Enhancing the Efficacy of CAR-T Cell Production Using BX795 and Rosuvastatin in a Serum-Free Medium
Abstract
1. Introduction
2. Results
2.1. Proliferation and Central Memory Phenotype of T Cells in Different Media
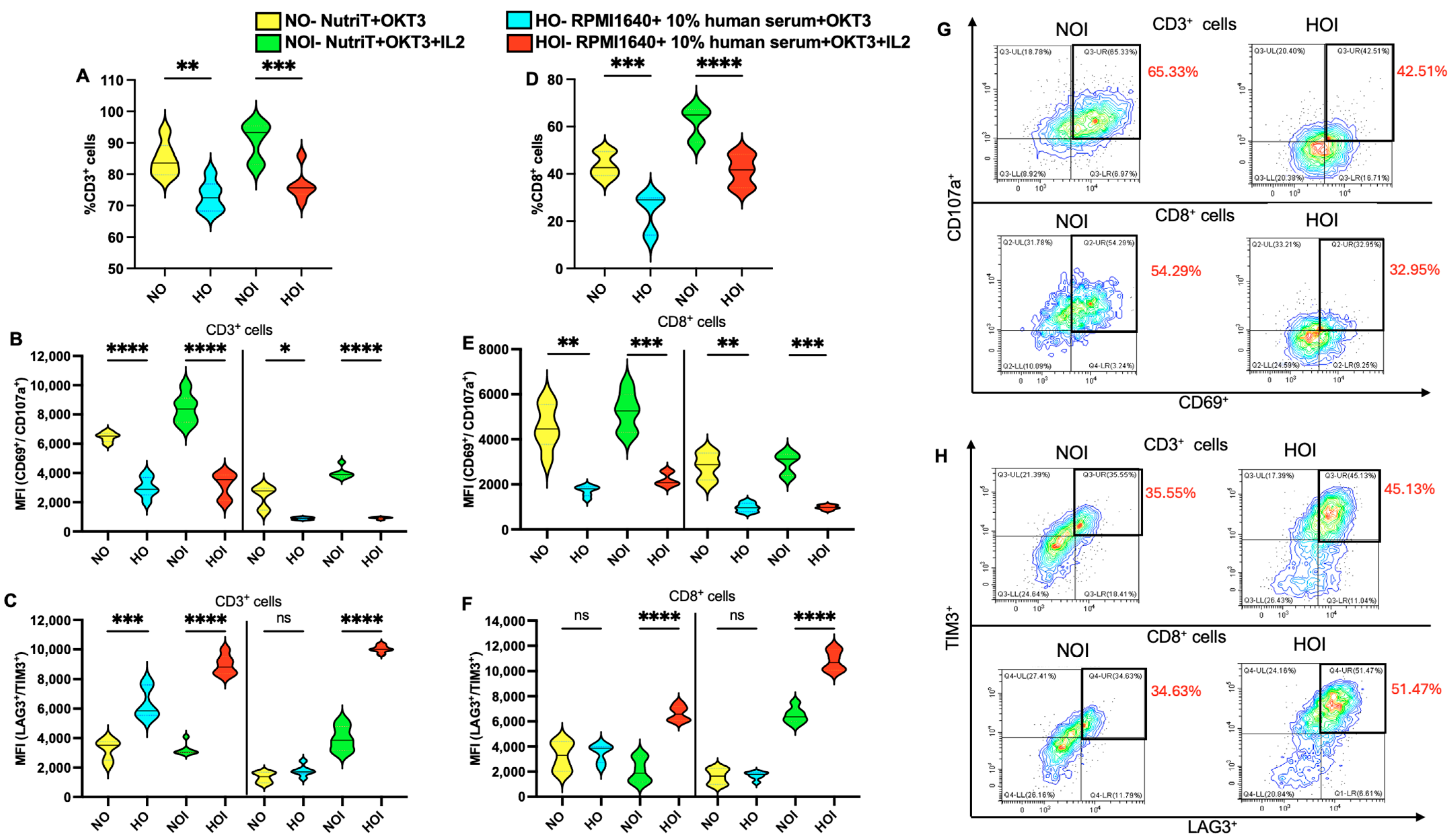
2.2. Activation and Exhaustion of T Cells in Different Media
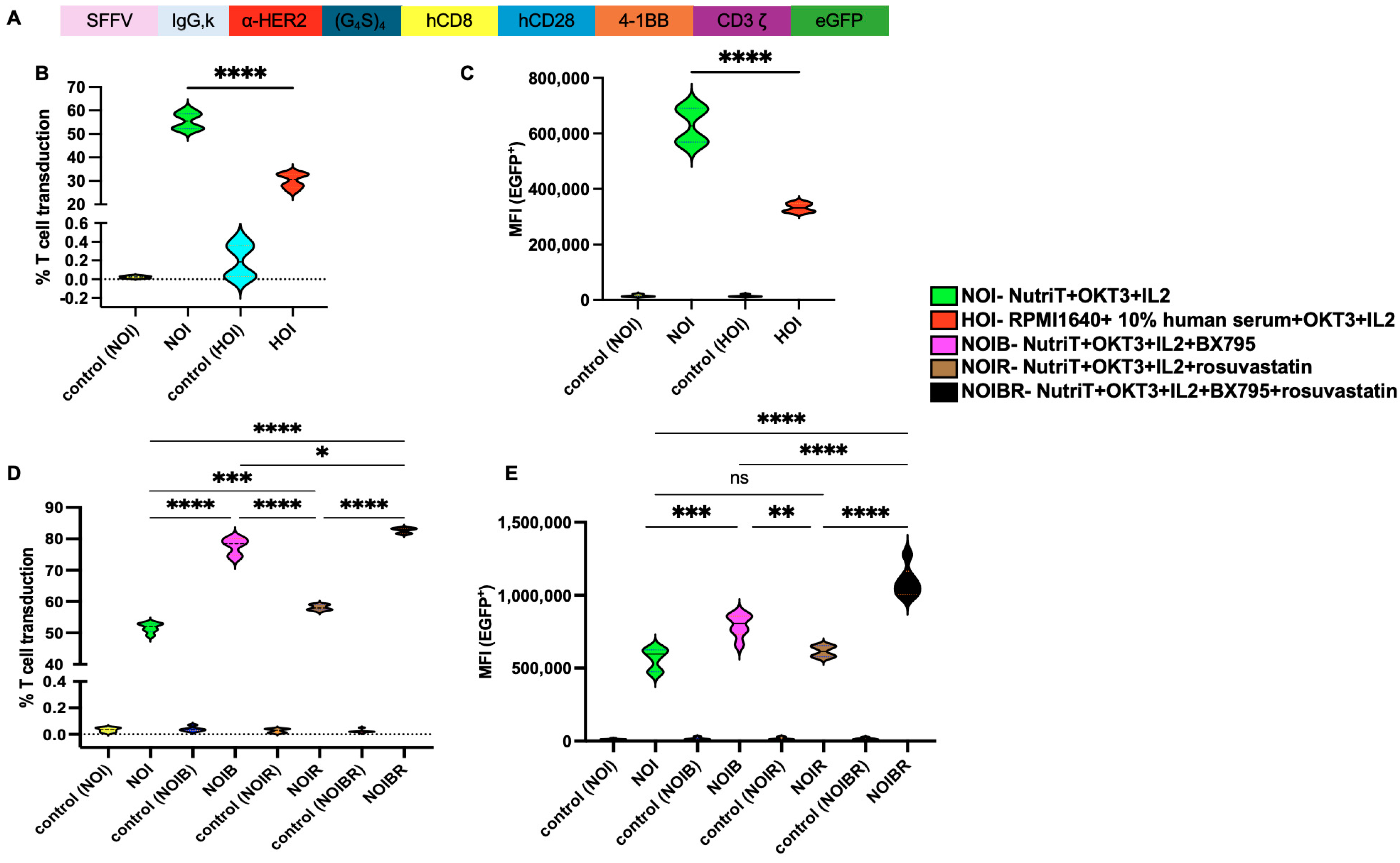
2.3. Effect of Nutri-T on Transduction Rate of CAR-HER2-EGFP Viruses Relative to T Cells
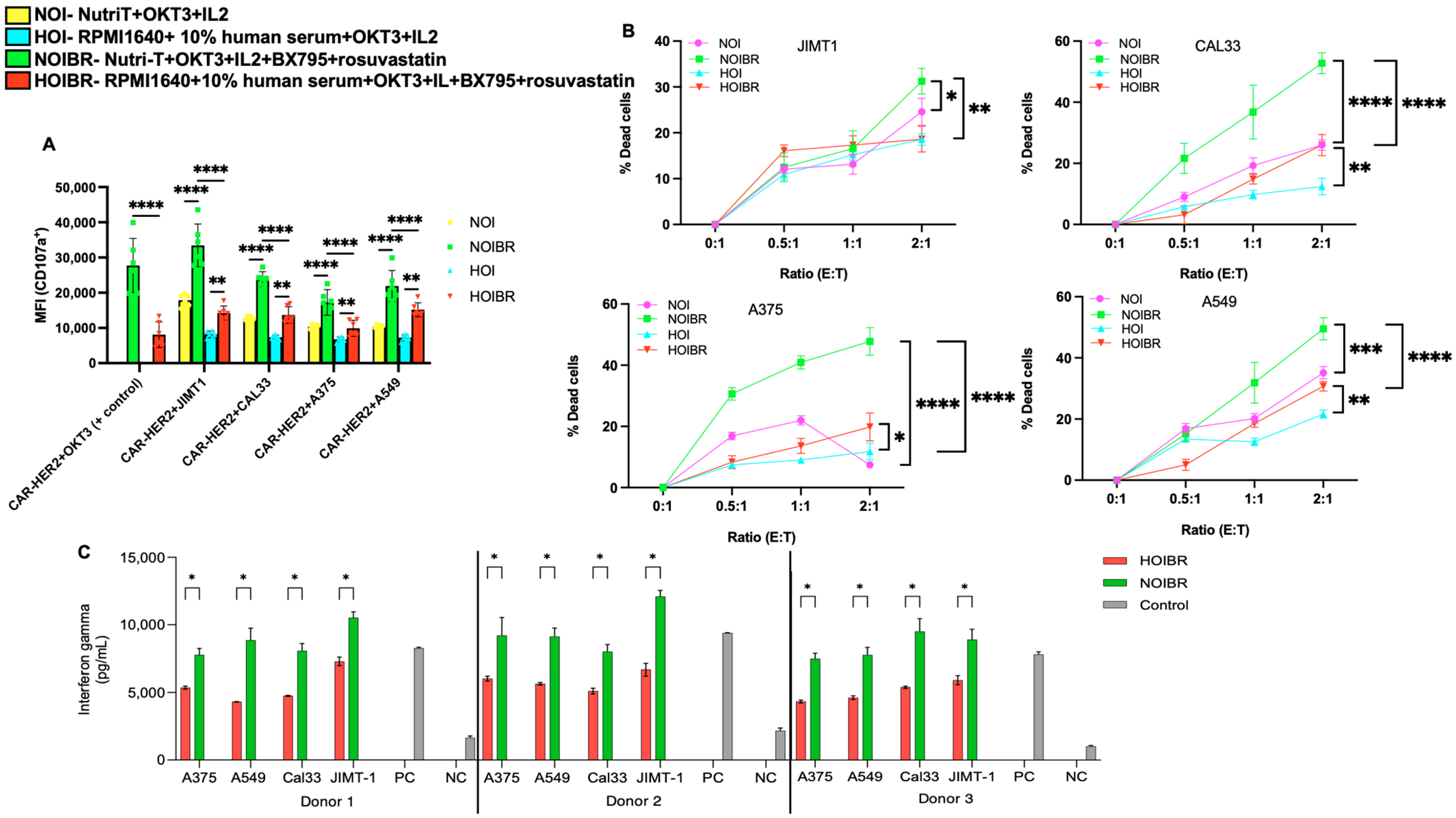
2.4. Evaluation of CAR-HER2-EGFP for Functional Activity
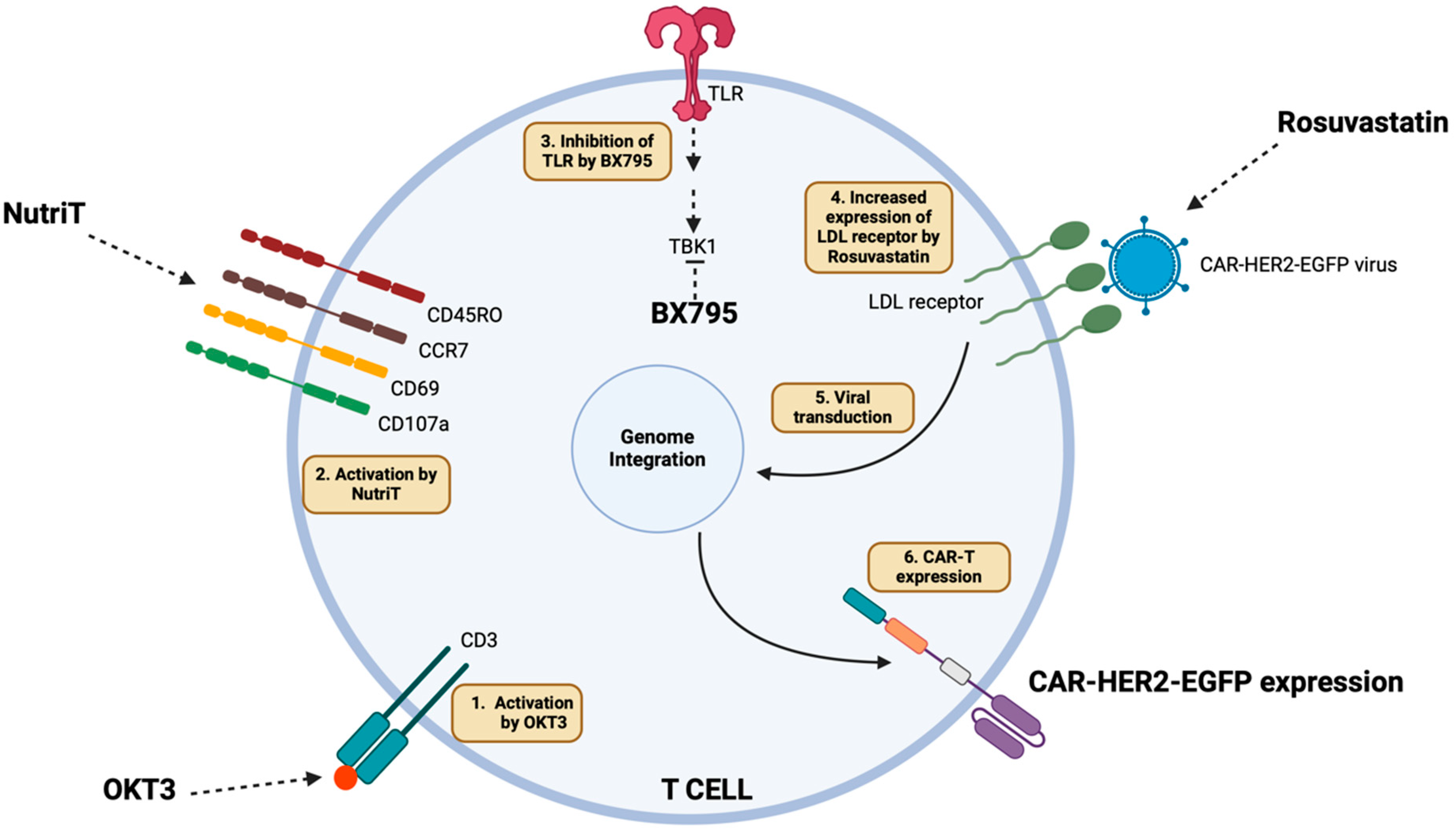
3. Discussion
4. Methods
4.1. Media Preparation and Reagents
4.2. PBMCs Extraction
4.3. T Cell Proliferation
4.4. Activation, Exhaustion, and Memory of T Cells
4.5. Lentivirus Preparation and Titration
4.6. Transduction in T Cells
4.7. Flow Cytometry
4.8. Degranulation Assay
4.9. Killing Assay
4.10. Mean Cell Volume
4.11. Interferon Gamma Assay
4.12. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Gross, G.; Waks, T.; Eshhar, Z. Expression of immunoglobulin-T-cell receptor chimeric molecules as functional receptors with antibody-type specificity. Proc. Natl. Acad. Sci. USA 1989, 86, 10024–10028. [Google Scholar] [CrossRef] [PubMed]
- Shimabukuro-Vornhagen, A.; Boll, B.; Schellongowski, P.; Valade, S.; Metaxa, V.; Azoulay, E.; von Bergwelt-Baildon, M. Critical care management of chimeric antigen receptor T-cell therapy recipients. CA Cancer J. Clin. 2022, 72, 78–93. [Google Scholar] [CrossRef] [PubMed]
- Park, J.H.; Riviere, I.; Gonen, M.; Wang, X.; Senechal, B.; Curran, K.J.; Sauter, C.; Wang, Y.; Santomasso, B.; Mead, E.; et al. Long-Term Follow-up of CD19 CAR Therapy in Acute Lymphoblastic Leukemia. N. Engl. J. Med. 2018, 378, 449–459. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Zhang, H.; Lan, H.; Wu, J.; Xiao, Y. CAR-T cell therapy in multiple myeloma: Current limitations and potential strategies. Front. Immunol. 2023, 14, 1101495. [Google Scholar] [CrossRef]
- Zhang, C.; Jones, A. Using serum-free media to streamline and optimize CAR T-cell manufacturing workflows. Cell Gene Ther. Insights 2020, 6, 1121–1126. [Google Scholar] [CrossRef]
- Alnabhan, R.; Gaballa, A.; Mork, L.M.; Mattsson, J.; Uhlin, M.; Magalhaes, I. Media evaluation for production and expansion of anti-CD19 chimeric antigen receptor T cells. Cytotherapy 2018, 20, 941–951. [Google Scholar] [CrossRef]
- Leney-Greene, M.A.; Boddapati, A.K.; Su, H.C.; Cantor, J.R.; Lenardo, M.J. Human Plasma-like Medium Improves T Lymphocyte Activation. iScience 2020, 23, 100759. [Google Scholar] [CrossRef]
- Medvec, A.R.; Ecker, C.; Kong, H.; Winters, E.A.; Glover, J.; Varela-Rohena, A.; Riley, J.L. Improved Expansion and In Vivo Function of Patient T Cells by a Serum-free Medium. Mol. Ther. Methods Clin. Dev. 2018, 8, 65–74. [Google Scholar] [CrossRef]
- Celebi Torabfam, G.; Yetisgin, A.A.; Erdem, C.; Cayli, A.; Kutlu, O.; Cetinel, S. A feasibility study of different commercially available serum-free mediums to enhance lentivirus and adeno-associated virus production in HEK 293 suspension cells. Cytotechnology 2022, 74, 635–655. [Google Scholar] [CrossRef]
- Gelinas, J.F.; Davies, L.A.; Gill, D.R.; Hyde, S.C. Assessment of selected media supplements to improve F/HN lentiviral vector production yields. Sci. Rep. 2017, 7, 10198. [Google Scholar] [CrossRef]
- Rogers, G.L.; Huang, C.; Clark, R.D.E.; Seclen, E.; Chen, H.Y.; Cannon, P.M. Optimization of AAV6 transduction enhances site-specific genome editing of primary human lymphocytes. Mol. Ther. Methods Clin. Dev. 2021, 23, 198–209. [Google Scholar] [CrossRef] [PubMed]
- Amadeo, F.; Hanson, V.; Murray, P.; Taylor, A. DEAE-Dextran Enhances the Lentiviral Transduction of Primary Human Mesenchymal Stromal Cells from All Major Tissue Sources Without Affecting Their Proliferation and Phenotype. Mol. Biotechnol. 2023, 65, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Fenard, D.; Ingrao, D.; Seye, A.; Buisset, J.; Genries, S.; Martin, S.; Kichler, A.; Galy, A. Vectofusin-1, a new viral entry enhancer, strongly promotes lentiviral transduction of human hematopoietic stem cells. Mol. Ther. Nucleic. Acids 2013, 2, e90. [Google Scholar] [CrossRef] [PubMed]
- Heffner, G.C.; Bonner, M.; Christiansen, L.; Pierciey, F.J.; Campbell, D.; Smurnyy, Y.; Zhang, W.; Hamel, A.; Shaw, S.; Lewis, G.; et al. Prostaglandin E(2) Increases Lentiviral Vector Transduction Efficiency of Adult Human Hematopoietic Stem and Progenitor Cells. Mol. Ther. 2018, 26, 320–328. [Google Scholar] [CrossRef]
- Amirache, F.; Lévy, C.; Costa, C.; Mangeot, P.E.; Torbett, B.E.; Wang, C.X.; Nègre, D.; Cosset, F.L.; Verhoeyen, E. Mystery solved: VSV-G-LVs do not allow efficient gene transfer into unstimulated T cells, B cells, and HSCs because they lack the LDL receptor. Blood 2014, 123, 1422–1424. [Google Scholar]
- Irving, M.; Lanitis, E.; Migliorini, D.; Ivics, Z.; Guedan, S. Choosing the Right Tool for Genetic Engineering: Clinical Lessons from Chimeric Antigen Receptor-T Cells. Hum. Gene. Ther. 2021, 32, 1044–1058. [Google Scholar] [CrossRef]
- Luvai, A.; Mbagaya, W.; Hall, A.S.; Barth, J.H. Rosuvastatin: A review of the pharmacology and clinical effectiveness in cardiovascular disease. Clin. Med. Insights Cardiol. 2012, 6, 17–33. [Google Scholar] [CrossRef]
- Gong, Y.; Klein Wolterink, R.G.J.; Janssen, I.; Groot, A.J.; Bos, G.M.J.; Germeraad, W.T.V. Rosuvastatin Enhances VSV-G Lentiviral Transduction of NK Cells via Upregulation of the Low-Density Lipoprotein Receptor. Mol. Ther. Methods Clin. Dev. 2020, 17, 634–646. [Google Scholar] [CrossRef]
- Karmaus, P.W.; Shi, M.; Perl, S.; Biancotto, A.; Candia, J.; Cheung, F.; Kotliarov, Y.; Young, N.; Fessler, M.B.; CHI Consortium. Effects of rosuvastatin on the immune system in healthy volunteers with normal serum cholesterol. JCI Insight 2019, 4, e131530. [Google Scholar] [CrossRef]
- Xu, H.; Yang, Y.J.; Qian, H.Y.; Tang, Y.D.; Wang, H.; Zhang, Q. Rosuvastatin treatment activates JAK-STAT pathway and increases efficacy of allogeneic mesenchymal stem cell transplantation in infarcted hearts. Circ. J. 2011, 75, 1476–1485. [Google Scholar] [CrossRef]
- Wang, J.; Liu, C.; Hu, R.; Wu, L.; Li, C. Statin therapy: A potential adjuvant to immunotherapies in hepatocellular carcinoma. Front. Pharmacol. 2024, 15, 1324140. [Google Scholar] [CrossRef]
- Sutlu, T.; Nystrom, S.; Gilljam, M.; Stellan, B.; Applequist, S.E.; Alici, E. Inhibition of intracellular antiviral defense mechanisms augments lentiviral transduction of human natural killer cells: Implications for gene therapy. Hum Gene Ther. 2012, 23, 1090–1100. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Gao, Y.; Srivastava, R.; Wang, W.; Xiong, Q.; Fang, Z.; Pelayo, A.; Denson, C.; Goswami, A.; Harari-Steinfeld, R.; et al. Lentiviral delivery of combinatorial CAR/CRISPRi circuit into human primary T cells is enhanced by TBK1/IKKvarepsilon complex inhibitor BX795. J. Transl Med. 2020, 18, 363. [Google Scholar] [CrossRef] [PubMed]
- Sartorius. Product Datasheet Product Information. Available online: https://www.sartorius.com/download/573796/4cell-nutri-t-medium-datasheet-en-b-2637243-sartorius-pdf-data.pdf (accessed on 1 November 2022).
- Greenshpan, Y.; Sharabi, O.; Yegodayev, K.M.; Novoplansky, O.; Elkabets, M.; Gazit, R.; Porgador, A. The Contribution of the Minimal Promoter Element to the Activity of Synthetic Promoters Mediating CAR Expression in the Tumor Microenvironment. Int. J. Mol. Sci. 2022, 23, 7431. [Google Scholar] [CrossRef]
- Ou, J.; Si, Y.; Tang, Y.; Salzer, G.E.; Lu, Y.; Kim, S.; Qin, H.; Zhou, L.; Liu, X. Novel biomanufacturing platform for large-scale and high-quality human T cells production. J. Biol. Eng. 2019, 13, 34. [Google Scholar] [CrossRef]
- Chicaybam, L.; Sodre, A.L.; Curzio, B.A.; Bonamino, M.H. An efficient low cost method for gene transfer to T lymphocytes. PLoS ONE 2013, 8, e60298. [Google Scholar] [CrossRef]
- Du, L.; Nai, Y.; Shen, M.; Li, T.; Huang, J.; Han, X.; Wang, W.; Pang, D.; Jin, A. IL-21 Optimizes the CAR-T Cell Preparation Through Improving Lentivirus Mediated Transfection Efficiency of T Cells and Enhancing CAR-T Cell Cytotoxic Activities. Front. Mol. Biosci. 2021, 8, 675179. [Google Scholar] [CrossRef]
- Eremenko, E.; Taylor, Z.V.; Khand, B.; Zaccai, S.; Porgador, A.; Monsonego, A. An optimized protocol for the retroviral transduction of mouse CD4 T cells. STAR Protoc. 2021, 2, 100719. [Google Scholar] [CrossRef]
- Rajabzadeh, A.; Hamidieh, A.A.; Rahbarizadeh, F. Spinoculation and retronectin highly enhance the gene transduction efficiency of Mucin-1-specific chimeric antigen receptor (CAR) in human primary T cells. BMC Mol. Cell Biol. 2021, 22, 57. [Google Scholar] [CrossRef]
- Watanabe, N.; Mo, F.; McKenna, M.K. Impact of Manufacturing Procedures on CAR T Cell Functionality. Front. Immunol. 2022, 13, 876339. [Google Scholar] [CrossRef]
- Sudarsanam, H.; Buhmann, R.; Henschler, R. Influence of Culture Conditions on Ex Vivo Expansion of T Lymphocytes and Their Function for Therapy: Current Insights and Open Questions. Front. Bioeng. Biotechnol. 2022, 10, 886637. [Google Scholar] [CrossRef]
- Ghassemi, S.; Nunez-Cruz, S.; O’Connor, R.S.; Fraietta, J.A.; Patel, P.R.; Scholler, J.; Barrett, D.M.; Lundh, S.M.; Davis, M.M.; Bedoya, F.; et al. Reducing Ex Vivo Culture Improves the Antileukemic Activity of Chimeric Antigen Receptor (CAR) T Cells. Cancer Immunol. Res. 2018, 6, 1100–1109. [Google Scholar] [CrossRef] [PubMed]
- Bryceson, Y.T.; Pende, D.; Maul-Pavicic, A.; Gilmour, K.C.; Ufheil, H.; Vraetz, T.; Chiang, S.C.; Marcenaro, S.; Meazza, R.; Bondzio, I.; et al. A prospective evaluation of degranulation assays in the rapid diagnosis of familial hemophagocytic syndromes. Blood 2012, 119, 2754–2763. [Google Scholar] [CrossRef]
- Li, Y.; Kurlander, R.J. Comparison of anti-CD3 and anti-CD28-coated beads with soluble anti-CD3 for expanding human T cells: Differing impact on CD8 T cell phenotype and responsiveness to restimulation. J. Transl. Med. 2010, 8, 1–15. [Google Scholar]
- Liu, X.; Jiang, S.; Fang, C.; Li, H.; Zhang, X.; Zhang, F.; June, C.H.; Zhao, Y. Novel T cells with improved in vivo anti-tumor activity generated by RNA electroporation. Protein Cell 2017, 8, 514–526. [Google Scholar] [CrossRef]
- Ghaffari, S.; Torabi-Rahvar, M.; Aghayan, S.; Jabbarpour, Z.; Moradzadeh, K.; Omidkhoda, A.; Ahmadbeigi, N. Optimizing interleukin-2 concentration, seeding density and bead-to-cell ratio of T-cell expansion for adoptive immunotherapy. BMC Immunol. 2021, 22, 43. [Google Scholar] [CrossRef]
- Shevach, E.M. Application of IL-2 therapy to target T regulatory cell function. Trends Immunol. 2012, 33, 626–632. [Google Scholar] [CrossRef]
- Bajnok, A.; Ivanova, M.; Rigó Jr, J.; Toldi, G. The Distribution of Activation Markers and Selectins on Peripheral T Lymphocytes in Preeclampsia. Mediat. Inflamm. 2017, 2017, 8045161. [Google Scholar] [CrossRef]
- McLane, L.M.; Abdel-Hakeem, M.S.; Wherry, E.J. CD8 T Cell Exhaustion During Chronic Viral Infection and Cancer. Annu. Rev. Immunol 2019, 37, 457–495. [Google Scholar] [CrossRef]
- Zappasodi, R.; Budhu, S.; Abu-Akeel, M.; Merghoub, T. In vitro assays for effector T cell functions and activity of immunomodulatory antibodies. Methods Enzym. 2020, 631, 43–59. [Google Scholar] [CrossRef]
- Wherry, E.J.; Kurachi, M. Molecular and cellular insights into T cell exhaustion. Nat. Rev. Immunol. 2015, 15, 486–499. [Google Scholar] [CrossRef] [PubMed]
- Okoye, I.; Namdar, A.; Xu, L.; Crux, N.; Elahi, S. Atorvastatin downregulates co-inhibitory receptor expression by targeting Ras-activated mTOR signalling. Oncotarget 2017, 8, 98215–98232. [Google Scholar] [PubMed]
- Janakiram, N.B.; Mohammed, A.; Bryant, T.; Zhang, Y.; Brewer, M.; Duff, A.; Biddick, L.; Singh, A.; Lightfoot, S.; Steele, V.E.; et al. Potentiating NK cell activity by combination of Rosuvastatin and Difluoromethylornithine for effective chemopreventive efficacy against Colon Cancer. Sci. Rep. 2016, 6, 37046. [Google Scholar] [CrossRef]
- Norouzi, A.; Taziki, S.; Najafipasandi, A.; Mohammadi, S.; Roshandel, G. Rosuvastatin Intervention Decreased the Frequencies of the TIM-3+ Population of NK Cells and NKT Cells among Patients with Chronic Hepatitis B. Iran. J. Immunol. 2022, 19, 255–262. [Google Scholar] [CrossRef]
- Clark, K.; Plater, L.; Peggie, M.; Cohen, P. Use of the pharmacological inhibitor BX795 to study the regulation and physiological roles of TBK1 and IkappaB kinase epsilon: A distinct upstream kinase mediates Ser-172 phosphorylation and activation. J. Biol. Chem. 2009, 284, 14136–14146. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Yassin, A.A.-K.; Banerji, R.; Bhattacharya, B.; Radinsky, O.; Hadad, U.; Kaufman, B.; Porgador, A. Enhancing the Efficacy of CAR-T Cell Production Using BX795 and Rosuvastatin in a Serum-Free Medium. Int. J. Mol. Sci. 2025, 26, 2988. https://doi.org/10.3390/ijms26072988
Yassin AA-K, Banerji R, Bhattacharya B, Radinsky O, Hadad U, Kaufman B, Porgador A. Enhancing the Efficacy of CAR-T Cell Production Using BX795 and Rosuvastatin in a Serum-Free Medium. International Journal of Molecular Sciences. 2025; 26(7):2988. https://doi.org/10.3390/ijms26072988
Chicago/Turabian StyleYassin, Abed Al-Kader, Rajashri Banerji, Baisali Bhattacharya, Olga Radinsky, Uzi Hadad, Bar Kaufman, and Angel Porgador. 2025. "Enhancing the Efficacy of CAR-T Cell Production Using BX795 and Rosuvastatin in a Serum-Free Medium" International Journal of Molecular Sciences 26, no. 7: 2988. https://doi.org/10.3390/ijms26072988
APA StyleYassin, A. A.-K., Banerji, R., Bhattacharya, B., Radinsky, O., Hadad, U., Kaufman, B., & Porgador, A. (2025). Enhancing the Efficacy of CAR-T Cell Production Using BX795 and Rosuvastatin in a Serum-Free Medium. International Journal of Molecular Sciences, 26(7), 2988. https://doi.org/10.3390/ijms26072988





