; Linhardt, R.J.; Harris, N.R. Effect of high glucose on glycosaminoglycans in cultured retinal endothelial cells and rat retina. Glycobiology 2022, 32, 720–734. [Google Scholar] [CrossRef]
- Meurot, C.; Jacques, C.; Martin, C.; Sudre, L.; Breton, J.; Rattenbach, R.; Bismuth, K.; Berenbaum, F. Targeting the GLP-1/GLP-1R axis to treat osteoarthritis: A new opportunity? J. Orthop. Transl. 2022, 32, 121–129. [Google Scholar] [CrossRef]
- Fu, G.K.; Xu, W.; Wilhelmy, J.; Mindrinos, M.N.; Davis, R.W.; Xiao, W.; Fodor, S.P. Molecular indexing enables quantitative targeted RNA sequencing and reveals poor efficiencies in standard library preparations. Proc. Natl. Acad. Sci. USA 2014, 111, 1891–1896. [Google Scholar] [CrossRef]
- Coenye, T. Do results obtained with RNA-sequencing require independent verification? Biofilm 2021, 3, 100043. [Google Scholar] [CrossRef]
- Page, R.S.; McGee, S.L.; Eng, K.; Brown, G.; Beattie, S.; Collier, F.; Gill, S.D. Adhesive capsulitis of the shoulder: Protocol for the adhesive capsulitis biomarker (AdCaB) study. BMC Musculoskelet. Disord. 2019, 20, 145. [Google Scholar] [CrossRef]
- Hamada, K.; Yamanaka, K.; Uchiyama, Y.; Mikasa, T.; Mikasa, M. A radiographic classification of massive rotator cuff tear arthritis. Clin. Orthop. Relat. Res. 2011, 469, 2452–2460. [Google Scholar] [CrossRef]
- Jiang, H.; Lei, R.; Ding, S.-W.; Zhu, S. Skewer: A fast and accurate adapter trimmer for next-generation sequencing paired-end reads. BMC Bioinform. 2014, 15, 182. [Google Scholar] [CrossRef]
- Ewels, P.; Magnusson, M.; Lundin, S.; Käller, M. MultiQC: Summarize analysis results for multiple tools and samples in a single report. Bioinformatics 2016, 32, 3047–3048. [Google Scholar] [CrossRef] [PubMed]
- Simon, A. A Quality Control Analysis Tool for High Throughput Sequencing Data. Available online: https://github.com/s-andrews/FastQC (accessed on 11 November 2023).
- Frankish, A.; Diekhans, M.; Jungreis, I.; Lagarde, J.; Loveland, J.E.; Mudge, J.M.; Sisu, C.; Wright, J.C.; Armstrong, J.; Barnes, I.; et al. GENCODE 2021. Nucleic Acids Res. 2020, 49, D916–D923. [Google Scholar] [CrossRef]
- Bray, N.L.; Pimentel, H.; Melsted, P.; Pachter, L. Near-optimal probabilistic RNA-seq quantification. Nat. Biotechnol. 2016, 34, 525–527. [Google Scholar] [CrossRef] [PubMed]
- Love, M.I.; Huber, W.; Anders, S. Moderated estimation of fold change and dispersion for RNA-seq data with DESeq2. Genome Biol. 2014, 15, 550. [Google Scholar] [CrossRef]
- Gillespie, M.; Jassal, B.; Stephan, R.; Milacic, M.; Rothfels, K.; Senff-Ribeiro, A.; Griss, J.; Sevilla, C.; Matthews, L.; Gong, C.; et al. The reactome pathway knowledgebase 2022. Nucleic Acids Res. 2022, 50, D687–D692. [Google Scholar] [CrossRef]
- Kaspi, A.; Ziemann, M. mitch: Multi-contrast pathway enrichment for multi-omics and single-cell profiling data. BMC Genom. 2020, 21, 447. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).