Apixaban Inhibits Progression of Experimental Diabetic Nephropathy by Blocking Advanced Glycation End Product-Receptor Axis
Abstract
1. Introduction
2. Results
2.1. General Characteristics of Experimental Animals
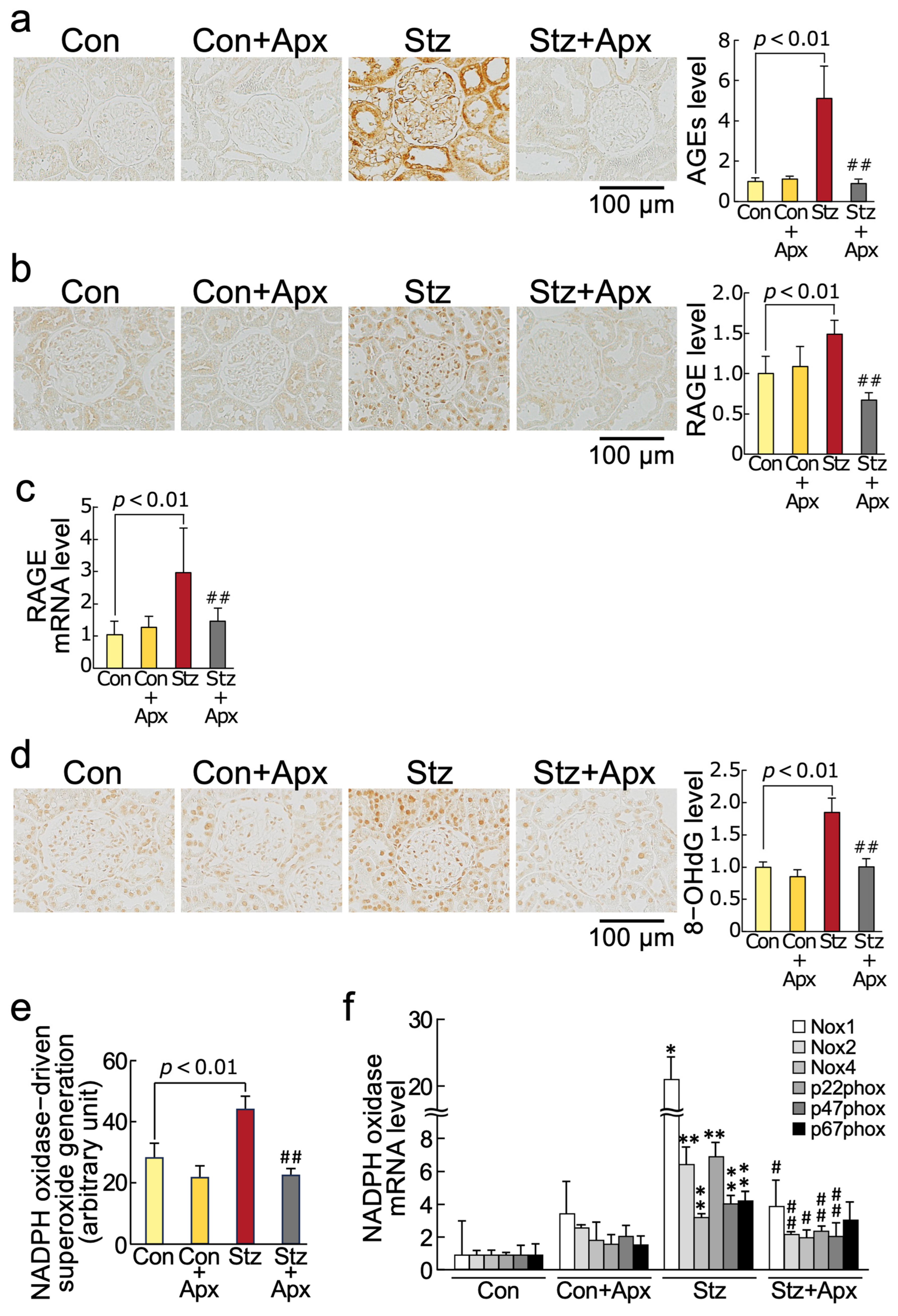
2.2. Effects of Apixaban on AGE-RAGE-Oxidative Stress Axis in the Kidneys of Streptozotocin-Induced Type 1 Diabetic Rats
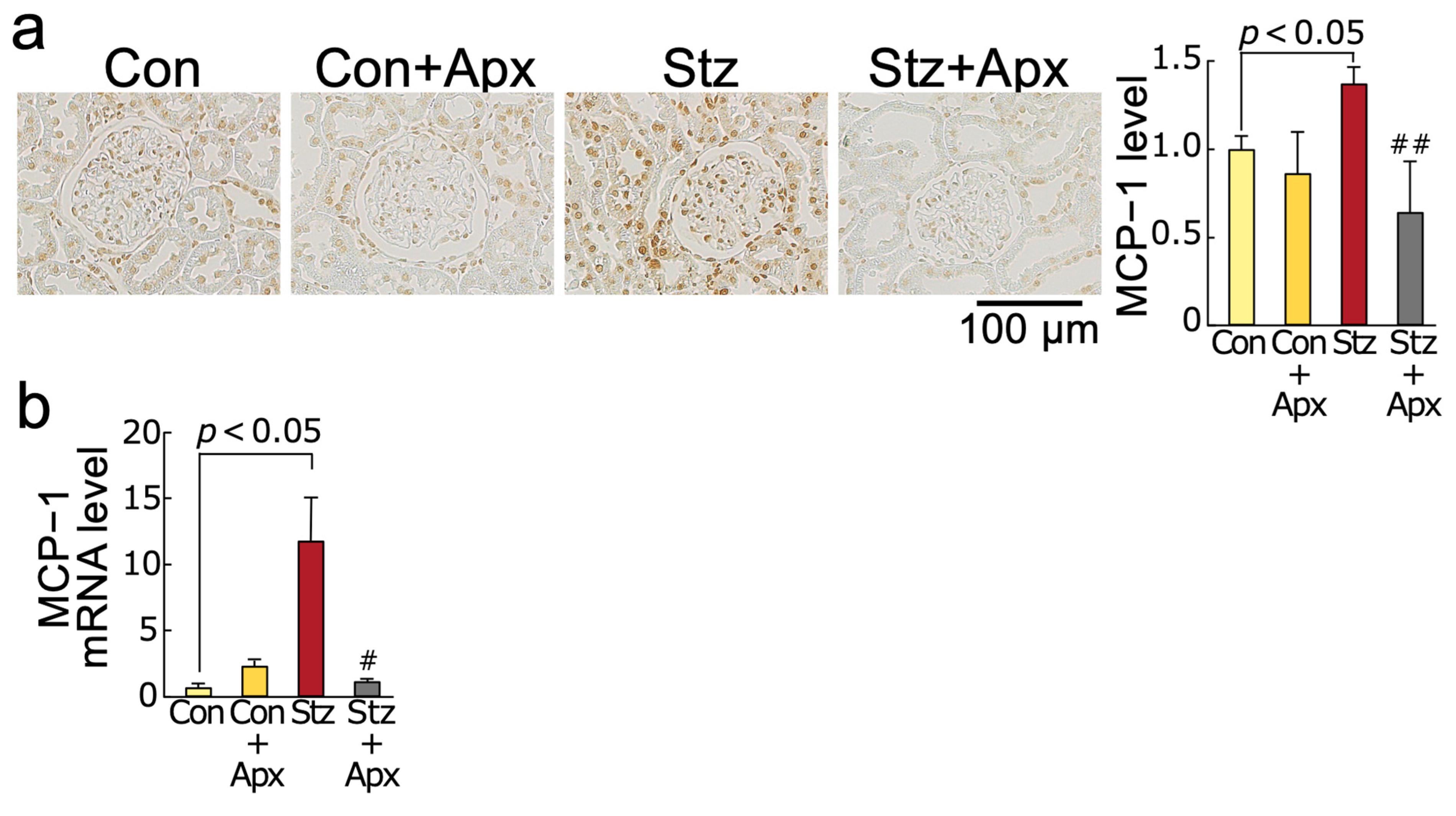
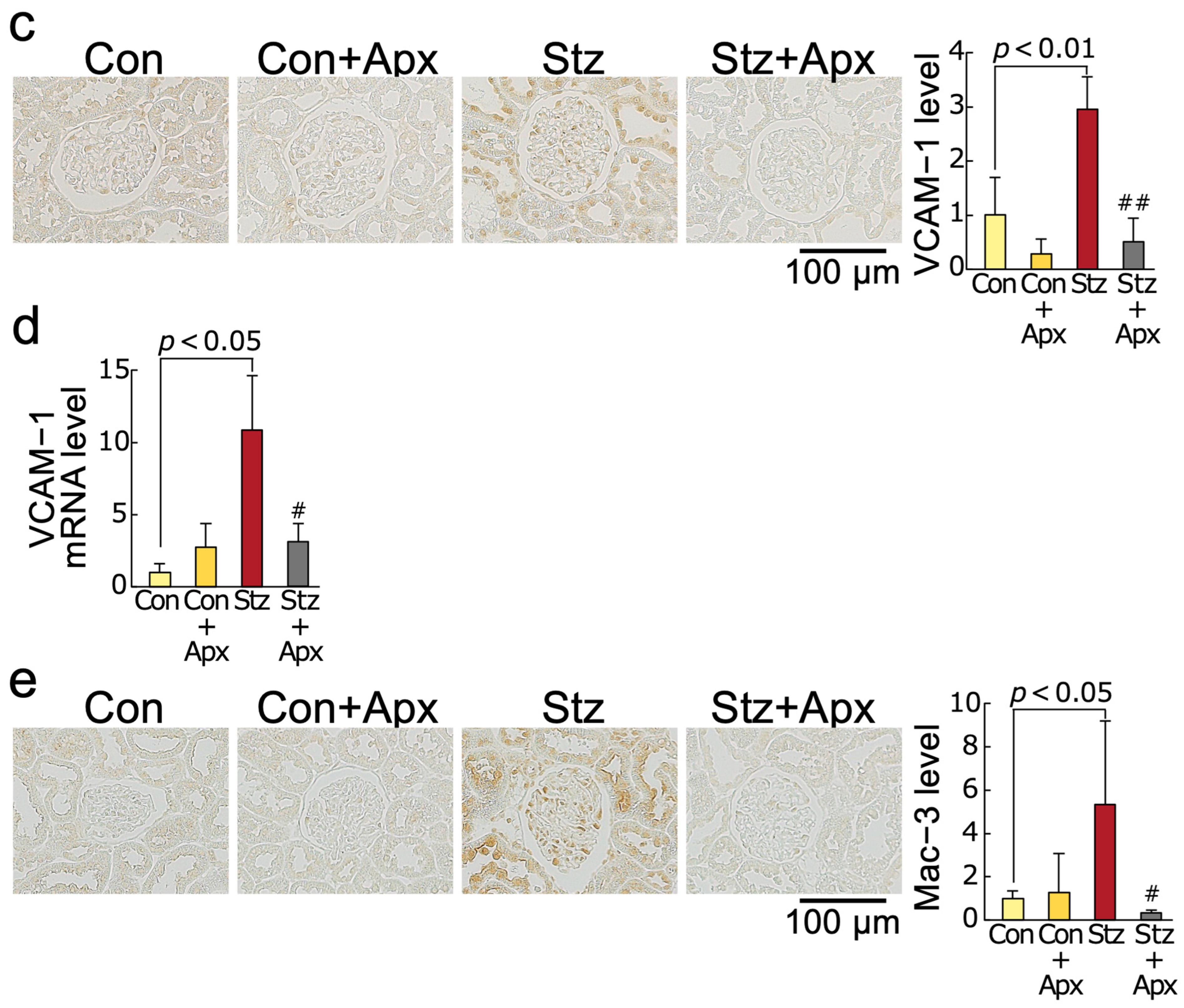
2.3. Effects of Apixaban on Inflammatory Reactions in the Kidneys of Streptozotocin-Induced Diabetic Rats
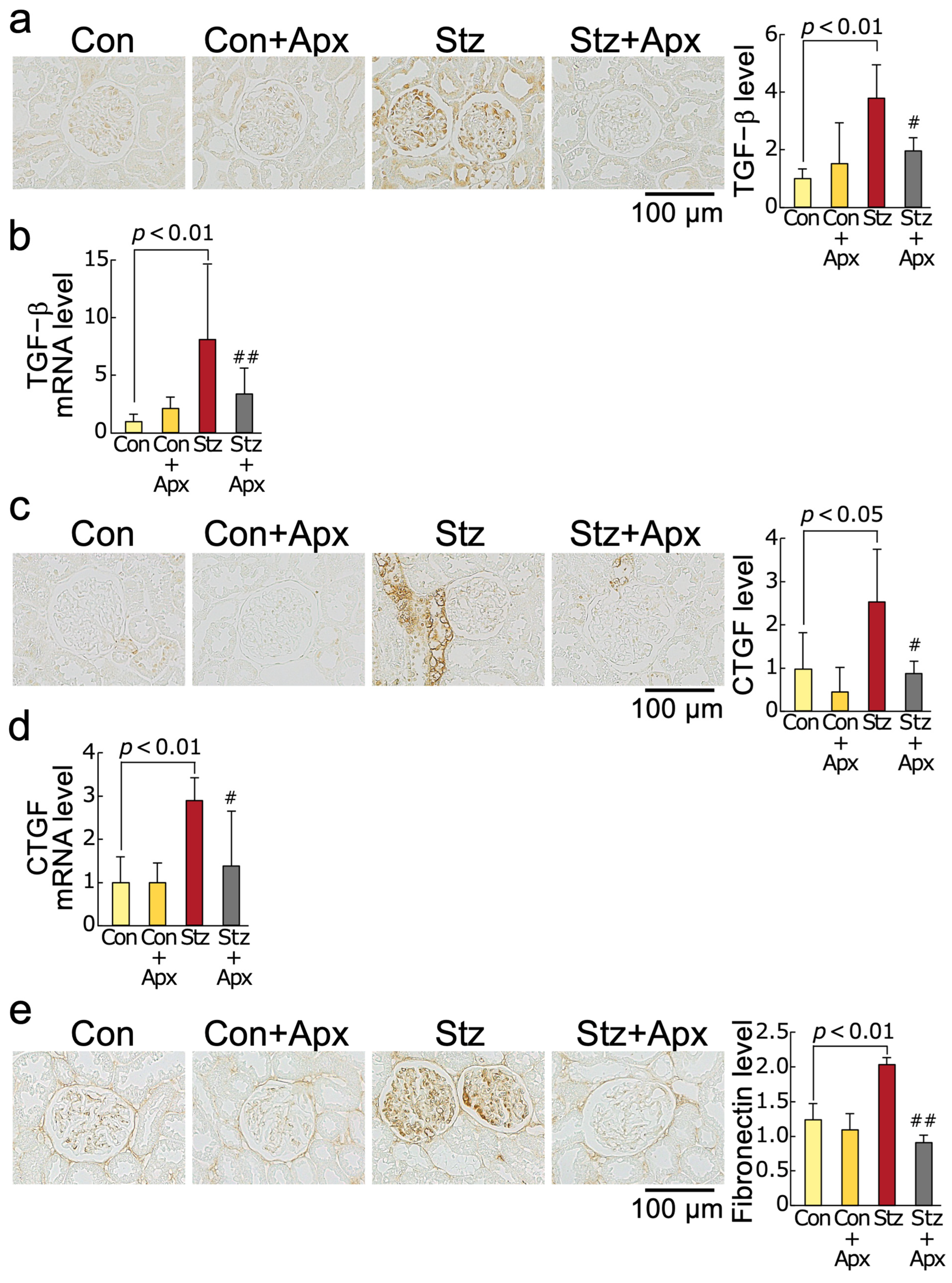
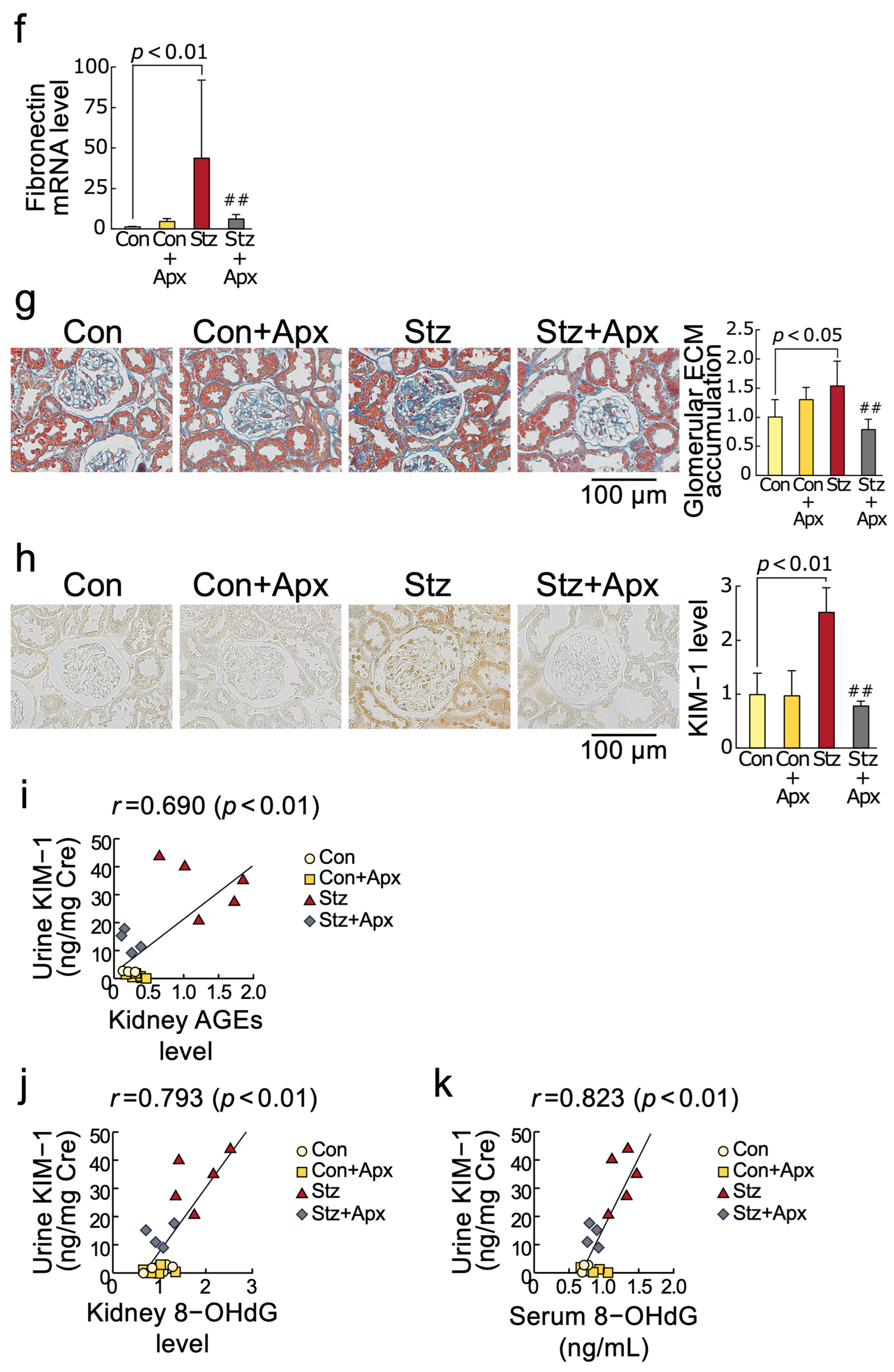
2.4. Effects of Apixaban on Fibrotic Reactions in the Kidneys of Streptozotocin-Induced Diabetic Rats
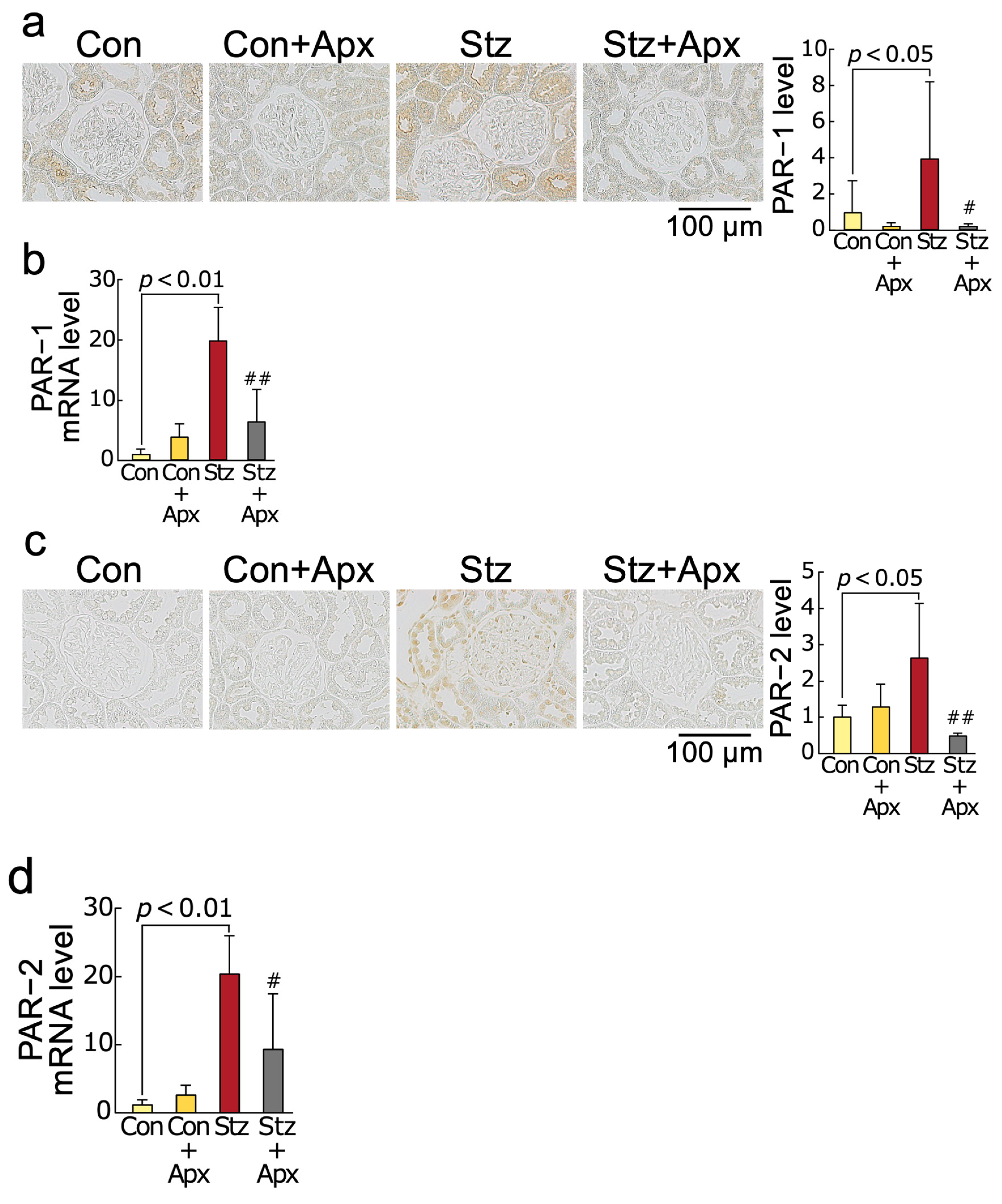
2.5. Effects of Apixaban on Protease-Activated Receptor-1 (PAR-1) and Protease-Activated Receptor-2 (PAR-2) Protein and mRNA Levels in the Kidneys of Streptozotocin-Induced Diabetic Rats
3. Discussion
4. Materials and Methods
4.1. Animal Experiments
4.2. Measurement of Urinary KIM-1
4.3. Measurement of NADPH Oxidase Activity
4.4. Immunostaining and Morphological Analysis
4.5. RT-PCR
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| AF | atrial fibrillation |
| AGEs | advanced glycation end products |
| RAGE | receptor for AGEs |
| 8-OHdG | 8-hydroxy-2′-deoxyguanosine |
| DOACs | direct oral anticoagulants |
| KIM-1 | kidney injury molecule-1 |
| BW | body weight |
| HbA1c | glycated hemoglobin |
| BP | blood pressure |
| HDL | high-density lipoprotein |
| BUN | blood urea nitrogen |
| MCP-1 | monocyte chemoattractant protein-1 |
| VCAM-1 | vascular cell adhesion molecule-1 |
| TGF-β | transforming growth factor-β |
| PAR-1 and PAR-2 | protease-activated receptor-1 and -2 |
| ELISA | enzyme-linked immunosorbent assay |
| Nox1, Nox2, and Nox4 | NADPH oxidase 1, NADPH oxidase 2, and NADPH oxidase 4 |
References
- Dwivedi, S.; Sikarwar, M.S. Diabetic Nephropathy: Pathogenesis, Mechanisms, and Therapeutic Strategies. Horm. Metab. Res. 2025, 57, 7–17. [Google Scholar] [CrossRef] [PubMed]
- Rossing, P.; Hansen, T.W.; Kümler, T. Cardiovascular and non-renal complications of chronic kidney disease: Managing risk. Diabetes Obes. Metab. 2024, 26 (Suppl. S6), 13–21. [Google Scholar] [CrossRef] [PubMed]
- Ma, Y.; Wang, X.; Lin, S.; King, L.; Liu, L. The Potential Role of Advanced Glycation End Products in the Development of Kidney Disease. Nutrients 2025, 17, 758. [Google Scholar] [CrossRef]
- Rahayu, I.; Arfian, N.; Kustanti, C.Y.; Wahyuningsih, M.S.H. The effectiveness of antioxidant agents in delaying progression of diabetic nephropathy: A systematic review of randomized controlled trials. Bioimpacts 2024, 15, 30129. [Google Scholar] [CrossRef]
- Sanglard, A.; Castello Branco Miranda, B.; França Vieira, A.L.; Miranda Macedo, M.V.; Lara Santos, R.; Stenner Rodrigues Radicchi Campos, A.; Campos Piva, A.; Simões, E.; Silva, A.C. The Role of Renin-Angiotensin System in Diabetic Nephropathy: An Update. Mini-Rev. Med. Chem. 2025, 25, 591–600. [Google Scholar] [CrossRef]
- Hauwanga, W.N.; Abdalhamed, T.Y.; Ezike, L.A.; Chukwulebe, I.S.; Ko Oo, A.; Wilfred, A.; Khan, A.R.A.K.A.; Chukwuwike, J.; Florial, E.; Lawan, H.; et al. The Pathophysiology and Vascular Complications of Diabetes in Chronic Kidney Disease: A Comprehensive Review. Cureus 2024, 16, e76498. [Google Scholar] [CrossRef] [PubMed]
- Fountoulakis, N.; Miyamoto, Y.; Pavkov, M.E.; Karalliedde, J.; Maltese, G. Pathophysiology of vascular ageing and the effect of novel cardio-renal protective medications in preventing progression of chronic kidney disease in people living with diabetes. Diabet. Med. 2025, 42, e15464. [Google Scholar] [CrossRef]
- Yang, S.Q.; Zhao, X.; Zhang, J.; Liu, H.; Wang, Y.H.; Wang, Y.G. Comparative efficacy and safety of SGLT2is and ns-MRAs in patients with diabetic kidney disease: A systematic review and network meta-analysis. Front. Endocrinol. 2024, 15, 1429261. [Google Scholar] [CrossRef]
- Felix, N.; Gauza, M.M.; Bittar, V.; Nogueira, A.; Costa, T.A.; Godoi, A.; Araújo de Lucena, L.; Ribeiro Gonçalves, O.; Santos Pinto, L.C.; Tramujas, L.; et al. Cardiovascular and Kidney Outcomes of Glucagon-like Peptide 1 Receptor Agonist Therapy in Type 2 Diabetes Mellitus and Chronic Kidney Disease: A Systematic Review and Meta-Analysis. Cardiorenal Med. 2025, 15, 98–107. [Google Scholar] [CrossRef]
- Venkatesan, K.; Cheryeth, M.M.J.; Verghese, A.T.; Mathews, A.M.; Ravisankar, N.; Unnikrishnan, P.; Prakash, V.; Harimohan, H.; Haroon, N.N.; James, S.; et al. Finerenone and diabetic renal disease: A narrative review. Endocrine 2024, 86, 882–889. [Google Scholar] [CrossRef]
- Magnocavallo, M.; Vetta, G.; Trivigno, S.; Mariani, M.V.; DI Lullo, L.; Bellasi, A.; Della Rocca, D.G.; Severino, P.; Piro, A.; Giunta, G.; et al. The connubium among diabetes, chronic kidney disease and atrial fibrillation. Minerva Cardiol. Angiol. 2022, 70, 393–402. [Google Scholar] [CrossRef] [PubMed]
- Parks, A.L.; Frankel, D.S.; Kim, D.H.; Ko, D.; Kramer, D.B.; Lydston, M.; Fang, M.C.; Shah, S.J. Management of atrial fibrillation in older adults. BMJ 2024, 386, e076246. [Google Scholar] [CrossRef] [PubMed]
- Verma, L.A.; Penson, P.E.; Akpan, A.; Lip, G.Y.H.; Lane, D.A. Managing older people with atrial fibrillation and preventing stroke: A review of anticoagulation approaches. Expert Rev. Cardiovasc. Ther. 2023, 21, 963–983. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.I.; Matsui, T.; Nakamura, K. Possible molecular mechanisms by which angiotensin II type 1 receptor blockers (ARBs) prevent the development of atrial fibrillation in insulin resistant patients. Horm. Metab. Res. 2008, 40, 640–644. [Google Scholar] [CrossRef]
- Ahmad, Y.; Lip, G.Y. Preventing stroke and systemic embolism in renal patients with atrial fibrillation: Focus on anticoagulation. Contrib. Nephrol. 2013, 179, 81–91. [Google Scholar] [CrossRef]
- Tsiachris, D.; Tsioufis, C.; Mazzone, P.; Katsiki, N.; Stefanadis, C. Atrial Fibrillation and Chronic Kidney Disease in Hypertension: A Common and Dangerous Triad. Curr. Vasc. Pharmacol. 2015, 13, 111–120. [Google Scholar] [CrossRef]
- Apostolakis, S.; Guo, Y.; Lane, D.A.; Buller, H.; Lip, G.Y. Renal function and outcomes in anticoagulated patients with non-valvular atrial fibrillation: The AMADEUS trial. Eur. Heart J. 2013, 34, 3572–3579. [Google Scholar] [CrossRef]
- Roldán, V.; Marín, F.; Manzano-Fernandez, S.; Fernández, H.; Gallego, P.; Valdés, M.; Vicente, V.; Lip, G.Y. Does chronic kidney disease improve the predictive value of the CHADS2 and CHA2DS2-VASc stroke stratification risk scores for atrial fibrillation? Thromb. Haemost. 2013, 109, 956–960. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Sotokawauchi, A.; Matsui, T. Pathological Role of Advanced Glycation End Products (AGEs) and their Receptor Axis in Atrial Fibrillation. Mini-Rev. Med. Chem. 2019, 19, 1040–1048. [Google Scholar] [CrossRef]
- Yamagishi, S.I. Concerns about clinical efficacy and safety of warfarin in diabetic patients with atrial fibrillation. Cardiovasc. Diabetol. 2019, 18, 12. [Google Scholar] [CrossRef]
- Hart, R.G.; Eikelboom, J.W.; Brimble, K.S.; McMurtry, M.S.; Ingram, A.J. Stroke prevention in atrial fibrillation patients with chronic kidney disease. Can. J. Cardiol. 2013, 29 (Suppl. S7), S71–S78. [Google Scholar] [CrossRef] [PubMed]
- Feldberg, J.; Patel, P.; Farrell, A.; Sivarajahkumar, S.; Cameron, K.; Ma, J.; Battistella, M. A systematic review of direct oral anticoagulant use in chronic kidney disease and dialysis patients with atrial fibrillation. Nephrol. Dial. Transplant. 2019, 34, 265–277. [Google Scholar] [CrossRef]
- Hohnloser, S.H.; Hijazi, Z.; Thomas, L.; Alexander, J.H.; Amerena, J.; Hanna, M.; Keltai, M.; Lanas, F.; Lopes, R.D.; Lopez-Sendon, J.; et al. Efficacy of apixaban when compared with warfarin in relation to renal function in patients with atrial fibrillation: Insights from the ARISTOTLE trial. Eur. Heart J. 2012, 33, 2821–2830. [Google Scholar] [CrossRef]
- Stacy, Z.A.; Richter, S.K. Direct oral anticoagulants for stroke prevention in atrial fibrillation: Treatment outcomes and dosing in special populations. Ther. Adv. Cardiovasc. Dis. 2018, 12, 247–262. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Nakamura, N.; Suematsu, M.; Kaseda, K.; Matsui, T. Advanced Glycation End Products: A Molecular Target for Vascular Complications in Diabetes. Mol. Med. 2015, 21 (Suppl. S1), S32–S40. [Google Scholar] [CrossRef] [PubMed]
- Yamagishi, S.I.; Matsui, T. Therapeutic Potential of DNA-aptamers Raised Against AGE-RAGE Axis in Diabetes-related Complications. Curr. Pharm. Des. 2018, 24, 2802–2809. [Google Scholar] [CrossRef] [PubMed]
- Fukami, K.; Taguchi, K.; Yamagishi, S.I.; Okuda, S. Receptor for advanced glycation endproducts and progressive kidney disease. Curr. Opin. Nephrol. Hypertens. 2015, 24, 54–60. [Google Scholar] [CrossRef]
- Egaña-Gorroño, L.; López-Díez, R.; Yepuri, G.; Ramirez, L.S.; Reverdatto, S.; Gugger, P.F.; Shekhtman, A.; Ramasamy, R.; Schmidt, A.M. Receptor for Advanced Glycation End Products (RAGE) and Mechanisms and Therapeutic Opportunities in Diabetes and Cardiovascular Disease: Insights From Human Subjects and Animal Models. Front. Cardiovasc. Med. 2020, 7, 37. [Google Scholar] [CrossRef]
- Asadipooya, K.; Uy, E.M. Advanced Glycation End Products (AGEs), Receptor for AGEs, Diabetes, and Bone: Review of the Literature. J. Endocr. Soc. 2019, 3, 1799–1818. [Google Scholar] [CrossRef]
- Yamagishi, S. Role of advanced glycation end products (AGEs) in osteoporosis in diabetes. Curr. Drug Targets 2011, 12, 2096–2102. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Fukami, K.; Matsui, T. Evaluation of tissue accumulation levels of advanced glycation end products by skin autofluorescence: A novel marker of vascular complications in high-risk patients for cardiovascular disease. Int. J. Cardiol. 2015, 185, 263–268. [Google Scholar] [CrossRef] [PubMed]
- Neuen, B.L.; Ostrominski, J.W.; Claggett, B.; Beldhuis, I.E.; Chatur, S.; McCausland, F.R.; Badve, S.V.; Arnott, C.; Heerspink, H.J.L.; Jun, M.; et al. Bidirectional Relationship Between Kidney Disease Progression and Cardiovascular Events in Type 2 Diabetes. J. Am. Coll. Cardiol. 2024, 84, 2246–2250. [Google Scholar] [CrossRef]
- Takenaka, K.; Yamagishi, S.I.; Matsui, T.; Nakamura, K.; Imaizumi, T. Role of advanced glycation end products (AGEs) in thrombogenic abnormalities in diabetes. Curr. Neurovasc. Res. 2006, 3, 73–77. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Imaizumi, T. Diabetic vascular complications: Pathophysiology, biochemical basis and potential therapeutic strategy. Curr. Pharm. Des. 2005, 11, 2279–2299. [Google Scholar] [CrossRef]
- Valencia, I.; Lumpuy-Castillo, J.; Magalhaes, G.; Sánchez-Ferrer, C.F.; Lorenzo, Ó.; Peiró, C. Mechanisms of endothelial activation, hypercoagulation and thrombosis in COVID-19: A link with diabetes mellitus. Cardiovasc. Diabetol. 2024, 23, 75. [Google Scholar] [CrossRef] [PubMed]
- Prídavková, D.; Samoš, M.; Bolek, T.; Škorňová, I.; Žolková, J.; Kubisz, P.; Staško, J.; Mokáň, M. Type 2 Diabetes, Atrial Fibrillation, and Direct Oral Anticoagulation. J. Diabetes Res. 2019, 2019, 5158308. [Google Scholar] [CrossRef] [PubMed]
- Matsui, T.; Higashimoto, Y.; Nishino, Y.; Nakamura, N.; Fukami, K.; Yamagishi, S.I. RAGE-Aptamer Blocks the Development and Progression of Experimental Diabetic Nephropathy. Diabetes 2017, 66, 1683–1695. [Google Scholar] [CrossRef]
- Wendt, T.M.; Tanji, N.; Guo, J.; Kislinger, T.R.; Qu, W.; Lu, Y.; Bucciarelli, L.G.; Rong, L.L.; Moser, B.; Markowitz, G.S.; et al. RAGE drives the development of glomerulosclerosis and implicates podocyte activation in the pathogenesis of diabetic nephropathy. Am. J. Pathol. 2003, 162, 1123–1137. [Google Scholar] [CrossRef]
- Kaida, Y.; Fukami, K.; Matsui, T.; Higashimoto, Y.; Nishino, Y.; Obara, N.; Nakayama, Y.; Ando, R.; Toyonaga, M.; Ueda, S.; et al. DNA aptamer raised against AGEs blocks the progression of experimental diabetic nephropathy. Diabetes 2013, 62, 3241–3250. [Google Scholar] [CrossRef]
- Thomas, M.C.; Forbes, J.M.; Cooper, M.E. Advanced glycation end products and diabetic nephropathy. Am. J. Ther. 2005, 12, 562–572. [Google Scholar] [CrossRef]
- Wu, X.Q.; Zhang, D.D.; Wang, Y.N.; Tan, Y.Q.; Yu, X.Y.; Zhao, Y.Y. AGE/RAGE in diabetic kidney disease and ageing kidney. Free Radic. Biol. Med. 2021, 171, 260–271. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Matsui, T.; Ueda, S.; Fukami, K.; Yamagishi, S. Advanced glycation end products potentiate citrated plasma-evoked oxidative and inflammatory reactions in endothelial cells by up-regulating protease-activated receptor-1 expression. Cardiovasc. Diabetol. 2014, 13, 60. [Google Scholar] [CrossRef] [PubMed]
- Ishibashi, Y.; Matsui, T.; Fukami, K.; Ueda, S.; Okuda, S.; Yamagishi, S. Rivaroxaban inhibits oxidative and inflammatory reactions in advanced glycation end product-exposed tubular cells by blocking thrombin/protease-activated receptor-2 system. Thromb. Res. 2015, 135, 770–773. [Google Scholar] [CrossRef]
- Ishibashi, Y.; Matsui, T.; Yamagishi, S. Apixaban exerts anti-inflammatory effects in mesangial cells by blocking thrombin/protease-activated receptor-1 system. Thromb. Res. 2014, 134, 1365–1367. [Google Scholar] [CrossRef]
- Ide, Y.; Matsui, T.; Ishibashi, Y.; Takeuchi, M.; Yamagishi, S. Pigment epithelium-derived factor inhibits advanced glycation end product-elicited mesangial cell damage by blocking NF-kappaB activation. Microvasc. Res. 2010, 80, 227–232. [Google Scholar] [CrossRef] [PubMed]
- Tan, A.L.; Sourris, K.C.; Harcourt, B.E.; Thallas-Bonke, V.; Penfold, S.; Andrikopoulos, S.; Thomas, M.C.; O’Brien, R.C.; Bierhaus, A.; Cooper, M.E.; et al. Disparate effects on renal and oxidative parameters following RAGE deletion, AGE accumulation inhibition, or dietary AGE control in experimental diabetic nephropathy. Am. J. Physiol. Renal Physiol. 2010, 298, F763–F770. [Google Scholar] [CrossRef]
- Yamagishi, S.I.; Matsui, T. Advanced glycation end products, oxidative stress and diabetic nephropathy. Oxid. Med. Cell. Longev. 2010, 3, 101–108. [Google Scholar] [CrossRef]
- Mitsui, S.; Oe, Y.; Sekimoto, A.; Sato, E.; Hashizume, Y.; Yamakage, S.; Kumakura, S.; Sato, H.; Ito, S.; Takahashi, N. Dual blockade of protease-activated receptor 1 and 2 additively ameliorates diabetic kidney disease. Am. J. Physiol. Renal Physiol. 2020, 318, F1067–F1073. [Google Scholar] [CrossRef]
- He, K.; Luettgen, J.M.; Zhang, D.; He, B.; Grace, J.E., Jr.; Xin, B.; Pinto, D.J.; Wong, P.C.; Knabb, R.M.; Lam, P.Y.; et al. Preclinical pharmacokinetics and pharmacodynamics of apixaban, a potent and selective factor Xa inhibitor. Eur. J. Drug Metab. Pharmacokinet. 2011, 36, 129–139. [Google Scholar] [CrossRef]
- Beretov, J.; Wasinger, V.C.; Schwartz, P.; Graham, P.H.; Li, Y. A standardized and reproducible urine preparation protocol for cancer biomarkers discovery. Biomark. Cancer 2014, 6, 21–27. [Google Scholar] [CrossRef]
| Control | Control + Apixaban | Streptozotocin | Streptozotocin + Apixaban | |
|---|---|---|---|---|
| Number | 6 | 5 | 5 | 4 |
| Body weight (g) | 467 ± 26 | 444 ± 30 | 189 ± 19 ** | 175 ± 59 |
| HR (beats/min) | 361 ± 31 | 366 ± 16 | 276 ± 21 ** | 263 ± 32 |
| SBP (mmHg) | 119 ± 10 | 115 ± 20 | 115 ± 8 | 102 ± 11 |
| DBP (mmHg) | 93 ± 6 | 92 ± 15 | 62 ± 11 ** | 56 ± 13 |
| BG (mg/dL) | 147 ± 37 | 134 ± 34 | 573 ± 98 ** | 666 ± 179 |
| HbA1c (%) | 6.1 ± 0.3 | 5.9 ± 0.3 | 10.4 ± 0.7 ** | 11.2 ± 0.5 |
| T-Chol (mg/dL) | 63 ± 13 | 63 ± 6 | 314 ± 133 ** | 239 ± 106 |
| TG (mg/dL) | 165 ± 52 | 121 ± 42 | 1132 ± 721 ** | 1053 ± 257 |
| HDL-C (mg/dL) | 35 ± 9 | 39 ± 4 | 99 ± 17 ** | 90 ± 12 |
| BUN (mg/dL) | 16.0 ± 1.5 | 17.3 ± 1.2 | 68.2 ± 20.5 ** | 51.2 ± 26.7 |
| Cre (mg/dL) | 0.2 ± 0.1 | 0.2 ± 0.0 | 0.3 ± 0.1 | 0.3 ± 0.1 |
| Serum 8-OHdG (ng/mL) | 0.73 ± 0.03 | 0.86 ± 0.16 | 1.27 ± 0.35 ** | 0.84 ± 0.09 ## |
| Urine protein (mg/mg Cre) | 0.6 ± 0.4 | 0.7 ± 0.3 | 5.9 ± 2.5 ** | 1.6 ± 0.7 ## |
| Urine KIM-1 (ng/mg Cre) | 1.7 ± 1.2 | 0.8 ± 0.8 | 33.2 ± 9.5 ** | 13.4 ± 3.8 ## |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Matsui, T.; Sotokawauchi, A.; Nishino, Y.; Koga, Y.; Yamagishi, S.-i. Apixaban Inhibits Progression of Experimental Diabetic Nephropathy by Blocking Advanced Glycation End Product-Receptor Axis. Int. J. Mol. Sci. 2025, 26, 3007. https://doi.org/10.3390/ijms26073007
Matsui T, Sotokawauchi A, Nishino Y, Koga Y, Yamagishi S-i. Apixaban Inhibits Progression of Experimental Diabetic Nephropathy by Blocking Advanced Glycation End Product-Receptor Axis. International Journal of Molecular Sciences. 2025; 26(7):3007. https://doi.org/10.3390/ijms26073007
Chicago/Turabian StyleMatsui, Takanori, Ami Sotokawauchi, Yuri Nishino, Yoshinori Koga, and Sho-ichi Yamagishi. 2025. "Apixaban Inhibits Progression of Experimental Diabetic Nephropathy by Blocking Advanced Glycation End Product-Receptor Axis" International Journal of Molecular Sciences 26, no. 7: 3007. https://doi.org/10.3390/ijms26073007
APA StyleMatsui, T., Sotokawauchi, A., Nishino, Y., Koga, Y., & Yamagishi, S.-i. (2025). Apixaban Inhibits Progression of Experimental Diabetic Nephropathy by Blocking Advanced Glycation End Product-Receptor Axis. International Journal of Molecular Sciences, 26(7), 3007. https://doi.org/10.3390/ijms26073007






