Effects of Deoxynivalenol Contamination on Growth Performance, Blood Biochemistry, Histology, Metabolomics, and the Microbiota: A Subacute Dose Oral Toxicity Study in Rats
Abstract
1. Introduction
2. Results
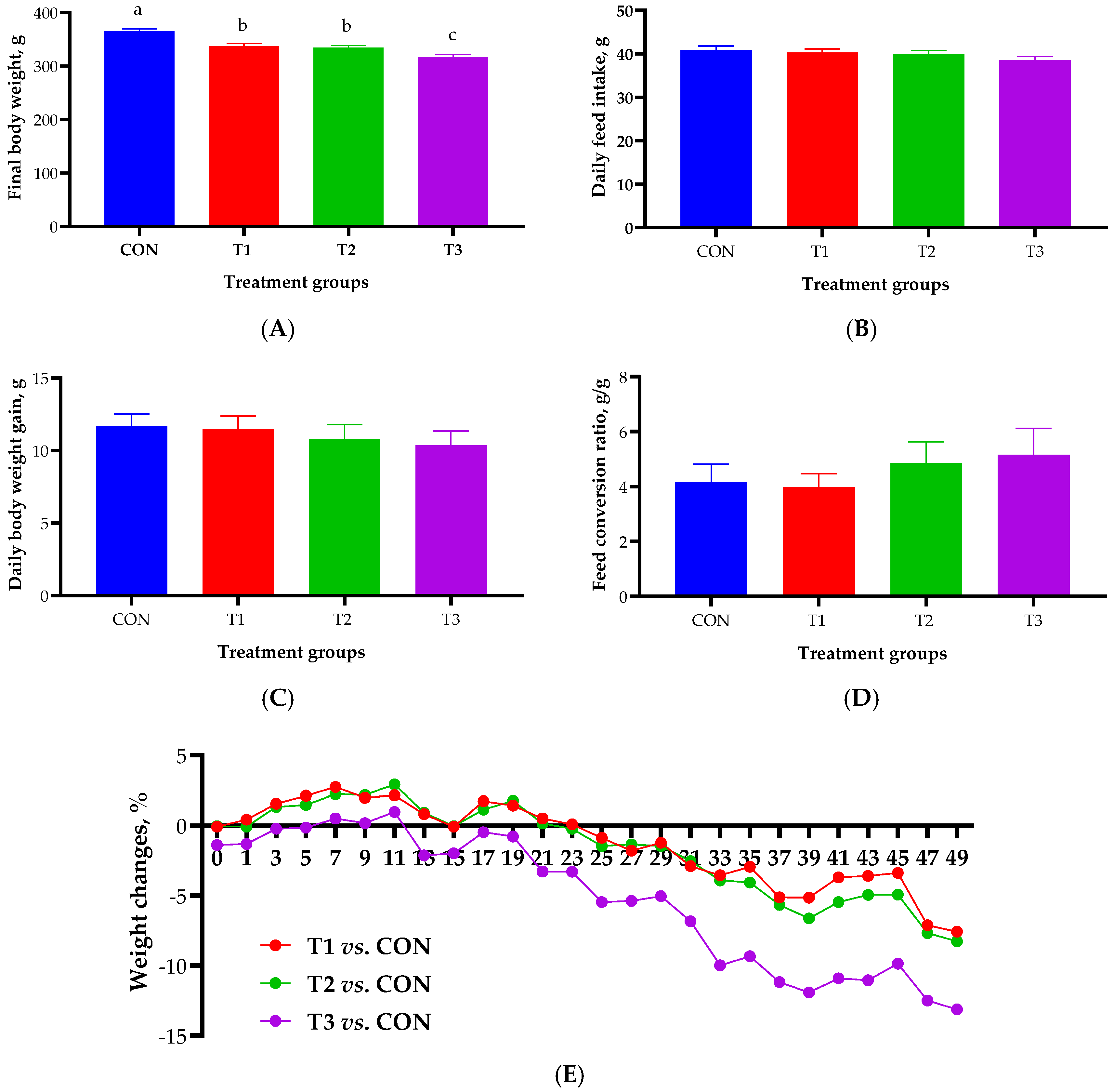
2.1. Growth Performance
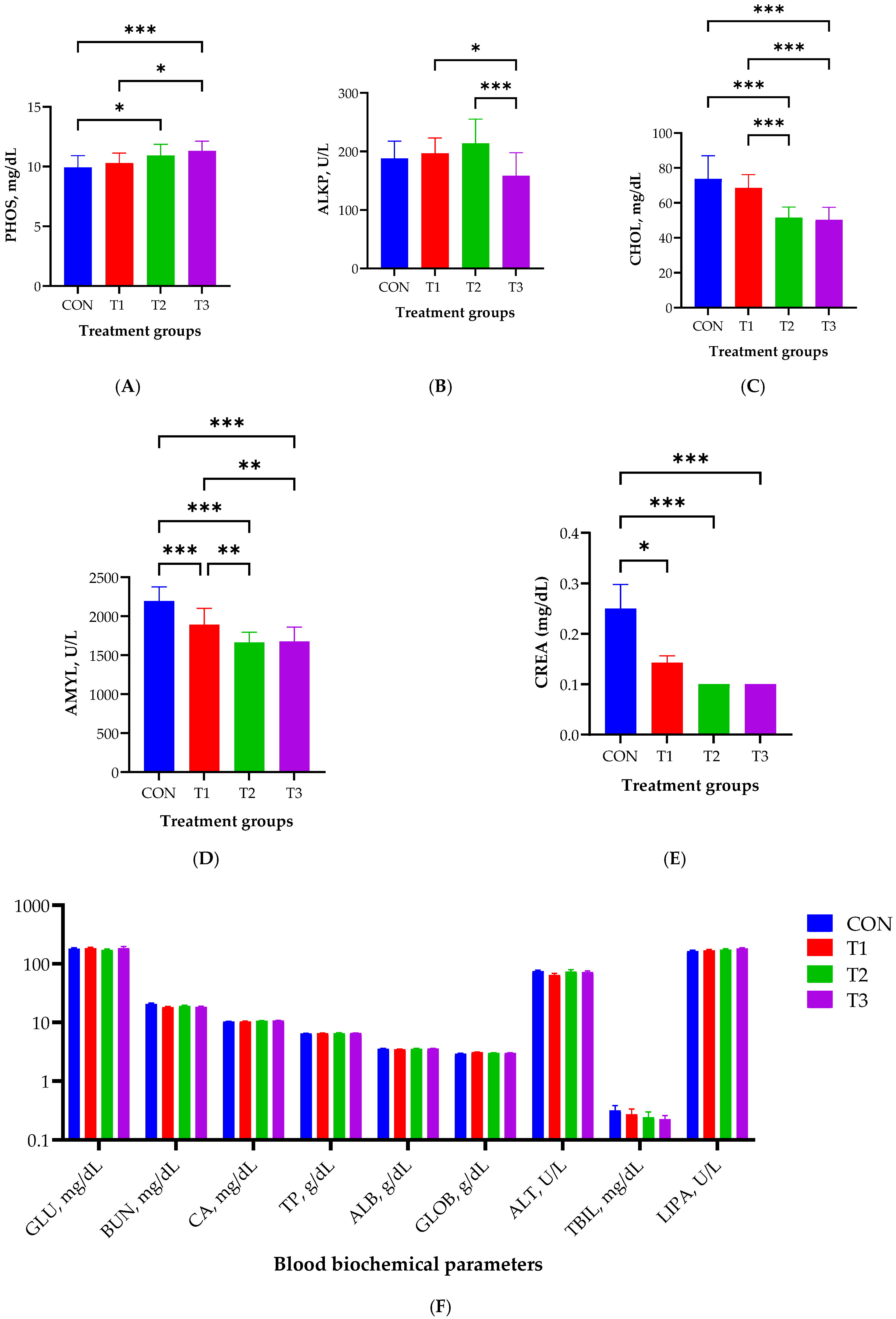
2.2. Blood Biochemistry
2.3. Histological Analysis in Different Tissues
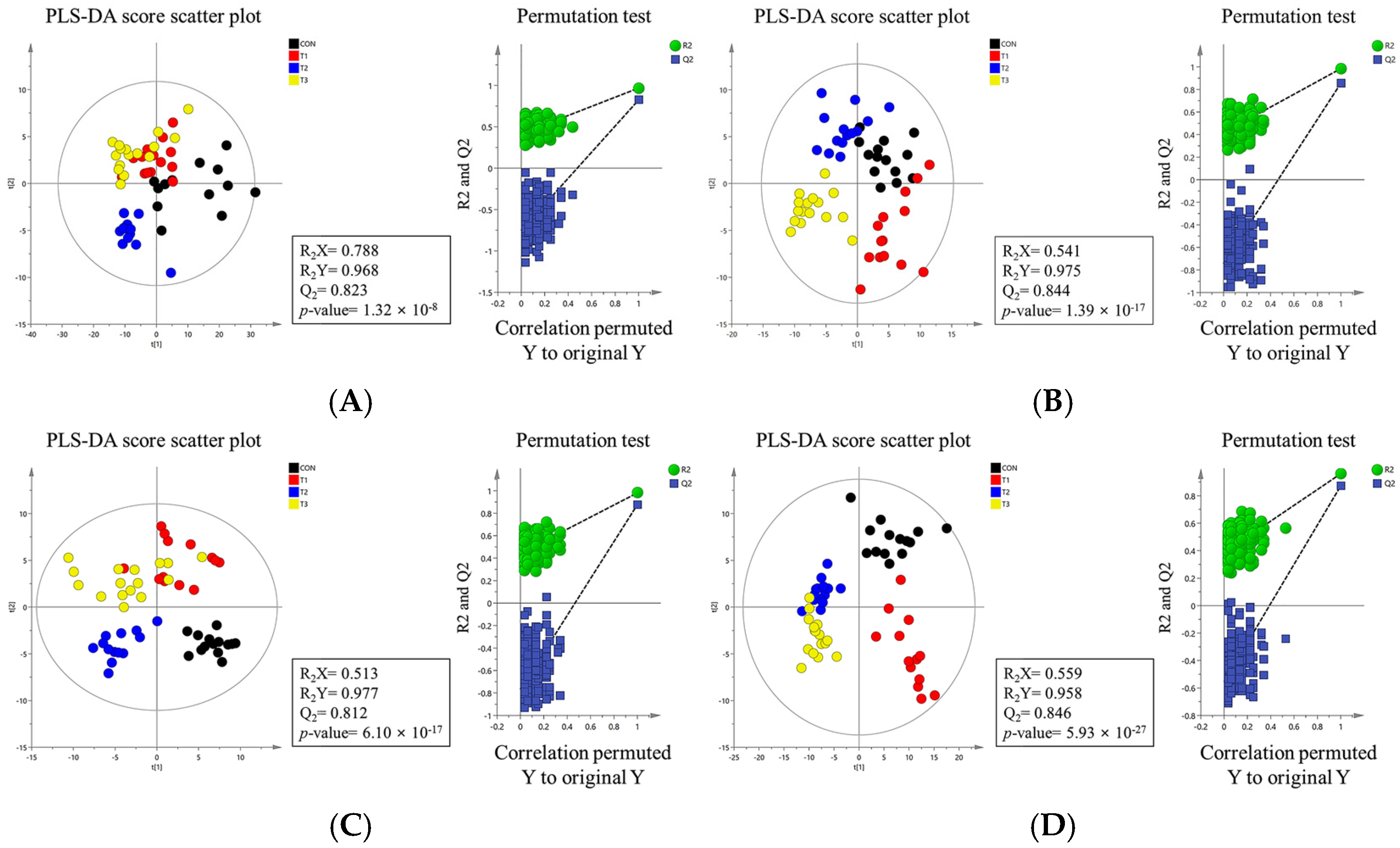
2.4. Alpha and Beta Diversity of the Gut Microbiome
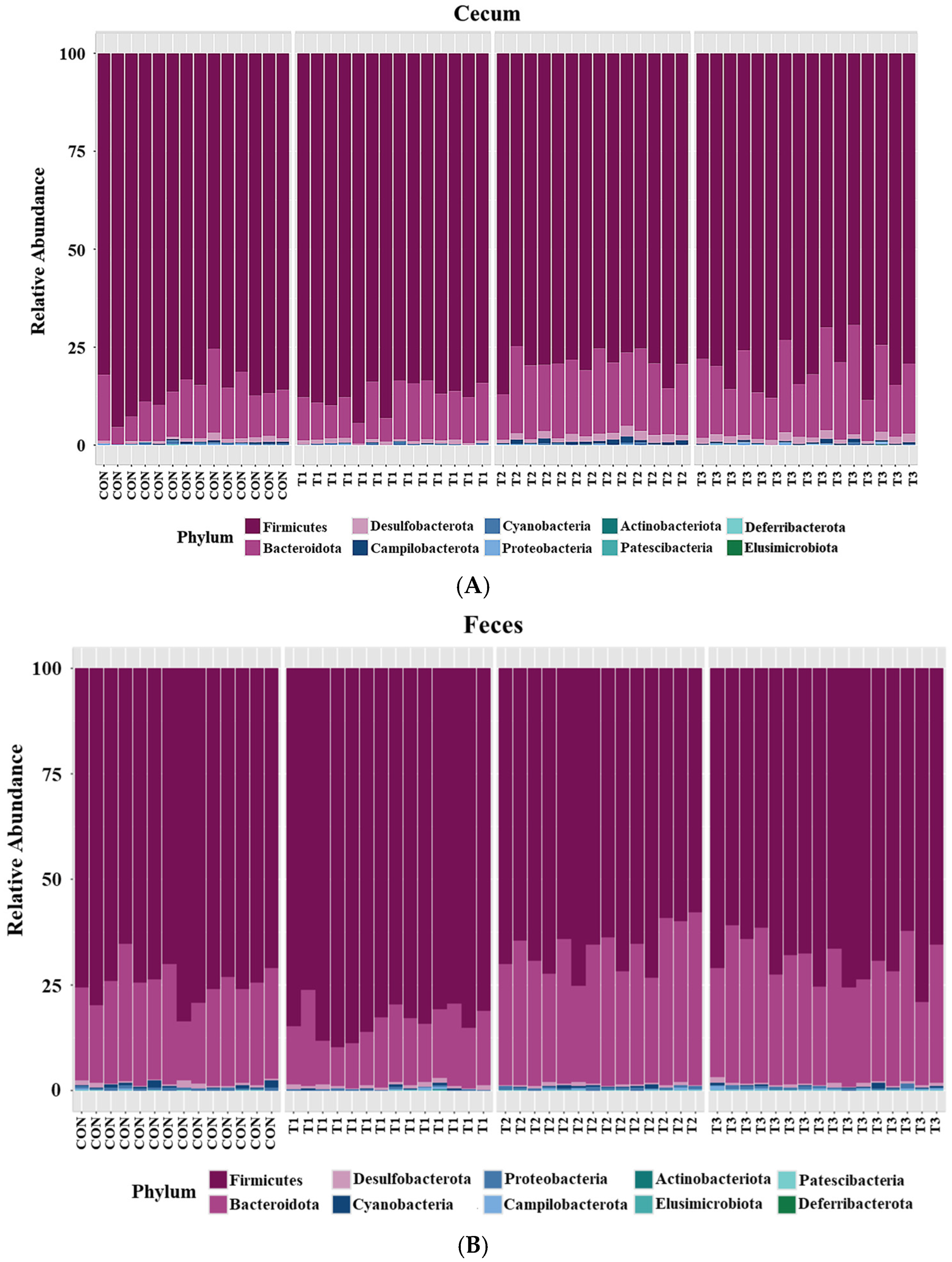
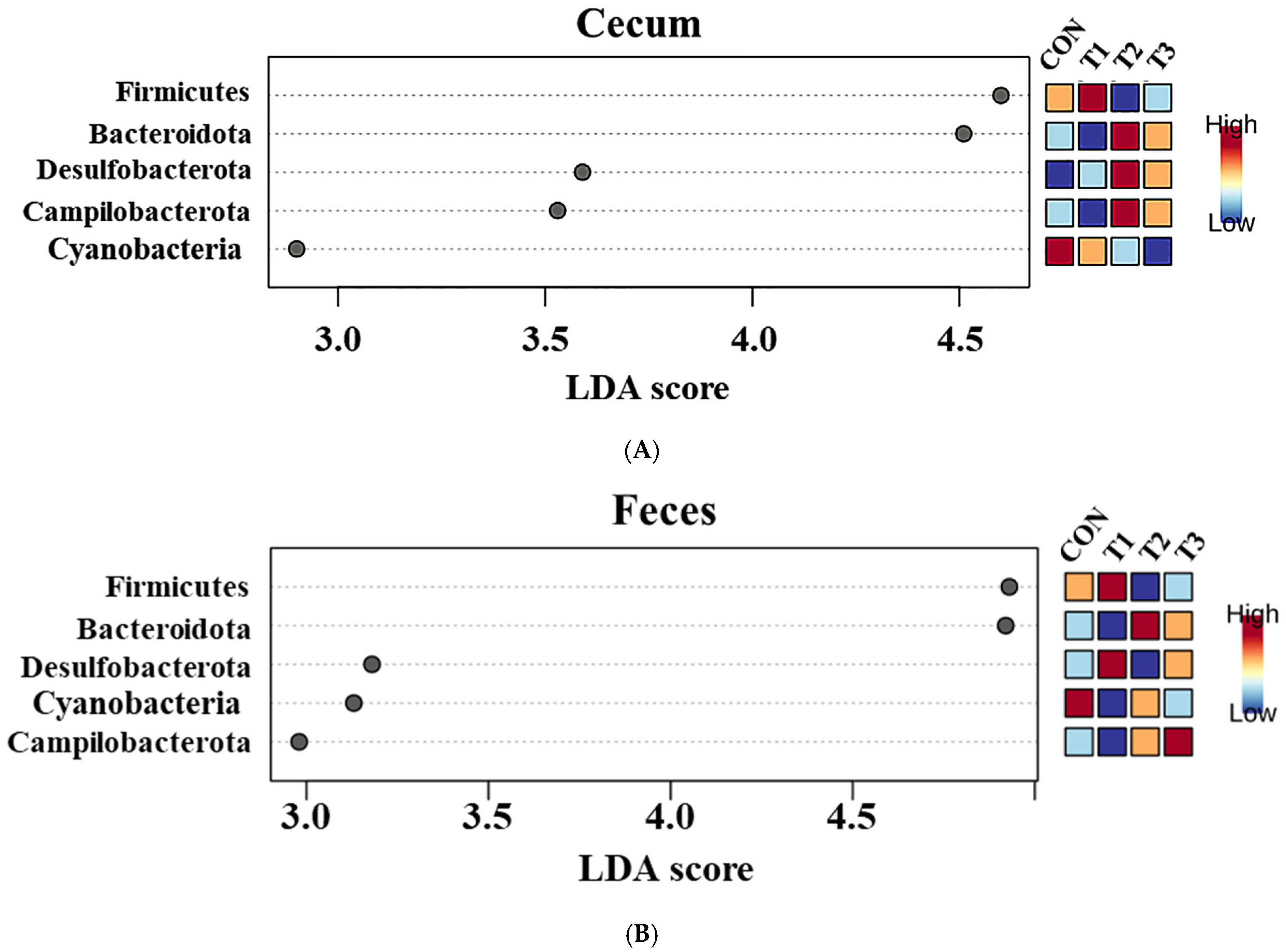
2.5. Cecal and Fecal Microbiota at the Phylum Level
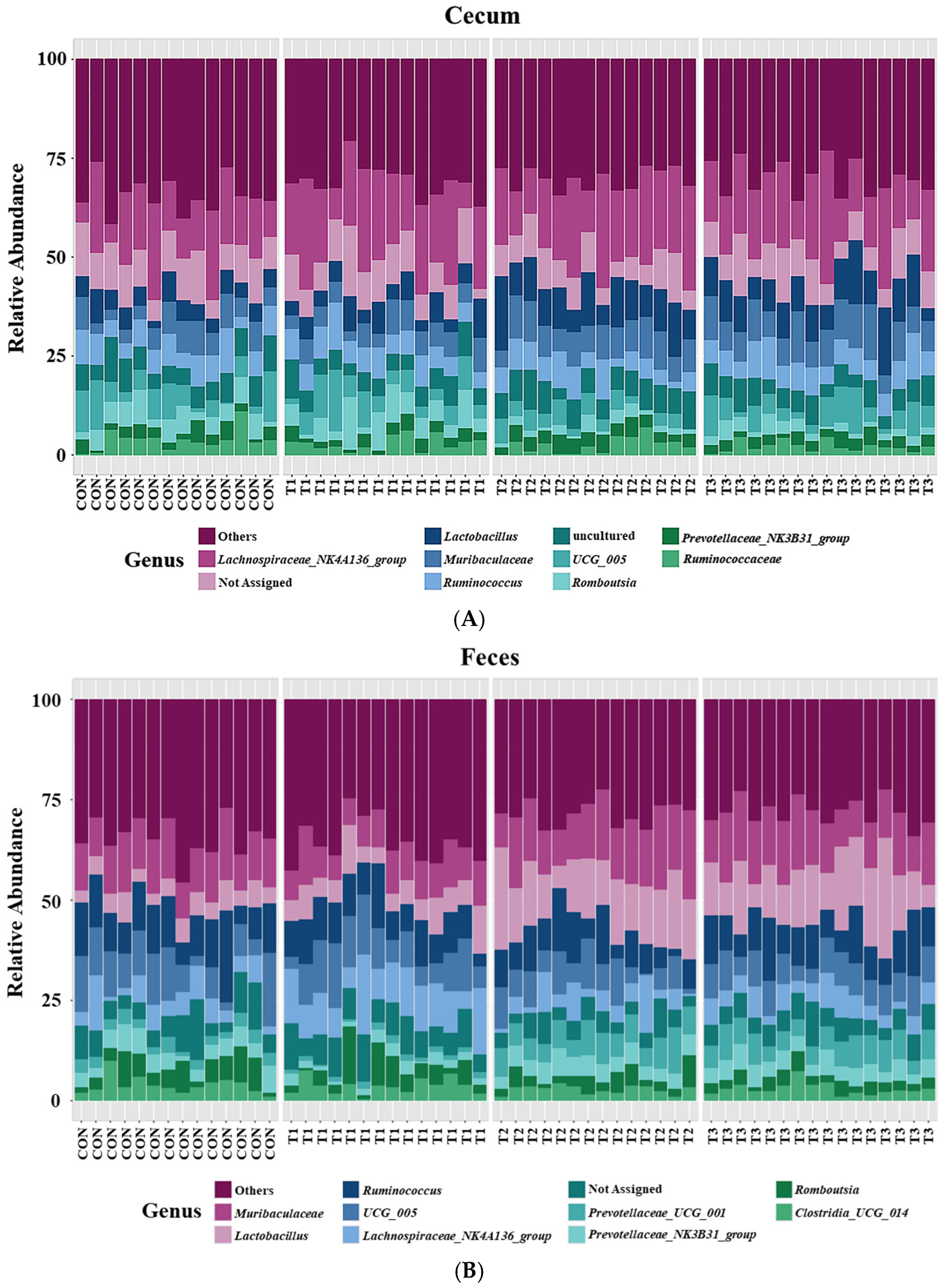
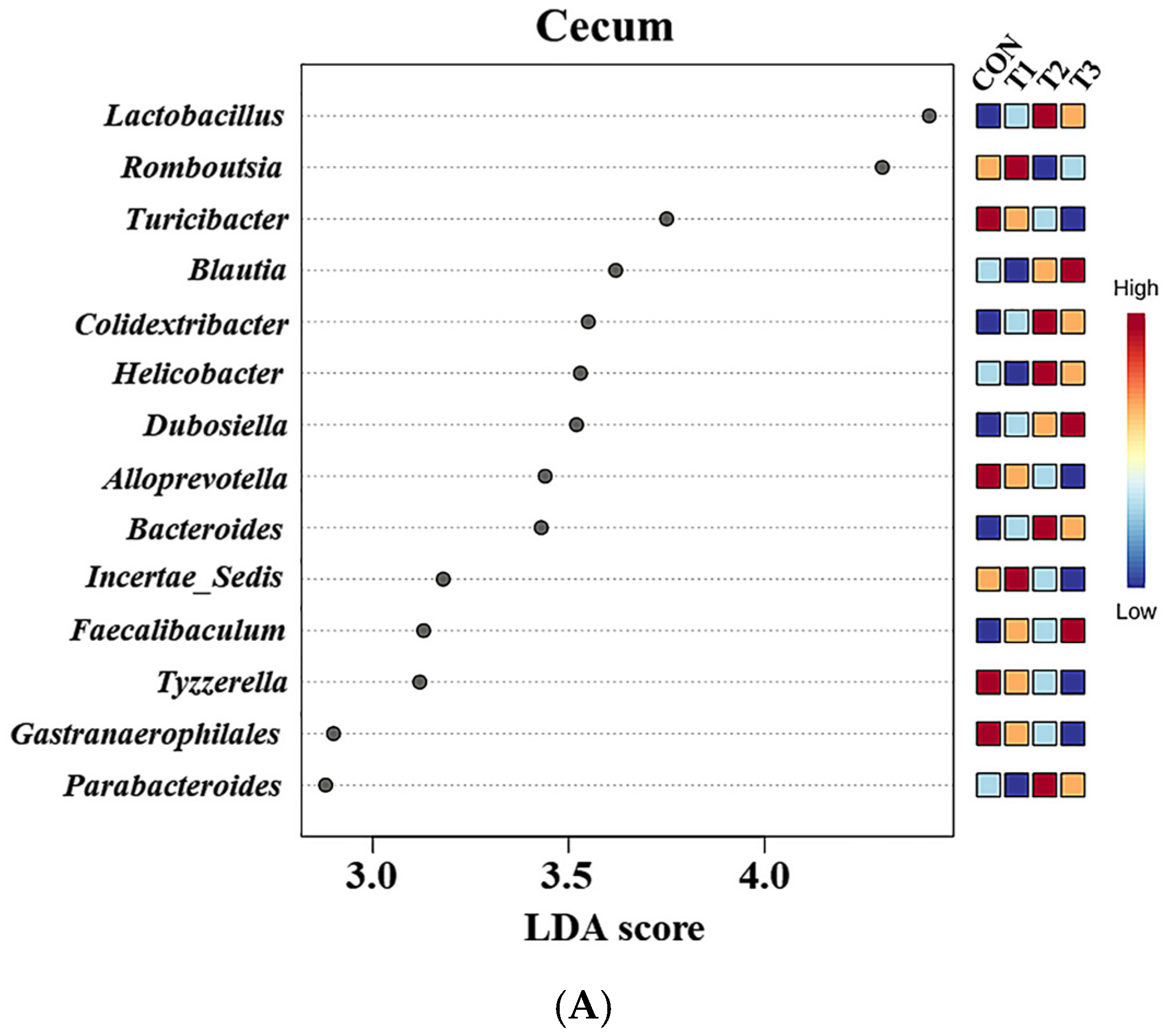
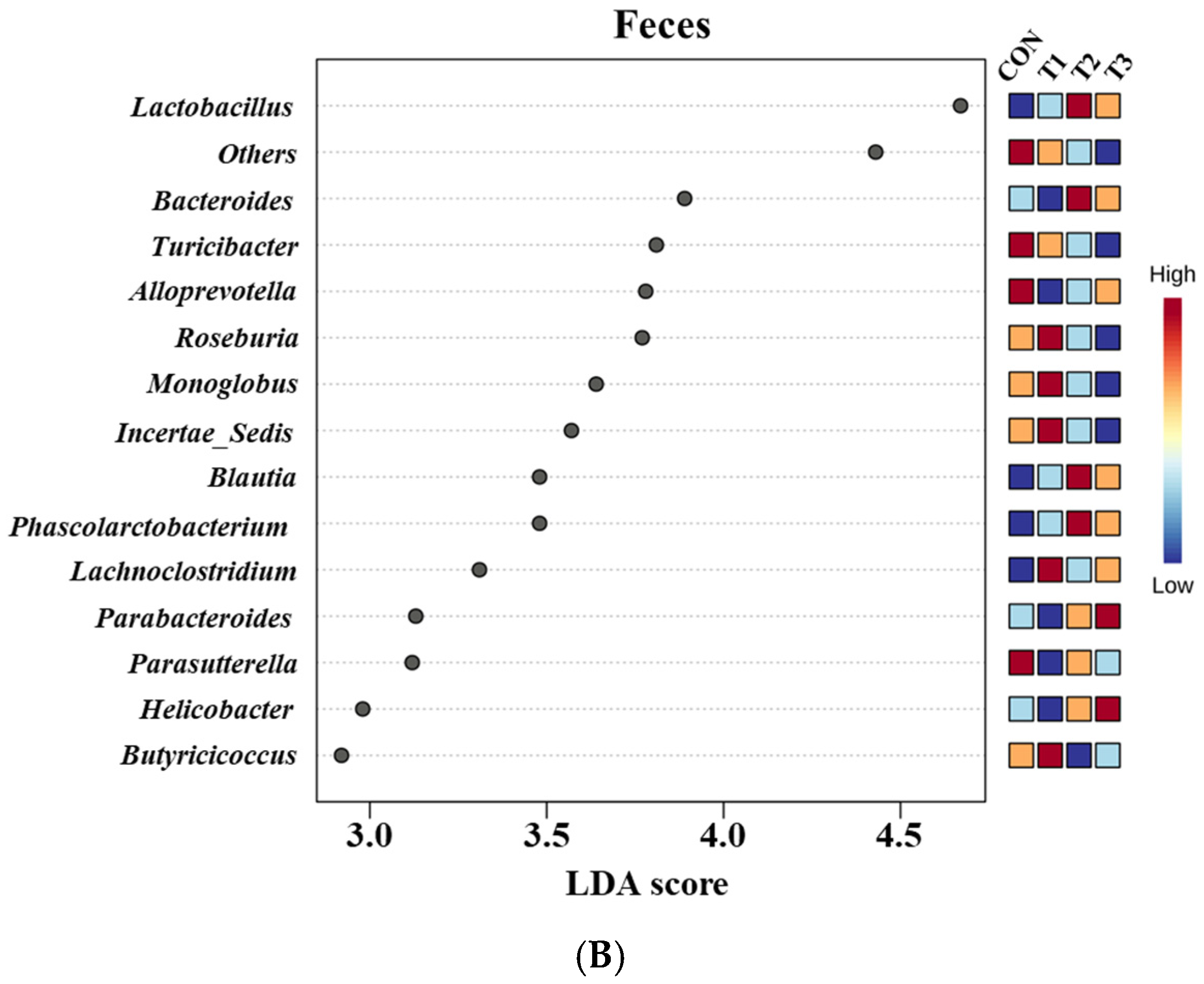
2.6. Cecal and Fecal Microbiota at the Genus Level
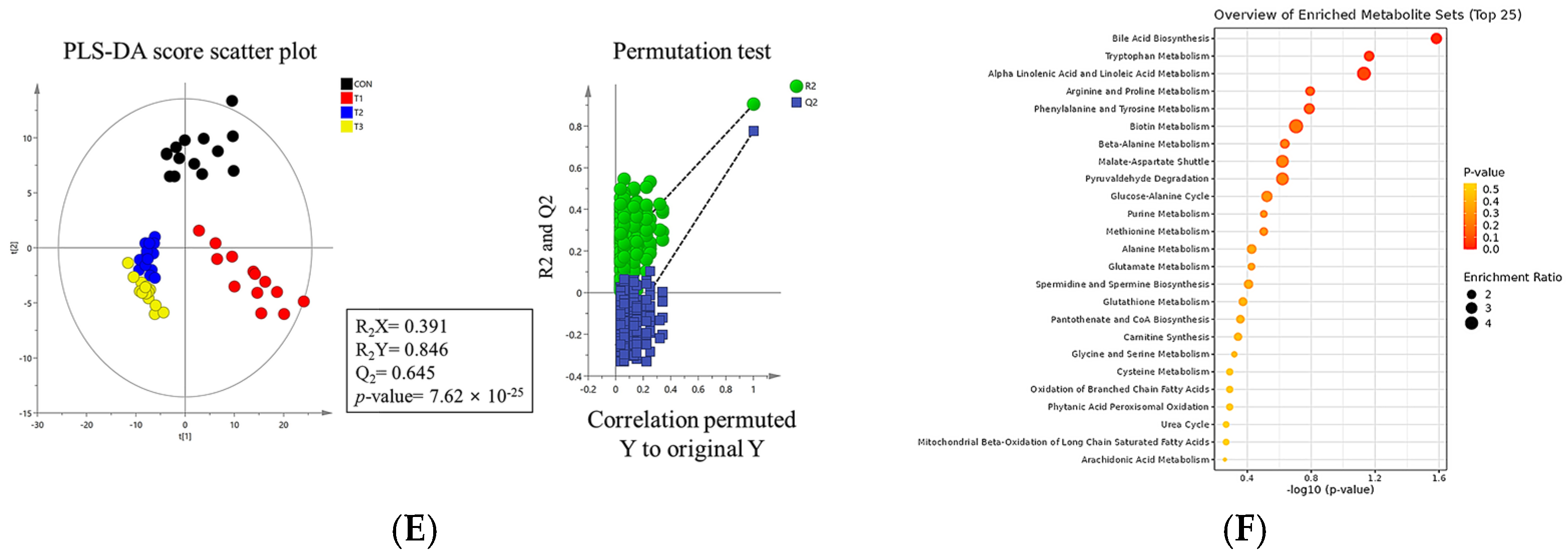
2.7. Metabolomic Profiling
2.8. Simple Linear Regression Analysis
3. Discussion
4. Materials and Methods
4.1. Ethics Statements
4.2. Animal and Study Design
4.3. Blood Biochemical Analysis
4.4. Histological Analysis
4.5. Cecal and Fecal Content Microbial Sequencing and Data Analysis
4.6. Metabolite Preparation and Analysis of Blood, Liver, Kidney, Cecum, and Feces
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Holanda, D.M.; Kim, S.W. Mycotoxin Occurrence, Toxicity, and Detoxifying Agents in Pig Production with an Emphasis on Deoxynivalenol. Toxins 2021, 13, 171. [Google Scholar] [CrossRef] [PubMed]
- Sun, L.H.; Lei, M.Y.; Zhang, N.Y.; Gao, X.; Li, C.; Krumm, C.S.; Qi, D.S. Individual and Combined Cytotoxic Effects of Aflatoxin B1, Zearalenone, Deoxynivalenol, and Fumonisin B1 on BRL 3A Rat Liver Cells. Toxicon 2015, 95, 6–12. [Google Scholar] [CrossRef] [PubMed]
- Dolenšek, T.; Švara, T.; Knific, T.; Gombač, M.; Luzar, B.; Jakovac-Strajn, B. The Influence of Fusarium Mycotoxins on the Liver of Gilts and Their Suckling Piglets. Animals 2021, 11, 2534. [Google Scholar] [CrossRef]
- Lattanzio, V.M.; Solfrizzo, M.; De Girolamo, A.; Chulze, S.N.; Torres, A.M.; Visconti, A. LC–MS/MS Characterization of the Urinary Excretion Profile of the Mycotoxin Deoxynivalenol in Human and Rat. J. Chromatogr. B 2011, 879, 707–715. [Google Scholar] [CrossRef]
- European Food Safety Authority (EFSA). Deoxynivalenol in Food and Feed: Occurrence and Exposure. EFSA J. 2013, 11, 3379. [Google Scholar]
- Gruber-Dorninger, C.; Jenkins, T.; Schatzmayr, G. Global Mycotoxin Occurrence in Feed: A Ten-Year Survey. Toxins 2019, 11, 375. [Google Scholar] [CrossRef]
- Mishra, S.; Srivastava, S.; Dewangan, J.; Divakar, A.; Kumar Rath, S. Global Occurrence of Deoxynivalenol in Food Commodities and Exposure Risk Assessment in Humans in the Last Decade: A Survey. Crit. Rev. Food Sci. Nutr. 2022, 60, 1346–1374. [Google Scholar] [CrossRef]
- Park, J.; Chang, H.; Kim, D.; Chung, S.; Lee, C. Long-Term Occurrence of Deoxynivalenol in Feed and Feed Raw Materials with a Special Focus on South Korea. Toxins 2018, 10, 127. [Google Scholar] [CrossRef]
- Zhao, L.; Zhang, L.; Xu, Z.; Liu, X.; Chen, L.; Dai, J.; Sun, L. Occurrence of Aflatoxin B1, Deoxynivalenol, and Zearalenone in Feeds in China during 2018–2020. J. Anim. Sci. Biotechnol. 2021, 12, 74. [Google Scholar] [CrossRef]
- Ren, Z.H.; Deng, H.D.; Deng, Y.T.; Deng, J.L.; Zuo, Z.C.; Yu, S.M.; Hu, Y.C. Effect of the Fusarium Toxins, Zearalenone and Deoxynivalenol, on the Mouse Brain. Environ. Toxicol. Pharmacol. 2016, 46, 62–70. [Google Scholar] [CrossRef]
- Kim, J.H.; Park, G.H.; Han, G.P.; Kil, D.Y. Effect of Feeding Corn Distillers Dried Grains with Solubles Naturally Contaminated with Deoxynivalenol on Growth Performance, Meat Quality, Intestinal Permeability, and Utilization of Energy and Nutrients in Broiler Chickens. Poult. Sci. 2021, 100, 101215. [Google Scholar] [CrossRef] [PubMed]
- Ghareeb, K.; Awad, W.A.; Böhm, J.; Zebeli, Q. Impacts of the Feed Contaminant Deoxynivalenol on the Intestine of Monogastric Animals: Poultry and Swine. J. Appl. Toxicol. 2015, 35, 327–337. [Google Scholar] [CrossRef] [PubMed]
- Miró-Abella, E.; Torrell, H.; Herrero, P.; Canela, N.; Arola, L.; Borrull, F.; Fontanals, N. Monitoring and Evaluation of the Interaction between Deoxynivalenol and Gut Microbiota in Wistar Rats by Mass Spectrometry-Based Metabolomics and Next-Generation Sequencing. Food Chem. Toxicol. 2018, 121, 124–130. [Google Scholar] [CrossRef] [PubMed]
- Gerez, J.R.; Verri, W.A.; Hohmann, M.S.; Flaiban, K.M.C.; Hasuda, A.L.; Gloria, E.M.; Bracarense, A.P.R.L. Animal Performance and Biochemical Parameters Are Sex-Dependent in Peripubertal Rats Exposed to Deoxynivalenol. Toxicon 2022, 220, 106944. [Google Scholar] [CrossRef]
- Saint-Cyr, M.J.; Perrin-Guyomard, A.; Manceau, J.; Houée, P.; Delmas, J.M.; Rolland, J.G.; Laurentie, M. Risk Assessment of Deoxynivalenol by Revisiting Its Bioavailability in Pig and Rat Models to Establish Which Is More Suitable. Toxins 2015, 7, 5167–5181. [Google Scholar] [CrossRef]
- Kang, T.H.; Kang, K.S.; Lee, S.I. Deoxynivalenol Induces Apoptosis via the FOXO3a-Signaling Pathway in Small-Intestinal Cells in Pigs. Toxics 2022, 10, 535. [Google Scholar] [CrossRef]
- Skiepko, N.; Przybylska-Gornowicz, B.; Gajęcka, M.; Gajęcki, M.; Lewczuk, B. Effects of Deoxynivalenol and Zearalenone on the Histology and Ultrastructure of Pig Liver. Toxins 2020, 12, 463. [Google Scholar] [CrossRef]
- Saenz, J.S.; Kurz, A.; Ruczizka, U.; Bünger, M.; Dippel, M.; Nagl, V.; Selberherr, E. Metaproteomics Reveals Alteration of the Gut Microbiome in Weaned Piglets due to the Ingestion of the Mycotoxins Deoxynivalenol and Zearalenone. Toxins 2021, 13, 583. [Google Scholar] [CrossRef]
- Wang, K.; Liao, M.; Zhou, N.; Bao, L.; Ma, K.; Zheng, Z.; Liu, H. Parabacteroides distasonis Alleviates Obesity and Metabolic Dysfunctions via Production of Succinate and Secondary Bile Acids. Cell Rep. 2019, 26, 222–235. [Google Scholar] [CrossRef]
- Dänicke, S.; Goyarts, T.; Döll, S.; Grove, N.; Spolders, M.; Flachowsky, G. Effects of the Fusarium Toxin Deoxynivalenol on Tissue Protein Synthesis in Pigs. Toxicol. Lett. 2006, 165, 297–311. [Google Scholar] [CrossRef]
- Pestka, J.J.; Smolinski, A.T. Deoxynivalenol: Toxicology and Potential Effects on Humans. J. Toxicol. Environ. Health B 2005, 8, 39–69. [Google Scholar] [CrossRef]
- Pestka, J.J. Deoxynivalenol: Toxicity, Mechanisms and Animal Health Risks. Anim. Feed Sci. Technol. 2007, 137, 283–298. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Kim, J.; Kim, M.; Park, S. Efficacy of High-Dose Synbiotic Additives for Deoxynivalenol Detoxification: Effects on Blood Biochemistry, Histology, and Intestinal Microbiome in Weaned Piglets. Biology 2024, 13, 889. [Google Scholar] [CrossRef] [PubMed]
- Wellington, M.O.; Bosompem, M.A.; Petracek, R.; Nagl, V.; Columbus, D.A. Effect of Long-Term Feeding of Graded Levels of Deoxynivalenol (DON) on Growth Performance, Nutrient Utilization, and Organ Health in Finishing Pigs and DON Content in Biological Samples. J. Anim. Sci. 2020, 98, skaa378. [Google Scholar] [CrossRef]
- Jeong, J.Y.; Kim, J.; Kim, M.; Shim, S.H.; Park, C.; Jung, S.; Jung, H. Effects of Increasing Oral Deoxynivalenol Gavage on Growth Performance, Blood Biochemistry, Metabolism, Histology, and Microbiome in Rats. Biology 2024, 13, 836. [Google Scholar] [CrossRef] [PubMed]
- Food and Drug Administration. Guidance for Industry and FDA: Advisory Levels for Deoxynivalenol (DON) in Finished Wheat Products for Human Consumption and Grains and Grain By-Products Used for Animal Feed. Available online: https://www.fda.gov/regulatory-information/search-fda-guidance-documents/guidance-industry-and-fda-advisory-levels-deoxynivalenol-don-finished-wheat-products-human (accessed on 17 September 2018).
- Holanda, D.M.; Kim, S.W. Efficacy of Mycotoxin Detoxifiers on Health and Growth of Newly-Weaned Pigs under Chronic Dietary Challenge of Deoxynivalenol. Toxins 2020, 12, 311. [Google Scholar] [CrossRef]
- Wu, L.; Liao, P.; He, L.; Ren, W.; Yin, J.; Duan, J.; Li, T. Growth Performance, Serum Biochemical Profile, Jejunal Morphology, and the Expression of Nutrient Transporter Genes in Deoxynivalenol (DON)-Challenged Growing Pigs. BMC Vet. Res. 2015, 11, 144. [Google Scholar] [CrossRef]
- Valgaeren, B.; Théron, L.; Croubels, S.; Devreese, M.; De Baere, S.; Van Pamel, E.; Antonissen, G. The Role of Roughage Provision on the Absorption and Disposition of the Mycotoxin Deoxynivalenol and Its Acetylated Derivatives in Calves: From Field Observations to Toxicokinetics. Arch. Toxicol. 2019, 93, 293–310. [Google Scholar] [CrossRef]
- Pestka, J.J.; Clark, E.S.; Schwartz-Zimmermann, H.E.; Berthiller, F. Sex Is a Determinant for Deoxynivalenol Metabolism and Elimination in the Mouse. Toxins 2017, 9, 240. [Google Scholar] [CrossRef]
- Pastorelli, H.; Van Milgen, J.; Lovatto, P.; Montagne, L. Meta-Analysis of Feed Intake and Growth Responses of Growing Pigs after a Sanitary Challenge. Animal 2012, 6, 952–961. [Google Scholar] [CrossRef]
- Pinton, P.; Nougayrède, J.P.; Del Rio, J.C.; Moreno, C.; Marin, D.E.; Ferrier, L.; Oswald, I.P. The Food Contaminant Deoxynivalenol Decreases Intestinal Barrier Permeability and Reduces Claudin Expression. Toxicol. Appl. Pharmacol. 2009, 237, 41–48. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Wang, W.; Yao, K.; Zhou, T.; Yin, J.; Li, T.; Yin, Y. Effects of Dietary Arginine and Glutamine on Alleviating the Impairment Induced by Deoxynivalenol Stress and Immune Relevant Cytokines in Growing Pigs. PLoS ONE 2013, 8, e69502. [Google Scholar] [CrossRef]
- Ji, X.; Tang, Z.; Zhang, F.; Zhou, F.; Wu, Y.; Wu, D. Dietary Taurine Supplementation Counteracts Deoxynivalenol-Induced Liver Injury via Alleviating Oxidative Stress, Mitochondrial Dysfunction, Apoptosis, and Inflammation in Piglets. Ecotoxicol. Environ. Saf. 2023, 253, 114705. [Google Scholar] [CrossRef] [PubMed]
- Hasuda, A.L.; Person, E.; Khoshal, A.K.; Bruel, S.; Puel, S.; Oswald, I.P.; Pinton, P. Deoxynivalenol Induces Apoptosis and Inflammation in the Liver: Analysis Using Precision-Cut Liver Slices. Food Chem. Toxicol. 2022, 163, 112930. [Google Scholar] [CrossRef]
- Chaytor, A.C.; See, M.T.; Hansen, J.A.; De Souza, A.L.P.; Middleton, T.F.; Kim, S.W. Effects of Chronic Exposure of Diets with Reduced Concentrations of Aflatoxin and Deoxynivalenol on Growth and Immune Status of Pigs. J. Anim. Sci. 2011, 89, 124–135. [Google Scholar] [CrossRef] [PubMed]
- Li, K.; Li, H.; Zhang, K.; Zhang, J.; Hu, P.; Li, Y.; Cai, D. Orphan Nuclear Receptor RORγ Modulates the Genome-Wide Binding of the Cholesterol Metabolic Genes during Mycotoxin-Induced Liver Injury. Nutrients 2021, 13, 2539. [Google Scholar] [CrossRef]
- Zong, Q.; Qu, H.; Zhao, Y.; Liu, H.; Wu, S.; Wang, S.; Cai, D. Sodium Butyrate Alleviates Deoxynivalenol-Induced Hepatic Cholesterol Metabolic Dysfunction via RORγ-Mediated Histone Acetylation Modification in Weaning Piglets. J. Anim. Sci. Biotechnol. 2022, 13, 133. [Google Scholar] [CrossRef]
- Wan, S.; Sun, N.; Li, H.; Khan, A.; Zheng, X.; Sun, Y.; Fan, R. Deoxynivalenol Damages the Intestinal Barrier and Biota of the Broiler Chickens. BMC Vet. Res. 2022, 18, 311. [Google Scholar] [CrossRef]
- Pierron, A.; Alassane-Kpembi, I.; Oswald, I.P. Impact of Two Mycotoxins, Deoxynivalenol and Fumonisin, on Pig Intestinal Health. Porcine Health Manag. 2016, 2, 21. [Google Scholar] [CrossRef]
- Liang, Z.; Ren, Z.; Gao, S.; Chen, Y.; Yang, Y.; Yang, D.; Shen, L. Individual and Combined Effects of Deoxynivalenol and Zearalenone on Mouse Kidney. Environ. Toxicol. Pharmacol. 2015, 40, 686–691. [Google Scholar] [CrossRef]
- Pasch, A.; Jahnen-Dechent, W.; Smith, E.R. Phosphate, Calcification in Blood, and Mineral Stress: The Physiologic Blood Mineral Buffering System and Its Association with Cardiovascular Risk. Int. J. Nephrol. 2018, 2018, 9182078. [Google Scholar] [CrossRef]
- Zhao, Q.; Zhang, S.; Feng, W.; Zhou, A.; Shi, L.; Zhang, J. Deoxynivalenol-Mediated Kidney Injury via Endoplasmic Reticulum Stress in Mice. Ecotoxicol. Environ. Saf. 2024, 286, 117243. [Google Scholar] [CrossRef] [PubMed]
- Lewczuk, B.; Przybylska-Gornowicz, B.; Gajęcka, M.; Targońska, K.; Ziółkowska, N.; Prusik, M.; Gajęcki, M. Histological Structure of Duodenum in Gilts Receiving Low Doses of Zearalenone and Deoxynivalenol in Feed. Exp. Toxicol. Pathol. 2016, 68, 157–166. [Google Scholar] [CrossRef]
- Kang, R.; Li, R.; Dai, P.; Li, Z.; Li, Y.; Li, C. Deoxynivalenol Induced Apoptosis and Inflammation of IPEC-J2 Cells by Promoting ROS Production. Environ. Pollut. 2019, 251, 689–698. [Google Scholar] [CrossRef]
- Xu, X.; Yan, G.; Chang, J.; Wang, P.; Yin, Q.; Liu, C.; Lu, F. Astilbin Ameliorates Deoxynivalenol-Induced Oxidative Stress and Apoptosis in Intestinal Porcine Epithelial Cells (IPEC-J2). J. Appl. Toxicol. 2020, 40, 1362–1372. [Google Scholar] [CrossRef]
- Panizo, S.; Martínez-Arias, L.; Alonso-Montes, C.; Cannata, P.; Martín-Carro, B.; Fernández-Martín, J.L.; Cannata-Andía, J.B. Fibrosis in Chronic Kidney Disease: Pathogenesis and Consequences. Int. J. Mol. Sci. 2021, 22, 408. [Google Scholar] [CrossRef] [PubMed]
- Lan, T.; Kisseleva, T.; Brenner, D.A. Deficiency of NOX1 or NOX4 Prevents Liver Inflammation and Fibrosis in Mice through Inhibition of Hepatic Stellate Cell Activation. PLoS ONE 2015, 10, e0129743. [Google Scholar] [CrossRef] [PubMed]
- Antar, S.A.; Ashour, N.A.; Marawan, M.E.; Al-Karmalawy, A.A. Fibrosis: Types, Effects, Markers, Mechanisms for Disease Progression, and Its Relation with Oxidative Stress, Immunity, and Inflammation. Int. J. Mol. Sci. 2023, 24, 4004. [Google Scholar] [CrossRef]
- Ranneh, Y.; Ali, F.; Akim, A.M.; Hamid, H.A.; Khazaai, H.; Fadel, A. Crosstalk between Reactive Oxygen Species and Pro-Inflammatory Markers in Developing Various Chronic Diseases: A Review. Appl. Biol. Chem. 2017, 60, 327–338. [Google Scholar] [CrossRef]
- Yao, Y.; Zhao, X.; Zheng, S.; Wang, S.; Liu, H.; Xu, S. Subacute Cadmium Exposure Promotes M1 Macrophage Polarization through Oxidative Stress-Evoked Inflammatory Response and Induces Porcine Adrenal Fibrosis. Toxicology 2021, 461, 152899. [Google Scholar] [CrossRef]
- Recharla, N.; Park, S.; Kim, M.; Kim, B.; Jeong, J.Y. Protective Effects of Biological Feed Additives on Gut Microbiota and the Health of Pigs Exposed to Deoxynivalenol: A Review. J. Anim. Sci. Technol. 2022, 64, 640. [Google Scholar] [CrossRef]
- Lkhagva, E.; Chung, H.J.; Ahn, J.S.; Hong, S.T. Host Factors Affect the Gut Microbiome More Significantly than Diet Shift. Microorganisms 2021, 9, 2520. [Google Scholar] [CrossRef] [PubMed]
- Zhang, F.; Chen, Z.; Jiang, L.; Chen, Z.; Sun, H. Response of Fecal Bacterial Flora to the Exposure of Fumonisin B1 in BALB/c Mice. Toxins 2021, 13, 612. [Google Scholar] [CrossRef]
- Bensch, H.M.; Tolf, C.; Waldenström, J.; Lundin, D.; Zöttl, M. Bacteroidetes to Firmicutes: Captivity Changes the Gut Microbiota Composition and Diversity in a Social Subterranean Rodent. Anim. Microbiome 2023, 5, 9. [Google Scholar] [CrossRef]
- Han, F.; Wang, Y.; Han, Y.; Zhao, J.; Han, F.; Song, G.; Miao, H. Effects of Whole-Grain Rice and Wheat on Composition of Gut Microbiota and Short-Chain Fatty Acids in Rats. J. Agric. Food Chem. 2018, 66, 6326–6335. [Google Scholar] [CrossRef] [PubMed]
- Parnell, J.A.; Reimer, R.A. Prebiotic Fibres Dose-Dependently Increase Satiety Hormones and Alter Bacteroidetes and Firmicutes in Lean and Obese JCR: LA-cp Rats. Br. J. Nutr. 2012, 107, 601–613. [Google Scholar] [CrossRef]
- Drobac, D.; Tokodi, N.; Lujić, J.; Marinović, Z.; Subakov-Simić, G.; Dulić, T.; Svirčev, Z. Cyanobacteria and Cyanotoxins in Fishponds and Their Effects on Fish Tissue. Harmful Algae 2016, 55, 66–76. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Qu, W.; Wang, H.; Yan, H. Antidepressants Fluoxetine and Amitriptyline Induce Alterations in Intestinal Microbiota and Gut Microbiome Function in Rats Exposed to Chronic Unpredictable Mild Stress. Transl. Psychiatry 2021, 11, 131. [Google Scholar] [CrossRef]
- Olli, K.; Saarinen, M.T.; Forssten, S.D.; Madetoja, M.; Herzig, K.H.; Tiihonen, K. Independent and Combined Effects of Lactitol, Polydextrose, and Bacteroides thetaiotaomicron on Postprandial Metabolism and Body Weight in Rats Fed a High-Fat Diet. Front. Nutr. 2016, 3, 15. [Google Scholar] [CrossRef]
- Hu, B.; He, X.; Tan, J.; Ma, Y.; Wang, G.; Liu, S.; Lv, X. Gender-Related Differences in the Effects of Inonotus obliquus Polysaccharide on Intestinal Microorganisms in SD Rats Model. Front. Vet. Sci. 2022, 9, 957053. [Google Scholar] [CrossRef]
- Ma, L.; Zhou, B.; Liu, H.; Chen, S.; Zhang, J.; Wang, T.; Wang, C. Dietary Rutin Improves the Antidiarrheal Capacity of Weaned Piglets by Improving Intestinal Barrier Function, Antioxidant Capacity, and Cecal Microbiota Composition. J. Sci. Food Agric. 2024, 104, 6262–6275. [Google Scholar] [CrossRef] [PubMed]
- Wei, X.; Xing, F.; Xu, Y.; Zhang, F.; Cheng, D.; Zhou, Y.; Zhang, W. Preoperative Gut Microbiota of POCD Patients Induces Pre- and Postoperative Cognitive Impairment and Systemic Inflammation in Rats. J. Neuroinflamm. 2024, 21, 221. [Google Scholar] [CrossRef]
- Cukovic-Cavka, S.; Likic, R.; Francetic, I.; Rustemovic, N.; Opacic, M.; Vucelic, B. Lactobacillus acidophilus as a Cause of Liver Abscess in a NOD2/CARD15-Positive Patient with Crohn’s Disease. Digestion 2006, 73, 107–110. [Google Scholar] [CrossRef]
- Shokryazdan, P.; Faseleh Jahromi, M.; Liang, J.B.; Kalavathy, R.; Sieo, C.C.; Ho, Y.W. Safety Assessment of Two New Lactobacillus Strains as Probiotic for Humans Using a Rat Model. PLoS ONE 2016, 11, e0159851. [Google Scholar] [CrossRef]
- Bhattamisra, S.K.; Yan, V.L.Y.; Lee, C.K.; Kuean, C.H.; Candasamy, M.; Liew, Y.K.; Sahu, P.S. Protective Activity of Geraniol against Acetic Acid- and Helicobacter pylori-Induced Gastric Ulcers in Rats. J. Tradit. Complement. Med. 2019, 9, 206–214. [Google Scholar] [CrossRef]
- Peng, W.; Zhao, X.; Li, X. Helicobacter bilis Contributes to the Occurrence of Inflammatory Bowel Disease by Inducing Host Immune Disorders. Biomed. Res. Int. 2022, 2022, 1837850. [Google Scholar] [CrossRef]
- Zafar, H.; Saier, M.H., Jr. Gut Bacteroides Species in Health and Disease. Gut Microbes 2021, 13, 1848158. [Google Scholar] [CrossRef]
- Gómez del Pulgar, E.M.; Benítez-Páez, A.; Sanz, Y. Safety Assessment of Bacteroides uniformis CECT 7771, a Symbiont of the Gut Microbiota in Infants. Nutrients 2020, 12, 551. [Google Scholar] [CrossRef]
- Liu, P.; Li, R.; Tian, X.; Zhao, Y.; Li, M.; Wang, M.; Yan, X. Co-Exposure to Fluoride and Arsenic Disrupts Intestinal Flora Balance and Induces Testicular Autophagy in Offspring Rats. Ecotoxicol. Environ. Saf. 2021, 222, 112506. [Google Scholar] [CrossRef]
- Wang, S.; Yang, J.; Zhang, B.; Zhang, L.; Wu, K.; Yang, A.; Qi, D. Potential Link between Gut Microbiota and Deoxynivalenol-Induced Feed Refusal in Weaned Piglets. J. Agric. Food Chem. 2019, 67, 4976–4986. [Google Scholar] [CrossRef]
- Jiang, D.; Kang, A.; Yao, W.; Lou, J.; Zhang, Q.; Bao, B.; Zhang, L. Euphorbia kansui Fry-Baked with Vinegar Modulates Gut Microbiota and Reduces Intestinal Toxicity in Rats. J. Ethnopharmacol. 2018, 226, 26–35. [Google Scholar] [CrossRef]
- Cao, Y.; Tao, F.; Yu, Y.; Song, L.; Zhang, R.; Feng, J.; Xue, P. Safety Evaluation of Rare Ginsenosides of Stems and Leaves from American Ginseng: 90-Day Exposure Toxicity Study Combined with Intestinal Flora Analysis and Metabonomics in Rats. Ecotoxicol. Environ. Saf. 2023, 264, 115429. [Google Scholar] [CrossRef] [PubMed]
- Qiu, X.; Li, Z.; Huang, S.; Cai, X.; Qu, S.; Zheng, Z.; Zhang, Z. Electroacupuncture Improves Depression-Like Behavior by Regulating the Abundance of Lactobacillus and Staphylococci in Mice. J. Integr. Neurosci. 2023, 22, 28. [Google Scholar] [CrossRef] [PubMed]
- Zhou, S.K.; Xu, J.D.; Gao, X.Q.; Zhang, R.J.; Cheng, F.F.; Yao, W.F.; Zhang, L. Fructus Jujubae Cooperated with Water-Expelling Members in Shizao Decoction Alleviated Intestinal Injury and Malignant Ascites by Modulating Gut Microbiota and Metabolic Homeostasis. Phytomedicine 2024, 133, 155895. [Google Scholar] [CrossRef] [PubMed]
- Miao, J.; Guo, L.; Cui, H.; Wang, L.; Zhu, B.; Lei, J.; Zhang, Z. Er-Chen Decoction Alleviates High-Fat Diet-Induced Nonalcoholic Fatty Liver Disease in Rats through Remodeling Gut Microbiota and Regulating the Serum Metabolism. Evid.-Based Complement. Altern. Med. 2022, 2022, 6221340. [Google Scholar] [CrossRef]
- Qin, R.; Wang, J.; Chao, C.; Yu, J.; Copeland, L.; Wang, S.; Wang, S. RS5 Produced More Butyric Acid through Regulating the Microbial Community of Human Gut Microbiota. J. Agric. Food Chem. 2021, 69, 3209–3218. [Google Scholar] [CrossRef]
- Huang, W.; Yau, Y.; Zhu, J.; Wang, Y.; Dai, Z.; Gan, H.; Yang, Z. Effect of Electroacupuncture at Zusanli (ST36) on Intestinal Microbiota in Rats with Chronic Atrophic Gastritis. Front. Genet. 2022, 13, 824739. [Google Scholar] [CrossRef]
- Zhang, J.; Song, L.; Wang, Y.; Liu, C.; Zhang, L.; Zhu, S.; Duan, L. Beneficial Effect of Butyrate-Producing Lachnospiraceae on Stress-Induced Visceral Hypersensitivity in Rats. J. Gastroenterol. Hepatol. 2019, 34, 1368–1376. [Google Scholar] [CrossRef]
- Ermolenko, E.; Simanenkova, A.; Voropaeva, L.; Lavrenova, N.; Kotyleva, M.; Minasian, S.; Karonova, T. Metformin Influence on the Intestinal Microbiota and Organism of Rats with Metabolic Syndrome. Int. J. Mol. Sci. 2022, 23, 6837. [Google Scholar] [CrossRef]
- Lin, Y.; Zeng, H.; Lin, J.; Peng, Y.; Que, X.; Wang, L.; Bai, N. Evaluating the Therapeutic Potential of Moxibustion on Polycystic Ovary Syndrome: A Rat Model Study on Gut Microbiota and Metabolite Interaction. Front. Cell. Infect. Microbiol. 2024, 14, 1328741. [Google Scholar] [CrossRef]
- He, X.; Liang, J.; Li, X.; Wang, Y.; Zhang, X.; Chen, D.; Wang, S. Dahuang Zhechong Pill Ameliorates Hepatic Fibrosis by Regulating Gut Microbiota and Metabolites. J. Ethnopharmacol. 2024, 321, 117402. [Google Scholar] [CrossRef] [PubMed]
- Guo, Y.; Liu, J.; Tuo, Q.; Zhang, D.; Wanapat, M.; Xin, G. The Effect of Dietary Supplementation of Lycium barbarum Leaves on the Growth Performance, Organ Indexes, and Intestinal Microflora of Rats. Front. Vet. Sci. 2024, 11, 1416793. [Google Scholar] [CrossRef]
- Wang, M.; Ma, Y.; Yu, G.; Zeng, B.; Yang, W.; Huang, C.; Dong, Y.; Tang, B.; Wu, Z. Integration of Microbiome, Metabolomics, and Transcriptome for In-Depth Understanding of Berberine Attenuates AOM/DSS-Induced Colitis-Associated Colorectal Cancer. Biomed. Pharmacother. 2024, 179, 117292. [Google Scholar] [CrossRef]
- Liu, R.; Zhang, F.; Li, S.; Liu, Q.; Pang, Y.; Li, L. Regulation of ROS Metabolism in Macrophage via Xanthine Oxidase Is Associated with Disease Progression in Pulmonary Tuberculosis. Metabolomics 2024, 20, 127. [Google Scholar] [CrossRef] [PubMed]
- Yu, Z.; Morrison, M. Improved Extraction of PCR-Quality Community DNA from Digesta and Fecal Samples. BioTechniques 2004, 36, 808–812. [Google Scholar] [CrossRef]
- Martin, M. Cutadapt Removes Adapter Sequences from High-Throughput Sequencing Reads. EMBnet J. 2011, 17, 10–12. [Google Scholar] [CrossRef]
- Bolyen, E.; Rideout, J.R.; Dillon, M.R.; Bokulich, N.A.; Abnet, C.C.; Al-Ghalith, G.A.; Alexander, H.; Alm, E.J.; Arumugam, M.; Asnicar, F. Reproducible, Interactive, Scalable and Extensible Microbiome Data Science Using QIIME 2. Nat. Biotechnol. 2019, 37, 852–857. [Google Scholar] [CrossRef]
- Callahan, B.J.; McMurdie, P.J.; Rosen, M.J.; Han, A.W.; Johnson, A.J.A.; Holmes, S.P. DADA2: High-Resolution Sample Inference from Illumina Amplicon Data. Nat. Methods 2016, 13, 581–583. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jeong, J.; Kim, J.; Kim, M.; Lee, B.; Park, C.; Kim, M. Effects of Deoxynivalenol Contamination on Growth Performance, Blood Biochemistry, Histology, Metabolomics, and the Microbiota: A Subacute Dose Oral Toxicity Study in Rats. Int. J. Mol. Sci. 2025, 26, 3086. https://doi.org/10.3390/ijms26073086
Jeong J, Kim J, Kim M, Lee B, Park C, Kim M. Effects of Deoxynivalenol Contamination on Growth Performance, Blood Biochemistry, Histology, Metabolomics, and the Microbiota: A Subacute Dose Oral Toxicity Study in Rats. International Journal of Molecular Sciences. 2025; 26(7):3086. https://doi.org/10.3390/ijms26073086
Chicago/Turabian StyleJeong, Jinyoung, Junsik Kim, Minji Kim, Boram Lee, Cheolju Park, and Minseok Kim. 2025. "Effects of Deoxynivalenol Contamination on Growth Performance, Blood Biochemistry, Histology, Metabolomics, and the Microbiota: A Subacute Dose Oral Toxicity Study in Rats" International Journal of Molecular Sciences 26, no. 7: 3086. https://doi.org/10.3390/ijms26073086
APA StyleJeong, J., Kim, J., Kim, M., Lee, B., Park, C., & Kim, M. (2025). Effects of Deoxynivalenol Contamination on Growth Performance, Blood Biochemistry, Histology, Metabolomics, and the Microbiota: A Subacute Dose Oral Toxicity Study in Rats. International Journal of Molecular Sciences, 26(7), 3086. https://doi.org/10.3390/ijms26073086





