Unveiling Matrix Metalloproteinase 13’s Dynamic Role in Breast Cancer: A Link to Physical Changes and Prognostic Modulation
Abstract
1. Introduction
2. Physical Characteristics and Prognosis of Breast Cancer
2.1. Stiffness Is an Indicator of Poor Prognosis in Breast Cancer
2.2. Mechanical and Structural Characteristics Influence Breast Cancer Metastasis
2.3. Thermal Properties: Increased Angiogenesis and Metabolism Lead to High Temperature
3. MMP13 Is the Key to Regulating These Physical Properties
3.1. The Background of MMP13
3.2. Effect of MMP13 on Tumour Stiffness
3.3. Effect of MMP13 on Tumour Stromal Structure
3.4. Effect of MMP13 on Tumour Stromal Temperature
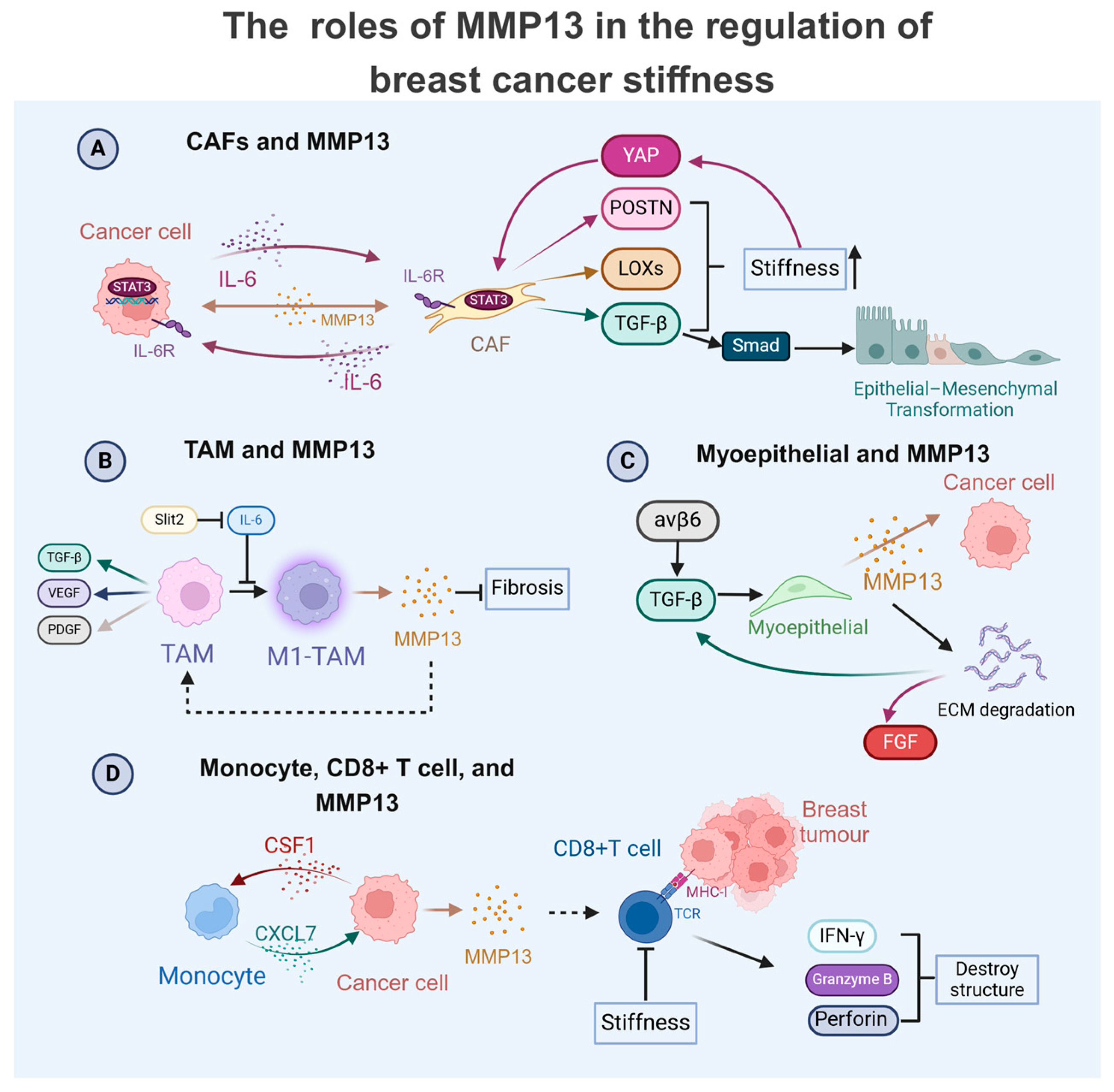
4. MMP13 as a Central Regulator in Breast Cancer TME: Crosstalk with Cells to Modulate Physical Properties
4.1. MMP13 in TAM-Mediated Breast Cancer Progression
4.2. MMP13 in CAF-Driven Breast Cancer Progression
4.3. MMP13 with Immune and Stromal Cells in Breast Cancer Progression
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BC | Breast cancer |
| ECM | Extracellular matrix |
| MMP13 | Matrix metalloproteinase 13 |
| TME | Tumour microenvironment |
| CAFs | Cancer-associated fibroblasts |
| TAMs | Tumour-associated macrophages |
| EMT | Epithelial–mesenchymal transition |
| CSCs | Cancer stem cells |
| TGF-β | Transforming growth factor-β |
| IL-6 | Interleukin-6 |
| YAP | Yes-associated protein |
| POSTN | Periostin |
| LOXs | Lysyl oxidase |
| STAT3 | Signal transducer and activator of transcription 3 |
| FGF | Fibroblast growth factor |
| DCIS | Ductal carcinoma in situ |
| IFN-γ | Interferon-γ |
| CSF1 | Colony-stimulating factor 1 |
| CXCL | CXC motif chemokine ligand |
| NF-κB | Nuclear factor κB |
| TNF-α | Tumour necrosis factor-α |
| VEGF | Vascular endothelial growth factor |
| HIF | Hypoxia inducible factor |
| AP-1 | Activator protein-1 |
| ETS/PEA-3 | E26 transformation-specific/polyomavirus enhancer activator-3 |
| ETV4 | ETS variant transcription factor 4 |
| TB | Tumour bone |
| ER | Oestrogen receptor |
| PNI | Perineural invasion |
| ILK | Integrin-linked kinase |
| TNBC | Triple-negative breast cancer |
| BCSCs | Breast cancer stem cells |
| SWE | Shear wave elastography |
| TACS | Tumour-associated collagen signature |
| BM | Basement membrane |
| STAT3 | Signal transducer and activator of transcription 3 |
| PDGF | Platelet-derived growth factor |
| bFGF/FGF2 | Basic fibroblast growth factor |
| NF1 | Neurofibromin 1 |
| ATF3 | Activating transcription factor 3 |
| NFATC2 | Nuclear factor of activated T cells 2 |
| Runx2 | Runt-related transcription factor 2 |
| ABL | Abelson tyrosine kinase |
| CLT | Codonolactone |
| RKIP | Raf kinase inhibitor protein |
| STEAP1 | Six transmembrane epithelial antigen of the prostate 1 |
| MSCs | Mesenchymal stem cells |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [PubMed]
- Valastyan, S.; Weinberg, R.A. Tumor metastasis: Molecular insights and evolving paradigms. Cell 2011, 147, 275–292. [Google Scholar]
- Park, M.; Kim, D.; Ko, S.; Kim, A.; Mo, K.; Yoon, H. Breast Cancer Metastasis: Mechanisms and Therapeutic Implications. Int. J. Mol. Sci. 2022, 23, 6806. [Google Scholar] [CrossRef]
- André, F.; Zielinski, C.C. Optimal strategies for the treatment of metastatic triple-negative breast cancer with currently approved agents. Ann. Oncol. 2012, 23 (Suppl. S6), vi46–vi51. [Google Scholar]
- Saraswathibhatla, A.; Indana, D.; Chaudhuri, O. Cell-extracellular matrix mechanotransduction in 3D. Nat. Rev. Mol. Cell Biol. 2023, 24, 495–516. [Google Scholar] [PubMed]
- Mancini, A.; Gentile, M.T.; Pentimalli, F.; Cortellino, S.; Grieco, M.; Giordano, A. Multiple aspects of matrix stiffness in cancer progression. Front. Oncol. 2024, 14, 1406644. [Google Scholar]
- Otsuka, K.; Yamamoto, Y.; Ochiya, T. Uncovering temperature-dependent extracellular vesicle secretion in breast cancer. J. Extracell. Vesicles 2020, 10, e12049. [Google Scholar]
- Esserman, L.J.; Thompson, I.M., Jr.; Reid, B. Overdiagnosis and overtreatment in cancer: An opportunity for improvement. Jama 2013, 310, 797–798. [Google Scholar]
- Akinpelu, A.; Akinsipe, T.; Avila, L.A.; Arnold, R.D.; Mistriotis, P. The impact of tumor microenvironment: Unraveling the role of physical cues in breast cancer progression. Cancer Metastasis Rev. 2024, 43, 823–844. [Google Scholar]
- Wang, H.; Li, X.; Xi, X.; Hu, B.; Zhao, L.; Liao, Y.; Tang, J. Effects of magnetic induction hyperthermia and radiotherapy alone or combined on a murine 4T1 metastatic breast cancer model. Int. J. Hyperth. 2011, 27, 563–572. [Google Scholar]
- Kessenbrock, K.; Plaks, V.; Werb, Z. Matrix metalloproteinases: Regulators of the tumor microenvironment. Cell 2010, 141, 52–67. [Google Scholar] [PubMed]
- Han, C.; Feng, Z.; Wang, Y.; Hu, M.; Xu, S.; Jiang, F.; Han, Y.; Liu, Z.; Li, Y. Copper metabolism-related signature for prognosis prediction and MMP13 served as malignant factor for breast cancer. Heliyon 2024, 10, e36445. [Google Scholar]
- Niland, S.; Eble, J.A. Hold on or Cut? Integrin- and MMP-Mediated Cell-Matrix Interactions in the Tumor Microenvironment. Int. J. Mol. Sci. 2020, 22, 238. [Google Scholar] [CrossRef] [PubMed]
- Howes, J.M.; Bihan, D.; Slatter, D.A.; Hamaia, S.W.; Packman, L.C.; Knauper, V.; Visse, R.; Farndale, R.W. The recognition of collagen and triple-helical toolkit peptides by MMP-13: Sequence specificity for binding and cleavage. J. Biol. Chem. 2014, 289, 24091–24101. [Google Scholar]
- Cuadriello, E.F.; Fernández-Guinea, Ó.; Eiró, N.; González, L.O.; Junquera, S.; Vizoso, F.J. Relationship between morphological features and kinetic patterns of enhancement of the dynamic breast magnetic resonance imaging and tumor expression of metalloproteases and their inhibitors in invasive breast cancer. Magn. Reson. Imaging 2016, 34, 1107–1113. [Google Scholar]
- Knäuper, V.; Smith, B.; López-Otin, C.; Murphy, G. Activation of progelatinase B (proMMP-9) by active collagenase-3 (MMP-13). Eur. J. Biochem. 1997, 248, 369–373. [Google Scholar] [CrossRef] [PubMed]
- Nannuru, K.C.; Futakuchi, M.; Varney, M.L.; Vincent, T.M.; Marcusson, E.G.; Singh, R.K. Matrix metalloproteinase (MMP)-13 regulates mammary tumor-induced osteolysis by activating MMP9 and transforming growth factor-beta signaling at the tumor-bone interface. Cancer Res. 2010, 70, 3494–3504. [Google Scholar] [PubMed]
- Kim, G.E.; Lee, J.S.; Choi, Y.D.; Lee, K.H.; Lee, J.H.; Nam, J.H.; Choi, C.; Kim, S.S.; Park, M.H.; Yoon, J.H.; et al. Expression of matrix metalloproteinases and their inhibitors in different immunohistochemical-based molecular subtypes of breast cancer. BMC Cancer 2014, 14, 959. [Google Scholar]
- Xie, J.; Yang, Y.; Sun, J.; Jiao, Z.; Zhang, H.; Chen, J. STEAP1 Inhibits Breast Cancer Metastasis and Is Associated With Epithelial-Mesenchymal Transition Procession. Clin. Breast Cancer 2019, 19, e195–e207. [Google Scholar]
- Sendon-Lago, J.; Seoane, S.; Eiro, N.; Bermudez, M.A.; Macia, M.; Garcia-Caballero, T.; Vizoso, F.J.; Perez-Fernandez, R. Cancer progression by breast tumors with Pit-1-overexpression is blocked by inhibition of metalloproteinase (MMP)-13. Breast Cancer Res. 2014, 16, 505. [Google Scholar]
- Akshaya, R.L.; Rohini, M.; He, Z.; Partridge, N.C.; Selvamurugan, N. MiR-4638-3p regulates transforming growth factor-β1-induced activating transcription factor-3 and cell proliferation, invasion, and apoptosis in human breast cancer cells. Int. J. Biol. Macromol. 2022, 222 Pt B, 1974–1982. [Google Scholar]
- Krajina, B.A.; LeSavage, B.L.; Roth, J.G.; Zhu, A.W.; Cai, P.C.; Spakowitz, A.J.; Heilshorn, S.C. Microrheology reveals simultaneous cell-mediated matrix stiffening and fluidization that underlie breast cancer invasion. Sci. Adv. 2021, 7, eabe1969. [Google Scholar]
- Chaudhuri, O.; Cooper-White, J.; Janmey, P.A.; Mooney, D.J.; Shenoy, V.B. Effects of extracellular matrix viscoelasticity on cellular behaviour. Nature 2020, 584, 535–546. [Google Scholar] [PubMed]
- Fasching, P.A.; Heusinger, K.; Loehberg, C.R.; Wenkel, E.; Lux, M.P.; Schrauder, M.; Koscheck, T.; Bautz, W.; Schulz-Wendtland, R.; Beckmann, M.W.; et al. Influence of mammographic density on the diagnostic accuracy of tumor size assessment and association with breast cancer tumor characteristics. Eur. J. Radiol. 2006, 60, 398–404. [Google Scholar]
- Acerbi, I.; Cassereau, L.; Dean, I.; Shi, Q.; Au, A.; Park, C.; Chen, Y.Y.; Liphardt, J.; Hwang, E.S.; Weaver, V.M. Human breast cancer invasion and aggression correlates with ECM stiffening and immune cell infiltration. Integr. Biol. 2015, 7, 1120–1134. [Google Scholar]
- Koay, E.J.; Lee, Y.; Cristini, V.; Lowengrub, J.S.; Kang, Y.; Lucas, F.A.S.; Hobbs, B.P.; Ye, R.; Elganainy, D.; Almahariq, M.; et al. A Visually Apparent and Quantifiable CT Imaging Feature Identifies Biophysical Subtypes of Pancreatic Ductal Adenocarcinoma. Clin. Cancer Res. 2018, 24, 5883–5894. [Google Scholar]
- Liu, T.; Babaniyi, O.A.; Hall, T.J.; Barbone, P.E.; Oberai, A.A. Noninvasive In-Vivo Quantification of Mechanical Heterogeneity of Invasive Breast Carcinomas. PLoS ONE 2015, 10, e0130258. [Google Scholar]
- Piersma, B.; Hayward, M.K.; Weaver, V.M. Fibrosis and cancer: A strained relationship. Biochim. Biophys. Acta Rev. Cancer 2020, 1873, 188356. [Google Scholar]
- Tian, H.; Shi, H.; Yu, J.; Ge, S.; Ruan, J. Biophysics Role and Biomimetic Culture Systems of ECM Stiffness in Cancer EMT. Glob. Chall. 2022, 6, 2100094. [Google Scholar]
- Zhang, Z.; Richmond, A.; Yan, C. Immunomodulatory Properties of PI3K/AKT/mTOR and MAPK/MEK/ERK Inhibition Augment Response to Immune Checkpoint Blockade in Melanoma and Triple-Negative Breast Cancer. Int. J. Mol. Sci. 2022, 23, 7353. [Google Scholar] [CrossRef]
- Na, T.Y.; Schecterson, L.; Mendonsa, A.M.; Gumbiner, B.M. The functional activity of E-cadherin controls tumor cell metastasis at multiple steps. Proc. Natl. Acad. Sci. USA 2020, 117, 5931–5937. [Google Scholar] [PubMed]
- Babaei, G.; Aziz, S.G.; Jaghi, N.Z.Z. EMT, cancer stem cells and autophagy; The three main axes of metastasis. Biomed. Pharmacother. 2021, 133, 110909. [Google Scholar]
- Han, B.; Guan, X.; Ma, M.; Liang, B.; Ren, L.; Liu, Y.; Du, Y.; Jiang, S.H.; Song, D. Stiffened tumor microenvironment enhances perineural invasion in breast cancer via integrin signaling. Cell. Oncol. 2024, 47, 867–882. [Google Scholar]
- Qin, X.; Lv, X.; Li, P.; Yang, R.; Xia, Q.; Chen, Y.; Peng, Y.; Li, L.; Li, S.; Li, T.; et al. Matrix stiffness modulates ILK-mediated YAP activation to control the drug resistance of breast cancer cells. Biochim. Biophys. Acta Mol. Basis Dis. 2020, 1866, 165625. [Google Scholar]
- Tzima, E.; del Pozo, M.A.; Shattil, S.J.; Chien, S.; Schwartz, M.A. Activation of integrins in endothelial cells by fluid shear stress mediates Rho-dependent cytoskeletal alignment. Embo J. 2001, 20, 4639–4647. [Google Scholar]
- So, J.Y.; Ohm, J.; Lipkowitz, S.; Yang, L. Triple negative breast cancer (TNBC): Non-genetic tumor heterogeneity and immune microenvironment: Emerging treatment options. Pharmacol. Ther. 2022, 237, 108253. [Google Scholar]
- Habanjar, O.; Diab-Assaf, M.; Caldefie-Chezet, F.; Delort, L. 3D Cell Culture Systems: Tumor Application, Advantages, and Disadvantages. Int. J. Mol. Sci. 2021, 22, 12200. [Google Scholar] [CrossRef]
- Zhang, R.; Tu, J.; Liu, S. Novel molecular regulators of breast cancer stem cell plasticity and heterogeneity. Semin. Cancer Biol. 2022, 82, 11–25. [Google Scholar]
- Butti, R.; Gunasekaran, V.P.; Kumar, T.V.S.; Banerjee, P.; Kundu, G.C. Breast cancer stem cells: Biology and therapeutic implications. Int. J. Biochem. Cell Biol. 2019, 107, 38–52. [Google Scholar]
- Wozniak, M.A.; Desai, R.; Solski, P.A.; Der, C.J.; Keely, P.J. ROCK-generated contractility regulates breast epithelial cell differentiation in response to the physical properties of a three-dimensional collagen matrix. J. Cell Biol. 2003, 163, 583–595. [Google Scholar]
- Paul, C.D.; Mistriotis, P.; Konstantopoulos, K. Cancer cell motility: Lessons from migration in confined spaces. Nat. Rev. Cancer 2017, 17, 131–140. [Google Scholar] [CrossRef] [PubMed]
- Wolf, K.; Te Lindert, M.; Krause, M.; Alexander, S.; Te Riet, J.; Willis, A.L.; Hoffman, R.M.; Figdor, C.G.; Weiss, S.J.; Friedl, P. Physical limits of cell migration: Control by ECM space and nuclear deformation and tuning by proteolysis and traction force. J. Cell Biol. 2013, 201, 1069–1084. [Google Scholar] [CrossRef] [PubMed]
- Raeber, G.P.; Lutolf, M.P.; Hubbell, J.A. Molecularly engineered PEG hydrogels: A novel model system for proteolytically mediated cell migration. Biophys. J. 2005, 89, 1374–1388. [Google Scholar] [CrossRef]
- Trappmann, B.; Baker, B.M.; Polacheck, W.J.; Choi, C.K.; Burdick, J.A.; Chen, C.S. Matrix degradability controls multicellularity of 3D cell migration. Nat. Commun. 2017, 8, 371. [Google Scholar] [PubMed]
- Münster, S.; Jawerth, L.M.; Leslie, B.A.; Weitz, J.I.; Fabry, B.; Weitz, D.A. Strain history dependence of the nonlinear stress response of fibrin and collagen networks. Proc. Natl. Acad. Sci. USA 2013, 110, 12197–12202. [Google Scholar] [CrossRef]
- Nam, S.; Hu, K.H.; Butte, M.J.; Chaudhuri, O. Strain-enhanced stress relaxation impacts nonlinear elasticity in collagen gels. Proc. Natl. Acad. Sci. USA 2016, 113, 5492–5497. [Google Scholar]
- Ban, E.; Franklin, J.M.; Nam, S.; Smith, L.R.; Wang, H.; Wells, R.G.; Chaudhuri, O.; Liphardt, J.T.; Shenoy, V.B. Mechanisms of Plastic Deformation in Collagen Networks Induced by Cellular Forces. Biophys. J. 2018, 114, 450–461. [Google Scholar]
- Wisdom, K.M.; Adebowale, K.; Chang, J.; Lee, J.Y.; Nam, S.; Desai, R.; Rossen, N.S.; Rafat, M.; West, R.B.; Hodgson, L.; et al. Matrix mechanical plasticity regulates cancer cell migration through confining microenvironments. Nat. Commun. 2018, 9, 4144. [Google Scholar]
- Schultz, K.M.; Kyburz, K.A.; Anseth, K.S. Measuring dynamic cell-material interactions and remodeling during 3D human mesenchymal stem cell migration in hydrogels. Proc. Natl. Acad. Sci. USA 2015, 112, E3757–E3764. [Google Scholar] [CrossRef]
- Fraley, S.I.; Wu, P.H.; He, L.; Feng, Y.; Krisnamurthy, R.; Longmore, G.D.; Wirtz, D. Three-dimensional matrix fiber alignment modulates cell migration and MT1-MMP utility by spatially and temporally directing protrusions. Sci. Rep. 2015, 5, 14580. [Google Scholar] [CrossRef]
- Wolf, K.; Friedl, P. Extracellular matrix determinants of proteolytic and non-proteolytic cell migration. Trends Cell Biol. 2011, 21, 736–744. [Google Scholar]
- Bera, K.; Kiepas, A.; Godet, I.; Li, Y.; Mehta, P.; Ifemembi, B.; Paul, C.D.; Sen, A.; Serra, S.A.; Stoletov, K.; et al. Extracellular fluid viscosity enhances cell migration and cancer dissemination. Nature 2022, 611, 365–373. [Google Scholar] [PubMed]
- Balleyguier, C.; Lakhdar, A.B.; Dunant, A.; Mathieu, M.C.; Delaloge, S.; Sinkus, R. Value of whole breast magnetic resonance elastography added to MRI for lesion characterization. NMR Biomed. 2018, 31, e3795. [Google Scholar]
- Li, H.; Flé, G.; Bhatt, M.; Qu, Z.; Ghazavi, S.; Yazdani, L.; Bosio, G.; Rafati, I.; Cloutier, G. Viscoelasticity Imaging of Biological Tissues and Single Cells Using Shear Wave Propagation. Front. Phys. 2021, 9, 666192. [Google Scholar]
- Pillai, A.; Voruganti, T.; Barr, R.; Langdon, J. Diagnostic Accuracy of Shear-Wave Elastography for Breast Lesion Characterization in Women: A Systematic Review and Meta-Analysis. J. Am. Coll. Radiol. 2022, 19, 625–634.e620. [Google Scholar] [PubMed]
- Xi, G.; Qiu, L.; Xu, S.; Guo, W.; Fu, F.; Kang, D.; Zheng, L.; He, J.; Zhang, Q.; Li, L.; et al. Computer-assisted quantification of tumor-associated collagen signatures to improve the prognosis prediction of breast cancer. BMC Med. 2021, 19, 273. [Google Scholar]
- Qi, W. Roles of collagen-like molecules in tumorigenesis. Chin. Bull. Life Sci. 2009, 21, 276–279. [Google Scholar]
- de Heer, E.C.; Jalving, M.; Harris, A.L. HIFs, angiogenesis, and metabolism: Elusive enemies in breast cancer. J. Clin. Investig. 2020, 130, 5074–5087. [Google Scholar]
- Etehadtavakol, M.; Ng, E.Y.K. Breast Thermography as a Potential Non-Contact Method in the Early Detection of Cancer: A Review. J. Mech. Med. Biol. 2013, 13, 1330001. [Google Scholar] [CrossRef]
- Ahuja, S.; Zaheer, S. Multifaceted TGF-β signaling, a master regulator: From bench-to-bedside, intricacies, and complexities. Cell Biol. Int. 2024, 48, 87–127. [Google Scholar] [CrossRef]
- Xia, L.; Tan, S.; Zhou, Y.; Lin, J.; Wang, H.; Oyang, L.; Tian, Y.; Liu, L.; Su, M.; Wang, H.; et al. Role of the NFκB-signaling pathway in cancer. Onco Targets Ther. 2018, 11, 2063–2073. [Google Scholar] [PubMed]
- Elayat, G.; Selim, A. Angiogenesis in breast cancer: Insights and innovations. Clin. Exp. Med. 2024, 24, 178. [Google Scholar] [PubMed]
- Kapoor, C.; Vaidya, S.; Wadhwan, V.; Kaur, G.; Pathak, A. Seesaw of matrix metalloproteinases (MMPs). J. Cancer Res. Ther. 2016, 12, 28–35. [Google Scholar]
- Visse, R.; Nagase, H. Matrix metalloproteinases and tissue inhibitors of metalloproteinases: Structure, function, and biochemistry. Circ. Res. 2003, 92, 827–839. [Google Scholar] [PubMed]
- Li, S.; Pritchard, D.M.; Yu, L.G. Regulation and Function of Matrix Metalloproteinase-13 in Cancer Progression and Metastasis. Cancers 2022, 14, 3263. [Google Scholar] [CrossRef]
- Lovejoy, B.; Welch, A.R.; Carr, S.; Luong, C.; Broka, C.; Hendricks, R.T.; Campbell, J.A.; Walker, K.A.; Martin, R.; Van Wart, H.; et al. Crystal structures of MMP-1 and -13 reveal the structural basis for selectivity of collagenase inhibitors. Nat. Struct. Biol. 1999, 6, 217–221. [Google Scholar]
- Coussens, L.M.; Fingleton, B.; Matrisian, L.M. Matrix metalloproteinase inhibitors and cancer: Trials and tribulations. Science 2002, 295, 2387–2392. [Google Scholar] [CrossRef]
- Liang, Y.; Zhang, H.; Song, X.; Yang, Q. Metastatic heterogeneity of breast cancer: Molecular mechanism and potential therapeutic targets. Semin. Cancer Biol. 2020, 60, 14–27. [Google Scholar]
- Kim, N.H.; Sung, N.J.; Shin, S.; Ryu, D.S.; Youn, H.S.; Park, S.A. Sauchinone inhibits the proliferation, migration and invasion of breast cancer cells by suppressing Akt-CREB-MMP13 signaling pathway. Biosci. Rep. 2021, 41, BSR20211067. [Google Scholar]
- Shimizu, Y.; Temma, T.; Sano, K.; Ono, M.; Saji, H. Development of membrane type-1 matrix metalloproteinase-specific activatable fluorescent probe for malignant tumor detection. Cancer Sci. 2011, 102, 1897–1903. [Google Scholar]
- Lijnen, H.R. Plasmin and matrix metalloproteinases in vascular remodeling. Thromb. Haemost. 2001, 86, 324–333. [Google Scholar] [CrossRef] [PubMed]
- Kotepui, M.; Punsawad, C.; Chupeerach, C.; Songsri, A.; Charoenkijkajorn, L.; Petmitr, S. Differential expression of matrix metalloproteinase-13 in association with invasion of breast cancer. Contemp. Oncol. 2016, 20, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Sung, N.J.; Kim, N.H.; Surh, Y.J.; Park, S.A. Gremlin-1 Promotes Metastasis of Breast Cancer Cells by Activating STAT3-MMP13 Signaling Pathway. Int. J. Mol. Sci. 2020, 21, 9227. [Google Scholar] [CrossRef]
- Löffek, S.; Schilling, O.; Cw, F. Biological role of matrix metalloproteinases: A critical balance. Eur. Respir. J. 2010, 38, 191–208. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Yin, P.; Wei, J.; Ding, Q. The role of matrix stiffness in breast cancer progression: A review. Front. Oncol. 2023, 13, 1284926. [Google Scholar] [CrossRef]
- Anlaş, A.A.; Nelson, C.M. Soft Microenvironments Induce Chemoresistance by Increasing Autophagy Downstream of Integrin-Linked Kinase. Cancer Res. 2020, 80, 4103–4113. [Google Scholar] [CrossRef]
- Brown, N.H. Extracellular matrix in development: Insights from mechanisms conserved between invertebrates and vertebrates. Cold Spring Harb. Perspect. Biol. 2011, 3, a005082. [Google Scholar] [CrossRef]
- Agrez, M.; Chen, A.; Cone, R.I.; Pytela, R.; Sheppard, D. The alpha v beta 6 integrin promotes proliferation of colon carcinoma cells through a unique region of the beta 6 cytoplasmic domain. J. Cell Biol. 1994, 127, 547–556. [Google Scholar] [CrossRef]
- Huang, M.; Fu, M.; Wang, J.; Xia, C.; Zhang, H.; Xiong, Y.; He, J.; Liu, J.; Liu, B.; Pan, S.; et al. TGF-β1-activated cancer-associated fibroblasts promote breast cancer invasion, metastasis and epithelial-mesenchymal transition by autophagy or overexpression of FAP-α. Biochem. Pharmacol. 2021, 188, 114527. [Google Scholar] [CrossRef]
- Chen, Y.; Terajima, M.; Yang, Y.; Sun, L.; Ahn, Y.H.; Pankova, D.; Puperi, D.S.; Watanabe, T.; Kim, M.P.; Blackmon, S.H.; et al. Lysyl hydroxylase 2 induces a collagen cross-link switch in tumor stroma. J. Clin. Investig. 2015, 125, 1147–1162. [Google Scholar] [CrossRef]
- Provenzano, P.P.; Eliceiri, K.W.; Campbell, J.M.; Inman, D.R.; White, J.G.; Keely, P.J. Collagen reorganization at the tumor-stromal interface facilitates local invasion. BMC Med. 2006, 4, 38. [Google Scholar]
- Fares, J.; Fares, M.Y.; Khachfe, H.H.; Salhab, H.A.; Fares, Y. Molecular principles of metastasis: A hallmark of cancer revisited. Signal Transduct. Target. Ther. 2020, 5, 28. [Google Scholar] [PubMed]
- Jechlinger, M.; Grunert, S.; Tamir, I.H.; Janda, E.; Lüdemann, S.; Waerner, T.; Seither, P.; Weith, A.; Beug, H.; Kraut, N. Expression profiling of epithelial plasticity in tumor progression. Oncogene 2003, 22, 7155–7169. [Google Scholar] [PubMed]
- Radisky, E.S.; Radisky, D.C. Matrix metalloproteinase-induced epithelial-mesenchymal transition in breast cancer. J. Mammary Gland. Biol. Neoplasia 2010, 15, 201–212. [Google Scholar]
- Gutman, A.; Wasylyk, B. The collagenase gene promoter contains a TPA and oncogene-responsive unit encompassing the PEA3 and AP-1 binding sites. EMBO J. 1990, 9, 2241–2246. [Google Scholar]
- Saiganesh, S.; Saathvika, R.; Udhaya, V.; Arumugam, B.; Vishal, M.; Selvamurugan, N. Matrix metalloproteinase-13: A special focus on its regulation by signaling cascades and microRNAs in bone. Int. J. Biol. Macromol. 2018, 109, 338–349. [Google Scholar]
- Gokulnath, M.; Swetha, R.; Thejaswini, G.; Shilpa, P.; Selvamurugan, N. Transforming growth factor-β1 regulation of ATF-3, c-Jun and JunB proteins for activation of matrix metalloproteinase-13 gene in human breast cancer cells. Int. J. Biol. Macromol. 2017, 94 Pt A, 370–377. [Google Scholar] [CrossRef]
- Baert, J.L.; Monté, D.; Musgrove, E.A.; Albagli, O.; Sutherland, R.L.; de Launoit, Y. Expression of the PEA3 group of ETS-related transcription factors in human breast-cancer cells. Int. J. Cancer 1997, 70, 590–597. [Google Scholar] [CrossRef]
- Ogawa, E.; Inuzuka, M.; Maruyama, M.; Satake, M.; Naito-Fujimoto, M.; Ito, Y.; Shigesada, K. Molecular cloning and characterization of PEBP2 beta, the heterodimeric partner of a novel Drosophila runt-related DNA binding protein PEBP2 alpha. Virology 1993, 194, 314–331. [Google Scholar]
- Banerjee, C.; McCabe, L.R.; Choi, J.Y.; Hiebert, S.W.; Stein, J.L.; Stein, G.S.; Lian, J.B. Runt homology domain proteins in osteoblast differentiation: AML3/CBFA1 is a major component of a bone-specific complex. J. Cell Biochem. 1997, 66, 1–8. [Google Scholar]
- Ducy, P.; Zhang, R.; Geoffroy, V.; Ridall, A.L.; Karsenty, G. Osf2/Cbfa1: A transcriptional activator of osteoblast differentiation. Cell 1997, 89, 747–754. [Google Scholar] [CrossRef] [PubMed]
- Selvamurugan, N.; Kwok, S.; Partridge, N.C. Smad3 interacts with JunB and Cbfa1/Runx2 for transforming growth factor-beta1-stimulated collagenase-3 expression in human breast cancer cells. J. Biol. Chem. 2004, 279, 27764–27773. [Google Scholar] [PubMed]
- Reuben, P.M.; Cheung, H.S. Regulation of matrix metalloproteinase (MMP) gene expression by protein kinases. Front. Biosci. 2006, 11, 1199–1215. [Google Scholar]
- Dumortier, M.; Ladam, F.; Damour, I.; Vacher, S.; Bièche, I.; Marchand, N.; de Launoit, Y.; Tulasne, D.; Chotteau-Lelièvre, A. ETV4 transcription factor and MMP13 metalloprotease are interplaying actors of breast tumorigenesis. Breast Cancer Res. 2018, 20, 73. [Google Scholar]
- Gokulnath, M.; Partridge, N.C.; Selvamurugan, N. Runx2, a target gene for activating transcription factor-3 in human breast cancer cells. Tumour Biol. 2015, 36, 1923–1931. [Google Scholar] [CrossRef]
- Wang, W.; Chen, B.; Zou, R.; Tu, X.; Tan, S.; Lu, H.; Liu, Z.; Fu, J. Codonolactone, a sesquiterpene lactone isolated from Chloranthus henryi Hemsl, inhibits breast cancer cell invasion, migration and metastasis by downregulating the transcriptional activity of Runx2. Int. J. Oncol. 2014, 45, 1891–1900. [Google Scholar] [PubMed]
- He, F.; Matsumoto, Y.; Asano, Y.; Yamamura, Y.; Katsuyama, T.; La Rose, J.; Tomonobu, N.; Komalasari, N.; Sakaguchi, M.; Rottapel, R.; et al. RUNX2 Phosphorylation by Tyrosine Kinase ABL Promotes Breast Cancer Invasion. Front. Oncol. 2021, 11, 665273. [Google Scholar]
- Taipaleenmäki, H.; Browne, G.; Akech, J.; Zustin, J.; van Wijnen, A.J.; Stein, J.L.; Hesse, E.; Stein, G.S.; Lian, J.B. Targeting of Runx2 by miR-135 and miR-203 Impairs Progression of Breast Cancer and Metastatic Bone Disease. Cancer Res. 2015, 75, 1433–1444. [Google Scholar] [CrossRef]
- Xu, L.-M.; Zhang, J.; Ma, Y.; Yuan, Y.-J.; Yu, H.; Wang, J.; Cao, X.-C.; Zhu, L.; Wang, P. MicroRNA-135 inhibits initiation of epithelial-mesenchymal transition in breast cancer by targeting ZNF217 and promoting m6A modification of NANOG. Oncogene 2022, 41, 1742–1751. [Google Scholar]
- Taube, J.H.; Malouf, G.G.; Lu, E.; Sphyris, N.; Vijay, V.; Ramachandran, P.P.; Ueno, K.R.; Gaur, S.; Nicoloso, M.S.; Rossi, S.; et al. Epigenetic silencing of microRNA-203 is required for EMT and cancer stem cell properties. Sci. Rep. 2013, 3, 2687. [Google Scholar] [CrossRef]
- Si, W.; Kan, C.; Zhang, L.; Li, F. Role of RUNX2 in breast cancer development and drug resistance (Review). Oncol. Lett. 2023, 25, 176. [Google Scholar] [CrossRef] [PubMed]
- Deryugina, E.I.; Quigley, J.P. Matrix metalloproteinases and tumor metastasis. Cancer Metastasis Rev. 2006, 25, 9–34. [Google Scholar]
- Hayward, M.K.; Allen, M.D.; Gomm, J.J.; Goulding, I.; Thompson, C.L.; Knight, M.M.; Marshall, J.F.; Jones, J.L. Mechanostimulation of breast myoepithelial cells induces functional changes associated with DCIS progression to invasion. NPJ Breast Cancer 2022, 8, 109. [Google Scholar] [CrossRef]
- Safaei, S.; Sajed, R.; Shariftabrizi, A.; Dorafshan, S.; Saeednejad Zanjani, L.; Dehghan Manshadi, M.; Madjd, Z.; Ghods, R. Tumor matrix stiffness provides fertile soil for cancer stem cells. Cancer Cell Int. 2023, 23, 143. [Google Scholar] [PubMed]
- Kim, M.K.; Jang, J.W.; Bae, S.C. DNA binding partners of YAP/TAZ. BMB Rep. 2018, 51, 126–133. [Google Scholar]
- LeBlanc, L.; Ramirez, N.; Kim, J. Context-dependent roles of YAP/TAZ in stem cell fates and cancer. Cell Mol. Life Sci. 2021, 78, 4201–4219. [Google Scholar]
- Dupont, S.; Morsut, L.; Aragona, M.; Enzo, E.; Giulitti, S.; Cordenonsi, M.; Zanconato, F.; Le Digabel, J.; Forcato, M.; Bicciato, S.; et al. Role of YAP/TAZ in mechanotransduction. Nature 2011, 474, 179–183. [Google Scholar]
- Ros, M.; Sala, M.; Saltel, F. Linking Matrix Rigidity with EMT and Cancer Invasion. Dev. Cell 2020, 54, 293–295. [Google Scholar] [PubMed]
- Wei, S.C.; Fattet, L.; Tsai, J.H.; Guo, Y.; Pai, V.H.; Majeski, H.E.; Chen, A.C.; Sah, R.L.; Taylor, S.S.; Engler, A.J.; et al. Matrix stiffness drives epithelial-mesenchymal transition and tumour metastasis through a TWIST1-G3BP2 mechanotransduction pathway. Nat. Cell Biol. 2015, 17, 678–688. [Google Scholar]
- Ghasemi, H.; Mousavibahar, S.H.; Hashemnia, M.; Karimi, J.; Khodadadi, I.; Mirzaei, F.; Tavilani, H. Tissue stiffness contributes to YAP activation in bladder cancer patients undergoing transurethral resection. Ann. N. Y. Acad. Sci. 2020, 1473, 48–61. [Google Scholar]
- Li, W.; Yang, C.; Li, J.; Li, X.; Zhou, P. MicroRNA-217 aggravates breast cancer through activation of NF1-mediated HSF1/ATG7 axis and c-Jun/ATF3/MMP13 axis. Hum. Cell 2023, 36, 377–392. [Google Scholar] [CrossRef] [PubMed]
- Insua-Rodríguez, J.; Oskarsson, T. The extracellular matrix in breast cancer. Adv. Drug Deliv. Rev. 2016, 97, 41–55. [Google Scholar]
- Rakha, E.A.; Miligy, I.M.; Gorringe, K.L.; Toss, M.S.; Green, A.R.; Fox, S.B.; Schmitt, F.C.; Tan, P.H.; Tse, G.M.; Badve, S.; et al. Invasion in breast lesions: The role of the epithelial-stroma barrier. Histopathology 2018, 72, 1075–1083. [Google Scholar] [CrossRef] [PubMed]
- Overall, C.M. Matrix metalloproteinase substrate binding domains, modules and exosites. Overview and experimental strategies. Methods Mol. Biol. 2001, 151, 79–120. [Google Scholar]
- Perry, S.W.; Schueckler, J.M.; Burke, K.; Arcuri, G.L.; Brown, E.B. Stromal matrix metalloprotease-13 knockout alters Collagen I structure at the tumor-host interface and increases lung metastasis of C57BL/6 syngeneic E0771 mammary tumor cells. BMC Cancer 2013, 13, 411. [Google Scholar]
- Cai, R.; Tressler, C.M.; Cheng, M.; Sonkar, K.; Tan, Z.; Paidi, S.K.; Ayyappan, V.; Barman, I.; Glunde, K. Primary breast tumor induced extracellular matrix remodeling in premetastatic lungs. Sci. Rep. 2023, 13, 18566. [Google Scholar]
- Solomonov, I.; Zehorai, E.; Talmi-Frank, D.; Wolf, S.G.; Shainskaya, A.; Zhuravlev, A.; Kartvelishvily, E.; Visse, R.; Levin, Y.; Kampf, N.; et al. Distinct biological events generated by ECM proteolysis by two homologous collagenases. Proc. Natl. Acad. Sci. USA 2016, 113, 10884–10889. [Google Scholar]
- Wang, Q.; Ji, C.; Ali, A.; Ding, I.; Wang, Y.; McCulloch, C.A. TRPV4 mediates IL-1-induced Ca(2+) signaling, ERK activation and MMP expression. FASEB J. 2024, 38, e23731. [Google Scholar]
- Cheng, T.; Chen, P.; Chen, J.; Deng, Y.; Huang, C. Landscape Analysis of Matrix Metalloproteinases Unveils Key Prognostic Markers for Patients With Breast Cancer. Front. Genet. 2021, 12, 809600. [Google Scholar]
- Baker, K.J.; Houston, A.; Brint, E. IL-1 Family Members in Cancer; Two Sides to Every Story. Front. Immunol. 2019, 10, 1197. [Google Scholar] [CrossRef]
- Madu, C.O.; Wang, S.; Madu, C.O.; Lu, Y. Angiogenesis in Breast Cancer Progression, Diagnosis, and Treatment. J. Cancer 2020, 11, 4474–4494. [Google Scholar] [PubMed]
- Wang, D.; Liu, X.; Hong, W.; Xiao, T.; Xu, Y.; Fang, X.; Tang, H.; Zheng, Q.; Meng, X. Muscone abrogates breast cancer progression through tumor angiogenic suppression via VEGF/PI3K/Akt/MAPK signaling pathways. Cancer Cell Int. 2024, 24, 214. [Google Scholar] [PubMed]
- Cendrowicz, E.; Sas, Z.; Bremer, E.; Rygiel, T.P. The Role of Macrophages in Cancer Development and Therapy. Cancers 2021, 13, 1946. [Google Scholar] [CrossRef] [PubMed]
- Robert, S.; Gicquel, T.; Victoni, T.; Valença, S.; Barreto, E.; Bailly-Maître, B.; Boichot, E.; Lagente, V. Involvement of matrix metalloproteinases (MMPs) and inflammasome pathway in molecular mechanisms of fibrosis. Biosci. Rep. 2016, 36, e00360. [Google Scholar]
- Quintero-Fabián, S.; Arreola, R.; Becerril-Villanueva, E.; Torres-Romero, J.C.; Arana-Argáez, V.; Lara-Riegos, J.; Ramírez-Camacho, M.A.; Alvarez-Sánchez, M.E. Role of Matrix Metalloproteinases in Angiogenesis and Cancer. Front. Oncol. 2019, 9, 1370. [Google Scholar] [CrossRef]
- Lugano, R.; Ramachandran, M.; Dimberg, A. Tumor angiogenesis: Causes, consequences, challenges and opportunities. Cell Mol. Life Sci. 2020, 77, 1745–1770. [Google Scholar]
- Libby, J.R.; Royce, H.; Walker, S.R.; Li, L. The role of extracellular matrix in angiogenesis: Beyond adhesion and structure. Biomater. Biosyst. 2024, 15, 100097. [Google Scholar]
- Yu, P.F.; Huang, Y.; Han, Y.Y.; Lin, L.Y.; Sun, W.H.; Rabson, A.B.; Wang, Y.; Shi, Y.F. TNFα-activated mesenchymal stromal cells promote breast cancer metastasis by recruiting CXCR2(+) neutrophils. Oncogene 2017, 36, 482–490. [Google Scholar]
- Raghu, H.; Sodadasu, P.K.; Malla, R.R.; Gondi, C.S.; Estes, N.; Rao, J.S. Localization of uPAR and MMP-9 in lipid rafts is critical for migration, invasion and angiogenesis in human breast cancer cells. BMC Cancer 2010, 10, 647. [Google Scholar]
- Sang, R.; Yu, X.; Xia, H.; Qian, X.; Yong, J.; Xu, Y.; Sun, Y.; Yao, Y.; Zhou, J.; Zhuo, S. NT5DC2 knockdown suppresses progression, glycolysis, and neuropathic pain in triple-negative breast cancer by blocking the EGFR pathway. Mol. Carcinog. 2024, 63, 785–796. [Google Scholar]
- Ribatti, D.; Nico, B.; Crivellato, E.; Vacca, A. Macrophages and tumor angiogenesis. Leukemia 2007, 21, 2085–2089. [Google Scholar]
- Riabov, V.; Gudima, A.; Wang, N.; Mickley, A.; Orekhov, A.; Kzhyshkowska, J. Role of tumor associated macrophages in tumor angiogenesis and lymphangiogenesis. Front. Physiol. 2014, 5, 75. [Google Scholar]
- Brocks, B.; Kraft, S.; Zahn, S.; Noll, S.; Pan, C.; Schauer, M.; Krebs, B. Generation and optimization of human antagonistic antibodies against TIMP-1 as potential therapeutic agents in fibrotic diseases. Hum. Antibodies 2006, 15, 115–124. [Google Scholar] [PubMed]
- Dou, J.; Cui, H.; Cui, Z.; Xuan, M.; Gao, C.; Li, Z.; Lian, L.; Nan, J.; Wu, Y. Pterostilbene exerts cytotoxicity on activated hepatic stellate cells by inhibiting excessive proliferation through the crosstalk of Sirt1 and STAT3 pathways. Food Chem. Toxicol. 2023, 181, 114042. [Google Scholar]
- Umehara, T.; Winstanley, Y.E.; Andreas, E.; Morimoto, A.; Williams, E.J.; Smith, K.M.; Carroll, J.; Febbraio, M.A.; Shimada, M.; Russell, D.L.; et al. Female reproductive life span is extended by targeted removal of fibrotic collagen from the mouse ovary. Sci. Adv. 2022, 8, eabn4564. [Google Scholar]
- Zhang, R.; Jing, W.; Chen, C.; Zhang, S.; Abdalla, M.; Sun, P.; Wang, G.; You, W.; Yang, Z.; Zhang, J.; et al. Inhaled mRNA Nanoformulation with Biogenic Ribosomal Protein Reverses Established Pulmonary Fibrosis in a Bleomycin-Induced Murine Model. Adv. Mater. 2022, 34, e2107506. [Google Scholar] [CrossRef]
- Guo, L.; Cheng, X.; Chen, H.; Chen, C.; Xie, S.; Zhao, M.; Liu, D.; Deng, Q.; Liu, Y.; Wang, X.; et al. Induction of breast cancer stem cells by M1 macrophages through Lin-28B-let-7-HMGA2 axis. Cancer Lett. 2019, 452, 213–225. [Google Scholar]
- Ahirwar, D.K.; Charan, M.; Mishra, S.; Verma, A.K.; Shilo, K.; Ramaswamy, B.; Ganju, R.K. Slit2 Inhibits Breast Cancer Metastasis by Activating M1-Like Phagocytic and Antifibrotic Macrophages. Cancer Res. 2021, 81, 5255–5267. [Google Scholar] [CrossRef] [PubMed]
- Avalle, L.; Raggi, L.; Monteleone, E.; Savino, A.; Viavattene, D.; Statello, L.; Camperi, A.; Stabile, S.A.; Salemme, V.; De Marzo, N.; et al. STAT3 induces breast cancer growth via ANGPTL4, MMP13 and STC1 secretion by cancer associated fibroblasts. Oncogene 2022, 41, 1456–1467. [Google Scholar]
- Folgueira, M.A.; Maistro, S.; Katayama, M.L.; Roela, R.A.; Mundim, F.G.; Nanogaki, S.; de Bock, G.H.; Brentani, M.M. Markers of breast cancer stromal fibroblasts in the primary tumour site associated with lymph node metastasis: A systematic review including our case series. Biosci. Rep. 2013, 33, e00085. [Google Scholar]
- Yu, Y.; Xiao, C.H.; Tan, L.D.; Wang, Q.S.; Li, X.Q.; Feng, Y.M. Cancer-associated fibroblasts induce epithelial-mesenchymal transition of breast cancer cells through paracrine TGF-β signalling. Br. J. Cancer 2014, 110, 724–732. [Google Scholar]
- Atsawasuwan, P.; Mochida, Y.; Katafuchi, M.; Kaku, M.; Fong, K.S.; Csiszar, K.; Yamauchi, M. Lysyl oxidase binds transforming growth factor-beta and regulates its signaling via amine oxidase activity. J. Biol. Chem. 2008, 283, 34229–34240. [Google Scholar] [PubMed]
- Csiszar, K. Lysyl oxidases: A novel multifunctional amine oxidase family. Prog. Nucleic Acid. Res. Mol. Biol. 2001, 70, 1–32. [Google Scholar]
- Decitre, M.; Gleyzal, C.; Raccurt, M.; Peyrol, S.; Aubert-Foucher, E.; Csiszar, K.; Sommer, P. Lysyl oxidase-like protein localizes to sites of de novo fibrinogenesis in fibrosis and in the early stromal reaction of ductal breast carcinomas. Lab. Investig. 1998, 78, 143–151. [Google Scholar]
- Szauter, K.M.; Cao, T.; Boyd, C.D.; Csiszar, K. Lysyl oxidase in development, aging and pathologies of the skin. Pathol. Biol. 2005, 53, 448–456. [Google Scholar] [PubMed]
- Bierie, B.; Moses, H.L. Tumour microenvironment: TGFbeta: The molecular Jekyll and Hyde of cancer. Nat. Rev. Cancer 2006, 6, 506–520. [Google Scholar] [PubMed]
- Wu, T.; Xiong, S.; Chen, M.; Tam, B.T.; Chen, W.; Dong, K.; Ma, Z.; Wang, Z.; Ouyang, G. Matrix stiffening facilitates the collective invasion of breast cancer through the periostin-integrin mechanotransduction pathway. Matrix Biol. 2023, 121, 22–40. [Google Scholar]
- Oshi, M.; Asaoka, M.; Tokumaru, Y.; Yan, L.; Matsuyama, R.; Ishikawa, T.; Endo, I.; Takabe, K. CD8 T Cell Score as a Prognostic Biomarker for Triple Negative Breast Cancer. Int. J. Mol. Sci. 2020, 21, 6968. [Google Scholar] [CrossRef]
- Tharp, K.M.; Kersten, K.; Maller, O.; Timblin, G.A.; Stashko, C.; Canale, F.P.; Menjivar, R.E.; Hayward, M.K.; Berestjuk, I.; Ten Hoeve, J.; et al. Tumor-associated macrophages restrict CD8(+) T cell function through collagen deposition and metabolic reprogramming of the breast cancer microenvironment. Nat. Cancer 2024, 5, 1045–1062. [Google Scholar]
- Wang, Y.H.; Shen, C.Y.; Lin, S.C.; Kuo, W.H.; Kuo, Y.T.; Hsu, Y.L.; Wang, W.C.; Lin, K.T.; Wang, L.H. Monocytes secrete CXCL7 to promote breast cancer progression. Cell Death Dis. 2021, 12, 1090. [Google Scholar]
- Gibson, S.V.; Tomas Bort, E.; Rodríguez-Fernández, L.; Allen, M.D.; Gomm, J.J.; Goulding, I.; Auf dem Keller, U.; Agnoletto, A.; Brisken, C.; Peck, B.; et al. TGFβ-mediated MMP13 secretion drives myoepithelial cell dependent breast cancer progression. NPJ Breast Cancer 2023, 9, 9. [Google Scholar] [CrossRef] [PubMed]
- Zhang, L.; Zhang, J.; Ni, C. Silencing of lncRNA PART1 inhibits proliferation, invasion and migration of breast cancer cells and promotes the efficacy of cisplatin in breast cancer cells. Gen. Physiol. Biophys. 2020, 39, 343–354. [Google Scholar] [CrossRef] [PubMed]
- Kothapalli, R.; Sivaraman Siveen, K.; Tan, T.Z.; Thiery, J.P.; Kumar, A.P.; Sethi, G.; Swaminathan, K. Functional characterization of selective exosite-binding inhibitors of matrix metalloproteinase-13 (MMP-13)—experimental validation in human breast and colon cancer. Biosci. Biotechnol. Biochem. 2016, 80, 2122–2131. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sun, X.; Hu, X. Unveiling Matrix Metalloproteinase 13’s Dynamic Role in Breast Cancer: A Link to Physical Changes and Prognostic Modulation. Int. J. Mol. Sci. 2025, 26, 3083. https://doi.org/10.3390/ijms26073083
Sun X, Hu X. Unveiling Matrix Metalloproteinase 13’s Dynamic Role in Breast Cancer: A Link to Physical Changes and Prognostic Modulation. International Journal of Molecular Sciences. 2025; 26(7):3083. https://doi.org/10.3390/ijms26073083
Chicago/Turabian StyleSun, Xiaomeng, and Xiaojuan Hu. 2025. "Unveiling Matrix Metalloproteinase 13’s Dynamic Role in Breast Cancer: A Link to Physical Changes and Prognostic Modulation" International Journal of Molecular Sciences 26, no. 7: 3083. https://doi.org/10.3390/ijms26073083
APA StyleSun, X., & Hu, X. (2025). Unveiling Matrix Metalloproteinase 13’s Dynamic Role in Breast Cancer: A Link to Physical Changes and Prognostic Modulation. International Journal of Molecular Sciences, 26(7), 3083. https://doi.org/10.3390/ijms26073083





