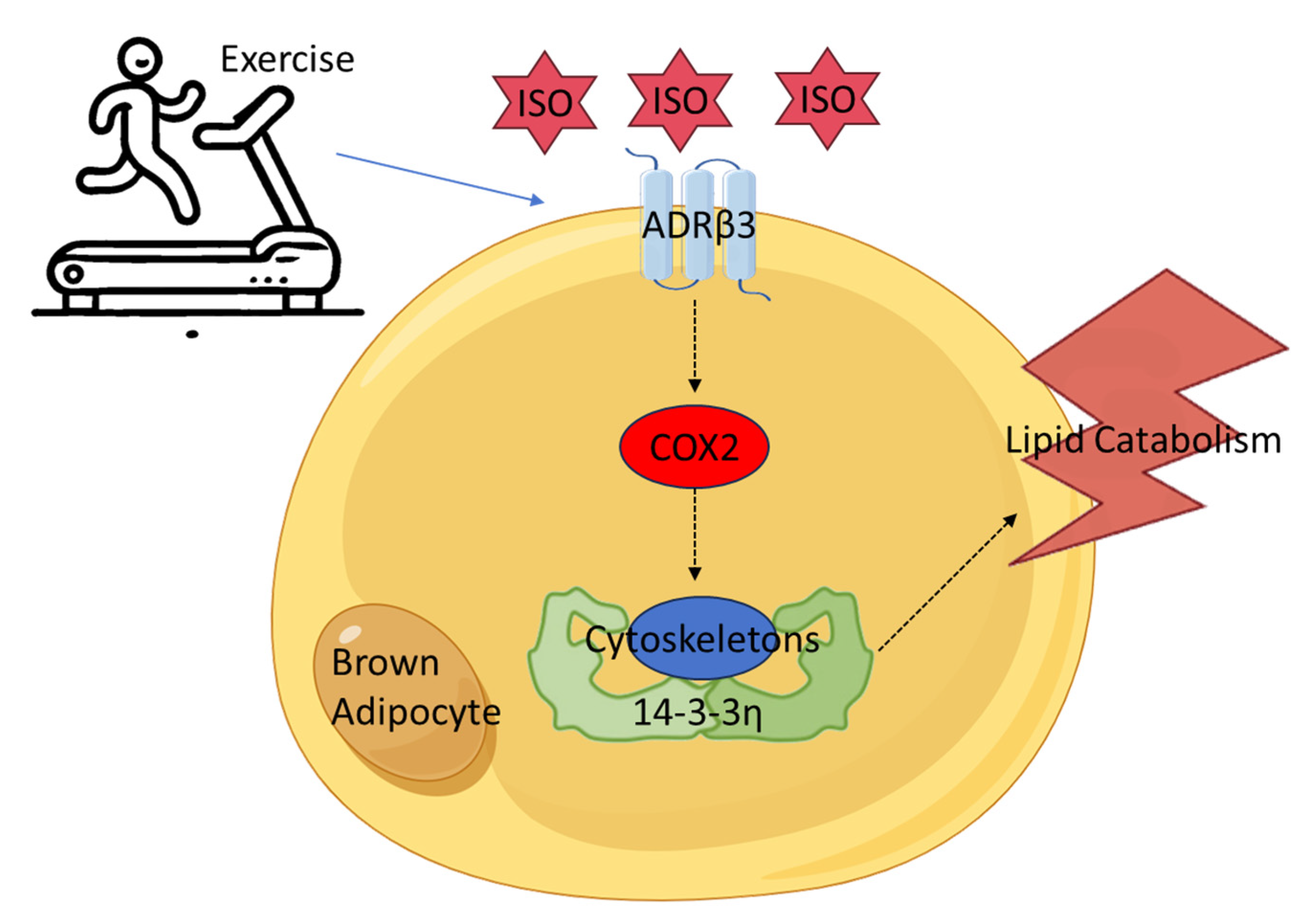
Exercise Improves the Cytoskeletal and Metabolic Functions of Brown Adipocytes Through the ADRβ3/COX2-Ywhah Axis
Abstract
1. Introduction
2. Results
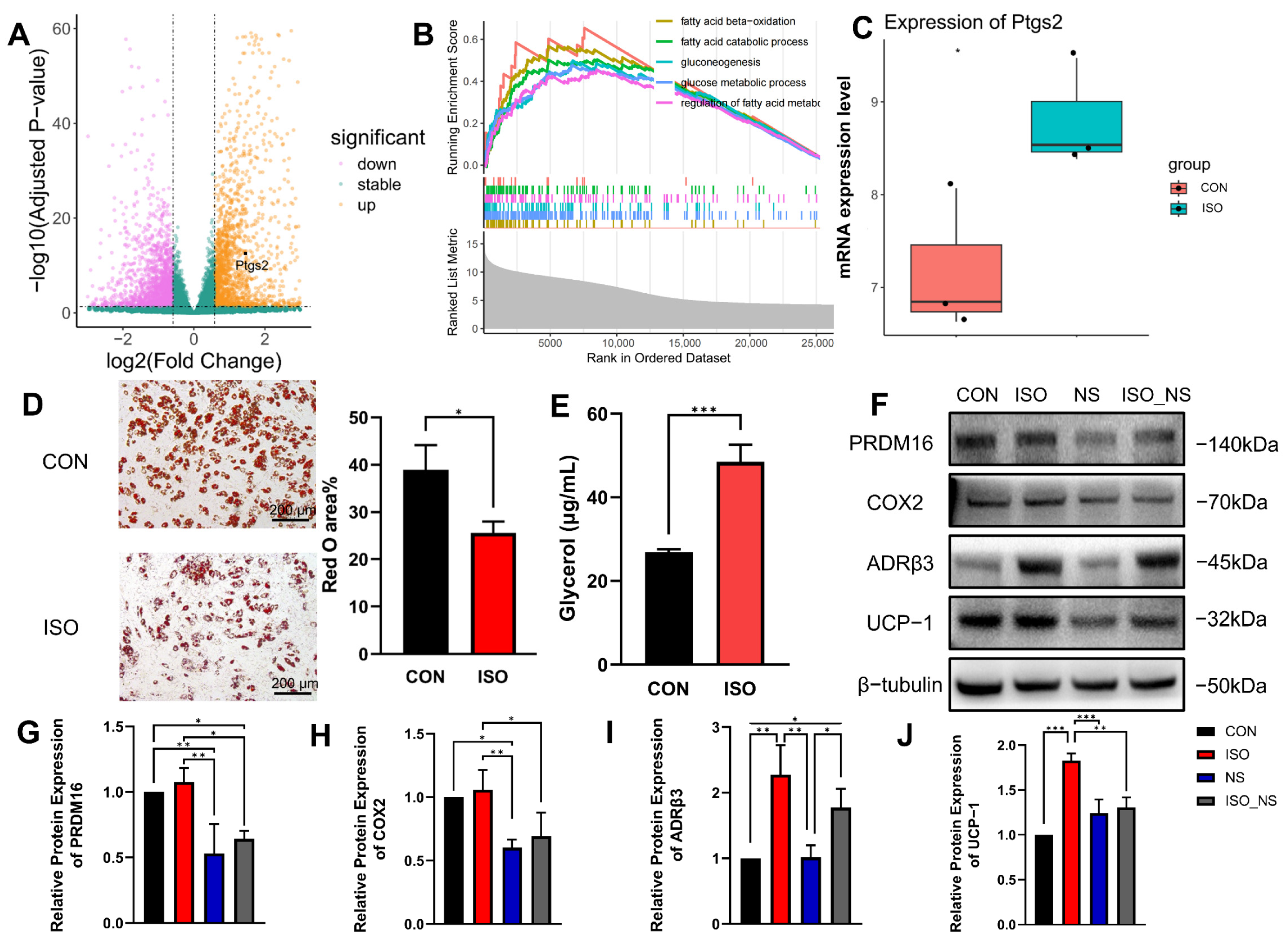
2.1. Exercise Enhances Lipid Metabolic Function in Brown Adipose Tissue
2.2. ADRβ3 Activation Promotes Lipolysis in Brown Adipocytes
2.3. Inhibition of COX2 Impairs the Metabolic Enhancement Effect Induced by ADRβ3 Activation in Brown Adipocytes
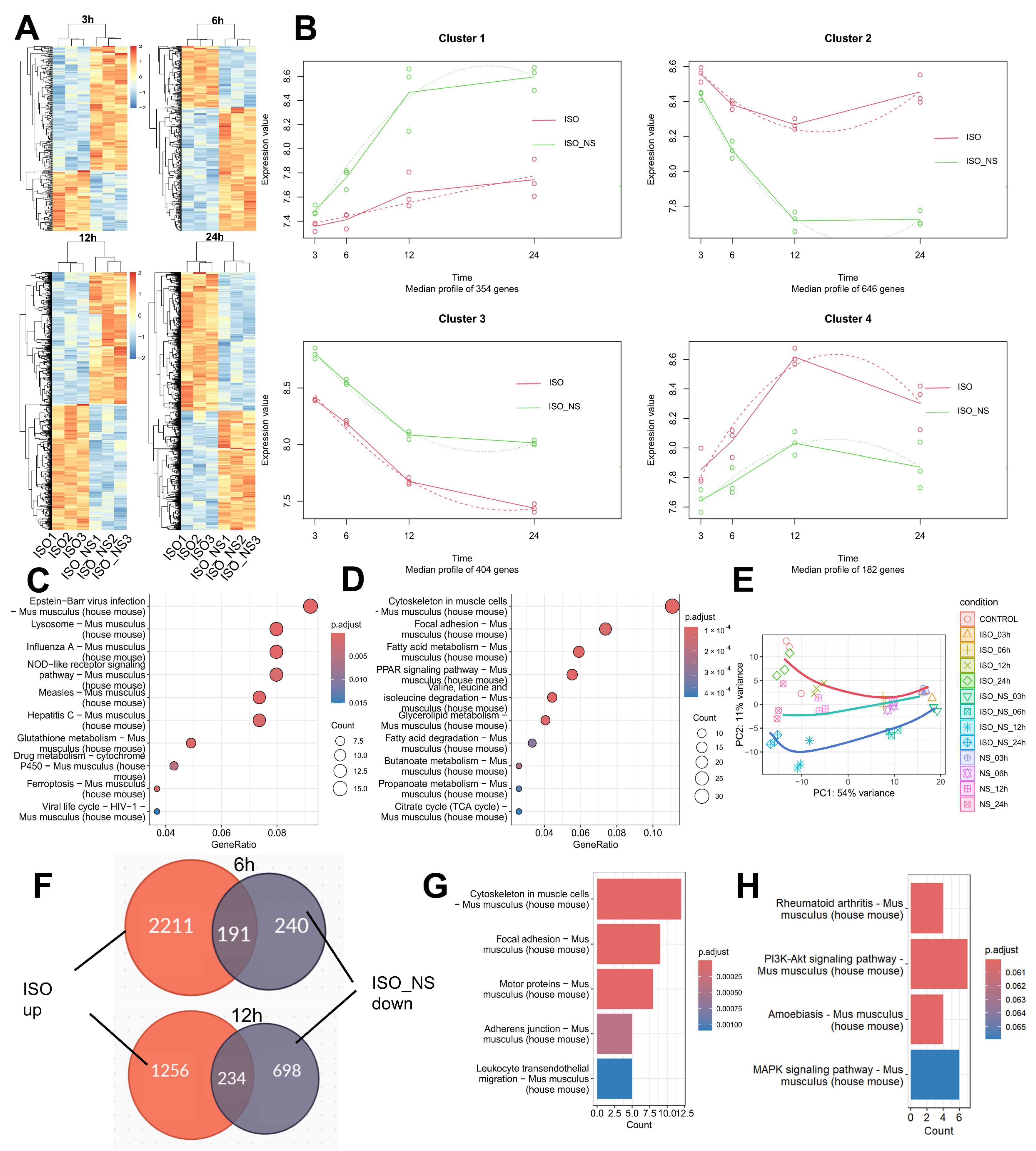
2.4. Time-Course Characteristics of RNA-Seq
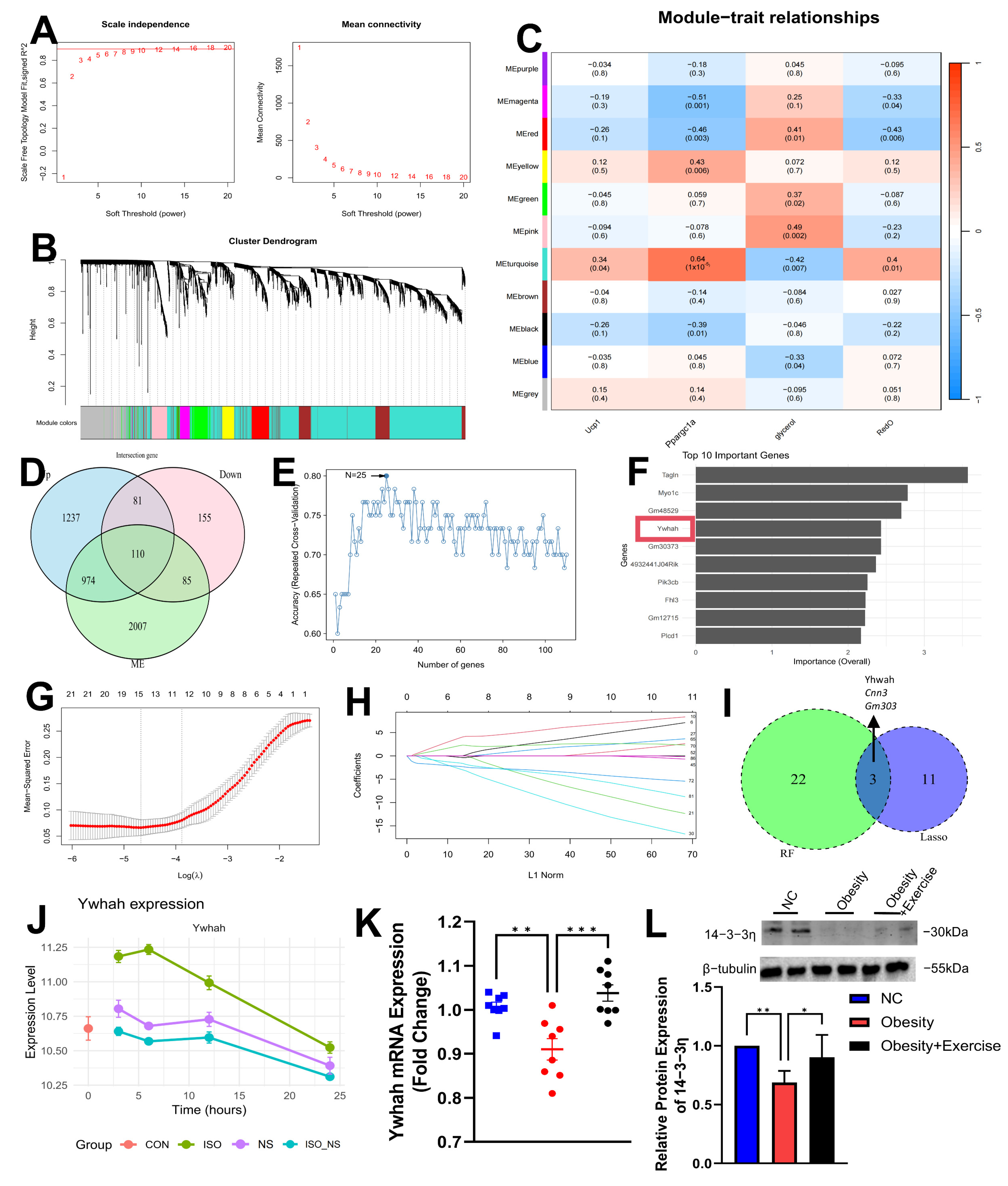
2.5. WGCNA and Machine Learning Identify Ywhah as a Key Feature Gene
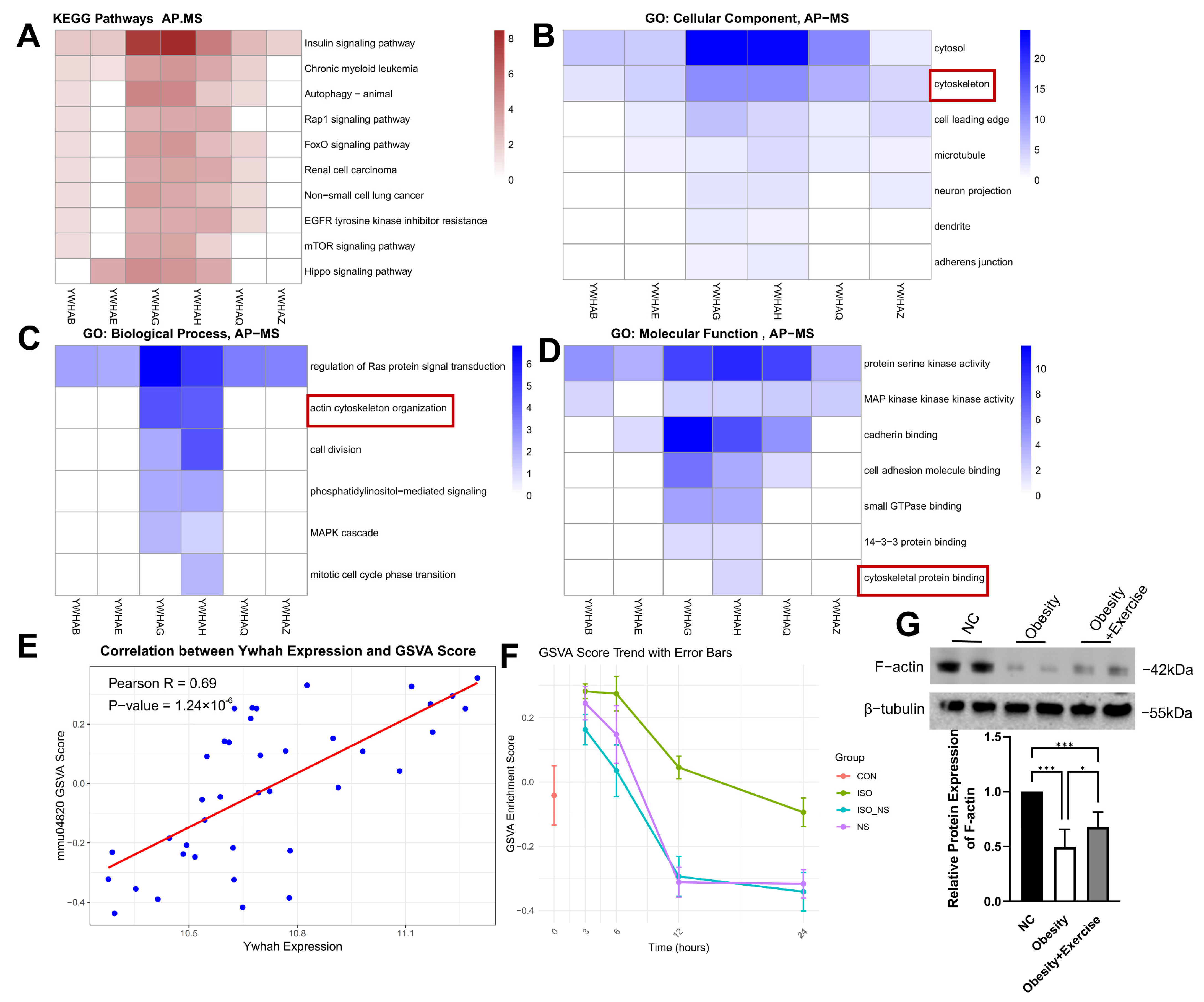
2.6. The 14-3-3η Protein Encoded by Ywhah Binding with Cytoskeletal Proteins
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Cell Culture
4.3. RNA-Seq
4.4. Glycerol Quantification Assay
4.5. Oil Red O Staining
4.6. Western Blotting
4.7. RT-qPCR
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
Abbreviations
| BAT | Brown Adipose Tissue |
| ADRβ3 | Adrenergic Receptor Beta-3 |
| COX2 | Cyclooxygenase-2 |
| ISO | Isoproterenol |
| WAT | White Adipose Tissue |
| Ywhah | 14-3-3η (a protein encoded by the Ywhah gene) |
| WGCNA | Weighted Gene Co-expression Network Analysis |
| GSEA | Gene Set Enrichment Analysis |
| GSVA | Gene Set Variation Analysis |
| KEGG | Kyoto Encyclopedia of Genes and Genomes |
References
- Prapaharan, B.; Lea, M.; Beaudry, J.L. Weighing in on the role of brown adipose tissue for treatment of obesity. J. Pharm. Pharm. Sci. 2024, 27, 13157. [Google Scholar] [CrossRef]
- Gunawardana, S.C.; Piston, D.W. Reversal of Type 1 Diabetes in Mice by Brown Adipose Tissue Transplant. Diabetes 2012, 61, 674–682. [Google Scholar] [CrossRef]
- Chand, S.; Tripathi, A.S.; Dewani, A.P.; Sheikh, N.W.A. Molecular targets for management of diabetes: Remodelling of white adipose to brown adipose tissue. Life Sci. 2024, 345, 122607. [Google Scholar] [CrossRef]
- Stanford, K.I.; Middelbeek, R.J.; Townsend, K.L.; An, D.; Nygaard, E.B.; Hitchcox, K.M.; Markan, K.R.; Nakano, K.; Hirshman, M.F.; Tseng, Y.-H.; et al. Brown adipose tissue regulates glucose homeostasis and insulin sensitivity. J. Clin. Investig. 2013, 123, 215–223. [Google Scholar] [CrossRef]
- Dewal, R.S.; Stanford, K.I. Effects of exercise on brown and beige adipocytes. Biochim. Biophys. Acta Mol. Cell Biol. Lipids 2019, 1864, 71–78. [Google Scholar] [CrossRef]
- da Silva, C.P.V.; Hernández-Saavedra, D.; White, J.D.; Stanford, K.I. Cold and Exercise: Therapeutic Tools to Activate Brown Adipose Tissue and Combat Obesity. Biology 2019, 8, 9. [Google Scholar] [CrossRef]
- Rodrigues, A.; Prímola-Gomes, T.; Peluzio, M.; Hermsdorff, H.; Natali, A. Aerobic exercise and lipolysis: A review of the β-adrenergic signaling pathways in adipose tissue. Sci. Sports 2021, 36, 16–26. [Google Scholar] [CrossRef]
- Fu, P.; Zhu, R.; Jia, J.; Hu, Y.; Wu, C.; Cieszczyk, P.; Holmberg, H.-C.; Gong, L. Aerobic exercise promotes the functions of brown adipose tissue in obese mice via a mechanism involving COX2 in the VEGF signaling pathway. Nutr. Metab. 2021, 18, 56. [Google Scholar] [CrossRef]
- Insel, P.A. Adrenergic Receptors—Evolving Concepts and Clinical Implications. N. Engl. J. Med. 1996, 334, 580–585. [Google Scholar] [CrossRef]
- Sakers, A.; De Siqueira, M.K.; Seale, P.; Villanueva, C.J. Adipose-tissue plasticity in health and disease. Cell 2022, 185, 419–446. [Google Scholar] [CrossRef]
- Degerman, E.; Smith, C.J.; Tornqvist, H.; Vasta, V.; Belfrage, P.; Manganiello, V.C. Evidence that insulin and isoprenaline activate the cGMP-inhibited low-Km cAMP phosphodiesterase in rat fat cells by phosphorylation. Proc. Natl. Acad. Sci. USA 1990, 87, 533–537. [Google Scholar] [CrossRef]
- Martín-Vázquez, E.; Cobo-Vuilleumier, N.; López-Noriega, L.; Lorenzo, P.I.; Gauthier, B.R. The PTGS2/COX2-PGE2 signaling cascade in inflammation: Pro or anti? A case study with type 1 diabetes mellitus. Int. J. Biol. Sci. 2023, 19, 4157–4165. [Google Scholar] [CrossRef]
- García-Alonso, V.; Clària, J. Prostaglandin E2signals white-to-brown adipogenic differentiation. Adipocyte 2014, 3, 290–296. [Google Scholar] [CrossRef]
- Carroll, C.C.; O’Connor, D.T.; Steinmeyer, R.; Del Mundo, J.D.; McMullan, D.R.; Whitt, J.A.; Ramos, J.E.; Gonzales, R.J. The influence of acute resistance exercise on cyclooxygenase-1 and -2 activity and protein levels in human skeletal muscle. Am. J. Physiol. Integr. Comp. Physiol. 2013, 305, R24–R30. [Google Scholar] [CrossRef]
- Madsen, L.; Pedersen, L.M.; Lillefosse, H.H.; Fjære, E.; Bronstad, I.; Hao, Q.; Petersen, R.K.; Hallenborg, P.; Ma, T.; De Matteis, R.; et al. UCP1 Induction during Recruitment of Brown Adipocytes in White Adipose Tissue Is Dependent on Cyclooxygenase Activity. PLoS ONE 2010, 5, e11391. [Google Scholar] [CrossRef]
- Paschos, G.K.; Tang, S.Y.; Theken, K.N.; Li, X.; Verginadis, I.; Lekkas, D.; Herman, L.; Yan, W.; Lawson, J.; FitzGerald, G.A. Cold-Induced Browning of Inguinal White Adipose Tissue Is Independent of Adipose Tissue Cyclooxygenase-2. Cell Rep. 2018, 24, 809–814. [Google Scholar] [CrossRef]
- Jin, J.; Smith, F.; Stark, C.; Wells, C.D.; Fawcett, J.P.; Kulkarni, S.; Metalnikov, P.; O’Donnell, P.; Taylor, P.; Taylor, L.; et al. Proteomic, Functional, and Domain-Based Analysis of In Vivo 14-3-3 Binding Proteins Involved in Cytoskeletal Regulation and Cellular Organization. Curr. Biol. 2004, 14, 1436–1450. [Google Scholar] [CrossRef]
- Sreedhar, R.; Arumugam, S.; Thandavarayan, R.A.; Karuppagounder, V.; Koga, Y.; Nakamura, T.; Harima, M.; Watanabe, K. Role of 14-3-3η protein on cardiac fatty acid metabolism and macrophage polarization after high fat diet induced type 2 diabetes mellitus. Int. J. Biochem. Cell Biol. 2017, 88, 92–99. [Google Scholar] [CrossRef]
- Potolitsyna, E.; Pickering, S.H.; Bellanger, A.; Germier, T.; Collas, P.; Briand, N. Cytoskeletal rearrangement precedes nucleolar remodeling during adipogenesis. Commun. Biol. 2024, 7, 458. [Google Scholar] [CrossRef]
- Wei, L.; Surma, M.; Yang, Y.; Tersey, S.; Shi, J. ROCK2 inhibition enhances the thermogenic program in white and brown fat tissue in mice. FASEB J. 2019, 34, 474–493. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, Z.; Lu, X.; Gong, H.; Liu, X.; Wang, H.; Cheng, H.; Gao, H.; Shi, X.; Li, Y.; et al. Transcriptomics Dissection of Calorie Restriction and Exercise Training in Brown Adipose Tissue and Skeletal Muscle. Nutrients 2023, 15, 1047. [Google Scholar] [CrossRef]
- Segal, D.; Maier, S.; Mastromarco, G.J.; Qian, W.; Nabeel-Shah, S.; Taneja, N.; Jansens, R.J.; Adams, C.; Gupta, R.; Dingar, D.; et al. Identification of a FoxO3-FOXM1 regulatory network that drives mitotic gene expression and proliferation of neural progenitor cells. Nucleic Acids Res. 2024, 52, 1430–1447. [Google Scholar] [CrossRef]
- Cero, C.; Lea, H.J.; Zhu, K.Y.; Shamsi, F.; Tseng, Y.-H.; Cypess, A.M. β3-Adrenergic receptors regulate human brown/beige adipocyte lipolysis and thermogenesis. J. Clin. Investig. 2021, 6, e139160. [Google Scholar] [CrossRef]
- Mottillo, E.P.; Balasubramanian, P.; Lee, Y.-H.; Weng, C.; Kershaw, E.E.; Granneman, J.G. Coupling of lipolysis and de novo lipogenesis in brown, beige, and white adipose tissues during chronic β3-adrenergic receptor activation. J. Lipid Res. 2014, 55, 2276–2286. [Google Scholar] [CrossRef]
- Paulsen, G.; Egner, I.M.; Drange, M.; Langberg, H.; Benestad, H.B.; Fjeld, J.G.; Hallén, J.; Raastad, T. A COX-2 inhibitor reduces muscle soreness, but does not influence recovery and adaptation after eccentric exercise. Scand. J. Med. Sci. Sports 2010, 20, e195–e207. [Google Scholar] [CrossRef]
- Tyagi, A.; Kamal, M.A.; Poddar, N.K. Integrated Pathways of COX-2 and mTOR: Roles in Cell Sensing and Alzheimer’s Disease. Front. Neurosci. 2020, 14, 693. [Google Scholar] [CrossRef]
- Baek, H.K.; Kim, P.S.; Song, J.A.; Choi, D.-H.; Kim, D.E.; Oh, S.I.; Park, S.-K.; Kim, S.-J.; Song, K.-D.; Hwang, I.K.; et al. Neuronal maturation in the hippocampal dentate gyrus via chronic oral administration of Artemisa annuaextract is independent of cyclooxygenase 2 signaling pathway in diet-induced obesity mouse model. J. Vet. Sci. 2017, 18, 119–127. [Google Scholar] [CrossRef]
- Medbo, J.I.; Mohn, A.C.; Tabata, I.; Bahr, R.; Vaage, O.; Sejersted, O.M. Anaerobic capacity determined by maximal accumulated O2 deficit. J. Appl. Physiol. 1988, 64, 50–60. [Google Scholar] [CrossRef]
- Goldstein, D.S.; Castillo, G.; Sullivan, P.; Sharabi, Y. Differential Susceptibilities of Catecholamines to Metabolism by Monoamine Oxidases. J. Pharmacol. Exp. Ther. 2021, 379, 253–259. [Google Scholar] [CrossRef] [PubMed]
- Thyfault, J.P.; Bergouignan, A. Exercise and metabolic health: Beyond skeletal muscle. Diabetologia 2020, 63, 1464–1474. [Google Scholar] [CrossRef]
- MoTrPAC Study Group; Analysts, L.; Amar, D.; Gay, N.R.; Jean-Beltran, P.M.; Generators, L.D.; Bae, D.; Dasari, S.; Dennis, C.; Evans, C.R.; et al. Temporal dynamics of the multi-omic response to endurance exercise training. Nature 2024, 629, 174–183. [Google Scholar] [CrossRef]
- Diallo, K.; Oppong, A.K.; Lim, G.E. Can 14-3-3 proteins serve as therapeutic targets for the treatment of metabolic diseases? Pharmacol. Res. 2019, 139, 199–206. [Google Scholar] [CrossRef]
- Lim, G.E.; Albrecht, T.; Piske, M.; Sarai, K.; Lee, J.T.C.; Ramshaw, H.S.; Sinha, S.; Guthridge, M.A.; Acker-Palmer, A.; Lopez, A.F.; et al. 14-3-3ζ coordinates adipogenesis of visceral fat. Nat. Commun. 2015, 6, 7671. [Google Scholar] [CrossRef]
- Oppong, A.K.; Diallo, K.; Frayne, I.R.; Rosiers, C.D.; Lim, G.E. Reducing 14-3-3ζ expression influences adipocyte maturity and impairs function. Am. J. Physiol. Metab. 2020, 319, E117–E132. [Google Scholar] [CrossRef]
- Wang, W.; Shakes, D.C. Molecular evolution of the 14-3-3 protein family. J. Mol. Evol. 1996, 43, 384–398. [Google Scholar] [CrossRef]


Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiao, J.; Xu, C.; Zhu, R.; Fu, P.; Jia, J.; Gong, L. Exercise Improves the Cytoskeletal and Metabolic Functions of Brown Adipocytes Through the ADRβ3/COX2-Ywhah Axis. Int. J. Mol. Sci. 2025, 26, 2978. https://doi.org/10.3390/ijms26072978
Xiao J, Xu C, Zhu R, Fu P, Jia J, Gong L. Exercise Improves the Cytoskeletal and Metabolic Functions of Brown Adipocytes Through the ADRβ3/COX2-Ywhah Axis. International Journal of Molecular Sciences. 2025; 26(7):2978. https://doi.org/10.3390/ijms26072978
Chicago/Turabian StyleXiao, Jingzhe, Chunyan Xu, Rongxin Zhu, Pengyu Fu, Jie Jia, and Lijing Gong. 2025. "Exercise Improves the Cytoskeletal and Metabolic Functions of Brown Adipocytes Through the ADRβ3/COX2-Ywhah Axis" International Journal of Molecular Sciences 26, no. 7: 2978. https://doi.org/10.3390/ijms26072978
APA StyleXiao, J., Xu, C., Zhu, R., Fu, P., Jia, J., & Gong, L. (2025). Exercise Improves the Cytoskeletal and Metabolic Functions of Brown Adipocytes Through the ADRβ3/COX2-Ywhah Axis. International Journal of Molecular Sciences, 26(7), 2978. https://doi.org/10.3390/ijms26072978






