Exploring the Toxicity and Therapeutic Potential of A. dahurica and A. pubescens in Zebrafish Larvae: Insights into Anxiety Treatment Mechanisms
Abstract
1. Introduction
2. Results
2.1. Chemical Profile of Methanolic Extracts from A. dahurica and Angelica pubescens
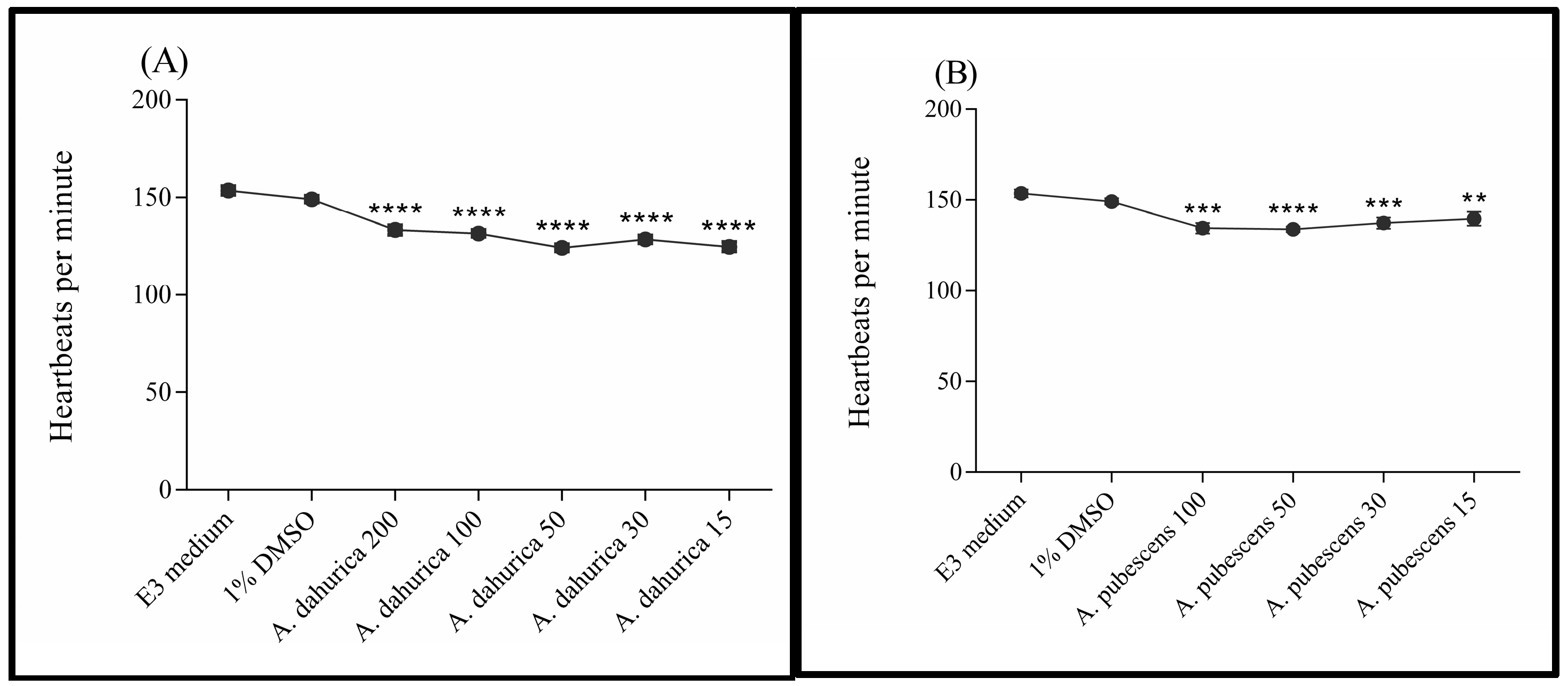
2.2. Toxicity Studies
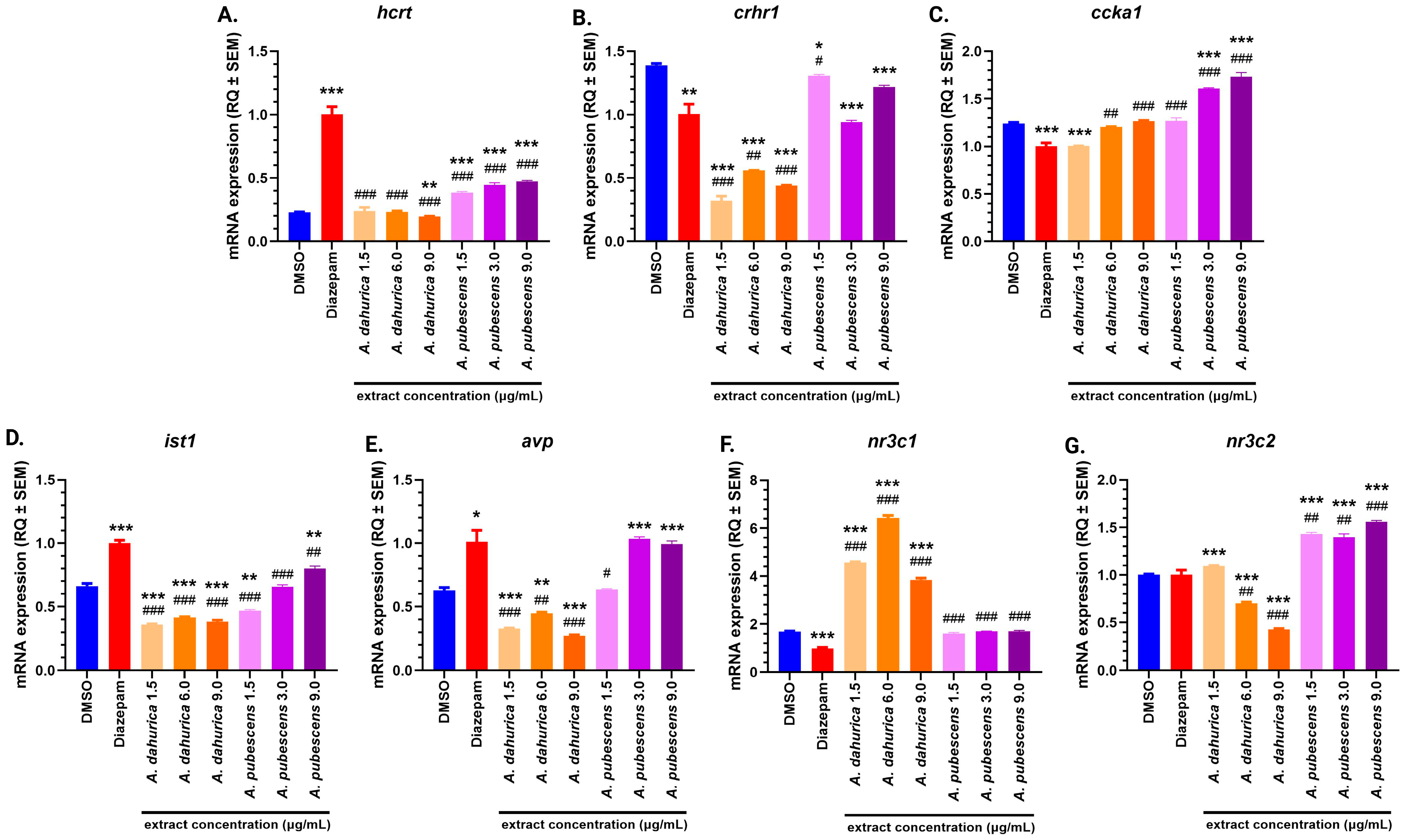
2.3. Molecular Studies
3. Discussion
3.1. Toxicity Studies
3.2. Hcrt Gene Expression and Its Behavioral Implications
3.3. Modulation of crhr1 Gene Expression and Anxiety
3.4. Ccka Gene Expression and Dose-Dependent Effects
3.5. Ist1 Gene Expression and Cognitive Implications
3.6. Avp Gene Expression and Stress Regulation
3.7. Nr3c1 and nr3c2 Genes Expression: Insights into HPA Axis Regulation
4. Materials and Methods
4.1. Plant Material and Extraction
4.2. HPLC/ESI-QTOF-MS
4.3. Animals
4.3.1. Toxicity Studies
4.3.2. Behavioral and Molecular Studies
4.3.3. Experimental Design
4.4. Real-Time Polymerase Chain Reaction (qPCR)
4.5. Statistical Analysis
5. Conclusions
Future Directions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Wu, Y.; Li, X.; Ji, X.; Ren, W.; Zhu, Y.; Chen, Z.; Du, X. Trends in the Epidemiology of Anxiety Disorders from 1990 to 2021: A Global, Regional, and National Analysis with a Focus on the Sociodemographic Index. J. Affect. Disord. 2024, 373, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Bandelow, B.; Michaelis, S. Epidemiology of Anxiety Disorders in the 21st Century. Dialogues Clin. Neurosci. 2015, 17, 327–335. [Google Scholar] [CrossRef] [PubMed]
- Massoni, L. Epigenetic and Mental Diseases: The Role of Psychotherapy. Int. J. Transl. Med. 2024, 4, 450–462. [Google Scholar] [CrossRef]
- Garakani, A.; Murrough, J.W.; Freire, R.C.; Thom, R.P.; Larkin, K.; Buono, F.D.; Iosifescu, D.V. Pharmacotherapy of Anxiety Disorders: Current and Emerging Treatment Options. Front. Psychiatry 2020, 11, 595584. [Google Scholar] [CrossRef]
- Sanabria, E.; Cuenca, R.E.; Esteso, M.Á.; Maldonado, M. Benzodiazepines: Their Use Either as Essential Medicines or as Toxics Substances. Toxics 2021, 9, 25. [Google Scholar] [CrossRef]
- Strawn, J.R.; Geracioti, L.; Rajdev, N.; Clemenza, K.; Levine, A. Pharmacotherapy for Generalized Anxiety Disorder in Adult and Pediatric Patients: An Evidence-Based Treatment Review. Expert. Opin. Pharmacother. 2018, 19, 1057–1070. [Google Scholar] [CrossRef]
- Bu, F.; Steptoe, A.; Fancourt, D. Depressive and Anxiety Symptoms in Adults during the COVID-19 Pandemic in England: A Panel Data Analysis over 2 Years. PLoS Med. 2023, 20, e1004144. [Google Scholar] [CrossRef]
- Kalin, N.H. The Critical Relationship Between Anxiety and Depression. Am. J. Psychiatry 2020, 177, 365–367. [Google Scholar] [CrossRef]
- Kessler, R.C.; Sampson, N.A.; Berglund, P.; Gruber, M.J.; Al-Hamzawi, A.; Andrade, L.; Bunting, B.; Demyttenaere, K.; Florescu, S.; de Girolamo, G.; et al. Anxious and Non-Anxious Major Depressive Disorder in the World Health Organization World Mental Health Surveys. Epidemiol. Psychiatr. Sci. 2015, 24, 210–226. [Google Scholar] [CrossRef]
- Hall, S.; Parr, B.-A.; Hussey, S.; Anoopkumar-Dukie, S.; Arora, D.; Grant, G.D. The Neurodegenerative Hypothesis of Depression and the Influence of Antidepressant Medications. Eur. J. Pharmacol. 2024, 983, 176967. [Google Scholar] [CrossRef]
- Chen, D.; Du, Z.; Lin, Z.; Su, P.; Huang, H.; Ou, Z.; Pan, W.; Huang, S.; Zhang, K.; Zheng, X.; et al. The Chemical Compositions of Angelica Pubescens Oil and Its Prevention of UV-B Radiation-Induced Cutaneous Photoaging. Chem. Biodivers. 2018, 15, e1800235. [Google Scholar] [CrossRef]
- Baek, S.C.; Kang, M.-G.; Park, J.-E.; Lee, J.P.; Lee, H.; Ryu, H.W.; Park, C.M.; Park, D.; Cho, M.-L.; Oh, S.-R.; et al. Osthenol, a Prenylated Coumarin, as a Monoamine Oxidase A Inhibitor with High Selectivity. Bioorg. Med. Chem. Lett. 2019, 29, 839–843. [Google Scholar] [CrossRef]
- Song, F.; Xie, M.-L.; Zhu, L.-J.; Zhang, K.-P.; Xue, J.; Gu, Z.-L. Experimental Study of Osthole on Treatment of Hyperlipidemic and Alcoholic Fatty Liver in Animals. World J. Gastroenterol. 2006, 12, 4359–4363. [Google Scholar] [CrossRef] [PubMed]
- Du, M.; Sun, Z.; Lu, Y.; Li, Y.-Z.; Xu, H.-R.; Zeng, C.-Q. Osthole Inhibits Proliferation and Induces Apoptosis in BV-2 Microglia Cells in Kainic Acid-Induced Epilepsy via Modulating PI3K/AKt/MTOR Signalling Way. Pharm. Biol. 2019, 57, 238–244. [Google Scholar] [CrossRef]
- Knöbel, M.; Busser, F.J.M.; Rico-Rico, A.; Kramer, N.I.; Hermens, J.L.M.; Hafner, C.; Tanneberger, K.; Schirmer, K.; Scholz, S. Predicting Adult Fish Acute Lethality with the Zebrafish Embryo: Relevance of Test Duration, Endpoints, Compound Properties, and Exposure Concentration Analysis. Environ. Sci. Technol. 2012, 46, 9690–9700. [Google Scholar] [CrossRef]
- Guo, J.; Hu, Z.; Yan, F.; Lei, S.; Li, T.; Li, X.; Xu, C.; Sun, B.; Pan, C.; Chen, L. Angelica Dahurica Promoted Angiogenesis and Accelerated Wound Healing in Db/Db Mice via the HIF-1α/PDGF-β Signaling Pathway. Free Radic. Biol. Med. 2020, 160, 447–457. [Google Scholar] [CrossRef]
- Cao, Y.; Liu, J.; Wang, Q.; Liu, M.; Cheng, Y.; Zhang, X.; Lin, T.; Zhu, Z. Antidepressive-like Effect of Imperatorin from Angelica Dahurica in Prenatally Stressed Offspring Rats through 5-Hydroxytryptamine System. Neuroreport 2017, 28, 426–433. [Google Scholar] [CrossRef] [PubMed]
- Fontana, B.D.; Gibbon, A.J.; Cleal, M.; Sudwarts, A.; Pritchett, D.; Miletto Petrazzini, M.E.; Brennan, C.H.; Parker, M.O. Moderate Early Life Stress Improves Adult Zebrafish (Danio Rerio) Working Memory but Does Not Affect Social and Anxiety-like Responses. Dev. Psychobiol. 2021, 63, 54–64. [Google Scholar] [CrossRef]
- Singh, R.; Biswas, D.A. Physiological Role of Orexin/Hypocretin in the Human Body in Motivated Behavior: A Comprehensive Review. Cureus 2023, 15, e34009. [Google Scholar] [CrossRef]
- Dyachuk, V. The Role and Mechanisms of the Hypocretin System in Zebrafish (Danio Rerio). Int. J. Mol. Sci. 2024, 26, 256. [Google Scholar] [CrossRef]
- Hernández-Díaz, Y.; Genis-Mendoza, A.D.; González-Castro, T.B.; Tovilla-Zárate, C.A.; Juárez-Rojop, I.E.; López-Narváez, M.L.; Nicolini, H. Association and Genetic Expression between Genes Involved in HPA Axis and Suicide Behavior: A Systematic Review. Genes 2021, 12, 1608. [Google Scholar] [CrossRef] [PubMed]
- Watkeys, O.J.; Kremerskothen, K.; Quidé, Y.; Fullerton, J.M.; Green, M.J. Glucocorticoid Receptor Gene (NR3C1) DNA Methylation in Association with Trauma, Psychopathology, Transcript Expression, or Genotypic Variation: A Systematic Review. Neurosci. Biobehav. Rev. 2018, 95, 85–122. [Google Scholar] [CrossRef] [PubMed]
- Rotzinger, S.; Vaccarino, F.J. Cholecystokinin Receptor Subtypes: Role in the Modulation of Anxiety-Related and Reward-Related Behaviours in Animal Models. J. Psychiatry Neurosci. 2003, 28, 171–181. [Google Scholar] [PubMed]
- Rigney, N.; de Vries, G.J.; Petrulis, A. Modulation of Social Behavior by Distinct Vasopressin Sources. Front. Endocrinol. 2023, 14, 1127792. [Google Scholar] [CrossRef]
- Lu, X.; Yuan, Z.-Y.; Yan, X.-J.; Lei, F.; Jiang, J.-F.; Yu, X.; Yang, X.-W.; Xing, D.-M.; Du, L.-J. Effects of Angelica Dahurica on Obesity and Fatty Liver in Mice. Chin. J. Nat. Med. 2016, 14, 641–652. [Google Scholar] [CrossRef]
- Luo, L.; Sun, T.; Yang, L.; Liu, A.; Liu, Q.; Tian, Q.; Wang, Y.; Zhao, M.; Yang, Q. Scopoletin Ameliorates Anxiety-like Behaviors in Complete Freund’s Adjuvant-Induced Mouse Model. Mol. Brain 2020, 13, 15. [Google Scholar] [CrossRef]
- Chen, K.; Wu, M.; Chen, C.; Xu, H.; Wu, X.; Qiu, X. Impacts of Chronic Exposure to Sublethal Diazepam on Behavioral Traits of Female and Male Zebrafish (Danio Rerio). Ecotoxicol. Environ. Saf. 2021, 208, 111747. [Google Scholar] [CrossRef]
- Hill, A.J.; Teraoka, H.; Heideman, W.; Peterson, R.E. Zebrafish as a Model Vertebrate for Investigating Chemical Toxicity. Toxicol. Sci. 2005, 86, 6–19. [Google Scholar] [CrossRef]
- Kinth, P.; Mahesh, G.; Panwar, Y. Mapping of Zebrafish Research: A Global Outlook. Zebrafish 2013, 10, 510–517. [Google Scholar] [CrossRef]
- Chahardehi, A.M.; Arsad, H.; Lim, V. Zebrafish as a Successful Animal Model for Screening Toxicity of Medicinal Plants. Plants 2020, 9, 1345. [Google Scholar] [CrossRef]
- Yang, L.; Hou, A.; Wang, S.; Zhang, J.; Man, W.; Guo, X.; Yang, B.; Jiang, H.; Kuang, H.; Wang, Q. A Review of the Botany, Traditional Use, Phytochemistry, Analytical Methods, Pharmacological Effects, and Toxicity of Angelicae Pubescentis Radix. Evid. Based Complement. Altern. Med. 2020, 2020, 7460781. [Google Scholar] [CrossRef]
- OECD. Test No. 236: Fish Embryo Acute Toxicity (FET) Test; OECD Guidelines for the Testing of Chemicals, Section 2; OECD: Paris, France, 2013; ISBN 978-92-64-20370-9. [Google Scholar]
- Sánchez-García, A.; Cabral-Pacheco, G.A.; Zomosa-Signoret, V.C.; Ortiz-López, R.; Camacho, A.; Tabera-Tarello, P.M.; Garnica-López, J.A.; Vidaltamayo, R. Modular Organization of a Hypocretin Gene Minimal Promoter. Mol. Med. Rep. 2018, 17, 2263–2270. [Google Scholar] [CrossRef] [PubMed]
- Schmeichel, B.E.; Matzeu, A.; Koebel, P.; Vendruscolo, L.F.; Sidhu, H.; Shahryari, R.; Kieffer, B.L.; Koob, G.F.; Martin-Fardon, R.; Contet, C. Knockdown of Hypocretin Attenuates Extended Access of Cocaine Self-Administration in Rats. Neuropsychopharmacology 2018, 43, 2373–2382. [Google Scholar] [CrossRef]
- Panula, P. Hypocretin/Orexin in Fish Physiology with Emphasis on Zebrafish. Acta Physiol. 2010, 198, 381–386. [Google Scholar] [CrossRef]
- Elbaz, I.; Yelin-Bekerman, L.; Nicenboim, J.; Vatine, G.; Appelbaum, L. Genetic Ablation of Hypocretin Neurons Alters Behavioral State Transitions in Zebrafish. J. Neurosci. 2012, 32, 12961–12972. [Google Scholar] [CrossRef]
- Seigneur, E.; de Lecea, L. Hypocretin (Orexin) Replacement Therapies. Med. Drug Discov. 2020, 8, 100070. [Google Scholar] [CrossRef]
- Scammell, T.E.; Winrow, C.J. Orexin Receptors: Pharmacology and Therapeutic Opportunities. Annu. Rev. Pharmacol. Toxicol. 2011, 51, 243–266. [Google Scholar] [CrossRef]
- Ten-Blanco, M.; Flores, Á.; Cristino, L.; Pereda-Pérez, I.; Berrendero, F. Targeting the Orexin/Hypocretin System for the Treatment of Neuropsychiatric and Neurodegenerative Diseases: From Animal to Clinical Studies. Front. Neuroendocrinol. 2023, 69, 101066. [Google Scholar] [CrossRef]
- Lee, H.B.; Schwab, T.L.; Sigafoos, A.N.; Gauerke, J.L.; Krug, R.G.; Serres, M.R.; Jacobs, D.C.; Cotter, R.P.; Das, B.; Petersen, M.O.; et al. Novel Zebrafish Behavioral Assay to Identify Modifiers of the Rapid, Nongenomic Stress Response. Genes Brain Behav. 2019, 18, e12549. [Google Scholar] [CrossRef]
- Soya, S.; Sakurai, T. Orexin as a Modulator of Fear-Related Behavior: Hypothalamic Control of Noradrenaline Circuit. Brain Res. 2020, 1731, 146037. [Google Scholar] [CrossRef]
- Sotnikov, S.V.; Chekmareva, N.Y.; Schmid, B.; Harbich, D.; Malik, V.; Bauer, S.; Kuehne, C.; Markt, P.O.; Deussing, J.M.; Schmidt, M.V.; et al. Enriched Environment Impacts Trimethylthiazoline-Induced Anxiety-Related Behavior and Immediate Early Gene Expression: Critical Role of Crhr1. Eur. J. Neurosci. 2014, 40, 2691–2700. [Google Scholar] [CrossRef] [PubMed]
- Savarese, A.; Lasek, A.W. Regulation of Anxiety-like Behavior and Crhr1 Expression in the Basolateral Amygdala by LMO3. Psychoneuroendocrinology 2018, 92, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Mehrhoff, E.A.; Booher, W.C.; Hutchinson, J.; Schumacher, G.; Borski, C.; Lowry, C.A.; Hoeffer, C.A.; Ehringer, M.A. Diazepam Effects on Anxiety-Related Defensive Behavior of Male and Female High and Low Open-Field Activity Inbred Mouse Strains. Physiol. Behav. 2023, 271, 114343. [Google Scholar] [CrossRef] [PubMed]
- Schroeder, M.; Weller, A. Anxiety-like Behavior and Locomotion in CCK1 Knockout Rats as a Function of Strain, Sex and Early Maternal Environment. Behav. Brain Res. 2010, 211, 198–207. [Google Scholar] [CrossRef]
- Wilson, J.; Markie, D.; Fitches, A. Cholecystokinin System Genes: Associations with Panic and Other Psychiatric Disorders. J. Affect. Disord. 2012, 136, 902–908. [Google Scholar] [CrossRef]
- Zwanzger, P.; Eser, D.; Aicher, S.; Schüle, C.; Baghai, T.C.; Padberg, F.; Ella, R.; Möller, H.-J.; Rupprecht, R. Effects of Alprazolam on Cholecystokinin-Tetrapeptide-Induced Panic and Hypothalamic–Pituitary–Adrenal-Axis Activity: A Placebo-Controlled Study. Neuropsychopharmacology 2003, 28, 979–984. [Google Scholar] [CrossRef]
- Rehfeld, J.F. Cholecystokinin and Panic Disorder: Reflections on the History and Some Unsolved Questions. Molecules 2021, 26, 5657. [Google Scholar] [CrossRef]
- Feng, Q.; Luo, Y.; Zhang, X.-N.; Yang, X.-F.; Hong, X.-Y.; Sun, D.-S.; Li, X.-C.; Hu, Y.; Li, X.-G.; Zhang, J.-F.; et al. MAPT/Tau Accumulation Represses Autophagy Flux by Disrupting IST1-Regulated ESCRT-III Complex Formation: A Vicious Cycle in Alzheimer Neurodegeneration. Autophagy 2020, 16, 641–658. [Google Scholar] [CrossRef]
- Frankel, E.B.; Shankar, R.; Moresco, J.J.; Yates, J.R.; Volkmann, N.; Audhya, A. Ist1 Regulates ESCRT-III Assembly and Function during Multivesicular Endosome Biogenesis in Caenorhabditis Elegans Embryos. Nat. Commun. 2017, 8, 1439. [Google Scholar] [CrossRef]
- Clippinger, A.K.; Naismith, T.V.; Yoo, W.; Jansen, S.; Kast, D.J.; Hanson, P.I. IST1 Regulates Select Recycling Pathways. Traffic 2024, 25, e12921. [Google Scholar] [CrossRef]
- Bajorek, M.; Morita, E.; Skalicky, J.J.; Morham, S.G.; Babst, M.; Sundquist, W.I. Biochemical Analyses of Human IST1 and Its Function in Cytokinesis. Mol. Biol. Cell 2009, 20, 1360–1373. [Google Scholar] [CrossRef] [PubMed]
- Landgraf, R. The Involvement of the Vasopressin System in Stress-Related Disorders. CNS Neurol. Disord. Drug Targets 2006, 5, 167–179. [Google Scholar] [CrossRef]
- Török, B.; Fazekas, C.L.; Szabó, A.; Zelena, D. Epigenetic Modulation of Vasopressin Expression in Health and Disease. Int. J. Mol. Sci. 2021, 22, 9415. [Google Scholar] [CrossRef] [PubMed]
- Barchiesi, R.; Chanthongdee, K.; Domi, E.; Gobbo, F.; Coppola, A.; Asratian, A.; Toivainen, S.; Holm, L.; Augier, G.; Xu, L.; et al. Stress-Induced Escalation of Alcohol Self-Administration, Anxiety-like Behavior, and Elevated Amygdala Avp Expression in a Susceptible Subpopulation of Rats. Addict. Biol. 2021, 26, e13009. [Google Scholar] [CrossRef] [PubMed]
- Zhao, H.; Feng, Y.-L.; Wang, M.; Wang, J.-J.; Liu, T.; Yu, J. The Angelica Dahurica: A Review of Traditional Uses, Phytochemistry and Pharmacology. Front. Pharmacol. 2022, 13, 896637. [Google Scholar] [CrossRef]
- Phootha, N.; Yongparnichkul, N.; Fang, Z.; Gan, R.-Y.; Zhang, P. Plants and Phytochemicals Potentials in Tackling Anxiety: A Systematic Review. Phytomedicine Plus 2022, 2, 100375. [Google Scholar] [CrossRef]
- Liu, P.Z.; Nusslock, R. How Stress Gets Under the Skin: Early Life Adversity and Glucocorticoid Receptor Epigenetic Regulation. Curr. Genom. 2018, 19, 653–664. [Google Scholar] [CrossRef]
- Cicchetti, D.; Handley, E.D. Methylation of the Glucocorticoid Receptor Gene, Nuclear Receptor Subfamily 3, Group C, Member 1 (NR3C1), in Maltreated and Nonmaltreated Children: Associations with Behavioral Undercontrol, Emotional Lability/Negativity, and Externalizing and Internalizing Symptoms. Dev. Psychopathol. 2017, 29, 1795–1806. [Google Scholar] [CrossRef]
- Raffetti, E.; Melas, P.A.; Landgren, A.J.; Andersson, F.; Forsell, Y.; Lavebratt, C.; Galanti, M.R. DNA Methylation of the Glucocorticoid Receptor Gene Predicts Substance Use in Adolescence: Longitudinal Data from over 1000 Young Individuals. Transl. Psychiatry 2021, 11, 477. [Google Scholar] [CrossRef]
- Lewis, C.R.; Tafur, J.; Spencer, S.; Green, J.M.; Harrison, C.; Kelmendi, B.; Rabin, D.M.; Yehuda, R.; Yazar-Klosinski, B.; Cahn, B.R. Pilot Study Suggests DNA Methylation of the Glucocorticoid Receptor Gene (NR3C1) Is Associated with MDMA-Assisted Therapy Treatment Response for Severe PTSD. Front. Psychiatry 2023, 14, 959590. [Google Scholar] [CrossRef]
- de Assis Pinheiro, J.; Freitas, F.V.; Borçoi, A.R.; Mendes, S.O.; Conti, C.L.; Arpini, J.K.; Dos Santos Vieira, T.; de Souza, R.A.; Dos Santos, D.P.; Barbosa, W.M.; et al. Alcohol Consumption, Depression, Overweight and Cortisol Levels as Determining Factors for NR3C1 Gene Methylation. Sci. Rep. 2021, 11, 6768. [Google Scholar] [CrossRef] [PubMed]
- Qing, L.; Gao, C.; Ji, A.; Lü, X.; Zhou, L.; Nie, S. Association of Mineralocorticoid Receptor Gene (NR3C2) Hypermethylation in Adult Males with Aggressive Behavior. Behav. Brain Res. 2021, 398, 112980. [Google Scholar] [CrossRef] [PubMed]
- Manuel, R.; Gorissen, M.; Piza Roca, C.; Zethof, J.; van de Vis, H.; Flik, G.; van den Bos, R. Inhibitory Avoidance Learning in Zebrafish (Danio Rerio): Effects of Shock Intensity and Unraveling Differences in Task Performance. Zebrafish 2014, 11, 341–352. [Google Scholar] [CrossRef]
- Galbally, M.; Watson, S.J.; van IJzendoorn, M.; Saffery, R.; Ryan, J.; de Kloet, E.R.; Oberlander, T.F.; Lappas, M.; Lewis, A.J. The Role of Glucocorticoid and Mineralocorticoid Receptor DNA Methylation in Antenatal Depression and Infant Stress Regulation. Psychoneuroendocrinology 2020, 115, 104611. [Google Scholar] [CrossRef]
- Chen, T.-H.; Wang, Y.-H.; Wu, Y.-H. Developmental Exposures to Ethanol or Dimethylsulfoxide at Low Concentrations Alter Locomotor Activity in Larval Zebrafish: Implications for Behavioral Toxicity Bioassays. Aquat. Toxicol. 2011, 102, 162–166. [Google Scholar] [CrossRef]
- Girardi, F.A.; Bruch, G.E.; Peixoto, C.S.; Dal Bosco, L.; Sahoo, S.K.; Gonçalves, C.O.F.; Santos, A.P.; Furtado, C.A.; Fantini, C.; Barros, D.M. Toxicity of Single-Wall Carbon Nanotubes Functionalized with Polyethylene Glycol in Zebrafish (Danio Rerio) Embryos. J. Appl. Toxicol. 2017, 37, 214–221. [Google Scholar] [CrossRef]
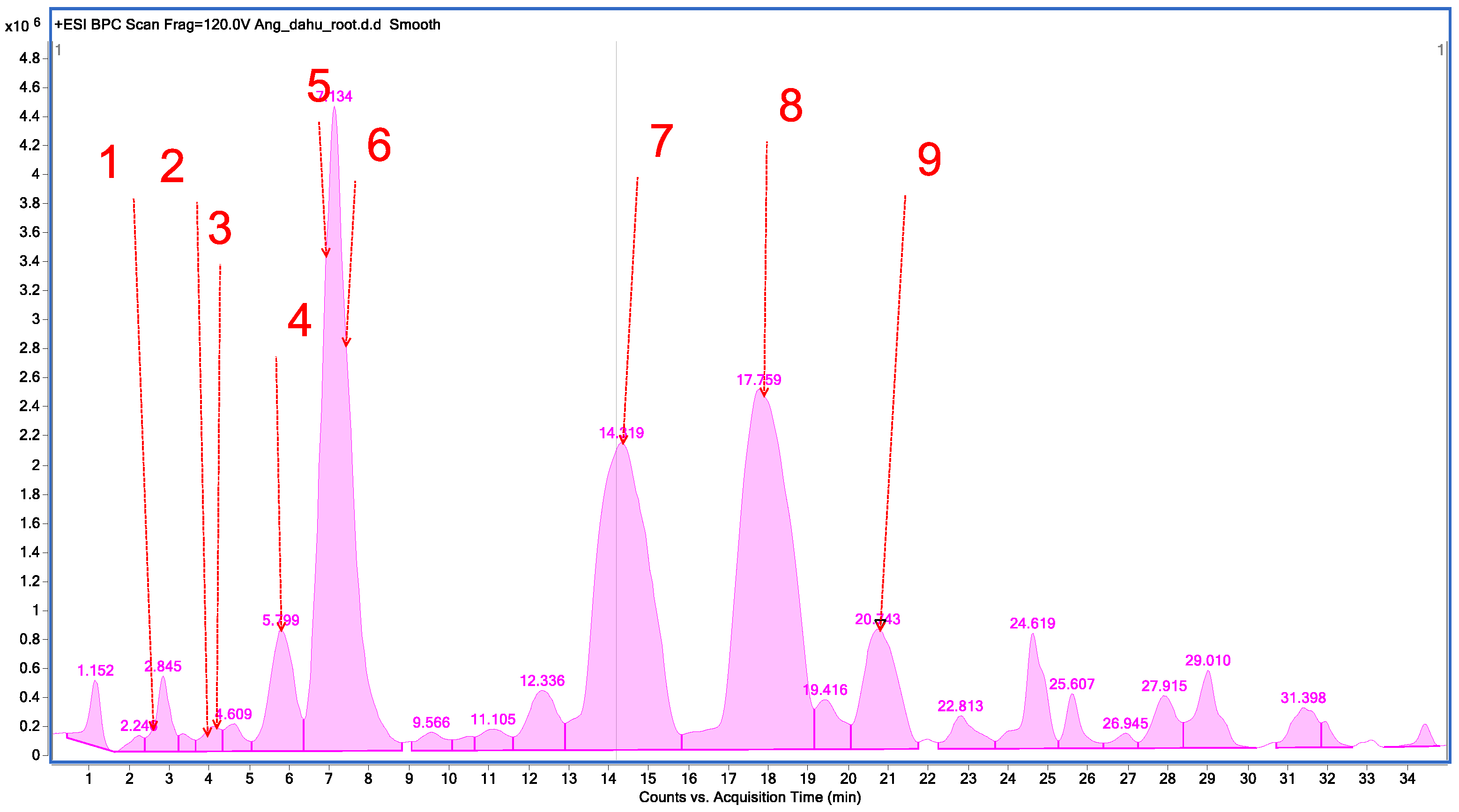
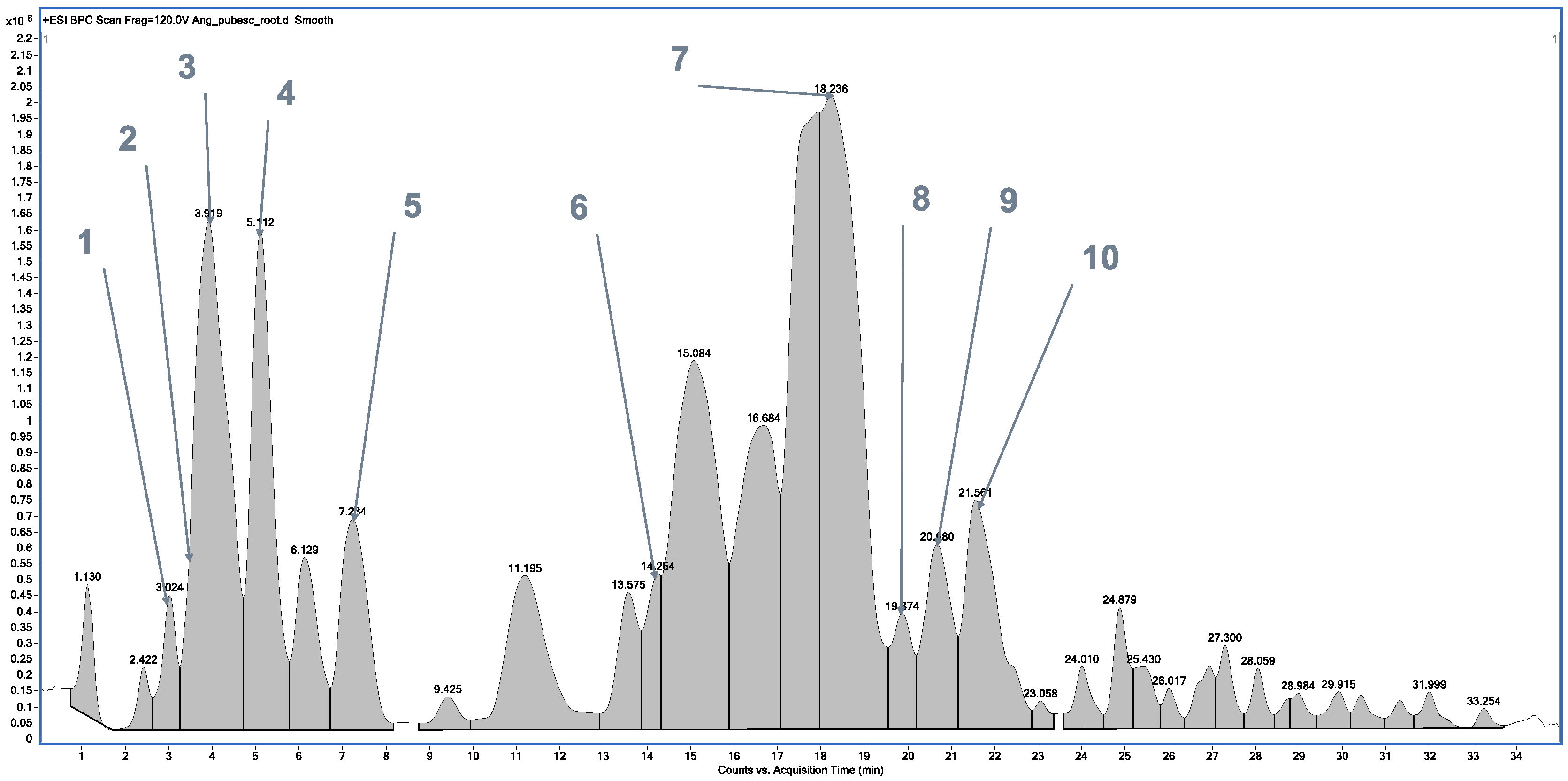
| No. | Tentatively Assignment | Formula | Retention Time | Molecular Ion [M + H]+ | Molecular Ion Sodium Adduct [M + Na]+ | Fragment Ion |
|---|---|---|---|---|---|---|
| 1. | Oxypeucedanin hydrate | C16H16O6 | 2.682 | 305.0920 | - | 203.0443; 175.0517; 159.0511; 147.0503; 131.0572. |
| 2. | Psoralene | C11H6O3 | 4.374 | 187.0323 | - | 143.0683; 131.0612; 115.0603; 103.0618. |
| 3. | 8-Methoxypsoralene | C12H8O4 | 4.483 | 217.0423 | - | 202.0372; 189.0630; 174.0414; 161.0688; 146.0434; 131.0556; 118.0470. |
| 4. | Oxypeucedanin | C16H14O5 | 5.871 | - | 309.0796 | 224.0125. |
| 5. | Byakangelicol | C17H16O6 | 6.885 | 317.0916 | - | 231.0265; 218.0191; 203.0316; 188.0104; 175.0383; 160.0143; 147.0389. |
| 6. | Heraclenin | C16H14O5 | 7.602 | 287.0828 | - | 203.0258; 175.0300; 159.0351; 147.0348; 131.0438. |
| 7. | Imperatorin | C16H16O4 | 14.866 | 271.0883 | - | 203.0291; 175.0370; 157.0256; 147.0437; 131.0457. |
| 8. | Phellopterin | C17H16O5 | 17.759 | 301.0978 | - | 233.0418; 218.0204; 190.0239; 162.0312. |
| 9. | Isoimperatorin | C16H16O4 | 20.743 | 271.0883 | - | 203.0317; 159.0429; 147.0424; 131.0495; 119.0464; 103.0535. |
| No. | Tentatively Assignment | Formula | Retention Time | Molecular Ion [M + H]+ | Molecular Ion Sodium Adduct [M + Na]+ | Fragment Ion |
|---|---|---|---|---|---|---|
| 1. | Nodakenetin | C14H14O4 | 2.871 | 247.0911 | - | 175.0259; 147.0333; 119.0492. |
| 2. | Auraptenol | C15H16O4 | 3.491 | 261.1100 | - | 243.0938; 213.0476; 185.0543; 131.0457; 103.0497. |
| 3. | Angelol G | C20H24O7 | 3.910 | 377.1552 | - | 277.1087; 259.0932; 231.0395; 219.0616; 205.0490; 191.0298; 175.0329; 160.0530. |
| 4. | 7-Methoxy-5-prenyloxycoumarin | C15H16O4 | 5.112 | - | 261.1042 | 189.0485; 131.0474; 103.0529. |
| 5. | Columbianetin acetate | C16H16O5 | 7.206 | 289.0978 | - | 229.0834; 187.0388; 175.0393; 159.0415; 147.0440; 131.0489. |
| 6. | Imperatorin | C16H16O4 | 14.087 | 271.0882 | - | 203.0339; 175.0350; 159.0396; 147.0416; 131.0444. |
| 7. | Osthol | C15H16O3 | 18.236 | 245.1096 | - | 189.0480; 131.0478; 103.0532. |
| 8. | Phellopterin | C17H16O5 | 19.882 | 301.0974 | - | 233.0317; 218.0089; 173.0150; 162.0222; 134.0329. |
| 9. | Isoimperatorin | C16H16O4 | 20.808 | 271.0882 | - | 203.0326; 159.0444; 147.0422; 131.0467; 119.0479; 103.0537. |
| Solution | 24 hpf | 48 hpf | 72 hpf | 96 hpf | |||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mortality | Mortality | Malformations | Hatching | Mortality | Malformations | Hatching | Mortality | Malformations | Hatching | ||
| E3 medium | 15% | 15% | 0 | 20.5% | 15% | 6% | 100% | 15% | 6% | 100% | |
| DMSO | 1% | 15% | 15% | 3% | 26.5% | 15% | 3% | 100% | 15% | 3% | 100% |
| A. dahurica extract | 200 µg/mL | 7.5% | 7.5% | 100% | 8% | 7.5% | 100% | 97% | 7.5% | 100% | 100% |
| 100 µg/mL | 22.5% | 22.5% | 100% | 9.5% | 22.5% | 100% | 96.5% | 22.5% | 100% | 100% | |
| 50 µg/mL | 5% | 5% | 47% | 10.5% | 5% | 100% | 100% | 5% | 100% | 100% | |
| 30 µg/mL | 15% | 15% | 0 | 11.5% | 15% | 67.5% | 94% | 15% | 67.5% | 100% | |
| 15 µg/mL | 15% | 15% | 0 | 11.5% | 15% | 3% | 94% | 15% | 3% | 100% | |
| A. pubescens extract | 200 µg/mL | 100% | 100% | - | - | 100% | - | - | 100% | - | - |
| 100 µg/mL | 22.5% | 22.5% | 100% | 32% | 27.5% | 100% | 93% | 75% | 100% | 100% | |
| 50 µg/mL | 7.5% | 7.5% | 100% | 54% | 10% | 100% | 100% | 10% | 100% | 100% | |
| 30 µg/mL | 12.5% | 12.5% | 71% | 60% | 15% | 73.5% | 100% | 15% | 73.5% | 100% | |
| 15 µg/mL | 17.5% | 17.5% | 0 | 60.5% | 20% | 0 | 100% | 20% | 0 | 100% | |
| 1. DMSO control | 100 µL DMSO + 9900 µL E3; 1.5 mL of 1% DMSO to each well |
| 2. Diazepam control | 5.7 µL diazepam + 100 µL DMSO + 9899.3 µL E3; 1.5 mL of solution to each well |
| 3. A. dahurica 1.5 µg/mL | 0.9 µL A. dahurica + 119.1 µL DMSO + 11,880 µL E3; 1.5 mL of solution to each well |
| 4. A. dahurica 6.0 µg/mL | 3.6 µL A. dahurica + 116.4 µL DMSO + 11,880 µL E3; 1.5 mL of solution to each well |
| 5. A. dahurica 9.0 µg/mL | 5.4 µL A. dahurica + 114.6 µL DMSO + 11,880 µL E3; 1.5 mL of solution to each well |
| 6. A. pubescens 1.5 µg/mL | 0.9 µL A. pubescens + 119.1 µL DMSO + 11,880 µL E3; 1.5 mL of solution to each well |
| 7. A. pubescens 6.0 µg/mL | 3.6 µL A. pubescens + 116.4 µL DMSO + 11,880 µL E3; 1.5 mL of solution to each well |
| 8. A. pubescens 9.0 µg/mL | 5.4 µL A. pubescens + 114.6 µL DMSO + 11,880 µL E3; 1.5 mL of solution to each well |
| Symbol of the Gene | Name of the Gene | Sequence 5′ -> 3′ | Amplicon Size (bp) | NCBI Reference Sequence | ACCESSION | |
|---|---|---|---|---|---|---|
| Forward | Reverse | |||||
| hcrt | hypocretin (orexin) neuropeptide precursor | GACGCAGAAACGACTCTTCC | GGCTTGATTCCGTGAGTTGT | 152 | NM_001077392.2 | NM_001077392 |
| ccka | cholecystokinin a | CCAGCTCTCTCTGCGTCTCT | GGTTTGGTCAGCAGGTTGAT | 217 | NM_001386383.1 | NM_001386383 XM_001346104 |
| crhr1 | Corticotropin-releasing hormone receptor 1 | CATAATTCGCCCTGCTGATT | GATGGAGGATGCGACTCATT | 197 | XM_691254.6 | XM_691254 |
| ist1 | IST1 factor associated with ESCRT-III | ACCTGAACACCAAAGGTTGC | GGAGCAGTGAAAGAGCAAGG | 237 | NM_212585.2 | NM_212585 XM_697451 |
| avp | arginine vasopressin | AGAGAGCTGCGCTGTAGACC | TTACAGTGATGTGGGGGACA | 157 | NM_178293.2 | NM_178293 |
| nr3c1 | nuclear receptor subfamily 3, group C, member 1 (glucocorticoid receptor) | TTCTACGTTGCTGACGATGC | CCGGTGTTCTCCTGTTTGAT | 239 | NM_001020711.3 | NM_001020711 XM_005173120 XM_696817 |
| nr3c2 | nuclear receptor subfamily 3, group C, member 2 | ATTGGGCCTAGTGCAAAATG | TCTCTGTTTGGCTCGGTCTT | 249 | NM_001100403.1 | NM_001100403 XM_685568 |
| eef1a1l1 | eukaryotic translation elongation factor 1 alpha 1, like 1 | GATGCACCACGAGTCTCTGA | TGACCTGAGCGTTGAAGTTG | 158 | NM_131263.1 | NM_131263 XM_001331218 |
| rpl8 | ribosomal protein L8 | GGAGCTCCTCTGGCTAAGGT | CAGGCTTCTCCTCCAGACAG | 199 | NM_200713.1 | NM_200713 |
| actb1 | actin, beta 1 | CTCTTCCAGCCTTCCTTCCT | CTTCTGCATACGGTCAGCAA | 165 | NM_131031.2 | NM_131031 |
| Sample | Concentration (µg RNA/mL) | A260/A280 |
|---|---|---|
| DMSO control | 788.4 | 1.875 |
| Diazepam control | 662.4 | 1.976 |
| A. dahurica 1.5 µg/mL | 718.4 | 2.000 |
| A. dahurica 6 µg/mL | 695.2 | 1.946 |
| A. dahurica 9 µg/mL | 842.4 | 1.892 |
| A. pubescens 1.5 µg/mL | 551.2 | 1.935 |
| A. pubescens 6 µg/mL | 629.6 | 1.931 |
| A. pubescens 9 µg/mL | 569.2 | 1.955 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2025 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Herbet, M.; Widelski, J.; Ostrowska-Leśko, M.; Serefko, A.; Wojtanowski, K.; Kurek, J.; Piątkowska-Chmiel, I. Exploring the Toxicity and Therapeutic Potential of A. dahurica and A. pubescens in Zebrafish Larvae: Insights into Anxiety Treatment Mechanisms. Int. J. Mol. Sci. 2025, 26, 2884. https://doi.org/10.3390/ijms26072884
Herbet M, Widelski J, Ostrowska-Leśko M, Serefko A, Wojtanowski K, Kurek J, Piątkowska-Chmiel I. Exploring the Toxicity and Therapeutic Potential of A. dahurica and A. pubescens in Zebrafish Larvae: Insights into Anxiety Treatment Mechanisms. International Journal of Molecular Sciences. 2025; 26(7):2884. https://doi.org/10.3390/ijms26072884
Chicago/Turabian StyleHerbet, Mariola, Jarosław Widelski, Marta Ostrowska-Leśko, Anna Serefko, Krzysztof Wojtanowski, Joanna Kurek, and Iwona Piątkowska-Chmiel. 2025. "Exploring the Toxicity and Therapeutic Potential of A. dahurica and A. pubescens in Zebrafish Larvae: Insights into Anxiety Treatment Mechanisms" International Journal of Molecular Sciences 26, no. 7: 2884. https://doi.org/10.3390/ijms26072884
APA StyleHerbet, M., Widelski, J., Ostrowska-Leśko, M., Serefko, A., Wojtanowski, K., Kurek, J., & Piątkowska-Chmiel, I. (2025). Exploring the Toxicity and Therapeutic Potential of A. dahurica and A. pubescens in Zebrafish Larvae: Insights into Anxiety Treatment Mechanisms. International Journal of Molecular Sciences, 26(7), 2884. https://doi.org/10.3390/ijms26072884







