Epigenetic Echoes: Bridging Nature, Nurture, and Healing Across Generations
Abstract
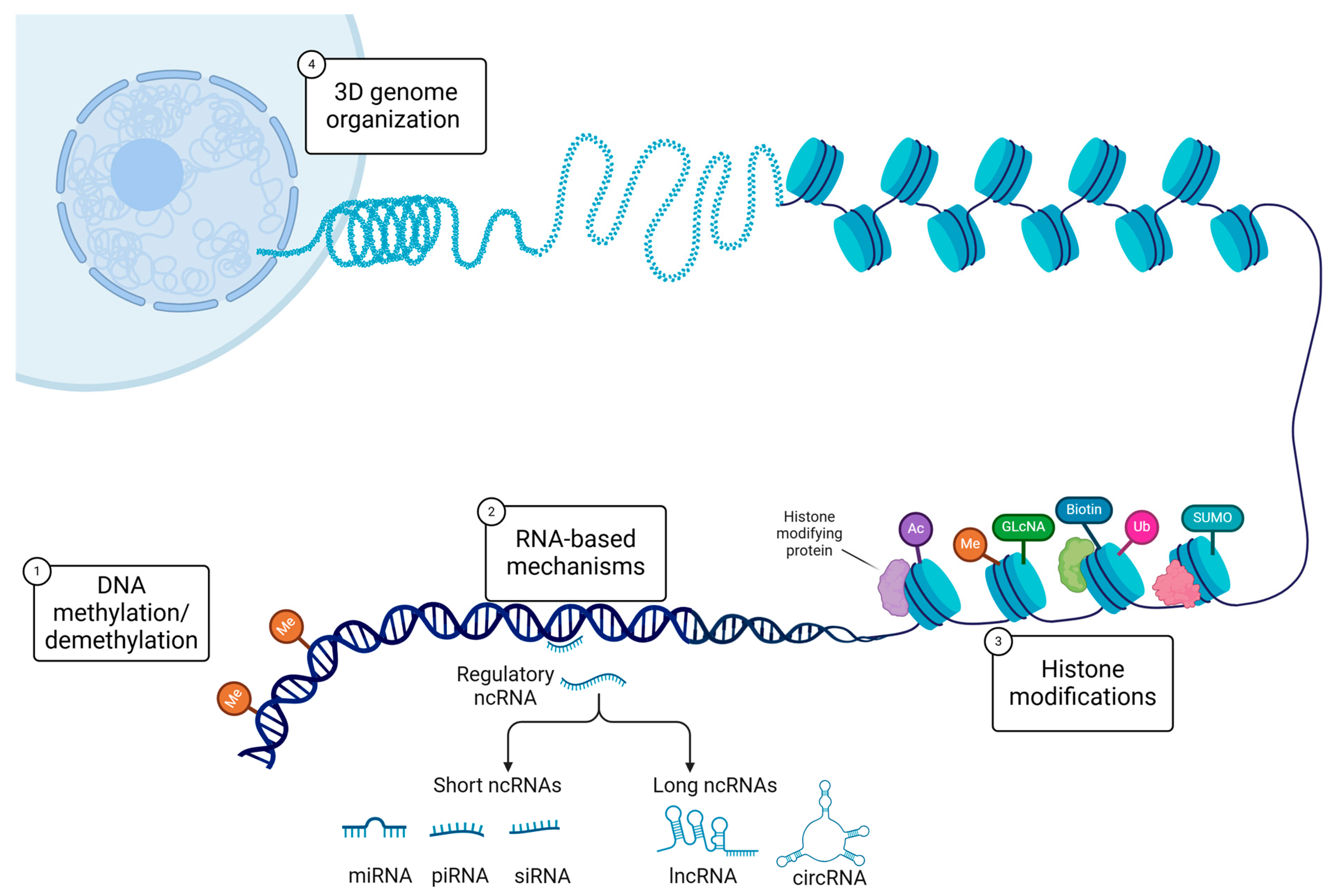
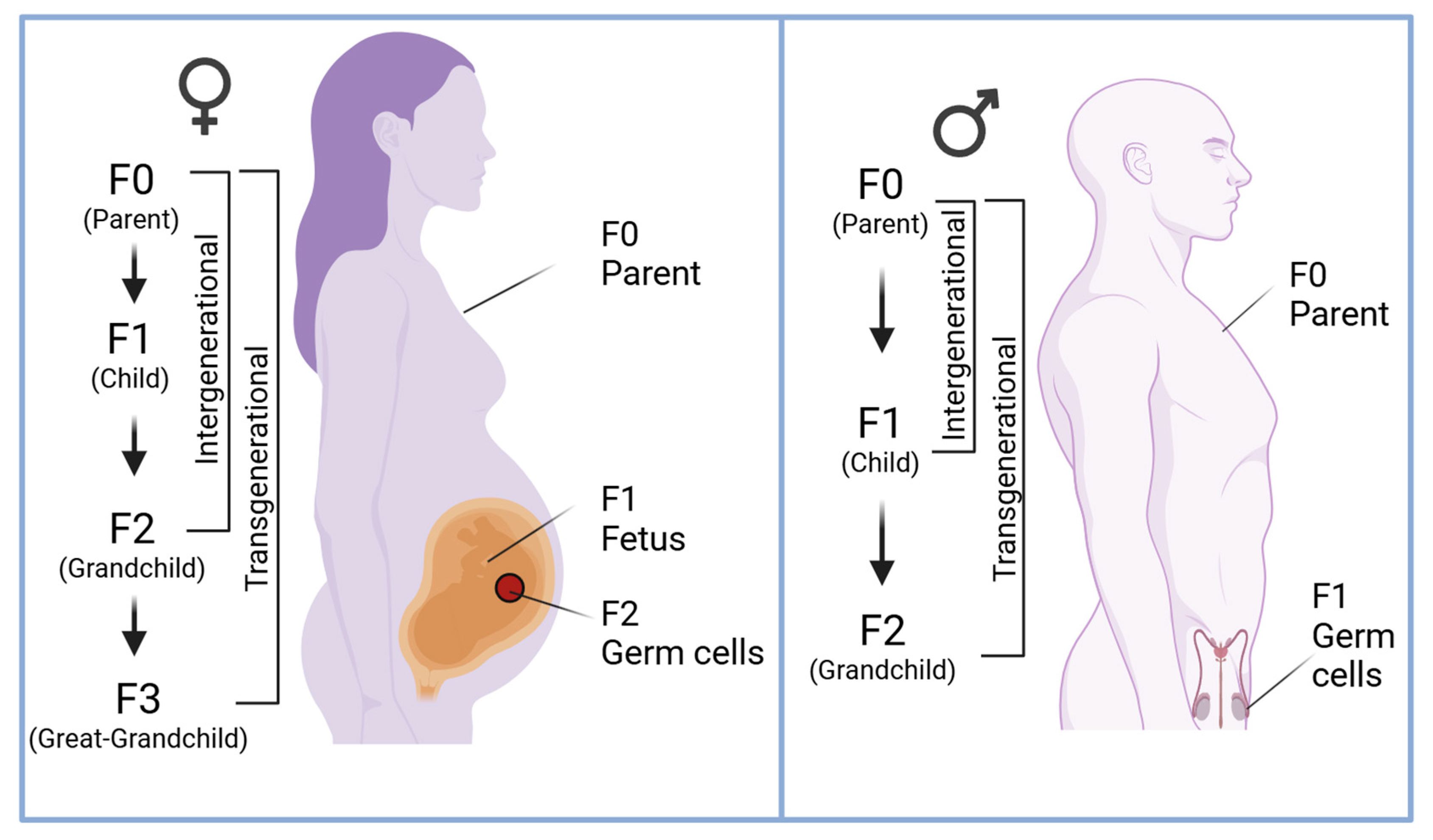
1. Introduction
2. DNA Methylation and Trauma Across Generations
3. Histone Modifications
4. Non-Coding RNAs in Transgenerational Inheritance
5. Challenges and Controversies in Studying Trauma Across Generations
Genetics/Epigenetics May Load the Gun, but the Environment Pulls the Trigger
6. Pathways to Healing: Reversing the Epigenetic Effects of Trauma
7. Ethical Considerations in Framing Trauma as Inheritable
8. Breaking Generational Chains: A Personal Reflection
9. Research Directions
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Waddington, C.H. Epigenetics and evolution. Symp. Soc. Exp. Biol. 1953, 7, 186–199. [Google Scholar]
- Millán-Zambrano, G.; Burton, A.; Bannister, A.J.; Schneider, R. Histone post-translational modifications—Cause and consequence of genome function. Nat. Rev. Genet. 2022, 23, 563–580. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.K.; Samaranayake, M.; Pradhan, S. Epigenetic mechanisms in mammals. Cell. Mol. Life Sci. 2009, 66, 596–612. [Google Scholar] [PubMed]
- Greally, J.M. A user’s guide to the ambiguous word ‘epigenetics’. Nat. Rev. Mol. Cell Biol. 2018, 19, 207–208. [Google Scholar] [CrossRef]
- Rando, O.J.; Chang, H.Y. Genome-wide views of chromatin structure. Annu. Rev. Biochem. 2009, 78, 245–271. [Google Scholar] [CrossRef]
- Jaenisch, R.; Bird, A. Epigenetic regulation of gene expression: How the genome integrates intrinsic and environmental signals. Nat. Genet. 2003, 33, 245–254. [Google Scholar]
- Bernstein, B.E.; Meissner, A.; Lander, E.S. The mammalian epigenome. Cell 2007, 128, 669–681. [Google Scholar] [CrossRef]
- Smith, Z.D.; Hetzel, S.; Meissner, A. DNA methylation in mammalian development and disease. Nat. Rev. Genet. 2024, 26, 7–30. [Google Scholar]
- Jones, P.A. Functions of DNA methylation: Islands, start sites, gene bodies and beyond. Nat. Rev. Genet. 2012, 13, 484–492. [Google Scholar] [CrossRef]
- Greenberg, M.V.C.; Bourc’his, D. The diverse roles of DNA methylation in mammalian development and disease. Nat. Rev. Mol. Cell Biol. 2019, 20, 590–607. [Google Scholar]
- Baubec, T.; Schübeler, D. Genomic patterns and context specific interpretation of DNA methylation. Curr. Opin. Genet. Dev. 2014, 25, 85–92. [Google Scholar] [CrossRef] [PubMed]
- Ambrosi, C.; Manzo, M.; Baubec, T. Dynamics and Context-Dependent Roles of DNA Methylation. J. Mol. Biol. 2017, 429, 1459–1475. [Google Scholar] [CrossRef] [PubMed]
- Mohn, F.; Schübeler, D. Genetics and epigenetics: Stability and plasticity during cellular differentiation. Trends Genet. 2009, 25, 129–136. [Google Scholar]
- Wu, X.; Zhang, Y. TET-mediated active DNA demethylation: Mechanism, function and beyond. Nat. Rev. Genet. 2017, 18, 517–534. [Google Scholar] [CrossRef]
- Wu, H.; Zhang, Y. Reversing DNA Methylation: Mechanisms, Genomics, and Biological Functions. Cell 2014, 156, 45–68. [Google Scholar] [CrossRef] [PubMed]
- He, Y.-F.; Li, B.-Z.; Li, Z.; Liu, P.; Wang, Y.; Tang, Q.; Ding, J.; Jia, Y.; Chen, Z.; Li, L. Tet-mediated formation of 5-carboxylcytosine and its excision by TDG in mammalian DNA. Science 2011, 333, 1303–1307. [Google Scholar]
- Tahiliani, M.; Koh, K.P.; Shen, Y.; Pastor, W.A.; Bandukwala, H.; Brudno, Y.; Agarwal, S.; Iyer, L.M.; Liu, D.R.; Aravind, L.; et al. Conversion of 5-methylcytosine to 5-hydroxymethylcytosine in mammalian DNA by MLL partner TET1. Science 2009, 324, 930–935. [Google Scholar] [CrossRef]
- Lee, S.-M. Detecting DNA hydroxymethylation: Exploring its role in genome regulation. BMB Rep. 2024, 57, 135. [Google Scholar] [PubMed]
- Balhorn, R. The protamine family of sperm nuclear proteins. Genome Biol. 2007, 8, 227. [Google Scholar] [CrossRef]
- Wang, T.; Gao, H.; Li, W.; Liu, C. Essential role of histone replacement and modifications in male fertility. Front. Genet. 2019, 10, 962. [Google Scholar]
- Bannister, A.J.; Kouzarides, T. Regulation of chromatin by histone modifications. Cell Res. 2011, 21, 381–395. [Google Scholar] [CrossRef] [PubMed]
- Kouzarides, T. Chromatin modifications and their function. Cell 2007, 128, 693–705. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.; Wu, J.; Guo, H.; Yao, W.; Li, S.; Lu, Y.; Jia, Y.; Liang, X.; Tang, J.; Zhang, H. Post-translational modifications of histones: Mechanisms, biological functions, and therapeutic targets. MedComm 2023, 4, e292. [Google Scholar] [CrossRef]
- Jenuwein, T.; Allis, C.D. Translating the histone code. Science 2001, 293, 1074–1080. [Google Scholar] [CrossRef] [PubMed]
- Lehtomaki, E.; Mackay, J.P. Post-Translational Modification of Histone Proteins. In Encyclopedia of Biophysics; Springer: Berlin/Heidelberg, Germany, 2013; pp. 1923–1926. [Google Scholar]
- Ramazi, S.; Allahverdi, A.; Zahiri, J. Evaluation of post-translational modifications in histone proteins: A review on histone modification defects in developmental and neurological disorders. J. Biosci. 2020, 45, 135. [Google Scholar] [CrossRef]
- Henikoff, S.; Shilatifard, A. Histone modification: Cause or cog? Trends Genet. 2011, 27, 389–396. [Google Scholar] [CrossRef]
- Rothbart, S.B.; Strahl, B.D. Interpreting the language of histone and DNA modifications. Biochim. Et. Biophys. Acta BBA-Gene Regul. Mech. 2014, 1839, 627–643. [Google Scholar] [CrossRef]
- Esteller, M. Non-coding RNAs in human disease. Nat. Rev. Genet. 2011, 12, 861–874. [Google Scholar] [CrossRef]
- Bartel, D.P. Metazoan MicroRNAs. Cell 2018, 173, 20–51. [Google Scholar] [CrossRef]
- Ji, P.; Diederichs, S.; Wang, W.; Böing, S.; Metzger, R.; Schneider, P.M.; Tidow, N.; Brandt, B.; Buerger, H.; Bulk, E. MALAT-1, a novel noncoding RNA, and thymosin β4 predict metastasis and survival in early-stage non-small cell lung cancer. Oncogene 2003, 22, 8031–8041. [Google Scholar] [CrossRef]
- Ponting, C.P.; Oliver, P.L.; Reik, W. Evolution and functions of long noncoding RNAs. Cell 2009, 136, 629–641. [Google Scholar] [CrossRef] [PubMed]
- Memczak, S.; Jens, M.; Elefsinioti, A.; Torti, F.; Krueger, J.; Rybak, A.; Maier, L.; Mackowiak, S.D.; Gregersen, L.H.; Munschauer, M.; et al. Circular RNAs are a large class of animal RNAs with regulatory potency. Nature 2013, 495, 333–338. [Google Scholar] [CrossRef]
- Kristensen, L.S.; Andersen, M.S.; Stagsted, L.V.W.; Ebbesen, K.K.; Hansen, T.B.; Kjems, J. The biogenesis, biology and characterization of circular RNAs. Nat. Rev. Genet. 2019, 20, 675–691. [Google Scholar] [CrossRef]
- Zhang, J.; Zhang, Y.-Z.; Jiang, J.; Duan, C.-G. The crosstalk between epigenetic mechanisms and alternative RNA processing regulation. Front. Genet. 2020, 11, 998. [Google Scholar] [CrossRef] [PubMed]
- Rinn, J.L.; Chang, H.Y. Genome regulation by long noncoding RNAs. Annu. Rev. Biochem. 2012, 81, 145–166. [Google Scholar] [CrossRef]
- Davidovich, C.; Zheng, L.; Goodrich, K.J.; Cech, T.R. Promiscuous RNA binding by Polycomb repressive complex 2. Nat. Struct. Mol. Biol. 2013, 20, 1250–1257. [Google Scholar] [PubMed]
- Bird, A. Perceptions of epigenetics. Nature 2007, 447, 396–398. [Google Scholar] [CrossRef]
- Schmitz, R.J.; Lewis, Z.A.; Goll, M.G. DNA Methylation: Shared and Divergent Features across Eukaryotes. Trends Genet. 2019, 35, 818–827. [Google Scholar] [CrossRef]
- Kundaje, A.; Meuleman, W.; Ernst, J.; Bilenky, M.; Yen, A.; Heravi-Moussavi, A.; Kheradpour, P.; Zhang, Z.; Wang, J.; Ziller, M.J. Integrative analysis of 111 reference human epigenomes. Nature 2015, 518, 317–330. [Google Scholar]
- Smith, Z.D.; Meissner, A. DNA methylation: Roles in mammalian development. Nat. Rev. Genet. 2013, 14, 204–220. [Google Scholar] [CrossRef]
- Smallwood, S.A.; Kelsey, G. De novo DNA methylation: A germ cell perspective. Trends Genet. 2012, 28, 33–42. [Google Scholar] [CrossRef]
- Cantone, I.; Fisher, A.G. Epigenetic programming and reprogramming during development. Nat. Struct. Mol. Biol. 2013, 20, 282–289. [Google Scholar] [CrossRef] [PubMed]
- Seumois, G.; Chavez, L.; Gerasimova, A.; Lienhard, M.; Omran, N.; Kalinke, L.; Vedanayagam, M.; Ganesan, A.P.V.; Chawla, A.; Djukanović, R. Epigenomic analysis of primary human T cells reveals enhancers associated with TH2 memory cell differentiation and asthma susceptibility. Nat. Immunol. 2014, 15, 777–788. [Google Scholar] [PubMed]
- Foster, S.L.; Hargreaves, D.C.; Medzhitov, R. Gene-specific control of inflammation by TLR-induced chromatin modifications. Nature 2007, 447, 972–978. [Google Scholar]
- Emilie, M.B.; Elsa, S.; Cécile, E.M. Histone acetylation in neuronal (dys)function. Biomol. Concepts 2016, 7, 103–116. [Google Scholar] [CrossRef]
- Singh, P.; Paramanik, V. DNA methylation, histone acetylation in the regulation of memory and its modulation during aging. Front. Aging 2025, 5, 1480932. [Google Scholar]
- Tiffon, C. The Impact of Nutrition and Environmental Epigenetics on Human Health and Disease. Int. J. Mol. Sci. 2018, 19, 3425. [Google Scholar] [CrossRef]
- Rodgers, A.B.; Morgan, C.P.; Leu, N.A.; Bale, T.L. Transgenerational epigenetic programming via sperm microRNA recapitulates effects of paternal stress. Proc. Natl. Acad. Sci. USA 2015, 112, 13699–13704. [Google Scholar] [CrossRef]
- Daxinger, L.; Whitelaw, E. Understanding transgenerational epigenetic inheritance via the gametes in mammals. Nat. Rev. Genet. 2012, 13, 153–162. [Google Scholar]
- Dunn, G.A.; Bale, T.L. Maternal high-fat diet effects on third-generation female body size via the paternal lineage. Endocrinology 2011, 152, 2228–2236. [Google Scholar] [CrossRef]
- Crews, D.; Gillette, R.; Scarpino, S.V.; Manikkam, M.; Savenkova, M.I.; Skinner, M.K. Epigenetic transgenerational inheritance of altered stress responses. Proc. Natl. Acad. Sci. USA 2012, 109, 9143–9148. [Google Scholar] [PubMed]
- Elgin, S.C.R.; Reuter, G. Position-effect variegation, heterochromatin formation, and gene silencing in Drosophila. Cold Spring Harb. Perspect. Biol. 2013, 5, a017780. [Google Scholar]
- Quadrana, L.; Colot, V. Plant transgenerational epigenetics. Annu. Rev. Genet. 2016, 50, 467–491. [Google Scholar] [PubMed]
- Greer, E.L.; Maures, T.J.; Ucar, D.; Hauswirth, A.G.; Mancini, E.; Lim, J.P.; Benayoun, B.A.; Shi, Y.; Brunet, A. Transgenerational epigenetic inheritance of longevity in Caenorhabditis elegans. Nature 2011, 479, 365–371. [Google Scholar] [CrossRef]
- Horsthemke, B. A critical view on transgenerational epigenetic inheritance in humans. Nat. Commun. 2018, 9, 2973. [Google Scholar] [CrossRef]
- Heard, E.; Martienssen, R.A. Transgenerational epigenetic inheritance: Myths and mechanisms. Cell 2014, 157, 95–109. [Google Scholar]
- Fitz-James, M.H.; Cavalli, G. Molecular mechanisms of transgenerational epigenetic inheritance. Nat. Rev. Genet. 2022, 23, 325–341. [Google Scholar] [CrossRef]
- Lacal, I.; Ventura, R. Epigenetic inheritance: Concepts, mechanisms and perspectives. Front. Mol. Neurosci. 2018, 11, 292. [Google Scholar]
- Krippner, S.; Barrett, D. Transgenerational Trauma The Role of Epigenetics. J. Mind Behav. 2019, 40, 53–62. [Google Scholar]
- Joubert, B.R.; Felix, J.F.; Yousefi, P.; Bakulski, K.M.; Just, A.C.; Breton, C.; Reese, S.E.; Markunas, C.A.; Richmond, R.C.; Xu, C.-J. DNA methylation in newborns and maternal smoking in pregnancy: Genome-wide consortium meta-analysis. Am. J. Hum. Genet. 2016, 98, 680–696. [Google Scholar] [CrossRef]
- Kaur, G.; Begum, R.; Thota, S.; Batra, S. A systematic review of smoking-related epigenetic alterations. Arch. Toxicol. 2019, 93, 2715–2740. [Google Scholar] [CrossRef] [PubMed]
- Grossniklaus, U.; Kelly, W.G.; Ferguson-Smith, A.C.; Pembrey, M.; Lindquist, S. Transgenerational epigenetic inheritance: How important is it? Nat. Rev. Genet. 2013, 14, 228–235. [Google Scholar] [CrossRef] [PubMed]
- Yehuda, R.; Daskalakis, N.P.; Bierer, L.M.; Bader, H.N.; Klengel, T.; Holsboer, F.; Binder, E.B. Holocaust exposure induced intergenerational effects on FKBP5 methylation. Biol. Psychiatry 2016, 80, 372–380. [Google Scholar] [CrossRef]
- Yehuda, R.; Lehrner, A. Intergenerational transmission of trauma effects: Putative role of epigenetic mechanisms. World Psychiatry 2018, 17, 243–257. [Google Scholar] [CrossRef]
- Heijmans, B.T.; Tobi, E.W.; Stein, A.D.; Putter, H.; Blauw, G.J.; Susser, E.S.; Slagboom, P.E.; Lumey, L.H. Persistent epigenetic differences associated with prenatal exposure to famine in humans. Proc. Natl. Acad. Sci. USA 2008, 105, 17046–17049. [Google Scholar] [CrossRef]
- Tobi, E.W.; Lumey, L.H.; Talens, R.P.; Kremer, D.; Putter, H.; Stein, A.D.; Slagboom, P.E.; Heijmans, B.T. DNA methylation differences after exposure to prenatal famine are common and timing-and sex-specific. Hum. Mol. Genet. 2009, 18, 4046–4053. [Google Scholar] [CrossRef]
- Lim, J.P.; Brunet, A. Bridging the transgenerational gap with epigenetic memory. Trends Genet. 2013, 29, 176–186. [Google Scholar] [CrossRef] [PubMed]
- Xavier, M.J.; Roman, S.D.; Aitken, R.J.; Nixon, B. Transgenerational inheritance: How impacts to the epigenetic and genetic information of parents affect offspring health. Hum. Reprod. Update 2019, 25, 519–541. [Google Scholar] [CrossRef]
- Youssef, N.A.; Lockwood, L.; Su, S.; Hao, G.; Rutten, B.P.F. The Effects of Trauma, with or without PTSD, on the Transgenerational DNA Methylation Alterations in Human Offsprings. Brain Sci. 2018, 8, 83. [Google Scholar] [CrossRef]
- Bohacek, J.; Mansuy, I.M. Molecular insights into transgenerational non-genetic inheritance of acquired behaviours. Nat. Rev. Genet. 2015, 16, 641–652. [Google Scholar] [CrossRef]
- Bierer, L.M.; Bader, H.N.; Daskalakis, N.P.; Lehrner, A.; Provençal, N.; Wiechmann, T.; Klengel, T.; Makotkine, I.; Binder, E.B.; Yehuda, R. Intergenerational effects of maternal holocaust exposure on FKBP5 methylation. Am. J. Psychiatry 2020, 177, 744–753. [Google Scholar]
- McGowan, P.O. Epigenomic mechanisms of early adversity and HPA dysfunction: Considerations for PTSD research. Front. Psychiatry 2013, 4, 110. [Google Scholar] [CrossRef]
- Dirven, B.C.J.; Homberg, J.R.; Kozicz, T.; Henckens, M. Epigenetic programming of the neuroendocrine stress response by adult life stress. J. Mol. Endocrinol. 2017, 59, R11–R31. [Google Scholar]
- Klengel, T.; Mehta, D.; Anacker, C.; Rex-Haffner, M.; Pruessner, J.C.; Pariante, C.M.; Pace, T.W.; Mercer, K.B.; Mayberg, H.S.; Bradley, B.; et al. Allele-specific FKBP5 DNA demethylation mediates gene-childhood trauma interactions. Nat. Neurosci. 2013, 16, 33–41. [Google Scholar] [CrossRef]
- Kang, J.I.; Kim, T.Y.; Choi, J.H.; So, H.S.; Kim, S.J. Allele-specific DNA methylation level of FKBP5 is associated with post-traumatic stress disorder. Psychoneuroendocrinology 2019, 103, 1–7. [Google Scholar] [CrossRef]
- Cao-Lei, L.; Laplante, D.P.; King, S. Prenatal Maternal Stress and Epigenetics: Review of the Human Research. Curr. Mol. Biol. Rep. 2016, 2, 16–25. [Google Scholar] [CrossRef]
- Oberlander, T.F.; Weinberg, J.; Papsdorf, M.; Grunau, R.; Misri, S.; Devlin, A.M. Prenatal exposure to maternal depression, neonatal methylation of human glucocorticoid receptor gene (NR3C1) and infant cortisol stress responses. Epigenetics 2008, 3, 97–106. [Google Scholar] [CrossRef]
- Öztürk, K.H.; Ünal, G.; Doğuç, D.K.; Toğay, V.A.; Koşar, P.A.; Sezik, M. Hypothalamic NR3C1 DNA methylation in rats exposed to prenatal stress. Mol. Biol. Rep. 2022, 49, 7921–7928. [Google Scholar] [CrossRef]
- Weaver, I.C.; Cervoni, N.; Champagne, F.A.; D’Alessio, A.C.; Sharma, S.; Seckl, J.R.; Dymov, S.; Szyf, M.; Meaney, M.J. Epigenetic programming by maternal behavior. Nat. Neurosci. 2004, 7, 847–854. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, L.; Czamara, D. Epigenetics of prenatal stress in humans: The current research landscape. Clin. Epigenetics 2024, 16, 20. [Google Scholar] [CrossRef] [PubMed]
- Lamadé, E.K.; Hendlmeier, F.; Wudy, S.A.; Witt, S.H.; Rietschel, M.; Coenen, M.; Gilles, M.; Deuschle, M. Rhythm of Fetoplacental 11β-Hydroxysteroid Dehydrogenase Type 2—Fetal Protection From Morning Maternal Glucocorticoids. J. Clin. Endocrinol. Metab. 2021, 106, 1630–1636. [Google Scholar] [CrossRef]
- Jensen Peña, C.; Monk, C.; Champagne, F.A. Epigenetic effects of prenatal stress on 11β-hydroxysteroid dehydrogenase-2 in the placenta and fetal brain. PLoS ONE 2012, 7, e39791. [Google Scholar] [CrossRef] [PubMed]
- Cottrell, E.C.; Seckl, J.R. Prenatal stress, glucocorticoids and the programming of adult disease. Front. Behav. Neurosci. 2009, 3, 19. [Google Scholar] [CrossRef]
- Lacagnina, S. The Developmental Origins of Health and Disease (DOHaD). Am. J. Lifestyle Med. 2020, 14, 47–50. [Google Scholar] [CrossRef]
- Musanabaganwa, C.; Wani, A.H.; Donglasan, J.; Fatumo, S.; Jansen, S.; Mutabaruka, J.; Rutembesa, E.; Uwineza, A.; Hermans, E.J.; Roozendaal, B.; et al. Leukocyte methylomic imprints of exposure to the genocide against the Tutsi in Rwanda: A pilot epigenome-wide analysis. Epigenomics 2022, 14, 11–25. [Google Scholar] [CrossRef]
- Mutesa, L. Predictive evidence of the relevance of epigenetics to PTSD. Nat. Rev. Genet. 2023, 24, 807. [Google Scholar] [CrossRef]
- Chou, P.-C.; Huang, Y.-C.; Yu, S. Mechanisms of Epigenetic Inheritance in Post-Traumatic Stress Disorder. Life 2024, 14, 98. [Google Scholar] [CrossRef]
- Zovkic, I.B.; Guzman-Karlsson, M.C.; Sweatt, J.D. Epigenetic regulation of memory formation and maintenance. Learn. Mem. 2013, 20, 61–74. [Google Scholar]
- Zannas, A.S.; Provençal, N.; Binder, E.B. Epigenetics of Posttraumatic Stress Disorder: Current Evidence, Challenges, and Future Directions. Biol. Psychiatry 2015, 78, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Wang, H.D.; Allard, P. Challenging dogmas: How transgenerational epigenetics reshapes our views on life. J. Exp. Zool. Part. A Ecol. Integr. Physiol. 2022, 337, 70–74. [Google Scholar] [CrossRef]
- Švorcová, J. Transgenerational Epigenetic Inheritance of Traumatic Experience in Mammals. Genes 2023, 14, 120. [Google Scholar] [CrossRef]
- Igolkina, A.A.; Zinkevich, A.; Karandasheva, K.O.; Popov, A.A.; Selifanova, M.V.; Nikolaeva, D.; Tkachev, V.; Penzar, D.; Nikitin, D.M.; Buzdin, A. H3K4me3, H3K9ac, H3K27ac, H3K27me3 and H3K9me3 Histone Tags Suggest Distinct Regulatory Evolution of Open and Condensed Chromatin Landmarks. Cells 2019, 8, 1034. [Google Scholar] [CrossRef]
- Bohacek, J.; Mansuy, I.M. Epigenetic inheritance of disease and disease risk. Neuropsychopharmacology 2013, 38, 220–236. [Google Scholar]
- González, C.R.; González, B. Exploring the Stress Impact in the Paternal Germ Cells Epigenome: Can Catecholamines Induce Epigenetic Reprogramming? Front. Endocrinol. 2021, 11, 630948. [Google Scholar] [CrossRef]
- Gagnidze, K.; Pfaff, D.W. Epigenetic Mechanisms: DNA Methylation and Histone Protein Modification. In Neuroscience in the 21st Century: From Basic to Clinical; Springer International Publishing: Cham, Switzerland, 2022; pp. 2677–2716. [Google Scholar]
- Weaver, I.C.G.; D’Alessio, A.C.; Brown, S.E.; Hellstrom, I.C.; Dymov, S.; Sharma, S.; Szyf, M.; Meaney, M.J. The transcription factor nerve growth factor-inducible protein a mediates epigenetic programming: Altering epigenetic marks by immediate-early genes. J. Neurosci. 2007, 27, 1756–1768. [Google Scholar]
- Terashima, M.; Barbour, S.; Ren, J.; Yu, W.; Han, Y.; Muegge, K. Effect of high fat diet on paternal sperm histone distribution and male offspring liver gene expression. Epigenetics 2015, 10, 861–871. [Google Scholar]
- Carone, B.R.; Fauquier, L.; Habib, N.; Shea, J.M.; Hart, C.E.; Li, R.; Bock, C.; Li, C.; Gu, H.; Zamore, P.D.; et al. Paternally induced transgenerational environmental reprogramming of metabolic gene expression in mammals. Cell 2010, 143, 1084–1096. [Google Scholar] [CrossRef]
- Soubry, A.; Hoyo, C.; Jirtle, R.L.; Murphy, S.K. A paternal environmental legacy: Evidence for epigenetic inheritance through the male germ line. Bioessays 2014, 36, 359–371. [Google Scholar]
- Lismer, A.; Siklenka, K.; Lafleur, C.; Dumeaux, V.; Kimmins, S. Sperm histone H3 lysine 4 trimethylation is altered in a genetic mouse model of transgenerational epigenetic inheritance. Nucleic Acids Res. 2020, 48, 11380–11393. [Google Scholar] [CrossRef] [PubMed]
- Siklenka, K.; Erkek, S.; Godmann, M.; Lambrot, R.; McGraw, S.; Lafleur, C.; Cohen, T.; Xia, J.; Suderman, M.; Hallett, M.; et al. Disruption of histone methylation in developing sperm impairs offspring health transgenerationally. Science 2015, 350, aab2006. [Google Scholar] [CrossRef]
- Gaydos, L.J.; Wang, W.; Strome, S. H3K27me and PRC2 transmit a memory of repression across generations and during development. Science 2014, 345, 1515–1518. [Google Scholar] [CrossRef]
- Greer, E.L.; Beese-Sims, S.E.; Brookes, E.; Spadafora, R.; Zhu, Y.; Rothbart, S.B.; Aristizábal-Corrales, D.; Chen, S.; Badeaux Aimee, I.; Jin, Q.; et al. A Histone Methylation Network Regulates Transgenerational Epigenetic Memory in C. elegans. Cell Rep. 2014, 7, 113–126. [Google Scholar] [CrossRef]
- Xiao, Y.; Bedet, C.; Robert, V.J.; Simonet, T.; Dunkelbarger, S.; Rakotomalala, C.; Soete, G.; Korswagen, H.C.; Strome, S.; Palladino, F. Caenorhabditis elegans chromatin-associated proteins SET-2 and ASH-2 are differentially required for histone H3 Lys 4 methylation in embryos and adult germ cells. Proc. Natl. Acad. Sci. USA 2011, 108, 8305–8310. [Google Scholar] [CrossRef]
- Greer, E.L.; Becker, B.; Latza, C.; Antebi, A.; Shi, Y. Mutation of C. elegans demethylase spr-5 extends transgenerational longevity. Cell Res. 2016, 26, 229–238. [Google Scholar] [CrossRef]
- Wan, Q.L.; Meng, X.; Wang, C.; Dai, W.; Luo, Z.; Yin, Z.; Ju, Z.; Fu, X.; Yang, J.; Ye, Q. Histone H3K4me3 modification is a transgenerational epigenetic signal for lipid metabolism in Caenorhabditis elegans. Nat. Commun. 2022, 13, 768. [Google Scholar]
- Rose, N.R.; Klose, R.J. Understanding the relationship between DNA methylation and histone lysine methylation. Biochim. Et. Biophys. Acta BBA-Gene Regul. Mech. 2014, 1839, 1362–1372. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Z. The silent guardian: Unraveling the roles of H3K9me3 in genome maintenance. Genome Instab. Dis. 2024, 5, 133–153. [Google Scholar] [CrossRef]
- Burgess, D.J. H3K27 methylation in transgenerational epigenetic memory. Nat. Rev. Genet. 2014, 15, 703. [Google Scholar] [CrossRef]
- Padilla, P.A.; Garcia, A.M.; Ladage, M.L.; Toni, L.S. Caenorhabditis elegans: An Old Genetic Model Can Learn New Epigenetic Tricks. Integr. Comp. Biol. 2014, 54, 52–60. [Google Scholar] [CrossRef]
- Greer, E.L.; Maures, T.J.; Hauswirth, A.G.; Green, E.M.; Leeman, D.S.; Maro, G.S.; Han, S.; Banko, M.R.; Gozani, O.; Brunet, A. Members of the H3K4 trimethylation complex regulate lifespan in a germline-dependent manner in C. elegans. Nature 2010, 466, 383–387. [Google Scholar] [CrossRef]
- Gapp, K.; von Ziegler, L.; Tweedie-Cullen, R.Y.; Mansuy, I.M. Early life epigenetic programming and transmission of stress-induced traits in mammals: How and when can environmental factors influence traits and their transgenerational inheritance? Bioessays 2014, 36, 491–502. [Google Scholar] [CrossRef]
- Sharma, U.; Conine, C.C.; Shea, J.M.; Boskovic, A.; Derr, A.G.; Bing, X.Y.; Belleannee, C.; Kucukural, A.; Serra, R.W.; Sun, F. Biogenesis and function of tRNA fragments during sperm maturation and fertilization in mammals. Science 2016, 351, 391–396. [Google Scholar]
- Bure, I.V.; Nemtsova, M.V.; Kuznetsova, E.B. Histone Modifications and Non-Coding RNAs: Mutual Epigenetic Regulation and Role in Pathogenesis. Int. J. Mol. Sci. 2022, 23, 5801. [Google Scholar] [CrossRef]
- Chen, Q.; Yan, W.; Duan, E. Epigenetic inheritance of acquired traits through sperm RNAs and sperm RNA modifications. Nat. Rev. Genet. 2016, 17, 733–743. [Google Scholar] [CrossRef]
- Liberman, N.; Wang, S.Y.; Greer, E.L. Transgenerational epigenetic inheritance: From phenomena to molecular mechanisms. Curr. Opin. Neurobiol. 2019, 59, 189–206. [Google Scholar] [CrossRef]
- Frías-Lasserre, D.; Villagra, C.A. The importance of ncRNAs as epigenetic mechanisms in phenotypic variation and organic evolution. Front. Microbiol. 2017, 8, 2483. [Google Scholar]
- Gapp, K.; Jawaid, A.; Sarkies, P.; Bohacek, J.; Pelczar, P.; Prados, J.; Farinelli, L.; Miska, E.; Mansuy, I.M. Implication of sperm RNAs in transgenerational inheritance of the effects of early trauma in mice. Nat. Neurosci. 2014, 17, 667–669. [Google Scholar] [CrossRef]
- Whalley, K. Early trauma alters sperm RNA. Nat. Rev. Neurosci. 2014, 15, 349. [Google Scholar] [CrossRef]
- Issler, O.; Haramati, S.; Paul, E.D.; Maeno, H.; Navon, I.; Zwang, R.; Gil, S.; Mayberg, H.S.; Dunlop, B.W.; Menke, A. MicroRNA 135 is essential for chronic stress resiliency, antidepressant efficacy, and intact serotonergic activity. Neuron 2014, 83, 344–360. [Google Scholar]
- Mannironi, C.; Camon, J.; De Vito, F.; Biundo, A.; De Stefano, M.E.; Persiconi, I.; Bozzoni, I.; Fragapane, P.; Mele, A.; Presutti, C. Acute stress alters amygdala microRNA miR-135a and miR-124 expression: Inferences for corticosteroid dependent stress response. PLoS ONE 2013, 8, e73385. [Google Scholar] [CrossRef]
- Guo, L.; Chao, S.B.; Xiao, L.; Wang, Z.B.; Meng, T.G.; Li, Y.Y.; Han, Z.M.; Ouyang, Y.C.; Hou, Y.; Sun, Q.Y.; et al. Sperm-carried RNAs play critical roles in mouse embryonic development. Oncotarget 2017, 8, 67394–67405. [Google Scholar] [CrossRef] [PubMed]
- Short, A.K.; Fennell, K.A.; Perreau, V.M.; Fox, A.; O’Bryan, M.K.; Kim, J.H.; Bredy, T.W.; Pang, T.Y.; Hannan, A.J. Elevated paternal glucocorticoid exposure alters the small noncoding RNA profile in sperm and modifies anxiety and depressive phenotypes in the offspring. Transl. Psychiatry 2016, 6, e837. [Google Scholar]
- Liu, Y.; Wang, Q.; Wen, J.; Wu, Y.; Man, C. MiR-375: A novel multifunctional regulator. Life Sci. 2021, 275, 119323. [Google Scholar]
- Wang, B.; Xia, L.; Zhu, D.; Zeng, H.; Wei, B.; Lu, L.; Li, W.; Shi, Y.; Liu, J.; Zhang, Y.; et al. Paternal High-Fat Diet Altered Sperm 5′tsRNA-Gly-GCC Is Associated with Enhanced Gluconeogenesis in the Offspring. Front. Mol. Biosci. 2022, 9, 857875. [Google Scholar] [CrossRef]
- Fullston, T.; Ohlsson Teague, E.M.; Palmer, N.O.; DeBlasio, M.J.; Mitchell, M.; Corbett, M.; Print, C.G.; Owens, J.A.; Lane, M. Paternal obesity initiates metabolic disturbances in two generations of mice with incomplete penetrance to the F2 generation and alters the transcriptional profile of testis and sperm microRNA content. FASEB J. 2013, 27, 4226–4243. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Zhang, X.; Shi, J.; Tuorto, F.; Li, X.; Liu, Y.; Liebers, R.; Zhang, L.; Qu, Y.; Qian, J. Dnmt2 mediates intergenerational transmission of paternally acquired metabolic disorders through sperm small non-coding RNAs. Nat. Cell Biol. 2018, 20, 535–540. [Google Scholar]
- Lee, S.-M.; Surani, M.A. Epigenetic reprogramming in mouse and human primordial germ cells. Exp. Mol. Med. 2024, 56, 2578–2587. [Google Scholar] [CrossRef]
- Cossetti, C.; Lugini, L.; Astrologo, L.; Saggio, I.; Fais, S.; Spadafora, C. Soma-to-germline transmission of RNA in mice xenografted with human tumour cells: Possible transport by exosomes. PLoS ONE 2014, 9, e101629. [Google Scholar] [CrossRef]
- Kowalczyk, A.; Wrzecińska, M.; Czerniawska-Piątkowska, E.; Kupczyński, R. Exosomes–Spectacular role in reproduction. Biomed. Pharmacother. 2022, 148, 112752. [Google Scholar]
- Phillips, D.; Noble, D. Bubbling beyond the barrier: Exosomal RNA as a vehicle for soma–germline communication. J. Physiol. 2024, 602, 2547–2563. [Google Scholar]
- Thomou, T.; Mori, M.A.; Dreyfuss, J.M.; Konishi, M.; Sakaguchi, M.; Wolfrum, C.; Rao, T.N.; Winnay, J.N.; Garcia-Martin, R.; Grinspoon, S.K. Adipose-derived circulating miRNAs regulate gene expression in other tissues. Nature 2017, 542, 450–455. [Google Scholar] [PubMed]
- Sun, Y.; Zhou, Y.; Shi, Y.; Zhang, Y.; Liu, K.; Liang, R.; Sun, P.; Chang, X.; Tang, W.; Zhang, Y.; et al. Expression of miRNA-29 in Pancreatic β Cells Promotes Inflammation and Diabetes via TRAF3. Cell Rep. 2021, 34, 108576. [Google Scholar] [CrossRef]
- Eaton, S.A.; Jayasooriah, N.; Buckland, M.E.; Martin, D.I.K.; Cropley, J.E.; Suter, C.M. Roll Over Weismann: Extracellular Vesicles in the Transgenerational Transmission of Environmental Effects. Epigenomics 2015, 7, 1165–1171. [Google Scholar] [CrossRef] [PubMed]
- Chan, J.C.; Morgan, C.P.; Adrian Leu, N.; Shetty, A.; Cisse, Y.M.; Nugent, B.M.; Morrison, K.E.; Jašarević, E.; Huang, W.; Kanyuch, N.; et al. Reproductive tract extracellular vesicles are sufficient to transmit intergenerational stress and program neurodevelopment. Nat. Commun. 2020, 11, 1499. [Google Scholar] [CrossRef]
- Marré, J.; Traver, E.C.; Jose, A.M. Extracellular RNA is transported from one generation to the next in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 2016, 113, 12496–12501. [Google Scholar] [CrossRef] [PubMed]
- Rechavi, O.; Houri-Ze’evi, L.; Anava, S.; Goh, W.S.S.; Kerk, S.Y.; Hannon, G.J.; Hobert, O. Starvation-induced transgenerational inheritance of small RNAs in C. elegans. Cell 2014, 158, 277–287. [Google Scholar] [CrossRef]
- Van Cauwenbergh, O.; Di Serafino, A.; Tytgat, J.; Soubry, A. Transgenerational epigenetic effects from male exposure to endocrine-disrupting compounds: A systematic review on research in mammals. Clin. Epigenetics 2020, 12, 65. [Google Scholar] [CrossRef]
- Nilsson, E.E.; Sadler-Riggleman, I.; Skinner, M.K. Environmentally induced epigenetic transgenerational inheritance of disease. Environ. Epigenetics 2018, 4, dvy016. [Google Scholar] [CrossRef]
- Guo, H.; Zhu, P.; Wu, X.; Li, X.; Wen, L.; Tang, F. Single-cell methylome landscapes of mouse embryonic stem cells and early embryos analyzed using reduced representation bisulfite sequencing. Genome Res. 2013, 23, 2126–2135. [Google Scholar]
- Conesa, A.; Madrigal, P.; Tarazona, S.; Gomez-Cabrero, D.; Cervera, A.; McPherson, A.; Szcześniak, M.W.; Gaffney, D.J.; Elo, L.L.; Zhang, X. A survey of best practices for RNA-seq data analysis. Genome Biol. 2016, 17, 13. [Google Scholar]
- Ha, M.; Kim, V.N. Regulation of microRNA biogenesis. Nat. Rev. Mol. Cell Biol. 2014, 15, 509–524. [Google Scholar] [CrossRef] [PubMed]
- Wessels, H.-H.; Méndez-Mancilla, A.; Guo, X.; Legut, M.; Daniloski, Z.; Sanjana, N.E. Massively parallel Cas13 screens reveal principles for guide RNA design. Nat. Biotechnol. 2020, 38, 722–727. [Google Scholar] [CrossRef] [PubMed]
- Fadul, S.M.; Arshad, A.; Mehmood, R. CRISPR-based epigenome editing: Mechanisms and applications. Epigenomics 2023, 15, 1137–1155. [Google Scholar] [CrossRef] [PubMed]
- Abudayyeh, O.O.; Gootenberg, J.S.; Franklin, B.; Koob, J.; Kellner, M.J.; Ladha, A.; Joung, J.; Kirchgatterer, P.; Cox, D.B.T.; Zhang, F. A cytosine deaminase for programmable single-base RNA editing. Science 2019, 365, 382–386. [Google Scholar] [CrossRef]
- Champagne, F.A. Epigenetic legacy of parental experiences: Dynamic and interactive pathways to inheritance. Dev. Psychopathol. 2016, 28, 1219–1228. [Google Scholar] [CrossRef]
- Mbiydzenyuy, N.E.; Hemmings, S.M.J.; Qulu, L. Prenatal maternal stress and offspring aggressive behavior: Intergenerational and transgenerational inheritance. Front. Behav. Neurosci. 2022, 16, 977416. [Google Scholar] [CrossRef]
- Hartman, S.; Belsky, J.; Pluess, M. Prenatal programming of environmental sensitivity. Transl. Psychiatry 2023, 13, 161. [Google Scholar] [CrossRef]
- Jablonka, E.; Raz, G. Transgenerational epigenetic inheritance: Prevalence, mechanisms, and implications for the study of heredity and evolution. Q. Rev. Biol. 2009, 84, 131–176. [Google Scholar] [CrossRef]
- Skinner, M.K.; Guerrero-Bosagna, C.; Haque, M.; Nilsson, E.; Bhandari, R.; McCarrey, J.R. Environmentally induced transgenerational epigenetic reprogramming of primordial germ cells and the subsequent germ line. PLoS ONE 2013, 8, e66318. [Google Scholar] [CrossRef]
- Reik, W.; Dean, W.; Walter, J. Epigenetic reprogramming in mammalian development. Science 2001, 293, 1089–1093. [Google Scholar] [CrossRef]
- Guibert, S.; Forné, T.; Weber, M. Global profiling of DNA methylation erasure in mouse primordial germ cells. Genome Res. 2012, 22, 633–641. [Google Scholar] [CrossRef] [PubMed]
- Bird, A. Transgenerational epigenetic inheritance: A critical perspective. Front. Epigenetics Epigenomics 2024, 2, 1434253. [Google Scholar] [CrossRef]
- Messerschmidt, D.M.; Knowles, B.B.; Solter, D. DNA methylation dynamics during epigenetic reprogramming in the germline and preimplantation embryos. Genes. Dev. 2014, 28, 812–828. [Google Scholar] [CrossRef] [PubMed]
- Seisenberger, S.; Andrews, S.; Krueger, F.; Arand, J.; Walter, J.; Santos, F.; Popp, C.; Thienpont, B.; Dean, W.; Reik, W. The dynamics of genome-wide DNA methylation reprogramming in mouse primordial germ cells. Mol. Cell 2012, 48, 849–862. [Google Scholar] [CrossRef]
- Gunasekara, C.J.; MacKay, H.; Scott, C.A.; Li, S.; Laritsky, E.; Baker, M.S.; Grimm, S.L.; Jun, G.; Li, Y.; Chen, R.; et al. Systemic interindividual epigenetic variation in humans is associated with transposable elements and under strong genetic control. Genome Biol. 2023, 24, 2. [Google Scholar] [CrossRef]
- Bertozzi, T.M.; Ferguson-Smith, A.C. Metastable epialleles and their contribution to epigenetic inheritance in mammals. Semin. Cell Dev. Biol. 2020, 97, 93–105. [Google Scholar] [CrossRef]
- Dolinoy, D.C. The agouti mouse model: An epigenetic biosensor for nutritional and environmental alterations on the fetal epigenome. Nutr. Rev. 2008, 66, S7–S11. [Google Scholar]
- Silvers, W.K. The Coat Colors of Mice: A Model for Mammalian Gene Action and Interaction; Springer Science & Business Media: Berlin/Heidelberg, Germany, 2012. [Google Scholar]
- Blewitt, M.E.; Vickaryous, N.K.; Paldi, A.; Koseki, H.; Whitelaw, E. Dynamic reprogramming of DNA methylation at an epigenetically sensitive allele in mice. PLoS Genet. 2006, 2, e49. [Google Scholar]
- Dolinoy, D.C.; Weidman, J.R.; Waterland, R.A.; Jirtle, R.L. Maternal genistein alters coat color and protects Avy mouse offspring from obesity by modifying the fetal epigenome. Environ. Health Perspect. 2006, 114, 567–572. [Google Scholar] [PubMed]
- Waterland, R.A.; Jirtle, R.L. Transposable elements: Targets for early nutritional effects on epigenetic gene regulation. Mol. Cell. Biol. 2003, 23, 5293–5300. [Google Scholar]
- Derakhshan, M.; Kessler, N.J.; Hellenthal, G.; Silver, M.J. Metastable epialleles in humans. Trends Genet. 2024, 40, 52–68. [Google Scholar] [CrossRef] [PubMed]
- Dominguez-Salas, P.; Moore, S.E.; Baker, M.S.; Bergen, A.W.; Cox, S.E.; Dyer, R.A.; Fulford, A.J.; Guan, Y.; Laritsky, E.; Silver, M.J.; et al. Maternal nutrition at conception modulates DNA methylation of human metastable epialleles. Nat. Commun. 2014, 5, 3746. [Google Scholar] [CrossRef]
- Jablonka, E.; Lamb, M.J. The changing concept of epigenetics. Ann. N. Y. Acad. Sci. 2002, 981, 82–96. [Google Scholar] [CrossRef]
- Morgan, H.D.; Santos, F.; Green, K.; Dean, W.; Reik, W. Epigenetic reprogramming in mammals. Hum. Mol. Genet. 2005, 14, R47–R58. [Google Scholar] [CrossRef] [PubMed]
- Xu, R.; Li, C.; Liu, X.; Gao, S. Insights into epigenetic patterns in mammalian early embryos. Protein Cell 2021, 12, 7–28. [Google Scholar] [CrossRef] [PubMed]
- Nilsson, E.E.; Maamar, M.B.; Skinner, M.K. Environmentally Induced Epigenetic Transgenerational Inheritance and the Weismann Barrier: The Dawn of Neo-Lamarckian Theory. J. Dev. Biol. 2020, 8, 28. [Google Scholar] [CrossRef]
- Rando, O.J.; Simmons, R.A. I’m eating for two: Parental dietary effects on offspring metabolism. Cell 2015, 161, 93–105. [Google Scholar] [CrossRef]
- Daskalakis, N.P.; Xu, C.; Bader, H.N.; Chatzinakos, C.; Weber, P.; Makotkine, I.; Lehrner, A.; Bierer, L.M.; Binder, E.B.; Yehuda, R. Intergenerational trauma is associated with expression alterations in glucocorticoid- and immune-related genes. Neuropsychopharmacology 2021, 46, 763–773. [Google Scholar] [CrossRef]
- Murgatroyd, C.; Bradburn, S. Translational Animal Models for the Study of Epigenetics and the Environment. In Epigenetics, the Environment, and Children’s Health Across Lifespans; Springer International Publishing: Cham, Switzerland, 2016; pp. 207–229. [Google Scholar]
- Zijlmans, M.A.C.; Riksen-Walraven, J.M.; de Weerth, C. Associations between maternal prenatal cortisol concentrations and child outcomes: A systematic review. Neurosci. Biobehav. Rev. 2015, 53, 1–24. [Google Scholar] [CrossRef]
- Tobi, E.W.; Slieker, R.C.; Luijk, R.; Dekkers, K.F.; Stein, A.D.; Xu, K.M.; Biobank-based Integrative Omics Studies Consortium; Slagboom, P.E.; van Zwet, E.W.; Lumey, L.H. DNA methylation as a mediator of the association between prenatal adversity and risk factors for metabolic disease in adulthood. Sci. Adv. 2018, 4, eaao4364. [Google Scholar] [CrossRef]
- Steegers-Theunissen, R.P.M.; Twigt, J.; Pestinger, V.; Sinclair, K.D. The periconceptional period, reproduction and long-term health of offspring: The importance of one-carbon metabolism. Hum. Reprod. Update 2013, 19, 640–655. [Google Scholar]
- Skvortsova, K.; Iovino, N.; Bogdanović, O. Functions and mechanisms of epigenetic inheritance in animals. Nat. Rev. Mol. Cell Biol. 2018, 19, 774–790. [Google Scholar] [PubMed]
- Glover, V.; O’connor, T.G.; O’Donnell, K. Prenatal stress and the programming of the HPA axis. Neurosci. Biobehav. Rev. 2010, 35, 17–22. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.; Strathearn, L. Trauma, Mothering, and Intergenerational Transmission: A Synthesis of Behavioral and Oxytocin Research. Psychoanal. Study Child 2017, 70, 200–223. [Google Scholar] [CrossRef]
- Lester, B.M.; Marsit, C.J. Epigenetic mechanisms in the placenta related to infant neurodevelopment. Epigenomics 2018, 10, 321–333. [Google Scholar]
- Hughes, V. Sperm RNA carries marks of trauma. Nature 2014, 508, 296–297. [Google Scholar] [CrossRef]
- Bryant, R.A.; Edwards, B.; Creamer, M.; O’Donnell, M.; Forbes, D.; Felmingham, K.L.; Silove, D.; Steel, Z.; Nickerson, A.; McFarlane, A.C.; et al. The effect of post-traumatic stress disorder on refugees’ parenting and their children’s mental health: A cohort study. Lancet Public Health 2018, 3, e249–e258. [Google Scholar] [CrossRef]
- Dekel, R.; Goldblatt, H. Is there intergenerational transmission of trauma? The case of combat veterans’ children. Am. J. Orthopsychiatry 2008, 78, 281–289. [Google Scholar] [CrossRef]
- Fazel, M.; Reed, R.V.; Panter-Brick, C.; Stein, A. Mental health of displaced and refugee children resettled in high-income countries: Risk and protective factors. Lancet 2012, 379, 266–282. [Google Scholar] [CrossRef]
- Blaze, J.; Roth, T.L. Evidence from clinical and animal model studies of the long-term and transgenerational impact of stress on DNA methylation. Semin. Cell Dev. Biol. 2015, 43, 76–84. [Google Scholar] [CrossRef]
- True, W.R.; Rice, J.; Eisen, S.A.; Heath, A.C.; Goldberg, J.; Lyons, M.J.; Nowak, J. A twin study of genetic and environmental contributions to liability for posttraumatic stress symptoms. Arch. Gen. Psychiatry 1993, 50, 257–264. [Google Scholar] [CrossRef] [PubMed]
- Almli, L.M.; Fani, N.; Smith, A.K.; Ressler, K.J. Genetic approaches to understanding post-traumatic stress disorder. Int. J. Neuropsychopharmacol. 2014, 17, 355–370. [Google Scholar] [CrossRef]
- Klengel, T.; Binder, E.B. Epigenetics of Stress-Related Psychiatric Disorders and Gene × Environment Interactions. Neuron 2015, 86, 1343–1357. [Google Scholar] [CrossRef] [PubMed]
- Allison, K.; Grilo, C.; Masheb, R.; Stunkard, A. Association of FKBP5 polymorphisms and childhood abuse with risk of posttraumatic stress disorder symptoms in adults. Behav. Res. Ther. 2007, 45, 2874–2883. [Google Scholar] [CrossRef] [PubMed]
- Großmann, N.L.; Weihs, A.; Kühn, L.; Sauer, S.; Röh, S.; Wiechmann, T.; Rex-Haffner, M.; Völzke, H.; Völker, U.; Binder, E.B.; et al. Methylation Patterns of the FKBP5 Gene in Association with Childhood Maltreatment and Depressive Disorders. Int. J. Mol. Sci. 2024, 25, 1485. [Google Scholar] [CrossRef]
- Mehta, D.; Binder, E.B. Gene × environment vulnerability factors for PTSD: The HPA-axis. Neuropharmacology 2012, 62, 654–662. [Google Scholar] [CrossRef]
- McEwen, B.S. The ever-changing brain: Cellular and molecular mechanisms for the effects of stressful experiences. Dev. Neurobiol. 2012, 72, 878–890. [Google Scholar] [CrossRef]
- Yehuda, R.; Bierer, L.M. The relevance of epigenetics to PTSD: Implications for the DSM-V. J. Trauma. Stress. 2009, 22, 427–434. [Google Scholar] [CrossRef]
- Goforth, A.N.; Pham, A.V.; Carlson, J.S. Diathesis-stress Model. In Encyclopedia of Child Behavior and Development; Springer: Boston, MA, USA, 2011; pp. 502–503. [Google Scholar]
- Zannas, A.S.; West, A.E. Epigenetics and the regulation of stress vulnerability and resilience. Neuroscience 2014, 264, 157–170. [Google Scholar] [CrossRef]
- Daud, A.; af Klinteberg, B.; Rydelius, P.-A. Resilience and vulnerability among refugee children of traumatized and non-traumatized parents. Child Adolesc. Psychiatry Ment. Health 2008, 2, 7. [Google Scholar] [CrossRef]
- Lancaster, C.L.; Teeters, J.B.; Gros, D.F.; Back, S.E. Posttraumatic Stress Disorder: Overview of Evidence-Based Assessment and Treatment. J. Clin. Med. 2016, 5, 105. [Google Scholar] [CrossRef]
- Liu, A.Y.; Gubbels, J.; Orobio de Castro, B. The Effectiveness of Trauma-Informed Parenting Programs for Traumatized Parents and Their Components: A Meta-Analytic Study. Clin. Child Fam. Psychol. Rev. 2024, 27, 1113–1143. [Google Scholar]
- Gómez-Cantarino, S.; García-Valdivieso, I.; Moncunill-Martínez, E.; Yáñez-Araque, B.; Ugarte Gurrutxaga, M.I. Developing a Family-Centered Care Model in the Neonatal Intensive Care Unit (NICU): A New Vision to Manage Healthcare. Int. J. Environ. Res. Public Health 2020, 17, 7197. [Google Scholar] [CrossRef] [PubMed]
- Wilker, S.; Vukojevic, V.; Schneider, A.; Pfeiffer, A.; Inerle, S.; Pauly, M.; Elbert, T.; Papassotiropoulos, A.; de Quervain, D.; Kolassa, I.-T. Epigenetics of traumatic stress: The association of NR3C1 methylation and posttraumatic stress disorder symptom changes in response to narrative exposure therapy. Transl. Psychiatry 2023, 13, 14. [Google Scholar] [CrossRef]
- Yehuda, R.; Daskalakis, N.P.; Desarnaud, F.; Makotkine, I.; Lehrner, A.L.; Koch, E.; Flory, J.D.; Buxbaum, J.D.; Meaney, M.J.; Bierer, L.M. Epigenetic biomarkers as predictors and correlates of symptom improvement following psychotherapy in combat veterans with PTSD. Front. Psychiatry 2013, 4, 118. [Google Scholar]
- Massoni, L. Epigenetic and Mental Diseases: The Role of Psychotherapy. Int. J. Transl. Med. 2024, 4, 450–462. [Google Scholar] [CrossRef]
- Vinkers, C.H.; Geuze, E.; van Rooij, S.J.H.; Kennis, M.; Schür, R.R.; Nispeling, D.M.; Smith, A.K.; Nievergelt, C.M.; Uddin, M.; Rutten, B.P.F.; et al. Successful treatment of post-traumatic stress disorder reverses DNA methylation marks. Mol. Psychiatry 2021, 26, 1264–1271. [Google Scholar] [CrossRef]
- Bleker, L.S.; Milgrom, J.; Sexton-Oates, A.; Roseboom, T.J.; Gemmill, A.W.; Holt, C.J.; Saffery, R.; Burger, H.; de Rooij, S.R. Exploring the effect of antenatal depression treatment on children’s epigenetic profiles: Findings from a pilot randomized controlled trial. Clin. Epigenetics 2019, 11, 18. [Google Scholar] [CrossRef]
- Bérard, A.; Sheehy, O.; Zhao, J.P.; Vinet, É.; Bernatsky, S.; Abrahamowicz, M. SSRI and SNRI use during pregnancy and the risk of persistent pulmonary hypertension of the newborn. Br. J. Clin. Pharmacol. 2017, 83, 1126–1133. [Google Scholar] [CrossRef]
- Huybrechts, K.F.; Palmsten, K.; Avorn, J.; Cohen, L.S.; Holmes, L.B.; Franklin, J.M.; Mogun, H.; Levin, R.; Kowal, M.; Setoguchi, S.; et al. Antidepressant use in pregnancy and the risk of cardiac defects. N. Engl. J. Med. 2014, 370, 2397–2407. [Google Scholar] [CrossRef]
- Brown, A.S.; Gyllenberg, D.; Malm, H.; McKeague, I.W.; Hinkka-Yli-Salomäki, S.; Artama, M.; Gissler, M.; Cheslack-Postava, K.; Weissman, M.M.; Gingrich, J.A.; et al. Association of Selective Serotonin Reuptake Inhibitor Exposure During Pregnancy With Speech, Scholastic, and Motor Disorders in Offspring. JAMA Psychiatry 2016, 73, 1163–1170. [Google Scholar] [CrossRef] [PubMed]
- Brown, H.K.; Ray, J.G.; Wilton, A.S.; Lunsky, Y.; Gomes, T.; Vigod, S.N. Association between serotonergic antidepressant use during pregnancy and autism spectrum disorder in children. JAMA 2017, 317, 1544–1552. [Google Scholar] [CrossRef]
- Oberlander, T.F.; Reebye, P.; Misri, S.; Papsdorf, M.; Kim, J.; Grunau, R.E. Externalizing and attentional behaviors in children of depressed mothers treated with a selective serotonin reuptake inhibitor antidepressant during pregnancy. Arch. Pediatr. Adolesc. Med. 2007, 161, 22–29. [Google Scholar] [CrossRef]
- Grove, K.; Lewis, A.J.; Galbally, M. Prenatal antidepressant exposure and child motor development: A meta-analysis. Pediatrics 2018, 142, e20180356. [Google Scholar] [CrossRef]
- Rommel, A.S.; Bergink, V.; Liu, X.; Munk-Olsen, T.; Molenaar, N.M. Long-Term Effects of Intrauterine Exposure to Antidepressants on Physical, Neurodevelopmental, and Psychiatric Outcomes: A Systematic Review. J. Clin. Psychiatry 2020, 81, 10661. [Google Scholar] [CrossRef]
- Pellicano, G.R.; Daniela, S.; Chiara, C.; Arianna, G.; Paola, A.; Carlo, L. Epigenetic correlates of the psychological interventions outcomes: A systematic review and meta-analysis. J. Affect. Disord. Rep. 2022, 7, 100310. [Google Scholar] [CrossRef]
- Bishop, J.R.; Lee, A.M.; Mills, L.J.; Thuras, P.D.; Eum, S.; Clancy, D.; Erbes, C.R.; Polusny, M.A.; Lamberty, G.J.; Lim, K.O. Methylation of FKBP5 and SLC6A4 in Relation to Treatment Response to Mindfulness Based Stress Reduction for Posttraumatic Stress Disorder. Front. Psychiatry 2018, 9, 418. [Google Scholar] [CrossRef]
- Wellington, N.J.; Boucas, A.P.; Lagopoulos, J.; Kuballa, A.V. Clinical potential of epigenetic and microRNA biomarkers in PTSD. J. Neurogenet. 2024, 38, 79–101. [Google Scholar] [CrossRef]
- Engelmann, J.; Zillich, L.; Frank, J.; Wagner, S.; Cetin, M.; Herzog, D.P.; Müller, M.B.; Tadic, A.; Foo, J.C.; Sirignano, L.; et al. Epigenetic signatures in antidepressant treatment response: A methylome-wide association study in the EMC trial. Transl. Psychiatry 2022, 12, 268. [Google Scholar] [CrossRef] [PubMed]
- Nestler, E.J.; Peña, C.J.; Kundakovic, M.; Mitchell, A.; Akbarian, S. Epigenetic Basis of Mental Illness. Neuroscientist 2016, 22, 447–463. [Google Scholar] [CrossRef]
- García-Giménez, J.L.; Mena-Mollá, S.; Beltrán-García, J.; Sanchis-Gomar, F. Challenges in the analysis of epigenetic biomarkers in clinical samples. Clin. Chem. Lab. Med. CCLM 2017, 55, 1474–1477. [Google Scholar] [PubMed]
- Abi-Dargham, A.; Moeller, S.J.; Ali, F.; DeLorenzo, C.; Domschke, K.; Horga, G.; Jutla, A.; Kotov, R.; Paulus, M.P.; Rubio, J.M.; et al. Candidate biomarkers in psychiatric disorders: State of the field. World Psychiatry 2023, 22, 236–262. [Google Scholar] [CrossRef]
- Roth, T.L. Epigenetic mechanisms in the development of behavior: Advances, challenges, and future promises of a new field. Dev. Psychopathol. 2013, 25, 1279–1291. [Google Scholar] [CrossRef]
- Champagne, F.A.; Curley, J.P. How social experiences influence the brain. Curr. Opin. Neurobiol. 2005, 15, 704–709. [Google Scholar] [CrossRef]
- Gudsnuk, K.; Champagne, F.A. Epigenetic Influence of Stress and the Social Environment. ILAR J. 2012, 53, 279–288. [Google Scholar] [CrossRef]
- Rosenzweig, M.R.; Bennett, E.L. Psychobiology of plasticity: Effects of training and experience on brain and behavior. Behav. Brain Res. 1996, 78, 57–65. [Google Scholar]
- Branchi, I.; Karpova, N.N.; D’Andrea, I.; Castrén, E.; Alleva, E. Epigenetic modifications induced by early enrichment are associated with changes in timing of induction of BDNF expression. Neurosci. Lett. 2011, 495, 168–172. [Google Scholar] [CrossRef]
- Gapp, K.; Bohacek, J.; Grossmann, J.; Brunner, A.M.; Manuella, F.; Nanni, P.; Mansuy, I.M. Potential of Environmental Enrichment to Prevent Transgenerational Effects of Paternal Trauma. Neuropsychopharmacology 2016, 41, 2749–2758. [Google Scholar] [CrossRef]
- Hegde, A.; Suresh, S.; Mitra, R. Early-life short-term environmental enrichment counteracts the effects of stress on anxiety-like behavior, brain-derived neurotrophic factor and nuclear translocation of glucocorticoid receptors in the basolateral amygdala. Sci. Rep. 2020, 10, 14053. [Google Scholar] [CrossRef]
- Heart, M.Y.; Chase, J.; Elkins, J.; Altschul, D.B. Historical trauma among Indigenous Peoples of the Americas: Concepts, research, and clinical considerations. J. Psychoact. Drugs 2011, 43, 282–290. [Google Scholar] [CrossRef]
- Kirmayer, L.J.; Gone, J.P.; Moses, J. Rethinking historical trauma. Transcult. Psychiatry 2014, 51, 299–319. [Google Scholar] [PubMed]
- Chandler, M.J.; Lalonde, C. Cultural Continuity as a Hedge against Suicide in Canada’s First Nations. Transcult. Psychiatry 1998, 35, 191–219. [Google Scholar] [CrossRef]
- Yashadhana, A.; Fields, T.; Liu, E.; Serova, N.; O’Leary, M.; Kenning, G.; Kuchelmeister, V.; Lockhart, J.; de Leeuw, E. Therapeutic aspects of Connection to Country and cultural landscapes among Aboriginal peoples from the Stolen Generations living in urban NSW, Australia. Public Health Res. Pract. 2023, 33, e3342332. [Google Scholar] [CrossRef]
- Fatima, Y.; Liu, Y.; Cleary, A.; Dean, J.; Smith, V.; King, S.; Solomon, S. Connecting the health of country with the health of people: Application of “caring for country” in improving the social and emotional well-being of Indigenous people in Australia and New Zealand. Lancet Reg. Health West. Pac. 2023, 31, 100648. [Google Scholar] [CrossRef]
- Gone, J.P. Redressing First Nations historical trauma: Theorizing mechanisms for indigenous culture as mental health treatment. Transcult. Psychiatry 2013, 50, 683–706. [Google Scholar] [CrossRef] [PubMed]
- Harper, L. Epigenetic inheritance and the intergenerational transfer of experience. Psychol. Bull. 2005, 131, 340. [Google Scholar] [CrossRef]
- Zaretsky, T.G.; Jagodnik, K.M.; Barsic, R.; Antonio, J.H.; Bonanno, P.A.; MacLeod, C.; Pierce, C.; Carney, H.; Morrison, M.T.; Saylor, C. The Psychedelic Future of Post-Traumatic Stress Disorder Treatment. Curr. Neuropharmacol. 2024, 22, 636–735. [Google Scholar]
- Nichols, D.E.; Barker, E.L. Psychedelics. Pharmacol. Rev. 2016, 68, 264–355. [Google Scholar]
- Carhart-Harris, R.L.; Goodwin, G.M. The Therapeutic Potential of Psychedelic Drugs: Past, Present, and Future. Neuropsychopharmacology 2017, 42, 2105–2113. [Google Scholar] [CrossRef]
- Mithoefer, M.C.; Grob, C.S.; Brewerton, T.D. Novel psychopharmacological therapies for psychiatric disorders: Psilocybin and MDMA. Lancet Psychiatry 2016, 3, 481–488. [Google Scholar] [CrossRef]
- Cameron, L.P.; Tombari, R.J.; Lu, J.; Pell, A.J.; Hurley, Z.Q.; Ehinger, Y.; Vargas, M.V.; McCarroll, M.N.; Taylor, J.C.; Myers-Turnbull, D. A non-hallucinogenic psychedelic analogue with therapeutic potential. Nature 2021, 589, 474–479. [Google Scholar] [PubMed]
- Ly, C.; Greb, A.C.; Cameron, L.P.; Wong, J.M.; Barragan, E.V.; Wilson, P.C.; Burbach, K.F.; Zarandi, S.S.; Sood, A.; Paddy, M.R. Psychedelics promote structural and functional neural plasticity. Cell Rep. 2018, 23, 3170–3182. [Google Scholar] [PubMed]
- Banushi, B.; Polito, V. A Comprehensive Review of the Current Status of the Cellular Neurobiology of Psychedelics. Biology 2023, 12, 1380. [Google Scholar] [CrossRef] [PubMed]
- Glowiak, A. Resurrecting Ancestral Familial Health: A Role for Psilocybin? Fam. J. 2024, 32, 563–572. [Google Scholar] [CrossRef]
- de la Fuente Revenga, M.; Zhu, B.; Guevara, C.A.; Naler, L.B.; Saunders, J.M.; Zhou, Z.; Toneatti, R.; Sierra, S.; Wolstenholme, J.T.; Beardsley, P.M.; et al. Prolonged epigenomic and synaptic plasticity alterations following single exposure to a psychedelic in mice. Cell Rep. 2021, 37, 109836. [Google Scholar] [CrossRef]
- Inserra, A.; Campanale, A.; Cheishvili, D.; Dymov, S.; Wong, A.; Marcal, N.; Syme, R.A.; Taylor, L.; De Gregorio, D.; Kennedy, T.E.; et al. Modulation of DNA methylation and protein expression in the prefrontal cortex by repeated administration of D-lysergic acid diethylamide (LSD): Impact on neurotropic, neurotrophic, and neuroplasticity signaling. Prog. Neuro-Psychopharmacol. Biol. Psychiatry 2022, 119, 110594. [Google Scholar] [CrossRef]
- Inserra, A. Hypothesis: The Psychedelic Ayahuasca Heals Traumatic Memories via a Sigma 1 Receptor-Mediated Epigenetic-Mnemonic Process. Front. Pharmacol. 2018, 9, 330. [Google Scholar] [CrossRef]
- Ruffell, S.G.D.; Netzband, N.; Tsang, W.; Davies, M.; Inserra, A.; Butler, M.; Rucker, J.J.H.; Tófoli, L.F.; Dempster, E.L.; Young, A.H. Ceremonial ayahuasca in amazonian retreats—Mental health and epigenetic outcomes from a six-month naturalistic study. Front. Psychiatry 2021, 12, 687615. [Google Scholar]
- Maia, L.O.; Daldegan-Bueno, D.; Wießner, I.; Araujo, D.B.; Tófoli, L.F. Ayahuasca’s therapeutic potential: What we know—And what not. Eur. Neuropsychopharmacol. 2023, 66, 45–61. [Google Scholar] [CrossRef]
- Hinkle, J.T.; Graziosi, M.; Nayak, S.M.; Yaden, D.B. Adverse Events in Studies of Classic Psychedelics: A Systematic Review and Meta-Analysis. JAMA Psychiatry 2024, 81, 1225–1235. [Google Scholar] [CrossRef]
- Romeo, B.; Kervadec, E.; Fauvel, B.; Strika-Bruneau, L.; Amirouche, A.; Verroust, V.; Piolino, P.; Benyamina, A. Safety and risk assessment of psychedelic psychotherapy: A meta-analysis and systematic review. Psychiatry Res. 2024, 335, 115880. [Google Scholar] [CrossRef] [PubMed]
- Johnson, M.W.; Richards, W.A.; Griffiths, R.R. Human hallucinogen research: Guidelines for safety. J. Psychopharmacol. 2008, 22, 603–620. [Google Scholar]
- Dos Santos, R.G.; Bouso, J.C.; Hallak, J.E.C. Ayahuasca, dimethyltryptamine, and psychosis: A systematic review of human studies. Ther. Adv. Psychopharmacol. 2017, 7, 141–157. [Google Scholar] [PubMed]
- Banushi, B.; Brendle, M.; Ragnhildstveit, A.; Murphy, T.; Moore, C.; Egberts, J.; Robison, R. Breathwork Interventions for Adults with Clinically Diagnosed Anxiety Disorders: A Scoping Review. Brain Sci. 2023, 13, 256. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Hargreaves, M. Epigenetics and Exercise. Trends Endocrinol. Metab. 2019, 30, 636–645. [Google Scholar] [CrossRef]
- Singh, B.; Olds, T.; Curtis, R.; Dumuid, D.; Virgara, R.; Watson, A.; Szeto, K.; O’Connor, E.; Ferguson, T.; Eglitis, E.; et al. Effectiveness of physical activity interventions for improving depression, anxiety and distress: An overview of systematic reviews. Br. J. Sports Med. 2023, 57, 1203–1209. [Google Scholar] [CrossRef]
- Janusek, L.W.; Tell, D.; Mathews, H.L. Mindfulness based stress reduction improves psychological well-being and restores immune function in women with breast cancer, from diagnosis through 6-months post-cancer treatment. Brain Behav. Immun. 2015, 49, e37–e38. [Google Scholar]
- van der Kolk Laura, B.A.; West, J.; Rhodes, A.; Emerson, D.; Suvak, M.; Spinazzola, J. Yoga as an adjunctive treatment for posttraumatic stress disorder: A randomized controlled trial. J. Clin. Psychiatry 2014, 75, 22573. [Google Scholar]
- Lee, A.; Thuras, P.; Baller, J.; Jiao, C.; Guo, B.; Erbes, C.R.; Polusny, M.A.; Liu, C.; Wu, B.; Lim, K.O. Serotonin Transporter (SLC6A4) and FK506-Binding Protein 5 (FKBP5) Genotype and Methylation Relationships with Response to Meditation in Veterans with PTSD. Mol. Neurobiol. 2024, 61, 9608–9622. [Google Scholar]
- Stoffel, M.; Aguilar-Raab, C.; Rahn, S.; Steinhilber, B.; Witt, S.H.; Alexander, N.; Ditzen, B. Effects of mindfulness-based stress prevention on serotonin transporter gene methylation. Psychother. Psychosom. 2019, 88, 317–319. [Google Scholar]
- Abomoelak, B.; Prather, R.; Pragya, S.U.; Pragya, S.C.; Mehta, N.D.; Uddin, P.; Veeramachaneni, P.; Mehta, N.; Young, A.; Kapoor, S.; et al. Cognitive Skills and DNA Methylation Are Correlating in Healthy and Novice College Students Practicing Preksha Dhyāna Meditation. Brain Sci. 2023, 13, 1214. [Google Scholar] [CrossRef]
- Abomoelak, B.; Pragya, S.U.; Griswold, A.J.; Mehta, N.; Uddin, P.; Veeramachaneni, P.; Mehta, N.; Pragya, S.C.; El Enshasy, H.A.; Mehta, D. Preksha Dhyāna meditation induces alterations at the transcriptome level in novice and healthy college students. Saudi J. Biol. Sci. 2022, 29, 2299–2305. [Google Scholar] [CrossRef] [PubMed]
- Pragya, S.U.; Pragya, S.C.; Griswold, A.J.; Gu, E.; Mehta, N.D.; Uddin, P.; Veeramachaneni, P.; Mehta, N.; Mehta, D.; Abomoelak, B. Preksha Dhyāna Meditation Effect on the DNA Methylation Signature in College Students. J. Integr. Complement. Med. 2023, 29, 224–233. [Google Scholar] [CrossRef] [PubMed]
- McGee, S.L.; Walder, K.R. Exercise and the Skeletal Muscle Epigenome. Cold Spring Harb. Perspect. Med. 2017, 7, a029876. [Google Scholar] [CrossRef] [PubMed]
- Tarnowski, M.; Kopytko, P.; Piotrowska, K. Epigenetic Regulation of Inflammatory Responses in the Context of Physical Activity. Genes 2021, 12, 1313. [Google Scholar] [CrossRef]
- Fernandes, J.; Arida, R.M.; Gomez-Pinilla, F. Physical exercise as an epigenetic modulator of brain plasticity and cognition. Neurosci. Biobehav. Rev. 2017, 80, 443–456. [Google Scholar] [CrossRef]
- Mew, E.J.; Nyhan, K.; Bonumwezi, J.L.; Blas, V.; Gorman, H.; Hennein, R.; Quach, K.; Shabanova, V.; Hawley, N.L.; Lowe, S.R. Psychosocial family-level mediators in the intergenerational transmission of trauma: Protocol for a systematic review and meta-analysis. PLoS ONE 2022, 17, e0276753. [Google Scholar] [CrossRef]
- Keaney, J.; Byrne, H.; Warin, M.; Kowal, E. Refusing epigenetics: Indigeneity and the colonial politics of trauma. Hist. Philos. Life Sci. 2023, 46, 1. [Google Scholar] [CrossRef]
- Szyf, M. Epigenetics, a key for unlocking complex CNS disorders? Therapeutic implications. Eur. Neuropsychopharmacol. 2015, 25, 682–702. [Google Scholar] [CrossRef]
- Milroy, H.; Kashyap, S.; Collova, J.; Mitchell, M.; Ryder, A.; Cox, Z.; Coleman, M.; Taran, M.; Cuesta Briand, B.; Gee, G. Walking together in friendship: Learning about cultural safety in mainstream mental health services through Aboriginal Participatory Action Research. Aust. N. Zeal. J. Psychiatry 2024, 58, 498–505. [Google Scholar] [CrossRef]
- Milroy, H.; Kashyap, S.; Collova, J.; Mitchell, M.; Derry, K.L.; Alexi, J.; Chang, E.P.; Dudgeon, P. Co-designing research with Aboriginal and Torres Strait Islander consumers of mental health services, mental health workers, elders and cultural healers. Aust. J. Rural. Health 2022, 30, 772–781. [Google Scholar]
- National, A.R. Epigenetics—Do you have trauma in your genes? ABC Listen 2018. [Google Scholar]
- Nestler, E.J. Transgenerational epigenetic contributions to stress responses: Fact or fiction? PLoS Biol. 2016, 14, e1002426. [Google Scholar]
- Rand, A.C.; Jain, M.; Eizenga, J.M.; Musselman-Brown, A.; Olsen, H.E.; Akeson, M.; Paten, B. Mapping DNA methylation with high-throughput nanopore sequencing. Nat. Methods 2017, 14, 411–413. [Google Scholar] [CrossRef] [PubMed]
- Jiang, S.; Postovit, L.; Cattaneo, A.; Binder, E.B.; Aitchison, K.J. Epigenetic modifications in stress response genes associated with childhood trauma. Front. Psychiatry 2019, 10, 808. [Google Scholar]
- Flanagan, J.M. Epigenome-Wide Association Studies (EWAS): Past, Present, and Future. In Cancer Epigenetics: Risk Assessment, Diagnosis, Treatment, and Prognosis; Springer: New York, NY, USA, 2015; pp. 51–63. [Google Scholar]
- Gladish, N.; Merrill, S.M.; Kobor, M.S. Childhood Trauma and Epigenetics: State of the Science and Future. Curr. Environ. Health Rep. 2022, 9, 661–672. [Google Scholar] [CrossRef] [PubMed]
- Turecki, G.; Meaney, M.J. Effects of the Social Environment and Stress on Glucocorticoid Receptor Gene Methylation: A Systematic Review. Biol. Psychiatry 2016, 79, 87–96. [Google Scholar] [CrossRef]
- O’Neill, L.; Fraser, T.; Kitchenham, A.; McDonald, V. Hidden Burdens: A Review of Intergenerational, Historical and Complex Trauma, Implications for Indigenous Families. J. Child. Adolesc. Trauma. 2018, 11, 173–186. [Google Scholar] [CrossRef]
- Monk, C.; Spicer, J.; Champagne, F.A. Linking prenatal maternal adversity to developmental outcomes in infants: The role of epigenetic pathways. Dev. Psychopathol. 2012, 24, 1361–1376. [Google Scholar] [CrossRef]
- Dunkel Schetter, C.; Tanner, L. Anxiety, depression and stress in pregnancy: Implications for mothers, children, research, and practice. Curr. Opin. Psychiatry 2012, 25, 141–148. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Banushi, B.; Collova, J.; Milroy, H. Epigenetic Echoes: Bridging Nature, Nurture, and Healing Across Generations. Int. J. Mol. Sci. 2025, 26, 3075. https://doi.org/10.3390/ijms26073075
Banushi B, Collova J, Milroy H. Epigenetic Echoes: Bridging Nature, Nurture, and Healing Across Generations. International Journal of Molecular Sciences. 2025; 26(7):3075. https://doi.org/10.3390/ijms26073075
Chicago/Turabian StyleBanushi, Blerida, Jemma Collova, and Helen Milroy. 2025. "Epigenetic Echoes: Bridging Nature, Nurture, and Healing Across Generations" International Journal of Molecular Sciences 26, no. 7: 3075. https://doi.org/10.3390/ijms26073075
APA StyleBanushi, B., Collova, J., & Milroy, H. (2025). Epigenetic Echoes: Bridging Nature, Nurture, and Healing Across Generations. International Journal of Molecular Sciences, 26(7), 3075. https://doi.org/10.3390/ijms26073075





