A Systematic Review on the Molecular Mechanisms of Resveratrol in Protecting Against Osteoporosis
Abstract
1. Introduction
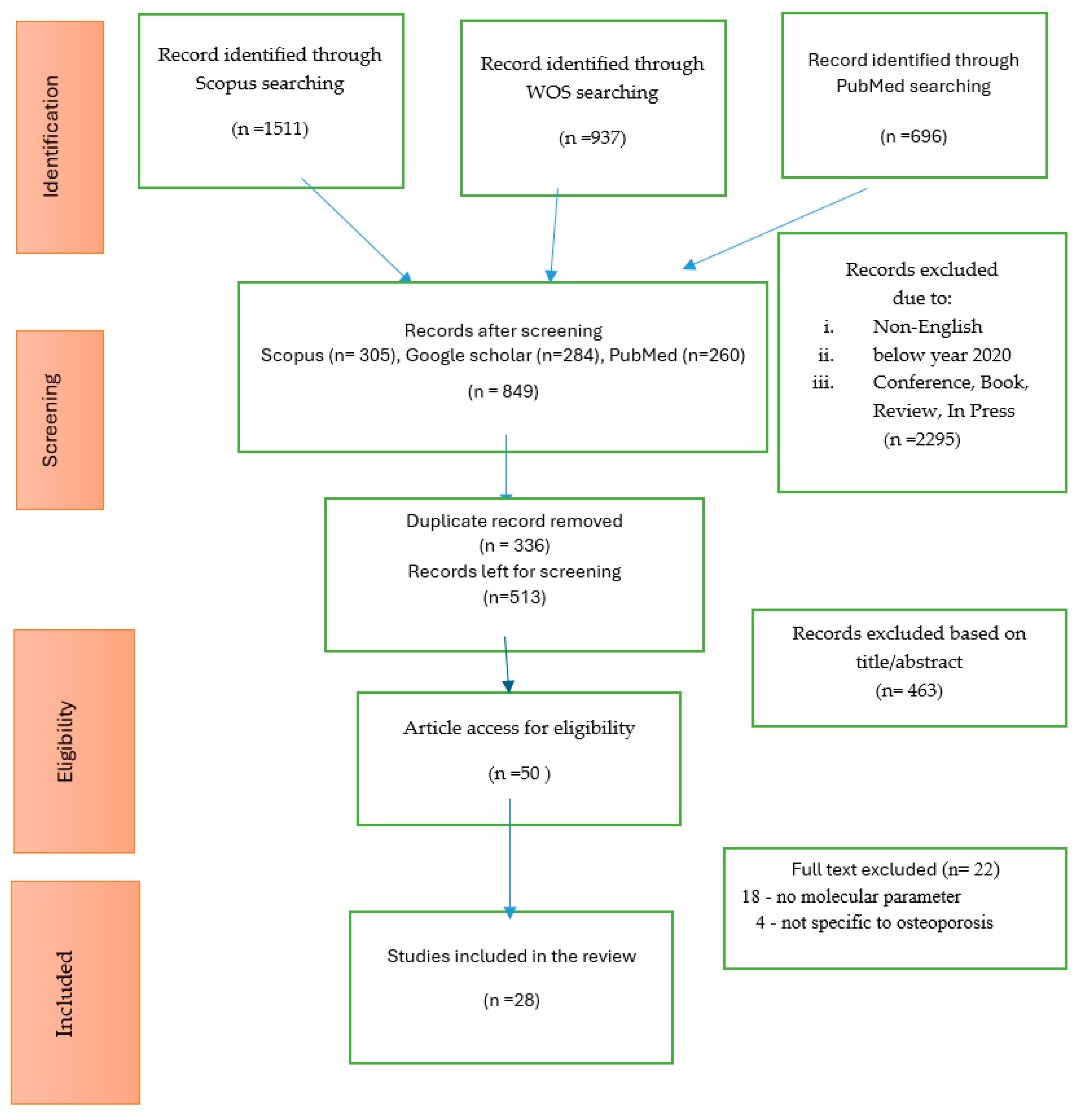
2. Methods
2.1. Search Strategy
- Peer-reviewed journal articles;
- Studies on resveratrol’s effects on bone that assess molecular parameters to determine its mechanisms in protecting against osteoporosis.
- Articles not published in English due to limited resources for translation;
- Reviews or meta-analyses without primary data;
- Letters, editorials, or case studies;
- Studies where resveratrol was combined with other agents or special carriers, making it difficult to determine its independent contribution to the mechanism;
- Studies that did not measure molecular parameters.
2.2. Selection Criteria
2.3. Data Extraction
2.4. Quality Assessment
2.5. Data Synthesis
2.6. Handling Missing Data
2.7. Ethical Considerations
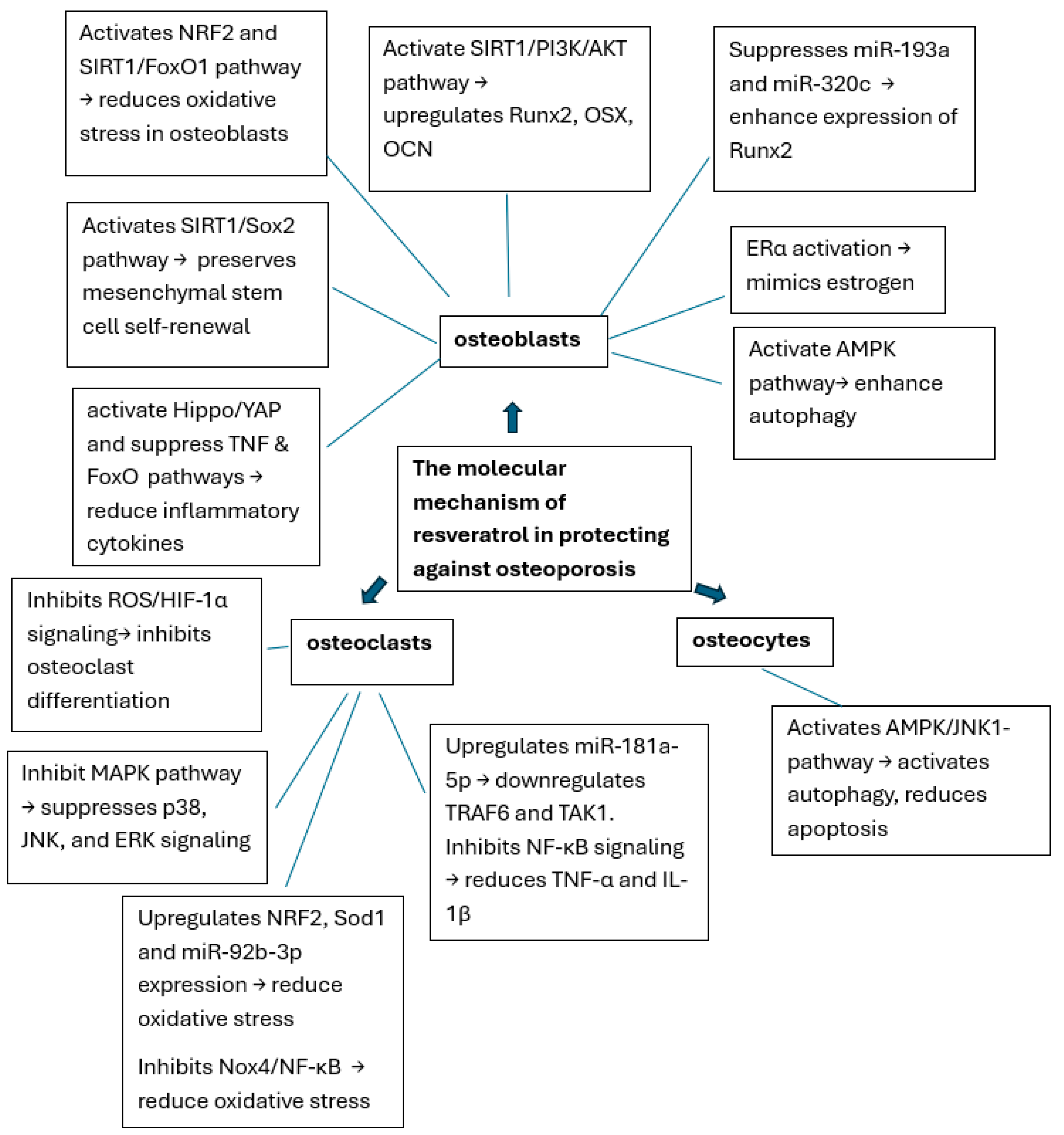
3. Results
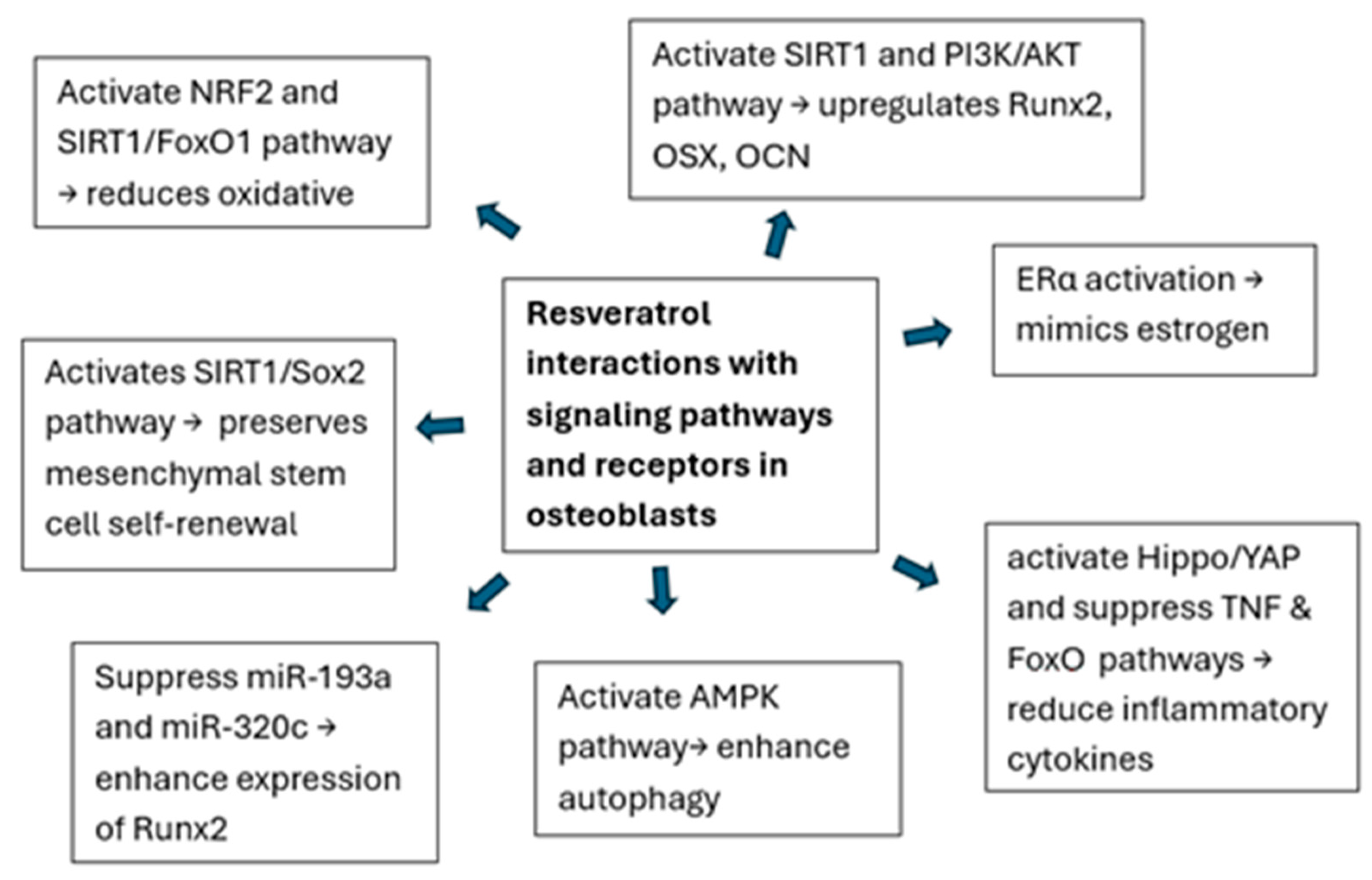
3.1. Effects of Resveratrol on Osteoblast (Table 2)
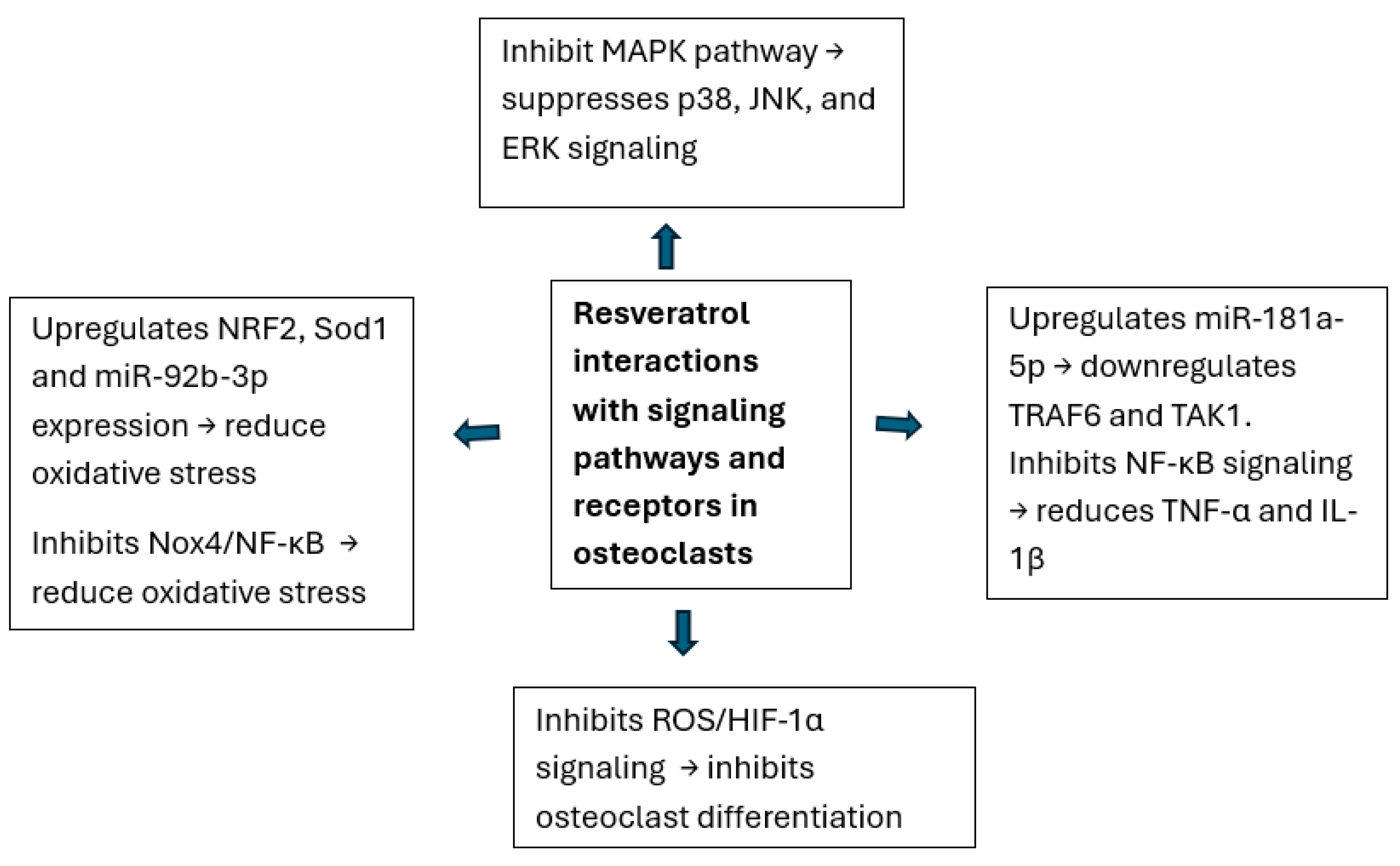
3.2. Effects of Resveratrol on Osteoclast (Table 3)
3.3. Role of Resveratrol in Osteoporosis Models and Pathways (Table 4)
| Author and Year | Types of Study | Objective | Molecular Parameters | Findings |
|---|---|---|---|---|
| Kuroyanagi et al., 2023 [18] | In vitro (MC3T3-E1 cells) | To investigate resveratrol’s effects on M-CSF synthesis. | M-CSF, OPG, Akt, PI3-kinase, and SIRT1 | Resveratrol reduces bFGF-induced M-CSF synthesis and OPG mRNA expression through the PI3-kinase/Akt pathway and SIRT1 activation in osteoblasts. |
| Han et al., 2024 [17] | In vivo (mice) and in vitro (BMSCs) | To study resveratrol’s effect on osteogenic differentiation of BMSCs. | Runx2, OSX, OCN, calcium deposition, ALP activity, SIRT1, and PI3K/AKT | Resveratrol enhances osteogenic differentiation in osteoporosis mice through the SIRT1/PI3K/AKT pathway, improving various bone parameters in vivo and in vitro. |
| Hwang et al., 2024 [19] | In vitro (MC3T3-E1 and hPDLF cells) | To explore resveratrol’s effect on bone formation under high-glucose conditions. | ALP activity, Col 1, TGF-β1, ALP, OCN, and RUNX2 | Resveratrol facilitates osteoblast function under high-glucose conditions, showing stage-specific effects on osteogenic differentiation in MC3T3-E1 cells and hPDLF cells. |
| Cai et al., 2023 [20] | In vitro (MC3T3-E1 cells) | To investigate resveratrol’s effects on proliferation and differentiation in MC3T3-E1 cells. | Runx2, OCN, and autophagy markers (LC3II/LC3I, and p62) | Resveratrol promotes osteogenic differentiation in MC3T3-E1 cells by activating autophagy. |
| He et al., 2024 [24] | In vitro (MC3T3-E1 cells) | To explore the mechanism by which resveratrol promotes the proliferation and differentiation of MC3T3-E1 cells. | Runx2, OPG, TNF, IL6, CASP3, and apoptosis-related proteins | Resveratrol promotes osteogenic differentiation by inhibiting apoptosis and regulating apoptosis-related proteins such as TNF and IL6, with potential implications for osteoporosis treatment. |
| Wang et al., 2022 [23] | In vitro (BMSCs) | To examine the effect of resveratrol on osteogenic differentiation in inflammatory conditions. | Inflammatory cytokines (IL-6, Mmp-9, and Il-1β), Hippo/YAP signaling, and YAP expression | Resveratrol reverses osteogenic decline by modulating inflammatory cytokines and restoring Hippo/YAP signaling in BMSCs. |
| Yahya et al., 2022 [25] | In vitro (Human BM-MSCs) | To investigate resveratrol’s effect on maintaining the stemness of human BM-MSCs. | SIRT1, Sox2, and stemness markers (CD73, CD90, and CD105) | Resveratrol maintains the stemness of human iliac BM-MSCs by activating SIRT1 and regulating Sox2 expression, promoting cell proliferation and reducing apoptosis and senescence. |
| Xuan et al., 2022 [22] | In vitro (Osteoblasts) | To explore resveratrol’s effect on osteoblast dysfunction under high glucose. | NRF2, AKT, GSK3β, FYN, and oxidative stress markers | Resveratrol alleviates osteoblast dysfunction under high glucose by activating NRF2 through the AKT/GSK3β/FYN axis, suppressing oxidative stress. |
| Liu et al., 2021 [35] | In vitro (MC3T3-E1 cells) | To explore resveratrol’s effect on osteoblast proliferation via GATA-1 and autophagy. | GATA-1, AMPKα, and autophagy markers | Resveratrol promotes osteoblast proliferation by activating GATA-1 and autophagy, with AMPKα acting as an upstream regulator. |
| Hioki et al., 2020 [26] | In vitro (MC3T3-E1 osteoblast-like cells) | To investigate the effects of resveratrol on IGF-I-induced osteoblast migration. | p44/p42 MAPK, Akt, SIRT1, IGF-I, and osteoblast migration | Resveratrol suppresses IGF-I-induced osteoblast migration via SIRT1 activation and the attenuation of the p44/p42 MAPK pathway. |
| Constanze et al., 2020 [27] | In vitro (MSC cultures) | To investigate the effect of TNF-β on MSC osteogenic differentiation and its reversal by resveratrol. | NF-κB, Sirt1, Runx2, β1-integrin, and osteogenic differentiation markers | Resveratrol reverses TNF-β-induced suppression of MSC osteogenesis by activating SIRT1 and Runx2, involving NF-κB modulation. |
| Authors and Year | Types of Study | Objective | Molecular Parameters | Findings |
|---|---|---|---|---|
| Lee et al., 2024 [36] | In vivo (OVX rat model) and in vitro (RAW 264.7 cell line) | To investigate the effect of oxyresveratrol on osteoclast differentiation and bone density in osteoporosis. | TRAP, MAPK (p38, JNK, and ERK), NFATc1, Cathepsin K, Bone mineral density (BMD), DPD, and TRAP activity | Oxyresveratrol inhibits osteoclast differentiation and MAPK phosphorylation and increases bone density in ovariectomized rats, suggesting a potential therapeutic use for osteoporosis. |
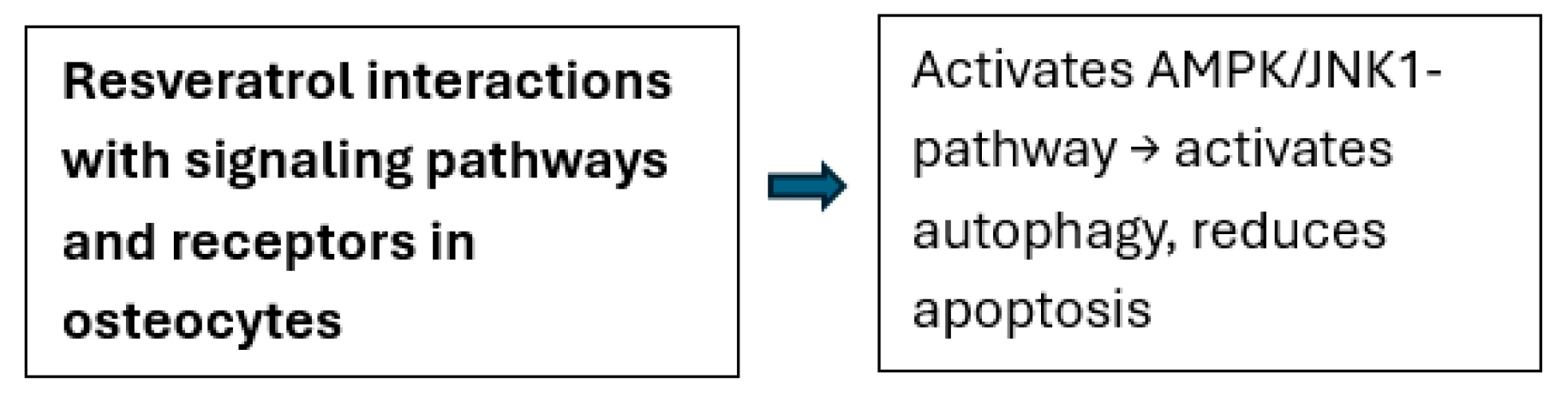
| Wei et al., 2023 [41] | In vivo (ovariectomized rat) and in vitro (MLO-Y4 osteocyte cells) | To investigate the protective effects of resveratrol on osteocytes against oxidative stress and apoptosis. | AMPK, JNK1, Beclin-1/Bcl-2 complex, autophagy markers, and osteocyte apoptosis markers | Resveratrol activates autophagy, reduces apoptosis, and protects osteocytes through the AMPK/JNK1-mediated pathway in ovariectomized rats and MLO-Y4 cells. |
| Poudel et al., 2022 [37] | In vitro (RAW 264.7 cells) and in vivo (zebrafish model) | To study resveratrol’s effect on doxorubicin-induced osteoclast differentiation and osteotoxicity. | Oc-stamp, RANK, TRAP, Ctsk, NRF2, SOD1, MitoTEMPO, and locomotion parameters | Resveratrol reverses DOX-induced osteoclast differentiation, reduces oxidative stress, and improves locomotion in zebrafish, suggesting protective effects against chemotherapy-induced bone loss. |
| Xue et al., 2023 [38] | In vitro (RAW 264.7 cell line) | To evaluate the effect of resveratrol on LPS-induced osteoclast differentiation through miR-181a-5p. | miR-181a-5p, TRAF6, P-IκB-α, NF-κB65, CTSK, MMP-9, and TRAP | Resveratrol inhibits osteoclast differentiation through miR-181a-5p-mediated suppression of the TRAF6/TAK1 pathway in LPS-stimulated RAW 264.7 cells. |
| Yan et al., 2022 [40] | In vivo (Wistar rat model) and in vitro (BMSCs and RAW264.7 cells) | To investigate resveratrol’s effect on high-altitude hypoxia-induced osteoporosis. | ROS, HIF-1α, RUNX2, ALP, OCN, CTX-I, TRAP, BMD, and BMSC proliferation and differentiation | Resveratrol mitigates osteoporosis caused by high-altitude hypoxia by suppressing the ROS/HIF-1α signaling pathway to inhibit osteoclastogenesis. |
| Zhang et al., 2020 [39] | In vivo (OVX rat model) and in vitro (bone tissue analysis) | To investigate resveratrol’s effect on estrogen deficiency-induced osteoporosis via NADPH oxidase and the NF-κB pathway. | miR-92b-3p, Nox4, NF-κBp65, BMP2, Smad7, RUNX2, Cathepsin K, BMD, and TRAP | Resveratrol alleviates estrogen deficiency-induced osteoporosis by enhancing miR-92b-3p expression, inhibiting Nox4/NF-κB signaling, and decreasing osteoclast activity. |
| Author and Year | Types of Study | Objective | Molecular Parameters | Findings |
|---|---|---|---|---|
| Zhang H. et al., 2024 [42] | In vivo (mice) | To investigate the skeletal toxicity of acrylamide (AA) and the protective effect of resveratrol (RVT). | RT-qPCR and histopathological analysis | AA induced osteogenesis and cartilage damage. RVT restored bone function and reduced apoptosis and oxidative stress in osteogenesis/cartilage. |
| Deng et al., 2023 [43] | In vitro (BMSC from T2DM patients) | To examine SIRT1 expression on osteogenic differentiation of BMSCs in type 2 diabetes mellitus (T2DM). | SIRT1 expression and BMSC osteogenic differentiation markers | Resveratrol increased SIRT1 expression, reduced BMSC apoptosis, and promoted osteogenic differentiation in T2DM patients. |
| Wang et al., 2023 [44] | Animal (ovariectomised rats) and cross-sectional study (postmenopausal women) | To examine the effects of resveratrol on bone mass in ovariectomized rats and the association of SIRT1 rs7896005 SNP with bone mass in women. | SIRT1, β-catenin, OPG, RANKL, and bone mineral density (BMD) | Resveratrol increased SIRT1, β-catenin, and BMD. Women with the A allele of SIRT1 rs7896005 had lower bone mass. |
| Chen et al., 2021 [49] | Animal study (rats) | To investigate the role of SIRT3 in glucocorticoid-induced osteonecrosis of the femoral head (GIONFH) and resveratrol’s effect. | SIRT3, oxidative stress markers, and the AMPK/PGC-1α/SIRT3 pathway | SIRT3 expression was reduced in GIONFH rats. Resveratrol activated SIRT3 and reduced oxidative stress, promoting osteogenic differentiation in BMSCs. |
| Yan et al., 2022 [40] | Animal and in vitro study (rats and BMSCs) | To investigate the effect of resveratrol on high-altitude hypoxia-induced osteoporosis. | ROS, HIF-1α, RUNX2, ALP, OCN, CTX-I, and TRAP | Resveratrol prevented high-altitude hypoxia-induced osteoporosis by promoting osteoblastogenesis and inhibiting osteoclastogenesis via ROS/HIF-1α suppression. |
| Ameen et al., 2020 [48] | Animal study (rats) | To investigate resveratrol’s therapeutic effect on aging-dependent male osteoporosis. | FoxO1, SIRT1, RANKL, OPG, and bone-specific markers | Resveratrol restored age-related osteoporotic changes by modulating inflammation, oxidative stress, and the gene expression of FoxO1 and SIRT1. |
| Ali et al., 2020 [47] | In vitro study (human BMSCs) | To examine resveratrol’s effect on adipocyte differentiation and cellular senescence in bone marrow stromal stem cells (BMSCs). | Adipocyte and osteoblast differentiation markers, ROS, SIRT1, FAK, and AKT | Resveratrol inhibited adipocyte differentiation and senescence, enhancing osteoblast differentiation in human BMSCs. |
| Louvet et al., 2020 [50] | Animal study (mice) | To investigate the role of SIRT1 in bone marrow adiposity (BMA) and its relationship with osteogenesis and adipogenesis. | SIRT1, Runx2, FoxO1, and acetylation levels | SIRT1 activation by resveratrol reduced BMA and promoted osteogenesis in an anorexia nervosa model by modulating the acetylation levels of Runx2 and FoxO1. |
| Shi et al., 2022 [46] | In vivo (OVX mice model) | To investigate the effect of resveratrol on osteoporosis in OVX mice through SFRP1-mediated osteogenesis. | SFRP1, ALP, osteogenesis-associated genes, and bone mineral density (BMD) | Resveratrol ameliorates bone loss in OVX mice by upregulating osteogenesis-associated genes and reducing SFRP1 levels, potentially useful for treating postmenopausal osteoporosis. |
| Liu et al., 2020 [45] | In vivo (nephrectomy mouse model) and in vitro (MC3T3-E1 cells) | To study the protective role of resveratrol against indoxyl sulfate (IS)-induced inhibition of osteoblastogenesis. | Runx2, ERK, p38 MAPK, AhR, SIRT1, and osteoblast differentiation markers | Resveratrol counteracts IS-induced inhibition of osteoblastogenesis through the AhR/MAPK/SIRT1 signaling pathway in vitro and in vivo. |
4. Discussion
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Charde, S.H.; Joshi, A.; Raut, J. A Comprehensive Review on Postmenopausal Osteoporosis in Women. Cureus 2023, 15, e48582. [Google Scholar] [CrossRef] [PubMed]
- Black, D.M.; Rosen, C.J. Postmenopausal Osteoporosis. N. Engl. J. Med. 2016, 374, 2095–2097. [Google Scholar] [CrossRef]
- An, R.; Luo, Q.; Li, L.; Cui, D.; Jin, J. The effects of resveratrol in animal models of primary osteoporosis: A systematic review and meta-analysis. J. Orthop. Surg. Res. 2024, 19, 137. [Google Scholar] [CrossRef]
- Chen, Y.; Zhou, F.; Liu, H.; Li, J.; Che, H.; Shen, J.; Luo, E. SIRT1, a promising regulator of bone homeostasis. Life Sci. 2021, 269, 119041. [Google Scholar] [CrossRef]
- Gomathi, K.; Akshaya, N.; Srinaath, N.; Moorthi, A.; Selvamurugan, N. Regulation of Runx2 by post-translational modifications in osteoblast differentiation. Life Sci. 2020, 245, 117389. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Buhrmann, C.; Mobasheri, A. Resveratrol-mediated SIRT-1 interactions with p300 modulate receptor activator of NF-κB ligand (RANKL) activation of NF-κB Signaling and inhibit osteoclastogenesis in bone-derived cells. J. Biol. Chem. 2011, 286, 11492–11505. [Google Scholar] [CrossRef] [PubMed]
- Hairi, H.A.; Jayusman, P.A.; Shuid, A.N. Revisiting Resveratrol as an Osteoprotective Agent: Molecular Evidence from In Vivo and In Vitro Studies. Biomedicines 2023, 11, 1453. [Google Scholar] [CrossRef]
- Salla, M.; Karaki, N.; El Kaderi, B.; Ayoub, A.J.; Younes, S.; Chahla, M.N.A.; Baksh, S.; El Khatib, S. Enhancing the Bioavailability of Resveratrol: Combine It, Derivatize It, or Encapsulate It? Pharmaceutics 2024, 16, 569. [Google Scholar] [CrossRef] [PubMed]
- Intagliata, S.; Modica, M.N.; Santagati, L.M.; Montenegro, L. Strategies to improve resveratrol systemic and topical bioavailability: An update. Antioxidants 2019, 8, 244. [Google Scholar] [CrossRef]
- Lushchak, O.; Strilbytska, O.; Koliada, A.; Zayachkivska, A.; Burdyliuk, N.; Yurkevych, I.; Storey, K.B.; Vaiserman, A. Nanodelivery of phytobioactive compounds for treating aging-associated disorders. GeroScience 2020, 42, 117–139. [Google Scholar] [CrossRef]
- Li, Q.; Yang, G.; Xu, H.; Tang, S.; Lee, W.Y.-W. Effects of resveratrol supplementation on bone quality: A systematic review and meta-analysis of randomized controlled trials. BMC Complement. Med. Ther. 2021, 21, 214. [Google Scholar] [CrossRef] [PubMed]
- Stroup, D.F.; Berlin, J.A.; Morton, S.C.; Olkin, I.; Williamson, G.D.; Rennie, D.; Moher, D.; Becker, B.J.; Sipe, T.A.; Thacker, S.B.; et al. Meta-analysis of observational studies in epidemiology: A proposal for reporting. JAMA 2000, 283, 2008–2012. [Google Scholar] [CrossRef]
- Shamseer, L.; Moher, D.; Clarke, M.; Ghersi, D.; Liberati, A.; Petticrew, M.; Shekelle, P.; Stewart, L.A.; PRISMA-P Group. Preferred reporting items for systematic review and meta-analysis protocols (prisma-p) 2015: Elaboration and explanation. BMJ 2015, 349, g7647. [Google Scholar] [CrossRef]
- Methley, A.M.; Campbell, S.; Chew-Graham, C.; McNally, R.; Cheraghi-Sohi, S. PICO, PICOS and SPIDER: A comparison study of specificity and sensitivity in three search tools for qualitative systematic reviews. BMC Health Serv. Res. 2014, 14, 579. [Google Scholar] [CrossRef] [PubMed]
- Hooijmans, C.R.; Rovers, M.M.; de Vries, R.B.M.; Leenaars, M.; Ritskes-Hoitinga, M.; Langendam, M.W. SYRCLE’s risk of bias tool for animal studies. BMC Med. Res. Methodol. 2014, 14, 43. [Google Scholar] [CrossRef]
- Wells, T.P. The Newcastle-Ottawa Scale (NOS) for assessing the quality of nonrandomised studies in meta-analyses. In Proceedings of the 3rd Symposium on Systematic Reviews: Beyond the Basics, Oxford, UK, 3–5 July 2000. [Google Scholar]
- Han, X.; Jia, G.-F.; Zhu, F. Resveratrol Alleviates Osteoporosis by Promoting Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells via SITR1/PI3K/AKT Pathway El Resveratrol Alivia la Osteoporosis al Promover la Diferenciación Osteogénica de las Células Madre Mesenquimales de la Médula Ósea a Través de la Vía SITR1/PI3K/AKT. Int. J. Morphol. 2024, 42, 216–224. [Google Scholar] [CrossRef]
- Kuroyanagi, G.; Hioki, T.; Tachi, J.; Matsushima-Nishiwaki, R.; Iida, H.; Tokuda, H.; Kozawa, O. Resveratrol inhibits basic fibroblast growth factor-induced macrophage colony-stimulating factor synthesis via the PI3-kinase/Akt pathway in osteoblasts. Biosci. Biotechnol. Biochem. 2023, 87, 1462–1469. [Google Scholar] [CrossRef] [PubMed]
- Hwang, S.-M.; Kim, T.-Y.; Kim, A.; Kim, Y.-G.; Park, J.-W.; Lee, J.-M.; Kim, J.-Y.; Suh, J.-Y. Resveratrol facilitates bone formation in high-glucose conditions. Front. Physiol. 2024, 15, 1347756. [Google Scholar] [CrossRef]
- Cai, W.; Sun, B.; Song, C.; Liu, F.; Wu, Z.; Liu, Z. Resveratrol induces proliferation and differentiation of mouse pre-osteoblast MC3T3-E1 by promoting autophagy. BMC Complement. Med. Ther. 2023, 23, 121. [Google Scholar] [CrossRef]
- Wang, L.; Li, Q.; Yan, H.; Jiao, G.; Wang, H.; Chi, H.; Zhou, H.; Chen, L.; Shan, Y.; Chen, Y. Resveratrol protects osteoblasts against dexamethasone-induced cytotoxicity through activation of amp-activated protein kinase. Drug Des. Dev. Ther. 2020, 14, 4451–4463. [Google Scholar] [CrossRef]
- Xuan, Y.; Wang, J.; Zhang, X.; Li, J.; Liu, Q.; Lu, G.; Xiao, M.; Gao, T.; Guo, Y.; Cao, C.; et al. Resveratrol Attenuates High Glucose-Induced Osteoblast Dysfunction via AKT/GSK3β/FYN-Mediated NRF2 Activation. Front. Pharmacol. 2022, 13, 862618. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Xia, C.; Chen, Y.; Jiang, T.; Hu, Y.; Gao, Y. Resveratrol Synergistically Promotes BMP9-Induced Osteogenic Differentiation of Mesenchymal Stem Cells. Stem Cells Int. 2022, 2022, 8124085. [Google Scholar] [CrossRef] [PubMed]
- He, Y.; Liu, F.; He, M.; Long, F.; Hu, D.; Chen, J.; Fang, M.; Wang, Z. Molecular mechanism of resveratrol promoting differentiation of preosteoblastic MC3T3-E1 cells based on network pharmacology and experimental validation. BMC Complement. Med. Ther. 2024, 24, 108. [Google Scholar] [CrossRef]
- Yahya, C.; Rohman, M.S.; Hidayat, M.; Nugraha, A.P.; Rantam, F.A. Resveratrol maintain Human Iliac Bone Marrow Mesenchymal Stem Cells Stemness through Sirtuin 1 Mediated Regulation of SRY-Box Transcription Factor 2: An in vitro and in silico study. Res. J. Pharm. Technol. 2022, 15, 2313–2319. [Google Scholar] [CrossRef]
- Hioki, T.; Kawabata, T.; Sakai, G.; Fujita, K.; Kuroyanagi, G.; Matsushima-Nishiwaki, R.; Kim, W.; Otsuka, T.; Iida, H.; Tokuda, H.; et al. Resveratrol suppresses insulin-like growth factor I-induced osteoblast migration: Attenuation of the p44/p42 MAP kinase pathway. Biosci. Biotechnol. Biochem. 2020, 84, 2428–2439. [Google Scholar] [CrossRef] [PubMed]
- Constanze, B.; Popper, B.; Aggarwal, B.B.; Shakibaei, M. Evidence that TNF-β suppresses osteoblast differentiation of mesenchymal stem cells and resveratrol reverses it through modulation of NF-κB, Sirt1 and Runx2. Cell Tissue Res. 2020, 381, 83–98. [Google Scholar] [CrossRef]
- Song, C.-Y.; Guo, Y.; Chen, F.-Y.; Liu, W.-G. Resveratrol Promotes Osteogenic Differentiation of Bone Marrow-Derived Mesenchymal Stem Cells Through miR-193a/SIRT7 Axis. Calcif. Tissue Int. 2022, 110, 117–130. [Google Scholar] [CrossRef]
- Zou, J.; Du, J.; Tu, H.; Chen, H.; Cong, K.; Bi, Z.; Sun, J. Resveratrol benefits the lineage commitment of bone marrow mesenchymal stem cells into osteoblasts via miR-320c by targeting Runx2. J. Tissue Eng. Regen. Med. 2021, 15, 347–360. [Google Scholar] [CrossRef]
- Jiang, Y.; Luo, W.; Wang, B.; Wang, X.; Gong, P.; Xiong, Y. Resveratrol promotes osteogenesis via activating SIRT1/FoxO1 pathway in osteoporosis mice. Life Sci. 2020, 246, 117422. [Google Scholar] [CrossRef]
- Mei, W.; Song, D.; Wu, Z.; Yang, L.; Wang, P.; Zhang, R.; Zhu, X. Resveratrol protects MC3T3-E1 cells against cadmium-induced suppression of osteogenic differentiation by modulating the ERK1/2 and JNK pathways. Ecotoxicol. Environ. Saf. 2021, 214, 112080. [Google Scholar] [CrossRef]
- Yu, T.; Wang, Z.; You, X.; Zhou, H.; He, W.; Li, B.; Xia, J.; Zhu, H.; Zhao, Y.; Yu, G.; et al. Resveratrol promotes osteogenesis and alleviates osteoporosis by inhibiting p53. Aging 2020, 12, 10359–10369. [Google Scholar] [CrossRef] [PubMed]
- Pervaiz, S. Resveratrol: From grapevines to mammalian biology. FASEB J. 2003, 17, 1975–1985. [Google Scholar] [CrossRef] [PubMed]
- Shah, A.A.; Kumar, A.; Lakra, A.; Singh, D.; Nayak, Y. Phytoestrogenic Potential of Resveratrol by Selective Activation of Estrogen Receptor-α in Osteoblast Cells. Rev. Bras. Farm. 2022, 32, 248–256. [Google Scholar] [CrossRef]
- Liu, Z.; Shui, C.; Huang, L.; Qu, Y. Resveratrol facilitates bone marrow mesenchymal stem cells (BMSCs) differentiation to prevent osteoporosis via restraining of secreted frizzled-related protein 1 expression. Mater. Express 2021, 11, 1636–1644. [Google Scholar] [CrossRef]
- Lee, Y.-J.; Ahn, J.-C.; Oh, C.-H. Oxyresveratrol attenuates bone resorption by inhibiting the mitogen-activated protein kinase pathway in ovariectomized rats. Nutr. Metab. 2024, 21, 7. [Google Scholar] [CrossRef]
- Poudel, S.; Martins, G.; Cancela, M.L.; Gavaia, P.J. Resveratrol-Mediated Reversal of Doxorubicin-Induced Osteoclast Differentiation. Int. J. Mol. Sci. 2022, 23, 15160. [Google Scholar] [CrossRef]
- Xue, H.-Y.; Liu, M.-W.; Yang, G. Resveratrol suppresses lipopolysaccharide-mediated activation of osteoclast precursor RAW 264.7 cells by increasing miR-181a-5p expression. Int. J. Immunopathol. Pharmacol. 2023, 37, 03946320231154995. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, M.-W.; He, Y.; Deng, N.; Chen, Y.; Huang, J.; Xie, W. Protective effect of resveratrol on estrogen deficiency-induced osteoporosis though attenuating NADPH oxidase 4/nuclear factor kappa B pathway by increasing miR-92b-3p expression. Int. J. Immunopathol. Pharmacol. 2020, 34, 2058738420941762. [Google Scholar] [CrossRef]
- Yan, C.; Wang, Z.; Liu, W.; Pu, L.; Li, R.; Ai, C.; Xu, H.; Zhang, B.; Wang, T.; Zhang, X.; et al. Resveratrol Ameliorates High Altitude Hypoxia-Induced Osteoporosis by Suppressing the ROS/HIF Signaling Pathway. Molecules 2022, 27, 5538. [Google Scholar] [CrossRef]
- Wei, L.; Chai, S.; Yue, C.; Zhang, H.; Li, J.; Qin, N. Resveratrol protects osteocytes against oxidative stress in ovariectomized rats through AMPK/JNK1-dependent pathway leading to promotion of autophagy and inhibition of apoptosis. Cell Death Discov. 2023, 9, 16. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, Y.; Wen, Y.; Wang, H.; Chen, L. The antioxidant protective effect of resveratrol on long-term exposure to acrylamide-induced skeletal toxicity in female mice. Toxicol. Res. 2024, 13, tfae109. [Google Scholar] [CrossRef]
- Deng, X.; Deng, L.; Xu, M.; Sun, Y.; Yang, M. Effects of SIRT1 on Osteogenic Differentiation of Bone Marrow Mesenchymal Stem Cells in Type 2 Diabetic Patients. Endocr. Metab. Immune Disord.-Drug Targets 2023, 23, 1077–1086. [Google Scholar] [CrossRef]
- Wang, X.; Lu, C.; Chen, Y.; Wang, Q.; Bao, X.; Zhang, Z.; Huang, X. Resveratrol promotes bone mass in ovariectomized rats and the SIRT1 rs7896005 SNP is associated with bone mass in women during perimenopause and early postmenopause. Climacteric 2023, 26, 25–33. [Google Scholar] [CrossRef]
- Liu, W.-C.; Shyu, J.-F.; Lin, Y.-F.; Chiu, H.-W.; Lim, P.S.; Lu, C.-L.; Zheng, C.-M.; Hou, Y.-C.; Chen, P.-H.; Lu, K.-C. Resveratrol rescue indoxyl sulfate-induced deterioration of osteoblastogenesis via the aryl hydrocarbon receptor /MAPK pathway. Int. J. Mol. Sci. 2020, 21, 7483. [Google Scholar] [CrossRef] [PubMed]
- Shi, Y.; Kong, C.; Li, Y. Resveratrol inhibits osteoporosis in mice model. Mater. Express 2022, 12, 939–947. [Google Scholar] [CrossRef]
- Ali, D.; Chen, L.; Kowal, J.M.; Okla, M.; Manikandan, M.; AlShehri, M.; AlMana, Y.; AlObaidan, R.; AlOtaibi, N.; Hamam, R.; et al. Resveratrol inhibits adipocyte differentiation and cellular senescence of human bone marrow stromal stem cells. Bone 2020, 133, 115252. [Google Scholar] [CrossRef]
- Ameen, O.; Yassien, R.I.; Naguib, Y.M. Activation of FoxO1/SIRT1/RANKL/OPG pathway may underlie the therapeutic effects of resveratrol on aging-dependent male osteoporosis. BMC Musculoskelet. Disord. 2020, 21, 375. [Google Scholar] [CrossRef]
- Chen, L.; Wang, B.-Z.; Xie, J.; Zhang, R.-Y.; Jin, C.; Chen, W.-K.; Fang, K.-H.; Hong, C.-X.; Xu, T.-H.; Huang, C.-B.; et al. Therapeutic effect of SIRT3 on glucocorticoid-induced osteonecrosis of the femoral head via intracellular oxidative suppression. Free Radic. Biol. Med. 2021, 176, 228–240. [Google Scholar] [CrossRef]
- Louvet, L.; Leterme, D.; Delplace, S.; Miellot, F.; Marchandise, P.; Gauthier, V.; Hardouin, P.; Chauveau, C.; Mhenni, O.G. Sirtuin 1 deficiency decreases bone mass and increases bone marrow adiposity in a mouse model of chronic energy deficiency. Bone 2020, 136, 115361. [Google Scholar] [CrossRef]
- Elesawy, B.H.; Sakr, H.F.; Abbas, A.M. Synergistic Protective Effects of Resveratrol and Estradiol on Estrogen Deficiency-Induced Osteoporosis Through Attenuating RANK Pathway. Int. J. Pharmacol. 2021, 17, 217–228. [Google Scholar] [CrossRef]
- Newman, D.J.; Cragg, G.M. Natural Products as Sources of New Drugs over the Nearly Four Decades from 01/1981 to 09/2019. J. Nat. Prod. 2020, 83, 770–803. [Google Scholar] [CrossRef] [PubMed]
- Atanasov, A.G.; Zotchev, S.B.; Dirsch, V.M.; Supuran, C.T. Natural products in drug discovery: Advances and opportunities. Nat. Rev. Drug Discov. 2021, 20, 200–216. [Google Scholar] [CrossRef]
- Pannu, N.; Bhatnagar, A. Resveratrol: From enhanced biosynthesis and bioavailability to multitargeting chronic diseases. Biomed. Pharmacother. 2019, 109, 2237–2251. [Google Scholar] [CrossRef]
- Walle, T. Bioavailability of resveratrol. Ann. N. Y. Acad. Sci. 2011, 1215, 9–15. [Google Scholar] [CrossRef]
- Singh, G. Resveratrol: Nanocarrier-Based Delivery Systems to Enhance its Therapeutic Potential. Nanomedicine 2020, 15, 2801–2817. [Google Scholar] [CrossRef] [PubMed]
- Shakibaei, M.; Shayan, P.; Busch, F.; Aldinger, C.; Buhrmann, C.; Lueders, C.; Mobasheri, A. Resveratrol Mediated Modulation of Sirt-1/Runx2 Promotes Osteogenic Differentiation of Mesenchymal Stem Cells: Potential Role of Runx2 Deacetylation. PLoS ONE 2012, 7, e35712. [Google Scholar] [CrossRef]
- Huang, M.; Wang, J.; Tan, C.; Ying, R.; Wu, X.; Chen, W.; Liu, J.; Ahmad, M. Liposomal co-delivery strategy to improve stability and antioxidant activity of trans-resveratrol and naringenin. Int. J. Food Sci. Technol. 2022, 57, 2701–2714. [Google Scholar] [CrossRef]
- Chopra, H.; Bibi, S.; Islam, F.; Ahmad, S.U.; Olawale, O.A.; Alhumaydhi, F.A.; Marzouki, R.; Baig, A.A.; Bin Emran, T. Emerging Trends in the Delivery of Resveratrol by Nanostructures: Applications of Nanotechnology in Life Sciences. J. Nanomater. 2022, 2022, 3083728. [Google Scholar] [CrossRef]
- Tian, B.; Liu, J. Resveratrol: A review of plant sources, synthesis, stability, modification and food application. J. Sci. Food Agric. 2020, 100, 1392–1404. [Google Scholar] [CrossRef]
| Inclusion | Exclusion | |
|---|---|---|
| Population | Cell line Animal model Human | - |
| Intervention | Resveratrol | - |
| Comparison | Group not receiving resveratrol Positive control and negative control groups | - |
| Outcome | Molecular parameters related to mechanisms of protection against osteoporosis | Did not measure molecular parameters |
| Study Type | In vitro studies, animal studies, randomized controlled studies, case–control studies, cohort studies | case reports, editorials, communications, reviews, meta-analysis |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Shuid, A.N.; Abdul Nasir, N.A.; Ab Azis, N.; Shuid, A.N.; Razali, N.; Ahmad Hairi, H.; Mohd Miswan, M.F.; Naina Mohamed, I. A Systematic Review on the Molecular Mechanisms of Resveratrol in Protecting Against Osteoporosis. Int. J. Mol. Sci. 2025, 26, 2893. https://doi.org/10.3390/ijms26072893
Shuid AN, Abdul Nasir NA, Ab Azis N, Shuid AN, Razali N, Ahmad Hairi H, Mohd Miswan MF, Naina Mohamed I. A Systematic Review on the Molecular Mechanisms of Resveratrol in Protecting Against Osteoporosis. International Journal of Molecular Sciences. 2025; 26(7):2893. https://doi.org/10.3390/ijms26072893
Chicago/Turabian StyleShuid, Ahmad Nazrun, Nurul Alimah Abdul Nasir, Norasikin Ab Azis, Ahmad Naqib Shuid, Norhafiza Razali, Haryati Ahmad Hairi, Mohd Fairudz Mohd Miswan, and Isa Naina Mohamed. 2025. "A Systematic Review on the Molecular Mechanisms of Resveratrol in Protecting Against Osteoporosis" International Journal of Molecular Sciences 26, no. 7: 2893. https://doi.org/10.3390/ijms26072893
APA StyleShuid, A. N., Abdul Nasir, N. A., Ab Azis, N., Shuid, A. N., Razali, N., Ahmad Hairi, H., Mohd Miswan, M. F., & Naina Mohamed, I. (2025). A Systematic Review on the Molecular Mechanisms of Resveratrol in Protecting Against Osteoporosis. International Journal of Molecular Sciences, 26(7), 2893. https://doi.org/10.3390/ijms26072893







