Mitigating Effect of Taurine Combined with Corona Dormancy on Oxidative Stress in Trachinotus ovatus Under Low-Temperature Stress
Abstract
1. Introduction
2. Results
2.1. Influence of Synergistic Anti-Stress Agents on Live Transportation
2.2. Influence of Corona Dormancy Combined with Taurine on the Serum Biochemical Indexes of Trachinotus ovatus
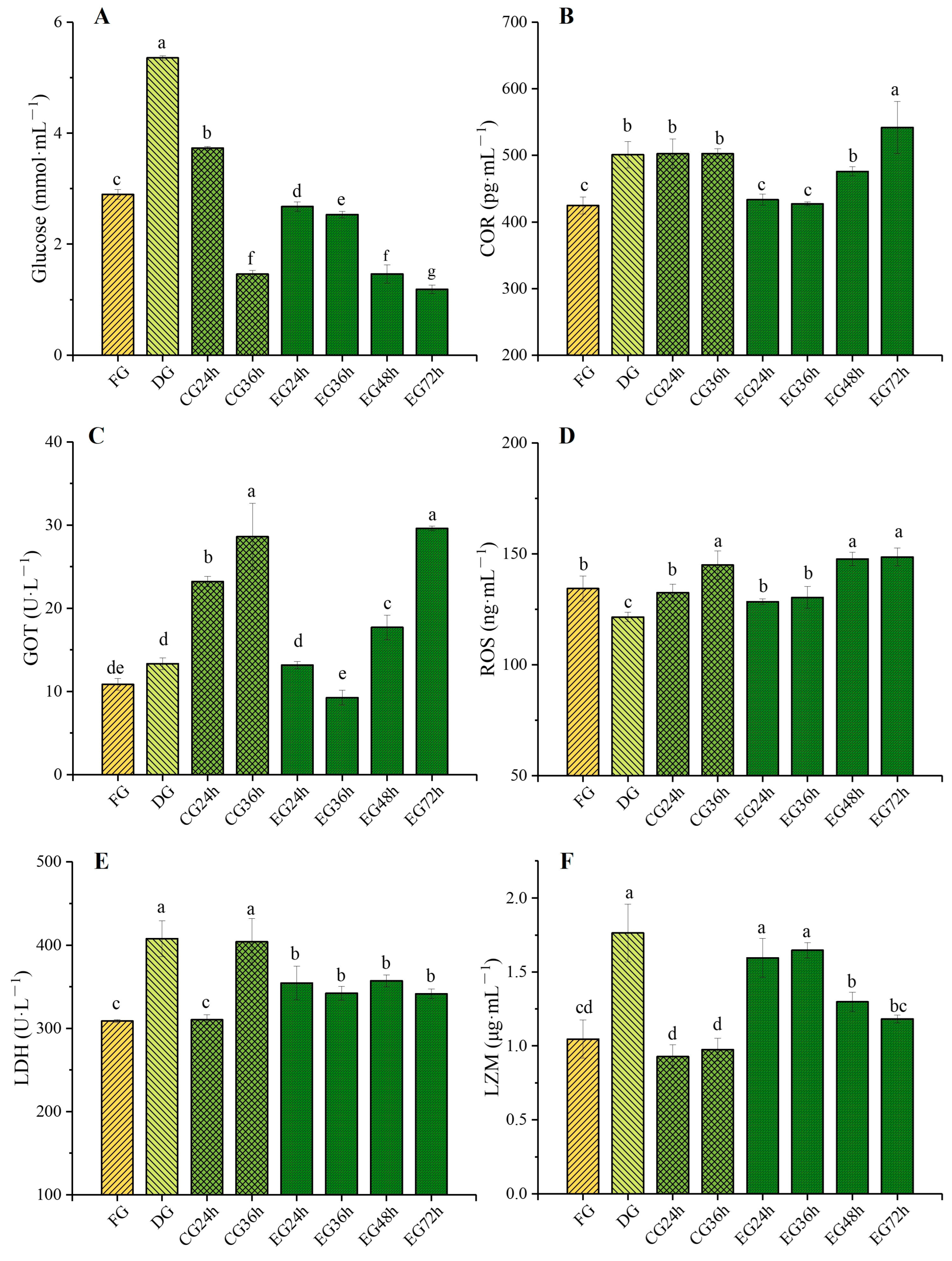
2.2.1. Glucose (GLU) and Cortisol (COR) Contents Changes
2.2.2. Glutamic Oxaloacetic Transaminase (GOT) and Reactive Oxygen Species (ROS) Contents Changes
2.2.3. Lactate Dehydrogenase (LDH) and Lysozyme (LZM) Contents Changes
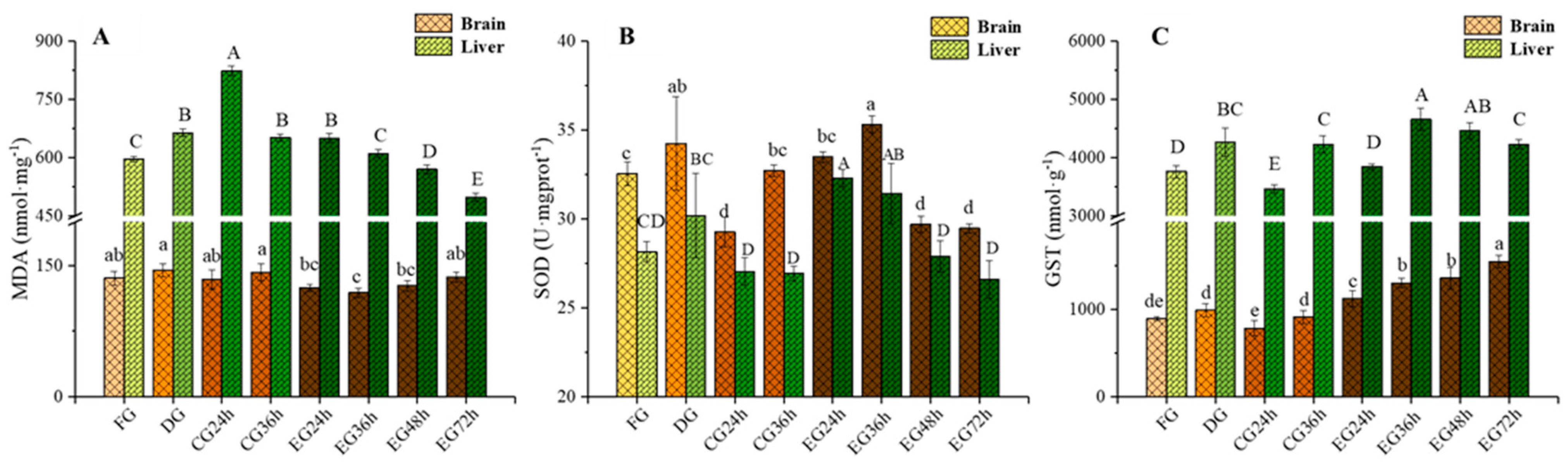
2.3. Influence of Corona Dormancy Combined with Taurine on Oxidative Stress Indexes (Malondialdehyde (MDA), Superoxide Dismutase (SOD), and Glutathione S-Transferase (GST)) in the Brain and Liver of Trachinotus ovatus
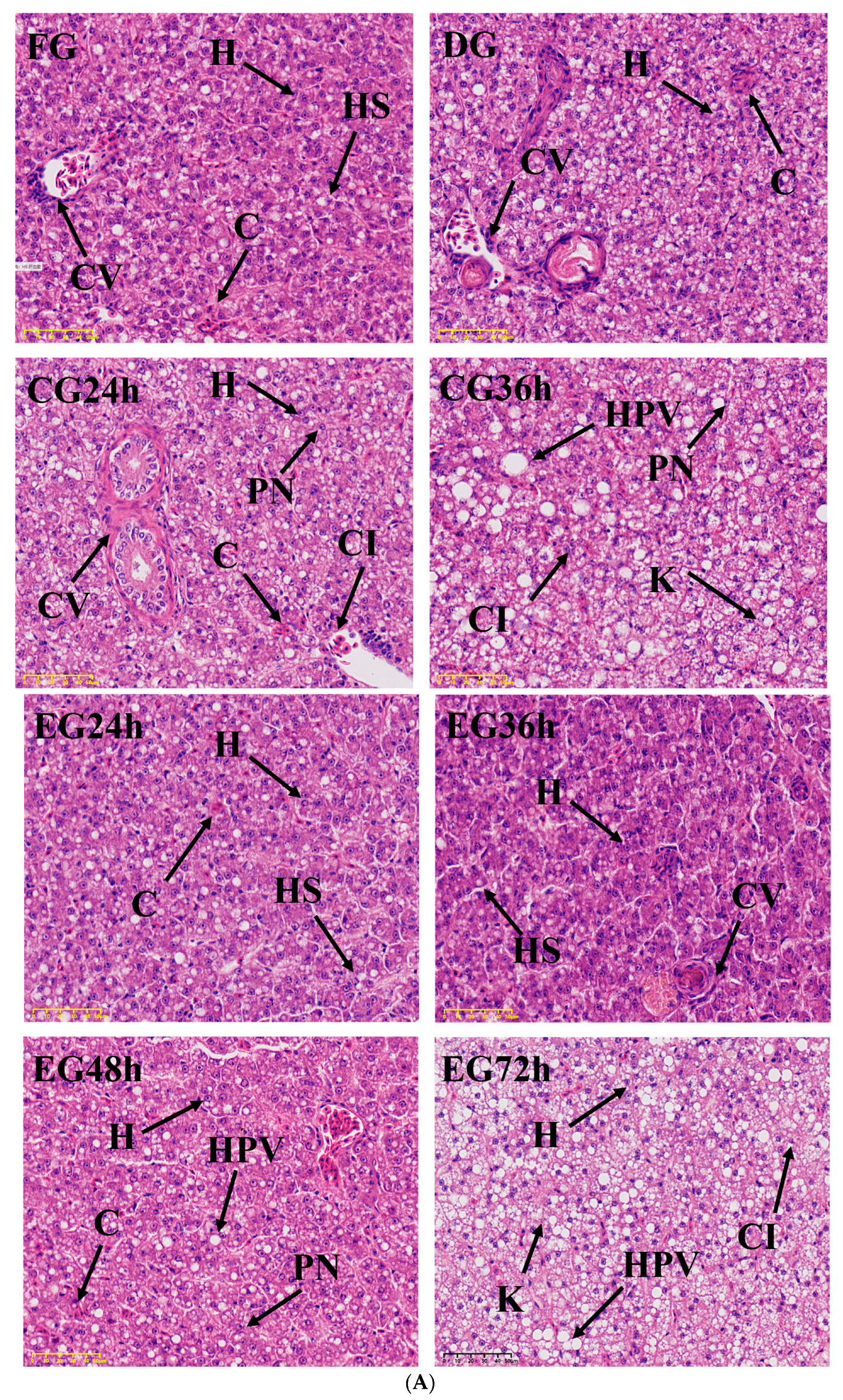
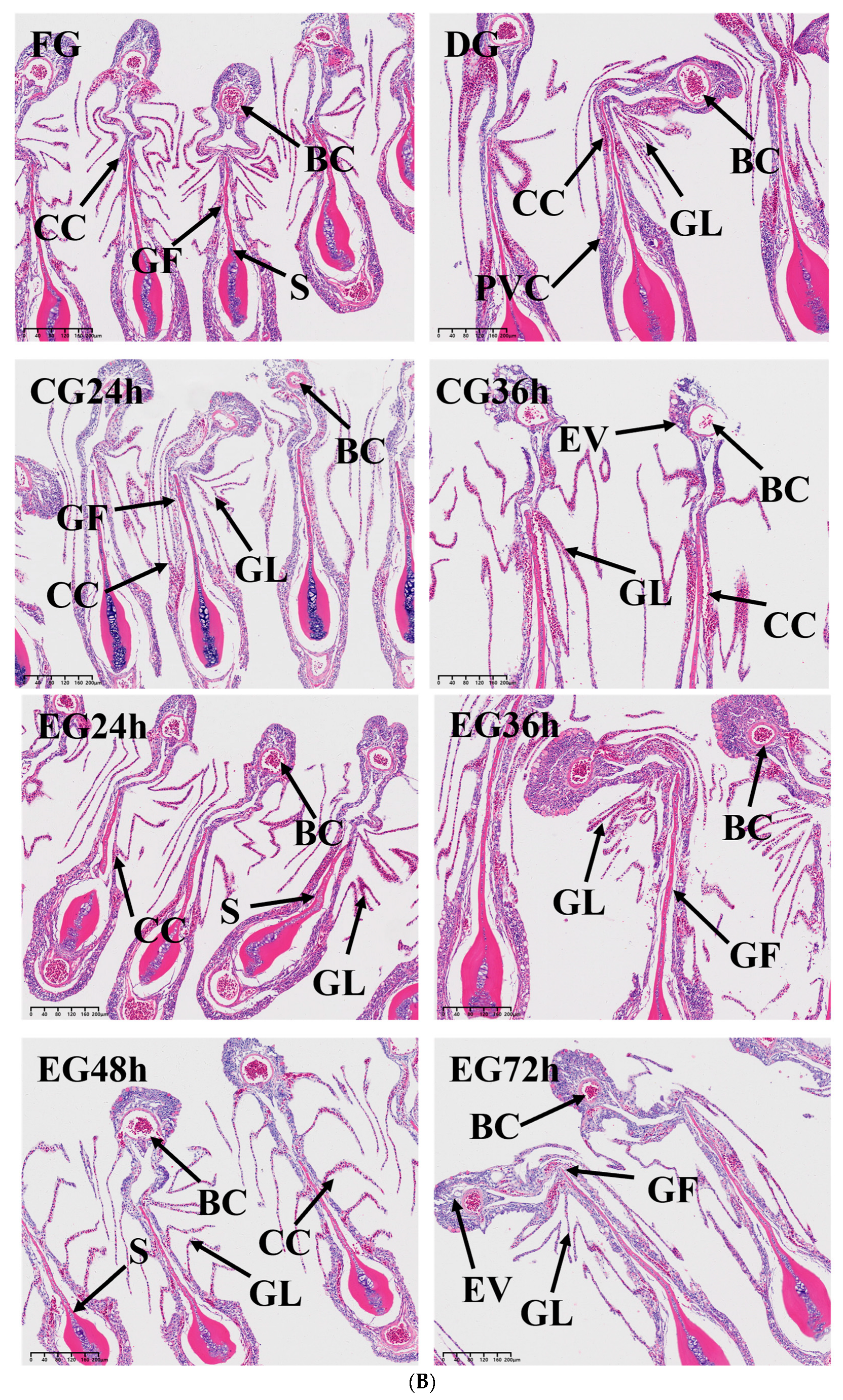
2.4. Histopathology Analysis
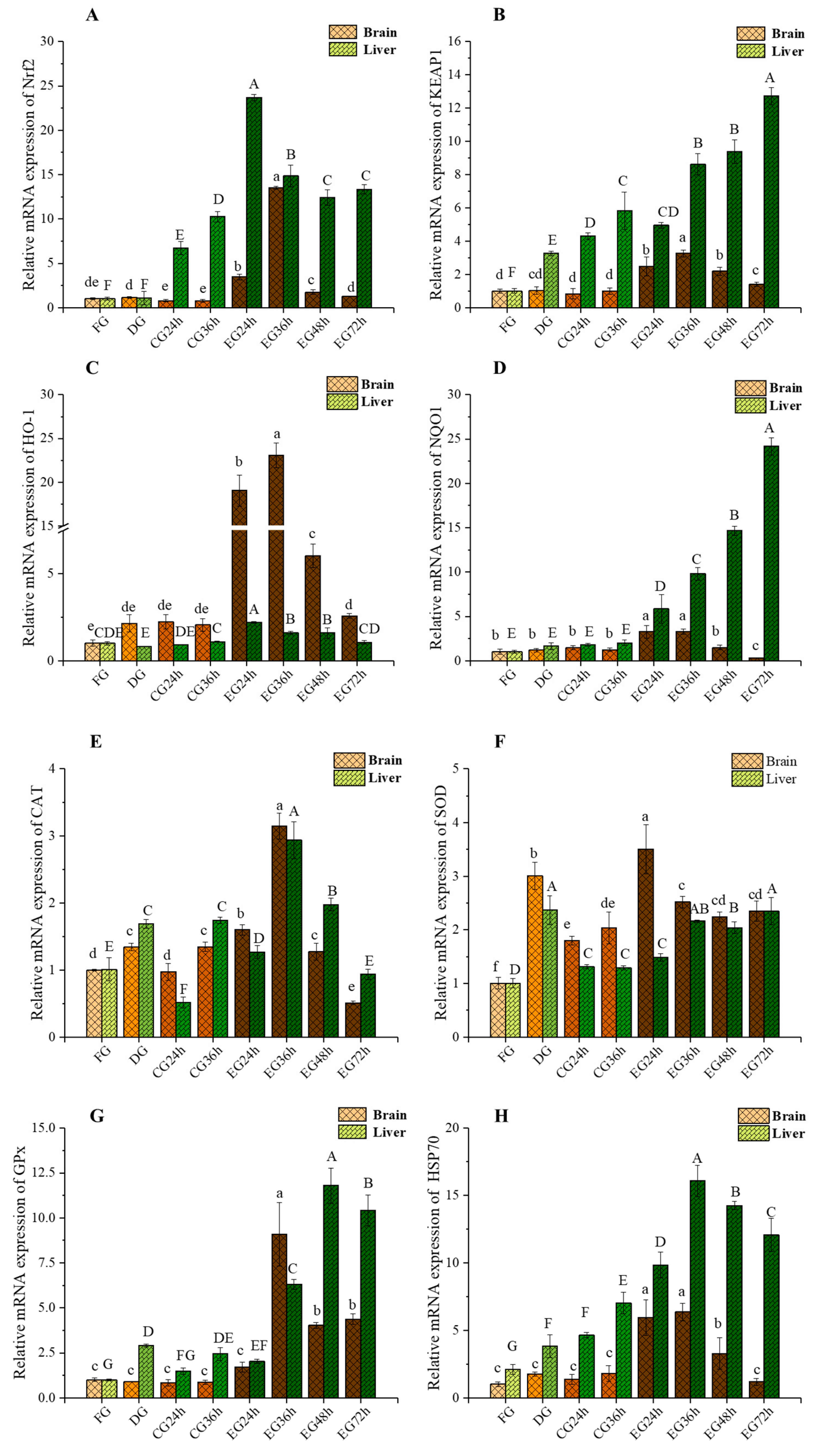
2.5. Impact of Corona Dormancy on the Nrf2-Keap1 Signaling Pathway and the Expression of Genes Related to Antioxidant Stress in the Brain and Liver of Trachinotus ovatus
2.5.1. mRNA Expression Levels of Nrf2 and Keap1 Changes
2.5.2. mRNA Expression Levels of Heme Oxygenase-1 (HO-1) and NAD(P)H: Quinone Oxidoreductase 1 (NQO1) Changes
2.5.3. mRNA Expression Levels of Catalase (CAT) and SOD Changes
2.5.4. mRNA Expression Levels of Glutathione Peroxidase (GPx) and Heat Shock Protein 70 (HSP70) Changes
3. Discussion
3.1. Effects of Different Anti-Stress Agents on the Live Transportation of Trachinotus ovatus
3.2. Effects of Synergistic Live Preservation on Serum Biochemical Indexes of Trachinotus ovatus
3.3. Effect of Synergistic Live Preservation on Oxidative Stress Indicators in the Brain and Liver of Trachinotus ovatus
3.4. Effect of Synergistic Live Preservation on the Liver and Gills of Trachinotus ovatus
3.5. Effect of Synergistic Live Preservation on the Nrf2-Keap1 Signaling Pathway and the Expression of Antioxidant Stress-Related Genes in Trachinotus ovatus
4. Materials and Methods
4.1. Materials and Chemicals
4.2. Temporary Rearing Treatment
4.3. Synergistic Live Transportation
4.4. Survival Rate and Survival Time of Trachinotus ovatus Under Different Anti-Stress Agents
4.5. Index Detection
4.5.1. Blood Sampling and Determination of Serum Biochemical Indexes
4.5.2. Liver and Brain Tissue Sampling and Determination of Oxidative Stress Indicators
4.5.3. Histopathology
4.5.4. Real-Time Quantitative PCR (qRT-PCR)
4.6. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Zhou, C.P.; Ge, X.P.; Niu, J.; Jin, H.Z.; Huang, Z.; Tan, X.H. Effect of dietary carbohydrate levels on growth performance, body composition, intestinal and hepatic enzyme activities, and growth hormone gene expression of juvenile golden pompano, Trachinotus ovatus. Aquaculture 2015, 437, 390–397. [Google Scholar] [CrossRef]
- Ke, C.L.; Liu, Q.; Li, L.; Chen, J.W.; Zhao, C.H.; Xu, J.P.; Huang, K.; Song, M.M.; Li, L.D. Residual levels and risk assessment of eugenol and its isomers in fish from China markets. Aquaculture 2018, 484, 338–342. [Google Scholar] [CrossRef]
- Zhang, Y.J.; Wang, W.S.; Yan, L.; Glamuzina, B.; Zhang, X.S. Development and evaluation of an intelligent traceability system for waterless live fish transportation. Food Control 2019, 95, 283–297. [Google Scholar] [CrossRef]
- Yang, Z.Y.; Li, S.; Chen, B.; Kang, H.Y.; Hang, M.H. China’s aquatic product processing industry: Policy evolution and economic performance. Trends Food Sci. Technol. 2016, 58, 149–154. [Google Scholar] [CrossRef]
- Fan, X.P.; Qin, X.M.; Zhang, C.H.; Zhu, Q.F.; Chen, J.P.; Chen, P.W. Metabolic and anti-oxidative stress responses to low temperatures during the waterless preservation of the hybrid grouper (Epinephelus fuscogutatus♀ × Epinephelus lanceolatus♂). Aquaculture 2019, 508, 10–18. [Google Scholar] [CrossRef]
- Refaey, M.M.; Li, D.; Tian, X.; Zhang, Z.M.; Zhang, X.; Li, L.; Tang, R. High stocking density alters growth performance, blood biochemistry, intestinal histology, and muscle quality of channel catfish Ictalurus punctatus. Aquaculture 2018, 492, 73–81. [Google Scholar] [CrossRef]
- Abram, Q.H.; Dixon, B.; Katzenback, B.A. Impacts of low temperature on the teleost immune system. Biology 2017, 6, 39. [Google Scholar] [CrossRef]
- Zeng, P.; Chen, T.; Shen, J. Effects of cold acclimation and storage temperature on crucian carp (Carassius auratus gibelio) in a waterless preservation. Fish Physiol. Biochem. 2014, 40, 973–982. [Google Scholar] [CrossRef]
- Cheng, C.H.; Guo, Z.X.; Wang, A.L. The protective effects of taurine on oxidative stress, cytoplasmic free-Ca2+ and apoptosis of pufferfish (Takifugu obscurus) under low temperature stress. Fish Shellfish Immunol. 2018, 77, 457–464. [Google Scholar] [CrossRef]
- Ventura, A.S.; Jerônimo, G.T.; de Oliveira, S.N.; de Araujo Gabriel, A.M.; Andrea, M.; Lima Cardoso, C.A.; Teodoro, G.C.; Corrêa Filho, R.A.C.; Aparecido Povh, J. Natural anesthetics in the transport of Nile tilapia: Hematological and biochemical responses and residual concentration in the fillet. Aquaculture 2020, 526, 735365. [Google Scholar] [CrossRef]
- Chakraborty, N.; De, A.; Ghosh, S.K.; De, T.K. Effect of direct current electroanaesthesia on Nile Tilapia (Oreochromis niloticus). Aquac. Eng. 2022, 99, 102286. [Google Scholar] [CrossRef]
- Faust, M.D.; Vandergoot, C.S.; Hostnik, E.T.; Binder, T.R.; Mida Hinderer, J.L.; Lves, J.T. Use of electricity to sedate Lake Trout for intracoelomic implantation of electronic transmitters. N. Am. J. Fish. Manag. 2017, 37, 768–777. [Google Scholar] [CrossRef]
- Bouwsema, J.A.; Ellis, M.A.; Lines, J.A.; Turnbull, J.F. In-water electric stunning as a humane commercial method for culling juvenile salmonids. Aquac. Eng. 2022, 99, 102286. [Google Scholar] [CrossRef]
- Liu, S.W.; Zhong, J.M.; Fan, X.P.; Qin, X.M.; Shen, J.; Xu, W.Q. The effect of corona dormancy on the physiological stress and main nutritional components in the transport of Trachinotus ovatus during survival. Prog. Fish. Sci. 2025, 46, 210–221. [Google Scholar] [CrossRef]
- Seidel, U.; Huebbe, P.; Rimbach, G. Taurine: A regulator of cellular redox homeostasis and skeletal muscle function. Mol. Nutr. Food Res. 2019, 63, e1800569. [Google Scholar] [CrossRef] [PubMed]
- Sun, B.J.; Maruta, H.; Ma, Y.; Yamashita, H. Taurine stimulates AMP-activated protein kinase and modulates the skeletal muscle functions in rats via the induction of intracellular calcium influx. Int. J. Mol. Sci. 2023, 24, 4125. [Google Scholar] [CrossRef] [PubMed]
- Salze, G.P.; Davis, D.A. Taurine: A critical nutrient for future fish feeds. Aquaculture 2015, 437, 215–229. [Google Scholar] [CrossRef]
- Che, Y.; Hou, L.; Sun, F.; Zhang, C.; Liu, X.F.; Piao, F.Y.; Zhang, D.; Li, H.H.; Wang, Q.S. Taurine protects dopaminergic neurons in a mouse Parkinson’s disease model through inhibition of microglial M1 polarization. Cell Death Dis. 2018, 9, 435. [Google Scholar] [CrossRef]
- Li, M.; Lai, H.; Li, Q.; Gong, S.Y.; Wang, R.X. Effects of dietary taurine on growth, immunity and hyperammonemia in juvenile yellow catfish Pelteobagrus fulvidraco fed all-plant protein diets. Aquaculture 2016, 450, 349–355. [Google Scholar] [CrossRef]
- Shi, Y.; Zhong, L.; Fan, Y.; Zhang, J.; Dai, J.; Zhong, H.; Fu, G.; Hu, Y. Taurine inhibits hydrogen peroxide-induced oxidative stress, inflammatory response and apoptosis in liver of Monopterus albus. Fish Shellfish Immunol. 2022, 128, 536–546. [Google Scholar] [CrossRef]
- Shi, Y.; Hu, Y.; Wang, Z.Q.; Zhou, J.C.; Zhang, J.Z.; Zhong, H.; Fu, G.H.; Zhong, L. The protective effect of taurine on oxidized fish-oil-induced liver oxidative stress and intestinal barrier-function impairment in juvenile Ictalurus punctatus. Antioxidants 2021, 10, 1690. [Google Scholar] [CrossRef]
- Zheng, P.L.; Ma, Z.H.; Guo, H.Y.; Li, Y.N.; Zhang, D.C.; Jiang, S.G. Ontogenetic development of caudal skeletons in Trachinotus ovatus larvae. South China Fish. Sci. 2014, 10, 45–50. [Google Scholar] [CrossRef]
- Wang, Q.H.; Wu, R.X.; Ji, J.N.; Zhang, J.; Niu, S.F.; Tang, B.G.; Miao, B.B.; Liang, Z.B. Integrated transcriptomics and metabolomics reveal changes in cell homeostasis and energy metabolism in Trachinotus ovatus in response to acute hypoxic stress. Int. J. Mol. Sci. 2024, 25, 1054. [Google Scholar] [CrossRef]
- Zhu, K.C.; Zhang, N.; Liu, B.S.; Guo, L.; Guo, H.Y.; Jiang, S.G.; Zhang, D.C. Transcription factor pparαb activates fads2s to promote LC-PUFA biosynthesis in the golden pompano Trachinotus ovatus (Linnaeus 1758). Int. J. Biol. Macromol. 2020, 161, 605–616. [Google Scholar] [CrossRef] [PubMed]
- Honrado, A.; Rubio, S.; Beltrán, J.A.; Calanche, J. Fish by-product valorization as source of bioactive compounds for food enrichment: Characterization, suitability and shelf life. Foods 2022, 11, 3656. [Google Scholar] [CrossRef] [PubMed]
- Pan, C.; Zhang, X.F.; Chen, S.; Xue, Y.; Wu, Y.Y.; Wang, Y.Q.; Wang, D. Analysis of quality-related proteins in golden pompano (Trachinotus ovatus) fillets with modified atmosphere packaging under superchilling storage. Food Sci. Hum. Wellness 2024, 13, 2253–2265. [Google Scholar] [CrossRef]
- Mohammadi Dehcheshmeh, A.; Khosravizadeh, M.; Mousavi, S.M.; Babadi, S.; Shiry, N. Electro-immobilisation and fish welfare: An investigation into stress, consciousness, and physiological aspects during slaughter. J. Appl. Anim. Welf. Sci. 2024, 28, 243–258. [Google Scholar] [CrossRef]
- Glover, C.N.; Bucking, C.; Wood, C.M. The skin of fish as a transport epithelium: A review. J. Comp. Physiol. B 2013, 183, 877–891. [Google Scholar] [CrossRef]
- Liu, B.L.; Jia, R.; Huang, B.; Lei, J.L. Interactive effect of ammonia and crowding stress on ion-regulation and expression of immune-related genes in juvenile turbot (Scophthalmus maximus). Mar. Freshw. Behav. Physiol. 2017, 50, 179–194. [Google Scholar] [CrossRef]
- Sadoul, B.; Geffroy, B. Measuring cortisol, the major stress hormone in fishes. J. Fish Biol. 2019, 94, 540–555. [Google Scholar] [CrossRef]
- Zhang, J.Z.; Che, C.B.; Cai, M.L.; Hu, Y. Taurine improves health of juvenile rice field eel (Monopterus albus) fed with oxidized fish oil: Involvement of lipid metabolism, antioxidant capacity, inflammatory response. Aquac. Rep. 2022, 27, 101388. [Google Scholar] [CrossRef]
- Tossetta, G.; Fantone, S.; Togni, L.; Santarelli, A.; Olivieri, F.; Marzioni, D.; Rippo, M.R. Modulation of nrf2/keap1 signaling by phytotherapeutics in periodontitis. Antioxidants 2024, 13, 1270. [Google Scholar] [CrossRef]
- Niu, X.; Zheng, S.; Liu, H.T.; Li, S. Protective effects of taurine against inflammation, apoptosis, and oxidative stress in brain injury. Mol. Med. Rep. 2018, 18, 4516–4522. [Google Scholar] [CrossRef]
- Bañuelos-Vargas, I.; López, L.M.; Pérez-Jiménez, A.; Peres, H. Effect of fishmeal replacement by soy protein concentrate with taurine supplementation on hepatic intermediary metabolism and antioxidant status of totoaba juveniles (Totoaba macdonaldi). Comp. Biochem. Phys. B 2014, 170, 18–25. [Google Scholar] [CrossRef] [PubMed]
- Almeida-Val, V.M.F.; Oliveira, A.R.; Silva, M.N.P.; Ferreira-Nozawa, M.S.; Araújo, R.M.; Val, A.L.; Nozawa, S.R. Anoxia-and hypoxia-induced expression of LDH-A* in the Amazon Oscar, Astronotus crassipinis. Genet. Mol. Biol. 2011, 34, 315–322. [Google Scholar] [CrossRef] [PubMed]
- Yang, S.; Wu, H.; He, K.; Yan, T.; Zhou, J.; Zhao, L.L.; Sun, J.L.; Lian, W.Q.; Zhang, D.M.; Du, Z.J.; et al. Response of AMP-activated protein kinase and lactate metabolism of largemouth bass (Micropterus salmoides) under acute hypoxic stress. Sci. Total Environ. 2019, 666, 1071–1079. [Google Scholar] [CrossRef]
- Hoseini, S.M.; Yousefi, M.; Abbasi, M.; Kulikov, E.V.; Drukovsky, S.G.; Petrov, A.K.; Krotova, E.A.; Hoseinifar, S.H.; Doan, H.V. Improvement of growth performance, hepatic and erythrocyte antioxidant capacity, innate immunity, and biochemical parameters of Persian sturgeon, Acipenser persicus, by sulfur amino acids’ supplementation. Aquac. Nutr. 2022, 1, 2025855. [Google Scholar] [CrossRef]
- Liu, J.X.; Guo, H.Y.; Zhu, K.C.; Liu, B.S.; Zhang, N.; Zhang, D.C. Effects of exogenous taurine supplementation on the growth, antioxidant capacity, intestine immunity, and resistance against Streptococcus agalactiae in juvenile golden pompano (Trachinotus ovatus) fed with a low-fishmeal diet. Front. Immunol. 2022, 13, 1036821. [Google Scholar] [CrossRef]
- Yang, H.J.; Tian, L.X.; Huang, J.W.; Liang, G.Y.; Liu, Y.J. Dietary taurine can improve the hypoxia-tolerance but not the growth performance in juvenile grass carp Ctenopharyngodon idellus. Fish Physiol. Biochem. 2013, 39, 1071–1078. [Google Scholar] [CrossRef] [PubMed]
- Qiang, J.; Zhang, Z.; Yu, J.; Xu, J.; Liu, H.; Zhang, Z.Y.; Xu, P. Water quality and physiological response of F1 hybrid seabream (Pagrus major♀× Acanthopagrus schlegelii♂) to transport stress at different densities. Aquac. Res. 2018, 49, 767–775. [Google Scholar] [CrossRef]
- Chang, C.Y.; Shen, C.Y.; Kang, C.K.; Sher, Y.P.; Sheu, W.H.; Lee, T.H. Taurine protects HK-2 cells from oxidized LDL-induced cytotoxicity via the ROS-mediated mitochondrial and p53-related apoptotic pathways. Toxicol. Appl. Pharm. 2014, 279, 351–363. [Google Scholar] [CrossRef]
- Islam, M.N.; Rauf, A.; Fahad, F.I.; Emran, T.B.; Mitra, S.; Olatunde, A.; Shariati, M.A.; Rebezov, M.; Rengasamy, K.R.; Mubarak, M.S. Superoxide dismutase: An updated review on its health benefits and industrial applications. Crit. Rev. Food. Sci. 2022, 62, 7282–7300. [Google Scholar] [CrossRef] [PubMed]
- Shao, X.; Zhang, M.; Chen, Y.; Sun, S.Y.; Yang, S.C.; Li, Q.S. Exosome-mediated delivery of superoxide dismutase for anti-aging studies in Caenorhabditis elegans. Int. J. Pharm. 2023, 641, 123090. [Google Scholar] [CrossRef] [PubMed]
- Olsvik, P.A.; Kristensen, T.; Waagbø, R.; Rosseland, B.O.; Tollefsen, K.-E.; Baeverfjord, G.; Berntssen, M.H.G. mRNA expression of antioxidant enzymes (SOD, CAT and GSH-Px) and lipid peroxidative stress in liver of Atlantic salmon (Salmo salar) exposed to hyperoxic water during smoltification. Comp. Biochem. Phys. C 2005, 141, 314–323. [Google Scholar] [CrossRef]
- Almeida, J.A.; Diniz, Y.S.; Marques, S.F.G.; Faine, L.A.; Ribas, B.O.; Burneiko, R.C.; Novelli, E.L.B. The use of the oxidative stress responses as biomarkers in Nile tilapia (Oreochromis niloticus) exposed to in vivo cadmium contamination. Environ. Int. 2002, 27, 673–679. [Google Scholar] [CrossRef]
- Sevcikova, M.; Modrá, H.; Slaninova, A.; Svobodova, Z. Metals as a cause of oxidative stress in fish: A review. Vet. Med. 2011, 56, 537–546. [Google Scholar]
- Mohamed, A.A.R.; El-Houseiny, W.; Abd Elhakeem, E.L.M.; Ebraheim, L.L.M.; Ahmed, A.I.; El-Hakim, Y.M. Effect of hexavalent chromium exposure on the liver and kidney tissues related to the expression of CYP450 and GST genes of Oreochromis niloticus fish: Role of curcumin supplemented diet. Ecotoxicol. Environ. Saf. 2020, 188, 109890. [Google Scholar] [CrossRef]
- Pretto, A.; Loro, V.L.; Baldisserotto, B.; Pavanato, M.A.; Moraes, B.S. Effects of water cadmium concentrations on bioaccumulation and various oxidative stress parameters in Rhamdia quelen. Arch. Environ. Contam. Toxicol. 2011, 60, 309–318. [Google Scholar] [CrossRef]
- Bruslé, J.; Anadon, G.G. The Structure and Function of Fish Liver//Fish Morphology; Routledge: London, UK, 2017; pp. 77–93. [Google Scholar]
- Henry, R.P.; Lucu, Č.; Onken, H.; Weihrauch, D. Multiple functions of the crustacean gill: Osmotic/ionic regulation, acid-base balance, ammonia excretion, and bioaccumulation of toxic metals. Front. Physiol. 2012, 3, 431. [Google Scholar] [CrossRef]
- Velmurugan, B.; Selvanayagam, M.; Cengiz, E.I.; Unlu, E. Histopathological changes in the gill and liver tissues of freshwater fish, Cirrhinus mrigala exposed to dichlorvos. Braz. Arch. Biol. Technol. 2009, 52, 1291–1296. [Google Scholar] [CrossRef]
- Ma, S.; Luo, S.; Zhang, K.; Liu, Y.X.; Wei, X.Z.; Cheng, J.H.; BI, Y.H.; Sun, Y.R.; Zhang, X.Y.; Yin, S.W.; et al. Mitigation of low temperature stress by increased salinity is associated with multiple physiological responses in the gills of Takifugu fasciatus. Mar. Biol. 2022, 169, 141. [Google Scholar] [CrossRef]
- Li, H.H.; Liao, T.; Bai, C.; Qiu, L.; Chu, X.Y.; Li, H.L.; Chen, L.P.; Xiong, G.Q.; Wang, J.G. Effects of Compound Additive on Biochemical Parameters and Tissue Structure of Simulated Transport of Juvenile Ictalurus punctatus. J. Guandong Ocean Univ. 2023, 43, 47–55. [Google Scholar] [CrossRef]
- Navarro, E.; Esteras, N. Multitarget Effects of Nrf2 Signalling in the Brain: Common and Specific Functions in Different Cell Types. Antioxidants 2024, 13, 1502. [Google Scholar] [CrossRef] [PubMed]
- Kaspar, J.W.; Niture, S.K.; Jaiswal, A.K. Nrf2: INrf2 (Keap1) signaling in oxidative stress. Free Radic. Biol. Med. 2009, 47, 1304–1309. [Google Scholar] [CrossRef]
- Fan, J.; Li, B.B.; Hong, Q.; Yan, Z.Y.; Yang, X.J.; Lu, K.C.; Chen, G.L.; Wang, L.; Chen, Y.H. A glutathione peroxidase gene from Litopenaeus vannamei is involved in oxidative stress responses and pathogen infection resistance. Int. J. Mol. Sci. 2022, 23, 567. [Google Scholar] [CrossRef]
- Verlecar, X.N.; Jena, K.B.; Chainy, G.B.N. Biochemical markers of oxidative stress in Perna viridis exposed to mercury and temperature. Chem.-Biol. Interact. 2007, 167, 219–226. [Google Scholar] [CrossRef] [PubMed]
- Azad, P.; Ryu, J.; Haddad, G.G. Distinct role of Hsp70 in Drosophila hemocytes during severe hypoxia. Free Radic. Biol. Med. 2011, 51, 530–538. [Google Scholar] [CrossRef]
- Sharma, J.G.; Singh, S.P.; Chakrabarti, R. Effect of temperature on digestive physiology, immune-modulatory parameters, and expression level of Hsp and LDH genes in Catla catla (Hamilton, 1822). Aquaculture 2017, 479, 134–141. [Google Scholar] [CrossRef]
- Setyarani, M.; Zinellu, A.; Carru, C.; Zulli, A. High dietary taurine inhibits myocardial apoptosis during an atherogenic diet: Association with increased myocardial HSP70 and HSF-1 but not caspase 3. Eur. J. Nutr. 2014, 53, 929–937. [Google Scholar] [CrossRef]
- Wan, J.J.; Ge, X.; Liu, B.; Xie, J.; Cui, S.; Zhou, M.; Xia, S.; Cheng, R. Effect of dietary vitamin C on non-specific immunity and mRNA expression of three heat shock proteins (HSPs) in juvenile Megalobrama amblycephala under pH stress. Aquaculture 2014, 434, 325–333. [Google Scholar] [CrossRef]
- Copple, I.M.; Goldring, C.E.; Kitteringham, N.R.; Park, B.K. The Nrf2-Keap1 defence pathway: Role in protection against drug-induced toxicity. Toxicology 2008, 246, 24–33. [Google Scholar] [CrossRef] [PubMed]
- Niture, S.K.; Kaspar, J.W.; Shen, J.; Jaiswal, A.K. Nrf2 signaling and cell survival. Toxicol. Appl. Pharmacol. 2010, 244, 37–42. [Google Scholar] [CrossRef] [PubMed]
- Valipour, J.; Taghizadeh, F.; Esfahani, R.; Ramesh, M.; Rastegar, T. Role of nuclear factor erythroid 2-related factor 2 (Nrf2) in female and male fertility. Heliyon 2024, 10, e29752. [Google Scholar] [CrossRef] [PubMed]
- Fan, X.P.; Qin, X.M.; Liu, S.C. A Method for the Live Transport of Golden Pomfret. China CN202111285195, 5, 24 February 2023. [Google Scholar]
- Li, Y.N. Study on Alleviation of Transport Stress in Cyprinus carpio by Adding Vitamin C to Water; Jiangnan University: Wuxi, China, 2016. [Google Scholar]
- Du, H. Study on Artificial Induced Dormancy Combined with Ecological Ice Temperature Keeping Alive Transportation Technology of Golden Pomfret; Guangdong Ocean University: Zhanjiang, China, 2022. [Google Scholar] [CrossRef]
- Qi, X. Effects of Density Stress on Fry of Yellow Catfish (Pelteobagrus fulvidraco) and Stress Mitigation Study of Additive-Assisted Transport; Huazhong Agricultural University: Wuhan, China, 2023. [Google Scholar]
- Li, R.X.; Amenyogbe, E.; Lu, Y.; Jin, J.H.; Xie, R.T.; Huang, J.S. Effects of low-temperature stress on intestinal structure, enzyme activities and metabolomic analysis of juvenile golden pompano (Trachinotus ovatus). Front. Mar. Sci. 2023, 10, 1114120. [Google Scholar] [CrossRef]
- Liu, M.J.; Guo, H.Y.; Liu, B.; Zhu, K.C.; Go, L.; Liu, B.S.; Zhang, N.; Yang, J.W.; Jiang, S.G.; Zhang, D.C. Gill oxidative damage caused by acute ammonia stress was reduced through the HIF-1α/NF-κb signaling pathway in golden pompano (Trachinotus ovatus). Ecotoxicol. Environ. Saf. 2021, 222, 112504. [Google Scholar] [CrossRef]
- Tan, X.H.; Sun, Z.; Huang, Z.; Zhou, C.; Lin, H.Z.; Tian, L.J.; Xun, P.W.; Huang, Q. Effects of dietary hawthorn extract on growth performance, immune responses, growth-and immune-related genes expression of juvenile golden pompano (Trachinotus ovatus) and its susceptibility to Vibrio harveyi infection. Fish Shellfish Immunol. 2017, 70, 656–664. [Google Scholar] [CrossRef]
- Liu, M.J.; Guo, H.Y.; Zhu, K.C.; Liu, B.S.; Liu, B.; Gou, L.; Zhang, N.; Yang, J.W.; Jiang, S.G.; Zhang, D.C. Effects of acute ammonia exposure and recovery on the antioxidant response and expression of genes in the Nrf2-Keap1 signaling pathway in the juvenile golden pompano (Trachinotus ovatus). Aquat. Toxicol. 2021, 240, 105969. [Google Scholar] [CrossRef] [PubMed]
- Xie, J.; He, X.; Fang, H.H.; Liao, S.Y.; Liu, Y.J.; Tian, L.X.; Niu, J. Identification of heme oxygenase-1 from golden pompano (Trachinotus ovatus) and response of Nrf2/HO-1 signaling pathway to copper-induced oxidative stress. Chemosphere 2020, 253, 126654. [Google Scholar] [CrossRef] [PubMed]

| Group | Concentration | pH | Keep Alive (h) | 24 h Survival Rate | 36 h Survival Rate | 48 h Survival Rate | 72 h Survival Rate | |
|---|---|---|---|---|---|---|---|---|
| 1 | CG | 0 | 7.3 | 36.4 ± 3.5 f | 87.50% | 37.50% | 0% | 0% |
| 2 | DG | 0 mg/L | 7.3 | 47.3 ± 2.4 de | 100% | 75% | 25.00% | 0% |
| 3 | VC | 10 mg/L | 6.9 | 55.7 ± 6.7 bc | 100% | 100% | 62.50% | 0% |
| 4 | 25 mg/L | 6.5 | 58.3 ± 8.1 bc | 100% | 100% | 62.50% | 0% | |
| 5 | 40 mg/L | 6.2 | 60.1 ± 5.5 b | 100% | 100% | 62.50% | 0% | |
| 6 | ASP | 25 mg/L | 7.3 | 56.5 ± 8.4 bc | 100% | 100% | 50% | 0% |
| 7 | 50 mg/L | 7.3 | 60.3 ± 7.1 ab | 100% | 100% | 62.50% | 0% | |
| 8 | 75 mg/L | 7.1 | 43.4 ± 3.7 ef | 100% | 75% | 0% | 0% | |
| 9 | Taurine | 20 mg/L | 7.3 | 61.4 ± 8.9 ab | 100% | 100% | 50% | 0% |
| 10 | 70 mg/L | 7.2 | 67.7 ± 10.0 a | 100% | 100% | 100% | 50% | |
| 11 | 120 mg/L | 7.2 | 56.2 ± 9.7 bc | 100% | 100% | 62.5% | 0% | |
| 12 | Thyme oil | 5 mg/L | 7.3 | 56.4 ± 6.2 bc | 100% | 100% | 50% | 0% |
| 13 | 10 mg/L | 7.3 | 52.3 ± 2.5 cd | 100% | 100% | 62.50% | 0% | |
| 14 | 15 mg/L | 7.4 | 44.4 ± 6.4 e | 100% | 50% | 37.50% | 0% | |
| 15 | Clove oil | 5 mg/L | 7.3 | 44.3 ± 2.5 e | 100% | 87.50% | 0% | 0% |
| 16 | 10 mg/L | 7.3 | 45.7 ± 2.7 de | 100% | 87.50% | 25% | 0% | |
| 17 | 15 mg/L | 7.2 | 44.5 ± 2.0 e | 100% | 87.50% | 0% | 0% | |
| Primer | Primer Sequence (5′-3′) | Source |
|---|---|---|
| CAT-F | GGATGGACAGCCTTCAAGTTCTCG | Liu et al. (2021) [70] |
| CAT-R | TGGACCGTTACAACAGTGCAGATG | |
| SOD-F | CCTCATCCCCCTGCTTGGTA | Liu et al. (2021) [70] |
| SOD-R | CCAGGGAGGGATGAGAGGTG | |
| GSH-PX-F | GCTGAGAGGCTGGTGCAAGTG | Liu et al. (2021) [70] |
| GSH-PX-R | TTCAAGCGTTACAGCAGGAGGTTC | |
| HSP70-F | TTGAGGAGGCTGCGCACAGCTTGTG | Tan et al. (2017) [71] |
| HSP70-R | ACGTCCAGCAGCAGCAGGTCCT | |
| Nrf2-F | TTGCCTGGACACAACTGCTGTTAC | Liu et al. (2021) [72] |
| Nrf2-R | TCTGTGACGGTGGCAGTGGAC | |
| HO-1-F | AGAAGATTCAGACAGCAGCAGAACAG | Xie et al. (2020) [73] |
| HO-1-R | TCATACAGCGAGCACAGGAGGAG | |
| Keap-1-F | CAGATAGACAGCGTGGTGAAGGC | Liu et al. (2021) [72] |
| Keap-1-R | GACAGTGAGACAGGTTGAAGAACTCC | |
| NQO1-F | TGGTCCAGGTGTCACGTCTTCC | Xie et al. (2020) [73] |
| NQO1-R | GACTTGGCGTGTAGTGCTTGG | |
| β-Actin-F | TACGAGCTGCCTGACGGACA | Xie et al. (2020) [73] |
| β-Actin-R | GGCTGTGATCTCCTTCTGCA |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, S.; Zhong, K.; Zhong, J.; Fan, X.; Qin, X. Mitigating Effect of Taurine Combined with Corona Dormancy on Oxidative Stress in Trachinotus ovatus Under Low-Temperature Stress. Int. J. Mol. Sci. 2025, 26, 2927. https://doi.org/10.3390/ijms26072927
Liu S, Zhong K, Zhong J, Fan X, Qin X. Mitigating Effect of Taurine Combined with Corona Dormancy on Oxidative Stress in Trachinotus ovatus Under Low-Temperature Stress. International Journal of Molecular Sciences. 2025; 26(7):2927. https://doi.org/10.3390/ijms26072927
Chicago/Turabian StyleLiu, Siwei, Kaicui Zhong, Jiamei Zhong, Xiuping Fan, and Xiaoming Qin. 2025. "Mitigating Effect of Taurine Combined with Corona Dormancy on Oxidative Stress in Trachinotus ovatus Under Low-Temperature Stress" International Journal of Molecular Sciences 26, no. 7: 2927. https://doi.org/10.3390/ijms26072927
APA StyleLiu, S., Zhong, K., Zhong, J., Fan, X., & Qin, X. (2025). Mitigating Effect of Taurine Combined with Corona Dormancy on Oxidative Stress in Trachinotus ovatus Under Low-Temperature Stress. International Journal of Molecular Sciences, 26(7), 2927. https://doi.org/10.3390/ijms26072927





