The Presence of Adipose Tissue in Aortic Valves Influences Inflammation and Extracellular Matrix Composition in Chronic Aortic Regurgitation
Abstract
1. Introduction
2. Results
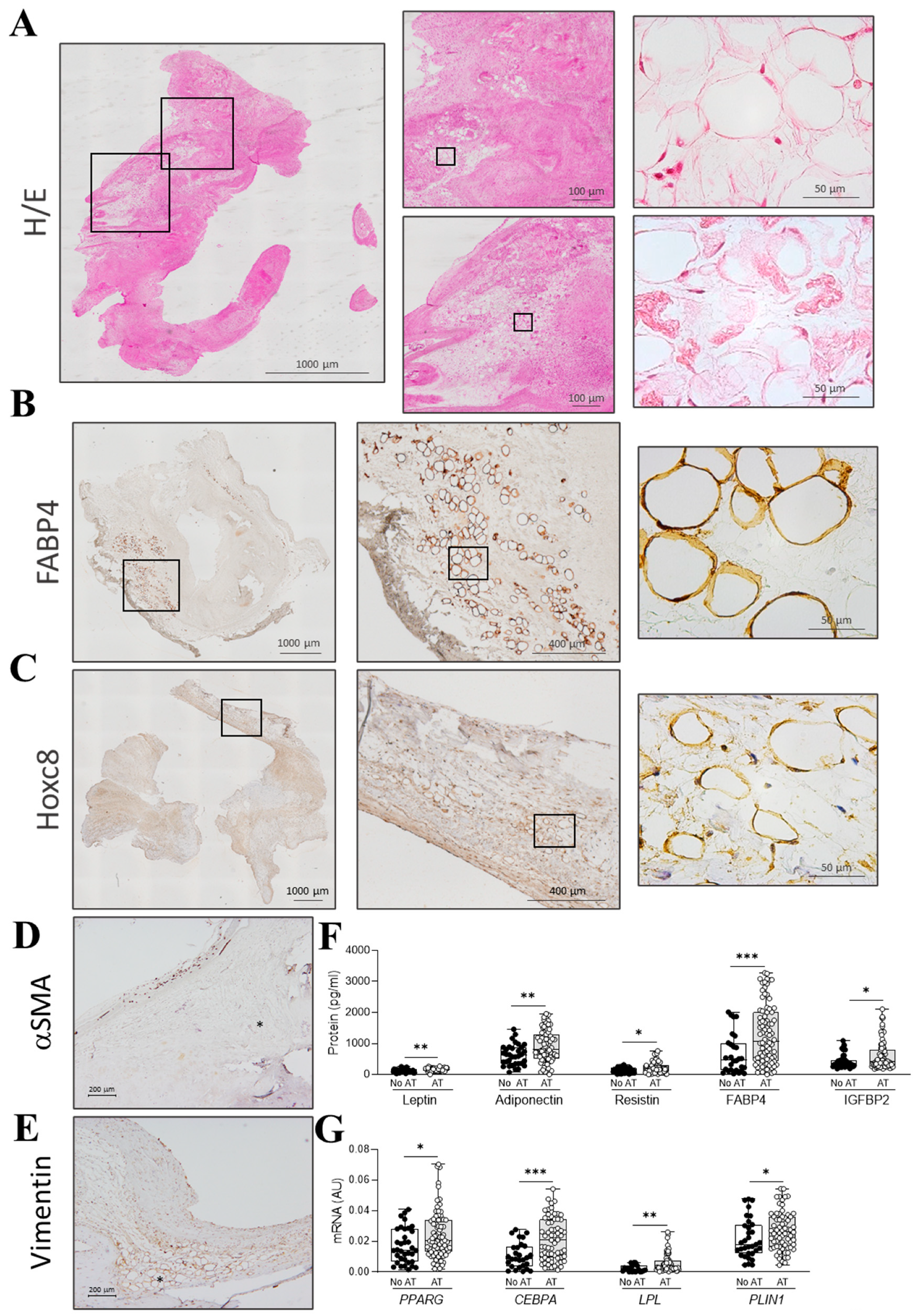
2.1. Clinical and Histological Parameters in AR Patients Presenting Adipocytes
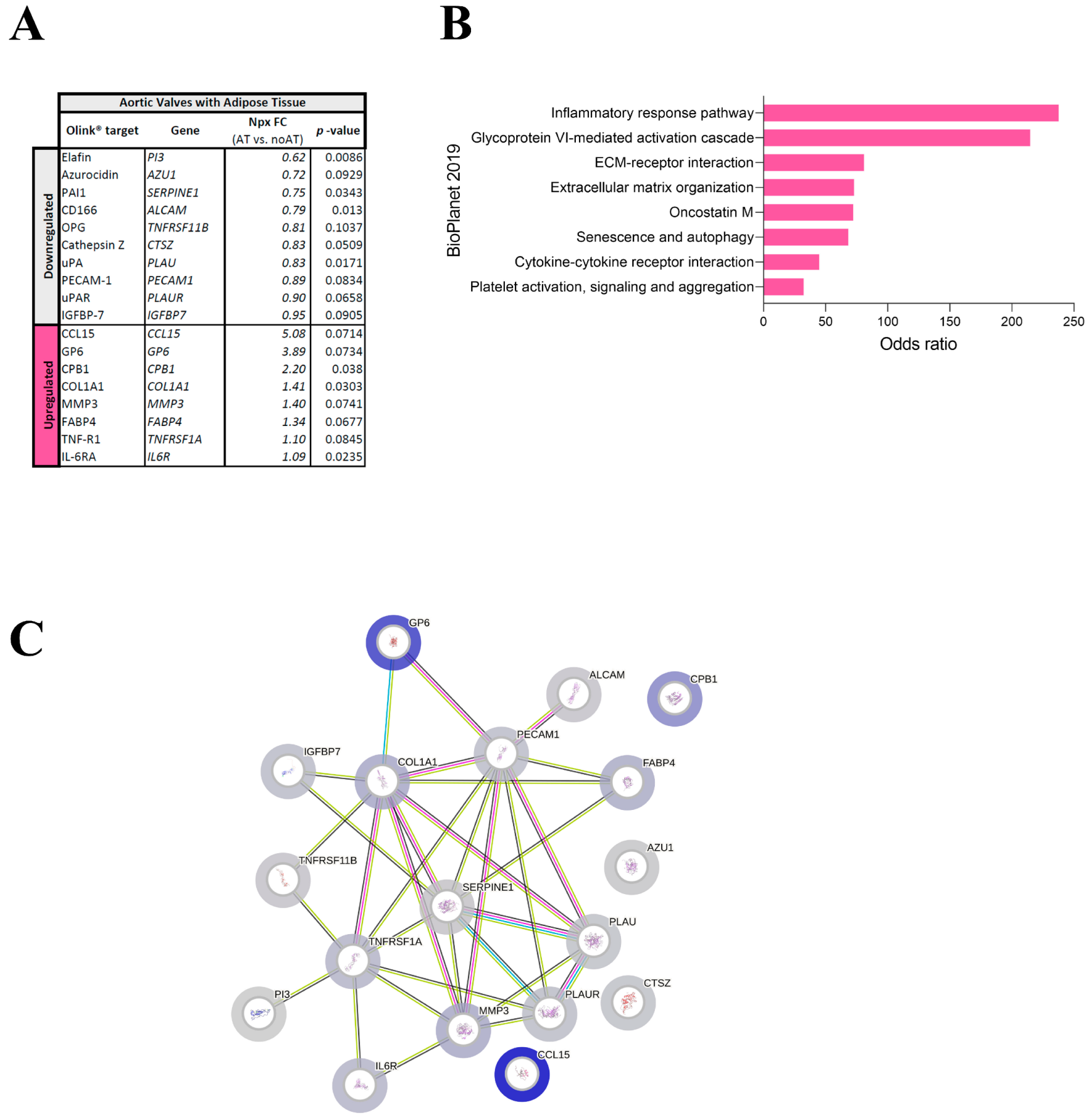
2.2. Targeted Proteomics Reveals Changes in ECM Molecules in AVs with Adipocytes
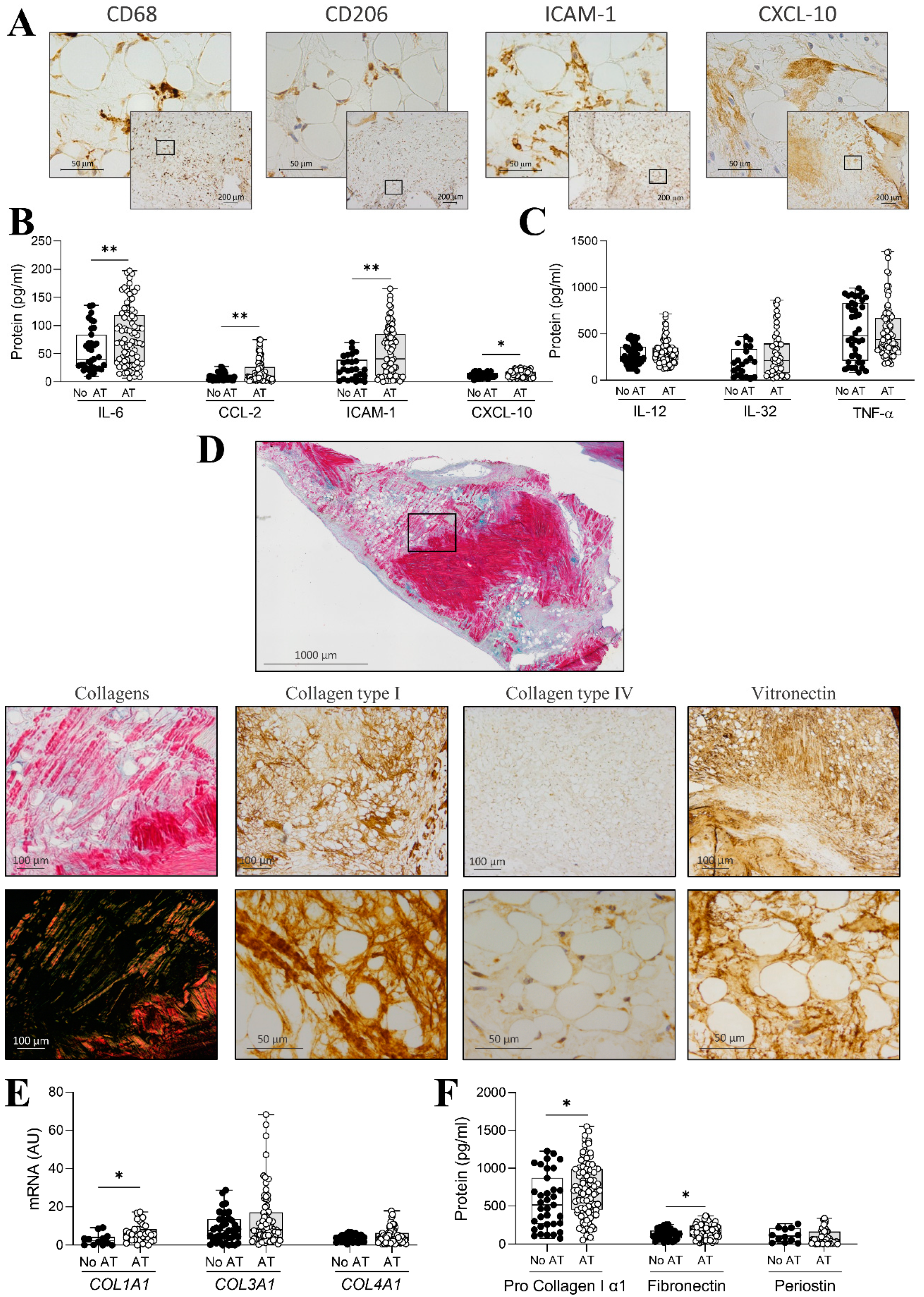
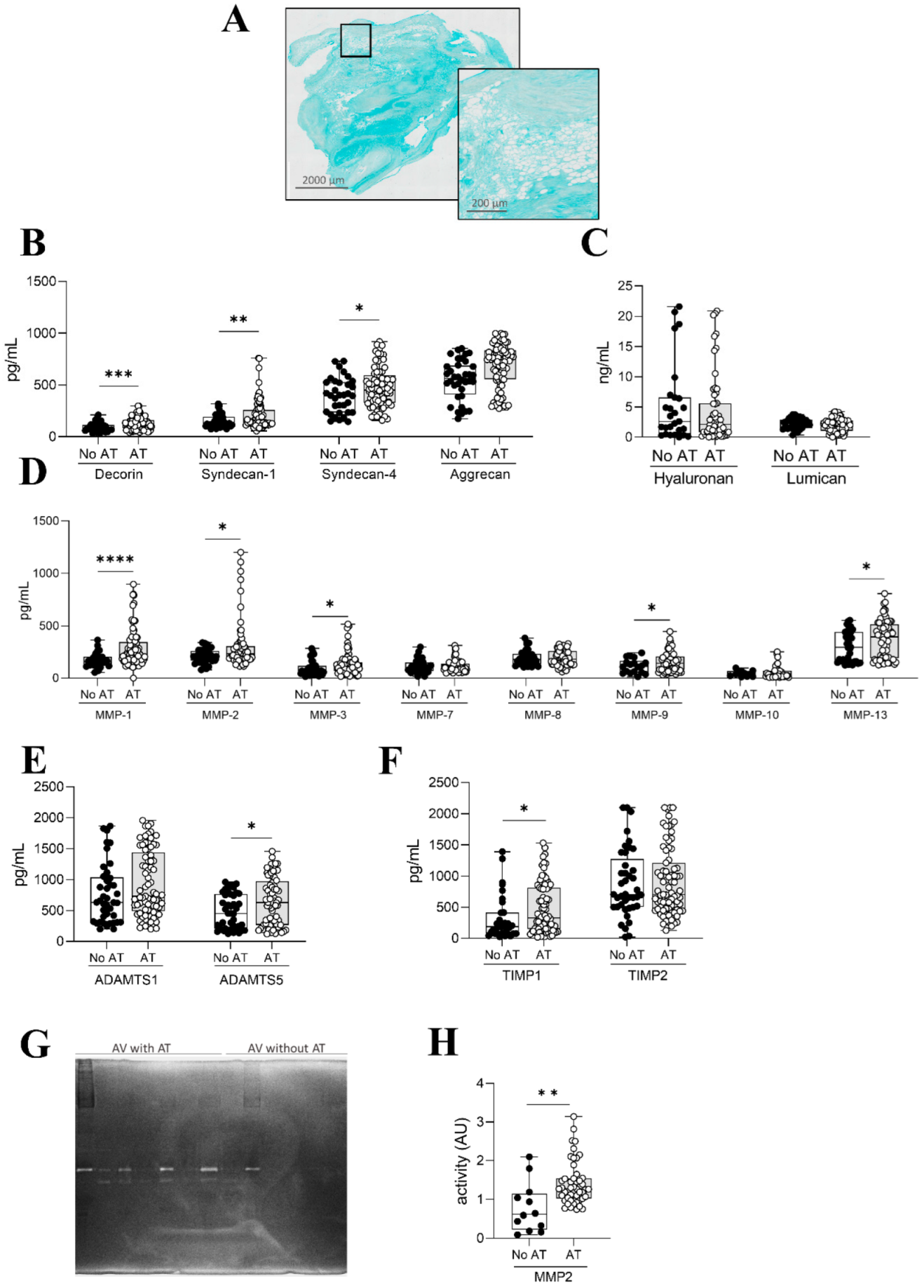
2.3. Inflammation and ECM Composition in AVs with Adipocytes
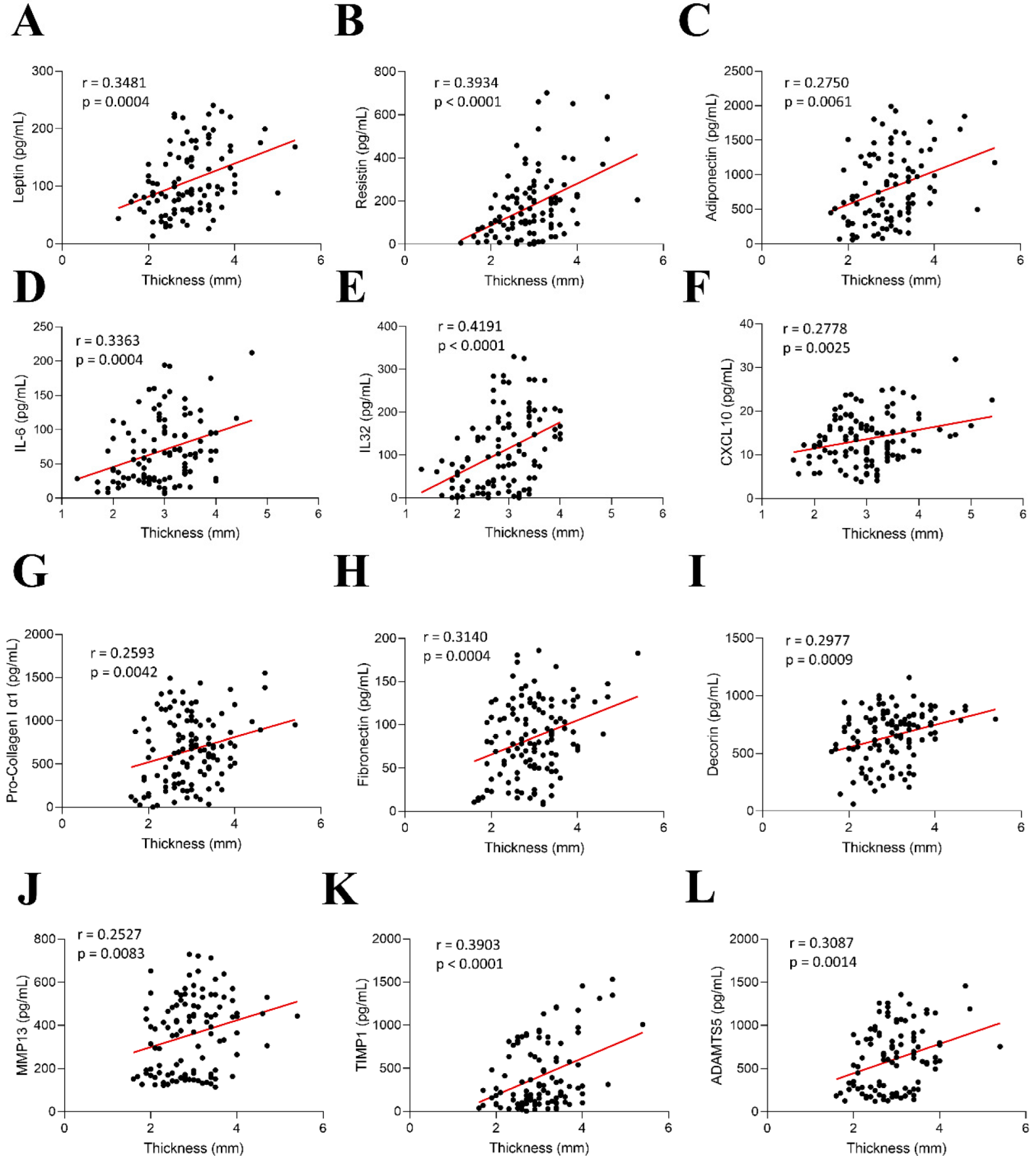
2.4. Correlations Between AV Thickness with Inflammation and ECM Components in AR Patients
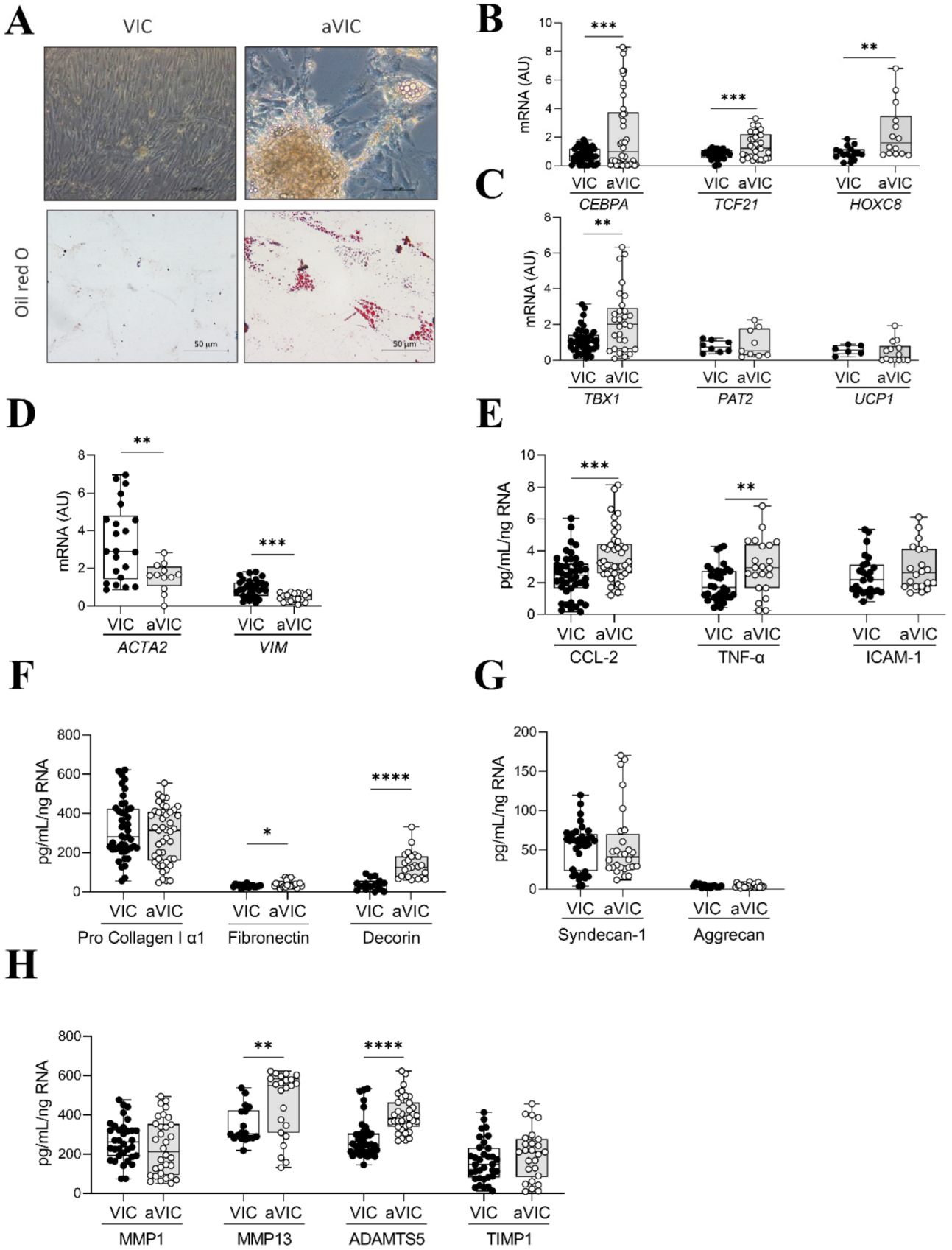
2.5. Characterization of Adipogenic Differentiation of VICs
2.6. Co-Culture of Adipogenic VICs with Control VICs
3. Discussion
4. Material and Methods
4.1. Patient Population
4.2. Echocardiographic and Multi-Detector Computed Tomography Measurements
4.3. Histology and Immunohistochemistry
4.4. AV Processing
4.5. Targeted Proteomics in AV Samples
4.6. VICs Experiments
4.7. Human White Adipocyte Experiments
4.8. Cell Culture Staining
4.9. Proteome Array Profile
4.10. Enzyme-Linked Immunosorbent Assays (ELISA)
4.11. Real-Time Reverse Transcription PCR
4.12. Gelatin Zymography
4.13. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Bekeredjian, R.; Grayburn, P.A. Valvular heart disease: Aortic regurgitation. Circulation 2005, 112, 125–134. [Google Scholar] [CrossRef]
- Singh, J.P.; Evans, J.C.; Levy, D.; Larson, M.G.; Freed, L.A.; Fuller, D.L.; Lehman, B.; Benjamin, E.J. Prevalence and clinical determinants of mitral, tricuspid, and aortic regurgitation (the Framingham Heart Study). Am. J. Cardiol. 1999, 83, 897–902. [Google Scholar] [CrossRef]
- Miura, K.; Katoh, H. Structural and Histochemical Alterations in the Aortic Valves of Elderly Patients: A Comparative Study of Aortic Stenosis, Aortic Regurgitation, and Normal Valves. BioMed Res. Int. 2016, 2016, 6125204. [Google Scholar] [CrossRef] [PubMed]
- Cabral-Pacheco, G.A.; Garza-Veloz, I.; Castruita-De la Rosa, C.; Ramirez-Acuña, J.M.; Perez-Romero, B.A.; Guerrero-Rodriguez, J.F.; Martinez-Avila, N.; Martinez-Fierro, M.L. The Roles of Matrix Metalloproteinases and Their Inhibitors in Human Diseases. Int. J. Mol. Sci. 2020, 21, 9739. [Google Scholar] [CrossRef]
- Herum, K.M.; Lunde, I.G.; Skrbic, B.; Louch, W.E.; Hasic, A.; Boye, S.; Unger, A.; Brorson, S.-H.; Sjaastad, I.; Tønnessen, T.; et al. Syndecan-4 is a key determinant of collagen cross-linking and passive myocardial stiffness in the pressure-overloaded heart. Cardiovasc. Res. 2015, 106, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Conklin, M.W.; Eickhoff, J.C.; Riching, K.M.; Pehlke, C.A.; Eliceiri, K.W.; Provenzano, P.P.; Friedl, A.; Keely, P.J. Aligned collagen is a prognostic signature for survival in human breast carcinoma. Am. J. Pathol. 2011, 178, 1221–1232. [Google Scholar] [CrossRef] [PubMed]
- Doll, S.; Dreßen, M.; Geyer, P.E.; Itzhak, D.N.; Braun, C.; Doppler, S.A.; Meier, F.; Deutsch, M.-A.; Lahm, H.; Lange, R.; et al. Region and cell-type resolved quantitative proteomic map of the human heart. Nat. Commun. 2017, 8, 1469. [Google Scholar] [CrossRef]
- Chen, J.-H.; Yip, C.Y.Y.; Sone, E.D.; Simmons, C.A. Identification and characterization of aortic valve mesenchymal progenitor cells with robust osteogenic calcification potential. Am. J. Pathol. 2009, 174, 1109–1119. [Google Scholar] [CrossRef]
- Taylor, P.M.; Batten, P.; Brand, N.J.; Thomas, P.S.; Yacoub, M.H. The cardiac valve interstitial cell. Int. J. Biochem. Cell Biol. 2003, 35, 113–118. [Google Scholar] [CrossRef]
- Pichler Sekulic, S.; Sekulic, M. Adipocytes and membranous fat necrosis within native cardiac valves: Clinicopathologic characterization of histologic constituents. Cardiovasc. Pathol. 2021, 50, 107276. [Google Scholar] [CrossRef]
- Pantanowitz, L.; Jiang, L. Fatty infiltration of the aortic valve. Int. J. Cardiol. 2009, 134, e59–e61. [Google Scholar] [CrossRef]
- Otto, C.M.; Kuusisto, J.; Reichenbach, D.D.; Gown, A.M.; O’Brien, K.D. Characterization of the early lesion of “degenerative” valvular aortic stenosis. Histological and immunohistochemical studies. Circulation 1994, 90, 844–853. [Google Scholar] [CrossRef] [PubMed]
- Anthony, S.R.; Guarnieri, A.R.; Gozdiff, A.; Helsley, R.N.; Phillip Owens, A.; Tranter, M. Mechanisms linking adipose tissue inflammation to cardiac hypertrophy and fibrosis. Clin. Sci. 2019, 133, 2329–2344. [Google Scholar] [CrossRef]
- Venteclef, N.; Guglielmi, V.; Balse, E.; Gaborit, B.; Cotillard, A.; Atassi, F.; Amour, J.; Leprince, P.; Dutour, A.; Clément, K.; et al. Human epicardial adipose tissue induces fibrosis of the atrial myocardium through the secretion of adipo-fibrokines. Eur. Heart J. 2015, 36, 795–805. [Google Scholar] [CrossRef] [PubMed]
- Riley, H.J.; Bradshaw, A.D. The Influence of the Extracellular Matrix in Inflammation: Findings from the SPARC-Null Mouse. Anat. Rec. 2020, 303, 1624–1629. [Google Scholar] [CrossRef]
- Rutkovskiy, A.; Malashicheva, A.; Sullivan, G.; Bogdanova, M.; Kostareva, A.; Stensløkken, K.-O.; Fiane, A.; Vaage, J. Valve Interstitial Cells: The Key to Understanding the Pathophysiology of Heart Valve Calcification. J. Am. Heart Assoc. 2017, 6, e006339. [Google Scholar] [CrossRef] [PubMed]
- Bogdanova, M.; Zabirnyk, A.; Malashicheva, A.; Semenova, D.; Kvitting, J.-P.E.; Kaljusto, M.-L.; Perez, M.D.M.; Kostareva, A.; Stensløkken, K.-O.; Sullivan, G.J.; et al. Models and Techniques to Study Aortic Valve Calcification in Vitro, ex Vivo and in Vivo. An Overview. Front. Pharmacol. 2022, 13, 835825. [Google Scholar] [CrossRef]
- Assmann, A.K.; Winnicki, V.; Sugimura, Y.; Chekhoeva, A.; Barth, M.; Assmann, A.; Lichtenberg, A.; Akhyari, P. Impact of PPAR-gamma activation on the durability of biological heart valve prostheses in hypercholesterolaemic rats. Eur. J. Cardiothorac. Surg. 2022, 63, ezad005. [Google Scholar] [CrossRef]
- Bogdanova, M.; Zabirnyk, A.; Malashicheva, A.; Enayati, K.Z.; Karlsen, T.A.; Kaljusto, M.-L.; Kvitting, J.-P.E.; Dissen, E.; Sullivan, G.J.; Kostareva, A.; et al. Interstitial cells in calcified aortic valves have reduced differentiation potential and stem cell-like properties. Sci. Rep. 2019, 9, 12934. [Google Scholar] [CrossRef]
- Kanaji, Y.; Ozcan, I.; Toya, T.; Gulati, R.; Young, M.; Kakuta, T.; Lerman, L.O.; Lerman, A. Circulating Progenitor Cells Are Associated With Bioprosthetic Aortic Valve Deterioration: A Preliminary Study. J. Am. Heart Assoc. 2023, 12, e027364. [Google Scholar] [CrossRef]
- Date, K.; Sakagami, H.; Yura, K. Regulatory properties of vitronectin and its glycosylation in collagen fibril formation and collagen-degrading enzyme cathepsin K activity. Sci. Rep. 2021, 11, 12023. [Google Scholar] [CrossRef] [PubMed]
- Engvall, E.; Ruoslahti, E.; Miller, E.J. Affinity of fibronectin to collagens of different genetic types and to fibrinogen. J. Exp. Med. 1978, 147, 1584–1595. [Google Scholar] [CrossRef] [PubMed]
- Robinson, K.A.; Sun, M.; Barnum, C.E.; Weiss, S.N.; Huegel, J.; Shetye, S.S.; Lin, L.; Saez, D.; Adams, S.M.; Iozzo, R.V.; et al. Decorin and biglycan are necessary for maintaining collagen fibril structure, fiber realignment, and mechanical properties of mature tendons. Matrix Biol. 2017, 64, 81–93. [Google Scholar] [CrossRef] [PubMed]
- Santamaria, S.; de Groot, R. ADAMTS proteases in cardiovascular physiology and disease. Open Biol. 2020, 10, 200333. [Google Scholar] [CrossRef]
- Fondard, O.; Detaint, D.; Iung, B.; Choqueux, C.; Adle-Biassette, H.; Jarraya, M.; Hvass, U.; Couetil, J.-P.; Henin, D.; Michel, J.-B.; et al. Extracellular matrix remodelling in human aortic valve disease: The role of matrix metalloproteinases and their tissue inhibitors. Eur. Heart J. 2005, 26, 1333–1341. [Google Scholar] [CrossRef]
- Kaden, J.J.; Dempfle, C.-E.; Grobholz, R.; Fischer, C.S.; Vocke, D.C.; Kiliç, R.; Sarikoç, A.; Piñol, R.; Hagl, S.; Lang, S.; et al. Inflammatory regulation of extracellular matrix remodeling in calcific aortic valve stenosis. Cardiovasc. Pathol. 2005, 14, 80–87. [Google Scholar] [CrossRef]
- Gupta, V.; Barzilla, J.E.; Mendez, J.S.; Stephens, E.H.; Lee, E.L.; Collard, C.D.; Laucirica, R.; Weigel, P.H.; Grande-Allen, K.J. Abundance and location of proteoglycans and hyaluronan within normal and myxomatous mitral valves. Cardiovasc. Pathol. 2009, 18, 191–197. [Google Scholar] [CrossRef]
- Rabkin, E.; Aikawa, M.; Stone, J.R.; Fukumoto, Y.; Libby, P.; Schoen, F.J. Activated interstitial myofibroblasts express catabolic enzymes and mediate matrix remodeling in myxomatous heart valves. Circulation 2001, 104, 2525–2532. [Google Scholar] [CrossRef]
- Santamaria, S. ADAMTS-5: A difficult teenager turning 20. Int. J. Exp. Pathol. 2020, 101, 4–20. [Google Scholar] [CrossRef]
- Théron, A.; Odelin, G.; Faure, E.; Avierinos, J.-F.; Zaffran, S. Krox20 heterozygous mice: A model of aortic regurgitation associated with decreased expression of fibrillar collagen genes. Arch. Cardiovasc. Dis. 2016, 109, 188–198. [Google Scholar] [CrossRef]
- Khang, A.; Buchanan, R.M.; Ayoub, S.; Rego, B.V.; Lee, C.-H.; Sacks, M.S. Biological Mechanics of the Heart Valve Interstitial Cell. In Advances in Heart Valve Biomechanics; Springer: Cham, Switzerland, 2018. [Google Scholar]
- Vahanian, A.; Beyersdorf, F.; Praz, F.; Milojevic, M.; Baldus, S.; Bauersachs, J.; Capodanno, D.; Conradi, L.; De Bonis, M.; De Paulis, R.; et al. 2021 ESC/EACTS Guidelines for the management of valvular heart disease: Developed by the Task Force for the management of valvular heart disease of the European Society of Cardiology (ESC) and the European Association for Cardio-Thoracic Surgery (EACTS). Eur. Heart J. 2021, 43, 561–632. [Google Scholar] [CrossRef]
- Lancellotti, P.; Tribouilloy, C.; Hagendorff, A.; Popescu, B.A.; Edvardsen, T.; Pierard, L.A.; Badano, L.; Zamorano, J.L. Recommendations for the echocardiographic assessment of native valvular regurgitation: An executive summary from the European Association of Cardiovascular Imaging. Eur. Heart J. Cardiovasc. Imaging 2013, 14, 611–644. [Google Scholar] [CrossRef] [PubMed]
- Sashida, Y.; Rodriguez, C.J.; Boden-Albala, B.; Jin, Z.; Elkind, M.S.V.; Liu, R.; Rundek, T.; Sacco, R.L.; DiTullio, M.R.; Homma, S. Ethnic differences in aortic valve thickness and related clinical factors. Am. Heart J. 2010, 159, 698–704. [Google Scholar] [CrossRef] [PubMed]
- Sahasakul, Y.; Edwards, W.D.; Naessens, J.M.; Tajik, A.J. Age-related changes in aortic and mitral valve thickness: Implications for two-dimensional echocardiography based on an autopsy study of 200 normal human hearts. Am. J. Cardiol. 1988, 62, 424–430. [Google Scholar] [CrossRef]
- Matilla, L.; Jover, E.; Garaikoetxea, M.; Martín-Nuñez, E.; Arrieta, V.; García-Peña, A.; Navarro, A.; Fernández-Celis, A.; Gainza, A.; Álvarez, V.; et al. Sex-Related Signaling of Aldosterone/Mineralocorticoid Receptor Pathway in Calcific Aortic Stenosis. Hypertension 2022, 79, 1724–1737. [Google Scholar] [CrossRef]

| Total | Presence of AT | Absence of AT | p-Value | |
|---|---|---|---|---|
| %, (N) | 100 (144) | 66.67 (96) | 33.33 (48) | |
| Baseline characteristics | ||||
| Male sex %, (N) | 71.53 (103) | 73.96 (71) | 66.67 (32) | 0.36 |
| Age [mean (SD)] | 67.07 (0.95) | 67.4 (1.17) | 66.35 (1.63) | 0.61 |
| Weight [mean (SD)] | 75.85 (1.28) | 76.19 (1.56) | 75.21 (2.27) | 0.72 |
| Height [mean (SD)] | 164.41 (1.87) | 165.86 (2.13) | 161.58 (3.66) | 0.28 |
| Body surface area [mean (SD)] | 1.87 (0.02) | 1.88 (0.02) | 1.85 (0.03) | 0.54 |
| Hypertension %, (N) | 70 (101) | 72 (69) | 67 (32) | 0.4 |
| Atrial fibrillation %, (N) | 26 (37) | 26 (25) | 25 (12) | 0.6 |
| Diabetes %, (N) | 12 (17) | 10 (10) | 15 (7) | 0.49 |
| Smokers %, (N) | 32 (46) | 32 (31) | 31 (15) | 0.84 |
| CAD %, (N) | 17 (24) | 16 (15) | 19 (9) | 0.67 |
| Drug medicines | ||||
| ACE/ARA %, (N) | 67 (96) | 64 (61) | 73 (35) | 0.33 |
| Beta Blockers %, (N) | 53 (76) | 49 (47) | 60 (29) | 0.24 |
| Statins %, (N) | 44 (63) | 42 (40) | 48 (23) | 0.54 |
| Furosemide %, (N) | 38 (55) | 38 (36) | 40 (19) | 0.88 |
| Biochemical analyses | ||||
| Total cholesterol (mg/dL) [mean (SD)] | 172.3 (3.4) | 169.2 (4.5) | 178.5 (5.1) | 0.21 |
| LDL (mg/mL) [mean (SD)] | 107.0 (3.0) | 105.8 (3.8) | 109.6 (4.7) | 0.54 |
| HDL (mg/mL) [mean (SD)] | 46.73 (1.62) | 46.29 (2.12) | 47.61 (2.41) | 0.7 |
| Triglycerides (mg/mL) [mean (SD)] | 108.2 (4.9) | 103.3 (5.2) | 118.7 (10.4) | 0.14 |
| Echocardiographic parameters | ||||
| LA diameter (mm) [mean (SD)] | 44.18 (0.89) | 44.5 (1.17) | 43.57 (1.33) | 0.63 |
| LVEDD (mm) [mean (SD)] | 61.52 (0.82) | 61.78 (1.03) | 60.98 (1.34) | 0.65 |
| LVESD (mm) [mean (SD)] | 43.33 (1) | 43.47 (1.28) | 43.05 (1.57) | 0.84 |
| LVEDV (mL) [mean (SD)] | 194.2 (9.78) | 195.45 (13.67) | 192.2 (13.35) | 0.87 |
| LVEF% [mean (SD)] | 53.24 (1.05) | 53.15 (1.34) | 53.40 (1.70) | 0.91 |
| SPAP (mmHg) [mean (SD)] | 37.58 (0.93) | 37.39 (1.11) | 38 (1.71) | 0.76 |
| Aortic dimensions (Echo) | ||||
| Annulus (mm) [mean (SD)] | 26.42 (0.79) | 27.07 (1.06) | 24.86 (1.76) | 0.2 |
| Sinus of Valsalva (mm) [mean (SD)] | 42.04 (0.82) | 42.81 (1.07) | 40.73 (1.23) | 0.22 |
| Sinotubular junction (mm) [mean (SD)] | 40.21 (1.54) | 40.43 (1.06) | 39.7 (1.65) | 0.71 |
| Ascending aorta (mm) [mean (SD)] | 45.69 (0.85) | 45.78 (0.97) | 45.53 (1.69) | 0.89 |
| Aortic dimensions (MDCT) | ||||
| Annulus (mm) [mean (SD)] | 26.84 (0.39) | 26.56 (0.39) | 27.41 (0.88) | 0.31 |
| Sinus of Valsalva (mm) [mean (SD)] | 42.87 (0.77) | 43.05 (0.97) | 42.48 (1.27) | 0.73 |
| Sinotubular junction (mm) [mean (SD)] | 36.17 (0.87) | 36.54 (1.12) | 35.45 (1.36) | 0.55 |
| Ascending aorta (mm) [mean (SD)] | 46.13 (1.02) | 46.81 (1.25) | 44.74 (1.77) | 0.34 |
| Bicuspid Valve %, (N) | 16 (23) | 15 (14) | 19 (9) | 0.68 |
| Valve thickness (Echo/MDCT) | ||||
| Thickened Valve %, (N) | 49 (70) | 61 (59) | 23 (11) | 0.000 |
| Valve thickness (mm) [mean (SD)] | 2.97 (0.06) | 3.10 (0.07) | 2.67 (0.09) | 0.001 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sádaba, A.; Garaikoetxea, M.; Tiraplegui, C.; San-Ildefonso-García, S.; Goñi-Olóriz, M.; Fernández-Celis, A.; Martín-Núñez, E.; Castillo, P.; Álvarez, V.; Sádaba, R.; et al. The Presence of Adipose Tissue in Aortic Valves Influences Inflammation and Extracellular Matrix Composition in Chronic Aortic Regurgitation. Int. J. Mol. Sci. 2025, 26, 3128. https://doi.org/10.3390/ijms26073128
Sádaba A, Garaikoetxea M, Tiraplegui C, San-Ildefonso-García S, Goñi-Olóriz M, Fernández-Celis A, Martín-Núñez E, Castillo P, Álvarez V, Sádaba R, et al. The Presence of Adipose Tissue in Aortic Valves Influences Inflammation and Extracellular Matrix Composition in Chronic Aortic Regurgitation. International Journal of Molecular Sciences. 2025; 26(7):3128. https://doi.org/10.3390/ijms26073128
Chicago/Turabian StyleSádaba, Alba, Mattie Garaikoetxea, Carolina Tiraplegui, Susana San-Ildefonso-García, Miriam Goñi-Olóriz, Amaya Fernández-Celis, Ernesto Martín-Núñez, Paula Castillo, Virginia Álvarez, Rafael Sádaba, and et al. 2025. "The Presence of Adipose Tissue in Aortic Valves Influences Inflammation and Extracellular Matrix Composition in Chronic Aortic Regurgitation" International Journal of Molecular Sciences 26, no. 7: 3128. https://doi.org/10.3390/ijms26073128
APA StyleSádaba, A., Garaikoetxea, M., Tiraplegui, C., San-Ildefonso-García, S., Goñi-Olóriz, M., Fernández-Celis, A., Martín-Núñez, E., Castillo, P., Álvarez, V., Sádaba, R., Jover, E., Navarro, A., & López-Andrés, N. (2025). The Presence of Adipose Tissue in Aortic Valves Influences Inflammation and Extracellular Matrix Composition in Chronic Aortic Regurgitation. International Journal of Molecular Sciences, 26(7), 3128. https://doi.org/10.3390/ijms26073128









