Overexpression of LcMYB90 Transcription Factor Enhances Drought and Salt Tolerance in Blue Honeysuckle (Lonicera caerulea L.) and Tobacco (Nicotiana tabacum L.)
Abstract
1. Introduction
2. Results
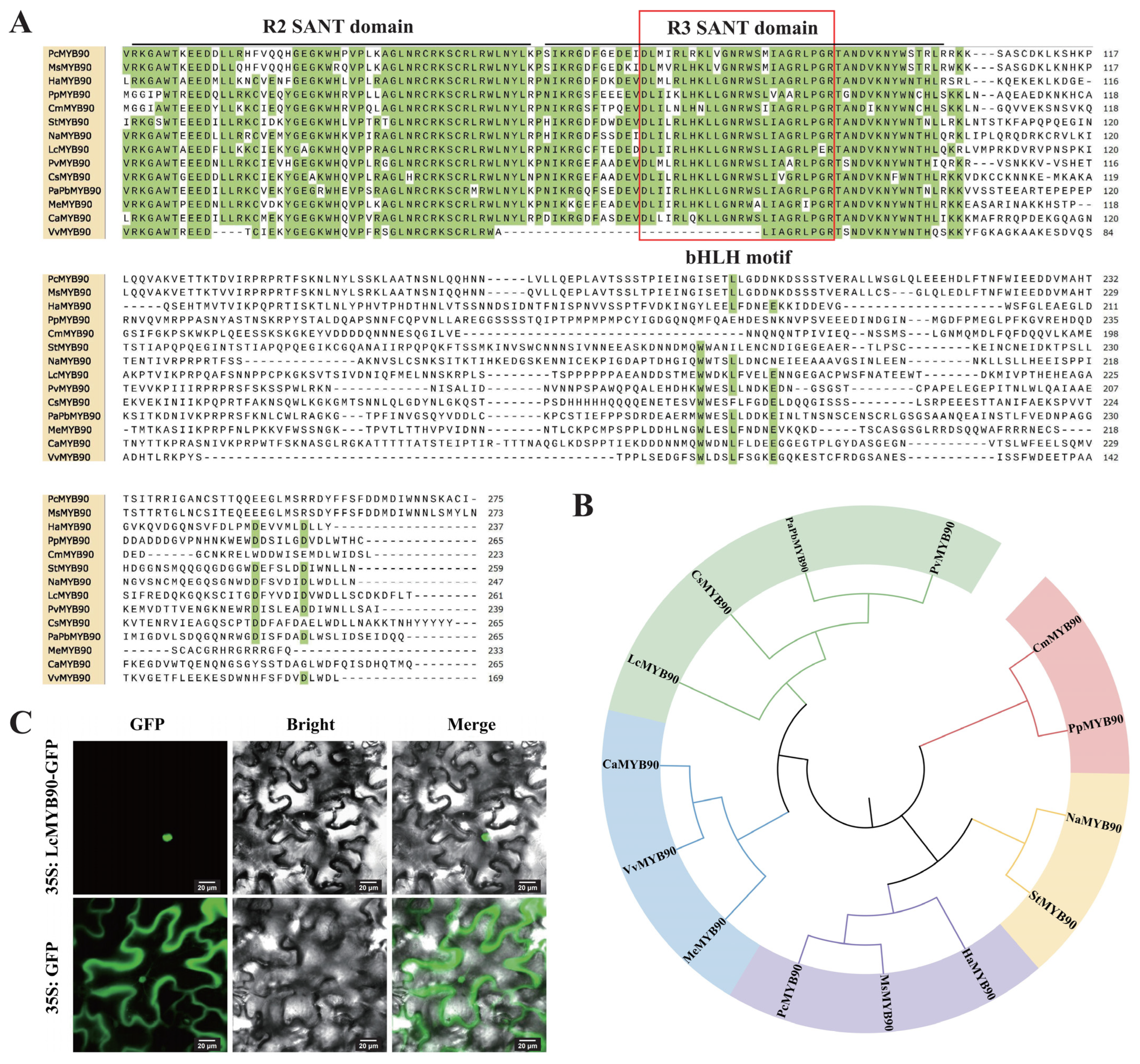
2.1. Bioinformatics Analysis of LcMYB90
2.2. LcMYB90 Is Localized in the Nucleus
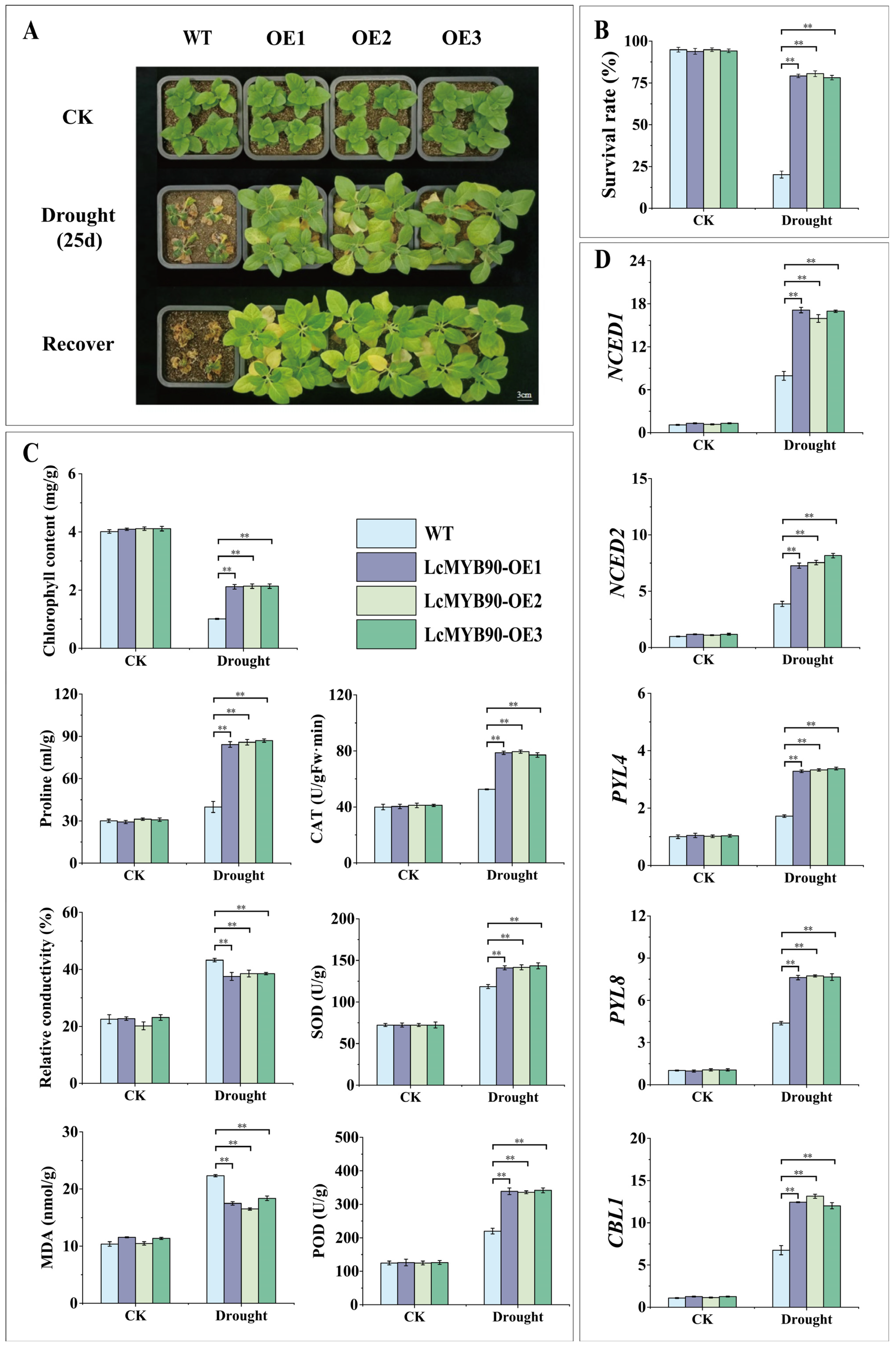
2.3. Physiological Changes in Transgenic Tobacco Under Drought Stress
2.4. Overexpression of LcMYB90 Increases the Expression of Drought-Related Genes in Transgenic Tobacco
2.5. Physiological Changes in Transgenic Tobacco Under Salt Stress
2.6. LcMYB90 Overexpression Enhances Salt-Related Gene Expression in Transgenic Tobacco
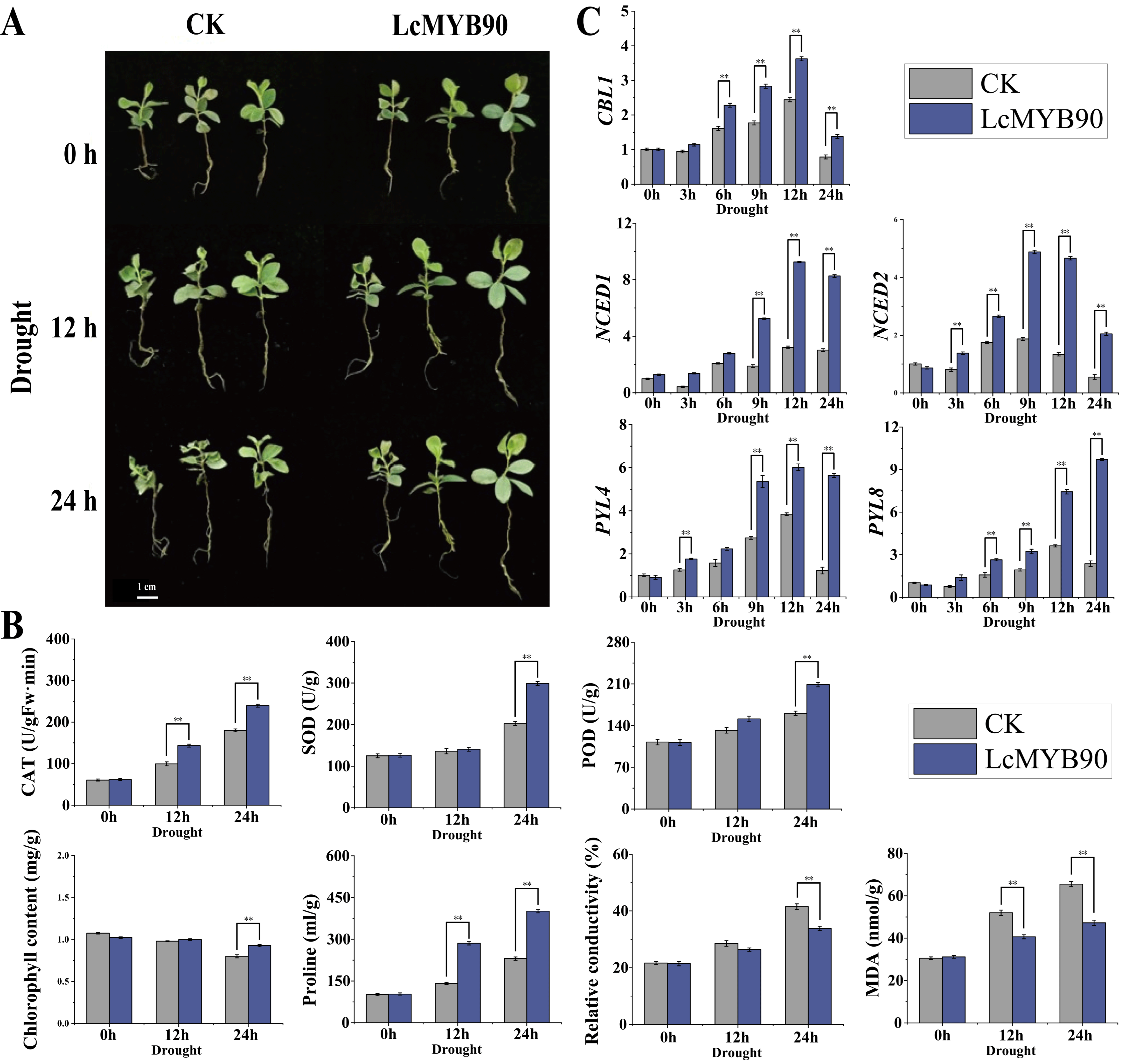
2.7. Reaction of Blue Honeysuckle with Dynamic Expression of LcMYB90 to Drought Conditions
2.8. Expression of Genes Related to Drought in Transiently Transformed LcMYB90 Blue Honeysuckle
2.9. Salt Stress Response of Transiently Transformed LcMYB90 in Blue Honeysuckle
2.10. Expression of Salt-Responsive Genes in Transiently Transformed LcMYB90 Blue Honeysuckle
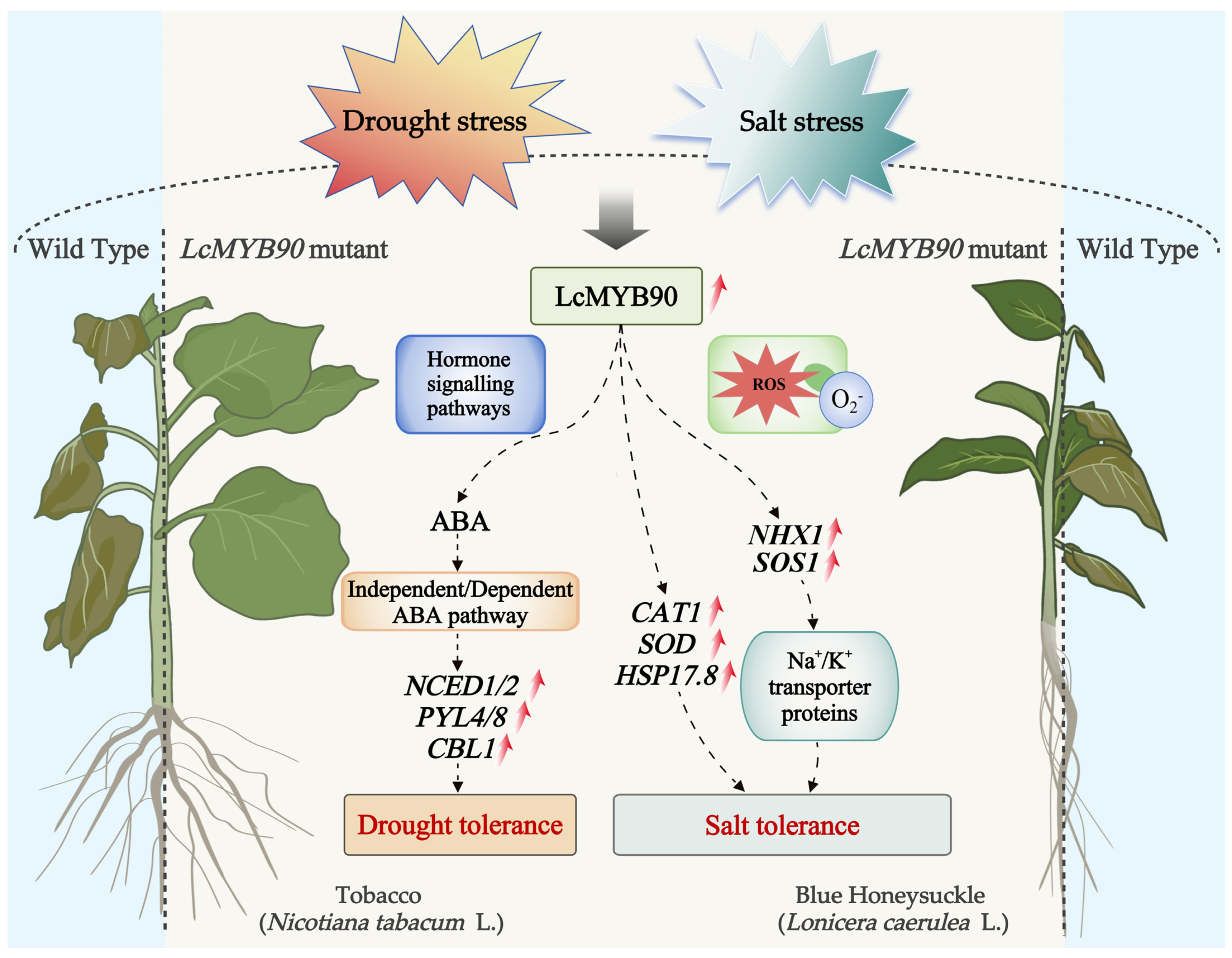
3. Discussion
3.1. Role of LcMYB90 in Drought Stress Response
3.1.1. Changes in Plant Physiological and Biochemical Indices Under Drought Conditions
3.1.2. Regulation of Drought-Stress-Related Genes by LcMYB90
3.2. Involvement of LcMYB90 in Response to Salt Stress
3.2.1. Changes in Plant Physiological and Biochemical Indices Under Salt Stress
3.2.2. Regulation of Salt-Stress-Related Genes by LcMYB90
4. Materials and Methods
4.1. Plant Materials
4.2. Phylogenetic Analysis
4.3. Genetic Transformation in Tobacco of LcMYB90
4.4. Temporary Transformation of Blue Honeysuckle
4.5. Drought Stress and Salt Treatment
4.6. Subcellular Localization
4.7. Real-Time Quantitative PCR
4.8. Measurement of Physiological Parameters
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
References
- Zhang, Y.; Xu, J.; Li, R.; Ge, Y.; Li, Y.; Li, R. Plants’ response to abiotic stress: Mechanisms and strategies. Int. J. Mol. Sci. 2023, 24, 10915. [Google Scholar] [CrossRef]
- Takasaki, H.; Maruyama, K.; Kidokoro, S.; Ito, Y.; Fujita, Y.; Shinozaki, K.; Yamaguchi-Shinozaki, K.; Nakashima, K. The abiotic stress-responsive NAC-type transcription factor OsNAC5 regulates stress-inducible genes and stress tolerance in rice. Mol. Genet. Genom. 2010, 284, 173–183. [Google Scholar] [CrossRef]
- Li, M.; Lin, L.; Zhang, Y.; Sui, N. ZmMYB31, a R2R3-MYB transcription factor in maize, positively regulates the expression of CBF genes and enhances resistance to chilling and oxidative stress. Mol. Biol. Rep. 2019, 46, 3937–3944. [Google Scholar] [CrossRef]
- Wang, M.; Hao, J.; Chen, X.; Zhang, X. SlMYB102 expression enhances low-temperature stress resistance in tomato plants. PeerJ 2020, 8, e10059. [Google Scholar] [CrossRef]
- An, J.P.; Li, R.; Qu, F.J.; You, C.X.; Wang, X.F.; Hao, Y.J. R2R3-MYB transcription factor Md MYB 23 is involved in the cold tolerance and proanthocyanidin accumulation in apple. Plant J. 2018, 96, 562–577. [Google Scholar] [CrossRef]
- Agarwal, M.; Hao, Y.; Kapoor, A.; Dong, C.-H.; Fujii, H.; Zheng, X.; Zhu, J.-K. A R2R3 type MYB transcription factor is involved in the cold regulation of CBF genes and in acquired freezing tolerance. J. Biol. Chem. 2006, 281, 37636–37645. [Google Scholar] [CrossRef]
- Shan, T.; Rong, W.; Xu, H.; Du, L.; Liu, X.; Zhang, Z. The wheat R2R3-MYB transcription factor TaRIM1 participates in resistance response against the pathogen Rhizoctonia cerealis infection through regulating defense genes. Sci. Rep. 2016, 6, 28777. [Google Scholar] [CrossRef]
- Mmadi, M.A.; Dossa, K.; Wang, L.; Zhou, R.; Wang, Y.; Cisse, N.; Sy, M.O.; Zhang, X. Functional characterization of the versatile MYB gene family uncovered their important roles in plant development and responses to drought and waterlogging in sesame. Genes 2017, 8, 362. [Google Scholar] [CrossRef]
- Chen, Y.; Yang, X.; He, K.; Liu, M.; Li, J.; Gao, Z.; Lin, Z.; Zhang, Y.; Wang, X.; Qiu, X.; et al. The MYB transcription factor superfamily of Arabidopsis: Expression analysis and phylogenetic comparison with the rice MYB family. Plant Mol. Biol. 2006, 60, 107–124. [Google Scholar] [CrossRef]
- Beathard, C.; Mooney, S.; Al-Saharin, R.; Goyer, A.; Hellmann, H. Characterization of Arabidopsis thaliana R2R3 S23 MYB transcription factors as novel targets of the ubiquitin proteasome-pathway and regulators of salt stress and abscisic acid response. Front. Plant Sci. 2021, 12, 629208. [Google Scholar] [CrossRef]
- Ding, Z.; Li, S.; An, X.; Liu, X.; Qin, H.; Wang, D. Transgenic expression of MYB15 confers enhanced sensitivity to abscisic acid and improved drought tolerance in Arabidopsis thaliana. J. Genet. Genom. 2009, 36, 17–29. [Google Scholar] [CrossRef]
- Cominelli, E.; Galbiati, M.; Vavasseur, A.; Conti, L.; Sala, T.; Vuylsteke, M.; Leonhardt, N.; Dellaporta, S.L.; Tonelli, C. A guard-cell-specific MYB transcription factor regulates stomatal movements and plant drought tolerance. Curr. Biol. 2005, 15, 1196–1200. [Google Scholar] [CrossRef]
- Shinozaki, K.; Yamaguchi-Shinozaki, K. Gene networks involved in drought stress response and tolerance. J. Exp. Bot. 2007, 58, 221–227. [Google Scholar] [CrossRef]
- Soma, F.; Takahashi, F.; Yamaguchi-Shinozaki, K.; Shinozaki, K. Cellular phosphorylation signaling and gene expression in drought stress responses: ABA-dependent and ABA-independent regulatory systems. Plants 2021, 10, 756. [Google Scholar] [CrossRef] [PubMed]
- Wasilewska, A.; Vlad, F.; Sirichandra, C.; Redko, Y.; Jammes, F.; Valon, C.; dit Frey, N.F.; Leung, J. An update on abscisic acid signaling in plants and more…. Mol. Plant 2008, 1, 198–217. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Jiang, J.; Zhao, T.; Xu, X.; Yang, H.; Li, J. Virus-induced gene silencing of SlPYL4 decreases the drought tolerance of tomato. Hortic. Plant J. 2022, 8, 361–368. [Google Scholar] [CrossRef]
- Mega, R.; Abe, F.; Kim, J.-S.; Tsuboi, Y.; Tanaka, K.; Kobayashi, H.; Sakata, Y.; Hanada, K.; Tsujimoto, H.; Kikuchi, J. Tuning water-use efficiency and drought tolerance in wheat using abscisic acid receptors. Nat. Plants 2019, 5, 153–159. [Google Scholar] [CrossRef]
- Albrecht, V.; Weinl, S.; Blazevic, D.; D’Angelo, C.; Batistic, O.; Kolukisaoglu, Ü.; Bock, R.; Schulz, B.; Harter, K.; Kudla, J. The calcium sensor CBL1 integrates plant responses to abiotic stresses. Plant J. 2003, 36, 457–470. [Google Scholar] [CrossRef]
- Wu, J.; Jiang, Y.; Liang, Y.; Chen, L.; Chen, W.; Cheng, B. Expression of the maize MYB transcription factor ZmMYB3R enhances drought and salt stress tolerance in transgenic plants. Plant Physiol. Biochem. 2019, 137, 179–188. [Google Scholar] [CrossRef]
- Alexander, R.D.; Wendelboe-Nelson, C.; Morris, P.C. The barley transcription factor HvMYB1 is a positive regulator of drought tolerance. Plant Physiol. Biochem. 2019, 142, 246–253. [Google Scholar] [CrossRef]
- Dossa, K.; A Mmadi, M.; Zhou, R.; Liu, A.; Yang, Y.; Diouf, D.; You, J.; Zhang, X. Ectopic expression of the sesame MYB transcription factor SiMYB305 promotes root growth and modulates ABA-mediated tolerance to drought and salt stresses in Arabidopsis. AoB PLANTS 2019, 12, plz081. [Google Scholar] [CrossRef] [PubMed]
- Deinlein, U.; Stephan, A.B.; Horie, T.; Luo, W.; Xu, G.; Schroeder, J.I. Plant salt-tolerance mechanisms. Trends Plant Sci. 2014, 19, 371–379. [Google Scholar] [CrossRef] [PubMed]
- Glenn, E.P.; Brown, J.J.; Blumwald, E. Salt tolerance and crop potential of halophytes. Crit. Rev. Plant Sci. 1999, 18, 227–255. [Google Scholar] [CrossRef]
- Barragán, V.; Leidi, E.O.; Andrés, Z.; Rubio, L.; De Luca, A.; Fernandez, J.A.; Cubero, B.; Pardo, J.M. Ion exchangers NHX1 and NHX2 mediate active potassium uptake into vacuoles to regulate cell turgor and stomatal function in Arabidopsis. Plant Cell 2012, 24, 1127–1142. [Google Scholar] [CrossRef]
- Sun, Y.; Zhao, J.; Li, X.; Li, Y. E2 conjugases UBC1 and UBC2 regulate MYB42-mediated SOS pathway in response to salt stress in Arabidopsis. New Phytol. 2020, 227, 455–472. [Google Scholar] [CrossRef]
- Kim, J.H.; Nguyen, N.H.; Jeong, C.Y.; Nguyen, N.T.; Hong, S.-W.; Lee, H. Loss of the R2R3 MYB, AtMyb73, causes hyper-induction of the SOS1 and SOS3 genes in response to high salinity in Arabidopsis. J. Plant Physiol. 2013, 170, 1461–1465. [Google Scholar] [CrossRef]
- Liu, M.; Wang, Z.; Xiao, H.-m.; Yang, Y. Characterization of TaDREB1 in wheat genotypes with different seed germination under osmotic stress. Hereditas 2018, 155, 1–9. [Google Scholar] [CrossRef]
- Song, Y.; Yang, W.; Fan, H.; Zhang, X.; Sui, N. TaMYB86B encodes a R2R3-type MYB transcription factor and enhances salt tolerance in wheat. Plant Sci. 2020, 300, 110624. [Google Scholar] [CrossRef]
- Han, J.; Dai, J.; Chen, Z.; Li, W.; Li, X.; Zhang, L.; Yao, A.; Zhang, B.; Han, D. Overexpression of a ‘Beta’ MYB Factor Gene, VhMYB15, Increases Salinity and Drought Tolerance in Arabidopsis thaliana. Int. J. Mol. Sci. 2024, 25, 1534. [Google Scholar] [CrossRef]
- Bian, C.; Wang, H.; Li, W.; Chen, J.; Ren, B.; Qin, D.; Liu, J.; Zang, S.; Li, J.; Ma, K. Expression Profiling of the 56 R2R3-MYB Family Genes in Response to Cold, Drought, and Salt Stress in Blue Honeysuckle (Lonicera caerulea L.). Plant Mol. Biol. Rep. 2024, 43, 103–120. [Google Scholar] [CrossRef]
- Horsch, R.B.; Fry, J.E.; Hoffmann, N.L.; Wallroth, M.; Eichholtz, D.; Rogers, S.G.; Fraley, R.T. A simple and general method for transferring genes into plants. Science 1985, 227, 1229–1231. [Google Scholar] [CrossRef]
- Sartory, D.P.; Grobbelaar, J.U. Extraction of chlorophyll a from freshwater phytoplankton for spectrophotometric analysis. Hydrobiologia 1984, 114, 177–187. [Google Scholar] [CrossRef]
- Wellburn, A.R. The spectral determination of chlorophylls a and b, as well as total carotenoids, using various solvents with spectrophotometers of different resolution. J. Plant Physiol. 1994, 144, 307–313. [Google Scholar] [CrossRef]
- Zhang, J.; Kirkham, M.B. Drought-stress-induced changes in activities of superoxide dismutase, catalase, and peroxidase in wheat species. Plant Cell Physiol. 1994, 35, 785–791. [Google Scholar] [CrossRef]
- Zhao, Y.; Xing, L.; Wang, X.; Hou, Y.-J.; Gao, J.; Wang, P.; Duan, C.-G.; Zhu, X.; Zhu, J.-K. The ABA Receptor PYL8 Promotes Lateral Root Growth by Enhancing MYB77-Dependent Transcription of Auxin-Responsive Genes. Sci. Signal. 2014, 7, ra53. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Wang, Y.; Wang, L.; Hu, P.; Wang, Y.; Jia, Y.; Zhang, C.; Zhang, Y.; Zhang, Y.; Wang, C. Expression of the MYB transcription factor gene BplMYB46 affects abiotic stress tolerance and secondary cell wall deposition in Betula platyphylla. Plant Biotechnol. J. 2017, 15, 107–121. [Google Scholar] [CrossRef]
- Zhang, L.; Zhao, G.; Jia, J.; Liu, X.; Kong, X. Molecular characterization of 60 isolated wheat MYB genes and analysis of their expression during abiotic stress. J. Exp. Bot. 2012, 63, 203–214. [Google Scholar] [CrossRef]
- Changan, S.S.; Ali, K.; Kumar, V.; Garg, N.K.; Tyagi, A. Abscisic acid biosynthesis under water stress: Anomalous behavior of the 9-cis-epoxycarotenoid dioxygenase1 (NCED1) gene in rice. Biol. Plant. 2018, 62, 663–670. [Google Scholar] [CrossRef]
- Neves, D.M.; Filho, M.A.C.; Bellete, B.S.; Silva, M.; Souza, D.T.; dos S. Soares Filho, W.; Costa, M.G.C.; Gesteira, A.S. Comparative study of putative 9-cis-epoxycarotenoid dioxygenase and abscisic acid accumulation in the responses of Sunki mandarin and Rangpur lime to water deficit. Mol. Biol. Rep. 2013, 40, 5339–5349. [Google Scholar] [CrossRef]
- Cheong, Y.H.; Kim, K.-N.; Pandey, G.K.; Gupta, R.; Grant, J.J.; Luan, S. CBL1, a calcium sensor that differentially regulates salt, drought, and cold responses in Arabidopsis. Plant Cell 2003, 15, 1833–1845. [Google Scholar] [CrossRef]
- Fang, Q.; Jiang, T.; Xu, L.; Liu, H.; Mao, H.; Wang, X.; Jiao, B.O.; Duan, Y.; Wang, Q.; Dong, Q. A salt-stress-regulator from the Poplar R2R3 MYB family integrates the regulation of lateral root emergence and ABA signaling to mediate salt stress tolerance in Arabidopsis. Plant Physiol. Biochem. 2017, 114, 100–110. [Google Scholar] [CrossRef] [PubMed]
- Lu, T.; Ai, Y.; Na, M.; Xu, S.; Li, X.; Zheng, X.; Zhou, J. Physiological and Biochemical Analysis of Selenium-Enriched Rice. Agronomy 2024, 14, 1715. [Google Scholar] [CrossRef]
- Wang, H.; Liang, X.; Huang, J.; Zhang, D.; Lu, H.; Liu, Z.; Bi, Y. Involvement of ethylene and hydrogen peroxide in induction of alternative respiratory pathway in salt-treated Arabidopsis calluses. Plant Cell Physiol. 2010, 51, 1754–1765. [Google Scholar] [CrossRef] [PubMed]
- Zhao, L.; Liu, F.; Xu, W.; Di, C.; Zhou, S.; Xue, Y.; Yu, J.; Su, Z. Increased expression of OsSPX1 enhances cold/subfreezing tolerance in tobacco and Arabidopsis thaliana. Plant Biotechnol. J. 2009, 7, 550–561. [Google Scholar] [CrossRef]
- Si, T.; Chen, H.; Qiu, Z.; Zhang, L.; Ohore, O.E.; Zhang, S. Bacterial succession in epiphytic biofilms and deciduous layer sediments during Hydrilla verticillata decay: A field investigation. J. Environ. Sci. 2020, 93, 193–201. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, J.; Bian, C.; Fu, C.; Zhang, Q.; Qin, D.; Hao, W.; Guo, M.; Huo, J.; Li, J.; Gang, H. Overexpression of LcMYB90 Transcription Factor Enhances Drought and Salt Tolerance in Blue Honeysuckle (Lonicera caerulea L.) and Tobacco (Nicotiana tabacum L.). Int. J. Mol. Sci. 2025, 26, 3124. https://doi.org/10.3390/ijms26073124
Chen J, Bian C, Fu C, Zhang Q, Qin D, Hao W, Guo M, Huo J, Li J, Gang H. Overexpression of LcMYB90 Transcription Factor Enhances Drought and Salt Tolerance in Blue Honeysuckle (Lonicera caerulea L.) and Tobacco (Nicotiana tabacum L.). International Journal of Molecular Sciences. 2025; 26(7):3124. https://doi.org/10.3390/ijms26073124
Chicago/Turabian StyleChen, Jing, Chunyang Bian, Chunlin Fu, Qian Zhang, Dong Qin, Wenjun Hao, Manman Guo, Junwei Huo, Jiangkuo Li, and Huixin Gang. 2025. "Overexpression of LcMYB90 Transcription Factor Enhances Drought and Salt Tolerance in Blue Honeysuckle (Lonicera caerulea L.) and Tobacco (Nicotiana tabacum L.)" International Journal of Molecular Sciences 26, no. 7: 3124. https://doi.org/10.3390/ijms26073124
APA StyleChen, J., Bian, C., Fu, C., Zhang, Q., Qin, D., Hao, W., Guo, M., Huo, J., Li, J., & Gang, H. (2025). Overexpression of LcMYB90 Transcription Factor Enhances Drought and Salt Tolerance in Blue Honeysuckle (Lonicera caerulea L.) and Tobacco (Nicotiana tabacum L.). International Journal of Molecular Sciences, 26(7), 3124. https://doi.org/10.3390/ijms26073124





