Expression of Fascin and DNA Topoisomerase 2-Alpha in Breast Carcinoma: Correlation with Histological Subtypes and Other Prognostic Markers
Abstract
1. Introduction
1.1. Fascin and Cancer
1.2. Topoisomerase II Alpha
2. Results
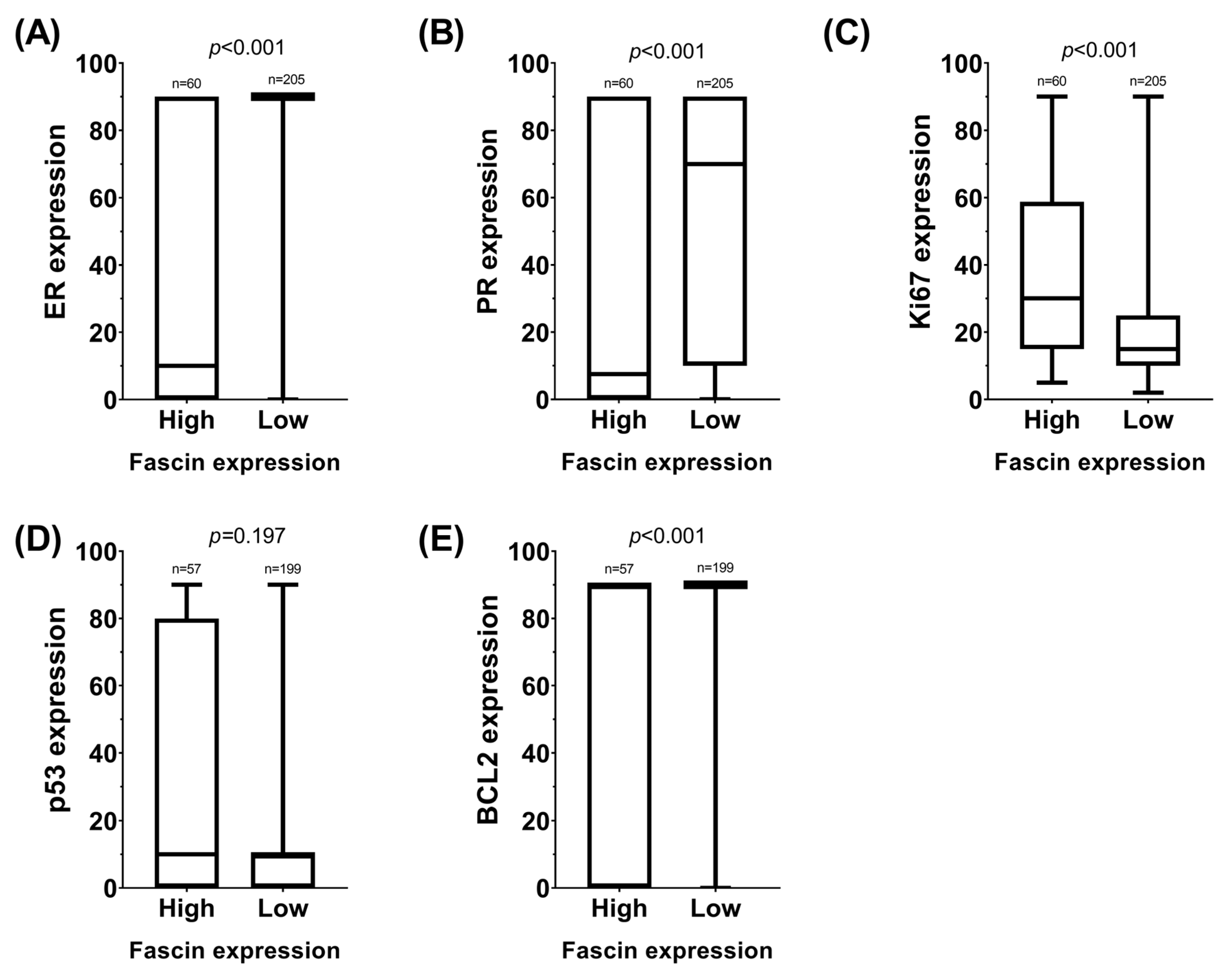
2.1. Expression of Fascin and TOP2A by Categories and Grouping
2.2. Fascin Expression in Different Histological and Molecular Subtypes of Breast Cancer
2.3. TOP2A/CEP17 Ratio in Different Histological and Molecular Subtypes of Breast Cancer
2.4. Fascin Expression and TOP2A Ratio Concerning Histological Grade and Pathological-Tumor Stage
2.5. Expression of Fascin and TOP2A Ratio Concerning Other Clinicopathological Variables
2.6. Correlation Analysis Between Continuous Variables and ROC Curves
2.7. Study of pCR in Relation to Fascin/TOP2A and Other IHC Markers
2.8. Overall Survival Analysis Concerning Fascin/TOP2A and Other IHC Markers
2.9. Disease-Free Survival Analysis Concerning Fascin/TOP2A and Other IHC Markers
2.10. Other Unassessed Histological Markers
3. Discussion
4. Materials and Methods
4.1. Selection Criteria
4.2. Histological and Molecular Subtype Classification, Histological Grade, and Slide Preparation for Additional Studies
4.3. Fascin and DNA Topoisomerase 2-Alpha Quantification
4.4. Other IHC Determinations
4.5. Clinical and Prognostic Variables
4.6. Statistical Analysis
5. Conclusions
Study Limitations
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| DNA | Deoxyribonucleic acid |
| TOP2A | Topoisomerase II alpha |
| ATP | Adenosine triphosphate |
| CEP17 | Centromere Enumeration Probe 17 |
| IHC | Immunohistochemical |
| HR | Hormone receptor |
| ER | Estrogen receptor |
| OS | Overall survival |
| DFS | Disease-free survival |
| CNB | Core needle biopsy |
| VAB | Vacuum-assisted biopsy |
| NOS | No other subtype |
| WHO | World Health Organization |
| CNS | Central nervous system |
| TDCC | TOP2A-DNA covalent complexes |
| FISH | Fluorescence in situ hybridization |
| TNBC | Triple-negative breast cancer |
| SBR | Scarff–Bloom–Richardson |
| PR | Progesterone receptor |
| pCR | Pathological complete response |
| mRNA | messenger ribonucleic acid |
| RT-PCR | Reverse transcription polymerase chain reaction |
| CISH | Chromogenic in situ hybridization |
| H&E | Hematoxylin and Eosin |
| AJCC | American Joint Committee on Cancer |
References
- Bray, F.; Laversanne, M.; Sung, H.; Ferlay, J.; Siegel, R.L.; Soerjomataram, I.; Jemal, A. Global cancer statistics 2022: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA. Cancer J. Clin. 2024, 74, 229–263. [Google Scholar] [CrossRef]
- Siegel, R.L.; Giaquinto, A.N.; Jemal, A. Cancer statistics, 2024. CA. Cancer J. Clin. 2024, 74, 12–49. [Google Scholar] [CrossRef] [PubMed]
- Sung, H.; Ferlay, J.; Siegel, R.L.; Laversanne, M.; Soerjomataram, I.; Jemal, A.; Bray, F. Global Cancer Statistics 2020: GLOBOCAN Estimates of Incidence and Mortality Worldwide for 36 Cancers in 185 Countries. CA. Cancer J. Clin. 2021, 71, 209–249. [Google Scholar] [CrossRef]
- Brewer, H.R.; Jones, M.E.; Schoemaker, M.J.; Ashworth, A.; Swerdlow, A.J. Family history and risk of breast cancer: An analysis accounting for family structure. Breast Cancer Res. Treat. 2017, 165, 193–200. [Google Scholar] [CrossRef]
- Yousef, A.J.A. Male Breast Cancer: Epidemiology and Risk Factors. Semin. Oncol. 2017, 44, 267–272. [Google Scholar] [CrossRef]
- Michaels, E.; Worthington, R.O.; Rusiecki, J. Breast Cancer: Risk Assessment, Screening, and Primary Prevention. Med. Clin. N. Am. 2023, 107, 271–284. [Google Scholar] [CrossRef]
- Sun, Y.S.; Zhao, Z.; Yang, Z.N.; Xu, F.; Lu, H.J.; Zhu, Z.Y.; Shi, W.; Jiang, J.; Yao, P.P.; Zhu, H.P. Risk factors and preventions of breast cancer. Int. J. Biol. Sci. 2017, 13, 1387–1397. [Google Scholar] [CrossRef] [PubMed]
- Koboldt, D.C.; Fulton, R.S.; McLellan, M.D.; Schmidt, H.; Kalicki-Veizer, J.; McMichael, J.F.; Fulton, L.L.; Dooling, D.J.; Ding, L.; Mardis, E.R.; et al. Comprehensive molecular portraits of human breast tumours. Nature 2012, 490, 61–70. [Google Scholar] [CrossRef]
- Curtis, C.; Shah, S.P.; Chin, S.F.; Turashvili, G.; Rueda, O.M.; Dunning, M.J.; Speed, D.; Lynch, A.G.; Samarajiwa, S.; Yuan, Y.; et al. The genomic and transcriptomic architecture of 2000 breast tumours reveals novel subgroups. Nature 2012, 486, 346–352. [Google Scholar] [CrossRef]
- Trayes, K.P.; Cokenakes, S.E.H. Breast Cancer Treatment. Am. Fam. Physician 2021, 104, 171–178. [Google Scholar] [CrossRef]
- Varnier, R.; Sajous, C.; de Talhouet, S.; Smentek, C.; Péron, J.; You, B.; Reverdy, T.; Freyer, G. Using breast cancer gene expression signatures in clinical practice: Unsolved issues, ongoing trials and future perspectives. Cancers 2021, 13, 4840. [Google Scholar] [CrossRef] [PubMed]
- Curigliano, G.; Burstein, H.J.; Gnant, M.; Loibl, S.; Cameron, D.; Regan, M.M.; Denkert, C.; Poortmans, P.; Weber, W.P.; Thürlimann, B.; et al. Understanding breast cancer complexity to improve patient outcomes: The St Gallen International Consensus Conference for the Primary Therapy of Individuals with Early Breast Cancer 2023. Ann. Oncol. 2023, 34, 970–986. [Google Scholar] [CrossRef] [PubMed]
- Mogilner, A.; Rubinstein, B. The physics of filopodial protrusion. Biophys. J. 2005, 89, 782–795. [Google Scholar] [CrossRef] [PubMed]
- Schoumacher, M.; Goldman, R.D.; Louvard, D.; Vignjevic, D.M. Actin, microtubules, and vimentin intermediate filaments cooperate for elongation of invadopodia. J. Cell Biol. 2010, 189, 541–556. [Google Scholar] [CrossRef]
- Adams, J.C. Roles of fascin in cell adhesion and motility. Curr. Opin. Cell Biol. 2004, 16, 590–596. [Google Scholar] [CrossRef]
- Holthuis, J.C.M.; Schoonderwoert, V.T.G.; Martens, G.J.M. A vertebrate homolog of the actin-bundling protein fascin. BBA-Gene Struct. Expr. 1994, 1219, 184–188. [Google Scholar] [CrossRef]
- Lin, S.; Taylor, M.D.; Singh, P.K.; Yang, S. How does fascin promote cancer metastasis? FEBS J. 2021, 288, 1434–1446. [Google Scholar] [CrossRef]
- Li, A.; Dawson, J.C.; Forero-Vargas, M.; Spence, H.J.; Yu, X.; König, I.; Anderson, K.; Machesky, L.M. The Actin-Bundling Protein Fascin Stabilizes Actin in Invadopodia and Potentiates Protrusive Invasion. Curr. Biol. 2010, 20, 339–345. [Google Scholar] [CrossRef]
- Li, C.H.; Chan, M.H.; Liang, S.M.; Chang, Y.C.; Hsiao, M. Fascin-1: Updated biological functions and therapeutic implications in cancer biology. BBA Adv. 2022, 2, 100052. [Google Scholar] [CrossRef]
- Chen, L.; Yang, S.; Jakoncic, J.; Zhang, J.J.; Huang, X.Y. Migrastatin analogues target fascin to block tumour metastasis. Nature 2010, 464, 1062–1066. [Google Scholar] [CrossRef]
- Zheng, S.; Zhong, Q.; Jiang, Q.; Mottamal, M.; Zhang, Q.; Zhu, N.; Burow, M.E.; Worthylake, R.A.; Wang, G. Discovery of a series of thiazole derivatives as novel inhibitors of metastatic cancer cell migration and invasion. ACS Med. Chem. Lett. 2013, 4, 191–196. [Google Scholar] [CrossRef] [PubMed]
- Alburquerque-González, B.; Bernabé-García, M.; Montoro-García, S.; Bernabé-García, Á.; Rodrigues, P.C.; Ruiz Sanz, J.; López-Calderón, F.F.; Luque, I.; Nicolas, F.J.; Cayuela, M.L.; et al. New role of the antidepressant imipramine as a Fascin1 inhibitor in colorectal cancer cells. Exp. Mol. Med. 2020, 52, 281–292. [Google Scholar] [CrossRef] [PubMed]
- Asensi-Cantó, A.; Rodríguez-Braun, E.; Beltrán-Videla, A.; Hurtado, A.M.; Conesa-Zamora, P. Effects of imipramine on cancer patients over-expressing Fascin1; description of the HITCLIF clinical trial. Front. Oncol. 2023, 13, 1238464. [Google Scholar] [CrossRef]
- Timilsina, S.; Rajamanickam, S.; Rao, A.D.; Subbarayalu, P.; Nirzhor, S.; Abdelfattah, N.; Viswanadhapalli, S.; Chen, Y.; Jatoi, I.; Brenner, A.; et al. The antidepressant imipramine inhibits breast cancer growth by targeting estrogen receptor signaling and DNA repair events. Cancer Lett. 2022, 540, 215717. [Google Scholar] [CrossRef]
- Rajamanickam, S.; Panneerdoss, S.; Gorthi, A.; Timilsina, S.; Onyeagucha, B.; Kovalskyy, D.; Ivanov, D.; Hanes, M.A.; Vadlamudi, R.K.; Chen, Y.; et al. Inhibition of FoxM1-Mediated DNA repair by imipramine blue suppresses breast cancer growth and metastasis. Clin. Cancer Res. 2016, 22, 3524–3536. [Google Scholar] [CrossRef]
- Alburquerque-gonzález, B.; Bernabé-garcía, Á.; Bernabé-garcía, M.; Ruiz-sanz, J.; López-calderón, F.F.; Gonnelli, L.; Banci, L.; Peña-garcía, J.; Luque, I.; Nicolás, F.J.; et al. The fda-approved antiviral raltegravir inhibits fascin1-dependent invasion of colorectal tumor cells in vitro and in vivo. Cancers 2021, 13, 861. [Google Scholar] [CrossRef]
- Proietti, S.; Catizone, A.; Masiello, M.G.; Dinicola, S.; Fabrizi, G.; Minini, M.; Ricci, G.; Verna, R.; Reiter, R.J.; Cucina, A.; et al. Increase in motility and invasiveness of MCF7 cancer cells induced by nicotine is abolished by melatonin through inhibition of ERK phosphorylation. J. Pineal Res. 2018, 64, e12467. [Google Scholar] [CrossRef]
- Mahmoud, A.; Elkhalifa, D.; Alali, F.; Al Moustafa, A.-E.; Khalil, A. Novel Polymethoxylated Chalcones as Potential Compounds Against KRAS-Mutant Colorectal Cancers. Curr. Pharm. Des. 2020, 26, 1622–1633. [Google Scholar] [CrossRef] [PubMed]
- Kim, M.J.; Park, K.S.; Kim, K.T.; Gil, E.Y. The inhibitory effect of curcumin via fascin suppression through JAK/STAT3 pathway on metastasis and recurrence of ovary cancer cells. BMC Women’s Health 2020, 20, 256. [Google Scholar] [CrossRef]
- Jabeen, A.; Sharma, A.; Gupta, I.; Kheraldine, H.; Vranic, S.; Al Moustafa, A.E.; Al Farsi, H.F. Elaeagnus angustifolia plant extract inhibits epithelial-mesenchymal transition and induces apoptosis via HER2 inactivation and JNK pathway in HER2-positive breast cancer cells. Molecules 2020, 25, 4240. [Google Scholar] [CrossRef]
- Cuya, S.M.; Bjornsti, M.A.; van Waardenburg, R.C.A.M. DNA topoisomerase-targeting chemotherapeutics: What’s new? Cancer Chemother. Pharmacol. 2017, 80, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Nitiss, J.L. Targeting DNA topoisomerase II in cancer chemotherapy. Nat. Rev. Cancer 2009, 9, 338–350. [Google Scholar] [CrossRef]
- Almeida, D.; Gerhard, R.; Leitão, D.; Davilla, C.; Damasceno, M.; Schmitt, F. Topoisomerase II-alfa gene as a predictive marker of response to anthracyclines in breast cancer. Pathol. Res. Pract. 2014, 210, 675–679. [Google Scholar] [CrossRef]
- Min, K.W.; Chae, S.W.; Kim, D.H.; Do, S.I.; Kim, K.; Lee, H.J.; Sohn, J.H.; Pyo, J.S.; Kim, D.H.; Oh, S.; et al. Fascin expression predicts an aggressive clinical course in patients with advanced breast cancer. Oncol. Lett. 2015, 10, 121–130. [Google Scholar] [CrossRef] [PubMed]
- Abbasi, A.; Noroozinia, F.; Anvar, S.; Abbasi, M.A.; Hosseinzadeh, S.; Mokhtari, S.A.S. Fascin overexpression is associated with higher grades of breast cancer. Polish J. Pathol. 2019, 70, 264–268. [Google Scholar] [CrossRef] [PubMed]
- Esnakula, A.K.; Ricks-Santi, L.; Kwagyan, J.; Kanaan, Y.M.; DeWitty, R.L.; Wilson, L.L.; Gold, B.; Frederick, W.A.I.; Naab, T.J. Strong association of fascin expression with triple negative breast cancer and basal-like phenotype in African-American women. J. Clin. Pathol. 2014, 67, 153–160. [Google Scholar] [CrossRef]
- Tsuchiya, H. Fascin is Expressed in Basal-Liketype Triple Negative Breast Cancer Associated with High Malignant Potential in Japanese Women. Int. J. Cancer Clin. Res. 2015, 2, 035. [Google Scholar] [CrossRef]
- Grothey, A.; Hashizume, R.; Sahin, A.A.; McCrea, P.D. Fascin, an actin-bundling protein associated with cell motility, is upregulated in hormone receptor negative breast cancer. Br. J. Cancer 2000, 83, 870–873. [Google Scholar] [CrossRef]
- Wang, C.Q.; Tang, C.H.; Chang, H.T.; Li, X.N.; Zhao, Y.M.; Su, C.M.; Hu, G.N.; Zhang, T.; Sun, X.X.; Zeng, Y.; et al. Fascin-1 as a novel diagnostic marker of triple-negative breast cancer. Cancer Med. 2016, 5, 1983–1988. [Google Scholar] [CrossRef]
- Rodríguez-Pinilla, S.M.; Sarrío, D.; Honrado, E.; Hardisson, D.; Calero, F.; Benitez, J.; Palacios, J. Prognostic significance of basal-like phenotype and fascin expression in node-negative invasive breast carcinomas. Clin. Cancer Res. 2006, 12, 1533–1539. [Google Scholar] [CrossRef]
- Yoder, B.J.; Tso, E.; Skacel, M.; Pettay, J.; Tarr, S.; Budd, T.; Tubbs, R.R.; Adams, J.C.; Hicks, D.G. The expression of fascin, an actin-bundling motility protein, correlates with hormone receptor-negative breast cancer and a more aggressive clinical course. Clin. Cancer Res. 2005, 11, 186–192. [Google Scholar] [CrossRef] [PubMed]
- Tampaki, E.C.; Tampakis, A.; Nonni, A.; von Flüe, M.; Patsouris, E.; Kontzoglou, K.; Kouraklis, G. Combined Fascin-1 and MAP17 Expression in Breast Cancer Identifies Patients with High Risk for Disease Recurrence. Mol. Diagn. Ther. 2019, 23, 635–644. [Google Scholar] [CrossRef]
- Lee, H.J.; An, H.J.; Kim, T.H.; Kim, G.; Kang, H.; Heo, J.H.; Kwon, A.Y.; Kim, S. Fascin expression is inversely correlated with breast cancer metastasis suppressor 1 and predicts a worse survival outcome in node-negative breast cancer patients. J. Cancer 2017, 8, 3122–3129. [Google Scholar] [CrossRef] [PubMed]
- Erdogan, G.; Peştereli, H.E.; Çolal, T.; Karaveli, F.; Şeyda, A.M. Fascin Expression in Invasive Ductal Carcinoma of Breast. Turk. J. Pathol. 2010, 26, 209. [Google Scholar] [CrossRef][Green Version]
- Al-Alwan, M.; Olabi, S.; Ghebeh, H.; Barhoush, E.; Tulbah, A.; Al-Tweigeri, T.; Ajarim, D.; Adra, C. Fascin is a key regulator of breast cancer invasion that acts via the modification of metastasis-associated molecules. PLoS ONE 2011, 6, e27339. [Google Scholar] [CrossRef]
- Eltohamy, M.I.; Badawy, O.M.; El kinaai, N.; Loay, I.; Nassar, H.R.; Allam, R.M.; Sakr, M.A. Topoisomerase II α Gene alteration in triple negative breast cancer and its predictive role for anthracycline-based chemotherapy (Egyptian NCI Patients). Asian Pac. J. Cancer Prev. 2018, 19, 3581–3589. [Google Scholar] [CrossRef]
- Kalogeraki, A.; Ieromonachou, P.; Kafousi, M.; Giannikaki, E.; Vrekoussis, T.; Zoras, O.; Tsiftsis, D.; Delides, G.; Stathopoulos, E. Topoisomerase II alpha expression in breast ductal invasive carcinomas and correlation with clinicopathological variables. Vivo 2005, 19, 837–840. [Google Scholar]
- Romero, A.; Martín, M.; Cheang, M.C.U.; García-Asenjo, J.A.L.; Oliva, B.; He, X.; De La Hoya, M.; Sáenz, J.Á.G.; Fernández, M.A.; Rubio, E.D.; et al. Assessment of topoisomerase II α status in breast cancer by quantitative PCR, gene expression microarrays, immunohistochemistry, and fluorescence in Situ hybridization. Am. J. Pathol. 2011, 178, 1453–1460. [Google Scholar] [CrossRef]
- An, X.; Xu, F.; Luo, R.; Zheng, Q.; Lu, J.; Yang, Y.; Qin, T.; Yuan, Z.; Shi, Y.; Jiang, W.; et al. The prognostic significance of topoisomerase II alpha protein in early stage luminal breast cancer. BMC Cancer 2018, 18, 331. [Google Scholar] [CrossRef]
- Fountzilas, G.; Valavanis, C.; Kotoula, V.; Eleftheraki, A.G.; Kalogeras, K.T.; Tzaida, O.; Batistatou, A.; Kronenwett, R.; Wirtz, R.M.; Bobos, M.; et al. HER2 and TOP2A in high-risk early breast cancer patients treated with adjuvant epirubicin-based dose-dense sequential chemotherapy. J. Transl. Med. 2012, 10, 10. [Google Scholar] [CrossRef]
- Shigematsu, H.; Ozaki, S.; Yasui, D.; Yamamoto, H.; Zaitsu, J.; Taniyama, D.; Saitou, A.; Kuraoka, K.; Hirata, T.; Taniyama, K. Overexpression of topoisomerase II alpha protein is a factor for poor prognosis in patients with luminal B breast cancer. Oncotarget 2018, 9, 26701–26710. [Google Scholar] [CrossRef]
- Glynn, R.W.; Miller, N.; Whelan, M.C.; Kerin, M.J. Topoisomerase 2 alpha and the case for individualized breast cancer therapy. Ann. Surg. Oncol. 2010, 17, 1392–1397. [Google Scholar] [CrossRef] [PubMed]
- O’Malley, F.P.; Chia, S.; Tu, D.; Shepherd, L.E.; Levine, M.N.; Bramwell, V.H.; Andrulis, I.L.; Pritchard, K.I. Topoisomerase II alpha and responsiveness of breast cancer to adjuvant chemotherapy. J. Natl. Cancer Inst. 2009, 101, 644–650. [Google Scholar] [CrossRef] [PubMed]
- Arriola, E.; Rodriguez-Pinilla, S.M.; Lambros, M.B.K.; Jones, R.L.; James, M.; Savage, K.; Smith, I.E.; Dowsett, M.; Reis-Filho, J.S. Topoisomerase II alpha amplification may predict benefit from adjuvant anthracyclines in HER2 positive early breast cancer. Breast Cancer Res. Treat. 2007, 106, 181–189. [Google Scholar] [CrossRef]
- Tubbs, R.; Barlow, W.E.; Budd, G.T.; Swain, E.; Porter, P.; Gown, A.; Yeh, I.T.; Sledge, G.; Shapiro, C.; Ingle, J.; et al. Outcome of patients with early-stage breast cancer treated with doxorubicin-based adjuvant chemotherapy as a function of HER2 and TOP2A status. J. Clin. Oncol. 2009, 27, 3881–3886. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.R.; Chien, H.P.; Chen, K.S.; Hwang, C.C.; Chen, H.Y.; Yeh, K.Y.; Hsieh, T.Y.; Chang, L.C.; Hsu, Y.C.; Lu, R.J.; et al. Amplification of HER2 and TOP2A and deletion of TOP2A genes in a series of Taiwanese breast cancer. Medicine 2017, 96, e5582. [Google Scholar] [CrossRef]
- Park, K.; Han, S.; Gwak, G.H.; Kim, H.J.; Kim, J.; Kim, K.M. Topoisomerase II-alpha gene deletion is not frequent as its amplification in breast cancer. Breast Cancer Res. Treat. 2006, 98, 337–342. [Google Scholar] [CrossRef]
- Engelstaedter, V.; Schiffers, J.; Kahlert, S.; Mainka, P.; Engel, J.; Kirchner, T.; Diebold, J.; Mayr, D. Her-2/neu and topoisomerase IIα in advanced breast cancer: A comprehensive FISH analysis of 245 cases. Diagn. Mol. Pathol. 2012, 21, 77–83. [Google Scholar] [CrossRef]
- Todorović-Raković, N.; Nešković-Konstantinović, Z.; Nikolić-Vukosavljević, D. Metastatic breast cancer survival according to HER2 and Topo2a gene status. Dis. Markers 2009, 26, 171–180. [Google Scholar] [CrossRef]
- Mrklić, I.; Pogorelić, Z.; Ćapkun, V.; Tomić, S. Expression of topoisomerase II-α in triple negative breast cancer. Appl. Immunohistochem. Mol. Morphol. 2014, 22, 182–187. [Google Scholar] [CrossRef]
- Guestini, F.; Ono, K.; Miyashita, M.; Ishida, T.; Ohuchi, N.; Nakagawa, S.; Hirakawa, H.; Tamaki, K.; Ohi, Y.; Rai, Y.; et al. Impact of Topoisomerase IIα, PTEN, ABCC1/MRP1, and KI67 on triple-negative breast cancer patients treated with neoadjuvant chemotherapy. Breast Cancer Res. Treat. 2019, 173, 275–288. [Google Scholar] [CrossRef] [PubMed]
- Neama, R.; Habib, M.; Ali, S.; Al-Khafaji, A.; Alqanbar, M. Assessment of topoisomerase II-alpha gene status by dual color chromogenic in situ hybridization in a set of Iraqi patients with invasive breast carcinoma. Indian J. Pathol. Microbiol. 2017, 60, 475–480. [Google Scholar] [CrossRef] [PubMed]
- Tokiniwa, H.; Horiguchi, J.; Takata, D.; Kikuchi, M.; Rokutanda, N.; Nagaoka, R.; Sato, A.; Odawara, H.; Tozuka, K.; Oyama, T.; et al. Topoisomerase II alpha expression and the Ki-67 labeling index correlate with prognostic factors in estrogen receptor-positive and human epidermal growth factor type-2-negative breast cancer. Breast Cancer 2012, 19, 309–314. [Google Scholar] [CrossRef]
- Şahin, S.; Işik Gönül, I.; Çaklr, A.; Seçkin, S.; Uluoǧlu, Ö. Clinicopathological Significance of the Proliferation Markers Ki67, RacGAP1, and Topoisomerase 2 Alpha in Breast Cancer. Int. J. Surg. Pathol. 2016, 24, 607–613. [Google Scholar] [CrossRef] [PubMed]
- Won, H.S.; Lee, K.E.; Sung, S.H.; Choi, M.Y.; Jo, J.Y.; Nam, E.M.; Mun, Y.C.; Seong, C.M.; Lee, S.N. Topoisomerase II alpha and microtubuleassociated protein-tau as a predictive marker in axillary lymph node positive breast cancer. Tumori 2014, 100, 80–86. [Google Scholar] [CrossRef]
- Honma, N.; Horii, R.; Ito, Y.; Saji, S.; Younes, M.; Iwase, T.; Akiyama, F. Differences in clinical importance of Bcl-2 in breast cancer according to hormone receptors status or adjuvant endocrine therapy. BMC Cancer 2015, 15, 698. [Google Scholar] [CrossRef]
- Min, K.W.; Kim, D.H.; Do, S.I.; Chae, S.W.; Kim, K.; Sohn, J.H.; Pyo, J.S.; Lee, H.J.; Kim, D.H.; Oh, S.; et al. Negative association between GATA3 and fascin could predict relapse-free and overall survival in patients with breast cancer. Virchows Arch. 2016, 468, 409–416. [Google Scholar] [CrossRef]
- Mrklić, I.; Spagnoli, G.C.; Juretić, A.; Pogorelić, Z.; Tomić, S. Co-expression of cancer testis antigens and topoisomerase 2-alpha in triple negative breast carcinomas. Acta Histochem. 2014, 116, 740–746. [Google Scholar] [CrossRef]
- Elston, C.W.; Ellis, I.O. Pathological prognostic factors in breast cancer. I. The value of histological grade in breast cancer: Experience from a large study with long-term follow-up. C. W. Elston & I. O. Ellis. Histopathology 1991; 19; 403-410—Author commentary. Histopathology 2002, 41, 151. [Google Scholar] [CrossRef]
- Varga, Z.; Moelans, C.B.; Zuerrer-Hardi, U.; Ramach, C.; Behnke, S.; Kristiansen, G.; Moch, H. Topoisomerase 2A gene amplification in breast cancer. Critical evaluation of different FISH probes. Breast Cancer Res. Treat. 2012, 133, 929–935. [Google Scholar] [CrossRef]
- Amin, M.B.; Greene, F.L.; Edge, S.B.; Compton, C.C.; Gershenwald, J.E.; Brookland, R.K.; Meyer, L.; Gress, D.M.; Byrd, D.R.; Winchester, D.P. The Eighth Edition AJCC Cancer Staging Manual: Continuing to build a bridge from a population-based to a more “personalized” approach to cancer staging. CA. Cancer J. Clin. 2017, 67, 93–99. [Google Scholar] [CrossRef] [PubMed]
| Pearson’s χ² p = 0.610 | TOP2A Expression | Total | ||||
|---|---|---|---|---|---|---|
| Amplif | Gain | Normal | Deletion | |||
| Histological subtypes | Adenoid cystic | 0 | 0 | 2 | 0 | 2 |
| Apocrine | 0 | 0 | 5 | 0 | 5 | |
| Infiltrating lobular | 3 | 2 | 15 | 0 | 20 | |
| Medullary | 0 | 0 | 8 | 1 | 9 | |
| Micropapillary | 0 | 1 | 3 | 0 | 4 | |
| Mucinous | 3 | 6 | 11 | 1 | 21 | |
| NOS (Not Otherwise Specified) | 6 | 8 | 72 | 4 | 90 | |
| Infiltrating papillary | 0 | 2 | 10 | 0 | 12 | |
| Tubular | 0 | 2 | 10 | 0 | 12 | |
| Total | 12 | 21 | 136 | 6 | 175 | |
| Fascin | TOP2A | |||||
|---|---|---|---|---|---|---|
| Characteristic | High n (%) | Low n (%) | p | Normal n (%) | Altered n (%) | p |
| Molecular subtype | <0.001 | 0.041 | ||||
| Luminal A | 8 (14) | 78 (39) | 42 (33) | 6 (16) | ||
| Luminal B HER2− | 16 (29) | 93 (47) | 55 (43) | 20 (53) | ||
| Luminal B HER2+ | 5 (9) | 14 (7) | 9 (7) | 8 (21) | ||
| HER2+ | 0 (0) | 2 (1) | 1 (1) | 0 (0) | ||
| TNBC | 27 (48) | 12 (6) | 20 (16) | 4 (10) | ||
| Histological subtype | <0.001 | 0.188 | ||||
| Adenoid cystic | 3 (5) | 0 (0) | 2 (2) | 0 (0) | ||
| Apocrine | 2 (3) | 3 (2) | 5 (4) | 0 (0) | ||
| Invasive lobular | 5 (8) | 42 (21) | 15 (11) | 5 (13) | ||
| Medullary | 6 (10) | 6 (3) | 8 (6) | 1 (3) | ||
| Micropapillary | 1 (2) | 5 (2) | 3 (2) | 1 (3) | ||
| Mucinous | 1 (2) | 23 (11) | 11 (8) | 10 (25) | ||
| NOS (Not Otherwise Specified) | 41 (68) | 92 (45) | 72 (53) | 18 (46) | ||
| Invasive papillary | 0 (0) | 15 (7) | 10 (7) | 2 (5) | ||
| Tubular | 1 (2) | 19 (9) | 10 (7) | 2 (5) | ||
| ER | <0.001 | 0.166 | ||||
| Positive | 31 (52) | 185 (90) | 109 (80) | 35 (90) | ||
| Negative | 29 (48) | 20 (10) | 27 (20) | 4 (10) | ||
| PR | <0.001 | 0.240 | ||||
| Positive | 31 (52) | 164 (80) | 99 (73) | 32 (82) | ||
| Negative | 29 (48) | 41 (20) | 37 (27) | 7 (18) | ||
| BCL2 | 0.003 | 0.494 | ||||
| Positive | 40 () | 173 () | 107 (82) | 32 (87) | ||
| Negative | 17 () | 26 () | 24 (18) | 5 (13) | ||
| pT a | 0.692 | 0.858 | ||||
| pTis | 0 (0) | 1 (1) | 1 (1) | 0 (0) | ||
| pT1 | 15 (44) | 73 (48) | 52 (53) | 11 (46) | ||
| pT2 | 15 (44) | 63 (42) | 38 (38) | 11 (46) | ||
| pT3 | 4 (12) | 10 (7) | 6 (6) | 2 (8) | ||
| pT4 | 0 (0) | 4 (3) | 2 (2) | 0 (0) | ||
| pN a | 0.478 | 0.659 | ||||
| pN0 | 17 (53) | 84 (59) | 55 (59) | 16 (70) | ||
| pN1 | 10 (31) | 49 (34) | 32 (35) | 5 (22) | ||
| pN2 | 3 (10) | 6 (4) | 4 (4) | 1 (4) | ||
| pN3 | 2 (6) | 4 (3) | 2 (2) | 1 (4) | ||
| pM a | 0.289 | 0.476 | ||||
| pM0 | 31 (100) | 137 (97) | 90 (98) | 23 (100) | ||
| pM1 | 0 (0) | 5 (3) | 2 (2) | 0 (0) | ||
| cT b | 0.716 | 0.722 | ||||
| cTis | 0 (0) | 0 (0) | 0 (0) | 0 (0) | ||
| cT1 | 2 (9) | 2 (7) | 3 (10) | 0 (0) | ||
| cT2 | 12 (54) | 12 (41) | 12 (42) | 4 (57) | ||
| cT3 | 5 (23) | 8 (28) | 7 (24) | 2 (29) | ||
| cT4 | 3 (14) | 7 (24) | 7 (24) | 1 (14) | ||
| cN b | 0.687 | 0.176 | ||||
| cN0 | 6 (27) | 8 (28) | 6 (21) | 4 (57) | ||
| cN1 | 9 (41) | 8 (28) | 10 (34) | 1 (14) | ||
| cN2 | 3 (14) | 4 (13) | 6 (21) | 0 (0) | ||
| cN3 | 4 (18) | 9 (31) | 7 (24) | 2 (29) | ||
| cM b | 0.823 | 0.211 | ||||
| cM0 | 18 (82) | 23 (79) | 26 (90) | 5 (71) | ||
| cM1 | 4 (18) | 6 (21) | 3 (10) | 2 (29) | ||
| p53 | 0.962 | 0.822 | ||||
| Positive | 40 (70) | 139 (70) | 96 (74) | 28 (76) | ||
| Negative | 17 (30) | 60 (30) | 34 (26) | 9 (24) | ||
| Ki67 | 0.002 | 0.048 | ||||
| <14% | 11 (18) | 82 (40) | 47 (35) | 7 (18) | ||
| ≥14% | 49 (82) | 123 (60) | 89 (65) | 32 (82) | ||
| E-Cadherin | 0.194 | 0.989 | ||||
| Positive | 52 (90) | 166 (83) | 118 (89) | 34 (90) | ||
| Negative | 6 (10) | 35 (17) | 14 (11) | 4 (10) | ||
| Fascin | 0.893 | |||||
| High | - | - | 30 (22) | 9 (23) | ||
| Low | - | - | 106 (78) | 30 (77) | ||
| TOP2A | 0.893 | |||||
| Altered | 9 (23) | 30 (22) | - | - | ||
| Normal | 30 (77) | 106 (78) | - | - | ||
| SBR c | <0.001 | 0.033 | ||||
| 3 | 0 (0) | 9 (5) | 5 (4) | 0 (0) | ||
| 4 | 4 (7) | 24 (12) | 14 (11) | 3 (8) | ||
| 5 | 3 (5) | 40 (20) | 24 (18) | 6 (15) | ||
| 6 | 12 (21) | 65 (32) | 32 (24) | 15 (38) | ||
| 7 | 17 (29) | 36 (18) | 24 (18) | 13 (33) | ||
| 8 | 18 (31) | 24 (12) | 29 (22) | 1 (3) | ||
| 9 | 4 (7) | 3 (1) | 3 (2) | 1 (3) | ||
| SBR c per grades | <0.001 | <0.001 | ||||
| Grade 1 | 7 (12) | 73 (36) | 43 (33) | 9 (23) | ||
| Grade 2 | 29 (50) | 101 (50) | 56 (43) | 28 (72) | ||
| Grade 3 | 22 (38) | 27 (14) | 32 (24) | 2 (5) | ||
| Binary Logistic Regression (pCR) | OR | CI 95% | p |
|---|---|---|---|
| Univariate analysis | |||
| ER | 1.029 | 1.009–1.050 | 0.005 |
| PR | 1.020 | 0.996–1.044 | 0.098 |
| p53 | 0.989 | 0.972–1.007 | 0.239 |
| BCL2 | 1.024 | 1.006–1.043 | 0.010 |
| Ki67 | 0.957 | 0.930–0.986 | 0.003 |
| E-Cadherin | 1.917 | 0.110–33.412 | 0.656 |
| Histological grade SBR | 0.434 | 0.196–0.963 | 0.040 |
| Menopause | 1.731 | 0.411–7.288 | 0.455 |
| Age | 1.022 | 0.941–1.067 | 0.956 |
| Clinical stage | 3.680 | 1.026–13.197 | 0.046 |
| Histological subtype | 0.871 | 0.528–1.436 | 0.588 |
| Molecular subtype | 0.169 | 0.045–0.641 | 0.009 |
| TNBC/NO TNBC | 7 | 1.350–36.306 | 0.021 |
| Fascin (+/−) | 2.667 | 0.664–10.704 | 0.167 |
| TOP2A | 0 | 0–0 | 0.999 |
| Multivariate analysis | |||
| Clinical stage | 9.066 | 0.875–93.880 | 0.065 |
| Histological grade SBR | 2.576 | 0.477–13.925 | 0.272 |
| Molecular subtype | 0.037 | 0.002–0.819 | 0.037 |
| Chi-Sqr | Degrees of Freedom | p | |
|---|---|---|---|
| Log Rank (Mantel-Cox) | |||
| Histological subtype | 21.642 | 8 | 0.006 |
| Molecular subtype | 5.857 | 4 | 0.210 |
| CK19 (+/−) | 0.368 | 1 | 0.544 |
| Menopause (Yes/No) | 3.727 | 1 | 0.054 |
| Pathological tumor stage | 14.805 | 3 | 0.002 |
| Tumor size (pathological T) | 21.895 | 4 | <0.001 |
| Affected lymph nodes (pathological n) | 15.377 | 3 | 0.002 |
| Metastasis (pathological M) | 6.477 | 1 | 0.011 |
| IHC HER2 | 5.382 | 3 | 0.146 |
| E-Cadherin (+/−) | 1.009 | 1 | 0.315 |
| ER (+/−) | 5.456 | 1 | 0.020 |
| PR (+/−) | 1.646 | 1 | 0.200 |
| p53 (+/−) | 3.585 | 1 | 0.058 |
| BCL2 (+/−) | 4.192 | 1 | 0.041 |
| Ki67(<14%/≥14%) | 3.836 | 1 | 0.050 |
| Age (<65/≥65) | 12.657 | 1 | <0.001 |
| Histological grade SBR (grouped) | 2.844 | 2 | 0.241 |
| TOP2A | 0.034 | 1 | 0.853 |
| Fascin | 1.783 | 1 | 0.181 |
| Cox regression (multivariate analysis) | |||
| HR | CI 95% | p | |
| Histological subtype | 2.437 | 0.648–9.162 | 0.187 |
| Pathological tumor stage | 2.803 | 1.467–5.356 | 0.002 |
| ER (+/−) | 3.120 | 0.157–61.854 | 0.455 |
| BCL2 (+/−) | 0.349 | 0.056–2.190 | 0.261 |
| Age (<65/≥65) | 5.098 | 0.627–41.410 | 0.128 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sánchez-Espinosa, A.; García-Rodríguez, J.; Alonso-Aguirre, V.; Acosta-Ortega, J.M.; Conesa-Zamora, P.; García-Solano, J.; Luengo-Gil, G. Expression of Fascin and DNA Topoisomerase 2-Alpha in Breast Carcinoma: Correlation with Histological Subtypes and Other Prognostic Markers. Int. J. Mol. Sci. 2025, 26, 3076. https://doi.org/10.3390/ijms26073076
Sánchez-Espinosa A, García-Rodríguez J, Alonso-Aguirre V, Acosta-Ortega JM, Conesa-Zamora P, García-Solano J, Luengo-Gil G. Expression of Fascin and DNA Topoisomerase 2-Alpha in Breast Carcinoma: Correlation with Histological Subtypes and Other Prognostic Markers. International Journal of Molecular Sciences. 2025; 26(7):3076. https://doi.org/10.3390/ijms26073076
Chicago/Turabian StyleSánchez-Espinosa, Alberto, José García-Rodríguez, Virginia Alonso-Aguirre, Jesús María Acosta-Ortega, Pablo Conesa-Zamora, José García-Solano, and Ginés Luengo-Gil. 2025. "Expression of Fascin and DNA Topoisomerase 2-Alpha in Breast Carcinoma: Correlation with Histological Subtypes and Other Prognostic Markers" International Journal of Molecular Sciences 26, no. 7: 3076. https://doi.org/10.3390/ijms26073076
APA StyleSánchez-Espinosa, A., García-Rodríguez, J., Alonso-Aguirre, V., Acosta-Ortega, J. M., Conesa-Zamora, P., García-Solano, J., & Luengo-Gil, G. (2025). Expression of Fascin and DNA Topoisomerase 2-Alpha in Breast Carcinoma: Correlation with Histological Subtypes and Other Prognostic Markers. International Journal of Molecular Sciences, 26(7), 3076. https://doi.org/10.3390/ijms26073076







