Molecular Profile (Estrogen Receptor, Progesterone Receptor, Bcl-2 and Ki-67) of the Ectopic Endometrium in Patients with Endometriosis
Abstract
1. Introduction
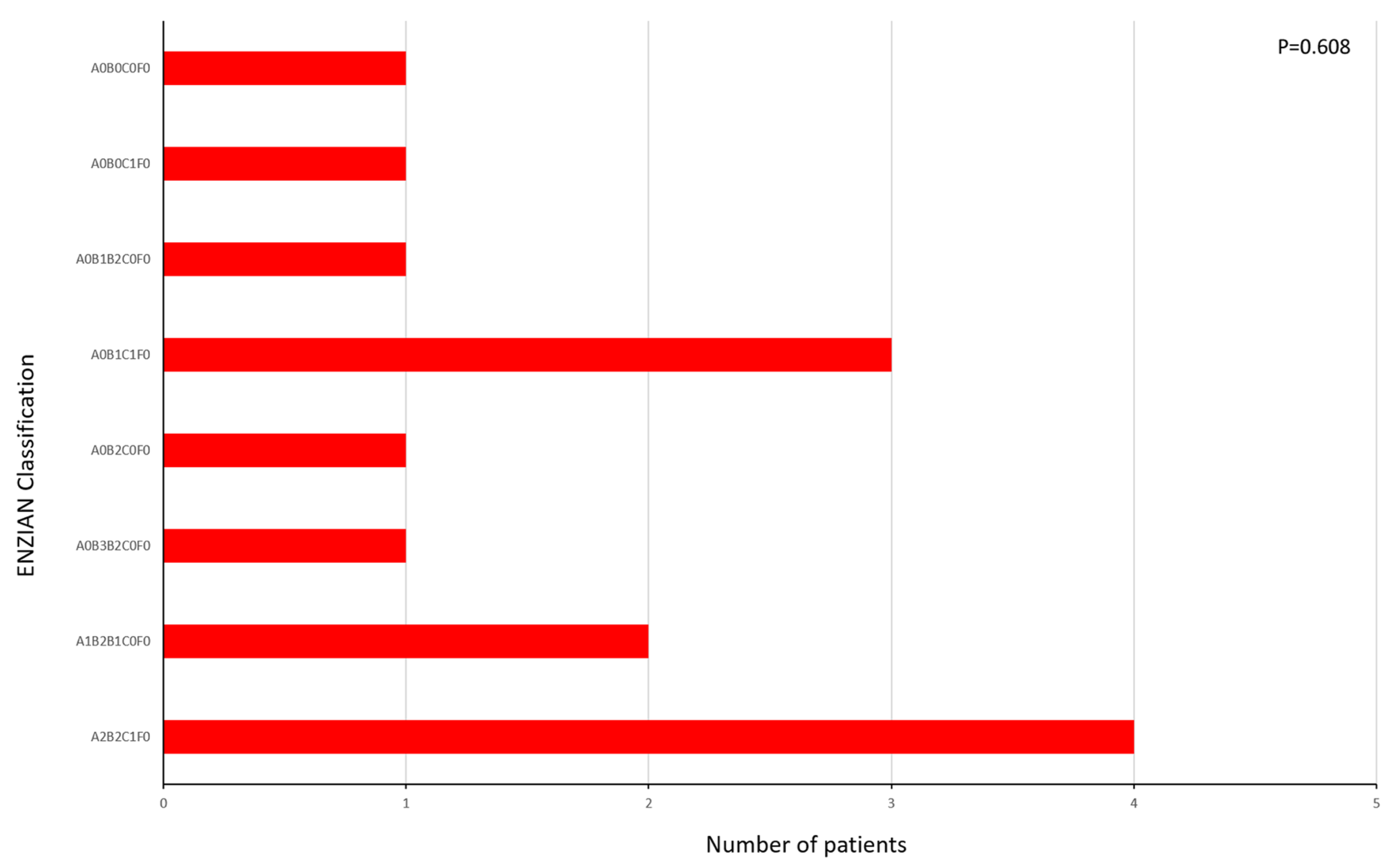
2. Results
3. Discussion
4. Materials and Methods
4.1. Patient Data and Selection Criteria
4.2. Surgical Procedure and Tissue Collection
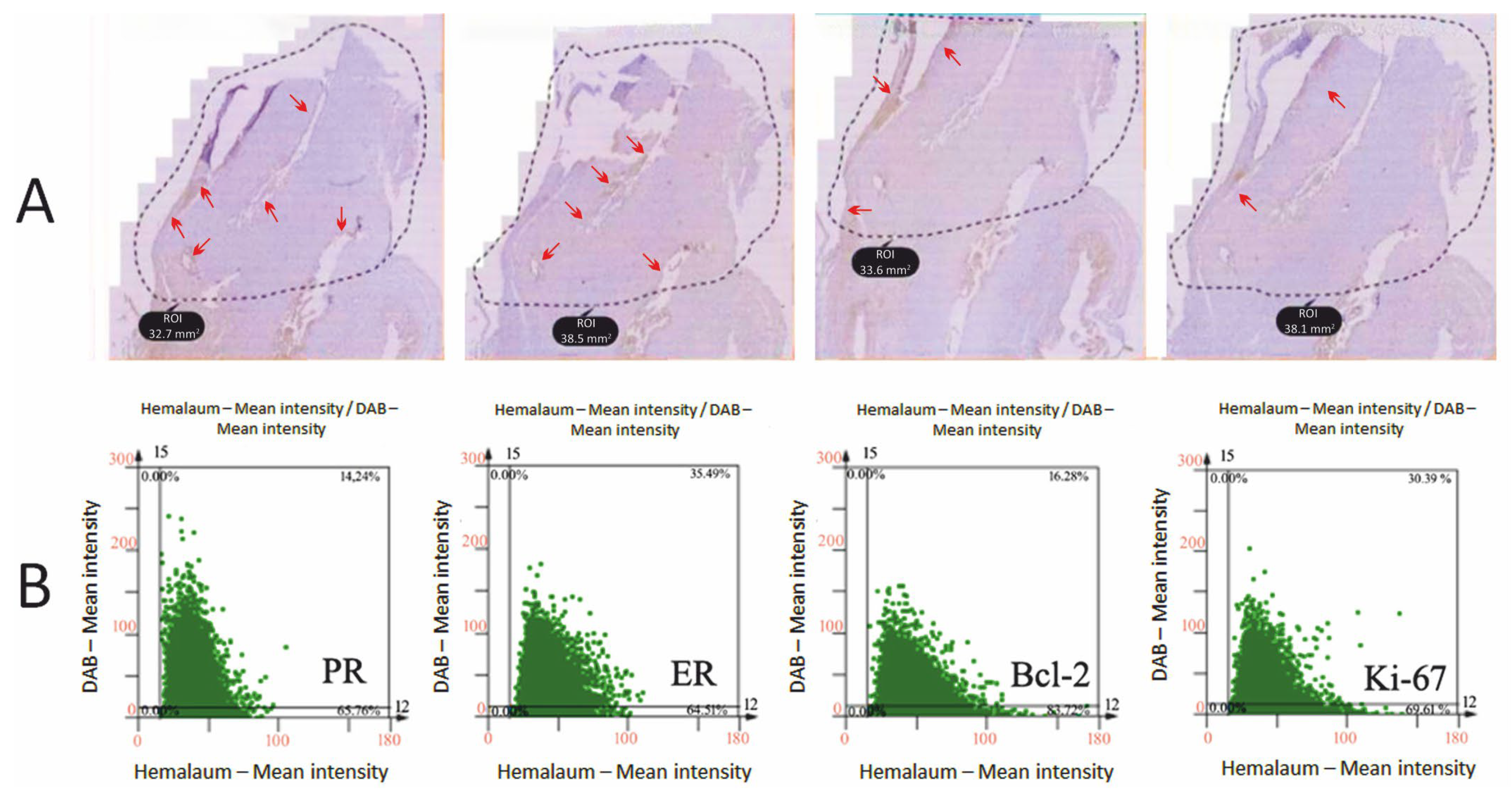
4.3. IHC
4.4. Biomarker Evaluation
4.5. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 17β-HSD | 17β-hydroxysteroid dehydrogenase |
| ANOVA | analysis of variance |
| Bcl-2 | B-cell lymphoma-2 |
| BSA | bovine serum albumin |
| DAB | diaminobenzidine |
| ER | estrogen receptor |
| IHC | immunohistochemistry |
| MRI | magnetic resonance imaging |
| PBS | phosphate buffered saline |
| PR | progesterone receptor |
| rASRM | revised American Society for Reproductive Medicine |
| TBS | Tris-buffered saline |
| VAS | visual analog scale |
References
- Chapron, C.; Marcellin, L.; Borghese, B.; Santulli, P. Rethinking mechanisms, diagnosis and management of endometriosis. Nat. Rev. Endocrinol. 2019, 15, 666–682. [Google Scholar] [PubMed]
- Vannuccini, S.; Clemenza, S.; Rossi, M.; Petraglia, F. Hormonal treatments for endometriosis: The endocrine background. Rev. Endocr. Metab. Disord. 2022, 23, 333–355. [Google Scholar]
- Bäumler, M.; Heiss, N.; Druckmann, R. Endometriosis at all ages: Diagnostic ultrasound. Horm. Mol. Biol. Clin. Investig. 2022, 43, 151–157. [Google Scholar] [CrossRef]
- Koninckx, P.R.; Ussia, A.; Adamyan, L.; Gomel, V.; Martin, D.C. Peritoneal fluid progesterone and progesterone resistance in superficial endometriosis lesions. Hum. Reprod. 2022, 37, 203–211. [Google Scholar] [PubMed]
- Agarwal, S.K.; Chapron, C.; Giudice, L.C.; Laufer, M.R.; Leyland, N.; Missmer, S.A.; Singh, S.S.; Taylor, H.S. Clinical diagnosis of endometriosis: A call to action. Am. J. Obstet. Gynecol. 2019, 220, 354.e1–354.e12. [Google Scholar] [CrossRef] [PubMed]
- Anupa, G.; Sharma, J.B.; Roy, K.K.; Sengupta, J.; Ghosh, D. An assessment of the multifactorial profile of steroid-metabolizing enzymes and steroid receptors in the eutopic endometrium during moderate to severe ovarian endometriosis. Reprod. Biol. Endocrinol. 2019, 17, 111. [Google Scholar]
- Lee, S.Y.; Koo, Y.J.; Lee, D.H. Classification of endometriosis. Yeungnam Univ. J. Med. 2021, 38, 10–18. [Google Scholar]
- Keckstein, J.; Saridogan, E.; Ulrich, U.A.; Sillem, M.; Oppelt, P.; Schweppe, K.W.; Krentel, H.; Janschek, E.; Exacoustos, C.; Malzoni, M.; et al. The #Enzian Classification: A Comprehensive Non-Invasive and Surgical Description System for Endometriosis. Acta Obstet. Gynecol. Scand. 2021, 100, 1165–1175. [Google Scholar]
- Vermeulen, N.; Abrao, M.S.; Einarsson, J.I.; Horne, A.W.; Johnson, N.P.; Lee, T.T.M.; Missmer, S.; Petrozza, J.; Tomassetti, C.; Zondervan, K.T.; et al. Endometriosis Classification, Staging and Reporting Systems: A Review on the Road to a Universally Accepted Endometriosis Classification. J. Minim. Invasive Gynecol. 2021, 28, 1822–1848. [Google Scholar]
- Yilmaz, B.D.; Bulun, S.E. Endometriosis and nuclear receptors. Hum. Reprod. Update 2019, 25, 473–485. [Google Scholar]
- Ahn, S.; Monsanto, S.; Miller, C.; Singh, S.; Thomas, R.; Tayade, C. Pathophysiology and Immune Dysfunction in Endometriosis. BioMed Res. Int. 2015, 2015, 795976. [Google Scholar] [CrossRef] [PubMed]
- Bouic, P.J. Endometriosis and infertility: The hidden link between endometritis, hormonal imbalances and immune dysfunctions preventing implantation! JBRA Assist. Reprod. 2023, 27, 144–146. [Google Scholar] [CrossRef]
- Evans, S.F.; Hull, M.L.; Hutchinson, M.R.; Rolan, P.E. Androgens, Endometriosis and Pain. Front. Reprod. Health 2021, 3, 792920. [Google Scholar] [CrossRef]
- Marquardt, R.M.; Kim, T.H.; Shin, J.H.; Jeong, J.W. Progesterone and estrogen signaling in the endometrium: What goes wrong in endometriosis? Int. J. Mol. Sci. 2019, 20, 3822. [Google Scholar] [CrossRef]
- Mishra, A.; Galvankar, M.; Singh, N.; Modi, D. Spatial and temporal changes in the expression of steroid hormone receptors in mouse model of endometriosis. J. Assist. Reprod. Genet. 2020, 37, 1069–1081. [Google Scholar] [CrossRef] [PubMed]
- Nasu, K.; Nishida, M.; Kawano, Y.; Tsuno, A.; Abe, W.; Yuge, A.; Takai, N.; Narahara, H. Aberrant expression of apoptosis-related molecules in endometriosis: A possible mechanism underlying the pathogenesis of endometriosis. Reprod. Sci. 2011, 18, 206–218. [Google Scholar] [CrossRef] [PubMed]
- Yalcin, S.E.; Ocal, I.; Yalcin, Y.; Selim, H.S.; Caltekin, M.D.; Aydogmus, H.; Kelekci, S. Evaluation of the Ki-67 proliferation index and urocortin expression in women with ovarian endometriomas. Eurasian J. Med. 2017, 49, 107–112. [Google Scholar] [CrossRef]
- Alif, M.; Anwar, R.; Pribadi, A. Ki-67 expression is correlated with cyst size and stage of endometriosis. Indones. J. Obstet. Gynecol. 2013, 1, 124–128. [Google Scholar] [CrossRef]
- Kahyaoglu, I.; Kahyaoglu, S.; Moraloglu, O.; Zergeroglu, S.; Sut, N.; Batioglu, S. Comparison of Ki-67 proliferative index between eutopic and ectopic endometrium: A case control study. Taiwan J. Obstet. Gynecol. 2012, 51, 393–396. [Google Scholar] [CrossRef]
- Alonzo, L.; Cannella, R.; Gullo, G.; Piombo, G.; Cicero, G.; Lopez, A.; Billone, V.; Andrisani, A.; Cucinella, G.; Lo Casto, A.; et al. Magnetic Resonance Imaging of Endometriosis: The Role of Advanced Techniques. J. Clin. Med. 2024, 13, 5783. [Google Scholar] [CrossRef]
- Dongye, H.; Ji, X.; Ma, X.; Song, J.; Yan, L. The Impact of Endometriosis on Embryo Quality in in-vitro Fertilization/Intracytoplasmic Sperm Injection: A Systematic Review and Meta-Analysis. Front. Med. 2021, 8, 669342. [Google Scholar]
- Gullo, G.; Basile, G.; Cucinella, G.; Greco, M.E.; Perino, A.; Chiantera, V.; Marinelli, S. Fresh vs. frozen embryo transfer in assisted reproductive techniques: A single center retrospective cohort study and ethical-legal implications. Eur. Rev. Med. Pharmacol. Sci. 2023, 27, 6809–6823. [Google Scholar] [PubMed]
- Robin, C.; Uk, A.; Decanter, C.; Behal, H.; Collinet, P.; Rubod, C.; Barbotin, A.L.; Robin, G. Impact of endometriosis on oocyte morphology in IVF-ICSI: Retrospective study of a cohort of more than 6000 mature oocytes. Reprod. Biol. Endocrinol. 2021, 19, 160. [Google Scholar] [CrossRef]
- Gullo, G.; Scaglione, M.; Cucinella, G.; Perino, A.; Chiantera, V.; D’Anna, R.; Laganà, A.S.; Buzzaccarini, G. Impact of assisted reproduction techniques on the neuro-psycho-motor outcome of newborns: A critical appraisal. J. Obs. Gynaecol. 2022, 42, 2583–2587. [Google Scholar]
- Attia, G.R.; Zeitoun, K.; Edwards, D.; Johns, A.; Carr, B.R.; Bulun, S.E. Progesterone receptor isoform A but not B is expressed in endometriosis. J. Clin. Endocrinol. Metab. 2000, 85, 2897–2902. [Google Scholar] [CrossRef] [PubMed]
- Nnoaham, K.E.; Hummelshoj, L.; Webster, P.; d’Hooghe, T.; de Cicco Nardone, F.; de Cicco Nardone, C.; Jenkinson, C.; Kennedy, S.H.; Zondervan, K.T. Impact of endometriosis on quality of life and work productivity: A multicenter study across ten countries. Fertil. Steril. 2011, 96, 366–373. [Google Scholar] [PubMed]
- Noble, L.S.; Takayama, K.; Zeitoun, K.M.; Putman, J.M.; Johns, D.A.; Hinshelwood, M.M.; Agarwal, V.R.; Zhao, Y.; Carr, B.R.; Bulun, S.E. Prostaglandin E2 stimulates aromatase expression in endometriosis-derived stromal cells. J. Clin. Endocrinol. Metab. 1997, 82, 600–606. [Google Scholar]
- Bergqvist, A.; Fernö, M. Estrogen and progesterone receptors in endometriotic tissue and endometrium: Comparison according to localization and recurrence. Fertil. Steril. 1993, 60, 63–68. [Google Scholar]
- Kirkin, V.; Joos, S.; Zornig, M. The role of Bcl-2 family members in tumorigenesis. Biochim. Biophys. Acta 2004, 1644, 229–249. [Google Scholar]
- Li, S.F.; Nakayama, K.; Masuzawa, H.; Fujii, S. The number of proliferating cell nuclear antigen positive cells in endometriotic lesions differs from that in the endometrium. Analysis of PCNA positive cells during the menstrual cycle and in post-menopause. Virchows. Arch. A Pathol. Anat. Histopathol. 1993, 423, 257–263. [Google Scholar]
- Matasariu, D.R.; Lozneanu, L.; Dumitraşcu, I.; Grigore, M.; Cristofor, A.E.; Mandici, C.E.; Bujor, I.E.; Ursache, A.; Brăila, A.D.; Bauşic, A.; et al. Hormonal, apoptotic, proliferative and inflammatory markers’ expression in Desogestrel-treated women with ovarian endometriosis. Rom. J. Morphol. Embryol. 2022, 63, 137–144. [Google Scholar] [CrossRef] [PubMed]
- Habib, N.; Buzzaccarini, G.; Centini, G.; Moawad, G.N.; Ceccaldi, P.F.; Gitas, G.; Alkatout, I.; Gullo, G.; Terzic, S.; Sleiman, Z. Impact of lifestyle and diet on endometriosis: A fresh look to a busy corner. Menopausal Rev. 2022, 21, 124–132. [Google Scholar] [CrossRef] [PubMed]
- Comănescu, M.; Arsene, D.; Ardeleanu, C.; Bussolati, G. The mandate for a proper preservation in histopathological tissues. Rom. J. Morphol. Embryol. 2012, 53, 233–242. [Google Scholar] [PubMed]
- Allred, D.C.; Harvey, J.M.; Berardo, M.; Clark, G.M. Prognostic and predictive factors in breast cancer by immunohistochemical analysis. Mod. Pathol. 1998, 11, 155–168. [Google Scholar]
- Suzuki, Y.; Honma, T.; Hayashi, S.; Ajioka, Y.; Asakura, H. Bcl-2 expression and frequency of apoptosis correlate with morphogenesis of colorectal neoplasia. J. Clin. Pathol. 2022, 55, 212–216. [Google Scholar] [CrossRef]
| Biomarker | Epithelial ER | Stromal ER | |||
|---|---|---|---|---|---|
| rASRM Stage | |||||
| rASRM stage II | Mean ± SD | range | Mean ± SD | range | |
| 14.7 ± 12.0% | 0–90% | 28.2 ± 23.0% | 0–100% | ||
| rASRM stage III | Mean ± SD | range | Mean ± SD | range | |
| 16.8 ± 21.2% | 0–90% | 7.5 ± 9.5% | 0–100% | ||
| rASRM stage IV | Mean ± SD | range | Mean ± SD | range | |
| 25.2 ± 20.5% | 0–90% | 45.2% ± 36.9% | 0–100% | ||
| p-value | p = 0.710 | p = 0.084 | |||
| Biomarker | Epithelial PR | Stromal PR | |||
|---|---|---|---|---|---|
| rASRM Stage | |||||
| rASRM stage II | Mean ± SD | range | Mean ± SD | range | |
| 13.2 ± 10.8% | 0–100% | 59.8 ± 40.1% | 5–100% | ||
| rASRM stage III | Mean ± SD | range | Mean ± SD | range | |
| 44.1 ± 42.6% | 0–100% | 54.4% ± 43.1% | 5–100% | ||
| rASRM stage IV | Mean ± SD | range | Mean ± SD | range | |
| 52.9 ± 38.5% | 0–100% | 70.7 ± 41.5% | 5–100% | ||
| p-value | p = 0.285 | p = 0.836 | |||
| Biomarker | Epithelial Bcl-2 | Stromal Bcl-2 | |||
|---|---|---|---|---|---|
| rASRM Stage | |||||
| rASRM stage II | Mean ± SD | range | Mean ± SD | range | |
| 13.6 ± 11.1% | 0–90% | 15.3 ± 12.5% | 0–90% | ||
| rASRM stage III | Mean ± SD | range | Mean ± SD | range | |
| 17.8 ± 22.5% | 0–90% | 20.0 ± 25.3% | 0–90% | ||
| rASRM stage IV | Mean ± SD | range | Mean ± SD | range | |
| 23.7 ± 19.4% | 0–90% | 26.7 ± 25.0% | 0–90% | ||
| p-value | p = 0.760 | p = 0.776 | |||
| Biomarker | Epithelial Ki-67 | Stromal Ki-67 | |||
|---|---|---|---|---|---|
| rASRM Stage | |||||
| rASRM stage II | Mean ± SD | range | Mean ± SD | range | |
| 3.8 ± 5.3% | 0–35% | 9.3 ± 13.2% | 0–90% | ||
| rASRM stage III | Mean ± SD | range | Mean ± SD | range | |
| 8.1 ± 10.2% | 0–35% | 8.4 ± 14.4% | 0–90% | ||
| rASRM stage IV | Mean ± SD | range | Mean ± SD | range | |
| 7.6 ± 14.9% | 0–35% | 20.1 ± 28.4% | 0–90% | ||
| p-value | p = 0.811 | p = 0.616 | |||
| Biomarker (pos./ neg.) | Epithelial ER (pos./ neg.) | Stromal ER (pos./ neg.) | Epithelial PR (pos./ neg.) | Stromal PR (pos./ neg.) | Epithelial Bcl-2 (pos./ neg.) | Stromal Bcl-2 (pos./ neg.) | Epithelial Ki-67 (pos./ neg.) | Stromal Ki-67 (pos./ neg.) | |
|---|---|---|---|---|---|---|---|---|---|
| rASRM Stage | |||||||||
| rASRM stage II | 1/3 | 3/1 | 3/1 | 4/0 | 2/2 | 2/2 | 3/1 | 3/1 | |
| rASRM stage III | 2/4 | 4/2 | 4/2 | 6/0 | 3/3 | 3/3 | 4/2 | 4/2 | |
| rASRM stage IV | 1/3 | 3/1 | 3/1 | 4/0 | 2/2 | 2/2 | 3/1 | 3/1 | |
| p-value | p = 0.943 | p = 0.943 | p = 0.943 | p = 1.000 | p = 1.000 | p = 0.943 | p = 0.943 | ||
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | |
|---|---|---|---|---|---|---|---|---|---|
| 1. rASRM stage | |||||||||
| 2. Epithelial ER | 0.431 | ||||||||
| 3. Stromal ER | 0.400 | 0.007 * | |||||||
| 4. Epithelial PR | 0.130 | 0.000 * | 0.040 * | ||||||
| 5. Stromal PR | 0.814 | 0.027 * | 0.405 | 0.060 | |||||
| 6. Epithelial Bcl-2 | 0.451 | 0.277 | 0.067 | 0.297 | 0.260 | ||||
| 7. Stromal Bcl-2 | 0.470 | 0.243 | 0.055 | 0.265 | 0.220 | 0.000 * | |||
| 8. Epithelial Ki-67 | 0.611 | 0.366 | 0.248 | 0.095 | 0.530 | 0.353 | 0.248 | ||
| 9. Stromal Ki-67 | 0.427 | 0.213 | 0.011 * | 0.151 | 0.920 | 0.221 | 0.139 | 0.003 * | - |
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | 10 | |
|---|---|---|---|---|---|---|---|---|---|---|
| 1. Personal history of endometriosis | ||||||||||
| 2. Previous surgeries for endometriosis | 0.025 * | |||||||||
| 3. Dysmenorrhea | 0.190 | 0.447 | ||||||||
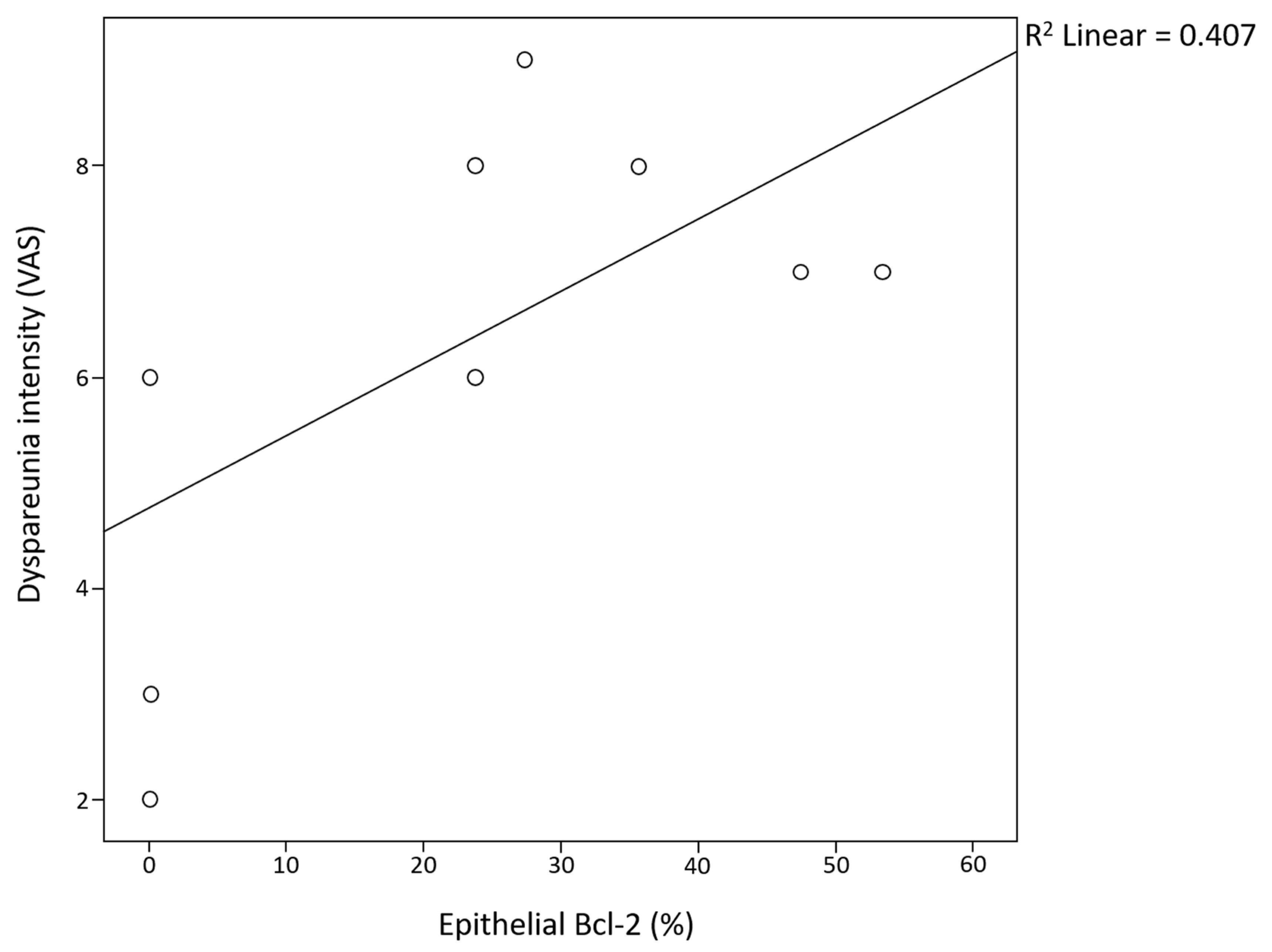
| 4. Dyspareunia | 0.519 | 0.302 | 0.549 | |||||||
| 5. Chronic pelvic pain | 0.746 | 0.361 | 0.288 | 0.249 | ||||||
| 6. Gastrointestinal symptoms | 0.630 | 0.124 | 0.549 | 0.156 | 0.803 | |||||
| 7. Epithelial Bcl-2 | 0.224 | 0.286 | 0.311 | 0.378 | 0.728 | 0.056 | ||||
| 8. Stromal Bcl-2 | 0.177 | 0.256 | 0.332 | 0.397 | 0.689 | 0.068 | 0.000 * | |||
| 9. Epithelial Ki-67 | 0.765 | 0.651 | 0.068 | 0.837 | 0.927 | 0.264 | 0.353 | 0.248 | ||
| 10. Stromal Ki-67 | 0.691 | 0.467 | 0.164 | 0.349 | 0.853 | 0.255 | 0.221 | 0.139 | 0.003 * | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Coroleucă, C.-A.; Coroleucă, C.-B.; Coroleucă, R.; Brătilă, P.C.; Nodiți, A.-R.; Roșca, I.; Brîndușe, L.A.; Brătilă, E.; Boț, M. Molecular Profile (Estrogen Receptor, Progesterone Receptor, Bcl-2 and Ki-67) of the Ectopic Endometrium in Patients with Endometriosis. Int. J. Mol. Sci. 2025, 26, 2983. https://doi.org/10.3390/ijms26072983
Coroleucă C-A, Coroleucă C-B, Coroleucă R, Brătilă PC, Nodiți A-R, Roșca I, Brîndușe LA, Brătilă E, Boț M. Molecular Profile (Estrogen Receptor, Progesterone Receptor, Bcl-2 and Ki-67) of the Ectopic Endometrium in Patients with Endometriosis. International Journal of Molecular Sciences. 2025; 26(7):2983. https://doi.org/10.3390/ijms26072983
Chicago/Turabian StyleCoroleucă, Ciprian-Andrei, Cătălin-Bogdan Coroleucă, Ruxandra Coroleucă, Petre Cornel Brătilă, Aniela-Roxana Nodiți, Ioana Roșca, Lăcrămioara Aurelia Brîndușe, Elvira Brătilă, and Mihaela Boț. 2025. "Molecular Profile (Estrogen Receptor, Progesterone Receptor, Bcl-2 and Ki-67) of the Ectopic Endometrium in Patients with Endometriosis" International Journal of Molecular Sciences 26, no. 7: 2983. https://doi.org/10.3390/ijms26072983
APA StyleCoroleucă, C.-A., Coroleucă, C.-B., Coroleucă, R., Brătilă, P. C., Nodiți, A.-R., Roșca, I., Brîndușe, L. A., Brătilă, E., & Boț, M. (2025). Molecular Profile (Estrogen Receptor, Progesterone Receptor, Bcl-2 and Ki-67) of the Ectopic Endometrium in Patients with Endometriosis. International Journal of Molecular Sciences, 26(7), 2983. https://doi.org/10.3390/ijms26072983







