RyhB Regulates Capsular Synthesis for Serum Resistance and Virulence of Avian Pathogenic Escherichia coli
Abstract
1. Introduction
2. Results
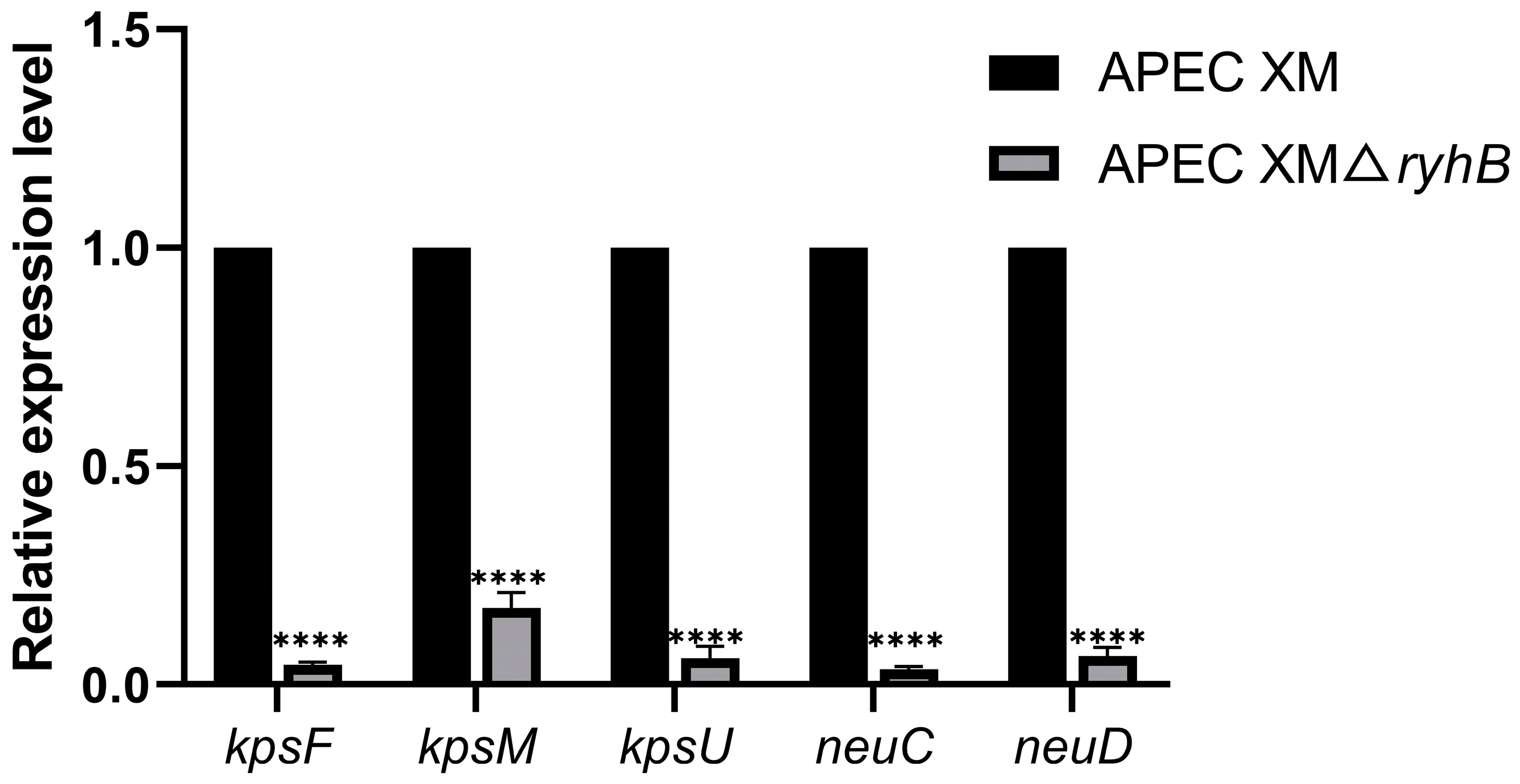
2.1. Deletion of ryhB Affects the Expression of Capsule Synthesis-Associated Genes
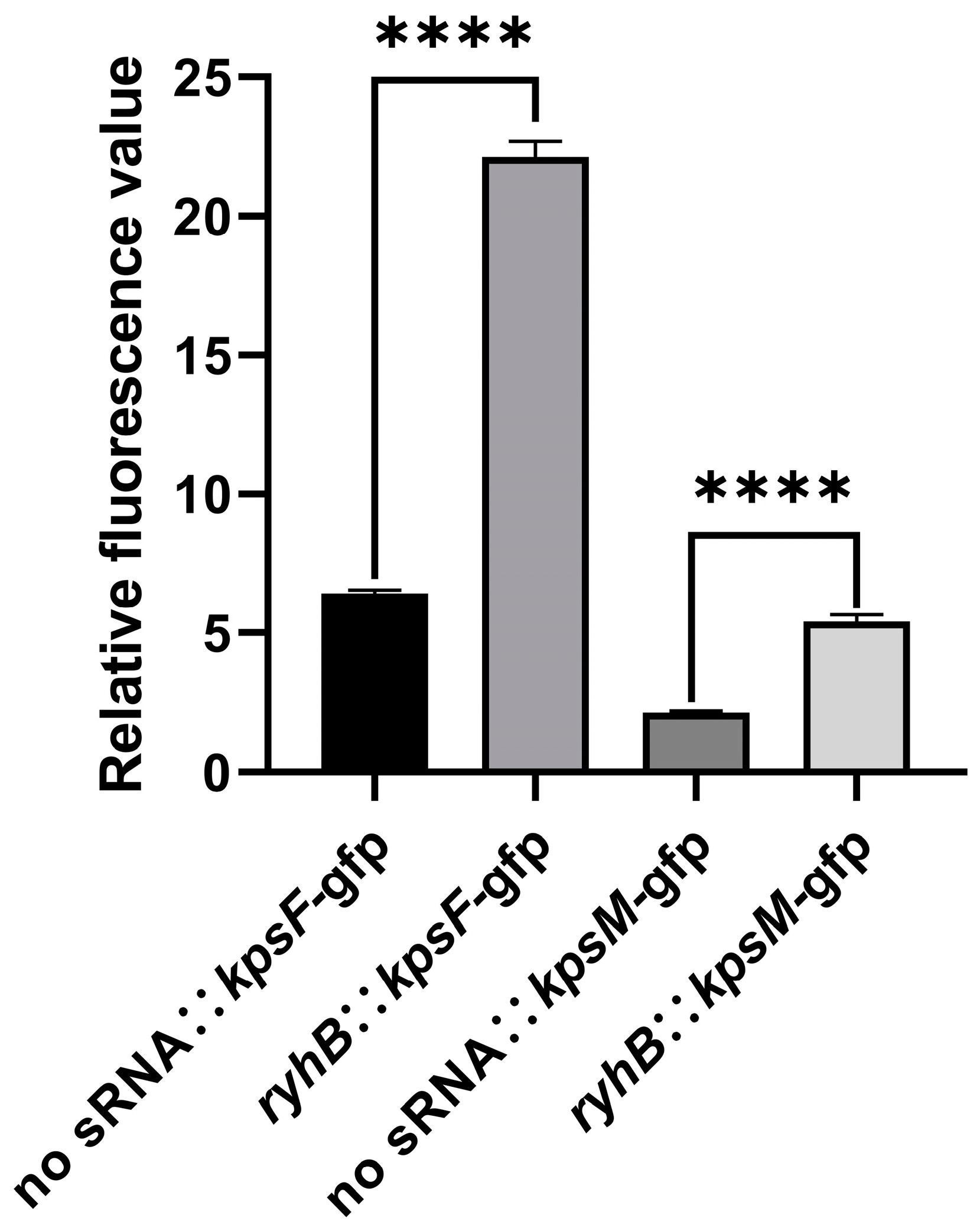
2.2. RyhB Directly Upregulates Capsular Gene Clusters by Interacting with the 5′ UTR of kpsF and kpsM
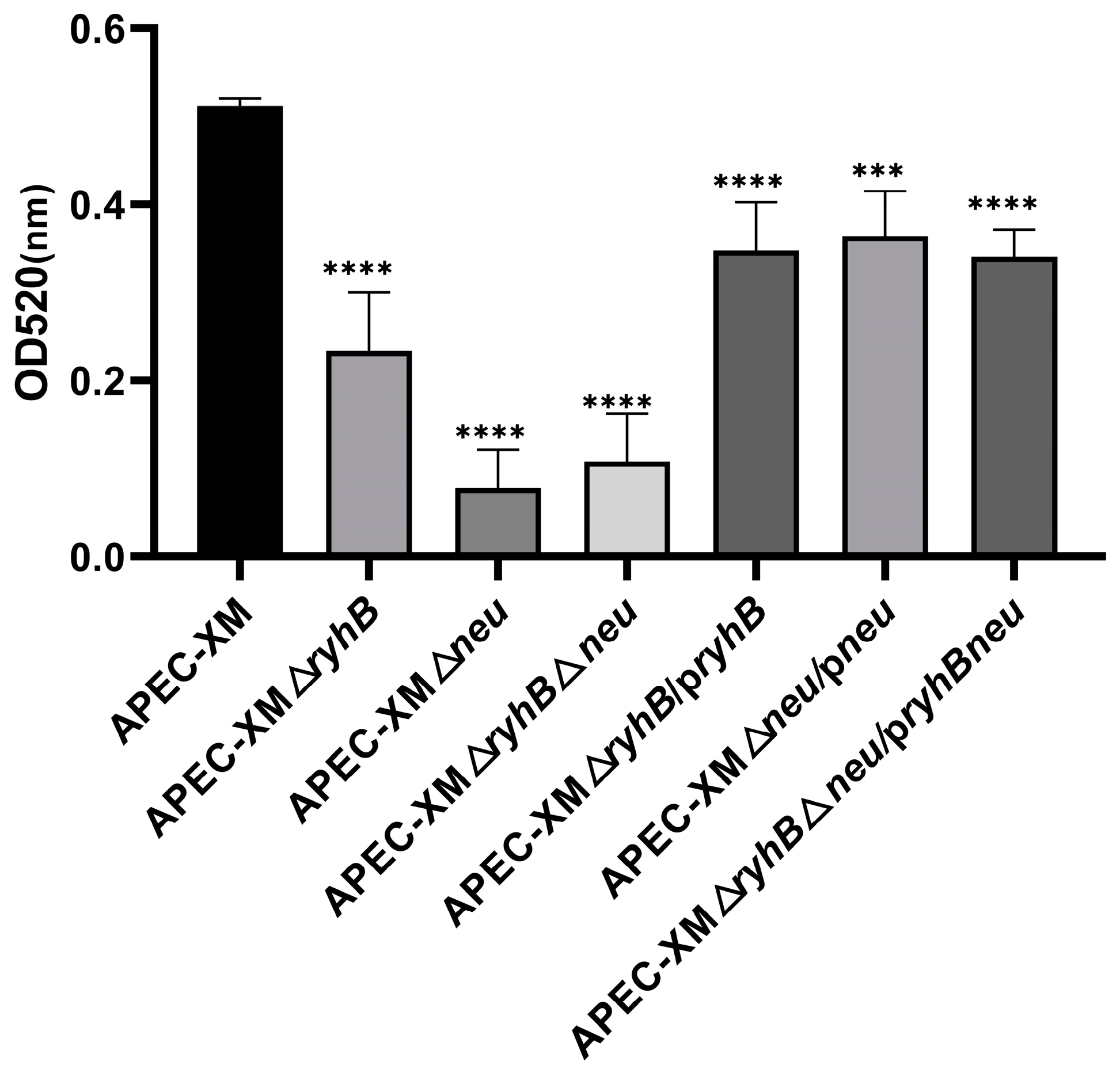
2.3. RyhB Contributes to the Production of Capsules
2.4. RyhB Is Required for Serum Resistance of APEC
2.5. RyhB Promotes Resistance to Phagocytosis
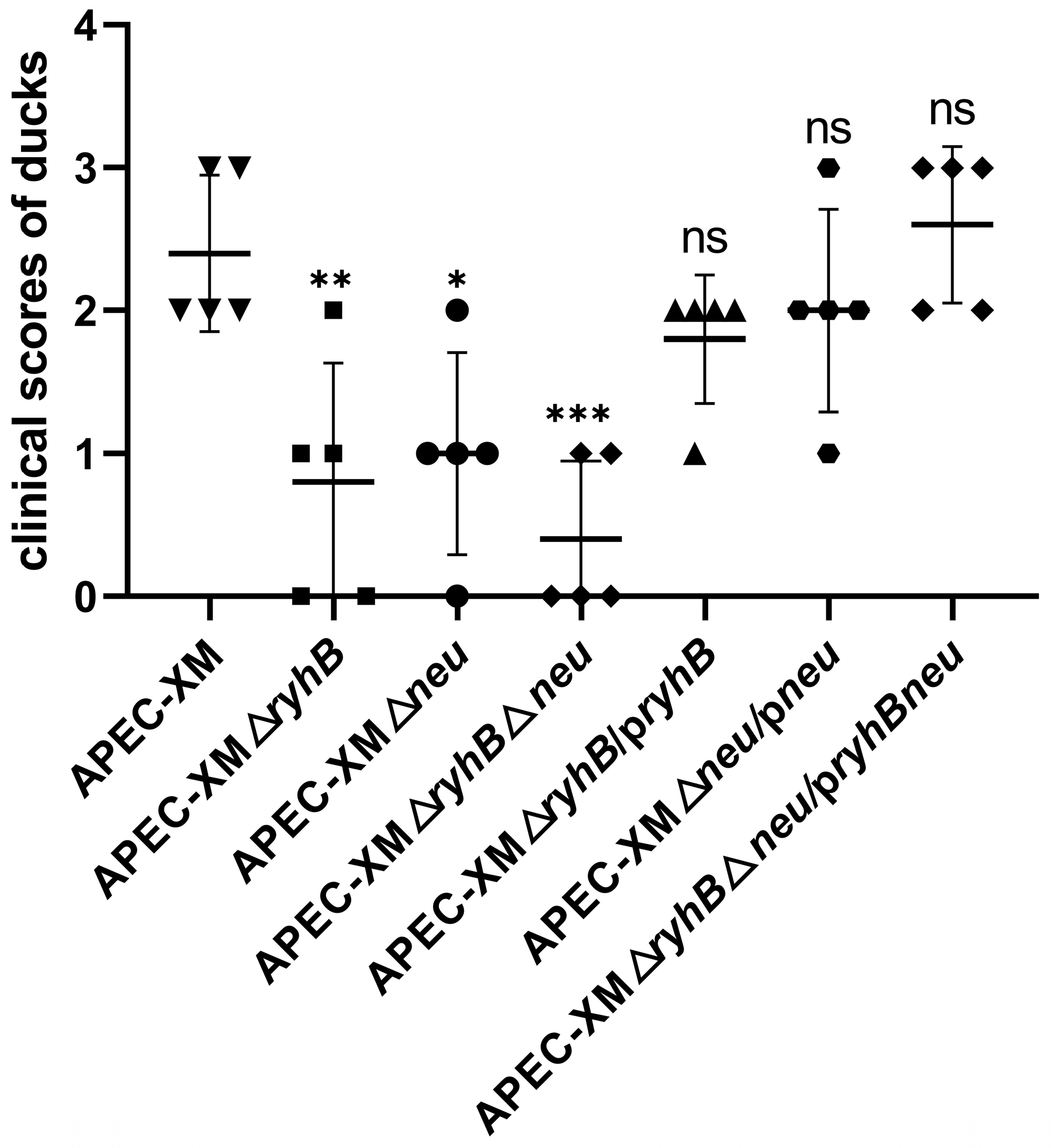
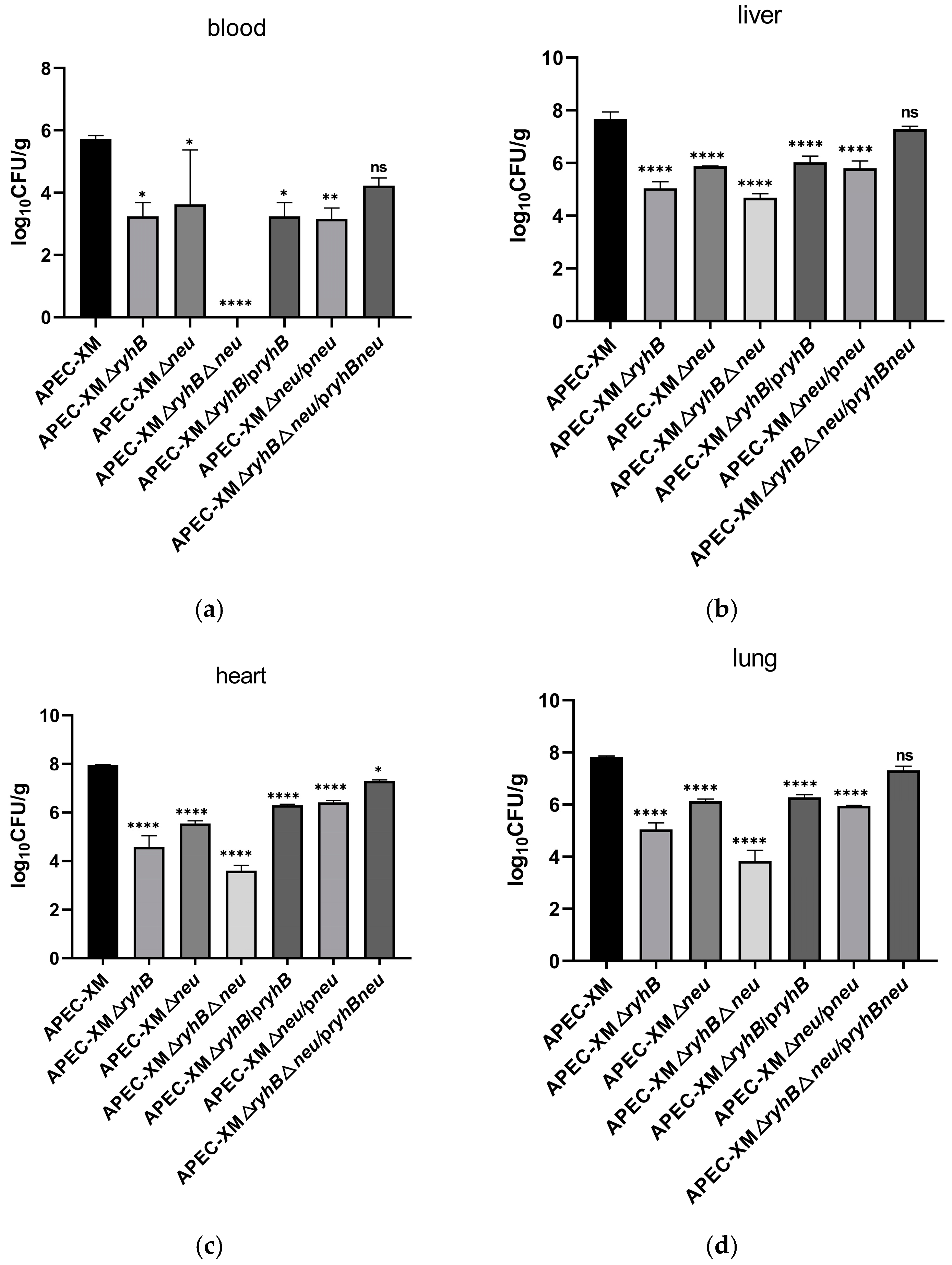
2.6. RyhB and Capsule Enhance Virulence of APEC in Ducks
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Plasmids, and Growth Conditions
4.2. Quantitative Real-Time PCR
4.3. Prediction of Interaction Sites and the Secondary Structure of Target mRNA
4.4. Validation of Interactions Between RyhB and Targets by a GFP-Based Reporter System
4.5. Construction of the Deletion Mutants and the Complemented Mutants
4.6. Extraction and Quantification of Capsules When APEC Resisted to Duck Serum
4.7. Survival of APEC in Duck Serum
4.8. Determination of Resistance to Phagocytosis
4.9. Animal Infections
4.10. Determination of Bacterial Loadings in the Tissues of Duck
4.11. LD50 Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| APEC | Avian pathogenic Escherichia coli |
| ExPEC | Extraintestinal pathogenic Escherichia coli |
| sRNA | small non-coding RNA |
| H-NS | histone-like nucleoid structuring protein |
| IHF | Integration Host Factor |
| kps | capsule gene clusters |
| qRT-PCR | quantitative real-time PCR |
| WT | wild type |
| UTR | untranslated region |
| SD | Shine–Dalgarno |
| ops | operon polarity suppressor |
| LB | Luria–Bertani |
| CFU | colony forming unit |
| LD50 | lethal dose 50% |
| RBS | ribosome binding site |
| DMEM | Dulbecco’s Modified Eagle Medium |
| FBS | fetal bovine serum |
| MOI | multiplicity of infection |
References
- Hu, J.; Afayibo, D.J.A.; Zhang, B.; Zhu, H.; Yao, L.; Guo, W.; Wang, X.; Wang, Z.; Wang, D.; Peng, H.; et al. Characteristics, pathogenic mechanism, zoonotic potential, drug resistance, and prevention of avian pathogenic Escherichia coli (APEC). Front. Microbiol. 2022, 13, 1049391. [Google Scholar]
- Krishnan, S.; Chang, A.C.; Hodges, J.; Couraud, P.-O.; Romero, I.A.; Weksler, B.; Nicholson, B.A.; Nolan, L.K.; Prasadarao, N.V. Serotype O18 avian pathogenic and neonatal meningitis Escherichia coli strains employ similar pathogenic strategies for the onset of meningitis. Virulence 2015, 6, 777–786. [Google Scholar] [PubMed]
- Zhu Ge, X.Z.; Jiang, J.; Pan, Z.; Hu, L.; Wang, S.; Wang, H.; Leung, F.C.; Dai, J.; Fan, H. Comparative Genomic Analysis Shows That Avian Pathogenic Escherichia coli Isolate IMT5155 (O2:K1:H5; ST Complex 95, ST140) Shares Close Relationship with ST95 APEC O1:K1 and Human ExPEC O18:K1 Strains. PLoS ONE 2014, 9, e112048. [Google Scholar] [CrossRef]
- Meng, X.; Chen, Y.; Wang, P.; He, M.; Shi, Y.; Lai, Y.; Zhu, G.; Wang, H. RyhB in Avian Pathogenic Escherichia coli Regulates the Expression of Virulence-Related Genes and Contributes to Meningitis Development in a Mouse Model. Int. J. Mol. Sci. 2022, 23, 15532. [Google Scholar] [CrossRef]
- Zhong, H.; Wang, P.; Chen, Y.; Wang, H.; Li, J.; Li, J.; Zhu, G.; Cui, L.; Meng, X. ClpV1 in avian pathogenic Escherichia coli is a crucial virulence factor contributing to meningitis in a mouse model in vivo. Vet. Microbiol. 2021, 263, 109273. [Google Scholar]
- Afayibo, D.J.A.; Zhu, H.; Zhang, B.; Yao, L.; Abdelgawad, H.A.; Tian, M.; Qi, J.; Liu, Y.; Wang, S. Isolation, Molecular Characterization, and Antibiotic Resistance of Avian Pathogenic Escherichia coli in Eastern China. Vet. Sci. 2022, 9, 319. [Google Scholar]
- Liu, C.; Diao, Y.; Wang, D.; Chen, H.; Tang, Y.; Diao, Y. Duck viral infection escalated the incidence of avian pathogenic Escherichia coli in China. Transbound. Emerg. Dis. 2019, 66, 929–938. [Google Scholar]
- Ma, J.; An, C.; Jiang, F.; Yao, H.; Logue, C.; Nolan, L.K.; Li, G. Extraintestinal pathogenic Escherichia coli increase extracytoplasmic polysaccharide biosynthesis for serum resistance in response to bloodstream signals. Mol. Microbiol. 2018, 110, 689–706. [Google Scholar]
- Leying, H.; Suerbaum, S.; Kroll, H.P.; Stahl, D.; Opferkuch, W. The capsular polysaccharide is a major determinant of serum resistance in K-1-positive blood culture isolates of Escherichia coli. Infect. Immun. 1990, 58, 222–227. [Google Scholar]
- Minh-Duy, P.; Peters, K.M.; Sarkar, S.; Lukowski, S.W.; Allsopp, L.P.; Moriel, D.G.; Achard, M.E.S.; Totsika, M.; Marshall, V.M.; Upton, M.; et al. The Serum Resistome of a Globally Disseminated Multidrug Resistant Uropathogenic Escherichia coli Clone. PLoS Genet. 2013, 9, e1003834. [Google Scholar]
- Burns, S.M.; Hull, S.I. Comparison of loss of serum resistance by defined lipopolysaccharide mutants and an acapsular mutant of uropathogenic Escherichia coli O75:K5. Infect. Immun. 1998, 66, 4244–4253. [Google Scholar] [CrossRef] [PubMed]
- Prasadarao, N.V.; Blom, A.M.; Villoutreix, B.O.; Linsangan, L.C. A novel interaction of outer membrane protein A with C4b binding protein mediates serum resistance of Escherichia coli K1. J. Immunol. 2002, 169, 6352–6360. [Google Scholar] [CrossRef] [PubMed]
- Mahendran, G.; Jayasinghe, O.T.; Thavakumaran, D.; Arachchilage, G.M.; Silva, G.N. Key players in regulatory RNA realm of bacteria. Biochem. Biophys. Rep. 2022, 30, 101276. [Google Scholar] [CrossRef] [PubMed]
- Jorgensen, M.G.; Pettersen, J.S.; Kallipolitis, B.H. sRNA-mediated control in bacteria: An increasing diversity of regulatory mechanisms. Biochim. Et Biophys. Acta-Gene Regul. Mech. 2020, 1863, 194504. [Google Scholar] [CrossRef]
- Chareyre, S.; Mandin, P. Bacterial Iron Homeostasis Regulation by sRNAs. Microbiol. Spectr. 2018, 6, rwr-0010-2017. [Google Scholar] [CrossRef]
- Calderon, I.L.; Morales, E.H.; Collao, B.; Calderon, P.F.; Chahuan, C.A.; Acuna, L.G.; Gil, F.; Saavedra, C.P. Role of Salmonella Typhimurium small RNAs RyhB-1 and RyhB-2 in the oxidative stress response. Res. Microbiol. 2014, 165, 30–40. [Google Scholar] [CrossRef]
- Calderon, P.F.; Morales, E.H.; Acuna, L.G.; Fuentes, D.N.; Gil, F.; Porwollik, S.; McClelland, M.; Saavedra, C.P.; Calderon, I.L. The small RNA RyhB homologs from Salmonella typhimurium participate in the response to S-nitrosoglutathione-induced stress. Biochem. Biophys. Res. Commun. 2014, 450, 641–645. [Google Scholar] [CrossRef]
- Oglesby, A.G.; Murphy, E.R.; Iyer, V.R.; Payne, S.M. Fur regulates acid resistance in Shigella flexneri via RyhB and ydeP. Mol. Microbiol. 2005, 58, 1354–1367. [Google Scholar] [CrossRef]
- Mey, A.R.; Craig, S.A.; Payne, S.M. Characterization of Vibrio cholerae RyhB: The RyhB regulon and role of ryhB in biofilm formation. Infect. Immun. 2005, 73, 5706–5719. [Google Scholar] [CrossRef]
- Meng, X.; He, M.; Chen, B.; Xia, P.; Wang, J.; Zhu, C.; Wang, H.; Zhu, G. RyhB Paralogs Downregulate the Expressions of Multiple Survival-Associated Genes and Attenuate the Survival of Salmonella Enteritidis in the Chicken Macrophage HD11. Microorganisms 2023, 11, 214. [Google Scholar] [CrossRef]
- Buckles, E.L.; Wang, X.; Lane, M.C.; Lockatell, C.V.; Johnson, D.E.; Rasko, D.A.; Mobley, H.L.T.; Donnenberg, M.S. Role of the K2 Capsule in Escherichia coli Urinary Tract Infection and Serum Resistance. J. Infect. Dis. 2009, 199, 1689–1697. [Google Scholar]
- Kim, K.S.; Itabashi, H.; Gemski, P.; Sadoff, J.; Warren, R.L.; Cross, A.S. The K1 capsule is the critical determinant in the development of Escherichia coli meningitis in the rat. J. Clin. Investig. 1992, 90, 897–905. [Google Scholar] [PubMed]
- Aldawood, E.; Roberts, I.S. Regulation of Escherichia coli Group 2 Capsule Gene Expression: A Mini Review and Update. Front. Microbiol. 2022, 13, 858767. [Google Scholar]
- Jia, J.; King, J.E.; Goldrick, M.C.; Aldawood, E.; Roberts, I.S. Three tandem promoters, together with IHF, regulate growth phase dependent expression of the Escherichia coli kps capsule gene cluster. Sci. Rep. 2017, 7, 17924. [Google Scholar] [CrossRef]
- Corbett, D.; Bennett, H.J.; Askar, H.; Green, J.; Roberts, I.S. SlyA and H-NS regulate transcription of the Escherichia coli K5 capsule gene cluster, and expression of slyA in Escherichia coli is temperature dependent, positively autoregulated, and independent of H-NS. J. Biol. Chem. 2007, 282, 33326–33335. [Google Scholar] [PubMed]
- Xue, P.; Corbett, D.; Goldrick, M.; Naylor, C.; Roberts, I.S. Regulation of Expression of the Region 3 Promoter of the Escherichia coli K5 Capsule Gene Cluster Involves H-NS, SlyA, and a Large 5′ Untranslated Region. J. Bacteriol. 2009, 191, 1838–1846. [Google Scholar]
- Rowe, S.; Hodson, N.; Griffiths, G.; Roberts, I.S. Regulation of the Escherichia coli K5 capsule gene cluster: Evidence for the roles of H-NS, BipA, and integration host factor in regulation of group 2 capsule gene clusters in pathogenic E-coli. J. Bacteriol. 2000, 182, 2741–2745. [Google Scholar]
- Navasa, N.; Rodriguez-Aparicio, L.B.; Ferrero, M.A.; Monteagudo-Mera, A.; Martinez-Blanco, H. Transcriptional control of RfaH on polysialic and colanic acid synthesis by Escherichia coli K92. Febs Lett. 2014, 588, 922–928. [Google Scholar]
- Urban, J.H.; Vogel, J. Two seemingly homologous noncoding RNAs act hierarchically to activate glmS mRNA translation. PLoS Biol. 2008, 6, 631–642. [Google Scholar]
- Prevost, K.; Salvail, H.; Desnoyers, G.; Jacques, J.-F.; Phaneuf, E.; Masse, E. The small RNA RyhB activates the translation of shiA mRNA encoding a permease of shikimate, a compound involved in siderophore synthesis. Mol. Microbiol. 2007, 64, 1260–1273. [Google Scholar]
- London, L.Y.; Aubee, J.I.; Nurse, J.; Thompson, K.M. Post-Transcriptional Regulation of RseA by Small RNAs RyhB and FnrS in Escherichia coli. Front. Mol. Biosci. 2021, 8, 668613. [Google Scholar]
- Balbontin, R.; Villagra, N.; Pardos de la Gandara, M.; Mora, G.; Figueroa-Bossi, N.; Bossi, L. Expression of IroN, the salmochelin siderophore receptor, requires mRNA activation by RyhB small RNA homologues. Mol. Microbiol. 2016, 100, 139–155. [Google Scholar] [PubMed]
- Stevens, M.P.; Clarke, B.R.; Roberts, I.S. Regulation of the Escherichia coli K5 capsule gene cluster by transcription antitermination. Mol. Microbiol. 1997, 24, 1001–1012. [Google Scholar]
- Mellata, M.; Dho-Moulin, M.; Dozois, C.M.; Curtiss, R.; Brown, P.K.; Arné, P.; Brée, A.; Desautels, C.; Fairbrother, J.M. Role of virulence factors in resistance of avian pathogenic Escherichia coli to serum and in pathogenicity. Infect. Immun. 2003, 71, 536–540. [Google Scholar] [PubMed]
- Li, G.; Tivendale, K.A.; Liu, P.; Feng, Y.; Wannemuehler, Y.; Cai, W.; Mangiamele, P.; Johnson, T.J.; Constantinidou, C.; Penn, C.W.; et al. Transcriptome Analysis of Avian Pathogenic Escherichia coli O1 in Chicken Serum Reveals Adaptive Responses to Systemic Infection. Infect. Immun. 2011, 79, 1951–1960. [Google Scholar]
- Kim, K.J.; Elliott, S.J.; Di Cello, F.; Stins, M.F.; Kim, K.S. The K1 capsule modulates trafficking of E-coli-containing vacuoles and enhances intracellular bacterial survival in human brain microvascular endothelial cells. Cell. Microbiol. 2003, 5, 245–252. [Google Scholar] [PubMed]
- Schwarz, F.; Landig, C.S.; Siddiqui, S.; Secundino, I.; Olson, J.; Varki, N.; Nizet, V.; Varki, A. Paired Siglec receptors generate opposite inflammatory responses to a humanspecific pathogen. Embo J. 2017, 36, 751–760. [Google Scholar]
- Yang, J.; Ma, W.; Wu, Y.; Zhou, H.; Song, S.; Cao, Y.; Wang, C.; Liu, X.; Ren, J.; Duan, J.; et al. O-Acetylation of Capsular Polysialic Acid Enables Escherichia coli K1 Escaping from Siglec-Mediated Innate Immunity and Lysosomal Degradation of E. coli-Containing Vacuoles in Macrophage-Like Cells. Microbiol. Spectr. 2021, 9, e00399-21. [Google Scholar]
- Datsenko, K.A.; Wanner, B.L. One-step inactivation of chromosomal genes in Escherichia coli K-12 using PCR products. Proc. Natl. Acad. Sci. USA 2000, 97, 6640–6645. [Google Scholar]
- Chen, B.; Meng, X.; Ni, J.; He, M.; Chen, Y.; Xia, P.; Wang, H.; Liu, S.; Zhu, G.; Meng, X. Positive regulation of Type III secretion effectors and virulence by RyhB paralogs in Salmonella enterica serovar Enteritidis. Vet. Res. 2021, 52, 44. [Google Scholar]
- Wright, P.R.; Georg, J.; Mann, M.; Sorescu, D.A.; Richter, A.S.; Lott, S.; Kleinkauf, R.; Hess, W.R.; Backofen, R. CopraRNA and IntaRNA: Predicting small RNA targets, networks and interaction domains. Nucleic Acids Res. 2014, 42, W119–W123. [Google Scholar] [CrossRef] [PubMed]
- Raden, M.; Ali, S.M.; Alkhnbashi, O.S.; Busch, A.; Costa, F.; Davis, J.A.; Eggenhofer, F.; Gelhausen, R.; Georg, J.; Heyne, S.; et al. Freiburg RNA tools: A central online resource for RNA-focused research and teaching. Nucleic Acids Res. 2018, 46, W25–W29. [Google Scholar] [CrossRef] [PubMed]
- Workbench, C.L.M. Available online: http://www.clcbio.com (accessed on 25 December 2018).
- Urban, J.H.; Vogel, J. Translational control and target recognition by Escherichia coli small RNAs in vivo. Nucleic Acids Res. 2007, 35, 1018–1037. [Google Scholar] [CrossRef] [PubMed]
- Wang, P.; Zhang, J.; Chen, Y.; Zhong, H.; Wang, H.; Li, J.; Zhu, G.; Xia, P.; Cui, L.; Li, J.; et al. ClbG in Avian Pathogenic Escherichia coli Contributes to Meningitis Development in a Mouse Model. Toxins 2021, 13, 546. [Google Scholar] [CrossRef]
- Meng, X.; Meng, X.; Zhu, C.; Wang, H.; Wang, J.; Nie, J.; Hardwidge, P.R.; Zhu, G. The RNA chaperone Hfq regulates expression of fimbrial-related genes and virulence of Salmonella enterica serovar Enteritidis. Fems Microbiol. Lett. 2013, 346, 90–96. [Google Scholar] [CrossRef]
- Van Der Velden AW, M.; Bumler, A.J.; Tsolis, R.M.; Heffron, F. Multiple fimbrial adhesins are required for full virulence of Salmonella typhimurium in mice. Abstr. Gen. Meet. Am. Soc. Microbiol. 1998, 98, 2803–2808. [Google Scholar] [CrossRef]

| Strain or Plasmid | Characteristic or Function | References |
|---|---|---|
| APEC XM | Virulent strain of APEC, isolated from the brain of duck | [4] |
| APEC XMΔryhB | Deletion mutant of ryhB with APEC XM background | [4] |
| APEC XMΔryhB/pryhB | APEC XM ΔryhB carrying the vector pBR-ryhB, Ampr | [4] |
| APEC XMΔneu | Deletion mutant of neuDBACES with APEC XM background | This study |
| APEC XMΔneu/pneu | APEC XM Δneu carrying the vector pACYC184-neu, Cmr | This study |
| APEC XMΔryhBΔneu | Deletion mutant of ryhB and neuDBACES | This study |
| APEC XMΔryhBΔneu/pryhBneu | APEC XMΔryhBΔneu carrying the vector pBR-ryhB and pACYC-neu | This study |
| pKD46 | Ampr, λ-red recombinase expression | [39] |
| pKD3 | Cmr, Cm cassette template | [39] |
| pCP20 | Ampr, Cmr, Flp recombinase expression | [39] |
| pBR-ryhB | Ampr, pBR322 carrying the full ryhB gene sequence | [4] |
| pACYC184-neu | Cmr, pACYC184 carrying the full neuDBACES sequence | This study |
| pJV-300 | Ampr, sRNA cloning vector | [40] |
| pXG-10SF | Cmr, target gene cloning vector with GFP | [40] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, Y.; Gao, M.; Xing, L.; Zhu, G.; Wang, H.; Meng, X. RyhB Regulates Capsular Synthesis for Serum Resistance and Virulence of Avian Pathogenic Escherichia coli. Int. J. Mol. Sci. 2025, 26, 3062. https://doi.org/10.3390/ijms26073062
Shi Y, Gao M, Xing L, Zhu G, Wang H, Meng X. RyhB Regulates Capsular Synthesis for Serum Resistance and Virulence of Avian Pathogenic Escherichia coli. International Journal of Molecular Sciences. 2025; 26(7):3062. https://doi.org/10.3390/ijms26073062
Chicago/Turabian StyleShi, Yuxing, Mingjuan Gao, Lin Xing, Guoqiang Zhu, Heng Wang, and Xia Meng. 2025. "RyhB Regulates Capsular Synthesis for Serum Resistance and Virulence of Avian Pathogenic Escherichia coli" International Journal of Molecular Sciences 26, no. 7: 3062. https://doi.org/10.3390/ijms26073062
APA StyleShi, Y., Gao, M., Xing, L., Zhu, G., Wang, H., & Meng, X. (2025). RyhB Regulates Capsular Synthesis for Serum Resistance and Virulence of Avian Pathogenic Escherichia coli. International Journal of Molecular Sciences, 26(7), 3062. https://doi.org/10.3390/ijms26073062





