Physicochemical Characterization, Skin Penetration, Anti-Melanogenesis and Safety Assessment of Flavokawain C Nanofibers
Abstract
1. Introduction
2. Results and Discussion
2.1. Material Characterization
2.2. Stability Tests
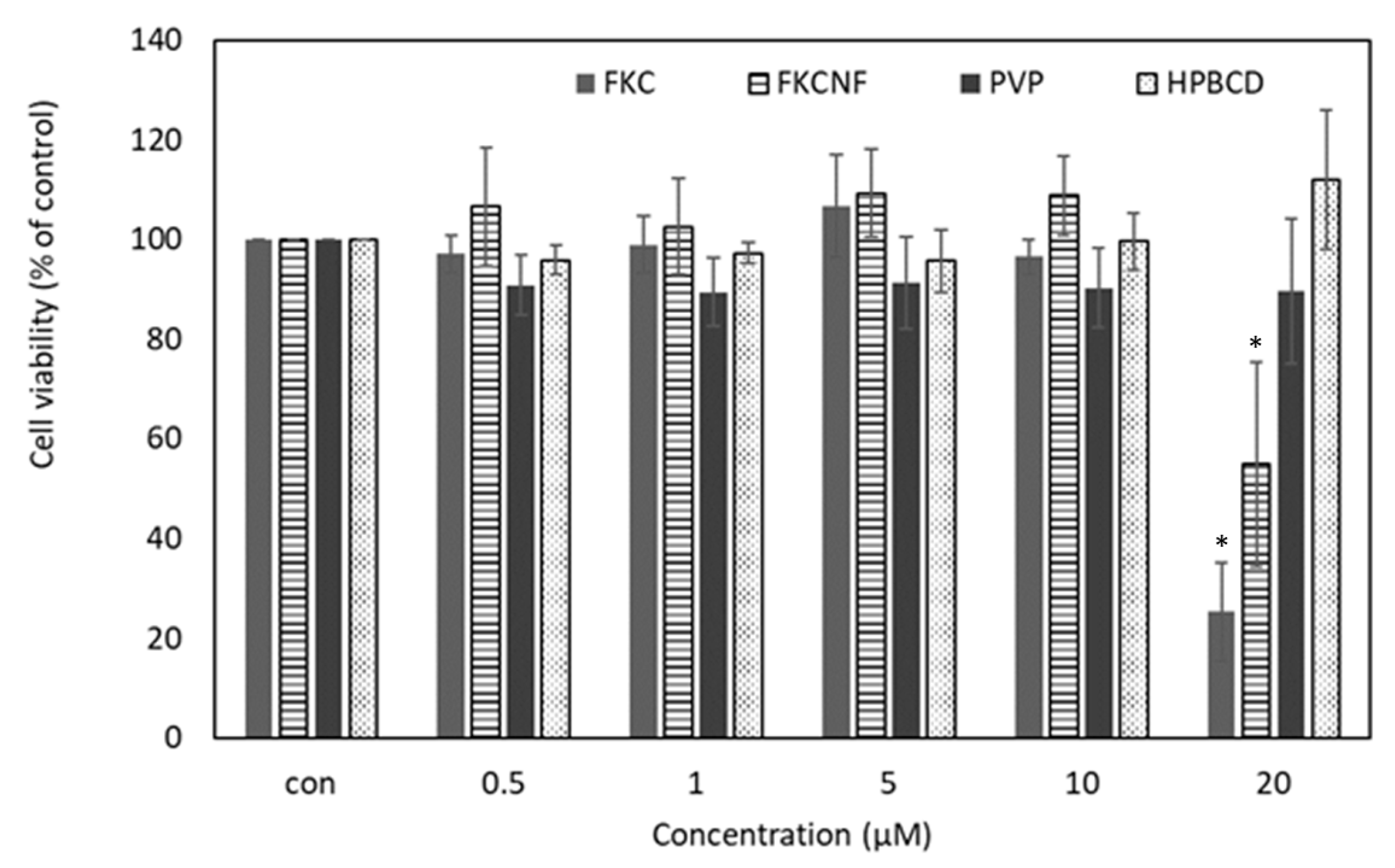
2.3. Cell Viability
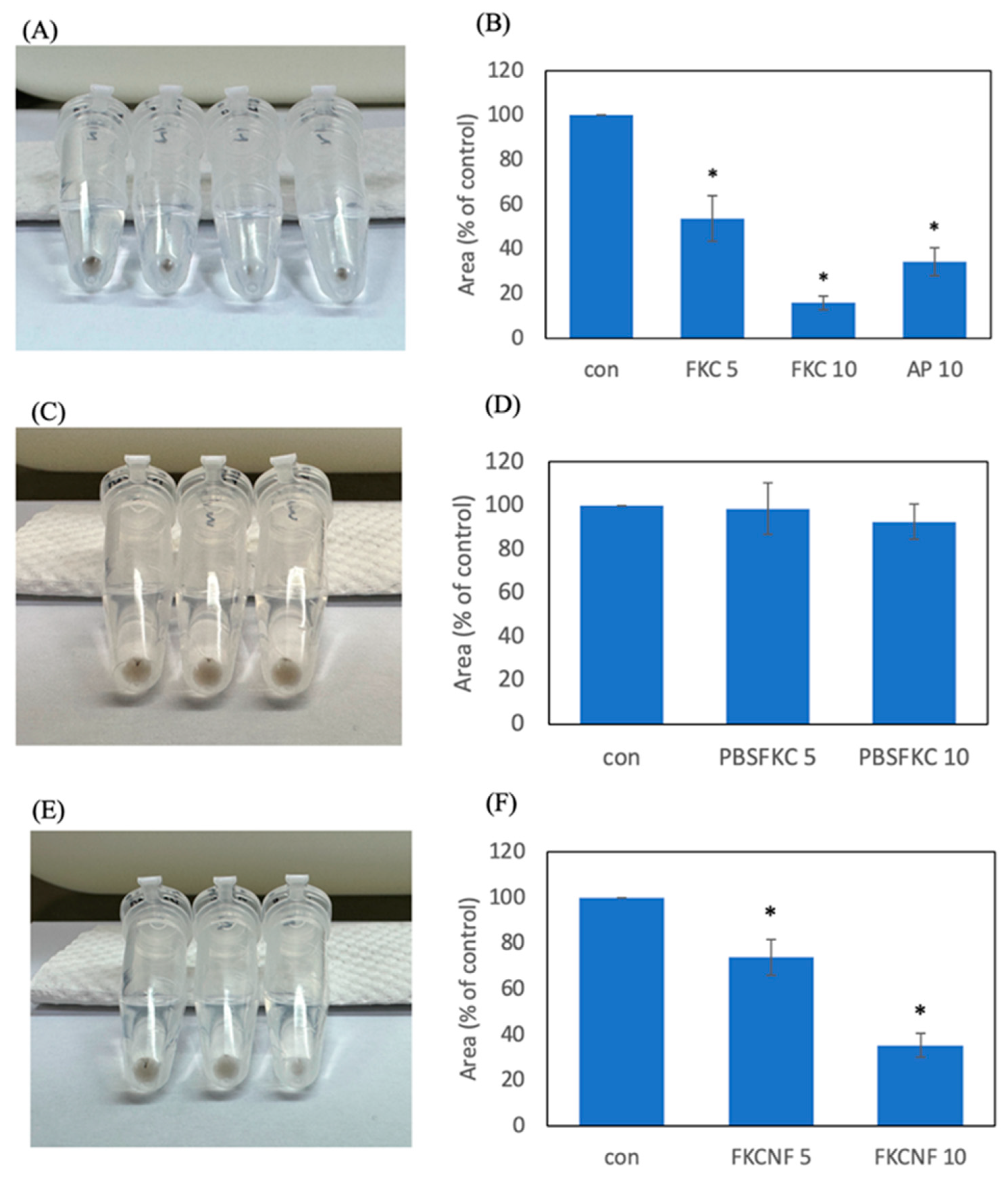
2.4. Melanogenesis Inhibition
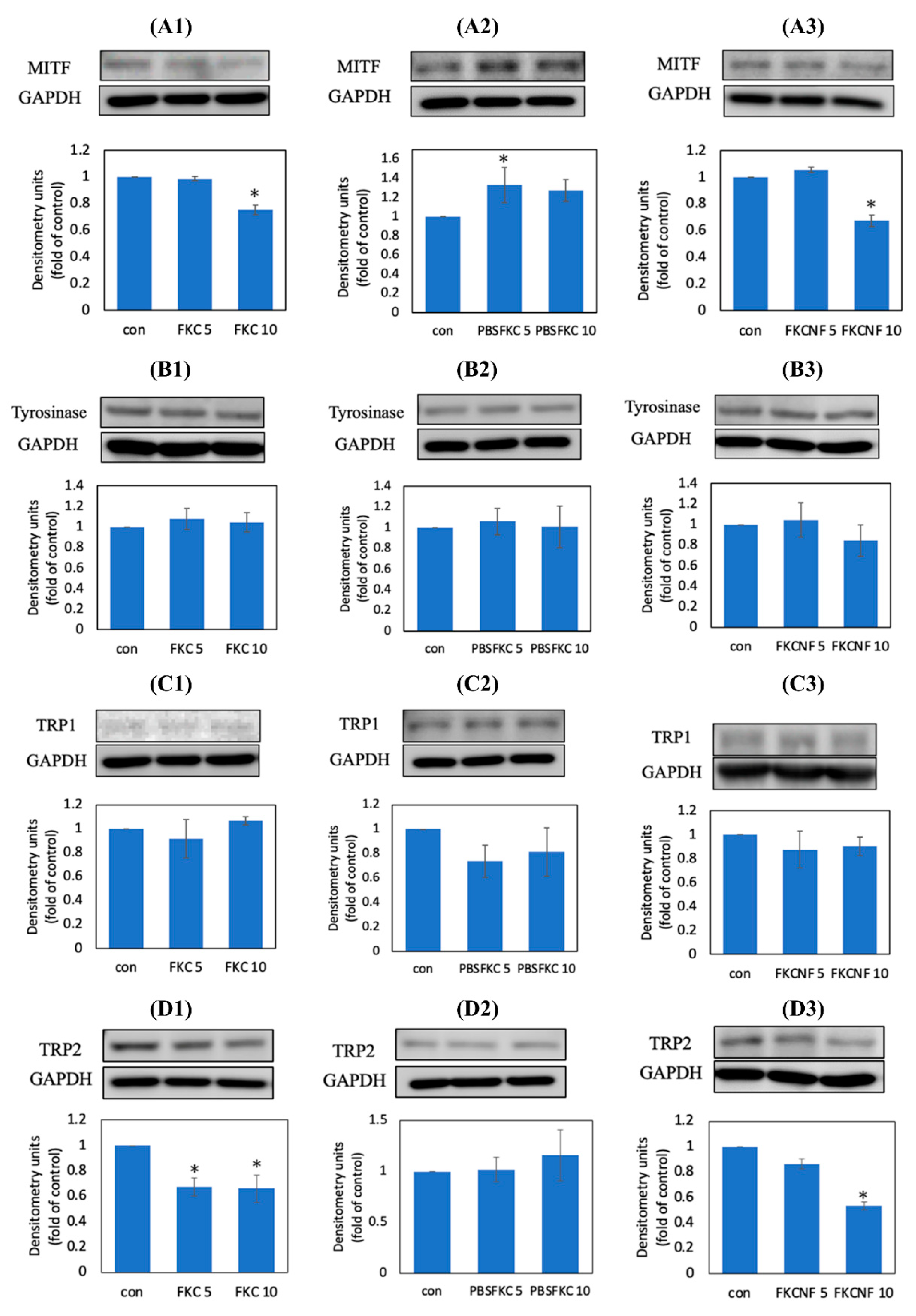
2.5. Expression of Melanogenesis-Related Proteins in B16F10 Cells
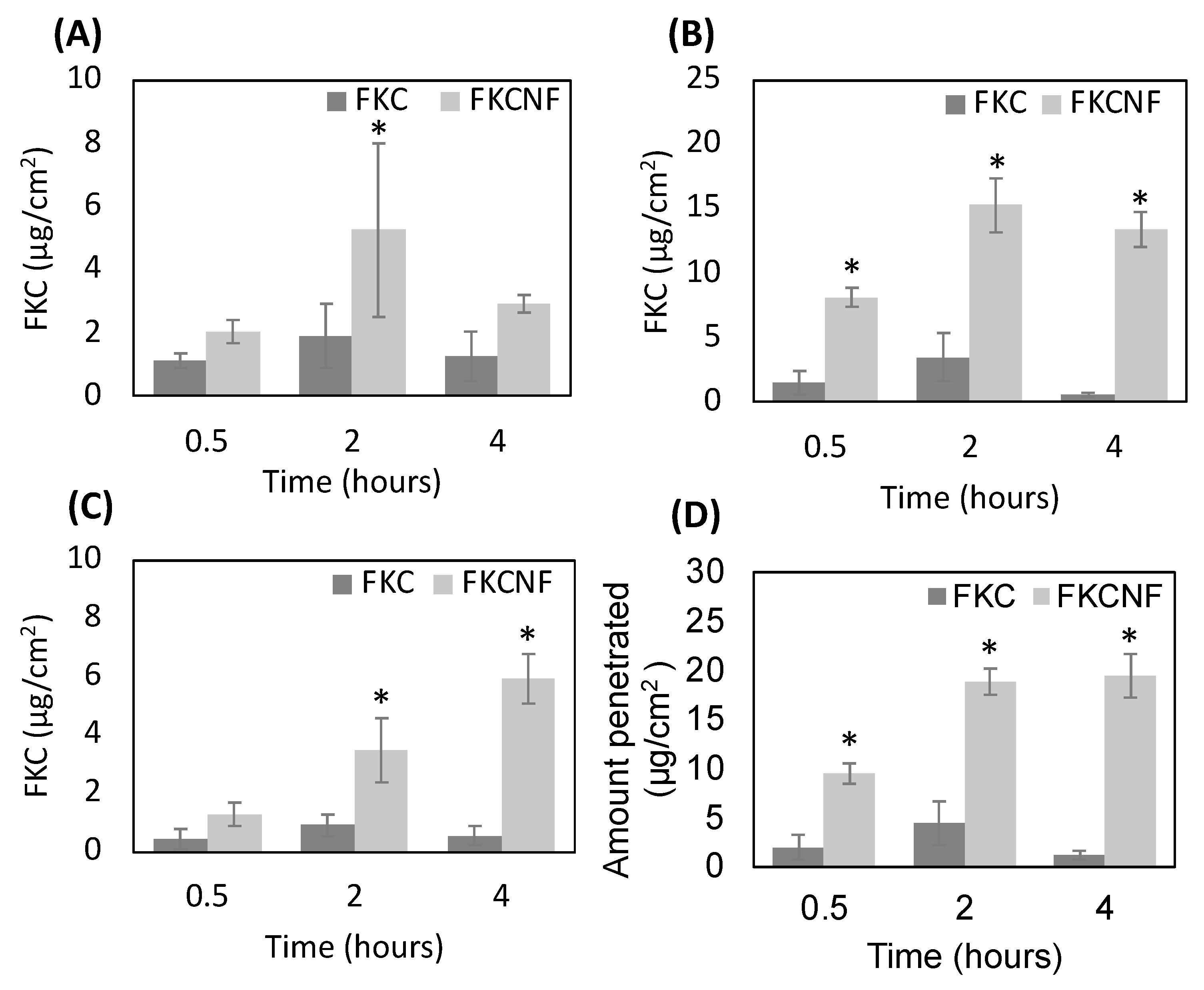
2.6. Percutaneous Absorption and Margin of Safety (MoS)
3. Materials and Methods
3.1. Materials
3.2. Preparation of FKCNF
3.3. FKC Content Analysis by HPLC
3.4. Material Characterization
3.5. Solubility Tests
3.6. Stability Tests
3.7. Cell Viability
3.8. Melanin Content Measurement
3.9. Western Blot Analysis
3.10. Safety Assessment
3.10.1. Percutaneous Absorption
3.10.2. Calculation for Margin of Safety
3.11. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Downregulation of melanogenesis: Drug discovery and therapeutic options. Drug Discov. Today 2017, 22, 282–298. [Google Scholar] [CrossRef]
- Vachtenheim, J.; Borovanský, J. “Transcription physiology” of pigment formation in melanocytes: Central role of MITF. Exp. Dermatol. 2010, 19, 617–627. [Google Scholar] [CrossRef]
- Hachiya, A.; Kobayashi, A.; Ohuchi, A.; Takema, Y.; Imokawa, G. The paracrine role of stem cell factor/c-kit signaling in the activation of human melanocytes in ultraviolet-B-induced pigmentation. J. Investig. Dermatol. 2001, 116, 578–586. [Google Scholar] [CrossRef] [PubMed]
- Kang, M.; Park, S.H.; Oh, S.W.; Lee, S.E.; Yoo, J.A.; Nho, Y.H.; Lee, S.; Han, B.S.; Cho, J.Y.; Lee, J. Anti-melanogenic effects of resorcinol are mediated by suppression of cAMP signaling and activation of p38 MAPK signaling. Biosci. Biotechnol. Biochem. 2018, 82, 1188–1196. [Google Scholar] [CrossRef]
- Orrarat, S.; Chulee, Y. Fermented unpolished black rice (Oryza sativa L.) inhibits melanogenesis via ERK, p38, and AKT phosphorylation in B16F10 melanoma cells. J. Microbiol. Biotechnol. 2020, 30, 1184–1194. [Google Scholar] [CrossRef]
- Song, Y.S.; Balcos, M.C.; Yun, H.Y.; Baek, K.J.; Kwon, N.S.; Kim, M.K.; Kim, D.S. ERK activation by fucoidan leads to inhibition of melanogenesis in Mel-Ab cells. Korean J. Physiol. Pharmacol. 2014, 19, 29–34. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Jung, S.H. Recent development of signaling pathways inhibitors of melanogenesis. Cell. Signal. 2017, 40, 99–115. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.F.; Huang, C.C.; Lee, M.Y.; Lin, Y.S. Fermented broth in tyrosinase and melanogenesis inhibition. Molecules 2014, 19, 13122–13135. [Google Scholar] [CrossRef]
- Scientific Committee on Consumer Safety: Opinion on Kojic Acid. 2022. Available online: https://health.ec.europa.eu/system/files/2023-08/sccs_o_259.pdf (accessed on 2 June 2023).
- Ephrem, E.; Elaissari, H.; Greige Gerges, H. Improvement of skin whitening agents efficiency through encapsulation: Current state of knowledge. Int. J. Pharm. 2017, 526, 50–68. [Google Scholar] [CrossRef]
- Jeon, J.S.; Kim, B.H.; Lee, S.H.; Kwon, H.J.; Bae, H.J.; Kim, S.K.; Park, J.A.; Shim, J.H.; Abd El Aty, A.M.; Shin, H.C. Simultaneous determination of arbutin and its decomposed product hydroquinone in whitening creams using high-performance liquid chromatography with photodiode array detection: Effect of temperature and pH on decomposition. Int. J. Cosmet. Sci. 2015, 37, 567–573. [Google Scholar] [CrossRef]
- Pillaiyar, T.; Manickam, M.; Namasivayam, V. Skin whitening agents: Medicinal chemistry perspective of tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2017, 32, 403–425. [Google Scholar]
- Zolghadri, S.; Bahrami, A.; Hassan Khan, M.T.; Munoz Munoz, J.; Garcia Molina, F.; Garcia Canovas, F.; Saboury, A.A. A comprehensive review on tyrosinase inhibitors. J. Enzym. Inhib. Med. Chem. 2019, 34, 279–309. [Google Scholar] [CrossRef]
- Bian, T.; Corral, P.; Wang, Y.; Botello, J.; Kingston, R.; Daniels, T.; Salloum, R.G.; Johnston, E.; Huo, Z.; Lu, J.; et al. Kava as a clinical nutrient: Promises and challenges. Nutrients 2020, 12, 44. [Google Scholar] [CrossRef]
- Sarris, J.; Stough, C.; Teschke, R.; Wahid, Z.T.; Bousman, C.A.; Murray, G.; Savage, K.M.; Mouatt, P.; Ng, C.; Schweitzer, I. Kava for the treatment of generalized anxiety disorder RCT: Analysis of adverse reactions, liver function, addiction, and sexual effects. Phytother. Res. 2013, 27, 1723–1728. [Google Scholar] [CrossRef] [PubMed]
- Fu, D.; Ramzan, I. Use of kava as a phytotherapeutic agent and kava-related hepatotoxicity. In Phytotherapies; John Wiley & Sons: Hoboken, NJ, USA, 2015; pp. 312–329. [Google Scholar]
- Phang, C.W.; Karsani, S.A.; Sethi, G.; Abd Malek, S.N. Flavokawain C inhibits cell cycle and promotes apoptosis, associated with endoplasmic reticulum stress and regulation of MAPKs and Akt signaling pathways in HCT 116 human colon carcinoma cells. PLoS ONE 2016, 11, e0148775. [Google Scholar] [CrossRef]
- Abu, N.; Ho, W.Y.; Yeap, S.K.; Akhtar, M.N.; Abdullah, M.P.; Omar, A.R.; Alitheen, N.B. The flavokawains: Uprising medicinal chalcones. Cancer Cell Int. 2013, 13, 102. [Google Scholar] [CrossRef] [PubMed]
- Phang, C.W.; Karsani, S.A.; Abd Malek, S.N. Induction of apoptosis and cell cycle arrest by flavokawain C on HT-29 human colon adenocarcinoma via enhancement of reactive oxygen species generation, upregulation of p21, p27, and GADD153, and inactivation of inhibitor of apoptosis proteins. Pharmacogn. Mag. 2017, 13, S321–s328. [Google Scholar] [CrossRef]
- Jeong, H.J.; Lee, C.S.; Choi, J.; Hong, Y.D.; Shin, S.S.; Park, J.S.; Lee, J.H.; Lee, S.; Yoon, K.D.; Ko, J. Flavokawains B and C, melanogenesis inhibitors, isolated from the root of Piper methysticum and synthesis of analogs. Bioorganic Med. Chem. Lett. 2015, 25, 799–802. [Google Scholar]
- Kim, H.D.; Choi, H.; Abekura, F.; Park, J.Y.; Yang, W.S.; Yang, S.H.; Kim, C.H. Naturally-occurring tyrosinase inhibitors classified by enzyme kinetics and copper chelation. Int. J. Mol. Sci. 2023, 24, 8226. [Google Scholar] [CrossRef]
- Guazelli, C.F.S.; Fattori, V.; Ferraz, C.R.; Borghi, S.M.; Casagrande, R.; Baracat, M.M.; Verri, W.A., Jr. Antioxidant and anti-inflammatory effects of hesperidin methyl chalcone in experimental ulcerative colitis. Chem.-Biol. Interact. 2021, 333, 109315. [Google Scholar] [CrossRef]
- Ferraz, C.R.; Carvalho, T.T.; Manchope, M.F.; Artero, N.A.; Rasquel Oliveira, F.S.; Fattori, V.; Casagrande, R.; Verri, W.A. Therapeutic potential of Flavonoids in pain and inflammation: Mechanisms of action, pre-clinical and clinical data, and pharmaceutical development. Molecules 2020, 25, 762. [Google Scholar] [CrossRef] [PubMed]
- Ouyang, Y.; Li, J.; Chen, X.; Fu, X.; Sun, S.; Wu, Q. Chalcone derivatives: Role in anticancer therapy. Biomolecules 2021, 11, 894. [Google Scholar] [CrossRef] [PubMed]
- Mohamed, M.F.A.; Abuo Rahma, G.E.A. Molecular targets and anticancer activity of quinoline-chalcone hybrids: Literature review. R. Soc. Chem. 2020, 10, 31139–31155. [Google Scholar] [CrossRef] [PubMed]
- Benavente Garcia, O.; Castillo, J.; Del Baño, M.; Lorente, J. Improved water solubility of neohesperidin dihydrochalcone in sweetener blends. J. Agric. Food Chem. 2001, 49, 189–191. [Google Scholar]
- Zhu, C.; Wang, R.; Zheng, W.; Chen, D.; Yue, X.; Cao, Y.; Qin, W.; Sun, H.; Wang, Y.; Liu, Z. Synthesis and evaluation of anticancer activity of BOC26P, an ortho-aryl chalcone sodium phosphate as water-soluble prodrugs in vitro and in vivo. Biomed. Pharmacother. 2017, 96, 551–562. [Google Scholar]
- Li, D.; Xia, Y. Electrospinning of nanofibers: Reinventing the wheel? Adv. Mater. 2004, 16, 1151–1170. [Google Scholar]
- Tan, R.; Yang, X.; Shen, Y. Robot-aided electrospinning toward intelligent biomedical engineering. Robot. Biomim. 2017, 4, 17. [Google Scholar]
- Mao, Y.; Shen, W.; Wu, S.; Ge, X.; Ao, F.; Ning, Y.; Luo, Y.; Liu, Z. Electrospun polymers: Using devices to enhance their potential for biomedical applications. React. Funct. Polym. 2023, 186, 105568. [Google Scholar] [CrossRef]
- Fu, H.; Wu, T.-H.; Ma, C.-P.; Yen, F.-L. Improving Water Solubility and Skin Penetration of Ursolic Acid through a Nanofiber Process to Achieve Better In Vitro Anti-Breast Cancer Activity. Pharmaceutics 2024, 16, 1147. [Google Scholar] [CrossRef]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Kaomongkolgit, R.; Opanasopit, P. Fast-acting clotrimazole composited PVP/HPβCD nanofibers for oral candidiasis application. Pharm. Res. 2014, 31, 1893–1906. [Google Scholar] [CrossRef]
- Tonglairoum, P.; Ngawhirunpat, T.; Rojanarata, T.; Panomsuk, S.; Kaomongkolgit, R.; Opanasopit, P. Fabrication of mucoadhesive chitosan coated polyvinylpyrrolidone/cyclodextrin/clotrimazole sandwich patches for oral candidiasis. Carbohydr. Polym. 2015, 132, 173–179. [Google Scholar] [CrossRef] [PubMed]
- Shin, S.; Ko, J.; Kim, M.; Song, N.; Park, K. Morin induces melanogenesis via activation of MAPK signaling pathways in B16F10 mouse melanoma cells. Molecules 2021, 26, 2150. [Google Scholar] [CrossRef] [PubMed]
- The SCCS Notes of Guidance for the testing of cosmetic ingredients and their safety evaluation, 11th revision, 30-31 March 2021, SCCS/1628/21. Regul. Toxicol. Pharmacol. 2021, 127, 105052. [CrossRef] [PubMed]
- Zi, X.; Simoneau, A.R. Flavokawain A, a novel chalcone from kava extract, induces apoptosis in bladder cancer cells by involvement of bax protein-dependent and mitochondria-dependent apoptotic pathway and suppresses tumor growth in mice. Cancer Res. 2005, 65, 3479–3486. [Google Scholar] [CrossRef]
- Cosmetics Europe. COLIPA Guidelines: Guidelines for Percutaneous Absorption/Penetration; Cosmetics Europe: Brussels, Belgium, 1997. [Google Scholar]
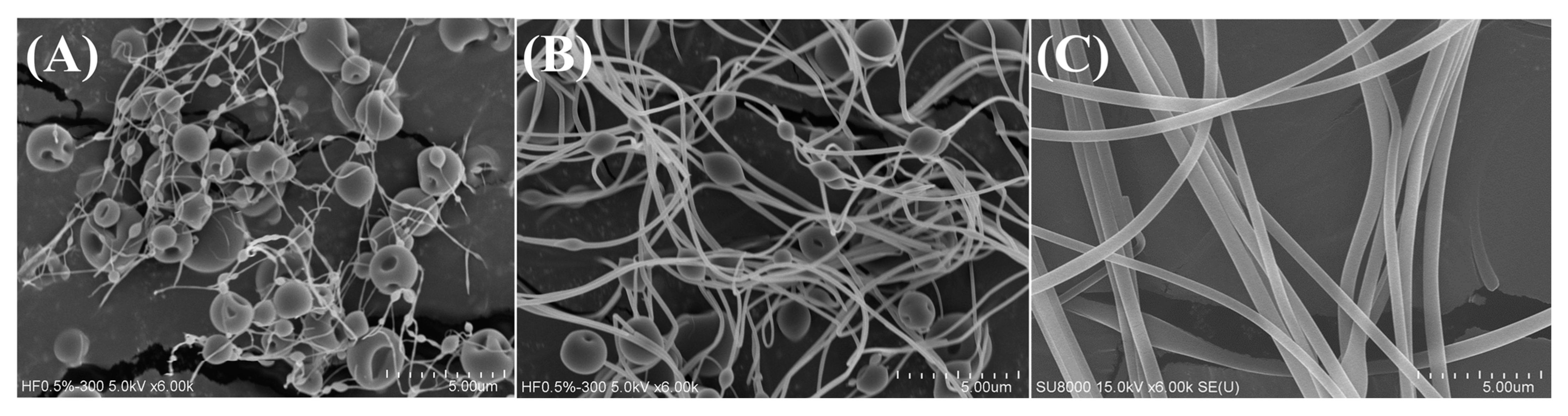
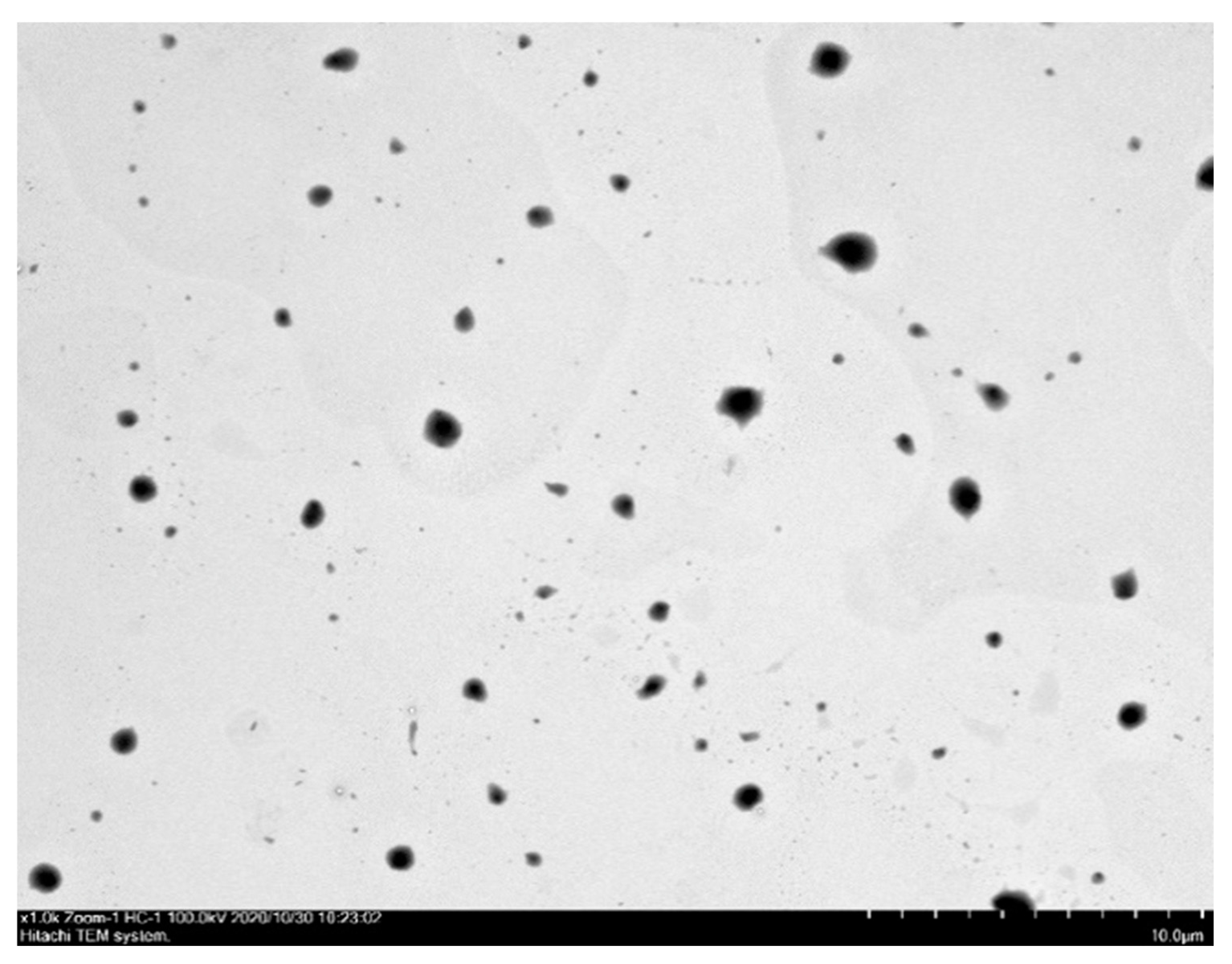
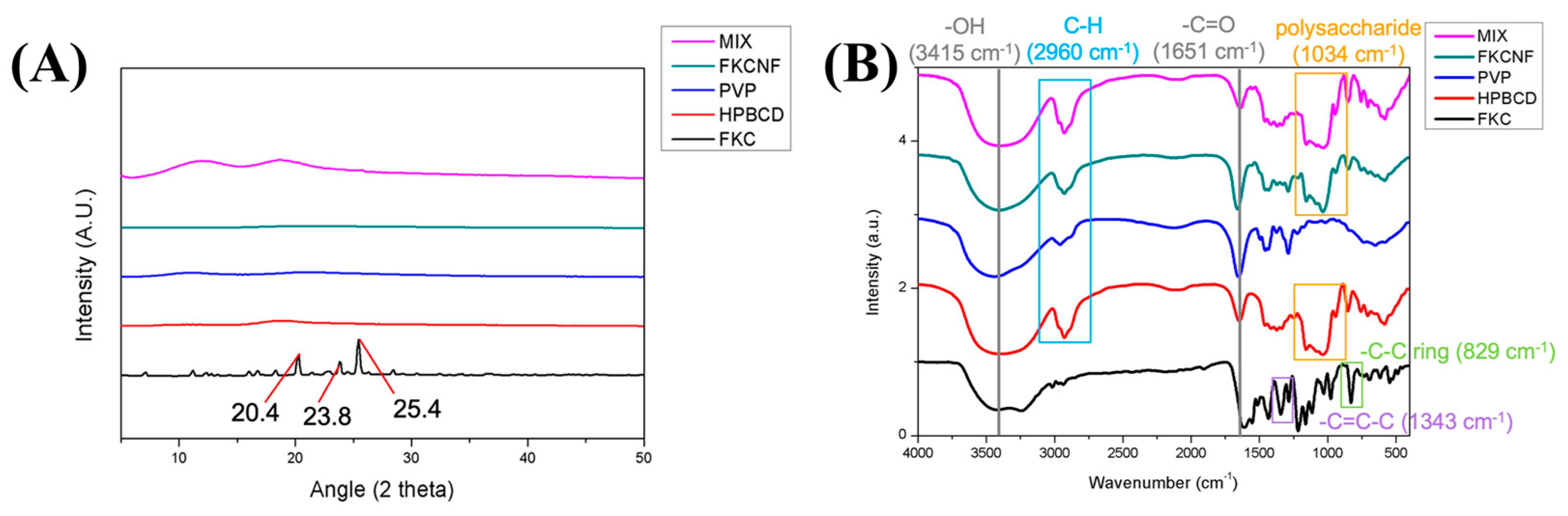
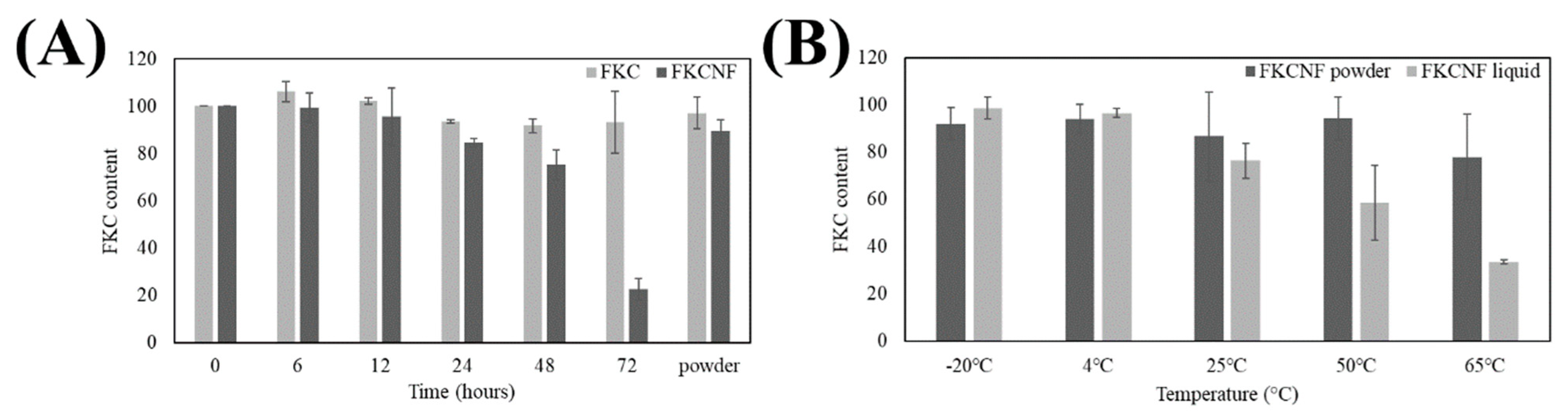
| Ratio (FKC:HPBCD:PVP) | Yield (%) | Solubility (μg/mL) |
|---|---|---|
| Raw FKC | – | <0.01 |
| 1:50:5 | 85.00 ± 0.80 | 402.74 ± 13.98 |
| 1:50:10 | 80.19 ± 0.51 | 436.39 ± 19.95 |
| 1:50:20 | 77.79 ± 0.63 | 515.32 ± 38.46 |
| Estimated daily exposure (face cream) | Eproduct = 24.14 mg/kg bw/day [35] |
| Concentration | C = 1.4% |
| Dermal absorption | DAp = 21.6% |
| Systemic exposure dose | SED = Eproduct × C × DAp = 0.07 |
| No observed adverse effect level (25-day, daily feeding) | NOAEL = 50 mg/kg bw/day |
| (90-day, oral) | Adjusted NOAEL = Dose × week × frequency = 13.89 mg/kg bw/day |
| Margin of safety | MOS = adjusted NOAEL/SED = 190.5 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
So, P.B.; Wang, Y.-C.; Huang, P.-H.; Wu, T.-H.; Yen, F.-L. Physicochemical Characterization, Skin Penetration, Anti-Melanogenesis and Safety Assessment of Flavokawain C Nanofibers. Int. J. Mol. Sci. 2025, 26, 2966. https://doi.org/10.3390/ijms26072966
So PB, Wang Y-C, Huang P-H, Wu T-H, Yen F-L. Physicochemical Characterization, Skin Penetration, Anti-Melanogenesis and Safety Assessment of Flavokawain C Nanofibers. International Journal of Molecular Sciences. 2025; 26(7):2966. https://doi.org/10.3390/ijms26072966
Chicago/Turabian StyleSo, Pamela Berilyn, Ying-Chu Wang, Pao-Hsien Huang, Tzu-Hui Wu, and Feng-Lin Yen. 2025. "Physicochemical Characterization, Skin Penetration, Anti-Melanogenesis and Safety Assessment of Flavokawain C Nanofibers" International Journal of Molecular Sciences 26, no. 7: 2966. https://doi.org/10.3390/ijms26072966
APA StyleSo, P. B., Wang, Y.-C., Huang, P.-H., Wu, T.-H., & Yen, F.-L. (2025). Physicochemical Characterization, Skin Penetration, Anti-Melanogenesis and Safety Assessment of Flavokawain C Nanofibers. International Journal of Molecular Sciences, 26(7), 2966. https://doi.org/10.3390/ijms26072966






