Urolithin A Protects Porcine Oocytes from Artificially Induced Oxidative Stress Damage to Enhance Oocyte Maturation and Subsequent Embryo Development
Abstract
1. Introduction
2. Results
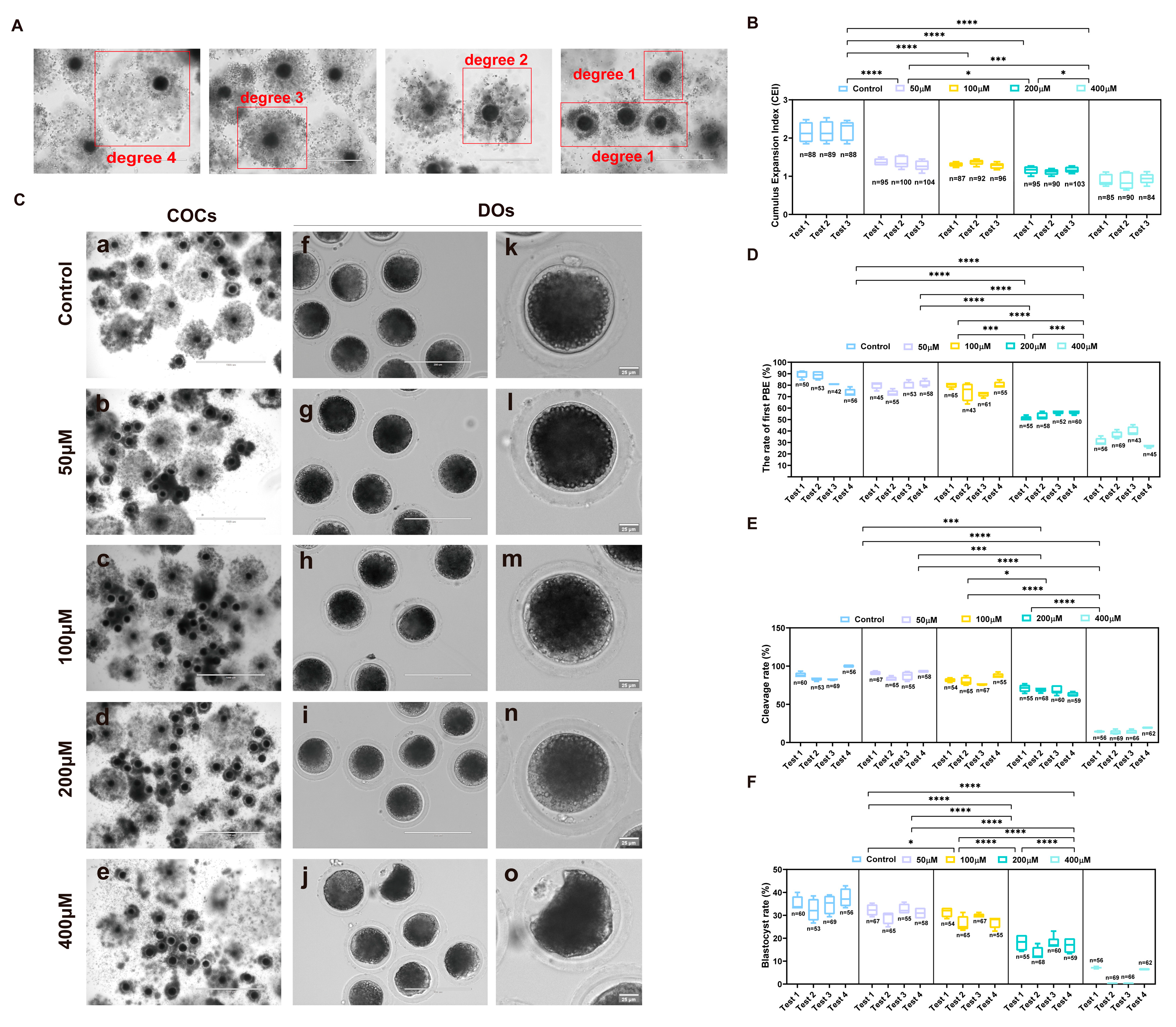
2.1. Establishment of an Artificially Induced Oxidative Stress Model in Porcine Oocytes
2.2. Effects of UA on Porcine Oocyte Maturation and Developmental Competence Under Artificially Induced Oxidative Stress Damage
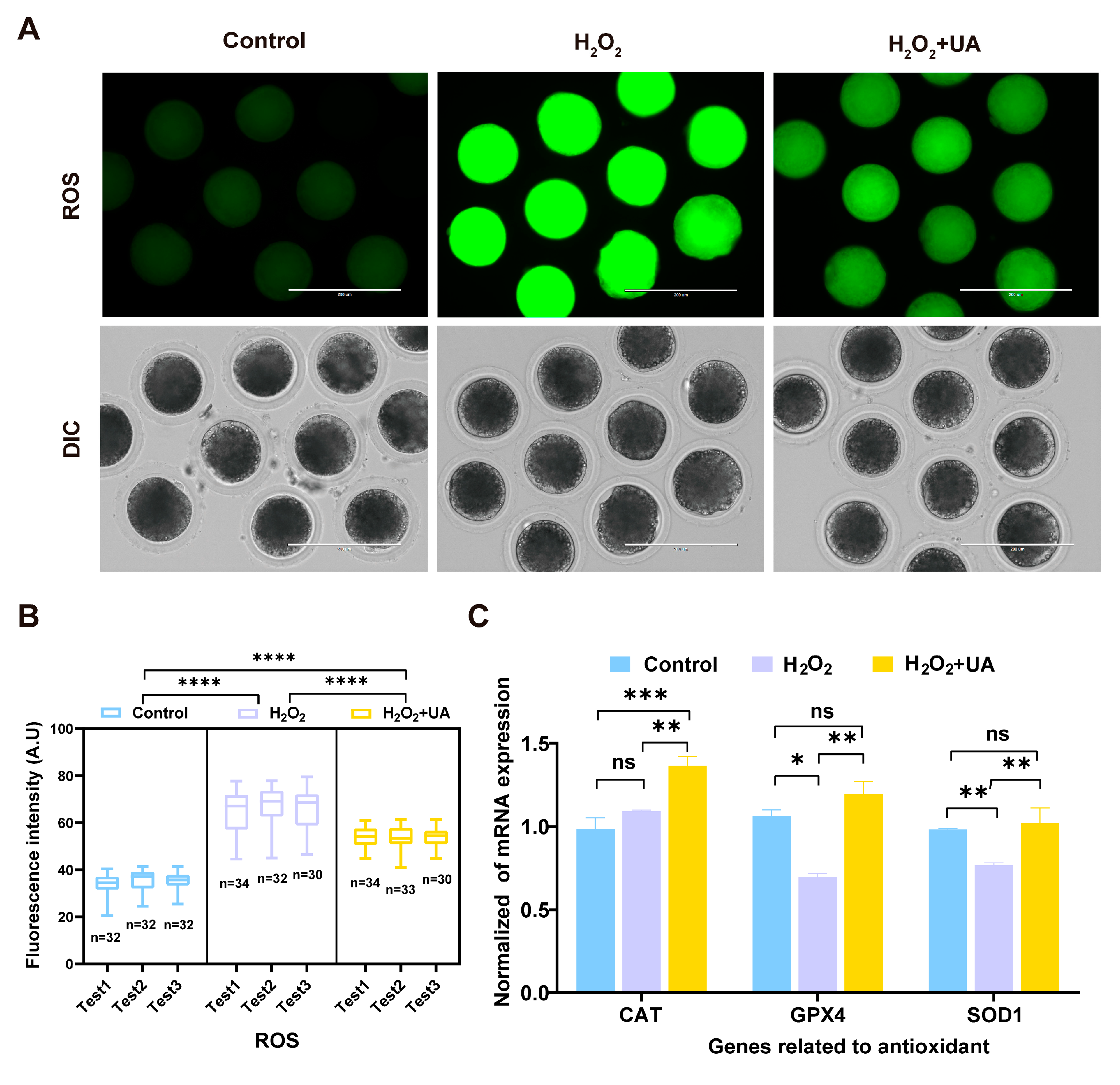
2.3. UA Supplementation Enhances Antioxidant Capacity in Porcine Oocytes with Artificially Induced Oxidative Stress Damage
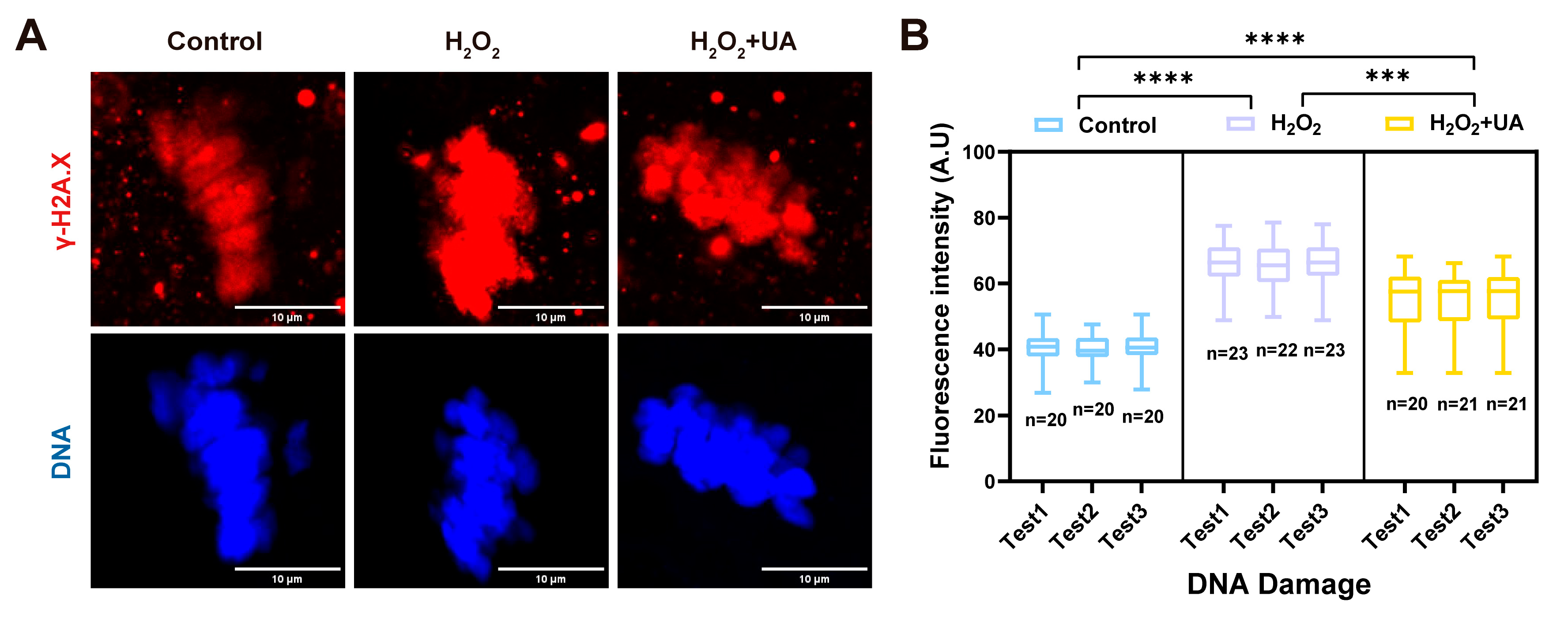
2.4. UA Supplementation Attenuates DNA Damage in Artificially Induced Oxidative Stress-Damaged Porcine Oocytes
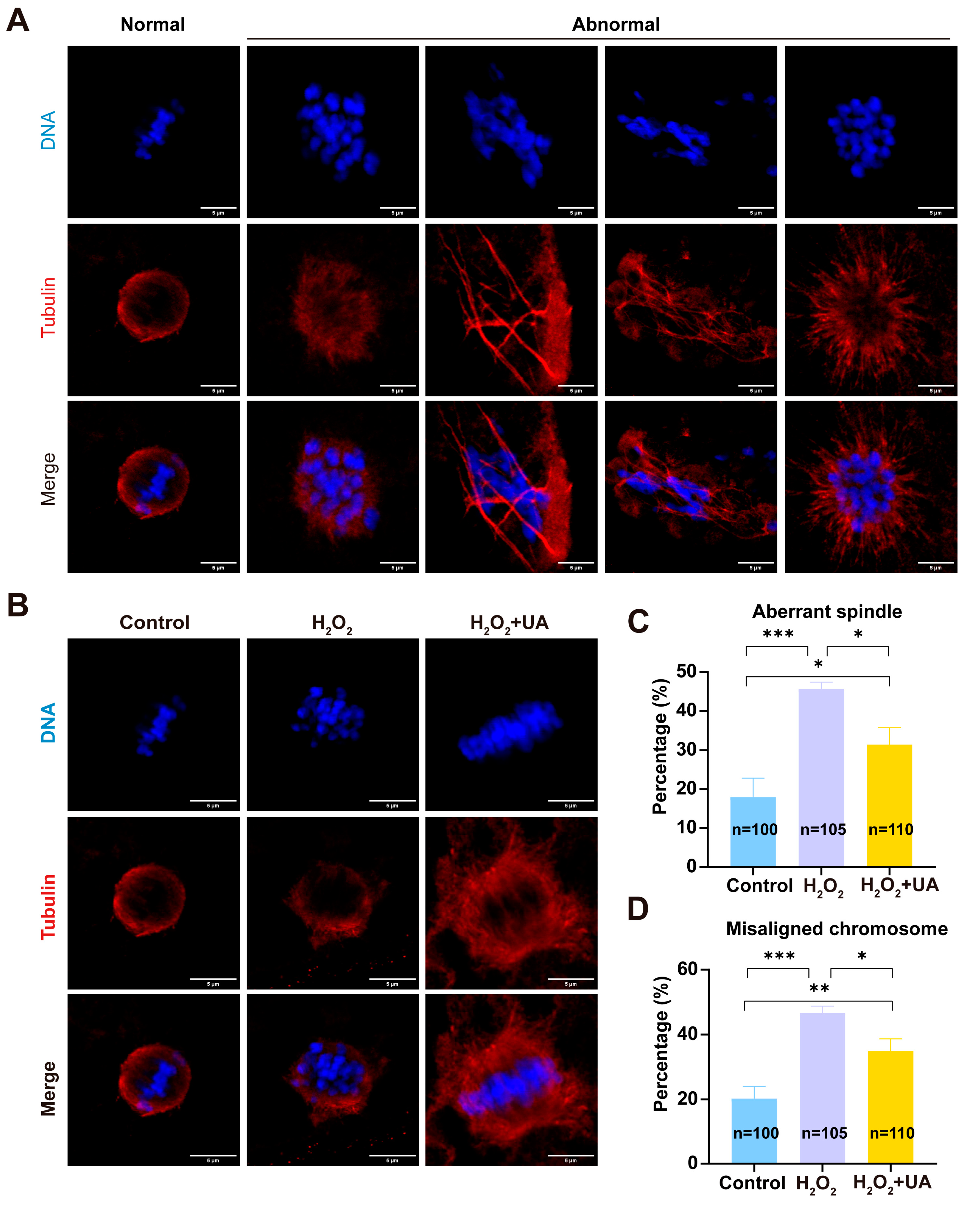
2.5. UA Supplementation Restores Spindle/Chromosome Defects in Artificially Induced Oxidative Stress-Damaged Porcine Oocytes
2.6. UA Supplementation Rescues Actin Polymerization Abnormalities in Artificially Induced Oxidative Stress-Damaged Porcine Oocytes
2.7. UA Supplementation Improves Mitochondrial Function in Artificially Induced Oxidative Stress-Damaged Porcine Oocytes
2.8. UA Rescues Cortical Granule Distribution in Artificially Induced Oxidative Stress-Damaged Porcine Oocytes
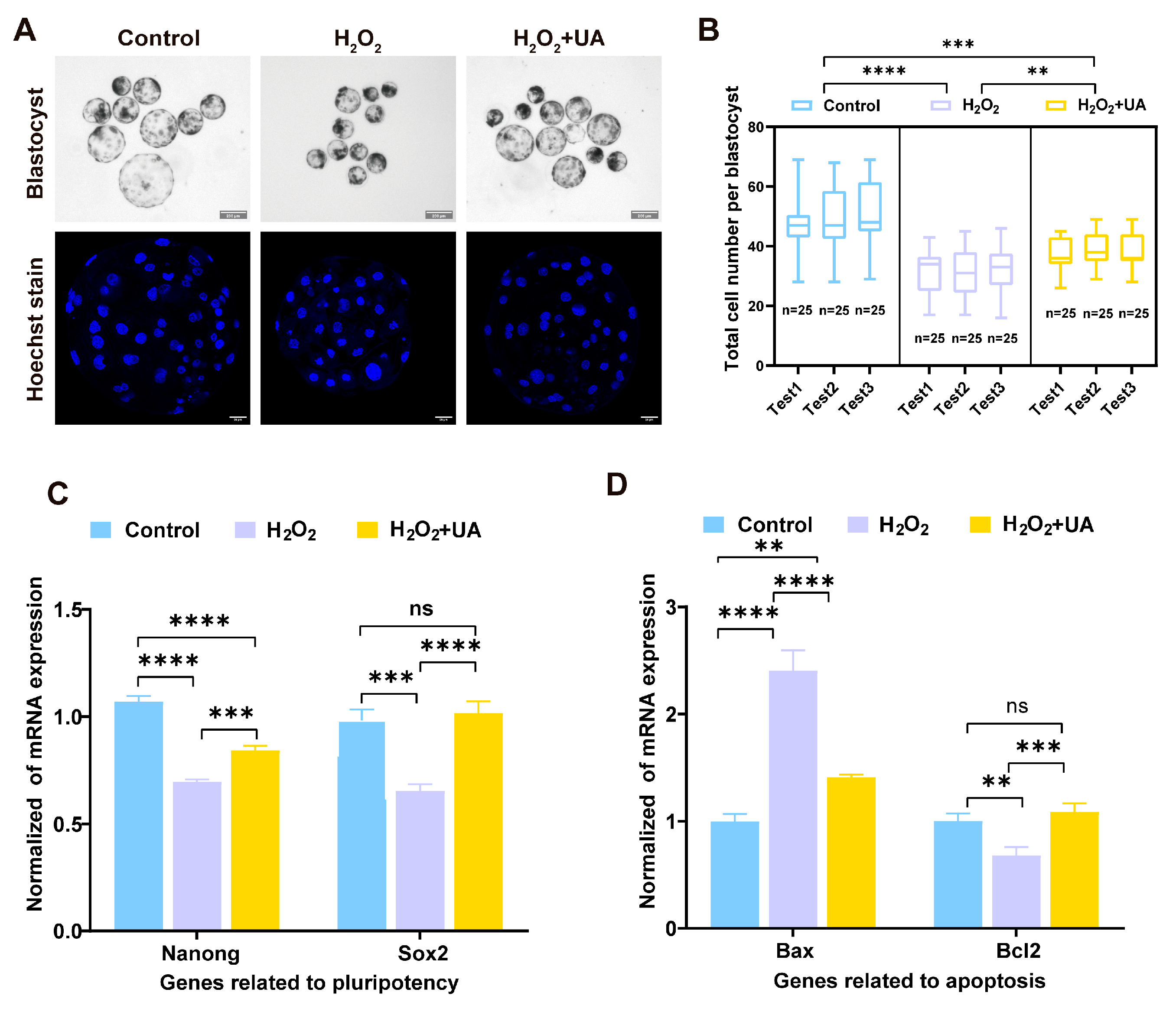
2.9. UA Supplementation Enhances the Quality of Parthenogenetic Embryo Development in Artificially Induced Oxidative Stress-Damaged Porcine Oocytes
3. Discussion
4. Materials and Methods
4.1. Oocyte Collection and Maturation In Vitro
4.2. Artificially Induced Oxidative Stress-Damage and Urolithin A Supplementation
4.3. Statistical Analysis of the Cumulus Cell Expansion Index
4.4. Parthenogenetic Activation and In Vitro Culturing of Porcine Oocytes
4.5. Fluorescence Staining
4.6. ATP Analysis in Oocytes
4.7. Oocyte Micro-Reverse Transcription and Real-Time Quantitative PCR Analysis
4.8. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Lunney, J.K. Advances in Swine Biomedical Model Genomics. Int. J. Biol. Sci. 2007, 3, 179–184. [Google Scholar] [CrossRef] [PubMed]
- Aigner, B.; Renner, S.; Kessler, B.; Klymiuk, N.; Kurome, M.; Wünsch, A.; Wolf, E. Transgenic Pigs as Models for Translational Biomedical Research. J. Mol. Med. 2010, 88, 653–664. [Google Scholar] [CrossRef] [PubMed]
- Abeydeera, L.R. In Vitro Production of Embryos in Swine. Theriogenology 2002, 57, 256–273. [Google Scholar] [CrossRef]
- Fang, X.; Xia, W.; Li, S.; Qi, Y.; Liu, M.; Yu, Y.; Li, H.; Li, M.; Tao, C.; Wang, Z.; et al. SIRT2 Is Critical for Sheep Oocyte Maturation through Regulating Function of Surrounding Granulosa Cells. Int. J. Mol. Sci. 2022, 23, 5013. [Google Scholar] [CrossRef]
- Wang, Y.; Qi, J.-J.; Yin, Y.-J.; Jiang, H.; Zhang, J.-B.; Liang, S.; Yuan, B. Ferulic Acid Enhances Oocyte Maturation and the Subsequent Development of Bovine Oocytes. Int. J. Mol. Sci. 2023, 24, 14804. [Google Scholar] [CrossRef]
- Lai, L.; Kolber-Simonds, D.; Park, K.-W.; Cheong, H.-T.; Greenstein, J.L.; Im, G.-S.; Samuel, M.; Bonk, A.; Rieke, A.; Day, B.N.; et al. Production of Alpha-1,3-Galactosyltransferase Knockout Pigs by Nuclear Transfer Cloning. Science 2002, 295, 1089–1092. [Google Scholar] [CrossRef]
- Zhang, L.; Zhang, Z.; Wang, J.; Lv, D.; Zhu, T.; Wang, F.; Tian, X.; Yao, Y.; Ji, P.; Liu, G. Melatonin Regulates the Activities of Ovary and Delays the Fertility Decline in Female Animals via MT1/AMPK Pathway. J. Pineal Res. 2019, 66, e12550. [Google Scholar] [CrossRef]
- Yong, W.; Ma, H.; Na, M.; Gao, T.; Zhang, Y.; Hao, L.; Yu, H.; Yang, H.; Deng, X. Roles of Melatonin in the Field of Reproductive Medicine. Biomed. Pharmacother. 2021, 144, 112001. [Google Scholar] [CrossRef]
- Igarashi, H.; Takahashi, T.; Nagase, S. Oocyte Aging Underlies Female Reproductive Aging: Biological Mechanisms and Therapeutic Strategies. Reprod. Med. Biol. 2015, 14, 159–169. [Google Scholar] [CrossRef]
- Khazaei, M.; Aghaz, F. Reactive Oxygen Species Generation and Use of Antioxidants during In Vitro Maturation of Oocytes. Int. J. Fertil. Steril. 2017, 11, 63–70. [Google Scholar] [CrossRef]
- Krisher, R.L. In Vivo and in Vitro Environmental Effects on Mammalian Oocyte Quality. Annu. Rev. Anim. Biosci. 2013, 1, 393–417. [Google Scholar] [CrossRef] [PubMed]
- Asano, A.; Niwa, K. Activation and Penetration in Vitro of Pig Oocytes Treated with Calcium Ionophore. J. Reprod. Dev. 2004, 50, 77–85. [Google Scholar] [CrossRef] [PubMed]
- Kawasaki, K.; Hirai, M.; Ishiki, Y.; Nagahama, A.; Konno, T.; Yamanaka, K.; Tatemoto, H. The Strong Anti-Hyaluronidase Effect of Ellagic Acid Markedly Decreases Polyspermy during In Vitro Fertilization, Resulting in Sustainment of the Developmental Potency in Porcine Oocytes. Theriogenology 2024, 215, 95–102. [Google Scholar] [CrossRef] [PubMed]
- Cui, Z.; Lu, Y.; Miao, Y.; Dai, X.; Zhang, Y.; Xiong, B. Transglutaminase 2 Crosslinks Zona Pellucida Glycoprotein 3 to Prevent Polyspermy. Cell Death Differ. 2022, 29, 1466–1473. [Google Scholar] [CrossRef]
- Rocha-Frigoni, N.A.S.; Leão, B.C.S.; Dall’Acqua, P.C.; Mingoti, G.Z. Improving the Cytoplasmic Maturation of Bovine Oocytes Matured in Vitro with Intracellular and/or Extracellular Antioxidants Is Not Associated with Increased Rates of Embryo Development. Theriogenology 2016, 86, 1897–1905. [Google Scholar] [CrossRef]
- Keane, J.A.; Ealy, A.D. An Overview of Reactive Oxygen Species Damage Occurring during In Vitro Bovine Oocyte and Embryo Development and the Efficacy of Antioxidant Use to Limit These Adverse Effects. Animals 2024, 14, 330. [Google Scholar] [CrossRef]
- Miao, Y.; Zhou, C.; Bai, Q.; Cui, Z.; ShiYang, X.; Lu, Y.; Zhang, M.; Dai, X.; Xiong, B. The Protective Role of Melatonin in Porcine Oocyte Meiotic Failure Caused by the Exposure to Benzo(a)Pyrene. Hum. Reprod. 2018, 33, 116–127. [Google Scholar] [CrossRef]
- Kim, W.-J.; Lee, S.-E.; Park, Y.-G.; Jeong, S.-G.; Kim, E.-Y.; Park, S.-P. Antioxidant Hesperetin Improves the Quality of Porcine Oocytes during Aging In Vitro. Mol. Reprod. Dev. 2019, 86, 32–41. [Google Scholar] [CrossRef]
- Yoon, J.-W.; Lee, S.-E.; Park, Y.-G.; Kim, W.-J.; Park, H.-J.; Park, C.-O.; Kim, S.-H.; Oh, S.-H.; Lee, D.-G.; Pyeon, D.-B.; et al. The Antioxidant Icariin Protects Porcine Oocytes from Age-Related Damage In Vitro. Anim. Biosci. 2021, 34, 546–557. [Google Scholar] [CrossRef]
- Pyeon, D.-B.; Lee, S.-E.; Yoon, J.-W.; Park, H.-J.; Park, C.-O.; Kim, S.-H.; Oh, S.-H.; Lee, D.-G.; Kim, E.-Y.; Park, S.-P. The Antioxidant Dieckol Reduces Damage of Oxidative Stress-Exposed Porcine Oocytes and Enhances Subsequent Parthenotes Embryo Development. Mol. Reprod. Dev. 2021, 88, 349–361. [Google Scholar] [CrossRef]
- Silva, B.R.; Silva, J.R.V. Mechanisms of Action of Non-Enzymatic Antioxidants to Control Oxidative Stress during in Vitro Follicle Growth, Oocyte Maturation, and Embryo Development. Anim. Reprod. Sci. 2023, 249, 107186. [Google Scholar] [CrossRef]
- D’Amico, D.; Andreux, P.A.; Valdés, P.; Singh, A.; Rinsch, C.; Auwerx, J. Impact of the Natural Compound Urolithin A on Health, Disease, and Aging. Trends Mol. Med. 2021, 27, 687–699. [Google Scholar] [CrossRef]
- Kim, K.B.; Lee, S.; Kim, J.H. Neuroprotective Effects of Urolithin A on H2O2-Induced Oxidative Stress-Mediated Apoptosis in SK-N-MC Cells. Nutr. Res. Pract. 2020, 14, 3–11. [Google Scholar] [CrossRef] [PubMed]
- Kallio, T.; Kallio, J.; Jaakkola, M.; Mäki, M.; Kilpeläinen, P.; Virtanen, V. Urolithins Display Both Antioxidant and Pro-Oxidant Activities Depending on Assay System and Conditions. J. Agric. Food Chem. 2013, 61, 10720–10729. [Google Scholar] [CrossRef] [PubMed]
- Ryu, D.; Mouchiroud, L.; Andreux, P.A.; Katsyuba, E.; Moullan, N.; Nicolet-Dit-Félix, A.A.; Williams, E.G.; Jha, P.; Lo Sasso, G.; Huzard, D.; et al. Urolithin A Induces Mitophagy and Prolongs Lifespan in C. Elegans and Increases Muscle Function in Rodents. Nat. Med. 2016, 22, 879–888. [Google Scholar] [CrossRef] [PubMed]
- Ávila-Gálvez, M.Á.; Espín, J.C.; González-Sarrías, A. Physiological Relevance of the Antiproliferative and Estrogenic Effects of Dietary Polyphenol Aglycones versus Their Phase-II Metabolites on Breast Cancer Cells: A Call of Caution. J. Agric. Food Chem. 2018, 66, 8547–8555. [Google Scholar] [CrossRef]
- Abdelazeem, K.N.M.; Kalo, M.Z.; Beer-Hammer, S.; Lang, F. The Gut Microbiota Metabolite Urolithin A Inhibits NF-κB Activation in LPS Stimulated BMDMs. Sci. Rep. 2021, 11, 7117. [Google Scholar] [CrossRef]
- Cota, V.; Sohrabi, S.; Kaletsky, R.; Murphy, C.T. Oocyte Mitophagy Is Critical for Extended Reproductive Longevity. PLoS Genet. 2022, 18, e1010400. [Google Scholar] [CrossRef]
- Jorge, M.; Ferreira, F.C.; Marques, C.C.; Batista, M.C.; Oliveira, P.J.; Lidon, F.; Duarte, S.C.; Teixeira, J.; Pereira, R.M.L.N. Effect of Urolithin A on Bovine Sperm Capacitation and In Vitro Fertilization. Animals 2024, 14, 2726. [Google Scholar] [CrossRef]
- Hong, Y.; Mu, X.; Ji, X.; Chen, X.; Geng, Y.; Zhang, Y.; Liu, Q.; Li, F.; Wang, Y.; He, J. In-Utero Exposure to HT-2 Toxin Affects Meiotic Progression and Early Oogenesis in Foetal Oocytes by Increasing Oxidative Stress. Environ. Pollut. 2021, 279, 116917. [Google Scholar] [CrossRef]
- Fonseca, É.; Marques, C.C.; Pimenta, J.; Jorge, J.; Baptista, M.C.; Gonçalves, A.C.; Pereira, R.M.L.N. Anti-Aging Effect of Urolithin A on Bovine Oocytes In Vitro. Animals 2021, 11, 2048. [Google Scholar] [CrossRef] [PubMed]
- Shen, P.-X.; Li, X.; Deng, S.-Y.; Zhao, L.; Zhang, Y.-Y.; Deng, X.; Han, B.; Yu, J.; Li, Y.; Wang, Z.-Z.; et al. Urolithin A Ameliorates Experimental Autoimmune Encephalomyelitis by Targeting Aryl Hydrocarbon Receptor. EBioMedicine 2021, 64, 103227. [Google Scholar] [CrossRef]
- Fu, X.; Gong, L.-F.; Wu, Y.-F.; Lin, Z.; Jiang, B.-J.; Wu, L.; Yu, K.-H. Urolithin A Targets the PI3K/Akt/NF-κB Pathways and Prevents IL-1β-Induced Inflammatory Response in Human Osteoarthritis: In Vitro and in Vivo Studies. Food Funct. 2019, 10, 6135–6146. [Google Scholar] [CrossRef] [PubMed]
- Guada, M.; Ganugula, R.; Vadhanam, M.; Ravi Kumar, M.N.V. Urolithin A Mitigates Cisplatin-Induced Nephrotoxicity by Inhibiting Renal Inflammation and Apoptosis in an Experimental Rat Model. J. Pharmacol. Exp. Ther. 2017, 363, 58–65. [Google Scholar] [CrossRef]
- Toney, A.M.; Fan, R.; Xian, Y.; Chaidez, V.; Ramer-Tait, A.E.; Chung, S. Urolithin A, a Gut Metabolite, Improves Insulin Sensitivity Through Augmentation of Mitochondrial Function and Biogenesis. Obesity 2019, 27, 612–620. [Google Scholar] [CrossRef]
- Merton, J.S.; de Roos, A.P.W.; Mullaart, E.; de Ruigh, L.; Kaal, L.; Vos, P.L.a.M.; Dieleman, S.J. Factors Affecting Oocyte Quality and Quantity in Commercial Application of Embryo Technologies in the Cattle Breeding Industry. Theriogenology 2003, 59, 651–674. [Google Scholar] [CrossRef]
- Gilchrist, R.B.; Smitz, J. Oocyte in Vitro Maturation: Physiological Basis and Application to Clinical Practice. Fertil. Steril. 2023, 119, 524–539. [Google Scholar] [CrossRef]
- Gong, X.; Li, H.; Zhao, Y. The Improvement and Clinical Application of Human Oocyte In Vitro Maturation (IVM). Reprod. Sci. 2022, 29, 2127–2135. [Google Scholar] [CrossRef]
- Lee, Y.; Shim, J.; Ko, N.; Kim, H.-J.; Kim, J.-H.; Kim, H.; Choi, K. Docosahexaenoic Acid Supplementation during Porcine Oocyte in Vitro Maturation Improves Oocyte Quality and Embryonic Development by Enhancing the Homeostasis of Energy Metabolism. Theriogenology 2024, 227, 49–59. [Google Scholar] [CrossRef]
- Wang, Y.-Q.; Dong, Y.-W.; Qu, H.-X.; Qi, J.-J.; Yan, C.-X.; Wei, H.-K.; Sun, H.; Sun, B.-X.; Liang, S. Oleanolic Acid Promotes Porcine Oocyte Maturation by Activating the Nrf2/HO-1 Signalling Pathway. Theriogenology 2024, 230, 203–211. [Google Scholar] [CrossRef]
- Bartolacci, A.; Busnelli, A.; Pagliardini, L.; de Girolamo, S.; De Santis, L.; Esposito, S.; Alteri, A.; Setti, P.E.L.; Papaleo, E. Assessing the Developmental Competence of Oocytes Matured Following Rescue in Vitro Maturation: A Systematic Review and Meta-Analysis. J. Assist. Reprod. Genet. 2024, 41, 1939–1950. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, A.; Said, T.M.; Bedaiwy, M.A.; Banerjee, J.; Alvarez, J.G. Oxidative Stress in an Assisted Reproductive Techniques Setting. Fertil. Steril. 2006, 86, 503–512. [Google Scholar] [CrossRef] [PubMed]
- Hardy, M.L.M.; Day, M.L.; Morris, M.B. Redox Regulation and Oxidative Stress in Mammalian Oocytes and Embryos Developed In Vivo and In Vitro. Int. J. Environ. Res. Public Health 2021, 18, 11374. [Google Scholar] [CrossRef] [PubMed]
- Halliwell, B.; Cross, C.E. Oxygen-Derived Species: Their Relation to Human Disease and Environmental Stress. Environ. Health Perspect. 1994, 102 (Suppl. S10), 5–12. [Google Scholar] [CrossRef]
- Sies, H.; Jones, D.P. Reactive Oxygen Species (ROS) as Pleiotropic Physiological Signalling Agents. Nat. Rev. Mol. Cell Biol. 2020, 21, 363–383. [Google Scholar] [CrossRef]
- Sies, H.; Berndt, C.; Jones, D.P. Oxidative Stress. Annu. Rev. Biochem. 2017, 86, 715–748. [Google Scholar] [CrossRef]
- Tesarik, J. Towards Personalized Antioxidant Use in Female Infertility: Need for More Molecular and Clinical Studies. Biomedicines 2021, 9, 1933. [Google Scholar] [CrossRef]
- Sovernigo, T.C.; Adona, P.R.; Monzani, P.S.; Guemra, S.; Barros, F.; Lopes, F.G.; Leal, C. Effects of Supplementation of Medium with Different Antioxidants during in Vitro Maturation of Bovine Oocytes on Subsequent Embryo Production. Reprod. Domest. Anim. Zuchthyg. 2017, 52, 561–569. [Google Scholar] [CrossRef]
- Shi, Y.-Q.; Zhu, X.-T.; Zhang, S.-N.; Ma, Y.-F.; Han, Y.-H.; Jiang, Y.; Zhang, Y.-H. Premature Ovarian Insufficiency: A Review on the Role of Oxidative Stress and the Application of Antioxidants. Front. Endocrinol. 2023, 14, 1172481. [Google Scholar] [CrossRef]
- Pan, C.; Chen, J.; Chen, Y.; Lu, Y.; Liang, X.; Xiong, B.; Lu, Y. Mogroside V Ameliorates the Oxidative Stress-Induced Meiotic Defects in Porcine Oocytes In Vitro. Reprod. Toxicol. 2022, 111, 148–157. [Google Scholar] [CrossRef]
- Schieber, M.; Chandel, N.S. ROS Function in Redox Signaling and Oxidative Stress. Curr. Biol. 2014, 24, R453–R462. [Google Scholar] [CrossRef] [PubMed]
- Juan, C.A.; Pérez de la Lastra, J.M.; Plou, F.J.; Pérez-Lebeña, E. The Chemistry of Reactive Oxygen Species (ROS) Revisited: Outlining Their Role in Biological Macromolecules (DNA, Lipids and Proteins) and Induced Pathologies. Int. J. Mol. Sci. 2021, 22, 4642. [Google Scholar] [CrossRef] [PubMed]
- Kirillova, A.; Smitz, J.E.J.; Sukhikh, G.T.; Mazunin, I. The Role of Mitochondria in Oocyte Maturation. Cells 2021, 10, 2484. [Google Scholar] [CrossRef] [PubMed]
- Chiang, J.L.; Shukla, P.; Pagidas, K.; Ahmed, N.S.; Karri, S.; Gunn, D.D.; Hurd, W.W.; Singh, K.K. Mitochondria in Ovarian Aging and Reproductive Longevity. Ageing Res. Rev. 2020, 63, 101168. [Google Scholar] [CrossRef]
- Yao, D.; Wang, P.; Zhang, J.; Fu, L.; Ouyang, L.; Wang, J. Deconvoluting the Relationships between Autophagy and Metastasis for Potential Cancer Therapy. Apoptosis Int. J. Program. Cell Death 2016, 21, 683–698. [Google Scholar] [CrossRef]
- Wang, L.; Tang, J.; Wang, L.; Tan, F.; Song, H.; Zhou, J.; Li, F. Oxidative Stress in Oocyte Aging and Female Reproduction. J. Cell. Physiol. 2021, 236, 7966–7983. [Google Scholar] [CrossRef]
- Ferreira, E.M.; Vireque, A.A.; Adona, P.R.; Meirelles, F.V.; Ferriani, R.A.; Navarro, P.A.A.S. Cytoplasmic Maturation of Bovine Oocytes: Structural and Biochemical Modifications and Acquisition of Developmental Competence. Theriogenology 2009, 71, 836–848. [Google Scholar] [CrossRef]
- Lundin, K.; Ahlström, A. Quality Control and Standardization of Embryo Morphology Scoring and Viability Markers. Reprod. Biomed. Online 2015, 31, 459–471. [Google Scholar] [CrossRef]
- Sciorio, R.; Meseguer, M. Focus on Time-Lapse Analysis: Blastocyst Collapse and Morphometric Assessment as New Features of Embryo Viability. Reprod. Biomed. Online 2021, 43, 821–832. [Google Scholar] [CrossRef]
- Lee, S.; Jin, J.-X.; Taweechaipaisankul, A.; Kim, G.A.; Ahn, C.; Lee, B.C. Melatonin Influences the Sonic Hedgehog Signaling Pathway in Porcine Cumulus Oocyte Complexes. J. Pineal Res. 2017, 63, e12424. [Google Scholar] [CrossRef]
- Miao, Y.-L.; Kikuchi, K.; Sun, Q.-Y.; Schatten, H. Oocyte Aging: Cellular and Molecular Changes, Developmental Potential and Reversal Possibility. Hum. Reprod. Update 2009, 15, 573–585. [Google Scholar] [CrossRef]
- Lenie, S.; Cortvrindt, R.; Eichenlaub-Ritter, U.; Smitz, J. Continuous exposure to bisphenol A during In Vitro follicular development induces meiotic abnormalities. Mutat. Res. Toxicol. Environ. Mutagen. 2008, 651, 71–81. [Google Scholar] [CrossRef]





Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shi, W.; Qin, C.; Yang, Y.; Yang, X.; Fang, Y.; Zhang, B.; Wang, D.; Feng, W.; Shi, D. Urolithin A Protects Porcine Oocytes from Artificially Induced Oxidative Stress Damage to Enhance Oocyte Maturation and Subsequent Embryo Development. Int. J. Mol. Sci. 2025, 26, 3037. https://doi.org/10.3390/ijms26073037
Shi W, Qin C, Yang Y, Yang X, Fang Y, Zhang B, Wang D, Feng W, Shi D. Urolithin A Protects Porcine Oocytes from Artificially Induced Oxidative Stress Damage to Enhance Oocyte Maturation and Subsequent Embryo Development. International Journal of Molecular Sciences. 2025; 26(7):3037. https://doi.org/10.3390/ijms26073037
Chicago/Turabian StyleShi, Wen, Chaobin Qin, Yanyan Yang, Xiaofen Yang, Yizhen Fang, Bing Zhang, Dong Wang, Wanyou Feng, and Deshun Shi. 2025. "Urolithin A Protects Porcine Oocytes from Artificially Induced Oxidative Stress Damage to Enhance Oocyte Maturation and Subsequent Embryo Development" International Journal of Molecular Sciences 26, no. 7: 3037. https://doi.org/10.3390/ijms26073037
APA StyleShi, W., Qin, C., Yang, Y., Yang, X., Fang, Y., Zhang, B., Wang, D., Feng, W., & Shi, D. (2025). Urolithin A Protects Porcine Oocytes from Artificially Induced Oxidative Stress Damage to Enhance Oocyte Maturation and Subsequent Embryo Development. International Journal of Molecular Sciences, 26(7), 3037. https://doi.org/10.3390/ijms26073037





