Bone Defect Treatment in Regenerative Medicine: Exploring Natural and Synthetic Bone Substitutes
Abstract
1. Introduction
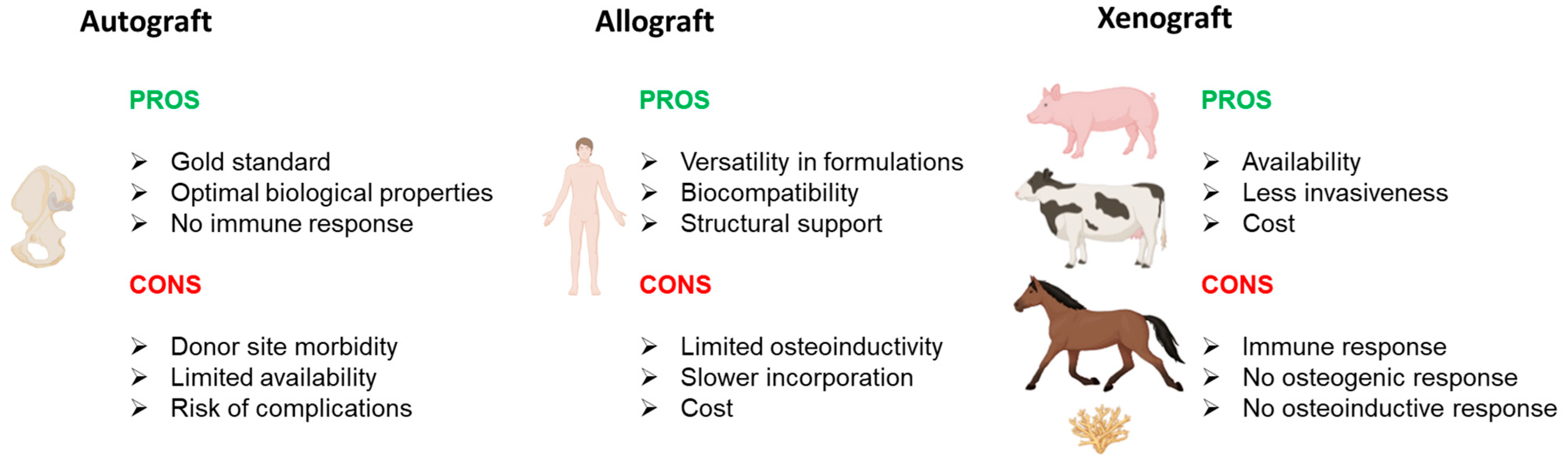
2. Bone Grafts
2.1. Natural Grafts
2.1.1. Autograft
2.1.2. Allograft
2.1.3. Xenograft
2.2. Future Perspectives on Natural Grafts
2.3. Market Availability
3. Tissue Scaffold
3.1. Ceramics
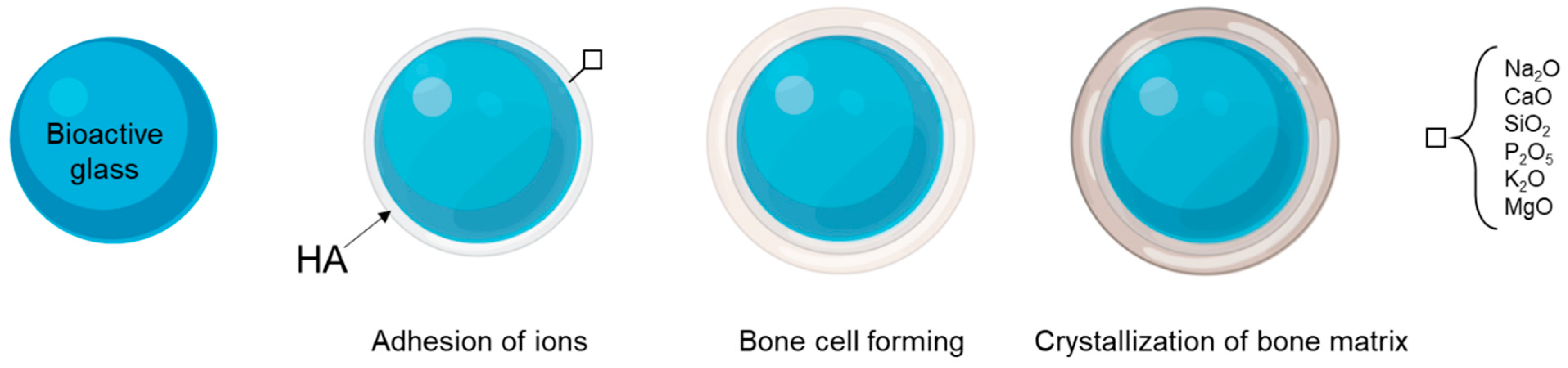
3.2. Bioactive Glasses
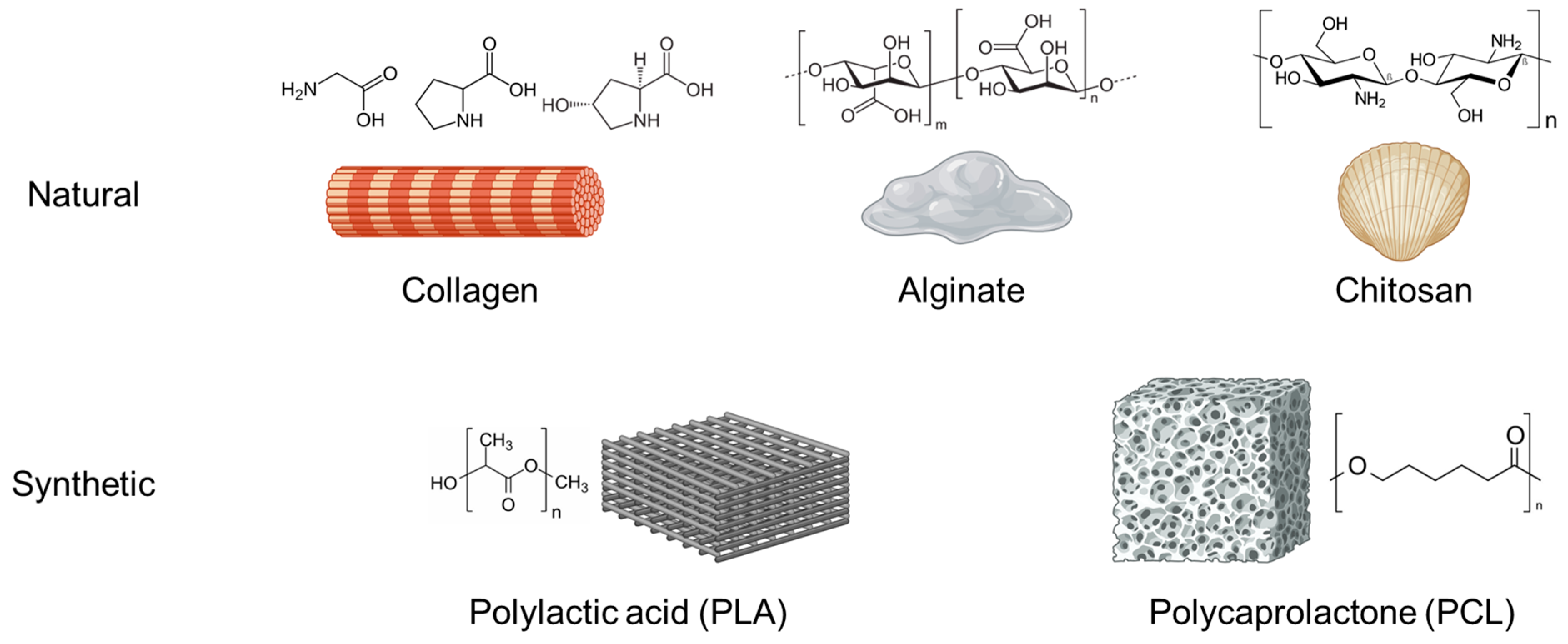
3.3. Polymers
3.3.1. Chitosan
3.3.2. Collagen
3.3.3. Alginate
3.3.4. Polylactic Acid (PLA)
3.3.5. Polycaprolactone (PCL)
3.3.6. Carbon Nanotubes (CNT)
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Fazzalari, N.L. Bone fracture and bone fracture repair. Osteoporos. Int. 2011, 22, 2003–2006. [Google Scholar] [CrossRef]
- Baht, G.S.; Vi, L.; Alman, B.A. The role of the immune cells in fracture healing. Curr. Osteoporos. Rep. 2018, 16, 138–145. [Google Scholar] [CrossRef] [PubMed]
- Hu, L.; Yin, C.; Zhao, F.; Ali, A.; Ma, J.; Qian, A. Mesenchymal stem cells: Cell fate decision to osteoblast or adipocyte and application in osteoporosis treatment. Int. J. Mol. Sci. 2018, 19, 360. [Google Scholar] [CrossRef] [PubMed]
- Granero-Moltó, F.; Weis, J.A.; Miga, M.I.; Landis, B.; Myers, T.J.; O’Rear, L.; Longobardi, L.; Jansen, E.D.; Mortlock, D.P.; Spagnoli, A. Regenerative effects of transplanted mesenchymal stem cells in fracture healing. Stem Cells 2009, 27, 1887–1898. [Google Scholar] [CrossRef]
- Katagiri, T.; Watabe, T. Bone morphogenetic proteins. Cold Spring Harb. Perspect. Biol. 2016, 8, a021899. [Google Scholar] [CrossRef] [PubMed]
- Halloran, D.; Durbano, H.W.; Nohe, A. Bone morphogenetic protein-2 in development and bone homeostasis. J. Dev. Biol. 2020, 8, 19. [Google Scholar] [CrossRef]
- Shen, B.; Wei, A.; Whittaker, S.; Williams, L.A.; Tao, H.; Ma, D.D.F.; Diwan, A.D. The role of BMP-7 in chondrogenic and osteogenic differentiation of human bone marrow multipotent mesenchymal stromal cells in vitro. J. Cell. Biochem. 2010, 109, 406–416. [Google Scholar] [CrossRef]
- Ramakrishnan, S.; Anand, V.; Roy, S. Vascular endothelial growth factor signaling in hypoxia and inflammation. J. Neuroimmune Pharmacol. 2014, 9, 142–160. [Google Scholar] [CrossRef]
- Dimitriou, R.; Tsiridis, E.; Giannoudis, P.V. Current concepts of molecular aspects of bone healing. Injury 2005, 36, 1392–1404. [Google Scholar] [CrossRef]
- Schindeler, A.; McDonald, M.M.; Bokko, P.; Little, D.G. Bone remodeling during fracture repair: The cellular picture. Semin. Cell Dev. Biol. 2008, 19, 459–466. [Google Scholar] [CrossRef]
- Grosso, A.; Burger, M.G.; Lunger, A.; Schaefer, D.J.; Banfi, A.; Di Maggio, N. It takes two to tango: Coupling of angiogenesis and osteogenesis for bone regeneration. Front. Bioeng. Biotechnol. 2017, 5, 68. [Google Scholar] [CrossRef]
- Zhao, Y.; Yu, B.; Wang, Y.; Tan, S.; Xu, Q.; Wang, Z.; Zhou, K.; Liu, H.; Ren, Z.; Jiang, Z. Ang-1 and VEGF: Central regulators of angiogenesis. Mol. Cell. Biochem. 2024, 480, 621–637. [Google Scholar] [CrossRef] [PubMed]
- Omorphos, N.P.; Gao, C.; Tan, S.S.; Sangha, M.S. Understanding angiogenesis and the role of angiogenic growth factors in the vascularisation of engineered tissues. Mol. Biol. Rep. 2021, 48, 941–950. [Google Scholar] [CrossRef]
- Wang, L.; You, X.; Zhang, L.; Zhang, C.; Zou, W. Mechanical regulation of bone remodeling. Bone Res. 2022, 10, 16. [Google Scholar] [CrossRef] [PubMed]
- Rowe, P.; Koller, A.; Sharma, S. Physiology, bone remodeling. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2018. [Google Scholar]
- Mao, A.S.; Mooney, D.J. Regenerative medicine: Current therapies and future directions. Proc. Natl. Acad. Sci. USA 2015, 112, 14452–14459. [Google Scholar] [CrossRef] [PubMed]
- Camponogara, F.; Zanotti, F.; Trentini, M.; Tiengo, E.; Zanolla, I.; Pishavar, E.; Soliani, E.; Scatto, M.; Gargiulo, P.; Zambito, Y. Biomaterials for Regenerative Medicine in Italy: Brief State of the Art of the Principal Research Centers. Int. J. Mol. Sci. 2022, 23, 8245. [Google Scholar] [CrossRef]
- Esdaille, C.J.; Washington, K.S.; Laurencin, C.T. Regenerative engineering: A review of recent advances and future directions. Regen. Med. 2021, 16, 495–512. [Google Scholar] [CrossRef]
- Tollemar, V.; Collier, Z.J.; Mohammed, M.K.; Lee, M.J.; Ameer, G.A.; Reid, R.R. Stem cells, growth factors and scaffolds in craniofacial regenerative medicine. Genes Dis. 2016, 3, 56–71. [Google Scholar] [CrossRef]
- Tweedell, K.S. The adaptability of somatic stem cells: A review. J. Stem Cells Regen. Med. 2017, 13, 3. [Google Scholar]
- Kim, Y.; Kim, I.; Shin, K. A new era of stem cell and developmental biology: From blastoids to synthetic embryos and beyond. Exp. Mol. Med. 2023, 55, 2127–2137. [Google Scholar] [CrossRef]
- Karami, Z.; Moradi, S.; Eidi, A.; Soleimani, M.; Jafarian, A. Induced pluripotent stem cells: Generation methods and a new perspective in COVID-19 research. Front. Cell Dev. Biol. 2023, 10, 1050856. [Google Scholar] [CrossRef]
- Cerneckis, J.; Cai, H.; Shi, Y. Induced pluripotent stem cells (iPSCs): Molecular mechanisms of induction and applications. Signal Transduct. Target. Ther. 2024, 9, 112. [Google Scholar] [CrossRef] [PubMed]
- Manuguerra-GagnÉ, R.; Boulos, P.R.; Ammar, A.; Leblond, F.A.; Krosl, G.; Pichette, V.; Lesk, M.R.; Roy, D.-C. Transplantation of mesenchymal stem cells promotes tissue regeneration in a glaucoma model through laser-induced paracrine factor secretion and progenitor cell recruitment. Stem Cells 2013, 31, 1136–1148. [Google Scholar] [CrossRef] [PubMed]
- Nöth, U.; Steinert, A.F.; Tuan, R.S. Technology insight: Adult mesenchymal stem cells for osteoarthritis therapy. Nat. Clin. Pract. Rheumatol. 2008, 4, 371–380. [Google Scholar] [CrossRef]
- Buonocore, M.; Grimaldi, M.; Santoro, A.; Covelli, V.; Marino, C.; Napolitano, E.; Novi, S.; Tecce, M.F.; Ciaglia, E.; Montella, F. Exploiting the Features of Short Peptides to Recognize Specific Cell Surface Markers. Int. J. Mol. Sci. 2023, 24, 15610. [Google Scholar] [CrossRef] [PubMed]
- Cillo, J.E., Jr.; Gassner, R.; Koepsel, R.R.; Buckley, M.J. Growth factor and cytokine gene expression in mechanically strained human osteoblast-like cells: Implications for distraction osteogenesis. Oral Surg. Oral Med. Oral Pathol. Oral Radiol. Endodontology 2000, 90, 147–154. [Google Scholar] [CrossRef]
- Bostrom, M.P.G.; Camacho, N.P. Potential role of bone morphogenetic proteins in fracture healing. Clin. Orthop. Relat. Res. 1998, 355, S274–S282. [Google Scholar] [CrossRef]
- Vidal, L.; Kampleitner, C.; Brennan, M.Á.; Hoornaert, A.; Layrolle, P. Reconstruction of large skeletal defects: Current clinical therapeutic strategies and future directions using 3D printing. Front. Bioeng. Biotechnol. 2020, 8, 61. [Google Scholar] [CrossRef]
- Ferraz, M.P. An Overview on the Big Players in Bone Tissue Engineering: Biomaterials, Scaffolds and Cells. Int. J. Mol. Sci. 2024, 25, 3836. [Google Scholar] [CrossRef]
- Krishani, M.; Shin, W.Y.; Suhaimi, H.; Sambudi, N.S. Development of scaffolds from bio-based natural materials for tissue regeneration applications: A review. Gels 2023, 9, 100. [Google Scholar] [CrossRef]
- Soucacos, P.N.; Johnson, E.O.; Babis, G. An update on recent advances in bone regeneration. Injury 2008, 39, S1–S4. [Google Scholar] [CrossRef]
- Kubosch, E.J.; Bernstein, A.; Wolf, L.; Fretwurst, T.; Nelson, K.; Schmal, H. Clinical trial and in-vitro study comparing the efficacy of treating bony lesions with allografts versus synthetic or highly-processed xenogeneic bone grafts. BMC Musculoskelet. Disord. 2016, 17, 77. [Google Scholar]
- Fernandez de Grado, G.; Keller, L.; Idoux-Gillet, Y.; Wagner, Q.; Musset, A.-M.; Benkirane-Jessel, N.; Bornert, F.; Offner, D. Bone substitutes: A review of their characteristics, clinical use, and perspectives for large bone defects management. J. Tissue Eng. 2018, 9, 2041731418776819. [Google Scholar] [CrossRef]
- Campana, V.; Milano, G.; Pagano, E.; Barba, M.; Cicione, C.; Salonna, G.; Lattanzi, W.; Logroscino, G. Bone substitutes in orthopaedic surgery: From basic science to clinical practice. J. Mater. Sci. Mater. Med. 2014, 25, 2445–2461. [Google Scholar] [CrossRef]
- Migliorini, F.; Cuozzo, F.; Torsiello, E.; Spiezia, F.; Oliva, F.; Maffulli, N. Autologous bone grafting in trauma and orthopaedic surgery: An evidence-based narrative review. J. Clin. Med. 2021, 10, 4347. [Google Scholar] [CrossRef] [PubMed]
- Banwart, J.C.; Asher, M.A.; Hassanein, R.S. Iliac crest bone graft harvest donor site morbidity: A statistical evaluation. Spine 1995, 20, 1055–1060. [Google Scholar] [CrossRef] [PubMed]
- Han, D.; Dai, K. Prefabrication of a vascularized bone graft with Beta tricalcium phosphate using an in vivo bioreactor. Artif. Organs 2013, 37, 884–893. [Google Scholar] [CrossRef]
- El-Ghannam, A. Bone reconstruction: From bioceramics to tissue engineering. Expert Rev. Med. Devices 2005, 2, 87–101. [Google Scholar] [CrossRef]
- Kokubo, T.; Kim, H.-M.; Kawashita, M. Novel bioactive materials with different mechanical properties. Biomaterials 2003, 24, 2161–2175. [Google Scholar] [CrossRef]
- Perinelli, D.R.; Fagioli, L.; Campana, R.; Lam, J.K.W.; Baffone, W.; Palmieri, G.F.; Casettari, L.; Bonacucina, G. Chitosan-based nanosystems and their exploited antimicrobial activity. Eur. J. Pharm. Sci. 2018, 117, 8–20. [Google Scholar] [CrossRef]
- Chang, P.C.; Liu, B.Y.; Liu, C.M.; Chou, H.H.; Ho, M.H.; Liu, H.C.; Wang, D.M.; Hou, L.T. Bone tissue engineering with novel rhBMP2-PLLA composite scaffolds. J. Biomed. Mater. Res. Part A 2007, 81, 771–780. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Wang, Y.; Zhou, Y.; Chen, J.; Wan, Q. The application of polycaprolactone in three-dimensional printing scaffolds for bone tissue engineering. Polymers 2021, 13, 2754. [Google Scholar] [CrossRef] [PubMed]
- Parenteau-Bareil, R.; Gauvin, R.; Berthod, F. Collagen-based biomaterials for tissue engineering applications. Materials 2010, 3, 1863–1887. [Google Scholar] [CrossRef]
- Aoki, K.; Usui, Y.; Narita, N.; Ogiwara, N.; Iashigaki, N.; Nakamura, K.; Kato, H.; Sano, K.; Ogiwara, N.; Kametani, K. A thin carbon-fiber web as a scaffold for bone-tissue regeneration. Small 2009, 5, 1540–1546. [Google Scholar] [CrossRef]
- Tanaka, M.; Aoki, K.; Haniu, H.; Kamanaka, T.; Takizawa, T.; Sobajima, A.; Yoshida, K.; Okamoto, M.; Kato, H.; Saito, N. Applications of carbon nanotubes in bone regenerative medicine. Nanomaterials 2020, 10, 659. [Google Scholar] [CrossRef]
- Assali, M.; Kittana, N.; Alhaj-Qasem, S.; Hajjyahya, M.; Abu-Rass, H.; Alshaer, W.; Al-Buqain, R. Noncovalent functionalization of carbon nanotubes as a scaffold for tissue engineering. Sci. Rep. 2022, 12, 12062. [Google Scholar] [CrossRef] [PubMed]
- Liu, D.; Shi, L.; Dai, Q.; Lin, X.; Mehmood, R.; Gu, Z.; Dai, L. Functionalization of carbon nanotubes for multifunctional applications. Trends Chem. 2024, 6, 186–210. [Google Scholar] [CrossRef]
- Khan, S.N.; Cammisa, F.P., Jr.; Sandhu, H.S.; Diwan, A.D.; Girardi, F.P.; Lane, J.M. The biology of bone grafting. JAAOS-J. Am. Acad. Orthop. Surg. 2005, 13, 77–86. [Google Scholar] [CrossRef]
- Daculsi, G.; Fellah, B.H.; Miramond, T.; Durand, M. Osteoconduction, osteogenicity, osteoinduction, what are the fundamental properties for a smart bone substitutes. Irbm 2013, 34, 346–348. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, Y.; Qian, Z. Nanostructured surface modification to bone implants for bone regeneration. J. Biomed. Nanotechnol. 2018, 14, 628–648. [Google Scholar] [CrossRef]
- Hasegawa, T.; Sasaki, A.; Saito, I.; Arimoto, S.; Yatagai, N.; Hiraoka, Y.; Takeda, D.; Kakei, Y.; Akashi, M. Success of dental implants in patients with large bone defect and analysis of risk factors for implant failure: A non-randomized retrospective cohort study. Clin. Oral Investig. 2021, 26, 2743–2750. [Google Scholar] [CrossRef]
- Huten, D.; Pasquier, G.; Lambotte, J.-C. Techniques for filling tibiofemoral bone defects during revision total knee arthroplasty. Orthop. Traumatol. Surg. Res. 2021, 107, 102776. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; See, C.W.; Li, X.; Zhu, D. Orthopedic implants and devices for bone fractures and defects: Past, present and perspective. Eng. Regen. 2020, 1, 6–18. [Google Scholar] [CrossRef]
- Friedenstein, A.Y. Induction of bone tissue by transitional epithelium. Clin. Orthop. Relat. Res. 1968, 59, 21–38. [Google Scholar] [CrossRef] [PubMed]
- Di Silvio, L.; Jayakumar, P. Cellular response to osteoinductive materials in orthopaedic surgery. In Cellular Response to Biomaterials; Elsevier: Amsterdam, The Netherlands, 2009; pp. 313–343. [Google Scholar]
- Villa, R.; Polimeni, G.; Wikesjö, U.M.E. Implant osseointegration in the absence of primary bone anchorage: A clinical report. J. Prosthet. Dent. 2010, 104, 282–287. [Google Scholar] [CrossRef] [PubMed]
- Albrektsson, T.; Johansson, C. Osteoinduction, osteoconduction and osseointegration. Eur. Spine J. 2001, 10, S96–S101. [Google Scholar]
- Davies, J.E. Understanding peri-implant endosseous healing. J. Dent. Educ. 2003, 67, 932–949. [Google Scholar] [CrossRef]
- Karlsson, J.; Sundell, G.; Thuvander, M.; Andersson, M. Atomically resolved tissue integration. Nano Lett. 2014, 14, 4220–4223. [Google Scholar] [CrossRef]
- Oryan, A.; Alidadi, S.; Moshiri, A.; Maffulli, N. Bone regenerative medicine: Classic options, novel strategies, and future directions. J. Orthop. Surg. Res. 2014, 9, 18. [Google Scholar] [CrossRef]
- Wang, W.; Yeung, K.W.K. Bone grafts and biomaterials substitutes for bone defect repair: A review. Bioact. Mater. 2017, 2, 224–247. [Google Scholar] [CrossRef]
- Fillingham, Y.; Jacobs, J. Bone grafts and their substitutes. Bone Jt. J. 2016, 98, 6–9. [Google Scholar] [CrossRef] [PubMed]
- Manyalich, M.; Navarro, A.; Koller, J.; Loty, B.; De Guerra, A.; Cornu, O.; Vabels, G.; Fornasari, P.M.; Costa, A.N.; Siska, I. European quality system for tissue banking. Transplant. Proc. 2009, 41, 2035–2043. [Google Scholar] [CrossRef] [PubMed]
- Gianluca, I.; Valente, N.A. Intraoral and extraoral autologous bone block graft techniques: A review of the recent literature. Int. J. Contemp. Dent. Med. Rev. 2016, 2016, 030316. [Google Scholar]
- Roberts, T.T.; Rosenbaum, A.J. Bone grafts, bone substitutes and orthobiologics: The bridge between basic science and clinical advancements in fracture healing. Organogenesis 2012, 8, 114–124. [Google Scholar] [CrossRef]
- Murshed, M. Mechanism of bone mineralization. Cold Spring Harb. Perspect. Med. 2018, 8, a031229. [Google Scholar] [CrossRef]
- Sohn, H.-S.; Oh, J.-K. Review of bone graft and bone substitutes with an emphasis on fracture surgeries. Biomater. Res. 2019, 23, 9. [Google Scholar] [CrossRef]
- McKee, M.D. Management of segmental bony defects: The role of osteoconductive orthobiologics. JAAOS-J. Am. Acad. Orthop. Surg. 2006, 14, S163–S167. [Google Scholar] [CrossRef]
- Flynn, J.M. Fracture repair and bone grafting. OKU 2011, 10, 11–21. [Google Scholar]
- Younger, E.M.; Chapman, M.W. Morbidity at bone graft donor sites. J. Orthop. Trauma 1989, 3, 192–195. [Google Scholar] [CrossRef]
- Schmidt, A.H. Autologous bone graft: Is it still the gold standard? Injury 2021, 52, S18–S22. [Google Scholar] [CrossRef]
- Riester, O.; Borgolte, M.; Csuk, R.; Deigner, H.-P. Challenges in bone tissue regeneration: Stem cell therapy, biofunctionality and antimicrobial properties of novel materials and its evolution. Int. J. Mol. Sci. 2020, 22, 192. [Google Scholar] [CrossRef] [PubMed]
- Orozco Delclós, L.; Soler Rich, R.; Arriaza Loureda, R.; Moreno García, A.; Gómez Barrena, E. Efficacy and safety of autologous or allogeneic mesenchymal stromal cells from adult adipose tissue expanded and combined with tricalcium phosphate biomaterial for the surgical treatment of atrophic nonunion of long bones: A phase II clinical trial. J. Transl. Med. 2024, 22, 493. [Google Scholar] [CrossRef]
- Dimitriou, R.; Jones, E.; McGonagle, D.; Giannoudis, P.V. Bone regeneration: Current concepts and future directions. BMC Med. 2011, 9, 66. [Google Scholar] [CrossRef]
- de Alencar, P.G.C.; Vieira, I.F.V. Bone banks. Rev. Bras. Ortop. (Engl. Ed.) 2010, 45, 524–528. [Google Scholar] [CrossRef]
- Pruß, A.; Kalus, U. Knochenbanken. Der Orthopäde 2018, 47, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Pruss, A.; Perka, C.; Degenhardt, P.; Maronna, U.; Büttner-Janz, K.; Paul, B.; Müller, K.; Klumpp, C.; Bruck, J.C.; Von Versen, R. Clinical efficacy and compatibility of allogeneic avital tissue transplants sterilized with a peracetic acid/ethanol mixture. Cell Tissue Bank. 2002, 3, 235–243. [Google Scholar] [CrossRef]
- Georgeanu, V.A.; Gingu, O.; Antoniac, I.V.; Manolea, H.O. Current options and future perspectives on bone graft and biomaterials substitutes for bone repair, from clinical needs to advanced biomaterials research. Appl. Sci. 2023, 13, 8471. [Google Scholar] [CrossRef]
- Tournier, P.; Guicheux, J.; Paré, A.; Veziers, J.; Barbeito, A.; Bardonnet, R.; Corre, P.; Geoffroy, V.; Weiss, P.; Gaudin, A. An extrudable partially demineralized allogeneic bone paste exhibits a similar bone healing capacity as the “Gold Standard” bone graft. Front. Bioeng. Biotechnol. 2021, 9, 658853. [Google Scholar] [CrossRef]
- Drosos, G.I.; Touzopoulos, P.; Ververidis, A.; Tilkeridis, K.; Kazakos, K. Use of demineralized bone matrix in the extremities. World J. Orthop. 2015, 6, 269. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, L.; Yang, X.G.; Wang, F.; Feng, J.T.; Hua, K.C.; Li, Q.; Hu, Y.C. Demineralized bone matrix carriers and their clinical applications: An overview. Orthop. Surg. 2019, 11, 725–737. [Google Scholar] [CrossRef]
- Haghwerdi, F.; Haririan, I.; Soleimani, M. Chondrogenic potential of PMSCs cultured on chondroitin sulfate/gelatin-modified DBM scaffold. BioImpacts 2024, 15, 30003. [Google Scholar] [CrossRef]
- Thiel, G.E.; Puga, T.B.; Perleberg, T.D.; Figuerres, B.F.; Dennis, J.F. Achilles Allograft Fiber Track Graft Preparation Technique for Anterior Cruciate Ligament Reconstruction. Arthrosc. Tech. 2024, 13, 102844. [Google Scholar] [CrossRef] [PubMed]
- Glowacki, J. A review of osteoinductive testing methods and sterilization processes for demineralized bone. Cell Tissue Bank. 2005, 6, 3–12. [Google Scholar] [CrossRef] [PubMed]
- Kumar, P.; Vinitha, B.; Fathima, G. Bone grafts in dentistry. J. Pharm. Bioallied Sci. 2013, 5, S125–S127. [Google Scholar] [CrossRef] [PubMed]
- Saito, N.; Takaoka, K. New synthetic biodegradable polymers as BMP carriers for bone tissue engineering. Biomaterials 2003, 24, 2287–2293. [Google Scholar] [CrossRef]
- Gautschi, O.P.; Frey, S.P.; Zellweger, R. Bone morphogenetic proteins in clinical applications. ANZ J. Surg. 2007, 77, 626–631. [Google Scholar] [CrossRef]
- Laurencin, C.T. Musculoskeletal allograft tissue banking and safety. In Bone Graft Substitutes; ASTM International: West Conshohocken, PA, USA, 2003; pp. 30–67. [Google Scholar]
- Doppelt, S.H.; Tomford, W.W.; Lucas, A.D.; Mankin, H.J. Operational and financial aspects of a hospital bone bank. J. Bone Jt. Surg. 1981, 63, 1472–1481. [Google Scholar] [CrossRef]
- Nemzek, J.A.; Arnoczky, S.P.; Swenson, C.L. Retroviral transmission by the transplantation of connective-tissue allografts. An experimental study. J. Bone Jt. Surg. 1994, 76, 1036–1041. [Google Scholar] [CrossRef]
- Tite, T.; Popa, A.-C.; Balescu, L.M.; Bogdan, I.M.; Pasuk, I.; Ferreira, J.M.F.; Stan, G.E. Cationic substitutions in hydroxyapatite: Current status of the derived biofunctional effects and their in vitro interrogation methods. Materials 2018, 11, 2081. [Google Scholar] [CrossRef]
- Benedini, L.; Laiuppa, J.; Santillán, G.; Baldini, M.; Messina, P. Antibacterial alginate/nano-hydroxyapatite composites for bone tissue engineering: Assessment of their bioactivity, biocompatibility, and antibacterial activity. Mater. Sci. Eng. C 2020, 115, 111101. [Google Scholar] [CrossRef]
- Falacho, R.I.; Palma, P.J.; Marques, J.A.; Figueiredo, M.H.; Caramelo, F.; Dias, I.; Viegas, C.; Guerra, F. Collagenated porcine heterologous bone grafts: Histomorphometric evaluation of bone formation using different physical forms in a rabbit cancellous bone model. Molecules 2021, 26, 1339. [Google Scholar] [CrossRef] [PubMed]
- Demers, C.; Hamdy, C.R.; Corsi, K.; Chellat, F.; Tabrizian, M.; Yahia, L.H. Natural coral exoskeleton as a bone graft substitute: A review. Bio-Med. Mater. Eng. 2002, 12, 15–35. [Google Scholar]
- Zhao, R.; Yang, R.; Cooper, P.R.; Khurshid, Z.; Shavandi, A.; Ratnayake, J. Bone grafts and substitutes in dentistry: A review of current trends and developments. Molecules 2021, 26, 3007. [Google Scholar] [CrossRef] [PubMed]
- Pistilli, R.; Signorini, L.; Pisacane, A.; Lizio, G.; Felice, P. Case of severe bone atrophy of the posterior maxilla rehabilitated with blocks of equine origin bone: Histological results. Implant. Dent. 2013, 22, 8–15. [Google Scholar] [CrossRef]
- Alqutub, M.N.; Mukhtar, A.H.; Alali, Y.; Vohra, F.; Abduljabbar, T. Osteogenic Differentiation of Periodontal Ligament Stem Cells Seeded on Equine-Derived Xenograft in Osteogenic Growth Media. Medicina 2022, 58, 1518. [Google Scholar] [CrossRef]
- Neto, A.; Ferreira, J.M. Synthetic and Marine-Derived Porous Bone Graft Substitutes. Materials 2018, 11, 1702. [Google Scholar] [CrossRef]
- Canullo, L.; Del Fabbro, M.; Khijmatgar, S.; Panda, S.; Ravidà, A.; Tommasato, G.; Sculean, A.; Pesce, P. Dimensional and histomorphometric evaluation of biomaterials used for alveolar ridge preservation: A systematic review and network meta-analysis. Clin. Oral Investig. 2022, 26, 141–158. [Google Scholar] [CrossRef]
- Tovar, N.; Jimbo, R.; Gangolli, R.; Perez, L.; Manne, L.; Yoo, D.; Lorenzoni, F.; Witek, L.; Coelho, P.G. Evaluation of bone response to various anorganic bovine bone xenografts: An experimental calvaria defect study. Int. J. Oral Maxillofac. Surg. 2014, 43, 251–260. [Google Scholar] [CrossRef]
- Dos Anjos, T.; De Molon, R.S.; Paim, P.R.F.; Marcantonio, E.; Marcantonio, E., Jr.; Faeda, R.S. Implant stability after sinus floor augmentation with deproteinized bovine bone mineral particles of different sizes: A prospective, randomized and controlled split-mouth clinical trial. Int. J. Oral Maxillofac. Surg. 2016, 45, 1556–1563. [Google Scholar] [CrossRef]
- Laurencin, C.T.; El-Amin, S.F. Xenotransplantation in orthopaedic surgery. JAAOS-J. Am. Acad. Orthop. Surg. 2008, 16, 4–8. [Google Scholar] [CrossRef]
- Borlongan, C.V.; Sanberg, P.R. Neural transplantation for treatment of Parkinson’s disease. Drug Discov. Today 2002, 7, 674–682. [Google Scholar] [CrossRef] [PubMed]
- Marmotti, A.; Mattia, S.; Mangiavini, L.; Bellato, E.; Ragni, E.; de Girolamo, L.; Peretti, G.M.; Blonna, D.; Bonasia, D.; Setti, S. Hamstring grafts are tenogenic constructs for ACL reconstruction and Pulsed Electromagnetic Fields improve tendon specific markers expression. An in-vitro study. J. Biol. Regul. Homeost. Agents 2020, 34, 363–376. [Google Scholar]
- James, A.W. Review of signaling pathways governing MSC osteogenic and adipogenic differentiation. Scientifica 2013, 2013, 684736. [Google Scholar] [CrossRef]
- Schubert, T.; Xhema, D.; Vériter, S.; Schubert, M.; Behets, C.; Delloye, C.; Gianello, P.; Dufrane, D. The enhanced performance of bone allografts using osteogenic-differentiated adipose-derived mesenchymal stem cells. Biomaterials 2011, 32, 8880–8891. [Google Scholar] [CrossRef] [PubMed]
- Jovic, T.H.; Combellack, E.J.; Jessop, Z.M.; Whitaker, I.S. 3D Bioprinting and the Future of Surgery. Front. Surg. 2020, 7, 609836. [Google Scholar] [CrossRef]
- Neishabouri, A.; Soltani Khaboushan, A.; Daghigh, F.; Kajbafzadeh, A.-M.; Majidi Zolbin, M. Decellularization in tissue engineering and regenerative medicine: Evaluation, modification, and application methods. Front. Bioeng. Biotechnol. 2022, 10, 805299. [Google Scholar] [CrossRef]
- Rana, D.; Zreiqat, H.; Benkirane-Jessel, N.; Ramakrishna, S.; Ramalingam, M. Development of decellularized scaffolds for stem cell-driven tissue engineering. J. Tissue Eng. Regen. Med. 2017, 11, 942–965. [Google Scholar] [CrossRef] [PubMed]
- Augustine, R.; Gezek, M.; Nikolopoulos, V.K.; Buck, P.L.; Bostanci, N.S.; Camci-Unal, G. Stem cells in bone tissue engineering: Progress, promises and challenges. Stem Cell Rev. Rep. 2024, 20, 1692–1731. [Google Scholar] [CrossRef]
- Rahman, M.S.; Akhtar, N.; Jamil, H.M.; Banik, R.S.; Asaduzzaman, S.M. TGF-β/BMP signaling and other molecular events: Regulation of osteoblastogenesis and bone formation. Bone Res. 2015, 3, 15005. [Google Scholar] [CrossRef]
- Zhang, H.; Ahmad, M.; Gronowicz, G. Effects of transforming growth factor-beta 1 (TGF-β1) on in vitro mineralization of human osteoblasts on implant materials. Biomaterials 2003, 24, 2013–2020. [Google Scholar] [CrossRef]
- Lupon, E.; Lellouch, A.G.; Acun, A.; Andrews, A.R.; Oganesyan, R.; Goutard, M.; Taveau, C.B.; Lantieri, L.A.; Cetrulo, C.L.; Uygun, B.E. Engineering vascularized composite allografts using natural scaffolds: A systematic review. Tissue Eng. Part B Rev. 2022, 28, 677–693. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Johnson, A.C.; Colakoglu, S.; Huang, C.A.; Mathes, D.W. Clinical and preclinical tolerance protocols for vascularized composite allograft transplantation. Arch. Plast. Surg. 2021, 48, 703–713. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Zhang, D.; Zhang, T.; Chen, C.; Song, Y.; Liu, S.; Su, Y.; Guo, S. Effect of the vascularized bone components on the survival of vascularized composite allografts. J. Surg. Res. 2018, 224, 132–138. [Google Scholar] [CrossRef]
- El-Hansi, N.S.; Said, H.H.; Desouky, O.S.; Khalaf, M.A.; Talaat, M.S.; Sallam, A.M. XRD and ATR-FTIR techniques for integrity assessment of gamma radiation sterilized cortical bone pretreated by antioxidants. Cell Tissue Bank. 2021, 22, 305–321. [Google Scholar] [CrossRef] [PubMed]
- Grieb, T.A.; Forng, R.-Y.; Stafford, R.E.; Lin, J.; Almeida, J.; Bogdansky, S.; Ronholdt, C.; Drohan, W.N.; Burgess, W.H. Effective use of optimized, high-dose (50ákGy) gamma irradiation for pathogen inactivation of human bone allografts. Biomaterials 2005, 26, 2033–2042. [Google Scholar] [CrossRef]
- Balsly, C.R.; Cotter, A.T.; Williams, L.A.; Gaskins, B.D.; Moore, M.A.; Wolfinbarger, L., Jr. Effect of low dose and moderate dose gamma irradiation on the mechanical properties of bone and soft tissue allografts. Cell Tissue Bank. 2008, 9, 289–298. [Google Scholar] [CrossRef]
- Tal, H.; Kozlovsky, A.; Artzi, Z.; Nemcovsky, C.E.; Moses, O. Long-term bio-degradation of cross-linked and non-cross-linked collagen barriers in human guided bone regeneration. Clin. Oral Implant. Res. 2008, 19, 295–302. [Google Scholar] [CrossRef]
- Sbricoli, L.; Guazzo, R.; Annunziata, M.; Gobbato, L.; Bressan, E.; Nastri, L. Selection of collagen membranes for bone regeneration: A literature review. Materials 2020, 13, 786. [Google Scholar] [CrossRef]
- Abpeikar, Z.; Alizadeh, A.A.; Ahmadyousefi, Y.; Najafi, A.A.; Safaei, M. Engineered cells along with smart scaffolds: Critical factors for improving tissue engineering approaches. Regen. Med. 2022, 17, 855–876. [Google Scholar] [CrossRef]
- Zielińska, A.; Karczewski, J.; Eder, P.; Kolanowski, T.; Szalata, M.; Wielgus, K.; Szalata, M.; Kim, D.; Shin, S.R.; Słomski, R. Scaffolds for drug delivery and tissue engineering: The role of genetics. J. Control. Release 2023, 359, 207–223. [Google Scholar] [CrossRef]
- Shen, M.; Wang, L.; Gao, Y.; Feng, L.; Xu, C.; Li, S.; Wang, X.; Wu, Y.; Guo, Y.; Pei, G. 3D bioprinting of in situ vascularized tissue engineered bone for repairing large segmental bone defects. Mater. Today Bio 2022, 16, 100382. [Google Scholar] [CrossRef] [PubMed]
- Kim, W.; Lee, H.; Roh, E.J.; An, S.B.; Han, I.-B.; Kim, G.H. A multicellular bioprinted cell construct for vascularized bone tissue regeneration. Chem. Eng. J. 2022, 431, 133882. [Google Scholar] [CrossRef]
- Palachur, D.; Rao, K.V.P.; Murthy, K.R.V.; Kishore, D.T.; Reddy, M.N.; Bhupathi, A. A comparative evaluation of bovine-derived xenograft (Bio-Oss Collagen) and type I collagen membrane (Bio-Gide) with bovine-derived xenograft (Bio-Oss Collagen) and fibrin fibronectin sealing system (TISSEEL) in the treatment of intrabony defects: A clinico-radiographic study. J. Indian Soc. Periodontol. 2014, 18, 336–343. [Google Scholar] [PubMed]
- Ashfaq, R.; Kovács, A.; Berkó, S.; Budai-Szűcs, M. Developments in alloplastic bone grafts and barrier membrane biomaterials for periodontal guided tissue and bone regeneration therapy. Int. J. Mol. Sci. 2024, 25, 7746. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, H.; Yue, Z.; Miao, L.; Han, Y.; Liu, K.; Hou, J. Modified minimally invasive surgical technique plus Bio-Oss Collagen for regenerative therapy of isolated interdental intrabony defects: Study protocol for a randomised controlled trial. BMJ Open 2020, 10, e040046. [Google Scholar] [CrossRef]
- Schallenberger, M.A.; Rossmeier, K.; Lovick, H.M.; Meyer, T.R.; Aberman, H.M.; Juda, G.A. Comparison of the osteogenic potential of OsteoSelect demineralized bone matrix putty to NovaBone calcium-phosphosilicate synthetic putty in a cranial defect model. J. Craniofacial Surg. 2014, 25, 657–661. [Google Scholar] [CrossRef]
- Bala, Y.; Farlay, D.; Boivin, G. Bone mineralization: From tissue to crystal in normal and pathological contexts. Osteoporos. Int. 2013, 24, 2153–2166. [Google Scholar] [CrossRef]
- Hench, L.L.; Wilson, J. An Introduction to Bioceramics; World Scientific: Singapore, 1993; Volume 1. [Google Scholar]
- Ana, I.D.; Matsuya, S.; Ishikawa, K. Engineering of carbonate apatite bone substitute based on composition-transformation of gypsum and calcium hydroxide. Engineering 2010, 2, 344. [Google Scholar] [CrossRef]
- Ostermann, P.A.; Seligson, D.; Henry, S.L. Local antibiotic therapy for severe open fractures. A review of 1085 consecutive cases. J. Bone Jt. Surg. Br. Vol. 1995, 77, 93–97. [Google Scholar] [CrossRef]
- Ferguson, J.Y.; Dudareva, M.; Riley, N.D.; Stubbs, D.; Atkins, B.L.; McNally, M.A. The use of a biodegradable antibiotic-loaded calcium sulphate carrier containing tobramycin for the treatment of chronic osteomyelitis: A series of 195 cases. Bone Jt. J. 2014, 96, 829–836. [Google Scholar] [CrossRef]
- Keating, J.F.; Blachut, P.A.; O’Brien, P.J.; Meek, R.N.; Broekhuyse, H. Reamed nailing of open tibial fractures: Does the antibiotic bead pouch reduce the deep infection rate? J. Orthop. Trauma 1996, 10, 298–303. [Google Scholar] [CrossRef]
- Xu, H.H.K.; Wang, P.; Wang, L.; Bao, C.; Chen, Q.; Weir, M.D.; Chow, L.C.; Zhao, L.; Zhou, X.; Reynolds, M.A. Calcium phosphate cements for bone engineering and their biological properties. Bone Res. 2017, 5, 17056. [Google Scholar] [CrossRef]
- Jeong, J.; Kim, J.H.; Shim, J.H.; Hwang, N.S.; Heo, C.Y. Bioactive calcium phosphate materials and applications in bone regeneration. Biomater. Res. 2019, 23, 4. [Google Scholar] [CrossRef]
- Szwed-Georgiou, A.; Płociński, P.; Kupikowska-Stobba, B.; Urbaniak, M.M.; Rusek-Wala, P.; Szustakiewicz, K.; Piszko, P.; Krupa, A.; Biernat, M.; Gazińska, M. Bioactive materials for bone regeneration: Biomolecules and delivery systems. ACS Biomater. Sci. Eng. 2023, 9, 5222–5254. [Google Scholar] [CrossRef]
- Hou, X.; Zhang, L.; Zhou, Z.; Luo, X.; Wang, T.; Zhao, X.; Lu, B.; Chen, F.; Zheng, L. Calcium phosphate-based biomaterials for bone repair. J. Funct. Biomater. 2022, 13, 187. [Google Scholar] [CrossRef]
- Zhao, D.; Huang, S.; Lu, F.; Wang, B.; Yang, L.; Qin, L.; Yang, K.; Li, Y.; Li, W.; Wang, W. Vascularized bone grafting fixed by biodegradable magnesium screw for treating osteonecrosis of the femoral head. Biomaterials 2016, 81, 84–92. [Google Scholar] [CrossRef]
- Sun, J.; Li, Z.; Liu, S.; Xia, T.; Shen, J. Biodegradable magnesium screw, titanium screw and direct embedding fixation in pedicled vascularized iliac bone graft transfer for osteonecrosis of the femoral head: A randomized controlled study. J. Orthop. Surg. Res. 2023, 18, 523. [Google Scholar] [CrossRef]
- Chevalier, J.; Gremillard, L. Ceramics for medical applications: A picture for the next 20 years. J. Eur. Ceram. Soc. 2009, 29, 1245–1255. [Google Scholar] [CrossRef]
- Rey, C.; Combes, C.; Drouet, C.; Glimcher, M.J. Bone mineral: Update on chemical composition and structure. Osteoporos. Int. 2009, 20, 1013–1021. [Google Scholar] [CrossRef]
- Fihri, A.; Len, C.; Varma, R.S.; Solhy, A. Hydroxyapatite: A review of syntheses, structure and applications in heterogeneous catalysis. Coord. Chem. Rev. 2017, 347, 48–76. [Google Scholar] [CrossRef]
- Barrère, F.; van Blitterswijk, C.A.; de Groot, K. Bone regeneration: Molecular and cellular interactions with calcium phosphate ceramics. Int. J. Nanomed. 2006, 1, 317. [Google Scholar]
- Shuai, C.; Li, P.; Liu, J.; Peng, S. Optimization of TCP/HAP ratio for better properties of calcium phosphate scaffold via selective laser sintering. Mater. Charact. 2013, 77, 23–31. [Google Scholar] [CrossRef]
- Boyan, B.D.; McMillan, J.; Lohmann, C.H.; Ranly, D.M.; Schwartz, Z. Basic information for successful clinical use with special focus on synthetic graft substitutes. In Bone Graft Substitutes; ASTM International: Philadelphia, PA, USA, 2002; pp. 231–259. [Google Scholar]
- Suchanek, W.; Yoshimura, M. Processing and properties of hydroxyapatite-based biomaterials for use as hard tissue replacement implants. J. Mater. Res. 1998, 13, 94–117. [Google Scholar] [CrossRef]
- Hing, K.A.; Best, S.M.; Tanner, K.E.; Bonfield, W.; Revell, P.A. Quantification of bone ingrowth within bone-derived porous hydroxyapatite implants of varying density. J. Mater. Sci. Mater. Med. 1999, 10, 663–670. [Google Scholar] [CrossRef]
- Gao, C.; Peng, S.; Feng, P.; Shuai, C. Bone biomaterials and interactions with stem cells. Bone Res. 2017, 5, 17059. [Google Scholar] [CrossRef]
- Mucalo, M.R. Animal-bone derived hydroxyapatite in biomedical applications. In Hydroxyapatite (HAp) for Biomedical Applications; Elsevier: Amsterdam, The Netherlands, 2015; pp. 307–342. [Google Scholar]
- Paul, W.; Sharma, C.P. Development of porous spherical hydroxyapatite granules: Application towards protein delivery. J. Mater. Sci. Mater. Med. 1999, 10, 383–388. [Google Scholar] [CrossRef]
- Weinlander, M.; Plenk, H., Jr.; Adar, F.; Holmes, R. TCP[Tricalcium Phosphate] Impurities in HA-Granules and Crystallinity Changes in Plasma Flame Sprayed HA-Coatings Detected by Spectroscopical Methods and Their Consequences. In Bioceramics and the Human Body; Springer, Dordrecht, The Netherlands, 1991.
- Siddiqui, H.A.; Pickering, K.L.; Mucalo, M.R. A review on the use of hydroxyapatite-carbonaceous structure composites in bone replacement materials for strengthening purposes. Materials 2018, 11, 1813. [Google Scholar] [CrossRef]
- Orlovskii, V.P.; Komlev, V.S.; Barinov, S.M. Hydroxyapatite and hydroxyapatite-based ceramics. Inorg. Mater. 2002, 38, 973–984. [Google Scholar] [CrossRef]
- Yamamoto, M.; Tabata, Y.; Kawasaki, H.; Ikada, Y. Promotion of fibrovascular tissue ingrowth into porous sponges by basic fibroblast growth factor. J. Mater. Sci. Mater. Med. 2000, 11, 213–218. [Google Scholar] [CrossRef] [PubMed]
- He, X.; Li, Y.; Zou, D.; Zu, H.; Li, W.; Zheng, Y. An overview of magnesium-based implants in orthopaedics and a prospect of its application in spine fusion. Bioact. Mater. 2024, 39, 456–478. [Google Scholar] [CrossRef] [PubMed]
- Yuan, Z.; Wan, Z.; Gao, C.; Wang, Y.; Huang, J.; Cai, Q. Controlled magnesium ion delivery system for in situ bone tissue engineering. J. Control. Release 2022, 350, 360–376. [Google Scholar] [CrossRef] [PubMed]
- Farraro, K.F.; Kim, K.E.; Woo, S.L.Y.; Flowers, J.R.; McCullough, M.B. Revolutionizing orthopaedic biomaterials: The potential of biodegradable and bioresorbable magnesium-based materials for functional tissue engineering. J. Biomech. 2014, 47, 1979–1986. [Google Scholar] [CrossRef]
- Wang, J.L.; Xu, J.K.; Hopkins, C.; Chow, D.H.K.; Qin, L. Biodegradable magnesium-based implants in orthopedics—A general review and perspectives. Adv. Sci. 2020, 7, 1902443. [Google Scholar] [CrossRef] [PubMed]
- Luo, Y.; Liu, F.; Chen, Z.; Luo, Y.; Li, W.; Wang, J. A magnesium screw with optimized geometry exhibits improved corrosion resistance and favors bone fracture healing. Acta Biomater. 2024, 178, 320–329. [Google Scholar] [CrossRef] [PubMed]
- Chaya, A.; Yoshizawa, S.; Verdelis, K.; Myers, N.; Costello, B.J.; Chou, D.-T.; Pal, S.; Maiti, S.; Kumta, P.N.; Sfeir, C. In vivo study of magnesium plate and screw degradation and bone fracture healing. Acta Biomater. 2015, 18, 262–269. [Google Scholar] [CrossRef]
- Jung, O.; Hesse, B.; Stojanovic, S.; Seim, C.; Weitkamp, T.; Batinic, M.; Goerke, O.; Kačarević, Ž.P.; Rider, P.; Najman, S. Biocompatibility analyses of HF-passivated magnesium screws for guided bone regeneration (GBR). Int. J. Mol. Sci. 2021, 22, 12567. [Google Scholar] [CrossRef]
- Diekmann, J.; Bauer, S.; Weizbauer, A.; Willbold, E.; Windhagen, H.; Helmecke, P.; Lucas, A.; Reifenrath, J.; Nolte, I.; Ezechieli, M. Examination of a biodegradable magnesium screw for the reconstruction of the anterior cruciate ligament: A pilot in vivo study in rabbits. Mater. Sci. Eng. C 2016, 59, 1100–1109. [Google Scholar] [CrossRef]
- Hench, L.L. The story of Bioglass®. J. Mater. Sci. Mater. Med. 2006, 17, 967–978. [Google Scholar] [CrossRef]
- Pereira, H.F.; Cengiz, I.F.; Silva, F.S.; Reis, R.L.; Oliveira, J.M. Scaffolds and coatings for bone regeneration. J. Mater. Sci. Mater. Med. 2020, 31, 27. [Google Scholar] [CrossRef]
- Hench, L.L.; Splinter, R.J.; Allen, W.C.; Greenlee, T.K. Bonding mechanisms at the interface of ceramic prosthetic materials. J. Biomed. Mater. Res. 1971, 5, 117–141. [Google Scholar] [CrossRef]
- Baino, F.; Hamzehlou, S.; Kargozar, S. Bioactive glasses: Where are we and where are we going? J. Funct. Biomater. 2018, 9, 25. [Google Scholar] [CrossRef]
- Pantulap, U.; Arango-Ospina, M.; Boccaccini, A.R. Bioactive glasses incorporating less-common ions to improve biological and physical properties. J. Mater. Sci. Mater. Med. 2022, 33, 3. [Google Scholar] [CrossRef] [PubMed]
- El-Rashidy, A.A.; Roether, J.A.; Harhaus, L.; Kneser, U.; Boccaccini, A.R. Regenerating bone with bioactive glass scaffolds: A review of in vivo studies in bone defect models. Acta Biomater. 2017, 62, 1–28. [Google Scholar] [CrossRef]
- Zhang, J.; Guan, J.; Zhang, C.; Wang, H.; Huang, W.; Guo, S.; Niu, X.; Xie, Z.; Wang, Y. Bioactive borate glass promotes the repair of radius segmental bone defects by enhancing the osteogenic differentiation of BMSCs. Biomed. Mater. 2015, 10, 065011. [Google Scholar] [CrossRef]
- Battafarano, G.; Rossi, M.; De Martino, V.; Marampon, F.; Borro, L.; Secinaro, A.; Del Fattore, A. Strategies for bone regeneration: From graft to tissue engineering. Int. J. Mol. Sci. 2021, 22, 1128. [Google Scholar] [CrossRef] [PubMed]
- Kaur, S.; Dhillon, G.S. The versatile biopolymer chitosan: Potential sources, evaluation of extraction methods and applications. Crit. Rev. Microbiol. 2014, 40, 155–175. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gómez, C.P.; Cecilia, J.A. Chitosan: A natural biopolymer with a wide and varied range of applications. Molecules 2020, 25, 3981. [Google Scholar] [CrossRef]
- Eze, F.N.; Jayeoye, T.J.; Singh, S. Fabrication of intelligent pH-sensing films with antioxidant potential for monitoring shrimp freshness via the fortification of chitosan matrix with broken Riceberry phenolic extract. Food Chem. 2022, 366, 130574. [Google Scholar] [CrossRef]
- Schmitz, C.; González Auza, L.; Koberidze, D.; Rasche, S.; Fischer, R.; Bortesi, L. Conversion of chitin to defined chitosan oligomers: Current status and future prospects. Mar. Drugs 2019, 17, 452. [Google Scholar] [CrossRef]
- Muxika, A.; Etxabide, A.; Uranga, J.; Guerrero, P.; De La Caba, K. Chitosan as a bioactive polymer: Processing, properties and applications. Int. J. Biol. Macromol. 2017, 105, 1358–1368. [Google Scholar] [CrossRef]
- Sukpaita, T.; Chirachanchai, S.; Pimkhaokham, A.; Ampornaramveth, R.S. Chitosan-based scaffold for mineralized tissues regeneration. Mar. Drugs 2021, 19, 551. [Google Scholar] [CrossRef] [PubMed]
- Di Martino, A.; Sittinger, M.; Risbud, M.V. Chitosan: A versatile biopolymer for orthopaedic tissue-engineering. Biomaterials 2005, 26, 5983–5990. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Ding, J.; Zhuang, X.; Chang, F.; Wang, J.; Chen, X. Chitosan-based scaffolds for cartilage regeneration. In Chitin and Chitosan for Regenerative Medicine; Springer: New Delhi, India, 2016; pp. 61–82. [Google Scholar]
- Yuan, Y.; Chesnutt, B.M.; Haggard, W.O.; Bumgardner, J.D. Deacetylation of chitosan: Material characterization and in vitro evaluation via albumin adsorption and pre-osteoblastic cell cultures. Materials 2011, 4, 1399–1416. [Google Scholar] [CrossRef]
- Guo, L.; Liang, Z.; Yang, L.; Du, W.; Yu, T.; Tang, H.; Li, C.; Qiu, H. The role of natural polymers in bone tissue engineering. J. Control. Release 2021, 338, 571–582. [Google Scholar] [CrossRef] [PubMed]
- Miyata, T.; Taira, T.; Noishiki, Y. Collagen engineering for biomaterial use. Clin. Mater. 1992, 9, 139–148. [Google Scholar] [CrossRef]
- Irastorza, A.; Zarandona, I.; Andonegi, M.; Guerrero, P.; de la Caba, K. The versatility of collagen and chitosan: From food to biomedical applications. Food Hydrocoll. 2021, 116, 106633. [Google Scholar] [CrossRef]
- Gelse, K.; Pöschl, E.; Aigner, T. Collagens—Structure, function, and biosynthesis. Adv. Drug Deliv. Rev. 2003, 55, 1531–1546. [Google Scholar] [CrossRef]
- Kirkness, M.W.H.; Lehmann, K.; Forde, N.R. Mechanics and structural stability of the collagen triple helix. Curr. Opin. Chem. Biol. 2019, 53, 98–105. [Google Scholar] [CrossRef]
- Chowdhury, S.R.; Mh Busra, M.F.; Lokanathan, Y.; Ng, M.H.; Law, J.X.; Cletus, U.C.; Binti Haji Idrus, R. Collagen type I: A versatile biomaterial. In Novel Biomaterials for Regenerative Medicine; Springer: Singapore, 2018; pp. 389–414. [Google Scholar]
- Sarrigiannidis, S.O.; Rey, J.M.; Dobre, O.; González-García, C.; Dalby, M.J.; Salmeron-Sanchez, M. A tough act to follow: Collagen hydrogel modifications to improve mechanical and growth factor loading capabilities. Mater. Today Bio 2021, 10, 100098. [Google Scholar] [CrossRef]
- Elango, J.; Hou, C.; Bao, B.; Wang, S.; Maté Sánchez de Val, J.E.; Wenhui, W. The molecular interaction of collagen with cell receptors for biological function. Polymers 2022, 14, 876. [Google Scholar] [CrossRef]
- Aoki, K.; Ideta, H.; Komatsu, Y.; Tanaka, A.; Kito, M.; Okamoto, M.; Takahashi, J.; Suzuki, S.; Saito, N. Bone-Regeneration Therapy Using Biodegradable Scaffolds: Calcium Phosphate Bioceramics and Biodegradable Polymers. Bioengineering 2024, 11, 180. [Google Scholar] [CrossRef] [PubMed]
- Baghersad, S.; Bolandi, B.; Imani, R.; Afaghi, S.; Davoudinia, S. An Overview of PRP-Delivering Scaffolds for Bone and Cartilage Tissue Engineering. J. Bionic Eng. 2024, 21, 674–693. [Google Scholar]
- Honvo, G.; Lengelé, L.; Charles, A.; Reginster, J.-Y.; Bruyère, O. Role of collagen derivatives in osteoarthritis and cartilage repair: A systematic scoping review with evidence mapping. Rheumatol. Ther. 2020, 7, 703–740. [Google Scholar]
- Cao, L.; Zhang, Z.; Yuan, D.; Yu, M.; Min, J. Tissue engineering applications of recombinant human collagen: A review of recent progress. Front. Bioeng. Biotechnol. 2024, 12, 1358246. [Google Scholar]
- Fernandes-Cunha, G.M.; Chen, K.M.; Chen, F.; Le, P.; Han, J.H.; Mahajan, L.A.; Lee, H.J.; Na, K.S.; Myung, D. In situ-forming collagen hydrogel crosslinked via multi-functional PEG as a matrix therapy for corneal defects. Sci. Rep. 2020, 10, 16671. [Google Scholar] [CrossRef]
- Chen, Y.; Kawazoe, N.; Chen, G. Preparation of dexamethasone-loaded biphasic calcium phosphate nanoparticles/collagen porous composite scaffolds for bone tissue engineering. Acta Biomater. 2018, 67, 341–353. [Google Scholar] [PubMed]
- Jäger, M.; Herten, M.; Fochtmann, U.; Fischer, J.; Hernigou, P.; Zilkens, C.; Hendrich, C.; Krauspe, R. Bridging the gap: Bone marrow aspiration concentrate reduces autologous bone grafting in osseous defects. J. Orthop. Res. 2011, 29, 173–180. [Google Scholar] [CrossRef]
- Govender, S.; Csimma, C.; Genant, H.K.; Valentin-Opran, A.; Amit, Y.; Arbel, R.; Aro, H.; Atar, D.; Bishay, M.; Börner, M.G. Recombinant human bone morphogenetic protein-2 for treatment of open tibial fractures: A prospective, controlled, randomized study of four hundred and fifty patients. J. Bone Jt. Surg. 2002, 84, 2123–2134. [Google Scholar]
- Calori, G.M.; Tagliabue, L.; Gala, L.; d’Imporzano, M.; Peretti, G.; Albisetti, W. Application of rhBMP-7 and platelet-rich plasma in the treatment of long bone non-unions: A prospective randomised clinical study on 120 patients. Injury 2008, 39, 1391–1402. [Google Scholar]
- Rico-Llanos, G.A.; Borrego-González, S.; Moncayo-Donoso, M.; Becerra, J.; Visser, R. Collagen type I biomaterials as scaffolds for bone tissue engineering. Polymers 2021, 13, 599. [Google Scholar] [CrossRef]
- Stender, E.G.P.; Andersen, C.D.; Fredslund, F.; Holck, J.; Solberg, A.; Teze, D.; Peters, G.H.J.; Christensen, B.E.; Aachmann, F.L.; Welner, D.H. Structural and functional aspects of mannuronic acid–specific PL6 alginate lyase from the human gut microbe Bacteroides cellulosilyticus. J. Biol. Chem. 2019, 294, 17915–17930. [Google Scholar] [CrossRef] [PubMed]
- Hasany, M.; Talebian, S.; Sadat, S.; Ranjbar, N.; Mehrali, M.; Wallace, G.G.; Mehrali, M. Synthesis, properties, and biomedical applications of alginate methacrylate (ALMA)-based hydrogels: Current advances and challenges. Appl. Mater. Today 2021, 24, 101150. [Google Scholar] [CrossRef]
- Lee, K.Y.; Mooney, D.J. Alginate: Properties and biomedical applications. Prog. Polym. Sci. 2012, 37, 106–126. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Tan, H. Alginate-based biomaterials for regenerative medicine applications. Materials 2013, 6, 1285–1309. [Google Scholar] [CrossRef]
- Dodero, A.; Pianella, L.; Vicini, S.; Alloisio, M.; Ottonelli, M.; Castellano, M. Alginate-based hydrogels prepared via ionic gelation: An experimental design approach to predict the crosslinking degree. Eur. Polym. J. 2019, 118, 586–594. [Google Scholar] [CrossRef]
- Hu, C.; Lu, W.; Mata, A.; Nishinari, K.; Fang, Y. Ions-induced gelation of alginate: Mechanisms and applications. Int. J. Biol. Macromol. 2021, 177, 578–588. [Google Scholar] [CrossRef]
- Brus, J.; Urbanova, M.; Czernek, J.; Pavelkova, M.; Kubova, K.; Vyslouzil, J.; Abbrent, S.; Konefal, R.; Horsky, J.; Vetchy, D. Structure and dynamics of alginate gels cross-linked by polyvalent ions probed via solid state NMR spectroscopy. Biomacromolecules 2017, 18, 2478–2488. [Google Scholar] [CrossRef]
- Farshidfar, N.; Iravani, S.; Varma, R.S. Alginate-based biomaterials in tissue engineering and regenerative medicine. Mar. Drugs 2023, 21, 189. [Google Scholar] [CrossRef]
- Lertwimol, T.; Sonthithai, P.; Hankamolsiri, W.; Kaewkong, P.; Uppanan, P. Development of chondrocyte-laden alginate hydrogels with modulated microstructure and properties for cartilage regeneration. Biotechnol. Prog. 2023, 39, e3322. [Google Scholar] [CrossRef]
- Łabowska, M.B.; Cierluk, K.; Jankowska, A.M.; Kulbacka, J.; Detyna, J.; Michalak, I. A review on the adaption of alginate-gelatin hydrogels for 3D cultures and bioprinting. Materials 2021, 14, 858. [Google Scholar] [CrossRef]
- Swetha, T.A.; Ananthi, V.; Bora, A.; Sengottuvelan, N.; Ponnuchamy, K.; Muthusamy, G.; Arun, A. A review on biodegradable polylactic acid (PLA) production from fermentative food waste-Its applications and degradation. Int. J. Biol. Macromol. 2023, 234, 123703. [Google Scholar]
- Taib, N.-A.A.B.; Rahman, M.R.; Huda, D.; Kuok, K.K.; Hamdan, S.; Bakri, M.K.B.; Julaihi, M.R.M.B.; Khan, A. A review on poly lactic acid (PLA) as a biodegradable polymer. Polym. Bull. 2023, 80, 1179–1213. [Google Scholar] [CrossRef]
- Shekhar, N.; Mondal, A. Synthesis, properties, environmental degradation, processing, and applications of Polylactic Acid (PLA): An overview. Polym. Bull. 2024, 81, 11421–11457. [Google Scholar]
- Khouri, N.G.; Bahú, J.O.; Blanco-Llamero, C.; Severino, P.; Concha, V.O.C.; Souto, E.B. Polylactic Acid (PLA): Properties, Synthesis, and Biomedical Applications-A Review of the Literature. J. Mol. Struct. 2024, 1309, 138243. [Google Scholar]
- Casalini, T.; Rossi, F.; Castrovinci, A.; Perale, G. A perspective on polylactic acid-based polymers use for nanoparticles synthesis and applications. Front. Bioeng. Biotechnol. 2019, 7, 259. [Google Scholar]
- Worch, J.C.; Prydderch, H.; Jimaja, S.; Bexis, P.; Becker, M.L.; Dove, A.P. Stereochemical enhancement of polymer properties. Nat. Rev. Chem. 2019, 3, 514–535. [Google Scholar]
- Farah, S.; Anderson, D.G.; Langer, R. Physical and mechanical properties of PLA, and their functions in widespread applications—A comprehensive review. Adv. Drug Deliv. Rev. 2016, 107, 367–392. [Google Scholar]
- Zhao, X.; Liu, J.; Li, J.; Liang, X.; Zhou, W.; Peng, S. Strategies and techniques for improving heat resistance and mechanical performances of poly (lactic acid)(PLA) biodegradable materials. Int. J. Biol. Macromol. 2022, 218, 115–134. [Google Scholar] [CrossRef]
- Ranakoti, L.; Gangil, B.; Mishra, S.K.; Singh, T.; Sharma, S.; Ilyas, R.A.; El-Khatib, S. Critical review on polylactic acid: Properties, structure, processing, biocomposites, and nanocomposites. Materials 2022, 15, 4312. [Google Scholar] [CrossRef]
- Narayanan, G.; Vernekar, V.N.; Kuyinu, E.L.; Laurencin, C.T. Poly (lactic acid)-based biomaterials for orthopaedic regenerative engineering. Adv. Drug Deliv. Rev. 2016, 107, 247–276. [Google Scholar]
- Gorrasi, G.; Pantani, R. Hydrolysis and Biodegradation of Poly (lactic acid). In Synthesis, Structure and Properties of Poly (Lactic Acid); Springer: Cham, Switzerland, 2018; pp. 119–151. [Google Scholar]
- Teixeira, S.; Eblagon, K.M.; Miranda, F.R.; Pereira, M.F.; Figueiredo, J.L. Towards controlled degradation of poly (lactic) acid in technical applications. C 2021, 7, 42. [Google Scholar] [CrossRef]
- Christen, M.O.; Vercesi, F. Polycaprolactone: How a well-known and futuristic polymer has become an innovative collagen-stimulator in esthetics. Clin. Cosmet. Investig. Dermatol. 2020, 13, 31–48. [Google Scholar] [CrossRef]
- Bartnikowski, M.; Dargaville, T.R.; Ivanovski, S.; Hutmacher, D.W. Degradation mechanisms of polycaprolactone in the context of chemistry, geometry and environment. Prog. Polym. Sci. 2019, 96, 1–20. [Google Scholar] [CrossRef]
- Kaluzynski, K.; Pretula, J.; Lewinski, P.; Kaźmierski, S.; Penczek, S. Synthesis and properties of functionalized poly (ε-caprolactone); chain polymerization followed by polycondensation in one pot with initiator and catalyst in one molecule. synthesis and molecular structures. Macromolecules 2022, 55, 2210–2221. [Google Scholar] [CrossRef]
- Jeon, H.; Kim, M.-S.; Park, S.B.; Kim, S.; Lee, M.; Park, S.-A.; Hwang, S.Y.; Koo, J.M.; Oh, D.X.; Park, J. Improved mechanical properties of biodegradable polycaprolactone nanocomposites prepared using cellulose nanocrystals. Cellulose 2023, 30, 11561–11574. [Google Scholar] [CrossRef]
- Zhang, Y.; Wu, Y.; Yang, M.; Zhang, G.; Ju, H. Thermal stability and dynamic mechanical properties of poly (ε-caprolactone)/chitosan composite membranes. Materials 2021, 14, 5538. [Google Scholar] [CrossRef]
- Mabrouk, M.; Beherei, H.H.; Das, D.B. Recent progress in the fabrication techniques of 3D scaffolds for tissue engineering. Mater. Sci. Eng. C 2020, 110, 110716. [Google Scholar] [CrossRef]
- Joseph, B.; Augustine, R.; Kalarikkal, N.; Thomas, S.; Seantier, B.; Grohens, Y. Recent advances in electrospun polycaprolactone based scaffolds for wound healing and skin bioengineering applications. Mater. Today Commun. 2019, 19, 319–335. [Google Scholar] [CrossRef]
- Azari, A.; Golchin, A.; Maymand, M.M.; Mansouri, F.; Ardeshirylajimi, A. Electrospun polycaprolactone nanofibers: Current research and applications in biomedical application. Adv. Pharm. Bull. 2022, 12, 658. [Google Scholar] [CrossRef]
- Leroux, A.; Ngoc Nguyen, T.; Rangel, A.; Cacciapuoti, I.; Duprez, D.; Castner, D.G.; Migonney, V. Long-term hydrolytic degradation study of polycaprolactone films and fibers grafted with poly (sodium styrene sulfonate): Mechanism study and cell response. Biointerphases 2020, 15, 061006. [Google Scholar] [CrossRef]
- Dwivedi, R.; Kumar, S.; Pandey, R.; Mahajan, A.; Nandana, D.; Katti, D.S.; Mehrotra, D. Polycaprolactone as biomaterial for bone scaffolds: Review of literature. J. Oral Biol. Craniofacial Res. 2020, 10, 381–388. [Google Scholar]
- Raina, N.; Pahwa, R.; Khosla, J.K.; Gupta, P.N.; Gupta, M. Polycaprolactone-based materials in wound healing applications. Polym. Bull. 2021, 79, 7041–7063. [Google Scholar]
- Soni, S.K.; Thomas, B.; Kar, V.R. A comprehensive review on CNTs and CNT-reinforced composites: Syntheses, characteristics and applications. Mater. Today Commun. 2020, 25, 101546. [Google Scholar]
- Nurazzi, N.M.; Sabaruddin, F.A.; Harussani, M.M.; Kamarudin, S.H.; Rayung, M.; Asyraf, M.R.M.; Aisyah, H.A.; Norrrahim, M.N.F.; Ilyas, R.A.; Abdullah, N. Mechanical performance and applications of CNTs reinforced polymer composites—A review. Nanomaterials 2021, 11, 2186. [Google Scholar] [CrossRef] [PubMed]
- de Almeida Barcelos, K.; Garg, J.; Soares, D.C.F.; de Barros, A.L.B.; Zhao, Y.; Alisaraie, L. Recent advances in the applications of CNT-based nanomaterials in pharmaceutical nanotechnology and biomedical engineering. J. Drug Deliv. Sci. Technol. 2023, 87, 104834. [Google Scholar]
- Huang, B. Carbon nanotubes and their polymeric composites: The applications in tissue engineering. Biomanufacturing Rev. 2020, 5, 3. [Google Scholar] [CrossRef]
- Choi, C.; Yun, T.G.; Hwang, B. Dispersion Stability of Carbon Nanotubes and Their Impact on Energy Storage Devices. Inorganics 2023, 11, 383. [Google Scholar] [CrossRef]
- Lavagna, L.; Nisticò, R.; Musso, S.; Pavese, M. Functionalization as a way to enhance dispersion of carbon nanotubes in matrices: A review. Mater. Today Chem. 2021, 20, 100477. [Google Scholar] [CrossRef]
- Benko, A.; Duch, J.; Gajewska, M.; Marzec, M.; Bernasik, A.; Nocuń, M.; Piskorz, W.; Kotarba, A. Covalently bonded surface functional groups on carbon nanotubes: From molecular modeling to practical applications. Nanoscale 2021, 13, 10152–10166. [Google Scholar] [CrossRef]
- Guiseppi-Elie, A.; Rahman, A.R.A.; Shukla, N.K. SAM-modified microdisc electrode arrays (MDEAs) with functionalized carbon nanotubes. Electrochim. Acta 2010, 55, 4247–4255. [Google Scholar]
- Chen, C.; Bai, X.; Ding, Y.; Lee, I.-S. Electrical stimulation as a novel tool for regulating cell behavior in tissue engineering. Biomater. Res. 2019, 23, 25. [Google Scholar] [CrossRef]
- Cheng, H.; Huang, Y.; Yue, H.; Fan, Y. Electrical stimulation promotes stem cell neural differentiation in tissue engineering. Stem Cells Int. 2021, 2021, 6697574. [Google Scholar] [CrossRef] [PubMed]
- Lovat, V.; Pantarotto, D.; Lagostena, L.; Cacciari, B.; Grandolfo, M.; Righi, M.; Spalluto, G.; Prato, M.; Ballerini, L. Carbon nanotube substrates boost neuronal electrical signaling. Nano Lett. 2005, 5, 1107–1110. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.-J.; Zhu, W.; Nowicki, M.; Lee, G.; Heo, D.N.; Kim, J.; Zuo, Y.Y.; Zhang, L.G. 3D printing nano conductive multi-walled carbon nanotube scaffolds for nerve regeneration. J. Neural Eng. 2018, 15, 016018. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.-R.; Ryu, S.; Kim, S.; Kim, B.-S. Behaviors of stem cells on carbon nanotube. Biomater. Res. 2015, 19, 3. [Google Scholar] [CrossRef]
- Kim, J.; Kim, H.D.; Park, J.; Lee, E.-s.; Kim, E.; Lee, S.S.; Yang, J.-K.; Lee, Y.-S.; Hwang, N.S. Enhanced osteogenic commitment of murine mesenchymal stem cells on graphene oxide substrate. Biomater. Res. 2018, 22, 1. [Google Scholar] [CrossRef]
- Zhang, L.L.; Zhao, X.S. Carbon-based materials as supercapacitor electrodes. Chem. Soc. Rev. 2009, 38, 2520–2531. [Google Scholar] [CrossRef]
- Zare, H.; Ahmadi, S.; Ghasemi, A.; Ghanbari, M.; Rabiee, N.; Bagherzadeh, M.; Karimi, M.; Webster, T.J.; Hamblin, M.R.; Mostafavi, E. Carbon nanotubes: Smart drug/gene delivery carriers. Int. J. Nanomed. 2021, 16, 1681–1706. [Google Scholar] [CrossRef]
- Vardharajula, S.; Ali, S.Z.; Tiwari, P.M.; Eroğlu, E.; Vig, K.; Dennis, V.A.; Singh, S.R. Functionalized carbon nanotubes: Biomedical applications. Int. J. Nanomed. 2012, 7, 5361–5374. [Google Scholar]
- Trzeciak, T.; Rybka, J.D.; Richter, M.; Kaczmarczyk, J.; Ramalingam, M.; Giersig, M. Cells and nanomaterial-based tissue engineering techniques in the treatment of bone and cartilage injuries. J. Nanosci. Nanotechnol. 2016, 16, 8948–8952. [Google Scholar] [CrossRef]
- Liu, H.; Chen, J.; Qiao, S.; Zhang, W. Carbon-based nanomaterials for bone and cartilage regeneration: A review. ACS Biomater. Sci. Eng. 2021, 7, 4718–4735. [Google Scholar] [CrossRef] [PubMed]
- Sonowal, L.; Gautam, S. Advancements and challenges in carbon nanotube-based drug delivery systems. Nano-Struct. Nano-Objects 2024, 38, 101117. [Google Scholar] [CrossRef]
- Ijaz, H.; Mahmood, A.; Abdel-Daim, M.M.; Sarfraz, R.M.; Zaman, M.; Zafar, N.; Alshehery, S.; Salem-Bekhit, M.M.; Ali, M.A.; Eltayeb, L.B. Review on carbon nanotubes (CNTs) and their chemical and physical characteristics, with particular emphasis on potential applications in biomedicine. Inorg. Chem. Commun. 2023, 155, 111020. [Google Scholar] [CrossRef]
- Murjani, B.O.; Kadu, P.S.; Bansod, M.; Vaidya, S.S.; Yadav, M.D. Carbon nanotubes in biomedical applications: Current status, promises, and challenges. Carbon Lett. 2022, 32, 1207–1226. [Google Scholar] [CrossRef]
- Krishnaveni, R.; Roobadoss, M.N.; Kumaran, S.; Kumar, A.A.; Geetha, K. Carbon Nanotubes in Regenerative Medicine. In Handbook of Carbon Nanotubes; Springer: Berlin/Heidelberg, Germany, 2022; pp. 1687–1737. [Google Scholar]
- Paratala, B.S.; Sitharaman, B. Carbon Nanotubes in Regenerative Medicine. Carbon Nanotubes for Biomedical Applications; Springer: Berlin/Heidelberg, Germany, 2011; pp. 27–39. [Google Scholar]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Santoro, A.; Voto, A.; Fortino, L.; Guida, R.; Laudisio, C.; Cillo, M.; D’Ursi, A.M. Bone Defect Treatment in Regenerative Medicine: Exploring Natural and Synthetic Bone Substitutes. Int. J. Mol. Sci. 2025, 26, 3085. https://doi.org/10.3390/ijms26073085
Santoro A, Voto A, Fortino L, Guida R, Laudisio C, Cillo M, D’Ursi AM. Bone Defect Treatment in Regenerative Medicine: Exploring Natural and Synthetic Bone Substitutes. International Journal of Molecular Sciences. 2025; 26(7):3085. https://doi.org/10.3390/ijms26073085
Chicago/Turabian StyleSantoro, Angelo, Andrea Voto, Luigi Fortino, Raffaella Guida, Carolina Laudisio, Mariarosaria Cillo, and Anna Maria D’Ursi. 2025. "Bone Defect Treatment in Regenerative Medicine: Exploring Natural and Synthetic Bone Substitutes" International Journal of Molecular Sciences 26, no. 7: 3085. https://doi.org/10.3390/ijms26073085
APA StyleSantoro, A., Voto, A., Fortino, L., Guida, R., Laudisio, C., Cillo, M., & D’Ursi, A. M. (2025). Bone Defect Treatment in Regenerative Medicine: Exploring Natural and Synthetic Bone Substitutes. International Journal of Molecular Sciences, 26(7), 3085. https://doi.org/10.3390/ijms26073085







