Fine Mapping of the QTL qRLP12 That Controls Root Length Under Polyethylene glycol-Induced Drought Stress During the Early Seedling Stage of Sesame
Abstract
1. Introduction
2. Results
2.1. Validation of qRLP12 for Root Length Under PEG Stress
2.2. Fine Mapping of qRLP12
2.3. Candidate Genes in qRLP12 Region
2.4. Expression Analysis of Candidate Genes
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Marker Development and Recombinant Screening
4.3. Phenotype Evaluation
4.4. Statistical Analysis
4.5. RNA Extraction and Quantitative PCR with Reverse Transcription
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| QTL | quantitative trait locus |
| JHM | jinhuangma |
| ZSB | zhushanbai |
| RIL | recombinant inbred line |
| PEG | polyethylene glycol |
| RLP | root length under PEG-induced drought stress |
| TFs | transcription factors |
| qRT-PCR | quantitative real-time PCR |
| GWAS | genome-wide association |
| GELP | GDSL esterase/lipase |
| InDels | insertions and deletions |
| dCAPS | derived cleaved amplified polymorphic sequences |
| CAPS | cleaved amplified polymorphic sequences |
| CALS7 | CALLOSE SYNTHASE 7 |
References
- Orimoloye, I.R.; Belle, J.A.; Orimoloye, Y.M.; Olusola, A.O.; Ololade, O.O. Drought: A Common Environmental Disaster. Atmosphere 2022, 13, 111. [Google Scholar] [CrossRef]
- Frolov, A.; Bilova, T.; Paudel, G.; Berger, R.; Balcke, G.U.; Birkemeyer, C.; Wessjohann, L.A. Early responses of mature Arabidopsis thaliana plants to reduced water potential in the agar-based polyethylene glycol infusion drought model. J. Plant Physiol. 2017, 208, 70–83. [Google Scholar] [CrossRef] [PubMed]
- Feng, X.; Jia, L.; Cai, Y.; Guan, H.; Zheng, D.; Zhang, W.; Xiong, H.; Zhou, H.; Wen, Y.; Hu, Y. ABA-inducible DEEPER ROOTING 1 improves adaptation of maize to water deficiency. Plant Biotechnol. J. 2022, 20, 2077–2088. [Google Scholar] [PubMed]
- Chen, X.; Wu, Q.; Gao, Y.; Zhang, J.; Wang, Y.; Zhang, R.; Zhou, Y.; Xiao, M.; Xu, W.; Huang, R. The role of deep roots in sorghum yield production under drought conditions. Agronomy 2020, 10, 611. [Google Scholar] [CrossRef]
- Bacher, H.; Montagu, A.; Herrmann, I.; Walia, H.; Schwartz, N.; Peleg, Z. Stress-induced deeper rooting introgression enhances wheat yield under terminal drought. J. Exp. Bot. 2023, 74, 4862–4874. [Google Scholar]
- Uga, Y.; Sugimoto, K.; Ogawa, S.; Rane, J.; Ishitani, M.; Hara, N.; Kitomi, Y.; Inukai, Y.; Ono, K.; Kanno, N. Control of root system architecture by DEEPER ROOTING 1 increases rice yield under drought conditions. Nat. Genet. 2013, 45, 1097–1102. [Google Scholar]
- Yadav, R.; Kalia, S.; Rangan, P.; Pradheep, K.; Rao, G.P.; Kaur, V.; Pandey, R.; Rai, V.; Vasimalla, C.C.; Langyan, S.; et al. Current Research Trends and Prospects for Yield and Quality Improvement in Sesame, an Important Oilseed Crop. Front. Plant Sci. 2022, 13, 863521. [Google Scholar] [CrossRef]
- Teboul, N.; Gadri, Y.; Berkovich, Z.; Reifen, R.; Peleg, Z. Genetic Architecture Underpinning Yield Components and Seed Mineral–Nutrients in Sesame. Genes 2020, 11, 1221. [Google Scholar] [CrossRef]
- Jeyaraj, S.; Beevy, S.S. Insights into the Drought Stress Tolerance Mechanisms of Sesame: The Queen of Oilseeds. J. Plant Growth Regul. 2024, 43, 3370–3391. [Google Scholar] [CrossRef]
- Dossa, K.; Wei, X.; Li, D.; Fonceka, D.; Zhang, Y.; Wang, L.; Yu, J.; Boshou, L.; Diouf, D.; Cissé, N.; et al. Insight into the AP2/ERF transcription factor superfamily in sesame and expression profiling of DREB subfamily under drought stress. BMC Plant Biol. 2016, 16, 171. [Google Scholar] [CrossRef]
- Chen, S.; Gong, B. Response and adaptation of agriculture to climate change: Evidence from China. J. Dev. Econ. 2021, 148, 102557. [Google Scholar] [CrossRef]
- Hermans, K.; McLeman, R. Climate change, drought, land degradation and migration: Exploring the linkages. Curr. Opin. Environ. Sustain. 2021, 50, 236–244. [Google Scholar]
- Boureima, S.; Diouf, S.; Amoukou, M.; Van Damme, P. Screening for sources of tolerance to drought in sesame induced mutants: Assessment of indirect selection criteria for seed yield. Int. J. Pure Appl. Biosci. 2016, 4, 45–60. [Google Scholar]
- Pandey, B.B.; Ratnakumar, P.; Usha Kiran, B.; Dudhe, M.Y.; Lakshmi, G.S.; Ramesh, K.; Guhey, A. Identifying traits associated with terminal drought tolerance in sesame (Sesamum indicum L.) genotypes. Front. Plant Sci. 2021, 12, 739896. [Google Scholar]
- Wang, L.; Yuan, X. Two types of flash drought and their connections with seasonal drought. Adv. Atmos. Sci. 2018, 35, 1478–1490. [Google Scholar]
- Deng, Y.; Wang, X.; Lu, T.; Du, H.; Ciais, P.; Lin, X. Divergent seasonal responses of carbon fluxes to extreme droughts over China. Agric. For. Meteorol. 2023, 328, 109253. [Google Scholar]
- Su, R.; Dossou, S.S.K.; Dossa, K.; Zhou, R.; Liu, A.; Zhong, Y.; Fang, S.; Zhang, X.; Wu, Z.; You, J. Genome-wide characterization and identification of candidate ERF genes involved in various abiotic stress responses in sesame (Sesamum indicum L.). BMC Plant Biol. 2022, 22, 256. [Google Scholar]
- Mmadi, M.A.; Dossa, K.; Wang, L.; Zhou, R.; Wang, Y.; Cisse, N.; Sy, M.O.; Zhang, X. Functional characterization of the versatile MYB gene family uncovered their important roles in plant development and responses to drought and waterlogging in sesame. Genes 2017, 8, 362. [Google Scholar] [CrossRef]
- Wang, Y.; Zhang, Y.; Zhou, R.; Dossa, K.; Yu, J.; Li, D.; Liu, A.; Mmadi, M.A.; Zhang, X.; You, J. Identification and characterization of the bZIP transcription factor family and its expression in response to abiotic stresses in sesame. PLoS ONE 2018, 13, e0200850. [Google Scholar]
- Zhang, Y.; Li, D.; Wang, Y.; Zhou, R.; Wang, L.; Zhang, Y.; Yu, J.; Gong, H.; You, J.; Zhang, X. Genome-wide identification and comprehensive analysis of the NAC transcription factor family in Sesamum indicum. PLoS ONE 2018, 13, e0199262. [Google Scholar]
- Wei, M.; Liu, A.; Zhang, Y.; Zhou, Y.; Li, D.; Dossa, K.; Zhou, R.; Zhang, X.; You, J. Genome-wide characterization and expression analysis of the HD-Zip gene family in response to drought and salinity stresses in sesame. BMC Genom. 2019, 20, 748. [Google Scholar] [CrossRef] [PubMed]
- Dossa, K.; Mmadi, M.A.; Zhou, R.; Zhang, T.; Su, R.; Zhang, Y.; Wang, L.; You, J.; Zhang, X. Depicting the core transcriptome modulating multiple abiotic stresses responses in sesame (Sesamum indicum L.). Int. J. Mol. Sci. 2019, 20, 3930. [Google Scholar] [CrossRef] [PubMed]
- Dossa, K.; Mmadi, M.A.; Zhou, R.; Liu, A.; Yang, Y.; Diouf, D.; You, J.; Zhang, X. Ectopic expression of the sesame MYB transcription factor SiMYB305 promotes root growth and modulates ABA-mediated tolerance to drought and salt stresses in Arabidopsis. AoB Plants 2020, 12, plz081. [Google Scholar] [CrossRef]
- Rajput, P.; Agarwal, P.; Agarwal, P.K. A Sesamum indicum SiMYB77 Transcription Factor, Enhances Drought and Salt Tolerance in Transgenic Tobacco Via Maintaining Higher Osmolytes and ROS Homeostasis. J. Plant Growth Regul. 2024, in press. [Google Scholar] [CrossRef]
- Chowdhury, S.; Basu, A.; Kundu, S. Overexpression of a new osmotin-like protein gene (SindOLP) confers tolerance against biotic and abiotic stresses in sesame. Front. Plant Sci. 2017, 8, 410. [Google Scholar] [CrossRef]
- Dossa, K.; Li, D.; Zhou, R.; Yu, J.; Wang, L.; Zhang, Y.; You, J.; Liu, A.; Mmadi, M.A.; Fonceka, D. The genetic basis of drought tolerance in the high oil crop Sesamum indicum. Plant Biotechnol. J. 2019, 17, 1788–1803. [Google Scholar] [CrossRef]
- Dossa, K.; Zhou, R.; Li, D.; Liu, A.; Qin, L.; Mmadi, M.A.; Su, R.; Zhang, Y.; Wang, J.; Gao, Y. A novel motif in the 5’-UTR of an orphan gene ‘Big Root Biomass’ modulates root biomass in sesame. Plant Biotechnol. J. 2021, 19, 1065–1079. [Google Scholar] [CrossRef]
- Baghery, M.A.; Kazemitabar, S.K.; Dehestani, A.; Mehrabanjoubani, P.; Naghizadeh, M.M.; Masoudi-Nejad, A. Transcription Factors and microRNA Genes Regulatory Network Construction Under Drought Stress in Sesame (Sesamum indicum L.). Res. Sq. 2020. [Google Scholar] [CrossRef]
- Baghery, M.A.; Kazemitabar, S.K.; Dehestani, A.; Mehrabanjoubani, P.; Naghizadeh, M.M.; Masoudi-Nejad, A. Insight into gene regulatory networks involved in sesame (Sesamum indicum L.) drought response. Biologia 2022, 77, 1181–1196. [Google Scholar] [CrossRef]
- Song, Q.; Joshi, M.; Wang, S.; Johnson, C.D.; Joshi, V. Comparative analysis of root transcriptome profiles of sesame (Sesamum indicum L.) in response to osmotic stress. J. Plant Growth Regul. 2021, 40, 1787–1801. [Google Scholar] [CrossRef]
- Dossa, K.; Li, D.; Wang, L.; Zheng, X.; Liu, A.; Yu, J.; Wei, X.; Zhou, R.; Fonceka, D.; Diouf, D. Transcriptomic, biochemical and physio-anatomical investigations shed more light on responses to drought stress in two contrasting sesame genotypes. Sci. Rep. 2017, 7, 8755. [Google Scholar]
- You, J.; Zhang, Y.; Liu, A.; Li, D.; Wang, X.; Dossa, K.; Zhou, R.; Yu, J.; Zhang, Y.; Wang, L. Transcriptomic and metabolomic profiling of drought-tolerant and susceptible sesame genotypes in response to drought stress. BMC Plant Biol. 2019, 19, 267. [Google Scholar]
- Liang, J.; Sun, J.; Ye, Y.; Yan, X.; Yan, T.; Rao, Y.; Zhou, H.; Le, M. QTL mapping of PEG-induced drought tolerance at the early seedling stage in sesame using whole genome re-sequencing. PLoS ONE 2021, 16, e0247681. [Google Scholar]
- Wang, L.; Yu, S.; Tong, C.; Zhao, Y.; Liu, Y.; Song, C.; Zhang, Y.; Zhang, X.; Wang, Y.; Hua, W. Genome sequencing of the high oil crop sesame provides insight into oil biosynthesis. Genome Biol. 2014, 15, R39. [Google Scholar]
- Siddiqui, M.N.; Léon, J.; Naz, A.A.; Ballvora, A. Genetics and genomics of root system variation in adaptation to drought stress in cereal crops. J. Exp. Bot. 2021, 72, 1007–1019. [Google Scholar] [PubMed]
- Courtois, B.; Ahmadi, N.; Khowaja, F.; Price, A.H.; Rami, J.-F.; Frouin, J.; Hamelin, C.; Ruiz, M. Rice root genetic architecture: Meta-analysis from a drought QTL database. Rice 2009, 2, 115–128. [Google Scholar] [CrossRef]
- Comas, L.H.; Becker, S.R.; Cruz, V.M.V.; Byrne, P.F.; Dierig, D.A. Root traits contributing to plant productivity under drought. Front. Plant Sci. 2013, 4, 442. [Google Scholar]
- Meister, R.; Rajani, M.; Ruzicka, D.; Schachtman, D.P. Challenges of modifying root traits in crops for agriculture. Trends Plant Sci. 2014, 19, 779–788. [Google Scholar]
- Su, R.; Zhou, R.; Mmadi, M.A.; Li, D.; Qin, L.; Liu, A.; Wang, J.; Gao, Y.; Wei, M.; Shi, L. Root diversity in sesame (Sesamum indicum L.): Insights into the morphological, anatomical and gene expression profiles. Planta 2019, 250, 1461–1474. [Google Scholar]
- Hamedani, N.G.; Gholamhoseini, M.; Bazrafshan, F.; Amiri, B.; Habibzadeh, F. Variability of root traits in sesame genotypes under different irrigation regimes. Rhizosphere 2020, 13, 100190. [Google Scholar]
- Li, D.; Dossa, K.; Zhang, Y.; Wei, X.; Wang, L.; Zhang, Y.; Liu, A.; Zhou, R.; Zhang, X. GWAS uncovers differential genetic bases for drought and salt tolerances in sesame at the germination stage. Genes 2018, 9, 87. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Ma, B.; Duan, K.-X.; Li, X.-K.; Lu, X.; Yin, C.-C.; Tao, J.-J.; Wei, W.; Zhang, W.-K.; Xin, P.-Y. The GDSL lipase MHZ11 modulates ethylene signaling in rice roots. Plant Cell 2020, 32, 1626–1643. [Google Scholar] [CrossRef] [PubMed]
- Su, H.-G.; Zhang, X.-H.; Wang, T.-T.; Wei, W.-L.; Wang, Y.-X.; Chen, J.; Zhou, Y.-B.; Chen, M.; Ma, Y.-Z.; Xu, Z.-S. Genome-wide identification, evolution, and expression of GDSL-type esterase/lipase gene family in soybean. Front. Plant Sci. 2020, 11, 726. [Google Scholar]
- Liu, D.; Zhao, X.; Liu, Y.; Tian, M.; Zhao, J.; Bai, N.; Huang, R.; Li, M.; Sui, S. The GDSL lipase CpGLIP1 from Chimonanthus praecox improves drought and cold tolerance in Arabidopsis and poplar. Ind. Crop. Prod. 2024, 215, 118636. [Google Scholar]
- Stührwohldt, N.; Bühler, E.; Sauter, M.; Schaller, A. Phytosulfokine (PSK) precursor processing by subtilase SBT3. 8 and PSK signaling improve drought stress tolerance in Arabidopsis. J. Exp. Bot. 2021, 72, 3427–3440. [Google Scholar]
- Zhao, X.; Goher, F.; Chen, L.; Song, J.; Zhao, J. Genome-wide identification, phylogeny and expression analysis of subtilisin (SBT) gene family under wheat biotic and abiotic stress. Plants 2023, 12, 3065. [Google Scholar] [CrossRef]
- Laloum, T.; De Mita, S.; Gamas, P.; Baudin, M.; Niebel, A. CCAAT-box binding transcription factors in plants: Y so many? Trends Plant Sci. 2013, 18, 157–166. [Google Scholar]
- Shi, X.; Han, X.; Lu, T.-g. Callose synthesis during reproductive development in monocotyledonous and dicotyledonous plants. Plant Signal. Behav. 2016, 11, e1062196. [Google Scholar]
- Ušák, D.; Haluška, S.; Pleskot, R. Callose synthesis at the center point of plant development—An evolutionary insight. Plant Physiol. 2023, 193, 54–69. [Google Scholar] [CrossRef]
- Nishimura, M.T.; Stein, M.; Hou, B.-H.; Vogel, J.P.; Edwards, H.; Somerville, S.C. Loss of a callose synthase results in salicylic acid-dependent disease resistance. Science 2003, 301, 969–972. [Google Scholar] [CrossRef]
- Li, N.; Lin, Z.; Yu, P.; Zeng, Y.; Du, S.; Huang, L.-J. The multifarious role of callose and callose synthase in plant development and environment interactions. Front. Plant Sci. 2023, 14, 1183402. [Google Scholar] [CrossRef] [PubMed]
- Diana, S.-G.; Kamila, G.-J.; Ewa, K.; Małgorzata, K.-K.; Monika, T.; Emilia, G.; Kaja, S.; Magdalena, R.; Karolina, U.; Monika, K. The effect of silicon supplementation and drought stress on the deposition of callose and chemical components in the cell walls of the Brassica napus roots. BMC Plant Biol. 2024, 24, 1249. [Google Scholar] [CrossRef]
- Vatén, A.; Dettmer, J.; Wu, S.; Stierhof, Y.-D.; Miyashima, S.; Yadav, S.R.; Roberts, C.J.; Campilho, A.; Bulone, V.; Lichtenberger, R. Callose biosynthesis regulates symplastic trafficking during root development. Dev. cell 2011, 21, 1144–1155. [Google Scholar] [CrossRef]
- Kalmbach, L.; Bourdon, M.; Belevich, I.; Safran, J.; Lemaire, A.; Heo, J.-o.; Otero, S.; Blob, B.; Pelloux, J.; Jokitalo, E. Putative pectate lyase PLL12 and callose deposition through polar CALS7 are necessary for long-distance phloem transport in Arabidopsis. Curr. Biol. 2023, 33, 926–939.e9. [Google Scholar] [CrossRef]
- Ehrhardt-Brocardo, N.C.M.; Coelho, C.M.M.; Souza, C.A.; Mathias, V. Callose accumulation in roots of soybean seedlings under water deficit. Theor. Exp. Plant Physiol. 2019, 31, 475–481. [Google Scholar] [CrossRef]
- Ahmed, I.M.; Nadira, U.A.; Cao, F.; He, X.; Zhang, G.; Wu, F. Physiological and molecular analysis on root growth associated with the tolerance to aluminum and drought individual and combined in Tibetan wild and cultivated barley. Planta 2016, 243, 973–985. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, I.M.; Nadira, U.A.; Bibi, N.; Cao, F.; He, X.; Zhang, G.; Wu, F. Secondary metabolism and antioxidants are involved in the tolerance to drought and salinity, separately and combined, in Tibetan wild barley. Environ. Exp. Bot. 2015, 111, 1–12. [Google Scholar] [CrossRef]
- You, J.; Li, D.; Yang, L.; Dossou, S.S.K.; Zhou, R.; Zhang, Y.; Wang, L. CRISPR/Cas9-mediated efficient targeted mutagenesis in sesame (Sesamum indicum L.). Front. Plant Sci. 2022, 13, 935825. [Google Scholar] [CrossRef]
- Porebski, S.; Bailey, L.G.; Baum, B.R. Modification of a CTAB DNA extraction protocol for plants containing high polysaccharide and polyphenol components. Plant Mol. Biol. Report. 1997, 15, 8–15. [Google Scholar] [CrossRef]
- Van Ooijen, J. JoinMap 4 ® Software for the Calculation of Genetic Linkage Maps in Experimental Populations; Kyazma B.V.: Wageningen, The Netherlands, 2006. [Google Scholar]
- Voorrips, R. MapChart: Software for the graphical presentation of linkage maps and QTLs. J. Hered. 2002, 93, 77–78. [Google Scholar] [CrossRef]
- Wei, L.; Miao, H.; Zhao, R.; Han, X.; Zhang, T.; Zhang, H. Identification and testing of reference genes for Sesame gene expression analysis by quantitative real-time PCR. Planta 2013, 237, 873–889. [Google Scholar] [PubMed]
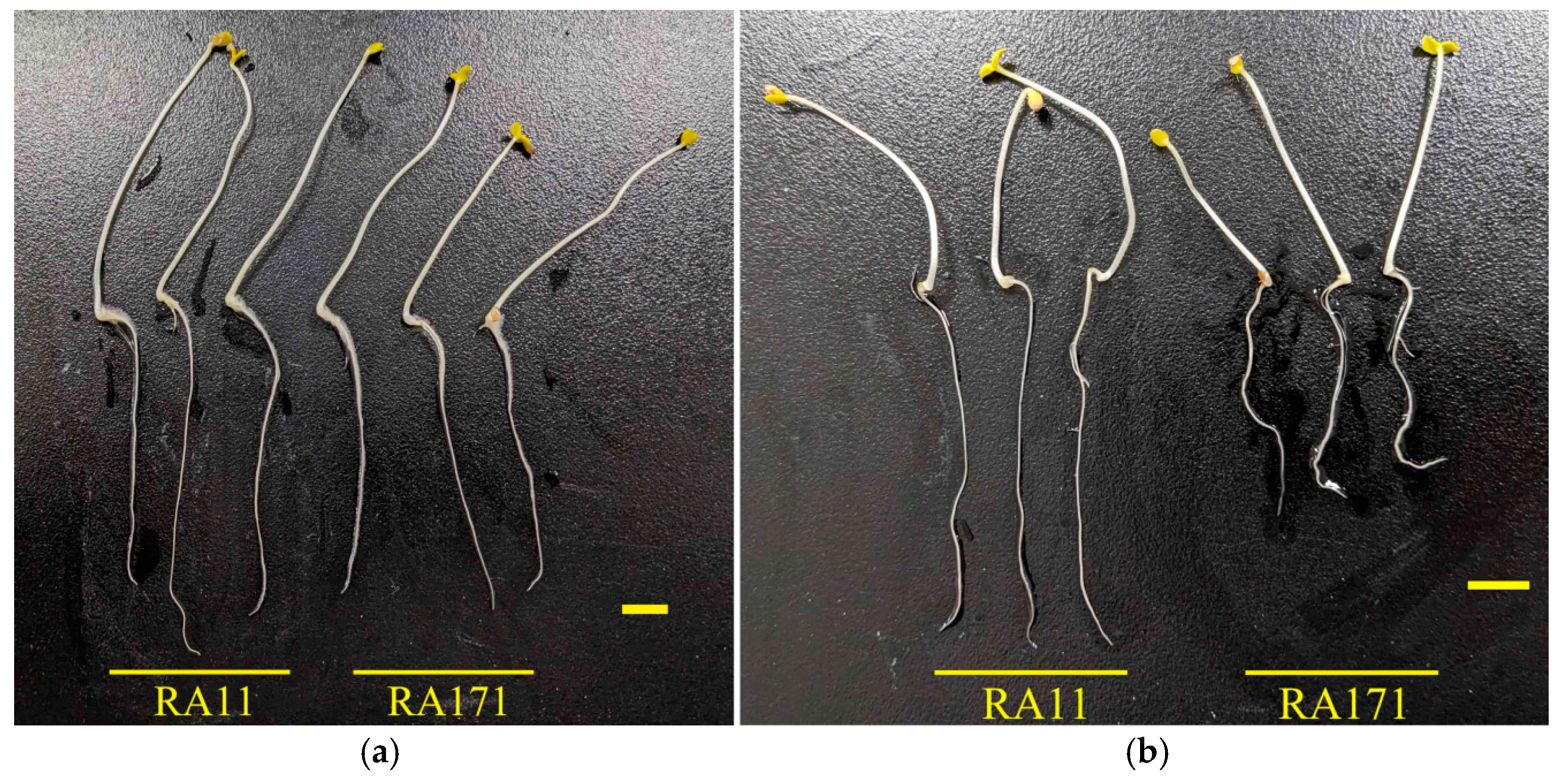
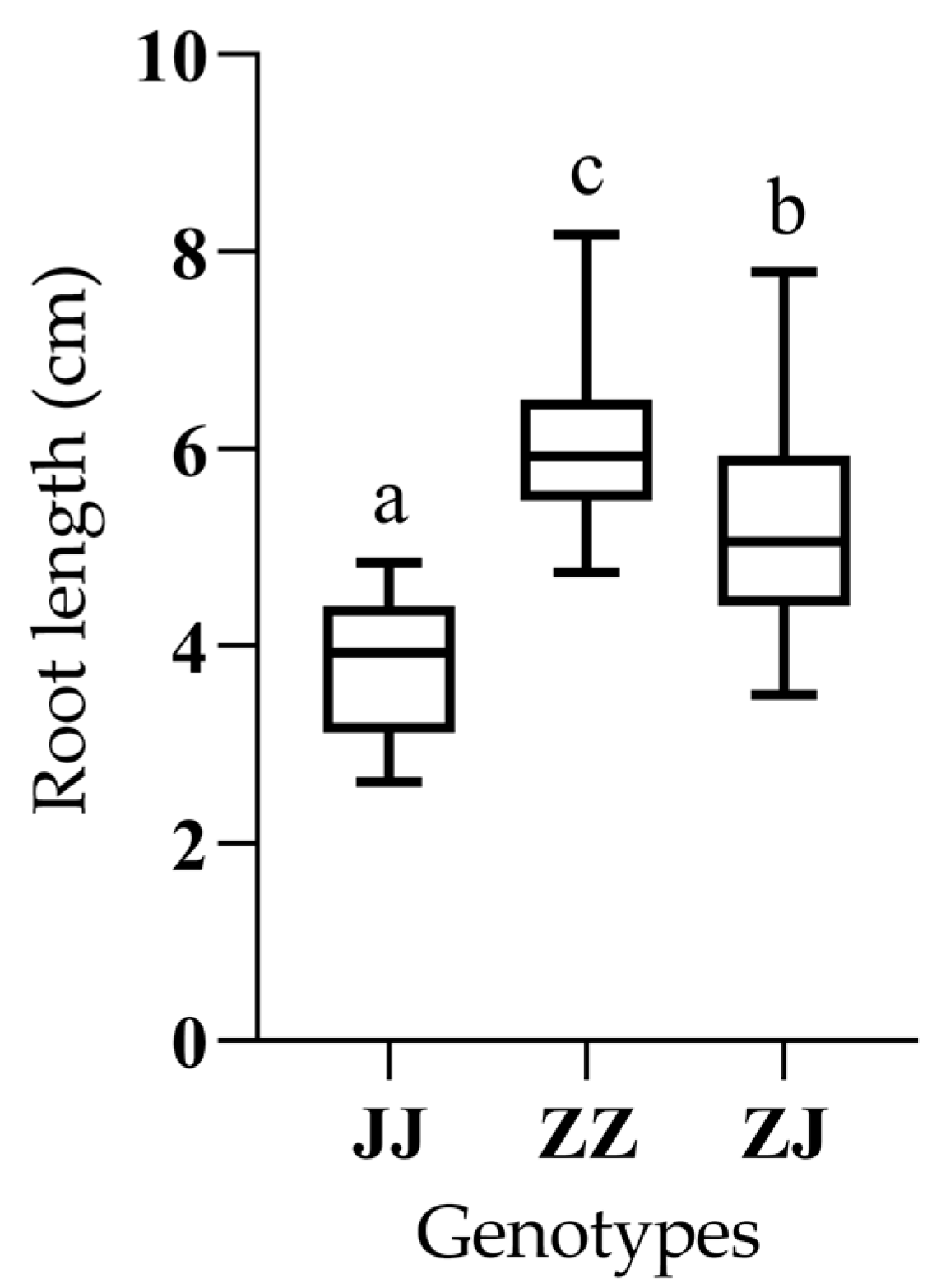

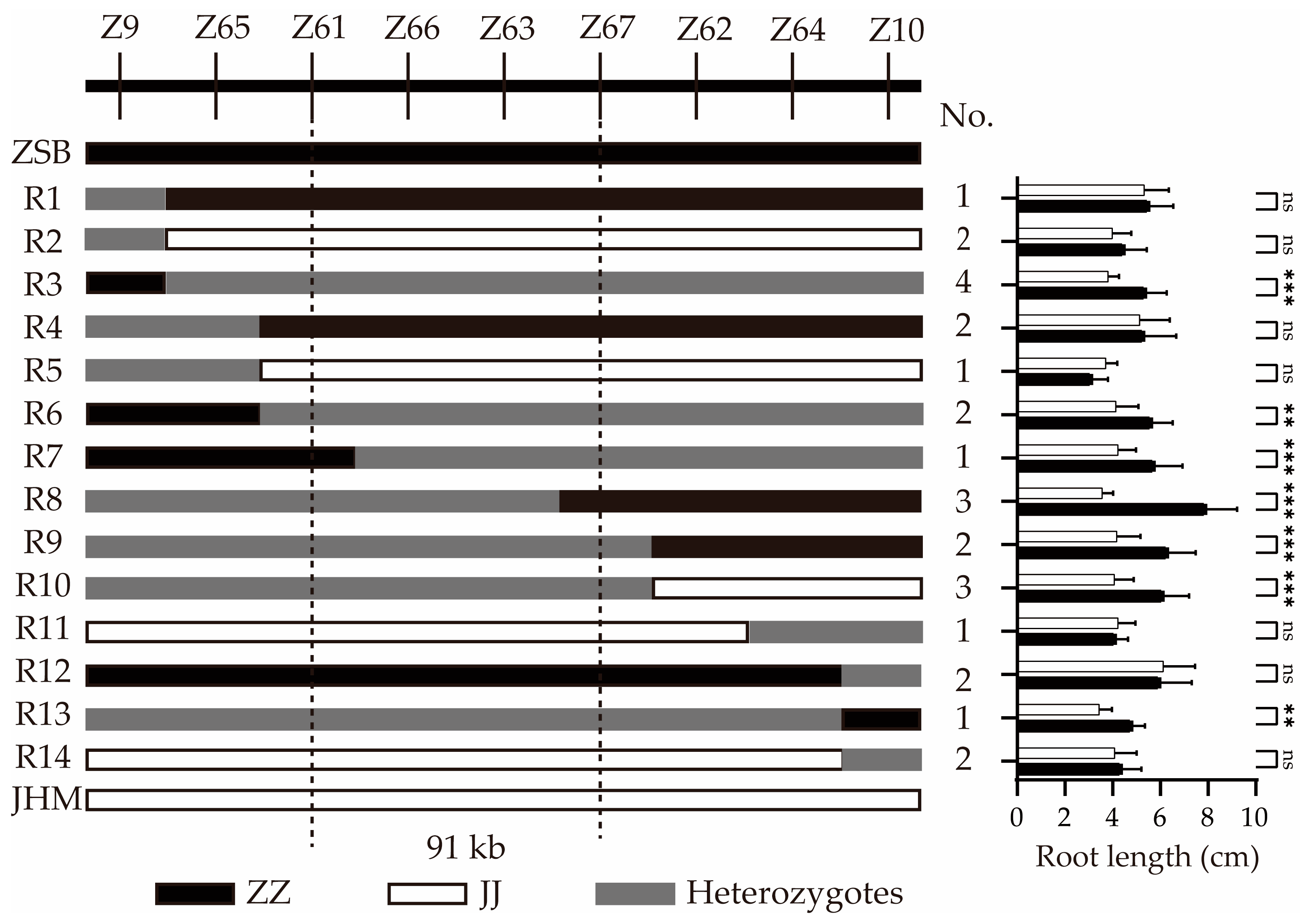
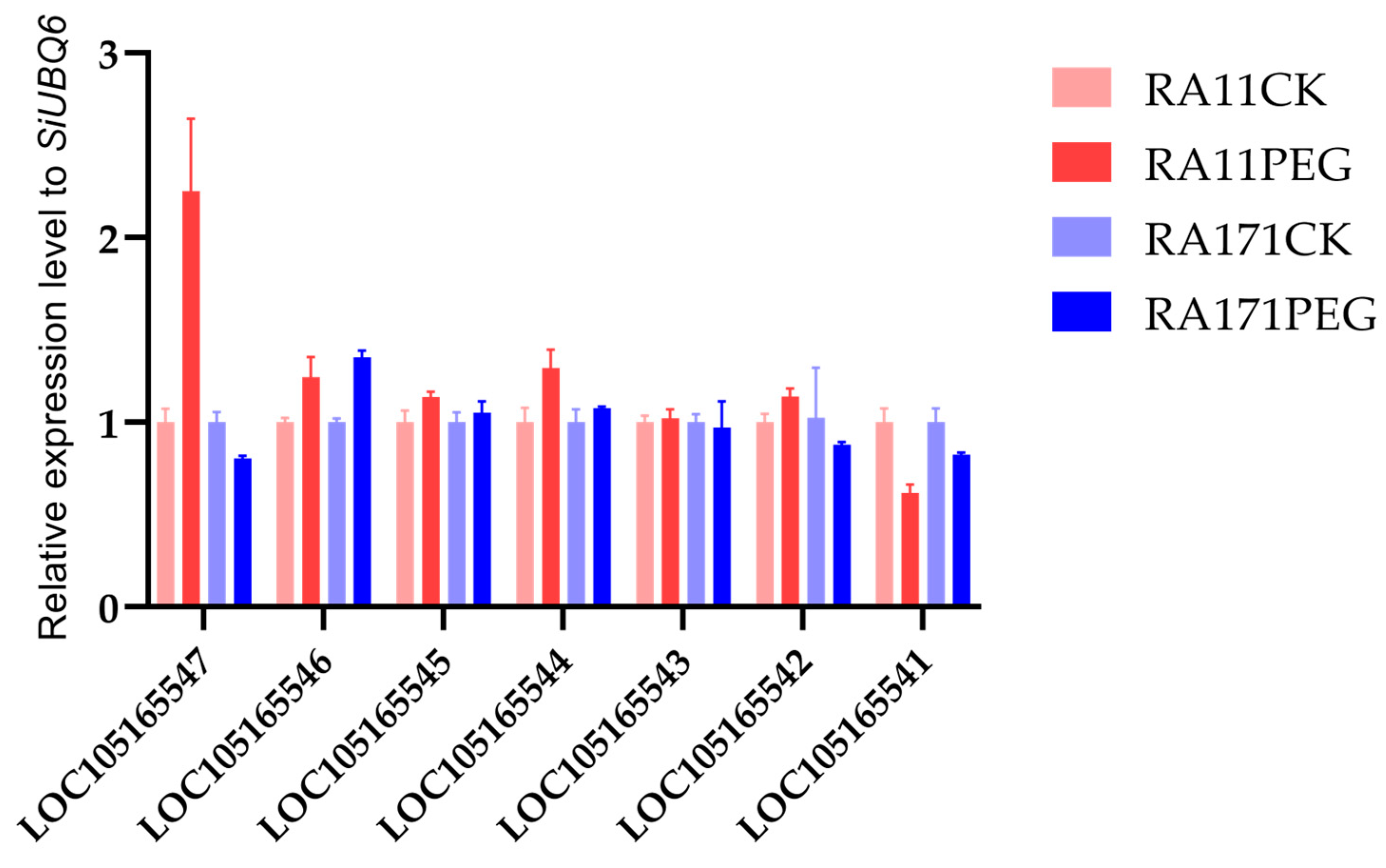
| Genotype 1 | No. 2 | RLP 3 (cm) |
|---|---|---|
| JJ | 23 | 3.80 ± 0.68 a |
| ZJ | 56 | 5.26 ± 1.05 b |
| ZZ | 27 | 6.05 ± 0.87 c |
| Gene ID 1 | Annotation | Variation 2 |
|---|---|---|
| LOC105165541 | subtilisin-like protease SBT5.3 | NA |
| LOC105165542 | heavy metal-associated isoprenylated plant protein 16-like | NA |
| LOC105165543 | 3-hydroxyisobutyryl-CoA hydrolase-like protein 5 | NA |
| LOC105165544 | protein VACUOLELESS1 | NA |
| LOC105165545 | GDSL esterase/lipase | NA |
| LOC105165546 | conserved oligomeric Golgi complex subunit 5 | NA |
| LOC105165547 | callose synthase 7 | Exon14: A1558G (Thr520Ala); Exon42: 5679indel35bp, 5684indel37bp; Exon42: T5696C (Val1910Ala) |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liang, J.; Deng, Y.; Yan, X.; Wang, Z.; Zeng, P.; Le, M.; Zhou, H.; Sun, J. Fine Mapping of the QTL qRLP12 That Controls Root Length Under Polyethylene glycol-Induced Drought Stress During the Early Seedling Stage of Sesame. Int. J. Mol. Sci. 2025, 26, 2886. https://doi.org/10.3390/ijms26072886
Liang J, Deng Y, Yan X, Wang Z, Zeng P, Le M, Zhou H, Sun J. Fine Mapping of the QTL qRLP12 That Controls Root Length Under Polyethylene glycol-Induced Drought Stress During the Early Seedling Stage of Sesame. International Journal of Molecular Sciences. 2025; 26(7):2886. https://doi.org/10.3390/ijms26072886
Chicago/Turabian StyleLiang, Junchao, Yanxin Deng, Xiaowen Yan, Zhiqi Wang, Pan Zeng, Meiwang Le, Hongying Zhou, and Jian Sun. 2025. "Fine Mapping of the QTL qRLP12 That Controls Root Length Under Polyethylene glycol-Induced Drought Stress During the Early Seedling Stage of Sesame" International Journal of Molecular Sciences 26, no. 7: 2886. https://doi.org/10.3390/ijms26072886
APA StyleLiang, J., Deng, Y., Yan, X., Wang, Z., Zeng, P., Le, M., Zhou, H., & Sun, J. (2025). Fine Mapping of the QTL qRLP12 That Controls Root Length Under Polyethylene glycol-Induced Drought Stress During the Early Seedling Stage of Sesame. International Journal of Molecular Sciences, 26(7), 2886. https://doi.org/10.3390/ijms26072886






