Polyoxometalate Etching of NMO@NF for Highly Efficient Oxygen Evolution Reaction in Water Splitting
Abstract
1. Introduction
2. Results and Discussion
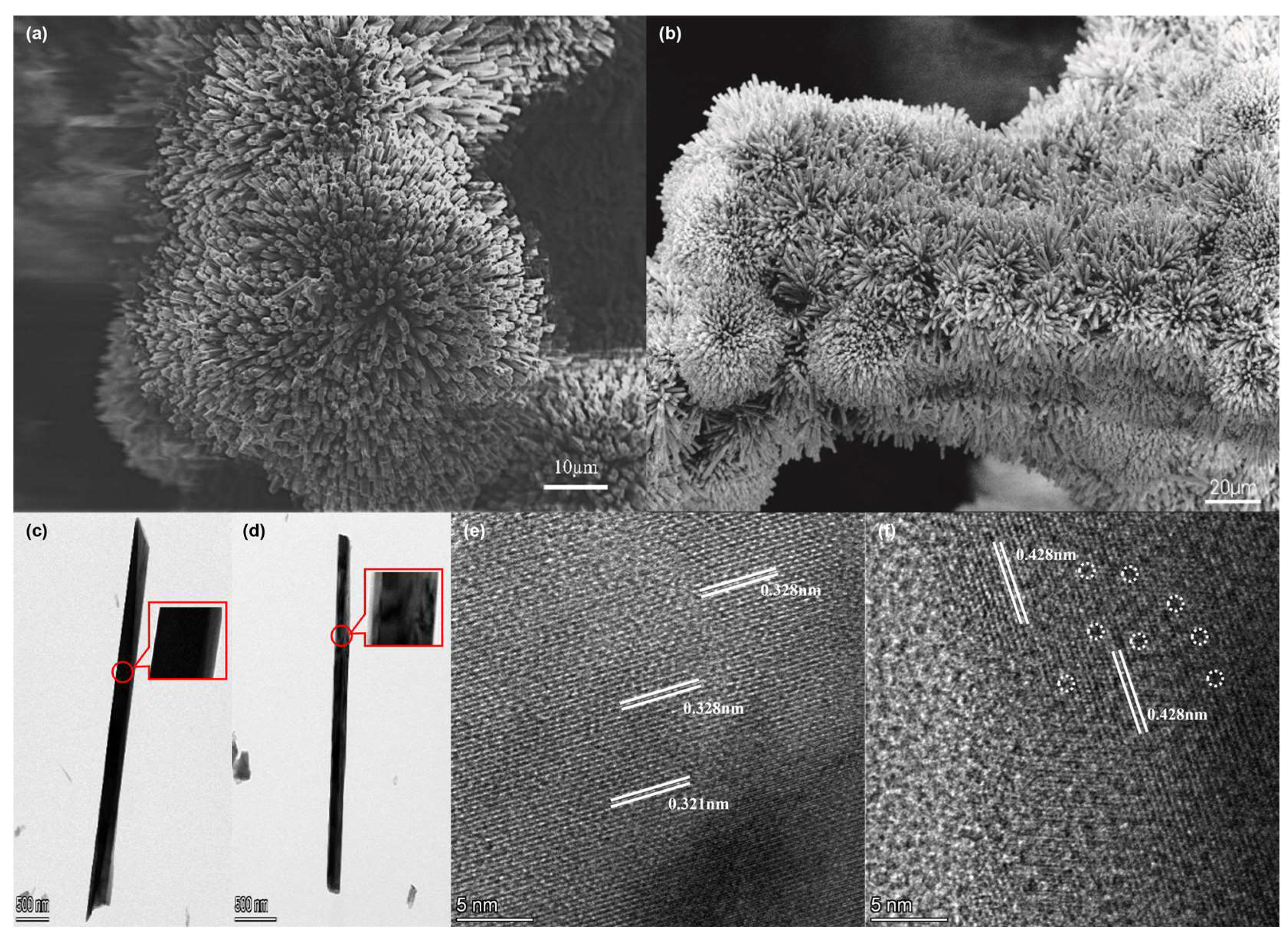
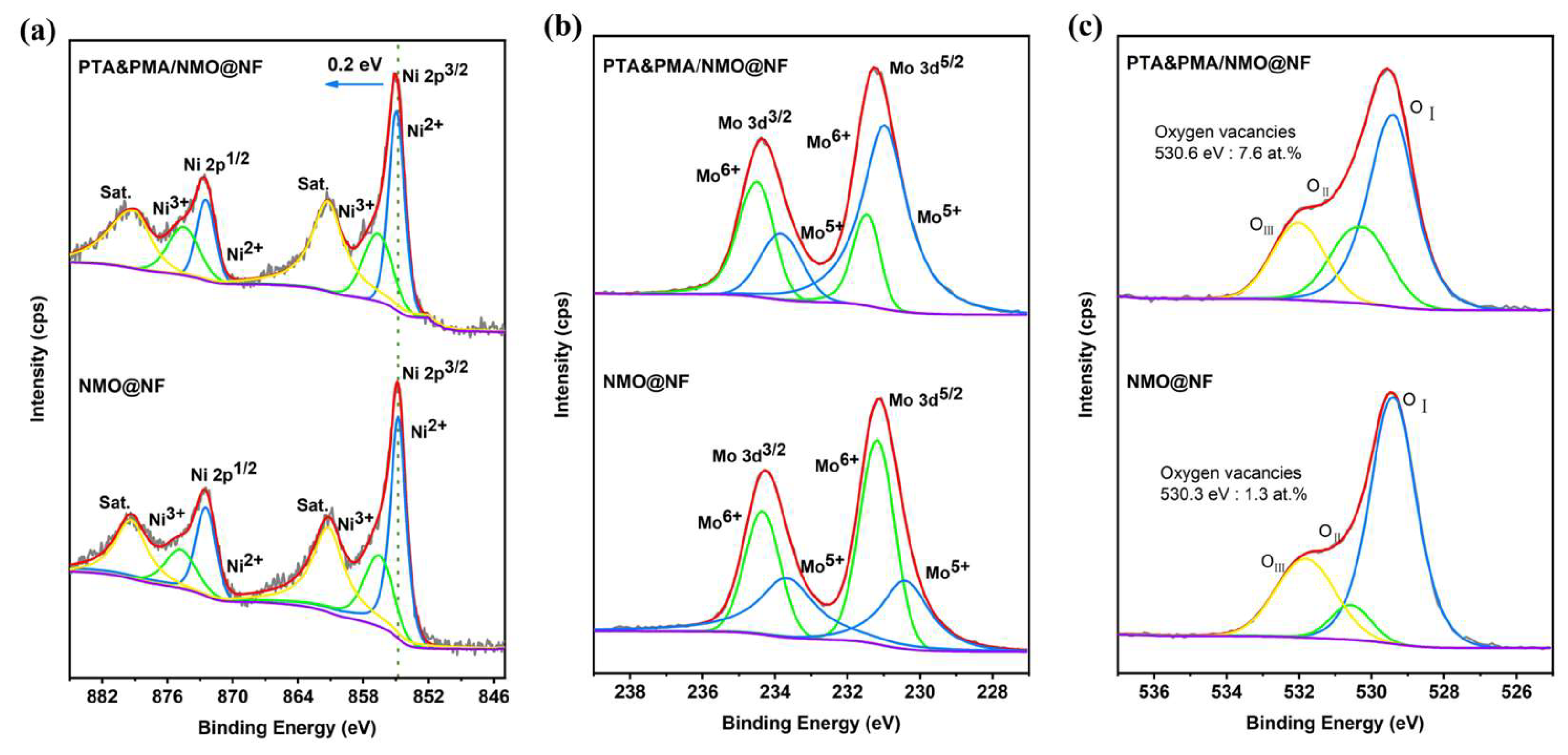
2.1. Material Synthesis and Characterization
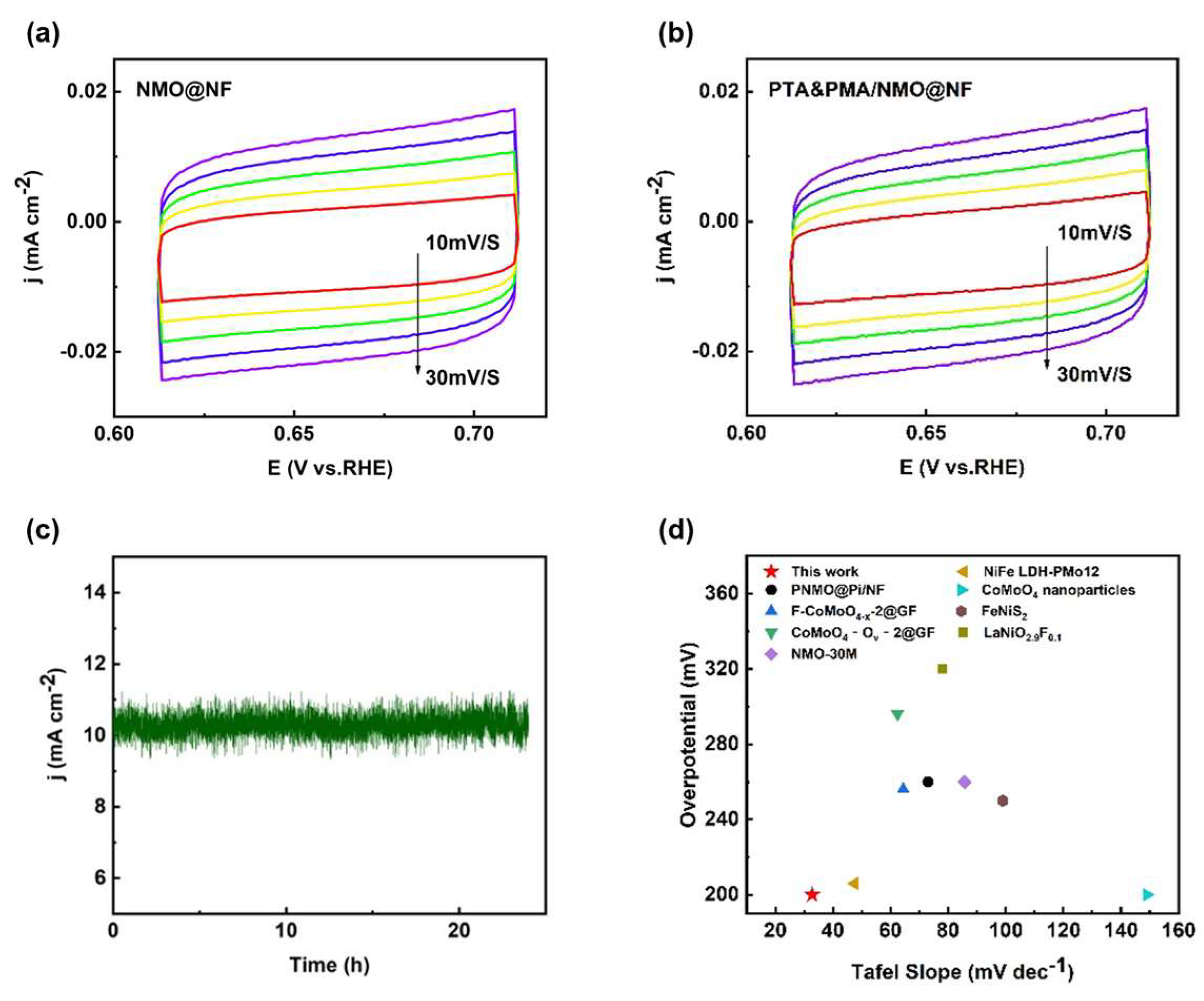
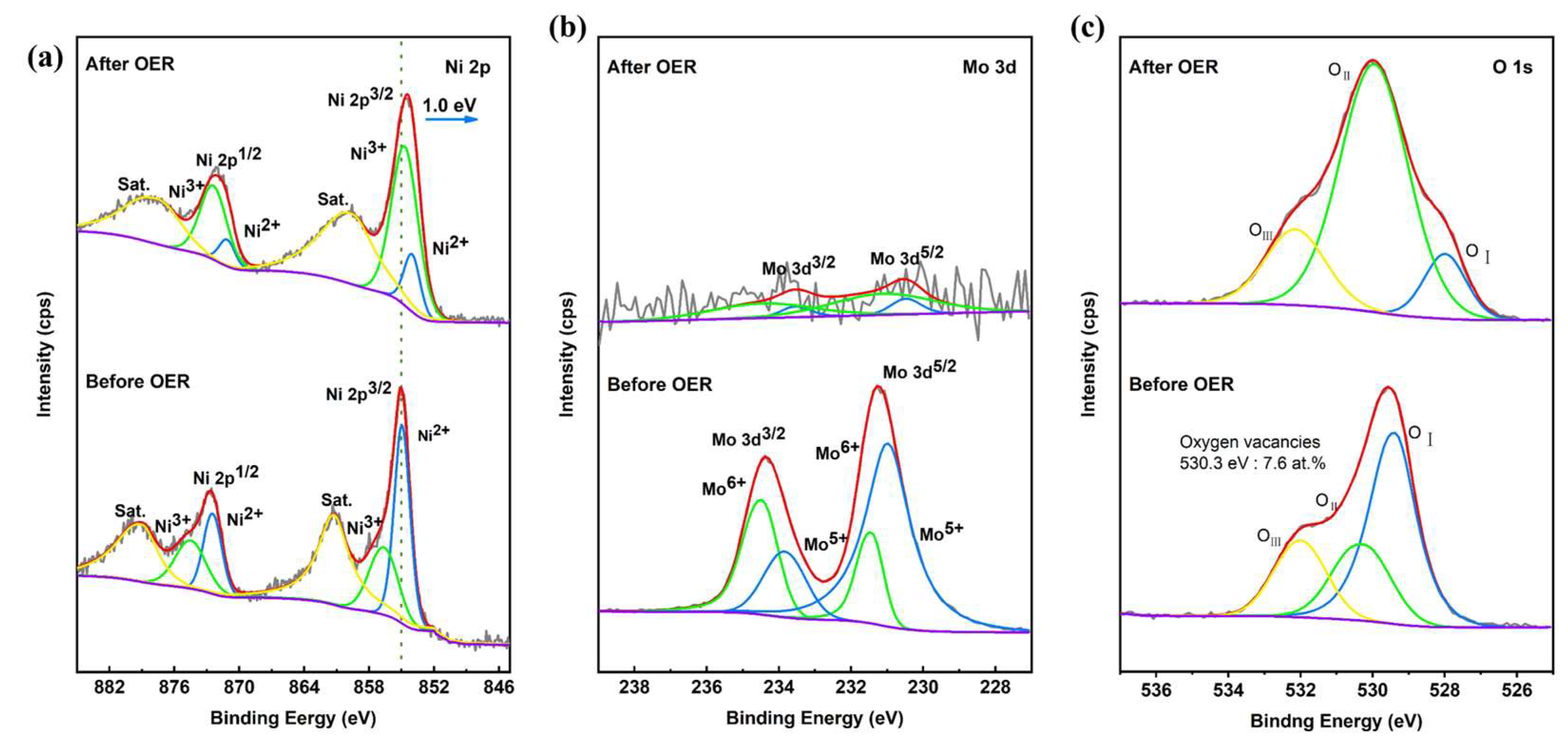
2.2. Electrochemical OER Performance
3. Materials and Methods
3.1. Material
3.2. Synthesis of NMO@NF
3.3. Synthesis of PMA/NMO@NF, PTA/NMO@NF, and PTA&PMA/NMO@NF
3.4. Material Characterization
3.5. Electrochemical Characterization
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Xie, W.; Huang, J.; Huang, L.; Geng, S.; Song, S.; Tsiakaras, P.; Wang, Y. Novel fluorine-doped cobalt molybdate nanosheets with enriched oxygen-vacancies for improved oxygen evolution reaction activity. Appl. Catal. B Environ. 2022, 303, 120871. [Google Scholar]
- Tian, L.; Wang, Q.; Li, Y.; Ren, X.; Wei, Q.; Wu, D. A hierarchical CoMoO4@CoFe-LDH heterostructure as a highly effective catalyst to boost electrocatalytic water oxidation. Dalton Trans. 2022, 51, 10552–10557. [Google Scholar] [PubMed]
- Stoerzinger, K.A.; Diaz-Morales, O.; Kolb, M.; Rao, R.R.; Frydendal, R.; Qiao, L.; Wang, X.R.; Halck, N.B.; Rossmeisl, J.; Hansen, H.A.; et al. Orientation-dependent oxygen evolution on RuO2 without lattice exchange. ACS Energy Lett. 2017, 2, 876–881. [Google Scholar] [CrossRef]
- Zhang, Y.; Fu, J.; Zhao, H.; Jiang, R.; Tian, F.; Zhang, R. Tremella-like Ni3S2/MnS with ultrathin nanosheets and abundant oxygen vacancies directly used for high speed overall water splitting. Appl. Catal. B Environ. 2019, 257, 117899. [Google Scholar]
- Liang, Y.; Wang, H.; Zhou, J.; Li, Y.; Wang, J.; Regier, T.; Dai, H. Covalent hybrid of spinel manganese–cobalt oxide and graphene as advanced oxygen reduction electrocatalysts. J. Am. Chem. Soc. 2012, 134, 3517–3523. [Google Scholar]
- Ma, T.Y.; Dai, S.; Jaroniec, M.; Qiao, S.Z. Metal–organic framework derived hybrid Co3O4-carbon porous nanowire arrays as reversible oxygen evolution electrodes. J. Am. Chem. Soc. 2014, 136, 13925–13931. [Google Scholar]
- An, L.; Feng, J.; Zhang, Y.; Wang, R.; Liu, H.; Wang, G.C.; Cheng, F.; Xi, P. Epitaxial heterogeneous interfaces on N-NiMoO4/NiS2 nanowires/nanosheets to boost hydrogen and oxygen production for overall water splitting. Adv. Funct. Mater. 2019, 29, 1805298. [Google Scholar]
- Liu, Z.; Yuan, C.; Teng, F. Crystal facets-predominated oxygen evolution reaction activity of earth abundant CoMoO4 electrocatalyst. J. Alloys Compd. 2019, 781, 460–466. [Google Scholar]
- Xu, J.; Xiao, T.; Tan, X.; Xiang, P.; Jiang, L.; Wu, D.; Li, J.; Wang, S. A new asymmetric aqueous supercapacitor: Co3O4//Co3O4@polypyrrole. J. Alloys Compd. 2017, 706, 351–357. [Google Scholar]
- Wang, Z.; Zheng, K.; Liu, S.; Dai, Z.; Xu, Y.; Li, X.; Wang, H.; Wang, L. Electrocatalytic Nitrogen Reduction to Ammonia by Fe2O3 Nanorod Array on Carbon Cloth. ACS Sustain. Chem. Eng. 2019, 7, 11754–11759. [Google Scholar]
- Salvador, G.M.; Silva, A.L.; Silva, L.P.; Passos, F.B.; Carvalho, N.M. Enhanced activity of Pd/α-MnO2 for electrocatalytic oxygen evolution reaction. Int. J. Hydrogen Energy 2021, 46, 26976–26988. [Google Scholar] [CrossRef]
- Zhang, X.; Su, H.; Du, X. A Nickel molybdenum oxide nanoarray as an efficient and stable electrocatalyst for overall water splitting. New J. Chem. 2020, 44, 8176–8182. [Google Scholar]
- Ratha, S.; Samantara, A.K.; Singha, K.K.; Gangan, A.S.; Chakraborty, B.; Jena, B.K.; Rout, C.S. Urea-assisted room temperature stabilized metastable β-NiMoO4: Experimental and theoretical insights into its unique bifunctional activity toward oxygen evolution and supercapacitor. ACS Appl. Mater. Interfaces 2017, 9, 9640–9653. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Xiang, J.; Zhang, W.; Chen, C.; Xu, H.; Huang, Y. 3D interconnected porous NiMoO4 nanoplate arrays on Ni foam as high-performance binder-free electrode for supercapacitors. J. Mater. Chem. A 2015, 3, 22081–22087. [Google Scholar]
- Wang, Z.; Wei, G.; Du, K.; Zhao, X.; Liu, M.; Wang, S.; Zhou, Y.; An, C.; Zhang, J. Ni foam-supported carbon-sheathed NiMoO4 nanowires as integrated electrode for high-performance hybrid supercapacitors. ACS Sustain. Chem. Eng. 2017, 5, 5964–5971. [Google Scholar] [CrossRef]
- Xu, Y.; Xuan, H.; Gao, J.; Liang, T.; Han, X.; Yang, J.; Zhang, Y.; Li, H.; Han, P.; Du, Y. Hierarchical three-dimensional NiMoO4-anchored rGO/Ni foam as advanced electrode material with improved supercapacitor performance. J. Mater. Sci. 2018, 53, 8483–8498. [Google Scholar]
- Murugan, E.; Govindaraju, S.; Santhoshkumar, S. Hydrothermal synthesis, characterization and electrochemical behavior of NiMoO4 nanoflower and NiMoO4/rGO nanocomposite for high-performance supercapacitors. Electrochim. Acta 2021, 392, 138973. [Google Scholar] [CrossRef]
- Yao, P.; Li, C.; Yu, J.; Zhang, S.; Zhang, M.; Liu, H.; Ji, M.; Cong, G.; Zhang, T.; Zhu, C.; et al. High performance flexible energy storage device based on copper foam supported NiMoO4 nanosheets-CNTs-CuO nanowires composites with core–shell holey nanostructure. J. Mater. Sci. Technol. 2021, 85, 87–94. [Google Scholar]
- Zhu, D.; Sun, X.; Yu, J.; Liu, Q.; Liu, J.; Chen, R.; Zhang, H.; Song, D.; Li, R.; Wang, J. Three-dimensional heterostructured polypyrrole/nickel molybdate anchored on carbon cloth for high-performance flexible supercapacitors. J. Colloid Interface Sci. 2020, 574, 355–363. [Google Scholar]
- Hao, Y.; Huang, H.; Wang, Q.; Wang, Q.; Zhou, G. Nitrogen-doped carbon/NiMoO4 nanospheres assembled by nanosheets and ultrasmall nanoparticles for supercapacitors. Chem. Phys. Lett. 2019, 728, 215–223. [Google Scholar]
- Tong, B.; Wei, W.; Chen, X.; Wang, J.; Ye, W.; Cui, S.; Chen, W.; Mi, L. Designed synthesis of porous NiMoO4/C composite nanorods for asymmetric supercapacitors. CrystEngComm 2019, 21, 5492–5499. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, S.; Ma, M.; Mu, X.; Zhang, Y.; Du, J.; Hu, Q.; Huang, B.; Hua, X.; Liu, G.; et al. Manganese-doped nickel molybdate nanostructures for high-performance asymmetric supercapacitors. Chem. Eng. J. 2019, 372, 452–461. [Google Scholar] [CrossRef]
- Yuan, J.; Yao, D.; Jiang, L.; Tao, Y.; Che, J.; He, G.; Chen, H. Mn-doped NiMoO4 mesoporous nanorods/reduced graphene oxide composite for high-performance all-solid-state supercapacitor. ACS Appl. Energy Mater. 2020, 3, 1794–1803. [Google Scholar] [CrossRef]
- Wang, F.; Ma, K.; Tian, W.; Dong, J.; Han, H.; Wang, H.; Deng, K.; Yue, H.; Zhang, Y.X.; Jiang, W.; et al. P-Doped NiMoO4 parallel arrays anchored on cobalt carbonate hydroxide with oxygen vacancies and mass transfer channels for supercapacitors and oxygen evolution. J. Mater. Chem. A 2019, 7, 19589–19596. [Google Scholar] [CrossRef]
- Sharma, P.; Minakshi Sundaram, M.; Watcharatharapong, T.; Laird, D.; Euchner, H.; Ahuja, R. Zn metal atom doping on the surface plane of one-dimesional NiMoO4 nanorods with improved redox chemistry. ACS Appl. Mater. Interfaces 2020, 12, 44815–44829. [Google Scholar] [CrossRef]
- Cui, S.; Wang, F.; Sun, K.; Wang, X.; Hu, Q.; Peng, H.; Ma, G.; Lei, Z. High-performance hybrid supercapacitors based on Ce-doped NiMoO4 nanosheets and Fe3O4@Bi2O3 nanoarrays. J. Phys. Chem. C 2021, 125, 18129–18140. [Google Scholar] [CrossRef]
- Li, P.; Ruan, C.; Xu, J.; Xie, Y. Supercapacitive performance of CoMoO4 with oxygen vacancy porous nanosheet. Electrochim. Acta 2020, 330, 135334. [Google Scholar] [CrossRef]
- Zhang, X.; Wei, L.; Guo, X. Ultrathin mesoporous NiMoO4-modified MoO3 core/shell nanostructures: Enhanced capacitive storage and cycling performance for supercapacitors. Chem. Eng. J. 2018, 353, 615–625. [Google Scholar] [CrossRef]
- Shen, J.; Wang, Q.; Zhang, K.; Wang, S.; Li, L.; Dong, S.; Zhao, S.; Chen, J.; Sun, R.; Wang, Y.; et al. Flexible carbon cloth based solid-state supercapacitor from hierarchical holothurian-morphological NiCo2O4@NiMoO4/PANI. Electrochim. Acta 2019, 320, 134578. [Google Scholar] [CrossRef]
- Xu, R.; Lin, J.; Wu, J.; Huang, M.; Fan, L.; Xu, Z.; Song, Z. A high-performance pseudocapacitive electrode material for supercapacitors based on the unique NiMoO4/NiO nanoflowers. Appl. Surf. Sci. 2019, 463, 721–731. [Google Scholar]
- Yu, D.; Zhang, Z.; Teng, Y.; Meng, Y.; Wu, Y.; Liu, X.; Hua, Y.; Zhao, X.; Liu, X. Fabrication of CuO@NiMoO4 core-shell nanowire arrays on copper foam and their application in high-performance all-solid-state asymmetric supercapacitors. J. Power Sources 2019, 440, 227164. [Google Scholar]
- Liu, Y.; Ma, Z.; Xin, N.; Ying, Y.; Shi, W. High-performance supercapacitor based on highly active P-doped one-dimension/two-dimension hierarchical NiCo2O4/NiMoO4 for efficient energy storage. J. Colloid Interface Sci. 2021, 601, 793–802. [Google Scholar]
- Zeng, Y.; Liao, J.; Wei, B.; Huang, Z.; Zhu, W.; Zheng, J.; Liang, H.; Zhang, Y.; Wang, Z. Tuning the electronic structure of NiMoO4 by coupling with SnO2 for high-performance hybrid supercapacitors. Chem. Eng. J. 2021, 409, 128297. [Google Scholar] [CrossRef]
- Chen, C.; Yan, D.; Luo, X.; Gao, W.; Huang, G.; Han, Z.; Zeng, Y.; Zhu, Z. Construction of core–shell NiMoO4@Ni-Co-S nanorods as advanced electrodes for high-performance asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2018, 10, 4662–4671. [Google Scholar] [CrossRef] [PubMed]
- Chen, F.; Ji, S.; Liu, Q.; Wang, H.; Liu, H.; Brett, D.J.; Wang, G.; Wang, R. Rational design of hierarchically core–shell structured Ni3S2@NiMoO4 nanowires for electrochemical energy storage. Small 2018, 14, 1800791. [Google Scholar] [CrossRef] [PubMed]
- Acharya, J.; Ojha, G.P.; Kim, B.-S.; Pant, B.; Park, M. Modish designation of hollow-tubular rGO–NiMoO4@Ni–Co–S hybrid core–shell electrodes with multichannel superconductive pathways for high-performance asymmetric supercapacitors. ACS Appl. Mater. Interfaces 2021, 13, 17487–17500. [Google Scholar]
- Kong, X.; Peng, H.; Bu, S.; Gao, Q.; Jiao, T.; Cheng, J.; Liu, B.; Hong, G.; Lee, C.; Zhang, W. Defect engineering of nanostructured electrocatalysts for enhancing nitrogen reduction. J. Mater. Chem. A 2020, 8, 7457–7473. [Google Scholar]
- Cai, Z.; Wang, P.; Zhang, J.; Chen, A.; Zhang, J.; Yan, Y.; Wang, X. Reinforced layered double hydroxide oxygen-evolution electrocatalysts: A polyoxometallic acid wet-etching approach and synergistic mechanism. Adv. Mater. 2022, 34, 2110696. [Google Scholar]
- Jiang, R.; Zhao, D.; Fan, H.; Xie, Y.; Li, M.; Lin, H.; Wu, Z.-S. Phosphorus doping and phosphates coating for nickel molybdate/nickel molybdate hydrate enabling efficient overall water splitting. J. Colloid Interface Sci. 2022, 606, 384–392. [Google Scholar]
- Laura, E.B.; Graciela, T.B.; Horacio, J.T. The state of the art on Wells-Dawson heteropoly-compounds A review of their properties and applications. Appl. Catal. A 2003, 256, 37–50. [Google Scholar]
- Ghosh, D.; Giri, S.; Das, C.K. Synthesis, characterization and electrochemical performance of graphene decorated with 1D NiMoO4·nH2O nanorods. Nanoscale 2013, 5, 10428–10437. [Google Scholar] [PubMed]
- Barraclough, C.; Lewis, J.; Nyholm, R. 713. The stretching frequencies of metal–oxygen double bonds. J. Chem. Soc. (Resumed) 1959, 11, 3552–3555. [Google Scholar] [CrossRef]
- Abdel-Dayem, H.M. Dynamic phenomena during reduction of α-NiMoO4 in different atmospheres: In-situ thermo-Raman spectroscopy study. Ind. Eng. Chem. Res. 2007, 46, 2466–2472. [Google Scholar] [CrossRef]
- Zhang, J.; Qian, J.; Ran, J.; Xi, P.; Yang, L.; Gao, D. Engineering lower coordination atoms onto NiO/Co3O4 heterointerfaces for boosting oxygen evolution reactions. ACS Catal. 2020, 10, 12376–12384. [Google Scholar] [CrossRef]
- Guan, X.; Yang, L.; Zhu, G.; Wen, H.; Zhang, J.; Sun, X.; Feng, H.; Tian, W.; Chen, X.; Yao, Y. A hierarchical CoMoO4 nanoparticle decorated nanoplate array as an electrocatalyst toward improved alkaline oxygen evolution reaction. Sustain. Energy Fuels 2020, 4, 1595–1599. [Google Scholar] [CrossRef]
- Shin, H.; Xiao, H.; Goddard, W.A., III. In silico discovery of new dopants for Fe-doped Ni oxyhydroxide (Ni1–xFexOOH) catalysts for oxygen evolution reaction. J. Am. Chem. Soc. 2018, 140, 6745–6748. [Google Scholar]
- Zhu, J.; Qian, J.; Peng, X.; Xia, B.; Gao, D. Etching-induced surface reconstruction of NiMoO4 for oxygen evolution reaction. Nano-Micro Lett. 2023, 15, 30. [Google Scholar]
- Wachs, I.E. Raman and IR studies of surface metal oxide species on oxide supports: Supported metal oxide catalysts. Catal. Today 1996, 27, 437–455. [Google Scholar]
- Zhuang, L.; Ge, L.; Yang, Y.; Li, M.; Jia, Y.; Yao, X.; Zhu, Z. Ultrathin iron-cobalt oxide nanosheets with abundant oxygen vacancies for the oxygen evolution reaction. Adv. Mater. 2017, 29, 1606793. [Google Scholar] [CrossRef]
- Bao, J.; Zhang, X.; Fan, B.; Zhang, J.; Zhou, M.; Yang, W.; Hu, X.; Wang, H.; Pan, B.; Xie, Y. Ultrathin spinel-structured nanosheets rich in oxygen deficiencies for enhanced electrocatalytic water oxidation. Angew. Chem. 2015, 127, 7507–7512. [Google Scholar]
- Tong, Y.; Chen, P.; Zhang, M.; Zhou, T.; Zhang, L.; Chu, W.; Wu, C.; Xie, Y. Oxygen vacancies confined in nickel molybdenum oxide porous nanosheets for promoted electrocatalytic urea oxidation. Acs Catal. 2018, 8, 1–7. [Google Scholar] [CrossRef]
- Yan, D.; Li, Y.; Huo, J.; Chen, R.; Dai, L.; Wang, S. Defect chemistry of nonprecious-metal electrocatalysts for oxygen reactions. Adv. Mater. 2017, 29, 1606459. [Google Scholar]
- Emiliana, F.; Maarten, N.; Tobias, B.; Xi, C.; Bae-Jung, K.; Julien, D.; Francesco, B.; Thomas, G.; Robin, S.; Luke, W.; et al. Dynamic surface self-reconstruction is the key of highly active perovskite nano-electrocatalysts for water splitting. Nat. Mater. 2017, 16, 925–931. [Google Scholar]
- Marcel, R.; Alexis, G.; Kevin, J.M.; Kelsey, A.S.; Tina, J.C.; Azzam, N.M.; Yang, S. Structural changes of cobalt-based perovskites upon water oxidation investigated by EXAFS. J. Phys. Chem. C 2013, 117, 8628–8635. [Google Scholar]
- Xi, C.; Bae-Jung, K.; Emiliana, F.; Thomas, J.S. Co/Fe oxyhydroxides supported on perovskite oxides as oxygen evolution reaction catalyst systems. ACS Appl. Mater. Interfaces 2019, 11, 34787–34795. [Google Scholar]
- Jiang, T.; Xie, W.; Geng, S.; Li, R.; Song, S.; Wang, Y. Constructing oxygen vacancy-regulated cobalt molybdate nanoflakes for efficient oxygen evolution reaction catalysis. Chin. J. Catal. 2022, 43, 2434–2442. [Google Scholar]
- Ray, S.K.; Bastakoti, B.P. Improved supercapacitor and oxygen evolution reaction performances of morphology-controlled cobalt molybdate. Int. J. Hydrogen Energy 2024, 51, 1109–1118. [Google Scholar] [CrossRef]
- Dalai, N.; Jena, B. Iron nickel sulfide nanorods for oxygen and hydrogen evolution reaction. ChemistrySelect 2023, 8, e202204370. [Google Scholar]
- Choi, S.; Kim, S.-J.; Han, S.; Wang, J.; Kim, J.; Koo, B.; Ryabin, A.A.; Kunze, S.; Hyun, H.; Han, J.; et al. Enhancing Oxygen Evolution Reaction via a Surface Reconstruction-Induced Lattice Oxygen Mechanism. ACS Catal. 2024, 14, 15096–15107. [Google Scholar]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, T.; Han, X.; Wang, Z.; Li, C.; Li, M.; Lan, X.; Ning, Y.; Wang, J.; Liu, P. Polyoxometalate Etching of NMO@NF for Highly Efficient Oxygen Evolution Reaction in Water Splitting. Int. J. Mol. Sci. 2025, 26, 3107. https://doi.org/10.3390/ijms26073107
Chen T, Han X, Wang Z, Li C, Li M, Lan X, Ning Y, Wang J, Liu P. Polyoxometalate Etching of NMO@NF for Highly Efficient Oxygen Evolution Reaction in Water Splitting. International Journal of Molecular Sciences. 2025; 26(7):3107. https://doi.org/10.3390/ijms26073107
Chicago/Turabian StyleChen, Ting, Xiang Han, Zefen Wang, Chaoying Li, Mei Li, Xiongdiao Lan, Yingying Ning, Jingxin Wang, and Pengru Liu. 2025. "Polyoxometalate Etching of NMO@NF for Highly Efficient Oxygen Evolution Reaction in Water Splitting" International Journal of Molecular Sciences 26, no. 7: 3107. https://doi.org/10.3390/ijms26073107
APA StyleChen, T., Han, X., Wang, Z., Li, C., Li, M., Lan, X., Ning, Y., Wang, J., & Liu, P. (2025). Polyoxometalate Etching of NMO@NF for Highly Efficient Oxygen Evolution Reaction in Water Splitting. International Journal of Molecular Sciences, 26(7), 3107. https://doi.org/10.3390/ijms26073107





